Abstract
OBJECTIVE:
This random assignment experimental study examined the intersection of children’s coping and physiologic stress reactivity and recovery patterns in a sample of preadolescent boys and girls.
METHOD:
A sample of 82 fourth and fifth grade (Mage = 10.59 years old) child-parent dyads participated in the present study. Children participated in the Trier Social Stress Test (TSST-C) and were randomly assigned to one of two post-TSST-C experimental coping conditions; behavioral distraction and cognitive avoidance. Children’s characteristic ways of coping were examined as moderators of the effect of experimental coping condition on cortisol reactivity and recovery patterns.
RESULTS:
Multi-level modeling analyses indicated that children’s characteristic coping and experimental coping condition interacted to predict differential cortisol recovery patterns. Children who characteristically engaged in primary control engagement coping strategies were able to more quickly down-regulate salivary cortisol when primed to distract themselves than when primed to avoid and vice versa. The opposite pattern was true for characteristic disengagement coping in the context of coping condition, suggesting that regulatory fit between children’s characteristic ways of coping and cues from their coping environment may lead to more and less adaptive physiologic recovery profiles.
CONCLUSIONS:
This study provides some of the first evidence that coping “gets under the skin” and that children’s characteristic ways of coping may constrain or enhance a child’s ability to make use of environmental coping resources.
Keywords: coping, children, cortisol, stress, experiment
Development of the ability to regulate one’s own emotions, behaviors, cognitions, and physiology in the face of stress, threat or challenge is a critical task of childhood (Zimmer-Gembeck & Skinner, 2011). Successful coping can protect children from the negative effects of a wide array of stressful events and circumstances on physical and mental health (Compas, Connor-Smith, Saltzman, Thomsen, & Wadsworth, 2001). Conversely, inadequate or inappropriate coping abilities are implicated in current theories of the development of many forms of psychopathology (Boxer, Sloan-Power, Mercado, & Schappell, 2012). It also appears, however, that coping strategies are not universally adaptive or maladaptive, as the context in which coping occurs seems to impact its efficacy (Edlynn, Miller, Gaylord-Harden, & Richards, 2008; Wadsworth, 2015). An objective assessment of coping’s effectiveness, as it relates to the ability to down-regulate physiologic stress responses in the moment, is needed to fully explore both how and when children’s coping prevails and fails (Compas et al.). We currently know very little about which coping strategies are accompanied by successful physiologic regulation and situational coping cues or resources which facilitate or thwart their effectiveness. Child coping therefore remains a black box, stalling progress in understanding how to leverage these critical processes to better children’s health and well-being. The current study aims to examine preadolescents’ coping and coping-context fit using multiple levels of analysis, including reactivity and recovery acute stress physiology, self-reported characteristic coping, and experimentally manipulated coping context.
According to the Responses to Stress theoretical framework (RTS) (Connor-Smith, Compas, Wadsworth, Thomsen, & Saltzman, 2000), effortful coping responses which moderate the effects of psychosocial stress occur at the cognitive, behavioral, and physiologic levels. Few studies have integrated more than one of these levels of analysis to gain a more thorough and nuanced understanding of children’s stress-and-coping processes (for an exception, see Chen & Miller, 2012). Children’s biological stress reactivity and recovery can be indexed by measuring by-products of the body’s physiologic stress responses, especially the hypothalamic-pituitary-adrenal (HPA) axis (Hostinar & Gunnar, 2013). One of the mechanisms of HPA action is cortisol, which is secreted in response to significant stressful events.Cortisol is a reliable marker of physiologic stress activation in children. Atypical levels and patterning of cortisol have been implicated in a variety of stress-related physical and mental health disorders in children, including, for example, asthma and depression (McEwen, 1998). Following an acute stressor, cortisol levels eventually return to pre-stress levels, though there is considerable variability in how quickly children’s cortisol levels decline following a stressor.
Though much research has focused on reactivity to stress, it appears that the duration of post-stress recovery may be most critical for understanding risk for psychopathology. The available evidence suggests that a protracted recovery period following a stressful event is more strongly related to negative outcomes than is the magnitude of reactivity to the stressor (e.g., Brosschot & Thayer, 1998; Davidson, 2003; Javaras et al., 2012). Little is currently known about what determines the patterning or efficiency of child post-stress physiologic recovery. RTS theory (Connor-Smith et al., 2000) suggests that effortful coping strategies may drive physiologic down-regulation, at least in part. The “recovery period,” which captures the period from peak reactivity to return-to-baseline, is a time during which coping may be directly linked to the speed and efficiency of physiologic down-regulation. We reserve the term “reactivity” for the immediate response to threat, which is activated prior to conscious awareness and therefore prior to effortful coping. We propose that coping will be uniquely related to post-stress recovery physiologic patterning. This proposition remains largely theoretical because scant research has used mixed methods to investigate children’s coping, reactivity, and recovery profiles. As such, we also explore the extent to which coping is associated with reactivity phase physiologic patterning.
Certain types of effortful coping generally protect children from the negative effects of various minor and major stressors, including interpersonal conflict, victimization, and financial disruptions (Zimmer-Gembeck & Skinner, 2011), though there are important exceptions. Both primary and secondary control engagement coping have demonstrated positive effects for children facing stressors in multiple studies (Hall, Chipperfield, Perry, Ruthig, & Goetz, 2006; Jaser et al., 2008; Wadsworth & Santiago, 2008). Primary control coping entails direct efforts to manage a stressful situation or one’s reactions to it, and includes strategies such as problem solving and emotional expression. Secondary control coping involves adapting oneself to a stressful situation via both cognitive and behavioral strategies such as acceptance and distraction, respectively (Connor-Smith et al., 2000). Disengagement coping, which reflects efforts to cognitively and behaviorally avoid a stressor, is generally not found to buffer children from maladaptive stress responses. However, protective effects of disengagement coping have been observed in circumstances in which children’s objective control over stressors is extremely limited, such as dangerous living situations involving community violence (Edlynn, Miller, Gaylord-Harden, & Richards, 2008) and inter-parental conflict (O’Brien & Margolin, 1995). Despite evidence suggesting that primary and secondary control coping are effective in down-regulating stress responses (and that disengagement coping exacerbates the effects of stress) using non-physiologic measures, we lack evidence that engagement and disengagement coping operate differently at the physiologic level.
This lack of knowledge reflects, in part, significant limitations in our ability to assess coping in the moment, and in particular a dearth of experimental methods for examining coping. Most child coping research has relied on children’s self-reports (Compas, 2009), and to a lesser extent parent reports of child coping (Antal, Wysocki, Canas, Taylor, & Edney-White, 2011). Much of this research has assessed retrospective reports of coping, relied on children or their parents to define or quantify a stressor, or have had children report on coping “styles” irrespective of an anchoring stressful domain or event (Terranova, Boxer, & Morris, 2009). Findings are often difficult to compare across studies because neither the source of stress nor the opportunities for coping are standardized, thus limiting rigorous examination of coping contexts and physiologic correlates of different types of coping.
Experimental methods exist for reliably inducing stress in preadolescents in the laboratory, such as the TSST-C (Kirschbaum, Pirke, & Hellhammer, 1993). Variations in preadolescents’ cortisol levels in response to the TSST-C have been used to examine stress reactivity. We extend this methodology to examine the influence of two forms of coping on preadolescents’ cortisol levels following a stressor (i.e., recovery). First, to compare the effectiveness of two coping strategies, we randomly assigned preadolescents to one of two experimental coping conditions. After completing the TSST-C, children were taken to one of two rooms— 1) a behavioral distraction coping room full of toys, art supplies, and musical instruments where children were invited to play with the toys while they waited for the experimenter to return (hereafter referred to as the distraction condition), or 2) a cognitive avoidance coping room, which was empty except for a chair and table where children were instructed to try not to think about their performance while they waited for the experimenter (referred to as the avoidance condition). Saliva samples were taken before, during, and after the TSST-C and the coping period. Guided by RTS theory, we expected that preadolescents primed to behaviorally distract would exhibit more efficient cortisol recovery trajectories than preadolescents primed to cognitively avoid.
Preadolescents are not, however, blank coping slates when they enroll in this research. While children’s coping is not completely trait-like, there is cross-time and -situation consistency in their self-reports of coping (Donaldson, Prinstein, & Danovsky, 2000; Valiente, Eisenberg, Fabes, Spinrad, & Sulik, 2014; Vierhaus, Lohaus, & Ball, 2007). Children’s characteristic ways of managing stress should influence physiologic recovery patterning in their own right. However, the extent to which these learned predispositions to cope in certain ways match or “fit” with environmental coping cues and resources may enhance or restrict children’s ability to efficiently down-regulate stress physiology. For example, individuals who characteristically cope with a stressor by actively engaging with their environment (e.g., primary control) may have difficulty down-regulating stress physiology in circumstances which restrict the means for doing so; waiting idly in a boring room while their TSST-C performance is being judged (Martin & Tesser, 2006; Wrosch, Scheier, & Miller, 2013). Alternatively, children’s characteristic cognitive and behavioral avoidance (e.g., disengagement) may contribute to the consumption of executive resources needed to reallocate attention to engagement coping opportunities; being invited to behaviorally distract (Gotlib & Joorman, 2010, Levens, Muhtadie, & Gotlib, 2009). Thus, we additionally examined whether there are limits posed by regulatory fit that lead to individual differences in the ability to make use of different types of coping cues and resulting stress physiology.
We target the preadolescent period for several reasons. First, this is a critical age for the development of coping and self-regulation (Losoya, Eisenberg, & Fabes, 1998; Seiffge-Krenke, Aunola, & Nurmi, 2009). Preadolescents are just beginning to acquire the skills and abilities for metacognition, abstraction, and self-reflection (Windle et al., 2008). Their ability to recognize stressors and their causes matures, and repertoires for coping with stress grow in terms of size, flexibility, and complexity (Seiffge-Krenke et al., 2009). Due to their burgeoning cognitive sophistication, preadolescents become capable of reflecting on internal states of distress and well-being, learning increasingly complex coping such as secondary control (Losoya et al., 1998; Seiffge-Krenke et al., 2009), and benefitting from structured interventions to improve coping (Raviv & Wadsworth, 2010). Second, preadolescents are poised to enter a developmental period of increased demands, challenges, and stressors, during which they benefit from a wide and flexible array of coping and self-regulation strategies. Third, this period is critical to the development of the physiologic stress response system (Hostinar, Sullivan, & Gunnar, 2014), which shapes and is shaped by stress (Stroud, Papandonatos, Williamson, & Dahl, 2011).
The current study examined how different forms of coping (e.g., experimentally-manipulated, characteristic) influence physiologic reactivity and recovery patterns in a community-based sample of preadolescent boys and girls. We adopt an analytic approach conducive to testing two hypotheses about preadolescents’ coping experience following exposure to the TSST-C (i.e., recovery phase). As an exploratory aim, we also examined the influence of reactivity coping (i.e., children’s self-report of strategies engaged in during the TSST-C) and characteristic coping on reactivity phase cortisol growth trajectories. Hypothesis 1: As RTS theory suggests that coping should manifest in post-stressor physiology, we expected that preadolescents’ characteristic coping and randomly assigned experimental coping condition would predict HPA recovery patterns in particular ways. We expected quicker cortisol down-regulation for characteristic primary and secondary control coping compared to disengagement coping. Also, we expected quicker cortisol down-regulation for children in the distraction condition compared to the avoidance condition. We operationalized quicker down-regulation as a steeper cortisol recovery trajectory, modeled by a significant coping by linear or quadratic time interaction. Hypothesis 2: Next, to examine the limitations on coping posed by regulatory fit, we anticipated that preadolescents’ characteristic ways of coping would interact with experimental coping condition in predicting recovery phase stress physiology. Specifically, we expected that high levels of characteristic engagement coping (e.g., primary control) would enhance efficient cortisol recovery for children asked to behaviorally distract, but interfere with efficient cortisol recovery for children who were primed to avoid. Furthermore, we expected that high levels of characteristic disengagement coping would contribute to efficient cortisol recovery for children primed for cognitive avoidance and would interfere with the ability to make use of the behavioral distraction activities (i.e., less efficient cortisol recovery).
Method
Participants
Eighty-two fourth and fifth grade children (Mage = 10.59 years, SD = .79) and one of their parents were recruited from elementary schools in both a large metropolitan area in the western U.S. (n = 30) and a smaller metropolitan area in the northeastern U.S. (n = 52). Child gender for the sample was evenly distributed (45.1% male). Mothers (85.4%) tended to be the primary adult respondents. Median annual household income for the sample was $75,000. The majority of participants identified as White (96.3% of children and caregivers), followed by Asian (3% of both children and caregivers).
Procedure
Children brought home recruitment letters from school directing caregivers to the study website. First, caregivers enrolled their children and completed the online parent portion of the study. During this initial portion, parents provided consent to participate, completed online questionnaires, and submitted a request to be contacted by the research team in order to schedule the in-person child portion of the study. Subsequently, all child participants were scheduled for 1.5 hour sessions that took place in the afternoon between the hours of 3:00 pm and 5:30 pm. Upon arrival to the lab (western US) or neighborhood elementary school (northeastern US), each child was assigned an experimenter for the duration of the session and an initial saliva sample was obtained (T1). Children then completed pre-assessment measures for 40 minutes to allow cortisol levels to return to baseline, after which a second saliva sample was taken (T2). Next, children were taken to a separate room to administer the Trier Social Stress Test (TSST-C; Buske-Kirschbaum et al., 1997). Specifically, the experimenter informed children that they would need to prepare and deliver a speech to a panel of expert judges (who were unaware of randomly-assigned coping condition) who would evaluate their performance on the speech. Following the speech, the panel of judges instructed children to perform an oral arithmetic task. A third saliva sample was taken immediately after the TSST-C (T3). Children were then taken to one of two coping rooms consistent with their randomly-assigned coping condition and told by an experimenter, “I need you to wait here for a few minutes while they discuss how well you did and score your performance. I will be back to let you know how you did in a few minutes.” In the avoidance condition (n = 40), children we taken to an empty room where they were they were additionally instructed by the experimenter, “While you are waiting, try not to think about how well you did or did not do on those tasks.” In the distraction condition (n = 42), children were taken to a room full of toys where they were also told by the experimenter, “While you are waiting, feel free to play around with any of the materials we have here for you: Legos, puzzles, instruments, and arts supplies. You can take home what you create.” After spending 10 minutes in their coping rooms, a fourth saliva sample was taken (T4). A 10 minute structured interview was then administered to each child to obtain their self-report of the coping strategies they used during and after the TSST-C, after which a fifth saliva sample was collected (T5). Finally, a sixth saliva sample was obtained following a debriefing and 10 minute guided progressive muscle relaxation (T6). Figure 1 offers a visual depiction of salivary cortisol sample timing.
Figure 1.
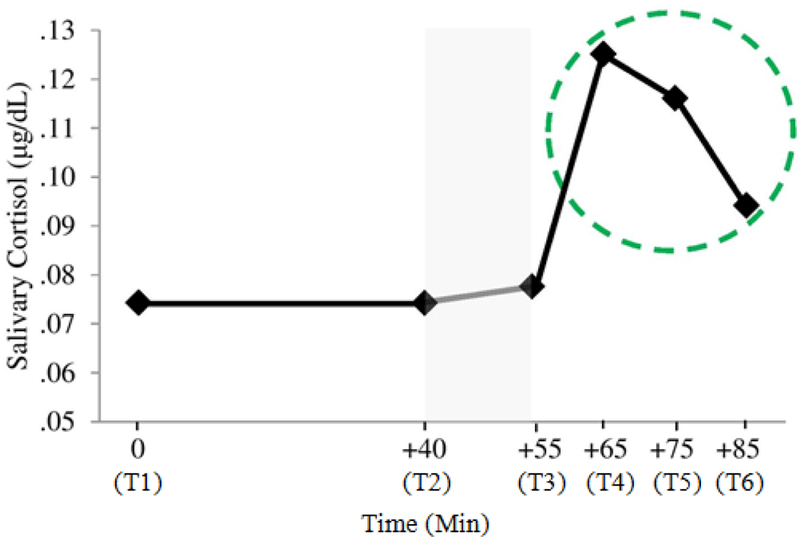
Untransformed mean salivary cortisol levels across the six time points of the study. Shaded region denotes TSST-C phase of the experimental protocol. The recovery phase time points are circled (N =80).
Measures
Child salivary cortisol.
HPA activation was assessed via salivary cortisol sampling. Samples were collected via passive drool through a straw directly into vials (Davis, Bruce, & Gunnar, 2002). Children were instructed to refrain from eating a large meal, brushing their teeth, or drinking sugary/acidic beverages within the hour prior to their lab appointment. Current medications were noted. Samples were stored in a medical grade ultra-low temperature freezer and were transported on ice via courier to the Behavioral Immunology and Endocrinology Lab at the University of Colorado. Cortisol levels were determined using a commercial expanded-range high-sensitivity enzyme immunosorbent assay kit (No. 1–3002/1–3012; Salimetrics, LLC, State College, PA) that detects cortisol levels in the range of 0.003 to 3.0 Kg/dL (range, 0.08Y82.77 nmol/L).
As preadolescents’ cortisol levels decrease during the 40 minute questionnaire period prior to the TSST-C (Abelson et al., 2014), we estimated that salivary cortisol sampled at T2 would index baseline cortisol levels. Also, due to the characteristic 20-minute delay of cortisol’s appearance in saliva, we estimated that T4 (25 minutes after starting TSST-C—hence, capturing the level of stress 5 minutes into the TSST-C procedure) would index peak reactivity (De Kloet, Joëls, & Holsboer, 2005). Lastly, salivary cortisol at T5 (10 minutes following completion of coping condition) and T6 (following debrief and PMR) were thought to reflect cortisol levels in response to children’s coping efforts.
Child characteristic coping.
During the 40 minutes prior to the TSST-C, preadolescents completed a social stress version of the Responses to Stress Questionnaire (RSQ; Connor-Smith et al., 2000), which is a 57 item self-report measure that assesses 10 types of coping and 9 types of involuntary stress responses. These 19 scales are aggregated into 3 coping factors (primary control, secondary control, disengagement) and 2 involuntary stress response factors (involuntary engagement and disengagement). Primary control coping consists of items related to problem solving and emotional regulation/expression (e.g., “I try to think of different ways to change the problem or fix the situation” and “I let someone or something know how I feel”, secondary control coping consists of items related to acceptance, distraction, and cognitive restructuring (e.g., “I realize that I just have to live with things the way they are” and “I think about the things that I am learning from the situation, or something good that will come from it”), and disengagement coping consists of items reflecting avoidance, denial, and wishful thinking (e.g., “I deal with the problem by wishing it would just go away”). Involuntary engagement and disengagement responses are not used in the current study. RSQ factor scores are computed as ratio proportion scores—i.e., the raw primary control factor scores divided by the raw total score on all items. Hence, the scores reflect the proportion of a child’s responses allocated to primary control coping, for example. Within this sample, primary control coping (α = .73), secondary control coping (α = .76), and disengagement coping (α = .70) demonstrated acceptable internal consistencies.
Child reactivity and experimental coping condition.
Directly following the 10 minutes in the coping room, preadolescents were administered a semi-structured interview during which they were asked to describe in their own words what they did (a) to cope while they were delivering the speech and completing the oral arithmetic task (reactivity coping), and (b) while they were alone in the room followingthe TSST-C (experimental coping condition). Follow-up probes asked specifically about things they did to make themselves feel better or make the situation better, even if they thought it did not work. Children’s qualitative coping responses were coded using the Responses to Stress Coding Manual for In Vivo Coping(Wadsworth, 2013). Coders gave each coping response a mutually-exclusive binary occurrence code in accordance with the 10 effortful coping scales of the RSQ. Inter-coder reliability was established by comparing two raters’ coding of 30% of the responses for the three coping factors (κ = .91). For reactivity coping, the total number of codes for each factor was used in all reactivity analyses: primary control (e.g., “Wrote down what I was going to say”), secondary control (e.g., “I tried to be calm and tell myself calming thoughts”), and disengagement (e.g., “I just tried to ignore it, doing whatever I could do to ignore it”). For the experimental coping condition, children’s qualitative responses reflected only secondary control (e.g., “I drew a tiger and colored”) and disengagement (e.g., “I tried not to think about what I did in there”) factors. A chi-square test was used to determine whether random assignment to the two experimental coping conditions successfully primed the target form of coping. The relationship between experimental coping condition and child reports of coping was significant, χ2 (1,80) = 33.21, p< .001. In the behavioral distraction condition, 90.5% reported compliance with the distraction instructions. In the cognitive avoidance condition, 73.7% of preadolescents reported trying to think about something other than their performance. Thus, our experimental coping condition was successful at priming the intended effortful coping response.
Covariates
Child gender.
Boys received a code of zero while girls received a code of 1.
Income-to-needs ratio (INR).
Parents reported family income.INR serves as an index of annual household income relative to national poverty line norms. An INR of 1.0 indicates poverty while middle income status is indicated by an INR of 3.0. The median INR for sample was 2.99.
Child puberty.
Pubertal status was assessed with four of the five items from the Pubertal Development Scale (Petersen, Crockett, Richards, & Boxer, 1988)—skin changes was excluded. For girls, parents indicated whether menstruation had begun, and the extent of height, body hair, and breast growth (α = .71). For boys, parents indicated the degree which the voice had deepened, and the extent of height, body hair, and facial hair growth (α = .61). All items were rated on either a 4- point (0 – 3) Likert-type scale, aside from the menstruation item, which was scored dichotomously (no = 0; yes = 3). A total puberty score was created by summing the four items for each child (Petersen et al.).
Child medication use.
Following Granger and colleagues (2009), parent-reported medications were rated on each of 11 mechanisms/pathways which might influence cortisol reactivity and regulation. Ratings were then aggregated within-person to yield a total medication-related risk score for each child.
Analytic Plan
Data reduction and preprocessing.
Participants whose age was atypical for fourth and fifth grade (n = 1, age = 7.13 years) and who did not complete the in-person child portion of the study (n = 1) were excluded from the present analyses. Following the recommendations of Gunnar, Morison, Chisholm, and Schuder (2001) as well as others (Davis, Bruce, & Gunnar, 2002; Dozier et al., 2006), cortisol values (n = 9; T1, n = 2; T2, n = 1; T3, n = 1; T4, n = 1; T5, n = 2; T6, n = 2) as well as income-to-needs ratio values (n = 2) greater than 3 SD from the sample mean were excluded from analyses. Cortisol values remained positively skewed. Thus, a fourth-root transformation (Miller & Plessow, 2013) was applied to all salivary cortisol data. A log10 transformation was sufficient to normalize family INR values. As is typical with count data, reactivity coping scores were positively skewed, zero-inflated, and thus dichotomized to reflect whether the child endorsed (1) or did not endorse (0) respective coping strategies. MANOVA post-hoc tests of geographic location (western US, northeastern US) indicated locations differed only with respect to family INR (F = 18.04, p< .001) and child pubertal status (F = 6.53, p< .05). These variables were controlled for in all subsequent analyses.
Missing data.
Missing value analysis (SAS 9.3) was conducted for all key demographic and study variables used in the present set of analyses. Little’s MCAR (missing completely at random) test was non-significant (Χ2 (153) = 128.83, p = .92) suggesting that the data could be MCAR. The total percentage of missing data points for key demographic and study variables was 2.5%. Despite the relatively small total percentage of missingness, 24 (30.00%) participants had missing data on at least one study variable to be entered into our analyses. Thus, Markov Chain Monte Carlo (MCMC) multiple imputation methods (PROC MI, SAS 9.3) were used to address missing data values and circumvent power loss associated with listwise deletion approaches. As less than 10% of data values were missing, five imputations were performed (Graham, Olchowski, & Gilreath, 2007; Yuan, SAS) which included all variables to be used in analyses. Interaction variables were mean centered to reduce issues with multicollinearity and their interactions terms computed prior to imputation. Parameter estimates were pooled across the five imputations using PROC MIANALYZE (SAS 9.3).
Bivariate associations.
Fisher’s Z adjusted Pearson correlations were used to examine bivariate associations between study variables. As previous research has demonstrated an association between gender, pubertal status and cortisol response (Stroud et al., 2011), partial correlations controlling for these variables in addition to income-to-needs ratio and medication use were conducted.
Multilevel models.
Salivary cortisol reactivity and recovery patterns were analyzed with multilevel modeling (MLM) methods (SAS 9.3, PROC MIXED) using respective reactivity (T2 – T4) and recovery phase (T4 – T6) sample time as a level-one within-subjects factor and respective coping as level-two between-subjects factors. A more through description of our MLM analytic approach for reactivity and recover phase aims follows in the Results section. Random intercepts and linear slopes models were used for all MLM analyses (best model fit data available upon request) with Kenward-Rogers corrected degrees of freedom. Child gender, pubertal status, income-to-needs-ratio, and medication use scores were included as covariates and retained despite statistical nonsignificance. As the group-level plot in Figure 1 indicated non-linearity in recovery phase cortisol trajectories and since one aspect of our definition of inefficient down-regulation is operationalized by a significant quadratic effect, we test for nonlinear effects and inefficiency by analyzing the quadratic effect of time. In the interest of symmetry, quadratic time effects were also included in all reactivity phase cortisol analyses.
Results
Preliminary Results
Descriptive statistics and correlations between our key study variables controlling for our identified covariates are shown in Table 1. Girls (Mpuberty = 3.41, SD = 2.31) were further along in their pubertal development than boys (Mpuberty = 1.81, SD = 1.47); Welch’s t (73.92) = −3.77, p < .001). Nine participants endorsed using steroid or anti-inflammatory medications (sample Mrisk = .74, SD = 2.44). As expected, salivary cortisol levels at each time point during the experiment were highly correlated.
Table 1.
Descriptives and Pooled Partial Correlation Estimates for Child Coping, Coping Condition, and Salivary Cortisol Concentrations
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | (10) | (11) | (12) | (13) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (1) Characteristic PCEC | — | ||||||||||||
| (2) Characteristic SCEC | .47* | — | |||||||||||
| (3) Characteristic DC | -.29* | -.45* | — | ||||||||||
| (4) Reactivity PCECa | .19 | .10 | .01 | — | |||||||||
| (5) Reactivity SCECa | -.01 | -.11 | .06 | -.56* | — | ||||||||
| (6) Reactivity DCa | -.07 | .13 | -.23* | -.10 | -.23* | — | |||||||
| (7) Coping condition a | -.07 | -.07 | -.13 | -.16 | .06 | .16 | — | ||||||
| (8) Salivary cortisol - T1 | -.03 | -.08 | .07 | .17 | -.12 | -.01 | .01 | — | |||||
| (9) Salivary cortisol - T2 | .06 | .11 | -.06 | .24* | -.10 | -.04 | -.05 | .54* | — | ||||
| (10) Salivary cortisol - T3 | .01 | -.01 | -.01 | .28* | -.16 | -.13 | -.01 | .48* | .76* | — | |||
| (11) Salivary cortisol - T4 | .06 | .09 | -.02 | .35* | -.24* | -.03 | -.15 | .05 | .38* | .56* | — | ||
| (12) Salivary cortisol - T5 | .07 | .03 | .01 | .33* | -.23* | -.03 | -.10 | .09 | .37* | .52* | .92* | — | |
| (13) Salivary cortisol - T6 | -.04 | -.03 | .10 | .27* | -.12 | -.05 | -.06 | .22 | .42* | .62* | .83* | .88* | — |
| M | .21 | .26 | .15 | .58 | .44 | .08 | .54 | .07 | .07 | .08 | .13 | .12 | .09 |
| SD | .04 | .05 | .03 | .50 | .50 | .27 | .50 | .04 | .05 | .04 | .08 | .08 | .07 |
| Range | .13-.32 | .14-.39 | .09-.23 | 0-1 | 0-1 | 0-1 | 0-1 | .01-.20 | .01-.29 | .01-.21 | .01-.35 | .01-.38 | .01-.31 |
Note.N = 80;
= Spearman’s rho; PCEC = Primary Control Engagement Coping; SCEC = Secondary Control Engagement Coping; DC = Disengagement Coping; T = Time. Coping condition was coded 0 for cognitive avoidance and 1 for behavioral distraction.
p<.05.
Characteristic primary control, secondary control, and disengagement coping were correlated in expected ways. Though characteristic coping and experimental coping condition were not significantly associated with salivary cortisol values at the bivariate level, reactivity primary control and secondary control coping were associated with salivary cortisol levels. Higher reactivity primary control was associated with higher cortisol across the duration of the experiment. Reactivity secondary control was correlated with reactivity disengagement coping in the expected direction. However, reactivity phase primary and secondary control were unexpectedly inversely correlated, as were characteristic and reactivity disengagement coping. Despite multiple prompts to report a variety of coping strategies in the interview, most children reported either primary or secondary control coping, but not both. Therefore, the codes from the interview reflect that the presence of one type of coping (e.g., primary control) was accompanied the absence of the other type (e.g., secondary control), thus contributing to an inverse association. Reactivity disengagement coping codes were restricted in range and zero inflated, which also contributed to the inverse association. This was likely due to the constraints on reactivity coping given the nature of the TSST-C (e.g., little opportunity for disengagement coping).
Reactivity Multilevel Models
Analyses.
We conducted a series of exploratory MLM analyses predicting salivary cortisol reactivity patterns to determine whether characteristic and reactivity coping influenced reactivity cortisol trajectories. In brief, sample time (T2 – T4) was entered as a level-one within-subjects factor and child coping was entered as a level-two between-subjects factor. Experimental coping condition was not included in the reactivity analyses given that cortisol in response to the manipulation takes 20-minutes to appear in saliva (De Kloet et al., 2005), an effect which should manifest at T5 and thereafter1. Reactivity MLM’s were conducted for each of the three characteristic coping factors and three reactivity coping variables. Main effects (covariates, baseline cortisol (T2), and coping main effects) and two-way interaction terms composed of coping and linear (time)/quadratic (time2) time (coping x time, coping x time2) were included in a single step in each of the six models. Non-significant (α> .05) quadratic interactions were systematically removed from each model, while linear interaction terms were retained.
Characteristic coping.
For all three characteristic coping models, child gender (β’s = −.008 – −.006, ps > .068), family INR (β’s = −.004 – −.003, ps > .250), pubertal status (β’s = −.001, ps > .250), and medication use (β’s = .001, ps > .250) were not significantly associated with overall reactivity phase salivary cortisol levels. Significant main effects for linear (β = .036, ps < .001) and quadratic (β = .009, ps < .001) time emerged, suggesting that children’s salivary cortisol levels did change (increase) over the course of the reactivity phase and that the shape of the effect was curvilinear. For all three models, child characteristic coping by linear (time) and quadratic (time2) interactions were non-significant; β’s = −.114 – −.008, ps > .250, β’s = −.081 – .008, ps > .065, respectively. Thus, there was insufficient evidence to suggest that children’s characteristic coping contributed to their cortisol reactivity trajectories.
Reactivity coping.
Similar results were obtained in all three reactivity coping models. That is, child gender (β’s = −.008 – −.006, ps > .066), family INR (β’s = −.003 – −.002, ps > .250), pubertal status (β’s = −.001, ps > .250), and medication use (β’s = .001, ps > .250) were not significantly associated with overall reactivity cortisol levels, with one exception. In the reactivity disengagement model, girls had lower overall reactivity cortisol levels than boys (β = −.009, p< .05). Paralleling the characteristic coping results, significant main effects for linear (β’s = .030 - .042, ps < .001) and quadratic (β’s = .005 - .011, ps < .001) time emerged. However, child reactivity coping by linear (time) and quadratic (time2) interactions were not significant; β’s = −.014 – .009, ps > .250, β’s = −.005 – .006, ps > .164. Our results did not indicate that children’s reactivity coping influenced their cortisol reactivity trajectories.
Recovery Multilevel Models
Analyses.
Three MLM building series were conducted to examine whether characteristic coping and experimental coping condition were associated with cortisol recovery trajectories (Hypothesis 1). Each series examined one of the three characteristic coping types (i.e. primary control, secondary control, disengagement) as a between-subjects factor along with experimental coping condition (distraction = 1, avoidance = 0). Two-way interaction terms composed of characteristic coping, experimental coping condition and linear (time)/quadratic (time2) time were added to covariates and main effects in the first step in each of the three series: characteristic coping x time, coping condition x time, characteristic coping x coping condition, characteristic coping x time2, coping condition x time2. To examine whether the effect of coping condition on recovery cortisol trajectories differed as a function of characteristic coping (Hypothesis 2), three-way interactions between characteristic coping, coping condition, and (time)/quadratic (time2) time were entered in the second-step in each of the three series: characteristic coping x coping condition x time, characteristic coping x coping condition x time2. At each step, non-significant (α> .05) interactions were systematically removed from each model, starting with the highest order polynomial interaction. This strategy preserved model parsimony and permitted and examination of possible linear time effects (which were retained in all models) and interactions which may have been masked by quadratic terms. Non-significant terms removed in the first step, but required to examine possible three-way interactions, were reinserted in the second step. All recovery multilevel models controlled for cortisol reactivity to account for the possibility that reactivity might influence cortisol recovery trajectories. Following Pruessner, Kirschbaum, Meinlschmid, and Hellhammer (2003), area-under-the-curve with respect to increase (AUCi) cortisol scores were computed for cortisol reactivity across T2 and T4, entered into each recovery model as a covariate.
Table 2 provides parameter estimates and fit statistics for the three final recovery multilevel models. Significant interactions were probed at 2 SD above and below the mean, as recommended by Roisman and colleagues (2012). In instances where children’s characteristic ways of coping interacted with experimental coping condition and time to predict recovery cortisol trajectories, we report and interpret characteristic coping effects within the context of those significant three-way interactions.
Table 2.
Parameter Estimates, (Standard Errors), and Fit Statistics for Final Multilevel Models Predicting Recovery Phase Salivary Cortisol Growth Trajectories
| Primary Control Engagement Coping | Secondary Control Engagement Coping | Disengagement Coping | |
|---|---|---|---|
| Main effects | |||
| Intercept | .576* (.032) | .590* (.031) | .584* (.032) |
| Child gender | -.017 (.024) | -.022 (.024) | -.025 (.024) |
| Child puberty | .001 (.005) | .001 (.006) | .001 (.006) |
| Family income-to-needs ratio | -.033 (.031) | -.040 (.030) | -.035 (.031) |
| Child medication use | -.002 (.004) | -.002 (.004) | -.001 (.004) |
| Cortisol AUCi reactivity | .035* (.010) | .038* (.010) | .032* (.010) |
| Characteristic coping | .688* (.301) | .137 (.203) | -.277 (.478) |
| Coping condition | -.009 (.019) | -.021 (.018) | -.006 (.018) |
| Linear time (time) | -.030* (.005) | -.024* (.004) | -.031* (.006) |
| Quadratic time (time2) | -.003 (.002) | -.003* (.001) | -.003* (.001) |
| Two-way interactions | |||
| Characteristic coping x coping condition | -1.251* (.518) | - | 1.373* (.602) |
| Characteristic coping x linear time | -.088 (.140) | - | .279* (.129) |
| Coping condition x linear time | .010 (.008) | - | .013 (.008) |
| Characteristic coping x quadratic time | -.103* (.050) | - | - |
| Coping condition x quadratic time | .001 (.003) | - | - |
| Three-way interactions | |||
| Characteristic coping x coping condition x linear time (time) | -.118 (.180) | - | - |
| Characteristic coping x coping condition x quadratic time (time2) | .165* (.070) | - | - |
| Random effects | |||
| Intercept | .006* (.001) | .006* (.001) | .006* (.001) |
| Slope | .001* (.001) | .001* (.001) | .001* (.001) |
| Covariance (Intercept, Slope) | .001* (.001) | .001* (.001) | .001* (.001) |
| Residual | .001* (.001) | .001* (.001) | .001* (.001) |
| Model fit | |||
| -2LL | -655.48 | -667.04 | -669.74 |
| AIC | -647.48 | -659.04 | -663.34 |
| BIC | -637.92 | -649.50 | -652.22 |
Note.N = 80;AUCi – Area Under the Curve – Increase.
p<.05.
Experimental coping condition.
For all three models, covariates were not significantly associated with recovery cortisol levels. Significant main effects for linear (β’s = −.031 – −.024, ps < .001) and quadratic (β’s = −.003 – −.004, ps < .05) time emerged, suggesting that child salivary cortisol levels decreased in a curvilinear manner over the course of the recovery phase. Interactions between coping condition and linear (β’s = .011 – .013, ps > .101) and quadratic (β’s = .001, ps > .250) time were non-significant. Thus, there was insufficient evidence to conclude that coping condition alone was associated with recovery phase salivary cortisol growth trajectories (Hypothesis 1).
Characteristic primary control coping.
Significant characteristic primary control x quadratic (time2) and characteristic primary control x coping condition interaction effects were better explained by a significant three-way interaction between characteristic primary control, coping condition, and quadratic time (β = .165, p< .05) (see Table 2 and Figure 2). In all figures, red lines depict typically maladaptive coping and blue lined represent typically adaptive coping. In support of Hypothesis 2, children with high, as opposed to low, characteristic primary control coping had a less quadratic and more linear (steeper down-regulation) salivary cortisol recovery trajectory when primed for distraction. Children with low characteristic primary control primed for distraction exhibited a more quadratic, less efficient elevated cortisol recovery trajectory, such that salivary cortisol levels continued to increase during the 10 minutes following peak reactivity to the TSST-C, which was then followed by a moderate decrease in salivary cortisol levels. The opposite was true for children primed for avoidance. That is, the recovery cortisol trajectories for children high in characteristic primary control who were in the avoidance condition were more quadratic and less efficient than the linear, steeper down-regulation observed in their low characteristic primary control counterparts.
Figure 2.
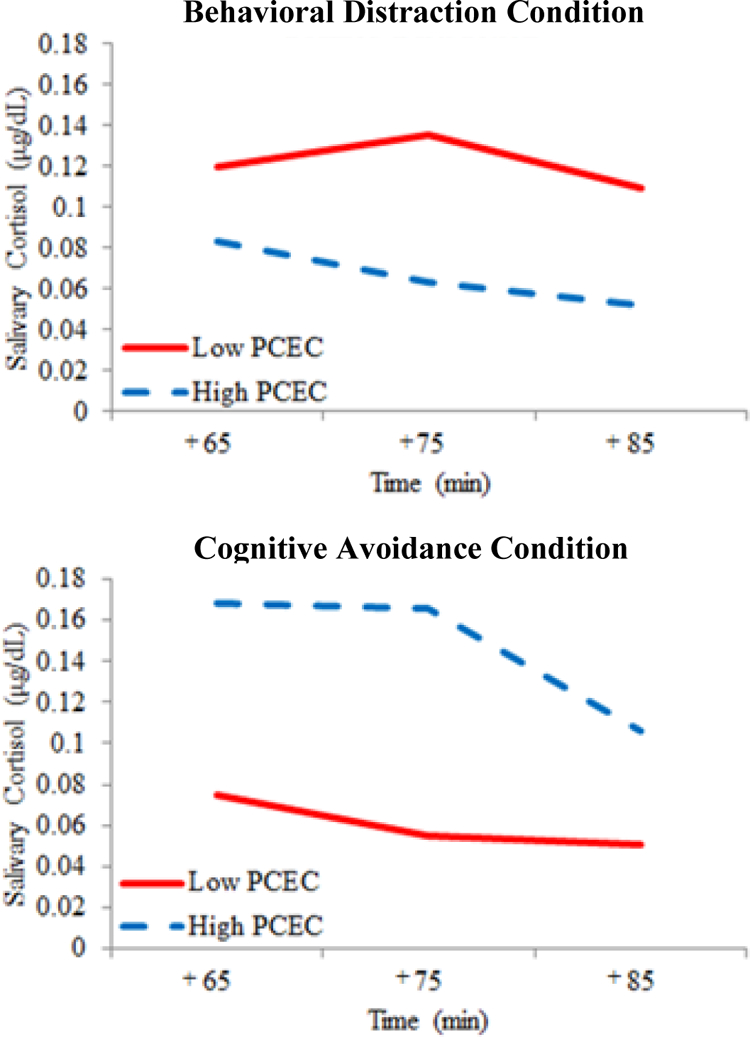
Predicted preadolescent salivary cortisol concentrations for high and low characteristic primary control engagement coping (PCEC) by experimental coping condition during the recovery phase (minutes 65 – 85) of the experiment. Analyses conducted with mean-centered coping values; predicted cortisol concentrations were plotted 2 standard deviations (SD) above and below mean coping scores and reverse transformed for illustrative purposes (N =80
Characteristic secondary control coping.
Apart from significant linear and quadratic time effects, no other predicted two way and three way interactions with characteristic secondary control coping, experimental coping condition, or linear (time) and quadratic (time2) emerged (Table 2). Thus, we found insufficient evidence to support the hypothesis that characteristic secondary control coping was associated with cortisol recovery trajectories (Hypothesis1) and that it differentially contributed to how children recovered from stress in each coping condition (Hypothesis 2).
Characteristic disengagement coping.
As depicted in Table 2, a significant characteristic disengagement by linear time interaction emerged (β = .279, p< .05). Consistent with Hypothesis 1, children who reported high characteristic disengagement coping levels had cortisol recovery trajectories which were more elevated and less steep than their low characteristic disengagement counterparts (Figure 3A). A significant characteristic disengagement by coping condition interaction emerged (β = 1.373, p< .05). Consistent with Hypothesis 2, children with higher characteristic disengagement had higher overall recovery salivary cortisol levels in the distraction condition (Figure 3B). The opposite was true for those primed for avoidance. That is, children with higher characteristic disengagement in the avoidance condition had lower overall levels of recovery phase salivary cortisol (simple slope = .087, SE = .037, p< .05). Also, children with low characteristic disengagement had lower recovery cortisol levels when primed for distraction, but when primed for avoidance had elevated cortisol levels (simple slope = −.099, SE = .037, p < .01). Both linear and quadratic three-way interactions were not significant in the second step of the disengagement model building series.
Figure 3.

Predicted preadolescent salivary cortisol concentrations for high and low characteristic disengagement coping (DC) during the recovery phase (minutes 65 – 85) of the experiment. Analyses conducted with mean-centered coping values; predicted salivary cortisol concentrations were plotted 2 standard deviations above and below mean coping scores and reverse transformed for illustrative purposes (N =80).
Discussion
Psychological research on children’s stress and coping processes has historically focused on prediction of psychological outcomes and understanding sources of risk and resilience (Cicchetti, 2010). This body of work has relied almost exclusively on retrospective self-reports of coping despite current theoretical emphasis on the multiple levels at which both stress and coping occur. In particular, stress research currently integrates biological and behavioral data as a matter of course, but coping research has lagged far behind. This study comprises the most comprehensive test of the Response to Stress model’s integrative psychobiological theoretical tenets. The results highlight the importance of regulatory fit between preadolescents’ characteristic ways of coping and their coping environment, as different physiologic recovery patterns were observed based on the intersection of both these aspects of coping. This study, therefore, has unlocked the coping black box and confirmed that preadolescent children’s coping “gets under the skin” and can be observed and measured at cognitive, behavioral, and physiologic levels of analysis (Slattery, Grieve, Ames, Armstrong, & Essex, 2013).
First, the findings support our proposition that preadolescents’ coping is specifically relevant to physiologic recovery. As predicted, neither characteristic nor reactivity coping contributed to cortisol reactivity trajectories. This is likely due to the neurobiology of reactivity, which occurs via a quick-acting chain of events that serves primarily to innervate muscles, heighten visual acuity, and enhance immune responses. This immediate stress response largely by-passes the central nervous system which undergirds effortful coping responses (Kaltsas & Chrousos, 2007). Thus, we would not expect children’s coping to play much of a role in physiologic stress reactivity. This underscores the importance of specifying the phases (i.e., reactivity versus recovery) of the stress response system in research on coping and post-stress regulation (e.g., Mills, Imm, Walling, & Weiler, 2008; Ramsay & Lewis, 2003).
In the recovery phase, significant two-way interactions between characteristic primary control coping and time as well as between characteristic disengagement coping and time emerged. While the primary control finding is best interpreted in the context of its three-way interaction with experimental coping condition, children with lower levels of characteristic disengagement had steeper down-regulation slopes from T4 to T6 than high disengagement peers. This suggests that preadolescents whose coping repertoires contain proportionally less characteristic disengagement coping are better able to regulate their recovery physiology following social stressors. Thus, children’s characteristic ways of coping appear to be especially relevant to the recovery phase of the acute response to social stress.
Next, our experimental coping manipulation was successful in creating two distinct coping environments, which were differentially associated with recovery phase cortisol growth trajectories when characteristic coping was simultaneously taken into account. These findings indicate that child-environment regulatory fit may determine a child’s physiologic recovery from stress, and therefore their risk for developing psychopathology in the face of stressors. Their characteristic ways of coping either help or hinder their ability to regulate—thus if the environment is consonant with primary control coping for example, characteristic skills will synergistically contribute to a child’s ability to springboard off a primary control related task and down-regulate stress physiology. When that environment is, however, at odds with what children typically do, poorer physiologic regulation ensues. Figure 2 shows that those children high in characteristic primary control in the avoidance condition fare much worse in terms of the curve of their slope and continued cortisol elevations compared to their low primary control peers. Children with high characteristic primary control levels who were primed for behavioral distraction regulated much better when they were able to manipulate their environments, which is presumably consistent with a more active and engaging coping style.
The characteristic coping and coping condition interactions are also notable as they suggest that primary control coping may not be universally adaptive. There may be circumstances which undermine its utility, such as those with little or no objective controllability. There may also be settings in which cognitive avoidance is related to better regulation, such as when thinking about a stressor overwhelms the ability to do anything else. Temporary cognitive avoidance may well be a useful strategy for modulating in-the-moment stress physiology for children who characteristically disengage. However, research has shown that even when avoidance has short-term salutary effects, there are eventual costs associated with its long-term use (e.g., Santiago & Wadsworth, 2009).
Bivariate correlations that were inconsistent with predictions further support this assertion. For example, reactivity phase primary control coping was associated with higher cortisol levels. Reactivity primary control coping indexed children’s active attempts to plan (e.g., “wrote down what to say”) and deliver (e.g., “said as many things as I could”) their speech successfully. As judges were unwavering in their assessment of children’s performance (e.g., stone-faced), engagement in reactivity phase primary control coping efforts in a context in which children had little objective controllability over the judge’s assessment may have contributed to additional frustration and higher cortisol levels. Conversely, higher reactivity secondary control coping was associated with lower recovery cortisol levels. Thus, engagement in coping which focused on adapting oneself (e.g., positive self-talk, relaxation) to an uncontrollable stressor (TSST-C) was associated with lower recovery cortisol levels.
Furthermore, our findings extend the literature which highlights that seemingly overlapping distraction and avoidance coping strategies are unique processes (e.g., Hampel & Petermann, 2005) with divergent outcomes (e.g., Compas et al., 2001). Indeed, our results support that these processes diverge even at the physiologic level, with characteristic disengagement coping contributing to higher and more persistent overall salivary cortisol levels in response to stress. This finding is in line with a larger body of research which suggests that disengagement coping strategies such as avoidance are often associated with poorer functioning in the face of stress, but can be effective under certain circumstances (Santiago & Wadsworth, 2009; Shelton & Harold, 2008). Our results expand on why disengagement coping is sometimes found to be associated with better functioning—environmental context may contribute to a more nuanced interplay between characteristic and environmentally influenced coping. Thus, coping that is maladaptive in a particular environment may well be adaptive in a different environment (Wadsworth, 2015). Hence, we propose that regulatory fit is critical for successful adaptation to stress.
Although these results offer a more nuanced understanding about preadolescent coping, evidence supporting our hypothesis that characteristic secondary control coping would influence HPA recovery did not emerge. As preadolescents are just beginning to develop the cognitive capacities needed for sophisticated secondary control coping strategies such as cognitive restructuring and acceptance, child-level moderating variables may help explain non-significant characteristic secondary control – cortisol recovery associations. Individual differences in children’s working memory and executive functioning capacities could, for example, differentiate children who are able to effectively use cognitively-sophisticated secondary control coping in the service of down-regulating stress physiology (Gur et al., 2012). Future studies should consider moderators of the relation between coping and HPA recovery patterning such as cognitive functioning or child gender (Galsworthy, Dionne, Dale, & Plomin, 2000).
Implications, Limitations, and Future Directions
Our study is among the first to provide support for the proposition that the effects of coping are evident at the physiologic level. Though these novel results will require replication, the results suggest that preadolescents’ coping is responsive to fairly subtle environmental influence, and that this is evident at the physiologic level. How long lasting such changes may be is beyond the scope of this investigation. This finding aligns with research showing this developmental period to be a critical period of neuroplasticity in the stress response system (Fisher, Gunnar, Dozier, Bruce, & Pears, 2006), which potentially opens up opportunities for interventions that can counteract the pernicious effects of stress and adversity on children’s development (Davidson & McEwen, 2012).
Coping, therefore, may represent a route to repair of physiologic stress response systems (e.g., HPA) that become damaged by chronic stress. Improving children’s abilities to cope with stress has the potential to break the cycle of damage, especially if the targeted coping has effects at the physiologic level. This study, therefore, suggests exciting directions for coping-focused intervention research (Raviv & Wadsworth, 2010) with the potential to evince physiologic change (Davidson & McEwen, 2012). The positive implications of psychobiological stress response system remediation are many and varied given the system’s role in a wide array of physical and mental health problems. Such knowledge and interventions could suggest solutions to mental and physical health disparities, which begin during childhood and stem from damage to these psychobiological stress systems (Wadsworth, 2015).
This study also has notable limitations. First, the relatively homogenous nature of our sample may limit how far we can generalize these findings. The current study was designed to help establish evidence of the existence of a physiologic base of child coping—as in a proof-of-principle study—a purpose for which a non-at risk sample such as this is well-suited (Gage, Smith, Zammit, Hickman, & Munafò, 2013). The RSQ has been successfully used to build an understanding of coping in a variety of at-risk populations, including multiple samples involving lower income ethnically diverse children, adolescents, and adults (Wadsworth & Santiago, 2008). Thus, complementary research demonstrating the applicability of these new findings to these types of diverse samples is needed (Brenner, Zimmerman, Bauermeister, & Caldwell, 2013). Second, the nature of the stressor in the present study may have constrained our ability to detect effects for reactivity coping strategies. Children were quite limited in what they could do to cope while performing the speech and conducting mental arithmetic. It is possible that coping may influence physiologic reactivity in the presence of a stressor which permits a wider range of coping options. In addition, by asking children to report “how they coped”, this assessment may have pulled for behavioral as opposed to cognitive coping strategies. Finally, though cortisol is well-researched in the developmental stress literature, it is not the only physiological indicator of stress responses relevant for understanding pathways to risk and resilience. Additional work in this area may benefit from expanding the range of physiologic indices (e.g. alpha amylase, galvanic skin response) to examine consistency of results across indices (Brody et al., 2013).
Conclusion
This study highlights the importance of using a multi method approach to understanding how preadolescents cope withstressful events and the effects of different types of coping on underlying physiology. Use of standardized pre and post assessment that incorporates both characteristic and in-the-momentcoping responses to stressful events (e.g., TSST-C) and physiologic reactivity and regulation data may invigorate intervention research as we become better able to accurately assess the degree to which an intervention has affected putative biological mechanisms of action. Until now, the ability to capture changes in stress regulation resulting from intervention has been limited by methodological constraints. This line of research therefore offers new directions for the next wave of preventive intervention research in which we look beyond broad, distal outcomes to proximal mechanisms of action with potential for repair of damaged stress response systems. This study highlights the substantial payoff that can be gained by looking under the skin and inside the black box, in terms of locating evidence for modifiable mechanisms of change that can be leveraged in designing effective psychosocial interventions (Davidson & McEwen, 2012).
Footnotes
An independent samples T-test suggested that there were no significant differences (t(78) = −.271, p = .790) between children’s salivary cortisol concentrations at T4 in the distraction condition compared to the avoidance condition. Thus,experimental coping conditiondid not significantly contribute to T4 cortisol values, likely due to the time lag in cortisol appearance (De Kloet et al., 2005).
References
- Abelson JL, Erickson TM, Mayer SE, Crocker J, Briggs H, Lopez-Duran NL, & Liberzon I (2014). Brief cognitive intervention can modulate neuroendocrine stress responses to the Trier Social Stress Test: Buffering effects of a compassionate goal orientation. Psychoneuroendocrinology , 44, 60–70. doi: 10.1016/j.psyneuen.2014.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antal H, Wysocki T, Canas JA, Taylor A, & Edney-White A (2011). Parent report and direct observation of injection-related coping behaviors in youth with type 1 diabetes. Journal of Pediatric Psychology , 36(3), 318–328. doi: 10.1093/jpepsy/jsq082 [DOI] [PubMed] [Google Scholar]
- Boxer P, Sloan-Power E, Mercado I, & Schappell A (2012). Coping with stress, coping with violence: Links to mental health outcomes among at-risk youth. Journal of Psychopathology and Behavioral Assessment , 34(3), 405–414. doi: 10.1007/s10862-012-9285-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner AB, Zimmerman MA, Bauermeister JA, & Caldwell CH (2013). Neighborhood context and perceptions of stress over time: An ecological model of neighborhood stressors and intrapersonal and interpersonal resources. American Journal of Community Psychology , 51(3–4), 544–556. doi: 10.1007/s10464-013-9571-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Yu T, Chen E, Miller GE, Kogan SM, & Beach SRH (2013). Is resilience only skin deep?: Rural African Americans’ socioeconomic status–related risk and competence in preadolescence and psychological adjustment and allostatic load at age 19. Psychological Science , 24(7), 1285–1293. doi: 10.1177/0956797612471954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buske-Kirschbaum A, Jobst S, Wustmans A, Kirschbaum C, Rauh W, & Hellhammer D (1997). Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosomatic Medicine , 59(4), 419–426. [DOI] [PubMed] [Google Scholar]
- Chen E, & Miller GE (2012). “Shift-and-Persist” strategies: Why being low in socioeconomic status isn’t always bad for health. Perspectives on Psychological Science , 7(2), 135–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D (2010). Resilience under conditions of extreme stress: a multilevel perspective. World Psychiatry , 9(3), 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compas BE (2009). Coping, regulation, and development during childhood and adolescence. New Dir Child and Adolescent Development , 124, 87–99. doi: 10.1002/cd.245 [DOI] [PubMed] [Google Scholar]
- Compas BE, Connor-Smith JK, Saltzman H, Thomsen AH, & Wadsworth ME (2001). Coping with stress during childhood and adolescence: Problems, progress, and potential in theory and research. Psychological Bulletin , 127(1), 87–127. [PubMed] [Google Scholar]
- Connor-Smith JK, Compas BE, Wadsworth ME, Thomsen AH, & Saltzman H (2000). Responses to stress in adolescence: measurement of coping and involuntary stress responses. Journal of Consulting and Clinical Psychology , 68(6), 976–992. [PubMed] [Google Scholar]
- Davidson RJ, & McEwen BS (2012). Social influences on neuroplasticity: Stress and interventions to promote well-being. Nature Neuroscience , 15(5), 689–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Bruce J, & Gunnar MR (2002). The anterior attention network: Associations with temperament and neuroendocrine activity in 6-year-old children. Developmental Psychobiology , 40(1), 43–56. doi: 10.1002/dev.10012 [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Joëls M, & Holsboer F (2005). Stress and the brain: from adaptation to disease. Nature Reviews Neuroscience , 6(6), 463–475. doi: 10.1038/nrn1683 [DOI] [PubMed] [Google Scholar]
- Donaldson D, Prinstein MJ, Danovsky M, & Spirito A (2000). Patterns of children’s coping with life stress: Implications for clinicians. American Journal of Orthopsychiatry , 70, 351–359. [DOI] [PubMed] [Google Scholar]
- Edlynn ES, Miller SA, Gaylord-Harden NK, & Richards MH (2008). African American inner-city youth exposed to violence: Coping skills as a moderator for anxiety. American Journal of Orthopsychiatry , 78(2), 249–258. doi: 10.1037/a0013948 [DOI] [PubMed] [Google Scholar]
- Gage SH, Smith GD, Zammit S, Hickman M, & Munafò MR (2013). Using Mendelian randomisation to infer causality in depression and anxiety research. Depression and Anxiety , 30(12), 1185–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galsworthy MJ, Dionne G, Dale PS, & Plomin R (2000). Sex differences in early verbal and non‐verbal cognitive development. Developmental Science , 3(2), 206–215. [Google Scholar]
- Gotlib IH, & Joormann J (2010). Cognition and depression: Current status and future directions. Annual Review of Clinical Psychology , 6, 285–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JW, Olchowski AE, & Gilreath TD (2007). How many imputations are really needed? Some practical clarifications of multiple imputation theory. Prevention Science , 8(3), 206–213. [DOI] [PubMed] [Google Scholar]
- Gur RC, Richard J, Calkins ME, Chiavacci R, Hansen JA, Bilker WB, . . . Gur, R. E. (2012). Age group and sex differences in performance on a computerized neurocognitive battery in children age 8−21. Neuropsychology , 26(2), 251–265. doi: 10.1037/a0026712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall NC, Chipperfield JG, Perry RP, Ruthig JC, & Goetz T (2006). Primary and secondary control in academic development: Gender-specific implications for stress and health in college students. Anxiety, Stress & Coping: An International Journal , 19(2), 189–210. [Google Scholar]
- Hampel P, & Petermann F (2005). Age and gender effects on coping in children and adolescents. Journal of Youth and Adolescence , 34(2), 73–83. [Google Scholar]
- Hostinar C, Sullivan R, & Gunnar M (2014). Psychobiological mechanisms underlying the social buffering of the hypothalamic-pituitary-adrenocortical axis: A review of animal models and human studies across development. Psychological Bulletin , 140(1), 256–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, & Gunnar MR (2013). The developmental effects of early life stress: An overview of current theoretical frameworks. Current Directions in Psychological Science , 22(5), 400–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaser SS, Fear JM, Reeslund KL, Champion JE, Reising MM, & Compas BE (2008). maternal sadness and adolescents’ responses to stress in offspring of mothers with and without a history of depression. Journal of Clinical Child & Adolescent Psychology , 37(4), 736–746. doi: 10.1080/15374410802359742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo B, & Muthén BO (2003). Longitudinal studies with intervention and noncompliance: Estimation of causal effects in growth mixture modeling Multilevel modeling: Methodological advances, issues, and applications . (pp. 112–139): Lawrence Erlbaum Associates Publishers, Mahwah, NJ. [Google Scholar]
- Kirschbaum C, Pirke K-M, & Hellhammer DH (1993). The Trier Social Stress Test - A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology , 28(1–2), 76–81. [DOI] [PubMed] [Google Scholar]
- Levens SM, Muhtadie L, & Gotlib IH (2009). Rumination and impaired resource allocation in depression. Journal of Abnormal Psychology , 118(4), 757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losoya S, Eisenberg N, & Fabes RA (1998). Developmental issues in the study of coping. International Journal of Behavioral Development , 22(2), 287–313. [Google Scholar]
- Martin LL, & Tesser A (2006). Extending the goal progress theory of rumination: Goal reevaluation and growth In Sanna LJ & Chang EC (Eds.), Judgments over time: The interplay of thoughts, feelings, and behaviors (pp. 145–162). New York, NY: Oxford University Press. [Google Scholar]
- McEwen BS (1998). Protective and damaging effects of stress mediators. The New England journal of medicine , 338(3), 171–179. [DOI] [PubMed] [Google Scholar]
- Miller R, & Plessow F (2013). Transformation techniques for cross-sectional and longitudinal endocrine data: Application to salivary cortisol concentrations. Psychoneuroendocrinology , 38(6), 941–946. [DOI] [PubMed] [Google Scholar]
- O’Brien M, & Margolin G (1995). Relation among marital conflict, child coping, and child adjustment. Journal of Clinical Child Psychology , 24(3), 346. [Google Scholar]
- Raviv T, & Wadsworth ME (2010). The efficacy of a pilot prevention program for children and caregivers coping with economic strain. Cognitive Therapy and Research , 34(3), 216–228. [Google Scholar]
- Roisman GI, Newman DA, Fraley RC, Haltigan JD, Groh AM, & Haydon KC (2012). Distinguishing differential susceptibility from diathesis–stress: Recommendations for evaluating interaction effects. Development and psychopathology , 24(02), 389–409. [DOI] [PubMed] [Google Scholar]
- Santiago CD, & Wadsworth ME (2009). Coping with family conflict: What’s helpful and what’s not for low-income adolescents. Journal of Child and Family Studies , 18(2), 192–202. [Google Scholar]
- Seiffge-Krenke I, Aunola K, & Nurmi J-E (2009). Changes in stress perception and coping during adolescence: The role of situational and personal factors. Child Development , 80(1), 259–279. [DOI] [PubMed] [Google Scholar]
- Shelton KH, & Harold GT (2008). Pathways between interparental conflict and adolescent psychological adjustment: Bridging links through children’s cognitive appraisals and coping strategies. The Journal of Early Adolescence , 28(4), 555–582. [Google Scholar]
- Slattery MJ, Grieve AJ, Ames ME, Armstrong JM, & Essex MJ (2013). Neurocognitive function and state cognitive stress appraisal predict cortisol reactivity to an acute psychosocial stressor in adolescents. Psychoneuroendocrinology , 38(8), 1318–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud LR, Papandonatos GD, Williamson DE, & Dahl RE (2011). Sex differences in cortisol response to corticotropin releasing hormone challenge over puberty: Pittsburgh Pediatric Neurobehavioral Studies. Psychoneuroendocrinology , 36(8), 1226–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terranova AM, Boxer P, & Morris AS (2009). Factors influencing the course of posttraumatic stress following a natural disaster: Children’s reactions to Hurricane Katrina. Journal of Applied Developmental Psychology, 30(3), 344–355. [Google Scholar]
- Valiente C, Eisenberg N, Fabes RA, Spinrad TL, & Sulik MJ (2014). Coping across the transition to adolescence: Evidence of interindividual consistency and mean-level change. Journal of Early Adolesence , 35(7), 1–19. [Google Scholar]
- Vierhaus M, Lohaus A, & Ball J (2007). Developmental changes in coping: Situational and methodological influences. Anxiety , Stress , and Coping , 20(3), 267–282. [DOI] [PubMed] [Google Scholar]
- Wadsworth ME (2013). Responses to Stress Coding Manual for in vivo Coping. The Pennsylvania State University. [Google Scholar]
- Wadsworth ME (2015). Development of maladaptive coping: A functional adaptation to chronic, uncontrollable stress. Child Development Perspectives , 9(2), 96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadsworth ME, & Santiago CD (2008). Risk and resiliency processes in ethnically diverse families in poverty. Journal of family psychology : JFP : journal of the Division of Family Psychology of the American Psychological Association (Division 43) , 22(3), 399–410. [DOI] [PubMed] [Google Scholar]
- Wrosch C, Scheier MF, & Miller GE (2013). Goal adjustment capacities, subjective well-being, and physical health. Social and Personality Psychology Compass , 7(12), 847–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle M, Spear LP, Fuligni AJ, Angold A, Brown JD, Pine D, . . . Dahl RE (2008). Transitions into underage and problem drinking: developmental processes and mechanisms between 10 and 15 years of age. Pediatrics , 121 Suppl 4, S273-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer-Gembeck M, & Skinner E (2011). The development of coping across childhood and adolescence: An integrative review and critique of research. International Journal of Behavioral Development , 35(1), 1–17. [Google Scholar]


