Abstract
Introduction:
Vitamin D (vit D) deficiency has defined as a health problem worldwide. World Health Organization (WHO) has declared that obesity is an epidemic of the 21st century. Previous studies have shown that obesity may increase the risk of Vit D deficiency. Furthermore, other studies have demonstrated that vit D insufficiency was accompanied with higher risk of type 2 diabetes, cardiovascular diseases, hypertension, and obesity. The aim of this study was to survey the effect of vit D supplementation on weight loss among overweight and obese women aged 20–40 years in Isfahan.
Methods:
This double-blind clinical trial was done on 50 overweight and obese women who were divided into two groups, in which one group received vit D supplements and the other group received placebo. Intervention group received vit D with dozes 50,000 IU/w for 6 weeks. The levels of total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-c), high-density lipoprotein cholesterol (HDL-c), fasting blood sugar (FBS), insulin (ins), homeostasis model assessment of ins resistance (IR), C-reactive protein (CRP), height, weight (WT), waist circumference (WC), hip circumference (HC), and blood pressure (BP) were measured before and after intervention.
Results:
After using vit D supplementation for 6 weeks, WT, WC, and body mass index (BMI) were decreased significantly and serum vit D increased significantly compared to control group (P < 0.001). Other factors including TC, TG, LDL-c, HDL-c, FBS, CRP, ins, IR, and waist to hip ratio (WHR) did not change significantly (P > 0.05).
Conclusions:
After 6 weeks of intervention, the means of WT, BMI, WC, and HC decreased significantly. Previous studies have shown that vit D deficiency was more prevalence in obese people and there was an inverse association among vit D with BMI and WC. The relationship between vit D and lipid profiles such as glycemic indexes, anthropometric indexes, CRP, and BP is not clear and needs more study in the future.
Keywords: Blood pressure, cholesterol, high-density lipoprotein, low-density lipoprotein, triglyceride, Vitamin D supplementation, weight loss, glycemic indices
Introduction
Vitamin D (vit D) deficiency is considered as a health problem worldwide. Nowadays, vit D deficiency has involved more than half of people worldwide.[1] The prevalence of vit D deficiency in Tehran and Isfahan was estimated about 81/3% and 70/16%, respectively.[2] vit D plays an important role in calcium metabolism, maintenance of the skeleton, control of cell proliferation and differentiation, and immunity.[3] Recently, it has been shown that vit D deficiency has a strong relationship with increased risk of type 2 diabetes, cardiovascular disease (CVD) as well as CVD risk factors such as hypertension and obesity.[4] Obesity was recognized as an epidemic of the 21st century by World Health Organization (WHO)[5] and it is a serious health problem worldwide.[5,6] Since 19th century, the prevalence of obesity has increased along with changes in diets and lifestyle factors.[7] WHO estimated that at least 300 million adults are obese and more than 1 billion are overweight worldwide.[7,8] It is proved that 3.4 million of obese and overweight people are died due to obesity and comorbidities including hypertension, type 2 diabetes, stroke, CVD, some type of cancer such as prostate, breast, ovary, cervix, colon, and gallbladder every year.[5,9] In addition, obesity is related with hypercholesterolemia, osteoarthritis, gastroesophageal reflux, sleep apnea, and kidney chronic disease.[10] Furthermore, this issue becomes a general health problem In Iran. The prevalence of obesity and overweight was 42% in men and 57% in women in 2005, and it was anticipated to reach 54% and 74% among men and women, respectively by 2015.[11] A study In Iran showed that obesity in women are more than twice than men.[12] In a performed study in Isfahan, all women over than 65 years had abdominal obesity.[13] Lifestyle modification such as proper exercise is the best and cheapest way for decreasing the obesity. In addition, diet plays a key role in weight loss programs.[7] Today's attention toward the role of vit D in chronic diseases such as obesity is increasing. Based on several studies, obese and overweight people mostly have a lower levels of vit D than those who have less body fat.[14] Some studies suggested that obesity increased risk of vit D deficiency[15] whereas other studies shown that insufficient levels of vit D could increase the risk of type 2 diabetes, CVD, and risks such as hypertension and obesity.[16] A previous study has shown that vit D deficiency is more common in obese people.
It was seen that there is an inverse association among vit D with body mass index (BMI) and waist circumference (WC).[4,17,18,19,20] However, conflicting results had been seen such as a study among Iranian individuals aged 20–64 years with BMI of 24.2 ± 3.8 (57% female) which has not shown significant association between serum levels of vit D and BMI.[4] In this study, we tried to perform a comprehensive assessment about the effects of vit D supplementation and body weight as well as other anthropometric measurements, BP, lipid profile, glycemic indices, and C-reactive protein (CRP) among Iranian women. Therefore, our main purpose was to examine the effect of vit D on weight loss in obese and overweight women aged 20–40 years in Isfahan.
Methods
Subjects
This double-blind clinical trial study was performed among overweight and obese women in Isfahan endocrine and metabolism center, and other participants were female students of Isfahan University of Medical Science. Convenient method was used to enroll participants to the study. The following inclusion criteria was used to select participants: 20–40 years females, BMI higher than 25 (obese and overweight), nonsmoking, no history of diabetes, no hyperthyroids and hypothyroids, no participation in other weight loss programs, no weight loss during two past months, regular menstrual cycle, and no pregnancy. The general questionnaire included information about demographic characteristics such as location, education level, marital status and the number of pregnancy and children. In addition, we asked other questions about physical activity, the duration and times of sleep, consumption of supplements, and being on a special of diet. After giving general overview about this study, all individuals provided informed written consent.
Study design
The aim of this double-blind clinical trial study was to evaluate the effect of vit D supplementation on weight loss in 20–40 years obese and overweight women in Isfahan. The enough sample size was 25 person in each group that calculated according to the following formula N = (z1+z2)2 2s2/d2. In this formula, α was considered 0.05 and β was 80%. Hence, we recruited 75 persons to compensate potential losses during 6 weeks at follow-up. After that, individuals were randomly divided into two groups (intervention and control) and received vit D supplements and placebo, respectively. The participants continued their usual diet during the study. The intervention follow-up was 6 weeks that began from May 21, 2013 to July 5, 2013. At the first visit, we gave 6 pearls of vit D supplements to intervention group and 6 pearls of placebo to the controls and we asked them to eat one per week. Supplements were made in Zahravi pharmaceutical company, Tabriz, Iran. The dozens of supplements were 50,000 IU and placebo had the same shape, color, and packaging with given supplement. In addition, at the first meeting, food record has been explained to the individuals and they were asked to prepare it for 3 days including one weekend and 2 week days. Furthermore, the individuals were asked to report their physical activity during one selected week. In addition, participants recorded their daily amount of sun exposure from sunrise till sunset. The levels of total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-c), high-density lipoprotein cholesterol (HDL-c), fasting blood sugar (FBS), insulin (ins), homeostasis model assessment of ins resistance (HOMA-IR), CRP, vit D, height (ht), WT, WC, blood pressure (BP), and BMI were measured at the beginning and the end of study. Furthermore, anthropometric indicators and BP were measured. ht was measured by tape without shoes, nearest to the 0.1 cm, and for weight, we used a Beshel model digital scale (Germany) nearest to the 0.1 kg that individuals wore light clothing with no shoes. BMI was calculated with this formula, BMI = weight (kg)/height2 (m). BMI between 24/9 and 29/9 was defined as overweight and more than 29/9 was defined obese. Individuals's BP was measured by trained personnel using a mercury sphygmomanometer, after 10 min of rest in a sitting position.
Biochemical analysis
Blood samples were taken in a sitting position following 12–14 h overnight fasting before and after the intervention. FBS, lipid profiles, and CRP were measured by biochemical autoanalyzer A15 with Biosystem kit (made by Spain). ELISA method was used to determine concentration of vit D and ins. In addition, we obtained the ins sensitivity with using this formula: (fasting ins [micro unit/ml] * fasting glucose [micro mol/l]/22.5).[8]
Statistical analysis
Normal distributions of all variables were analyzed by the Kolmogorov–Smirnov test and by evaluating the histogram curves. All variables had normal distribution. Analyses were performed with independent t-test and paired t-test. The data were analyzed with SPSS version 20 (IBM, Armonk, NY, USA). The significance was considered 0.05. Independent t-test and Chi-square was used to comparison the general characteristics of participants.
Results
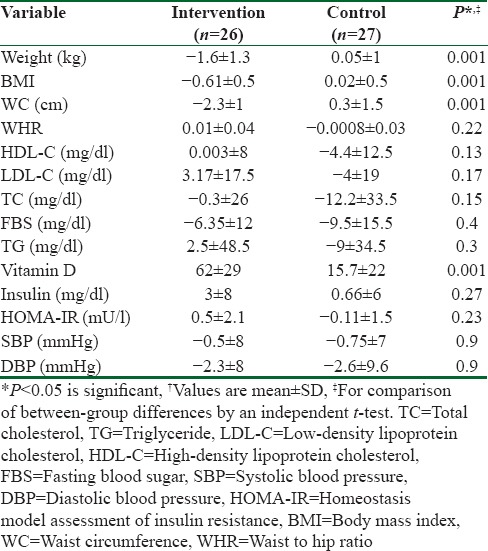
From 75 potentially eligible participants, 6 persons were excluded due to the personal reasons, 8 persons had normal serum levels of vit D, and 5 blood specimens were devastated due to the laboratory personnel mistake. We could not take blood from 3 participants because of high weight. Hence, they were eliminated. Finally, we had 53 individuals that 26 of them were assigned to intervention group and 27 of them were entered into control group. The baseline characteristics of 53 obese and overweight women are shown in Table 1. Anthropometric variables of participants are presented in Table 2. According to data, there were no significantly differences in baseline anthropometric variables between intervention and control groups except WC which was significantly higher (P = 0.04) in intervention group. The analysis showed that in intervention group, the means of weight, BMI, and WC were reduced significantly (73.2 ± 7.6–71.6 ± 7.7, 28 ± 2.7–27.2 ± 2.8, 90.4 ± 7.2–88 ± 7.5, respectively) (P < 0.001), but WHR (0.85 ± 0.05–0.84 ± 0.06) did not change significantly [Table 2]. As it has shown in Table 3, there were no differences in means of dietary energy, macronutrient, and micronutrient such as vit D of participants between two groups. Biochemical variables were reported in Table 4. The assessment of biochemical markers (HDL-c, LDL-c, TC, TG, FBS, Ins, HOMA-IR) in two groups shown that there were no significant differences in all of the biochemical variables (P > 0.05), except for the vit D that was 21.9 ± 6.5 in intervention and 18.1 ± 4.8 in control groups (P < 0.01). The mean of differences in anthropometric and laboratory variables were presented in Table 5. After calculating the mean of differences in two groups, it was cleared that intervention with the vit D (P < 0.001) decreased the means of weight (1.6 ± 1.3), BMI (0.6 ± 0.5), and WC (2.3 ± 1.1) and increased the mean of vit D (62 ± 29, P < 0.001). Furthermore, after adjusting for age, the means of the vit D was significant (83.49 ± 5.43, 34.2 ± 5.33 P = 0.001).
Table 1.
Baseline characteristics of the participants†
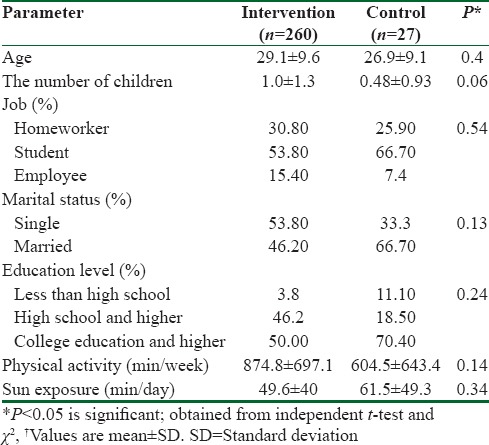
Table 2.
Anthropometric variables of the participants
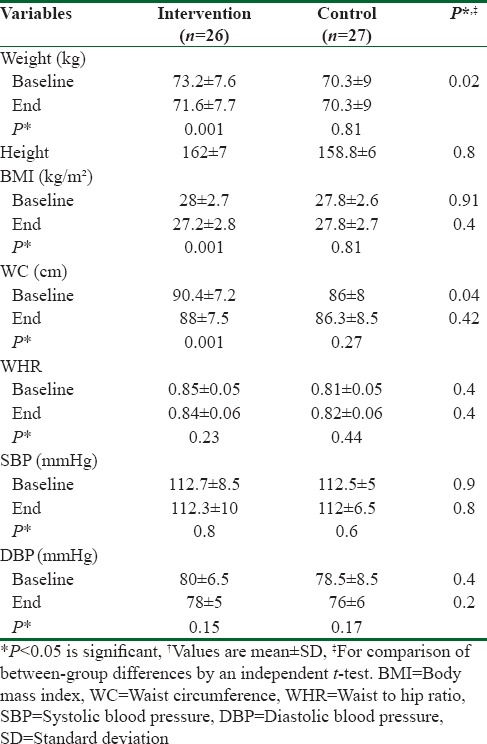
Table 3.
Dietary intake of participants before study†
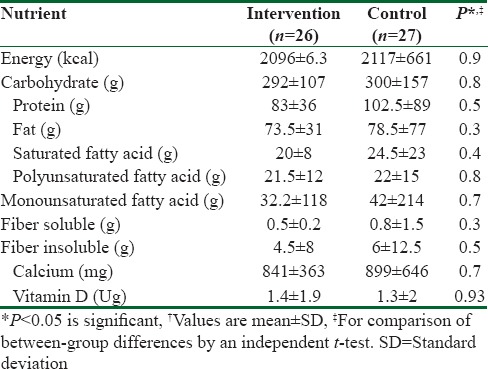
Table 4.
Biochemical variables in participants†
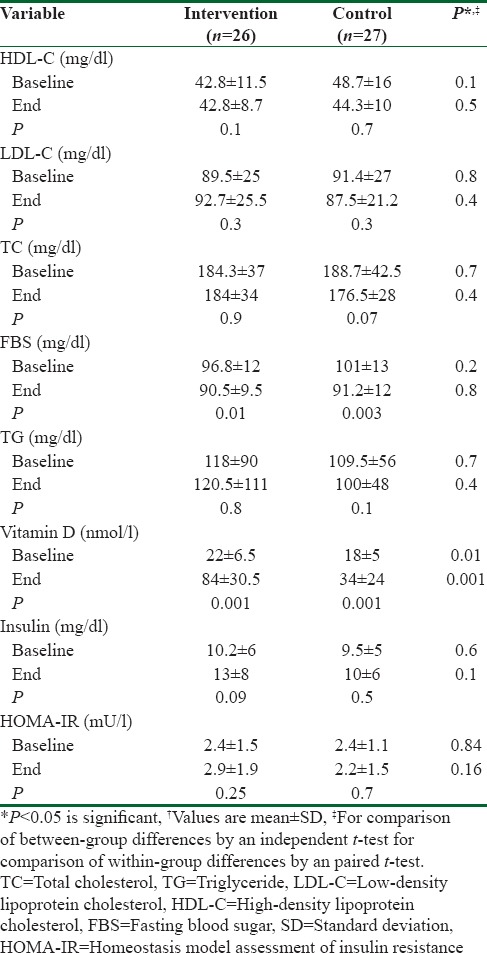
Table 5.
The mean of differences in anthropometric and biochemical variables†
Discussion
The findings of this double-blind clinical trial study in obese and overweight women aged 20–40 years showed that supplementation of the vit D with dozes 50,000 IU/w for 6 weeks reduced significantly the mean of BMI, weight, WC, and on the other hand, it increased significantly the level of vit D in comparison with the control group. However, there were no significant effect of the vit D on other factors such as hip circumference (HC), WHR, SBP, diastolic BP (DBP), lipid profiles, glycemic indexes, and CRP. Supplemental interventions on women aged 20–40 years were our study target because they were as mothers and had an important role in prevalence of chronic diseases and therefore health of society. The result of this study demonstrated that supplementation of the vit D in obese and overweight women reduced significantly the mean of weight, BMI, and WC. Previous studies had shown that the vit D deficiency is more prevalent in obese people and there was an inverse association between vit D, BMI, and WC.[3,4,9,11,13,14,15,16,17,18,19,20,21] Vashi et al. showed that 1 kg/m2 increase in BMI was associated with significantly reduced vit D level (42% ng/ml).[22] In another study, there was a significant inverse relationship after adjusting all confounding that associated with vit D and BMI.[23] However, conflicting results have been seen[3,17,24] such as a study on Iranian 20–64 years with BMI 24.2 ± 3.8 (57% female) which had not shown significant association between the level of vit D and BMI. Probably, its main reason was BMI, which was 24.2 and in the normal range. In addition, it could be another reason that only 48% of individuals had vit D deficiency.[17] In some studies, the inverse association between vit D and WC has been seen.[22,25] In one of these studies, vit D also had a significant association with HC.[25] In Seo's study only, this association between vit D and WC has been seen in women.[14]
Our study did not have a significant effect on FBS, ins, and HOMA-IR. A study in 2012 has not found any significant effect on FBS and HOMA-IR with supplementation of vit D with dozes 1000 IU/d during 1 year.[26] In another study, there was no significant relationship between vit D and HOMA-IR because of participants were healthy overweight adults with normal FBS.[3] From this aspect, it was similar to our study. Furthermore, in several studies, vit D did not have a relationship with fasting ins, IR, and fasting glucose.[20,27,28,29,30] Of course in some studies, vit D had an inverse effect on HOMA-IR, fasting ins, and FBS.[16,18] We could not find any significant effect of vit D; this effect may be seen in long-term studies. In this study, we could not find any role of vit D on the lipid profile such as TG, TC, HDL-c, and LDL-c. Result of this study were approved by previous studies.[31,32,33] In Moghassemi and Marjani's study of Iran, after 12 weeks supplementation with the vit D, lipid profile did not change significantly.[31] Also, in a randomly clinical trial on women has not found any changes in lipid profile that it maybe related to short duration of study and dose of the vit D.[23] In addition, some studies such as Women Health Initiative that had longer duration and dose of the vit D was 200 IU that take it twice a week for 7 years has not found changes in lipid profile.[33] In several studies, similar results were obtained.[20,22,26] However, some studies have found inverse results such as Kim et al. that after supplementation of the vit D only, HDL-c was reduced significantly.[30] in another study LDL-c and TG decreased significantly[34] but HDL-c increased significantly.[35] In a similar study, after consuming the supplementation of vit D with dozes 300 IU/d for 3 years, LDL-c and TG increased significantly but TC and HDL-c reduced significantly[36] A meta-analysis was conducted on the effects of vit D supplementation on lipid profile. showed that only LDL was changed significantly after the intervention.[25] Overall, there are contradictory results for the effect of the vit D on lipid profiles, so more investigations are necessary in the future. We have not seen a significant relationship between supplementation of the vit D and BP in our study. A clinical study in Germany supplementation of the vit D with dozes 100,000 IU did not cause any changes in BP.[37] In a meta-analysis that included 10 interventional studies, after supplementation of the vit D, no significant effects was found in systolic and DBP.[38] As well as several other studies have not reported the effect of vit D on BP.[26,39,40] Of course, a study has reported an inverse relationship between vit D levels and systolic BP in men,[19] but probably, its main reason was individuals’ age because the aim society in this study were the individuals with age higher than 65 years and mostly had a high BP but participants in our study were individuals with age 20–40 years that mostly had a normal BP. Another possible reason was the difference in gender.
We could not find any effect of the vit D on CRP in our study. In a study on the healthy population, vit D had no effect on CRP.[26] Forooghi et al. in Iran have not found effect of vit D on CRP too.[41] Furthermore, another studies had similar results. In some studies, an inverse association has been seen between vit D and CRP. It means that supplementation of the vit D can reduce CRP.[42,43] In an interventional study on obese people, CRP increased significantly.[16] According to the previous studies, the reason of the vit D deficiency in obesity has not cleared yet but some mechanisms has proposed these reasons for the relationship between vit D and obesity: trapped of the vit D in adipose tissue that makes less bioavailability for convert to the form of 1,25(OH)2D.[10] In a study vit D deficiency was accompanied with abdominal obesity. Therefore, vit D is lower in serum of obese people, and therefore, its bioavailability reduces for these individuals.[16] The role of vit D in causing IR has not cleared yet. Some studies have suggested that vit D may have beneficial effects on ins responsiveness by stimulating expression of ins receptors[44] or regulating calcium homeostasis which is necessary for intracellular ins-mediated processes.[45] As regard to the obesity is the most common cause of ins-resistance, the relation between vit D and IR might be the result of increased body size.[16] We could not find the effect of vit D on lipid profiles, glycemic indexes, CRP, and BP in this study. Some studies have reported these effects which were mostly due to the weight loss and BMI reduction that improved these factors. The possible effect of vit D on BP may be related to regulation the renin–angiotensin system, suppression the spread of proliferation of vascular smooth muscle cells, improvement IR, modification extended-dependent cells to endothelium and inhibition of anticoagulant activity and hypertrophy of myocardial cells.[46] Possibility mechanism for the effect of vit D in CRP reduction is that vit D receptors are in more than 37 body tissues that effect on these organs by their receptors and regulate pro-inflammatory mechanisms and systematic inflammation in the body. Vit D receptors are located in the nucleus of macrophages. Some of these macrophages produce cytokines, especially Tumor necrosis factor (TNF)-α. TNF-α expression significantly depends on the effect of NF-β. Increased vit D inhibits the protein expression of NF-β and reduces the expression of NF-β and thus reduces the level of TNF-α. In addition, vit D binds to receptors on monocytes and so produce inflammatory cytokines, CRP and reduces systemic inflammation.[41]
We performed this study as an interventional that gave us a more acceptable result. We included women aged 20–40 years that were at risk for vit D deficiency had more exposure to disease in the future.
Limitations
The short duration of the study and increase in the levels of the vit D in control group were our limitation. One of the possible causes of this increase can be attributed to the seasons. With the arrival of summer, the amount and intensity of the sun increases, and spontaneously, levels of vit D increases. Entrance healthy obese individuals with normal laboratory indexes may be the reason for not significant effects. The authors declared that there is no conflict of interest.
Conclusions
Overall supplementation of the vit D with dozes 50,000 IU/w for 6 weeks in obese and overweight women aged 20–40 years reduced in the mean of BMI, weight, and WC significantly and vit D increased significantly compared to the control group.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Holick MF, Chen TC. Vitamin D deficiency: A worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080S–6S. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- 2.Javidan AN, Sabour H, Latifi S, Vafa M, Shidfar F, Khazaeipour Z, et al. Calcium and Vitamin D plasma concentration and nutritional intake status in patients with chronic spinal cord injury: A referral center report. J Res Med Sci. 2014;19:881–4. [PMC free article] [PubMed] [Google Scholar]
- 3.Kim M, Na W, Sohn C. Correlation between Vitamin D and cardiovascular disease predictors in overweight and obese koreans. J Clin Biochem Nutr. 2013;52:167–71. doi: 10.3164/jcbn.12-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forsythe LK, Livingstone MB, Barnes MS, Horigan G, McSorley EM, Bonham MP, et al. Effect of adiposity on Vitamin D status and the 25-hydroxycholecalciferol response to supplementation in healthy young and older irish adults. Br J Nutr. 2012;107:126–34. doi: 10.1017/S0007114511002662. [DOI] [PubMed] [Google Scholar]
- 5.Barja-Fernandez S, Leis R, Casanueva FF, Seoane LM. Drug development strategies for the treatment of obesity: How to ensure efficacy, safety, and sustainable weight loss. Drug Des Devel Ther. 2014;8:2391–400. doi: 10.2147/DDDT.S53129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peirson L, Douketis J, Ciliska D, Fitzpatrick-Lewis D, Ali MU, Raina P, et al. Prevention of overweight and obesity in adult populations: A systematic review. CMAJ Open. 2014;2:E268–72. doi: 10.9778/cmajo.20140019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joharapurkar AA, Dhanesha NA, Jain MR. Inhibition of the methionine aminopeptidase 2 enzyme for the treatment of obesity. Diabetes Metab Syndr Obes. 2014;7:73–84. doi: 10.2147/DMSO.S56924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peirson L, Douketis J, Ciliska D, Fitzpatrick-Lewis D, Ali MU, Raina P, et al. Treatment for overweight and obesity in adult populations: A systematic review and meta-analysis. CMAJ Open. 2014;2:E306–17. doi: 10.9778/cmajo.20140012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarrafzadegan N, Talaei M, Sadeghi M, Mohammadifard N, Taheri M, Lotfizadeh M, et al. Determinants of weight change in a longitudinal study of iranian adults: Isfahan cohort study. Arch Iran Med. 2014;17:539–44. [PubMed] [Google Scholar]
- 10.Grethen E, McClintock R, Gupta CE, Jones R, Cacucci BM, Diaz D, et al. Vitamin D and hyperparathyroidism in obesity. J Clin Endocrinol Metab. 2011;96:1320–6. doi: 10.1210/jc.2010-2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veghari G, Sedaghat M, Maghsodlo S, Banihashem S, Moharloei P, Angizeh A, et al. Differences in the prevalence of obesity among fars-native, turkman, and sisstanish ethnic groups in Iranian Northern adults in 2010. Int Cardiovasc Res J. 2013;7:56–61. [PMC free article] [PubMed] [Google Scholar]
- 12.Salehpour A, Hosseinpanah F, Shidfar F, Vafa M, Razaghi M, Dehghani S, et al. A 12-week double-blind randomized clinical trial of Vitamin D3 supplementation on body fat mass in healthy overweight and obese women. Nutr J. 2012;11:78. doi: 10.1186/1475-2891-11-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sadeghi M, Talaei M, Oveisgharan S, Rabiei K, Dianatkhah M, Bahonar A, et al. The cumulative incidence of conventional risk factors of cardiovascular disease and their population attributable risk in an Iranian population: The isfahan cohort study. Adv Biomed Res. 2014;3:242. doi: 10.4103/2277-9175.145749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodríguez-Rodríguez E, Navia B, López-Sobaler AM, Ortega RM. Vitamin D in overweight/obese women and its relationship with dietetic and anthropometric variables. Obesity (Silver Spring) 2009;17:778–82. doi: 10.1038/oby.2008.649. [DOI] [PubMed] [Google Scholar]
- 15.Muscogiuri G, Sorice GP, Prioletta A, Policola C, Della Casa S, Pontecorvi A, et al. 25-hydroxyvitamin D concentration correlates with insulin-sensitivity and BMI in obesity. Obesity (Silver Spring) 2010;18:1906–10. doi: 10.1038/oby.2010.11. [DOI] [PubMed] [Google Scholar]
- 16.Moy FM, Bulgiba A. High prevalence of Vitamin D insufficiency and its association with obesity and metabolic syndrome among malay adults in Kuala Lumpur, Malaysia. BMC Public Health. 2011;11:735. doi: 10.1186/1471-2458-11-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baradaran A, Behradmanesh S, Nasri H. Association of body mass index and serum Vitamin D level in healthy Iranian adolescents. Endokrynol Pol. 2012;63:29–33. [PubMed] [Google Scholar]
- 18.Kang JH, Kim SS, Moon SS, Kim WJ, Bae MJ, Choi BG, et al. Adiposity in the relationship between serum Vitamin D level and insulin resistance in middle-aged and elderly korean adults: The Korea National Health and Nutrition Examination Survey 2008. Endocrinol Metab (Seoul) 2013;28:96–102. doi: 10.3803/EnM.2013.28.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seo JA, Cho H, Eun CR, Yoo HJ, Kim SG, Choi KM, et al. Association between visceral obesity and sarcopenia and Vitamin D deficiency in older Koreans: The ansan geriatric study. J Am Geriatr Soc. 2012;60:700–6. doi: 10.1111/j.1532-5415.2012.03887.x. [DOI] [PubMed] [Google Scholar]
- 20.Vilarrasa N, Vendrell J, Maravall J, Elío I, Solano E, San José P, et al. Is plasma 25(OH) D related to adipokines, inflammatory cytokines and insulin resistance in both a healthy and morbidly obese population? Endocrine. 2010;38:235–42. doi: 10.1007/s12020-010-9379-4. [DOI] [PubMed] [Google Scholar]
- 21.Johnson LK, Hofsø D, Aasheim ET, Tanbo T, Holven KB, Andersen LF, et al. Impact of gender on Vitamin D deficiency in morbidly obese patients: A cross-sectional study. Eur J Clin Nutr. 2012;66:83–90. doi: 10.1038/ejcn.2011.140. [DOI] [PubMed] [Google Scholar]
- 22.Vashi PG, Lammersfeld CA, Braun DP, Gupta D. Serum 25-hydroxyvitamin D is inversely associated with body mass index in cancer. Nutr J. 2011;10:51. doi: 10.1186/1475-2891-10-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Snijder MB, van Dam RM, Visser M, Deeg DJ, Dekker JM, Bouter LM, et al. Adiposity in relation to Vitamin D status and parathyroid hormone levels: A population-based study in older men and women. J Clin Endocrinol Metab. 2005;90:4119–23. doi: 10.1210/jc.2005-0216. [DOI] [PubMed] [Google Scholar]
- 24.Scragg R, Holdaway I, Singh V, Metcalf P, Baker J, Dryson E, et al. Serum 25-hydroxyvitamin D3 is related to physical activity and ethnicity but not obesity in a multicultural workforce. Aust N Z J Med. 1995;25:218–23. doi: 10.1111/j.1445-5994.1995.tb01526.x. [DOI] [PubMed] [Google Scholar]
- 25.Wang H, Xia N, Yang Y, Peng DQ. Influence of Vitamin D supplementation on plasma lipid profiles: A meta-analysis of randomized controlled trials. Lipids Health Dis. 2012;11:42. doi: 10.1186/1476-511X-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Breslavsky A, Frand J, Matas Z, Boaz M, Barnea Z, Shargorodsky M, et al. Effect of high doses of Vitamin D on arterial properties, adiponectin, leptin and glucose homeostasis in type 2 diabetic patients. Clin Nutr. 2013;32:970–5. doi: 10.1016/j.clnu.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 27.Mathieu C, Gysemans C, Giulietti A, Bouillon R. Vitamin D and diabetes. Diabetologia. 2005;48:1247–57. doi: 10.1007/s00125-005-1802-7. [DOI] [PubMed] [Google Scholar]
- 28.Beilfuss J, Berg V, Sneve M, Jorde R, Kamycheva E. Effects of a 1-year supplementation with cholecalciferol on interleukin-6, tumor necrosis factor-alpha and insulin resistance in overweight and obese subjects. Cytokine. 2012;60:870–4. doi: 10.1016/j.cyto.2012.07.032. [DOI] [PubMed] [Google Scholar]
- 29.Reis JP, von Mühlen D, Miller ER., 3rd Relation of 25-hydroxyvitamin D and parathyroid hormone levels with metabolic syndrome among US adults. Eur J Endocrinol. 2008;159:41–8. doi: 10.1530/EJE-08-0072. [DOI] [PubMed] [Google Scholar]
- 30.Kim HJ, Kang CK, Park H, Lee MG. Effects of Vitamin D supplementation and circuit training on indices of obesity and insulin resistance in T2D and Vitamin D deficient elderly women. J Exerc Nutrition Biochem. 2014;18:249–57. doi: 10.5717/jenb.2014.18.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moghassemi S, Marjani A. The effect of short-term Vitamin D supplementation on lipid profile and blood pressure in post-menopausal women: A randomized controlled trial. Iran J Nurs Midwifery Res. 2014;19:517–21. [PMC free article] [PubMed] [Google Scholar]
- 32.Wood AD, Secombes KR, Thies F, Aucott L, Black AJ, Mavroeidi A, et al. Vitamin D3 supplementation has no effect on conventional cardiovascular risk factors: A parallel-group, double-blind, placebo-controlled RCT. J Clin Endocrinol Metab. 2012;97:3557–68. doi: 10.1210/jc.2012-2126. [DOI] [PubMed] [Google Scholar]
- 33.Hsia J, Heiss G, Ren H, Allison M, Dolan NC, Greenland P, et al. Calcium/Vitamin D supplementation and cardiovascular events. Circulation. 2007;115:846–54. doi: 10.1161/CIRCULATIONAHA.106.673491. [DOI] [PubMed] [Google Scholar]
- 34.Zittermann A, Frisch S, Berthold HK, Götting C, Kuhn J, Kleesiek K, et al. Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular disease risk markers. Am J Clin Nutr. 2009;89:1321–7. doi: 10.3945/ajcn.2008.27004. [DOI] [PubMed] [Google Scholar]
- 35.Naharci I, Bozoglu E, Kocak N, Doganci S, Doruk H, Serdar M, et al. Effect of Vitamin D on insulin sensitivity in elderly patients with impaired fasting glucose. Geriatr Gerontol Int. 2012;12:454–60. doi: 10.1111/j.1447-0594.2011.00791.x. [DOI] [PubMed] [Google Scholar]
- 36.Heikkinen AM, Tuppurainen MT, Niskanen L, Komulainen M, Penttilä I, Saarikoski S, et al. Long-term Vitamin D3 supplementation may have adverse effects on serum lipids during postmenopausal hormone replacement therapy. Eur J Endocrinol. 1997;137:495–502. doi: 10.1530/eje.0.1370495. [DOI] [PubMed] [Google Scholar]
- 37.Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R, et al. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: A double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2006;83:754–9. doi: 10.1093/ajcn/83.4.754. [DOI] [PubMed] [Google Scholar]
- 38.Ke L, Mason RS, Kariuki M, Mpofu E, Brock KE. Vitamin D status and hypertension: A review. Integr Blood Press Control. 2015;8:13–35. doi: 10.2147/IBPC.S49958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seiki S, Chonchol M, Cheung AK, Kaufman JS, Greene T, Roberts WL, et al. 25-hydroxyvitamin D deficiency is associated with an increased risk of metabolic syndrome in patients with non-diabetic chronic kidney disease. Clin Nephrol. 2012;78:432–41. doi: 10.5414/CN107498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zittermann A, Schleithoff SS, Tenderich G, Berthold HK, Körfer R, Stehle P, et al. Low Vitamin D status: A contributing factor in the pathogenesis of congestive heart failure? J Am Coll Cardiol. 2003;41:105–12. doi: 10.1016/s0735-1097(02)02624-4. [DOI] [PubMed] [Google Scholar]
- 41.Foroughi M, Maghsoudi Z, Ghiasvand R, Iraj B, Askari G. Effect of Vitamin D supplementation on C-reactive protein in patients with nonalcoholic fatty liver. Int J Prev Med. 2014;5:969–75. [PMC free article] [PubMed] [Google Scholar]
- 42.Jorde R, Sneve M, Torjesen PA, Figenschau Y, Gøransson LG, Omdal R, et al. No effect of supplementation with cholecalciferol on cytokines and markers of inflammation in overweight and obese subjects. Cytokine. 2010;50:175–80. doi: 10.1016/j.cyto.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 43.Gholami K, Talasaz AH, Entezari-Maleki T, Salarifar M, Hadjibabaie M, Javadi MR, et al. The effect of high-dose Vitamin D3 on soluble P-selectin and hs-CRP level in patients with venous thromboembolism: A Randomized clinical trial. Clin Appl Thromb Hemost. 2016;22:483–9. doi: 10.1177/1076029614568715. [DOI] [PubMed] [Google Scholar]
- 44.Maestro B, Campión J, Dávila N, Calle C. Stimulation by 1,25-dihydroxyvitamin D3 of insulin receptor expression and insulin responsiveness for glucose transport in U-937 human promonocytic cells. Endocr J. 2000;47:383–91. doi: 10.1507/endocrj.47.383. [DOI] [PubMed] [Google Scholar]
- 45.Wright DC, Hucker KA, Holloszy JO, Han DH. Ca2+ and AMPK both mediate stimulation of glucose transport by muscle contractions. Diabetes. 2004;53:330–5. doi: 10.2337/diabetes.53.2.330. [DOI] [PubMed] [Google Scholar]
- 46.Pittas AG, Chung M, Trikalinos T, Mitri J, Brendel M, Patel K, et al. Systematic review: Vitamin D and cardiometabolic outcomes. Ann Intern Med. 2010;152:307–14. doi: 10.1059/0003-4819-152-5-201003020-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]


