Abstract
Background:
Although much attention has been paid to the pharmacokinetics (PKs) of different factor VIII (FVIII) concentrates in persons with hemophilia A (HA), limited information is available in young boys with severe HA. In this study, we aimed to assess the PK parameters of FVIII products in boys with severe HA in China.
Methods:
A total of 36 boys (plasma-derived [pd]-FVIII, n = 15; recombinant [r] FVIII, n = 21) were enrolled between January 2015 and May 2016 in Beijing Children's Hospital. PK characteristics of FVIII products were studied according to a reduced 4-sampling time point design (1 h, 9 h, 24 h, and 48 h postinfusion).
Results:
The mean FVIII half-life (t1/2) was 10.99 ± 3.45 h (range 5.52–20.02 h), the mean in vivo recovery (IVR) was 2.01 ± 0.42 IU/dl per IU/kg (range 1.24–3.02 IU/dl per IU/kg) and mean clearance (CL) of FVIII is 4.34 ± 1.58 ml·kg−1·h−1 (range 2.29–7.90 ml·kg−1·h−1). We also analyzed the influence of several parameters that potentially modulate FVIII PK. The age was closely associated with FVIII half-life (R2= 0.32, P < 0.01). The t1/2 of FVIII increased by 0.59 h per year. Besides age, von Willebrand factor antigen (VWF:Ag) also was associated with FVIII half-life (R2= 0.52, P < 0.01). Patients with blood Group O had a shorter FVIII half-life than patients with non-O blood group (9.40 ± 0.68 h vs. 12.3 ± 0.79 h, t = 2.70, P = 0.01). The FVIII IVR correlated with age (R2= 0.21, P < 0.01) and VWF:Ag level (R2= 0.28, P < 0.01). CL rates were faster in young patients and in those with low-VWF:Ag levels. CL rates of FVIII are higher in blood Group O versus non-blood Group O persons (5.02 ± 0.38 vs. 4.00 ± 0.32 ml·kg−1·h−1, t = 2.53, P = 0.02).
Conclusions:
Chinese boys with severe HA have similar PK values to other ethnic groups and large differences in FVIII PK between individual patients. Age, blood group, and VWF:Ag levels are important determining factors for FVIII CL.
Keywords: Boys, Factor VIII, Hemophilia A, Pharmacokinetics
摘要
背景:
关于血友病患者的FVIII的药代动力学(PK)研究有很多,但是针对儿童重型血友病患者的FVIII药代动力学研究很少。本研究旨在评估FVIII在中国重型血友病患儿中的药代动力学特征。
方法:
自2015年2月至2016年5月共纳入36例符合入组标准的重型血友病患儿(其中15例应用血浆来源的FVIII治疗,21例应用重组的FVIII治疗)。运用减量样本4个采样时间点(输注FVIII后1小时、9小时、24小时、48小时)的PK检测方案对FVIII的PK特征进行研究。
结果:
FVIII的半衰期为10.99 ± 3.45h (范围5.52-20.02 h),平均体外回收率为2.01± 0.42 IU/dl per IU/kg (范围1.24-3.02 IU/dl per IU/kg),平均清除率为4.34 ±1.58 ml·kg-1·h-1 (范围 2.29-7.90 ml·kg-1·h-1)。我们同时分析了PK的影响因素,研究结果显示FVIII的半衰期与年龄显著相关(R2=0.32, P <0.01),年龄每增长1岁,半衰期延长0.59小时。除年龄外,VWF抗原水平也与FVIII半衰期密切相关(R2=0.52, P <0.01)。FVIII在O型血患者的半衰期显著低于非O型血患者(9.40 ± 0.68 h vs. 12.3 ± 0.79h, t=2.70, P =0.01)。FVIII的体内回收率与年龄、VWF抗原水平相关(相关系数分别为R2=0.21、0.28, P均<0.01)。低年龄患儿及VWF抗原水平的患儿其FVIII清除速率加快。O型血患者体内的FVIII清除率高于非O型血患者(5.02±0.38 vs.4.00±0.32 ml·kg-1·h-1, t=2.53, P=0.02)。
结论:
中国重型血友病儿童FVIII的药代动力学特征与其他种族的特征相似。不同个体间的FVIII药代动力学参数差异较大,年龄、血型及VWF抗原水平是影响FVIII清除的重要因素。
INTRODUCTION
Hemophilia A (HA) is an inherited X-linked recessive bleeding disorder due to a deficiency in coagulation factor VIII (FVIII). In boys with severe or moderately severe HA (FVIII levels <2%), regular replacement therapy (“prophylaxis”) is a highly effective method for prevention of bleeding and is recommended as first-line therapy.[1] Standard high-dose prophylaxis in inhibitor-negative persons with severe HA targeted to maintain plasma FVIII coagulant (FVIII:C) activity above 1 IU/dl can achieve annualized spontaneous joint bleed rates of one or less if started early in life before the onset of joint damage.[2,3] The classic prophylaxis protocol (the Malmo protocol) involves the infusion of 20–40 FVIII IU/kg (average 30 FVIII IU/kg) on alternate days, minimum three times per week.[2] However, this protocol is very expensive and may require the placement of a central venous device to facilitate the frequent infusions of FVIII, especially in very young boys with HA. As a result alternate prophylaxis regimens with lower FVIII doses and frequency of infusion have been developed and tested in persons with severe HA.[4,5,6,7]
Calculation of FVIII dosing regimens based solely on body weight result in marked discrepancies in FVIII cover because of the marked variability in clearance (CL) of infused FVIII between individuals with severe HA.[8] Knowledge of pharmacokinetic (PK) profiles of individual patients could help to optimize the use of FVIII in prophylaxis regimens.[9] While some HA patients may need higher doses and more frequent infusions to achieve a target trough FVIII level, others could decrease their consumption of this expensive therapy based on individual PK results.[10] This is a potentially very important concept in countries such as China where there is currently constraints in access to expensive FVIII concentrates.
The purpose of this communication is to report the results of PK studies in Chinese boys with severe HA receiving both plasma-derived (pd) and recombinant (r) FVIII concentrates studied using the ISTH pediatric PK protocol. The results reported are important in the context of low-dose prophylaxis regimens that have been pioneered in China by Wu et al.,[6,7,11,12] and that are increasingly used for prophylaxis in young boys with severe HA in countries with limited access to expensive FVIII concentrates.
METHODS
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the Beijing Children's Hospital (No. 2013-81). Written informed consent was obtained from the parents or their legally authorized guardians before study enrollment.
Patients
A single-center, open-label study of 36 (pd FVIII [pd-FVIII], n = 15; recombinant FVIII [rFVIII] [3 products], n = 21) Chinese boys with severe HA was undertaken in Beijing Children's Hospital. The study conducted between January 2015 and May 2016. All enrolled patients were younger than 18 years old with severe HA (FVIII <1 IU/dl) without inhibitors (anti-FVIII antibody titer <0.6 Bethesda units [BU]). Patients primarily received low-dose prophylaxis or on-demand treatment, with more than 50 previous exposure days to pd-FVIII or rFVIII. A single-dose (FVIII 50 IU/kg) PK study comparing pd-FVIII and rFVIII concentrates were performed in all enrolled patients with HA.
Blood samples
Peripheral venous blood samples were drawn using BD (Becton, Dickinson and Company, USA) vacutainer blood collection tubes (3.2% Trisodium citrate). The blood samples were centrifuged immediately at 2500 ×g for 15 min at room temperature, and supernatant plasma was stored at −80°C in a Haier DW-86L-338 freezer (Haier, Qingdao, China) for later analysis. At baseline, the patients' blood group (ABO), FVIII activity, FVIII inhibitor, and levels of von Willebrand factor antigen (VWF:Ag) were determined.
Laboratory assays
FVIII activity was detected using a one-stage activated partial thromboplastin time (aPTT)-based assay (three dilutions of each sample), against an international plasma standard. All measurements were performed on an ACL TOP-500 analyzer (Instrumentation Laboratory, Bedford, MA, USA). The reagents of FVIII:C assay included HemosIL SynthASil and Calcium Chloride, HemosIL FVIII Deficient Plasma, HemosIL Calibration Plasma, HemosIL Normal Control 1 Unassayed, HemosIL Special Test Control Level 2, HemosIL Factor Diluent (Instrumentation Laboratory, Bedford, MA, USA). VWF:Ag determinations were also performed on an ACL TOP-500 analyzer with a latex particle-enhanced immunoturbidimetric assay using HemosIL von Willebrand factor antigen kit (Instrumentation Laboratory, Bedford, MA, USA). The Nijmegen modification of the Bethesda assay was used for determination of FVIII inhibitors.
Pharmacokinetic studies
PK studies were carried out after a minimum 72-h washout period with individuals in a nonbleeding state. Appropriate sampling times can be formally estimated using a PK model. Traditional PK studies in persons with HA required that the HA individuals be in a nonbleeding state, free of any infused FVIII for a minimum period of 72 h and that frequent postinfusion samples be collected for measurement of FVIII levels for a minimum period of 72 h.[13] This demanding protocol is impractical in very young boys with severe HA because of the need for multiple venipunctures and as a result limited data are available regarding PK parameters of infused FVIII in this very important population. To address this gap in information, the SSC recommended an abbreviated sampling schedule of five blood draws in pediatrics.[14] A reduced-points PK was suggested by Morfini et al.[15] and Björkman,[16] Blanchette et al.[17] Consistent with these recommendations, the PK characteristics of FVIII products were studied according to a reduced 4-point blood sampling design.[17] Blood samples for assay of plasma FVIII:C concentration were collected by venipuncture before dosing and, from the contralateral arm, at 1 h, 9 h, 24 h, and 48 h after the end of the FVIII infusion (4 sampling time points). The following PK parameters were analyzed by a noncompartment model using WinNonlin software (Pharsight Corp, Phoenix, Arizona, USA): the terminal phase half-life (t1/2), adjusted in vivo recovery (IVR), the area under the plasma concentration curve (AUC0–48 h), maximal plasma concentration (Cmax), CL, mean residence time (MRT), and volume of distribution at steady state (Vdss). In PK analyses, t1/2 was determined as previously described.[18] The area under the plasma concentration curve (AUC0–48 h) was computed by a linear trapezoidal method. The total area under the curve was estimated as the sum of AUC0–48 h and an additional area extrapolated by linear regression. CL was computed as dose divided by total AUC. MRT was computed by total AUC, and Vdss was calculated as CL × MRT. The observed peak FVIII concentration (Cmax) was measured 1 h postinfusion. The definition of adjusted IVR was determined as follows:[19] IVR (IU/dl per IU/kg) = (Cmax − preinfusion FVIII activity) (IU/dl)/(FVIII administered [IU]/body weight [kg]).
Statistical analysis
The statistical analyses were performed with JMP13.0 (SAS Institute Inc. Cary, NC, USA), and P < 0.05 was considered statistically significant. PK parameters were summarized using descriptive statistics. Quantitative data accordance with normal distribution were showed with mean and standard deviation, nonnormally distributed data were expressed as median and range. Student's t-test was conducted to evaluate differences when the quantitative variable was normal distribution, if it is not incompatible, Wilcoxon test was used. Chi-square test was used to test difference between categorical variables. Relationships between PK and demographic variables were evaluated by linear regression and linear correlation. Moreover, t-tests were conducted to evaluate differences in FVIII PK between type O blood group and non-O blood group.
RESULTS
Patient characteristics at baseline
The baseline patient data of 36 patients with severe HA (FVIII <1 IU/dl) were shown in Table 1. Age, blood type, and other baseline measurements (body mass index [BMI] and VWF:Ag) were not significantly different between the pd-FVIII and rFVIII groups [Table 1; P > 0.05].
Table 1.
Baseline characteristics of the 36 Chinese boys with severe HA
| Characteristics | All patients (n = 36) | pd-FVIII group (n = 15) | rFVIII group (n = 21) | Statistics | P |
|---|---|---|---|---|---|
| Age (years), median (range) | 7.8 (4.0–16.7) | 6.9 (4.0–13.1) | 9.4 (5.0–16.7) | −1.49* | 0.14 |
| BMI (kg/m2), mean ± SD | 16.9 ± 3.9 | 16.6 ± 2.4 | 17.1 ± 4.8 | 0.45† | 0.66 |
| VWF:Ag (%), mean ± SD | 98.9 ± 33.2 | 91.6 ± 31.5 | 104.0 ± 34.1 | 1.12† | 0.27 |
| Blood group, n (%) | |||||
| O type | 17 (47.2) | 7 (46.7) | 10 (47.6) | 0.003‡ | 0.95 |
| Non-O type | 19 (52.8) | 8 (53.3) | 11 (52.4) |
*Z values; †t values; ‡χ2 values. VWF:Ag: Von Willebrand factor antigen; BMI: Body mass index; FVIII: Factor VIII; pd-FVIII: Plasma-derived FVIII; rFVIII: Recombinant FVIII; SD: Standard deviation; HA: Hemophilia A.
Pharmacokinetic results of factor VIII in Chinese boys with severe hemophilia
Postinfusion FVIII levels exhibited a typical biphasic decay pattern [Figure 1]. The mean FVIII half-life (t1/2) was 10.99 ± 3.45 h (range 5.52–20.02 h), the mean IVR was 2.01 ± 0.42 IU/dl per IU/kg (range 1.24–3.02 IU/dl per IU/kg) and mean CL of FVIII is 4.34 ± 1.58 ml·kg−1·h−1 (range 2.29–7.90 ml·kg−1·h−1). PK parameters for the pd-FVIII and rFVIII were very similar. The CL of rFVIII had a slightly higher value than that of pd-FVIII [Table 2].
Figure 1.
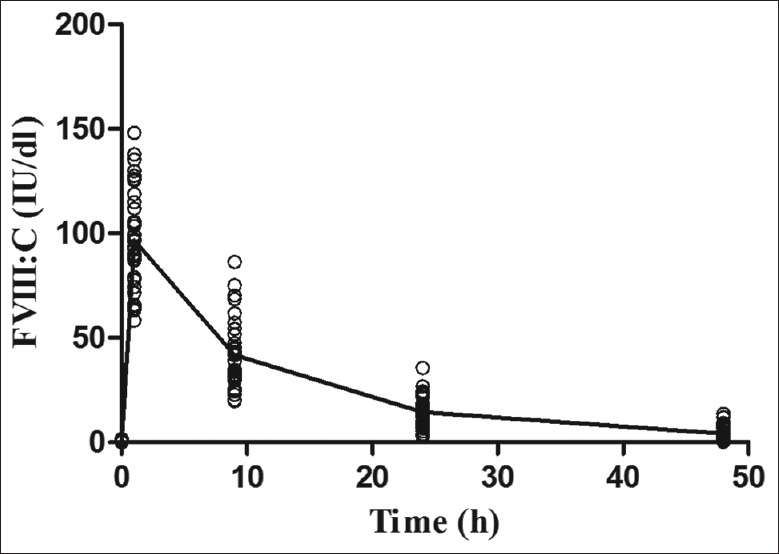
Time course of change in FVIII level (mean) after FVIII product infusion (n = 36). FVIII: Factor VIII.
Table 2.
Pharmacokinetic values of FVIII products in boys with HA
| PK parameters | All patients (n = 36) | pd-FVIII group (n = 15) | rFVIII group (n = 21) |
|---|---|---|---|
| t1/2 (h) | 10.99 ± 3.45 | 11.16 ± 0.78 | 10.87 ± 0.65 |
| AUC0–48 (IU·h−1·dl−1) | 1342.00 ± 583.61 | 1490.22 ± 135.62 | 1235.92 ± 113.61 |
| MRT (h) | 9.64 ± 2.15 | 9.75 ± 0.57 | 9.56 ± 0.48 |
| CL (ml·kg−1·h−1) | 4.34 ± 1.58 | 3.81 ± 0.36 | 4.69 ± 0.29 |
| Vdss (ml/kg) | 40.55 ± 10.78 | 37.22 ± 2.52 | 42.77 ± 2.04 |
| Cmax (IU/dl) | 104.30 ± 47.92 | 106.60 ± 11.86 | 102.66 ± 9.94 |
| IVR (IU/dl per IU/kg) | 2.11 ± 0.97 | 2.14 ± 0.24 | 2.10 ± 0.20 |
Values were shown as mean ± SD. PK: Pharmacokinetic; t1/2: Half-life; AUC: Area under the plasma concentration versus time curve; MRT: Mean residence time; CL: Clearance; Vdss: Volume of distribution at steady state; Cmax: Maximal plasma concentration; IVR: In vivo recovery; HA: Hemophilia A; SD: Standard deviation; FVIII: Factor VIII; pd-FVIII: Plasma-derived FVIII; rFVIII: Recombinant FVIII.
Correlation between pharmacokinetic parameters and cofactors
Factor VIII half-life
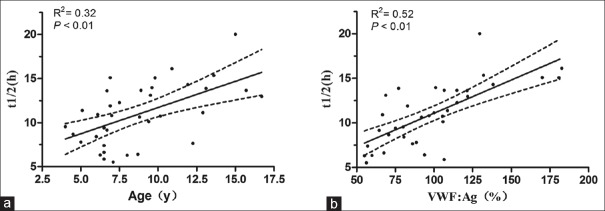
The age was significantly associated with FVIII half-life (t1/2=5.76 + 0.59 × age, R2= 0.32, P < 0.01) [Figure 2a]. Younger boys had a shorter FVIII half-life. The t1/2 of FVIII increased by 0.6 h per year. Preinfusion VWF:Ag levels were associated with FVIII half-life (R2= 0.52, P < 0.01) and patients with higher VWF:Ag levels showed a longer half-life [Figure 2b].
Figure 2.
Correlations between FVIII half-life and age, von Willebrand factor antigen (VWF:Ag) (n = 36). There were correlations between half-life and (a) age (P < 0.01), (b) VWF:Ag (P < 0.01). FVIII: Factor VIII; y: years.
Furthermore, patients with blood Group O showed significantly shorter FVIII half-life than patients with non-O blood group (9.40 ± 0.68 h vs. 12.30 ± 0.79 h, P = 0.01) [Table 3].
Table 3.
Parameters analyzed separately by blood groups
| Parameters | Blood group O (n = 17) | Blood group non-O (n = 19) | t | P |
|---|---|---|---|---|
| VWF:Ag (%) | 84.86 ± 6.58 | 111.4 ± 7.71 | 2.59 | 0.01 |
| t1/2 (h) | 9.40 ± 0.68 | 12.3 ± 0.79 | 2.70 | 0.01 |
| IVR (IU/dl per IU/kg) | 1.90 ± 0.12 | 1.91 ± 0.09 | 0.68 | 0.50 |
| CL (ml·kg−1·h−1) | 5.02 ± 0.38 | 4.00 ± 0.32 | 2.53 | 0.02 |
| MRT (h) | 8.50 ± 1.78 | 10.61 ± 2.01 | 3.23 | <0.01 |
VWF:Ag: Von Willebrand factor antigen; t1/2: Half-life; IVR: In vivo recovery; MRT: Mean residence time; CL: Clearance.
Factor VIII recovery
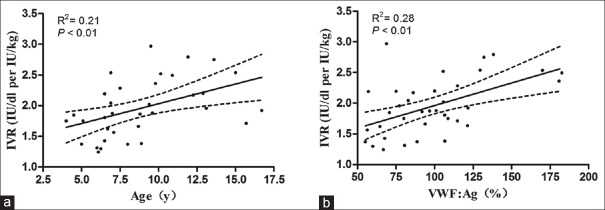
As shown in Figure 3, the FVIII recovery correlated with age (IVR = 1.39 + 0.06 × age, R2= 0.21, P = 0.005) and VWF:Ag (IVR = 0.23 + 0.007 × VWF:Ag, R2= 0.28, P < 0.01). Blood group had no effect on FVIII recovery. FVIII recovery in blood Group O patients was very similar to FVIII recovery in non-O blood groups (P > 0.05).
Figure 3.
Correlations between FVIII recovery and age, von Willebrand factor antigen (VWF:Ag) (n = 36). There were correlations between FVIII recovery and (a) age (P < 0.01), (b) VWF:Ag (P < 0.01). FVIII: Factor VIII; IVR: In vivo recovery; y: years.
Correlation between other pharmacokinetic parameters and baseline factors
In this study, we also analyzed other PK parameters and age, BMI, blood type, and VWF:Ag levels in all patients. The CL of FVIII was negatively correlated with age, VWF:Ag levels and BMI (CL = 6.53 − 0.25 × age, P < 0.01; CL = 7.66 − 0.03 × VWF:Ag, P < 0.01; CL = 6.69 − 0.14 × BMI, P < 0.05). There were obvious negative correlations of Vdss with age, VWF:Ag and BMI (P < 0.01). We also found that AUC0-48 and Cmax had a correlation with age and VWF:Ag (P < 0.01). Moreover, MRT and CL were affected by blood type [Table 3].
DISCUSSION
Knowledge of individual PK profiles of individuals with HA has the potential to play an important role in guiding the initiation, monitoring, and adjustment of prophylaxis regimens. However, there are few data about the PK of infused FVIII in boys with hemophilia in China. Although just 36 boys with severe HA were enrolled in this study, the total blood sampling size was more than 100 which met the requirement of population PK model.[20] We could find that some PK characteristics of hemophilia boys from this study were similar to other study. The FVIII is usually prescribed with 8–12 h half-life and an average IVR of 2 IU/dl per IU/kg. Our results showed that the mean half-life was 10.99 h and mean IVR was 2.01 IU/dl per IU/kg, but there are large differences in FVIII PK between individual patients. The FVIII half-life varied from 5.52 to 20.02 h and IVR range was 1.2–3.0 IU/dl per IU/kg. The target of prophylaxis in hemophilia is maintaining factor levels above 1 IU/dl, thereby converting the bleeding pattern of severe hemophilia into that of a milder type.[2] The tough level of FVIII is dependent on the patient's FVIII half-life and the factor dose/dosing frequency during factor prophylaxis. Longer FVIII half-life could lead to adapted prophylactic regimens for severe HA patients. The marked variation in PK parameters of infused FVIII in boys with severe HA suggests that the PK profile of individuals may be useful in developing optimal, individualized prophylaxis regimens in young boys with severe HA.
We also analyzed the influence of several parameters that potentially modulate FVIII PK in this study. Key parameters that influence the CL of infused FVIII in inhibitor negative persons with severe HA were patient age and the level of circulating VWF:Ag. We found that the shorter FVIII half-life and lower IVR were associated with younger age. Kepa et al.[21] and Björkman et al.[8] also found age to be an important predicting factor of FVIII half-life. In this study, FVIII half-life increased by 0.6 h per year. Blanchette et al.[17] also reported FVIII half-life increased by 0.4 h per year in children (<5 years) with severe HA. The median age of boys in the study was older than that of Blanchette's study. Above of all the results confirmed the effect of age on FVIII half-life.
VWF, the binding partner for FVIII, protect FVIII from protease degradation in the circulation as a protector protein for FVIII.[22] The low level or nonfunctional of VWF could lead to the FVIII activity decrease. All PK parameters had a correlation with VWF:Ag in the study. The lowest half-lives had been seen in cases with the lowest VWF:Ag levels. Preinfusion VWF:Ag levels were significantly associated with FVIII half-lives. Blood Group O was also an influence factor for PK. Patients with blood Group O showed significantly shorter FVIII half-life than patients with non-O blood group. Plasma VWF levels were significantly decreased in patients with blood Group O compared with those with non-O blood group. The FVIII half-life was shorter due to the lower VWF level in patients with blood Group O. Reflecting the fact that VWF:Ag levels are lower in persons who are blood Group O, CL rates of FVIII are higher in blood Group O versus non-blood Group O persons. Our results suggested a predict model that the hemophilia patients with low VWF:Ag levels and blood Group O might need more frequency FVIII infusion.
Other PK parameters were also affected by age, BMI, blood type, and VWF:Ag levels. These results are similar to the results reported by Blanchette et al. and Kepa et al.[17,21] Björkman's study also showed that IVR was lower, weight-adjusted CL was higher, and FVIII half-life was on average shorter in children than that in adults.[8,23] FVIII PK is affected by patient characteristics. We suggest repeated PK determination in HA boys and set an individual predict model for individual boy with hemophilia.
The present study has some limitations. The small number of patients enrolled in this study using different FVIII products did not allow the reaching of statistical significance, so we were unable to determine if there are differences between Chinese boys with hemophilia and other populations. Future studies involving larger numbers of individuals and more FVIIII concentrates are warranted.
PK of FVIII investigates the effects of the body on FVIII concentrates. Patients with the same FVIII dosage and infusion frequency might exhibit widely variable trough levels. The knowledge of individual PK might be a key factor for individually prophylaxis. If individual prophylaxis becomes available to the Chinese hemophilic population in the near future, this study about the PK characteristics of FVIII concentrates and evaluating whether some potentially FVIII-modulating parameters influence PK values would optimize individualized prophylaxis in China.
Financial support and sponsorship
This work was supported in part by grants from the Hemophilia Management System Project (No. IHECC2014HEM04), Novo Nordisk Haemophilia Research Fund in China, and Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (No. ZY201404).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We are grateful to Ms. Xiao-Lu Nie for analyzing the data.
Footnotes
Edited by: Yuan-Yuan Ji
REFERENCES
- 1.Fischer K, Ljung R. Primary prophylaxis in haemophilia care: Guideline update 2016. Blood Cells Mol Dis. 2017;67:81–5. doi: 10.1016/j.bcmd.2017.02.004. doi: 10.1016/j.bcmd.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Nilsson IM, Berntorp E, Löfqvist T, Pettersson H. Twenty-five years' experience of prophylactic treatment in severe haemophilia A and B. J Intern Med. 1992;232:25–32. doi: 10.1111/j.1365-2796.1992.tb00546.x. doi: 10.1111/j.1365-2796.1992.tb00546.x. [DOI] [PubMed] [Google Scholar]
- 3.Manco-Johnson MJ, Abshire TC, Shapiro AD, Riske B, Hacker MR, Kilcoyne R, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357:535–44. doi: 10.1056/NEJMoa067659. doi: 10.1056/NEJMoa067659. [DOI] [PubMed] [Google Scholar]
- 4.Fischer K, Steen Carlsson K, Petrini P, Holmström M, Ljung R, van den Berg HM, et al. Intermediate-dose versus high-dose prophylaxis for severe hemophilia: Comparing outcome and costs since the 1970s. Blood. 2013;122:1129–36. doi: 10.1182/blood-2012-12-470898. doi: 10.1182/blood-2012-12-470898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pasca S, Milan M, Sarolo L, Zanon E. PK-driven prophylaxis versus standard prophylaxis: When a tailored treatment may be a real and achievable cost-saving approach in children with severe hemophilia A. Thromb Res. 2017;157:58–63. doi: 10.1016/j.thromres.2017.07.003. doi: 10.1016/j.thromres.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Wu R, Luke KH, Poon MC, Wu X, Zhang N, Zhao L, et al. Low dose secondary prophylaxis reduces joint bleeding in severe and moderate haemophilic children: A pilot study in China. Haemophilia. 2011;17:70–4. doi: 10.1111/j.1365-2516.2010.02348.x. doi: 10.1111/j.1365-2516.2010.02348.x. [DOI] [PubMed] [Google Scholar]
- 7.Wu R, Sun J, Xiao J, Liu Y, Xue F, Wang H, et al. A prospective study of health-related quality of life of boys with severe haemophilia a in China: Comparing on-demand to prophylaxis treatment. Haemophilia. 2017;23:430–6. doi: 10.1111/hae.13198. doi: 10.1111/hae.13198. [DOI] [PubMed] [Google Scholar]
- 8.Björkman S, Blanchette VS, Fischer K, Oh M, Spotts G, Schroth P, et al. Comparative pharmacokinetics of plasma- and albumin-free recombinant factor VIII in children and adults: The influence of blood sampling schedule on observed age-related differences and implications for dose tailoring. J Thromb Haemost. 2010;8:730–6. doi: 10.1111/j.1538-7836.2010.03757.x. doi: 10.1111/j.1538-7836.2010.03757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ar MC, Vaide I, Berntorp E, Björkman S. Methods for individualising factor VIII dosing in prophylaxis. Eur J Haematol Suppl. 2014;76:16–20. doi: 10.1111/ejh.12370. doi: 10.1111/ejh.12370. [DOI] [PubMed] [Google Scholar]
- 10.Barnes C. Importance of pharmacokinetics in the management of hemophilia. Pediatr Blood Cancer. 2013;60(Suppl 1):S27–9. doi: 10.1002/pbc.24339. doi: 10.1002/pbc.24339. [DOI] [PubMed] [Google Scholar]
- 11.Tang L, Wu R, Sun J, Zhang X, Feng X, Zhang X, et al. Short-term low-dose secondary prophylaxis for severe/moderate haemophilia a children is beneficial to reduce bleed and improve daily activity, but there are obstacle in its execution: A multi-centre pilot study in China. Haemophilia. 2013;19:27–34. doi: 10.1111/j.1365-2516.2012.02926.x. doi: 10.1111/j.1365-2516.2012.02926.x. [DOI] [PubMed] [Google Scholar]
- 12.Yao W, Xiao J, Cheng X, Feng G, Li C, Zhang X, et al. The efficacy of recombinant FVIII low-dose prophylaxis in Chinese pediatric patients with severe hemophilia A: A retrospective analysis from the ReCARE study. Clin Appl Thromb Hemost. 2017;23:851–8. doi: 10.1177/1076029616679507. doi: 10.1177/1076029616679507. [DOI] [PubMed] [Google Scholar]
- 13.Morfini M, Lee M, Messori A. The design and analysis of half-life and recovery studies for factor VIII and factor IX. Factor VIII/Factor IX scientific and standardization committee of the international society for thrombosis and haemostasis. Thromb Haemost. 1991;66:384–6. [PubMed] [Google Scholar]
- 14.Scientific and Standardization Committee Communication. The Design and Analysis of Pharmacokinetic Studies of Coagulation Factors. 2001. [Last accessed on 2007 Aug 08]. pp. 1–9. Available from: http://www.med.unc.edu/isth/SSC/communications/factor8and9/FVIIIpharmaco.htm .
- 15.Morfini M, Marchesini E, Paladino E, Santoro C, Zanon E, Iorio A, et al. Pharmacokinetics of plasma-derived vs.recombinant FVIII concentrates: A comparative study. Haemophilia. 2015;21:204–9. doi: 10.1111/hae.12550. doi: 10.1111/hae.12550. [DOI] [PubMed] [Google Scholar]
- 16.Björkman S. Limited blood sampling for pharmacokinetic dose tailoring of FVIII in the prophylactic treatment of haemophilia A. Haemophilia. 2010;16:597–605. doi: 10.1111/j.1365-2516.2009.02191.x. doi: 10.1111/j.1365-2516.2009.02191.x. [DOI] [PubMed] [Google Scholar]
- 17.Blanchette VS, Shapiro AD, Liesner RJ, Hernández Navarro F, Warrier I, Schroth PC, et al. Plasma and albumin-free recombinant factor VIII: Pharmacokinetics, efficacy and safety in previously treated pediatric patients. J Thromb Haemost. 2008;6:1319–26. doi: 10.1111/j.1538-7836.2008.03032.x. doi: 10.1111/j.1538-7836.2008.03032.x. [DOI] [PubMed] [Google Scholar]
- 18.Lee ML, Poon WY, Kingdon HS. A two-phase linear regression model for biologic half-life data. J Lab Clin Med. 1990;115:745–8. [PubMed] [Google Scholar]
- 19.Lee ML, Gomperts ED, Kingdon HS. A note on the calculation of recovery for factor VIII infusions. Thromb Haemost. 1993;69:87. [PubMed] [Google Scholar]
- 20.Ogungbenro K, Aarons L. Sample-size calculations for multi-group comparison in population pharmacokinetic experiments. Pharm Stat. 2010;9:255–68. doi: 10.1002/pst.388. doi: 10.1002/pst.388. [DOI] [PubMed] [Google Scholar]
- 21.Kepa S, Horvath B, Reitter-Pfoertner S, Schemper M, Quehenberger P, Grundbichler M, et al. Parameters influencing FVIII pharmacokinetics in patients with severe and moderate haemophilia A. Haemophilia. 2015;21:343–50. doi: 10.1111/hae.12592. doi: 10.1111/hae.12592. [DOI] [PubMed] [Google Scholar]
- 22.Chen J, Schroeder JA, Luo X, Shi Q. The impact of von Willebrand factor on factor VIII memory immune responses. Blood Adv. 2017;1:1565–74. doi: 10.1182/bloodadvances.2017009209. doi: 10.1182/bloodadvances.2017009209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Björkman S, Oh M, Spotts G, Schroth P, Fritsch S, Ewenstein BM, et al. Population pharmacokinetics of recombinant factor VIII: The relationships of pharmacokinetics to age and body weight. Blood. 2012;119:612–8. doi: 10.1182/blood-2011-07-360594. doi: 10.1182/blood-2011-07-360594. [DOI] [PubMed] [Google Scholar]