Abstract
Background:
Central nervous system (CNS) involvement is found in many patients with hemophagocytic lymphohistiocytosis (HLH). In this study, we mainly analyzed neurological symptoms, imaging findings, cerebrospinal fluid (CSF), and their relationship with outcomes of HLH children.
Methods:
Related data of 179 Chinese pediatric patients with HLH admitted to our center from January 2010 to December 2015 were analyzed retrospectively. Diagnosis and treatment were based on the HLH-2004 protocol. Two-tailed Chi-squared test was used to compare between different groups, and Kaplan-Meier survival curves were used to analyze the overall survival (OS) of patients with HLH.
Results:
In the present study, 21.2% (38/179) of total patients had neurological symptoms including seizure, irritability, somnolence, and unconsciousness. There were 80 (50.0%, excluding 19 patients without imaging data) patients with cranial imaging abnormalities. There were 14.7% (17/116, excluding 63 patients who did not accept lumbar puncture) of patients with abnormal CSF results. CNS involvement is defined as abnormalities in one or more of CNS symptoms, radiological findings, and CSF. Thus, 60.3% of them had CNS involvement. As for the prognosis, the median follow-up time was 3.2 years (17 lost to follow-up). The probable 3-year OS of children was higher without CNS involvement (86.0% ± 4.6%) than those with CNS involvement (68.9% ± 4.9%, hazard ratio [HR] = 2.286, P = 0.019). Among them, the probable 3-year OS of children without CNS symptoms was 76.0% ± 3.8%, higher than with CNS symptoms (59.5% ± 8.1%, HR = 2.147, P = 0.047). The 3-year OS of children with abnormal CSF was 64.7% ± 11.6%, compared with normal CSF (85.1% ± 3.7%, HR = 0.255, P = 0.038).
Conclusions:
HLH patients with CNS involvement might have worse outcomes compared with those without CNS involvement, and CNS symptoms and CSF changes are more important to access the prognosis than imaging abnormality.
Keywords: Central Nervous System, Cerebrospinal Fluid, Hemophagocytic Lymphohistiocytosis
摘要
背景:
嗜血细胞性淋巴组织细胞增生症(Hemophagocytic Lymphohistiocytosis, HLH)是一种严重威胁患儿生命的临床综合征,许多HLH患儿合并中枢神经系统(CNS)受累。本文主要分析了HLH患儿的CNS症状、影像学表现、脑脊液和预后之间的关系。
方法:
回顾性分析了2010年1月至2015年12月之间就诊于我中心的179例HLH患儿的相关数据。诊断和治疗均根据HLH-2004方案。卡方检验用于分析各组之间的构成比,Kaplan-Meier生存曲线用于分析预后。
结果:
在这179例HLH患儿中,21.2%(38/179)存在神经系统症状,包括抽搐、易激惹、嗜睡、意识障碍等,80名患儿(50%,排除19例未做影像检查者)存在影像学异常,14.7%(17/116,排除63例未行腰穿者)具有脑脊液异常。存在症状、影像学、脑脊液三者其一异常即被认为具有CNS受累。因此,60.3%的HLH患儿具有CNS受累。预后方面,平均随访时间为3.2年(17名失访)。合并CNS受累的患儿3年生存率(68.9%±4.9%)明显低于无CNS受累组(86.0%±4.6%,风险比[HR]=2.286, p=0.019)。其中,存在CNS临床症状患儿的生存率(59.5%±8.1%)亦低于无CNS症状组(76.0%±3.8%,HR=2.147, p=0.047)。脑脊液异常患儿生存率(64.7%±11.6%)低于脑脊液正常组(85.1%±3.7%,HR=0.255, p=0.038)。
结论:
存在CNS受累的HLH患儿预后明显差于无CNS受累者,并且合并CNS临床症状和CSF异常相对于影像学改变更具意义。
INTRODUCTION
Hemophagocytic lymphohistiocytosis (HLH) is a life-threatening blood disorder, with high morbidity and mortality caused by the unbridled inflammation and immune dysregulation.[1,2] The disease manifests with persistent fever, hepatosplenomegaly, pancytopenia, hypofibrinogenemia, hypertriglyceridemia, hyperferritinemia, and sometimes hemophagocytosis. It is classified as primary or familial HLH and secondary HLH based on the disease etiology.[3]
Familial HLH is a congenital disease with an impaired immunomodulation resulted from autosomal or X-linked recessive gene defect; the presentation of the symptom is found commonly in infants during the 1st year of life. It is a congenital disorder with defects in immunomodulation.[4] While Epstein-Barr virus (EBV) infection is a well-known trigger for both primary and secondary HLH, it plays a prevailing role to induce secondary HLH onset.[5,6] Other than the EBV-associated secondary HLH (EBV-sHLH), the main subtype of secondary HLH, infections caused by other agents such as cytomegalovirus (CMV) or herpes simplex virus (HSV), could also present HLH symptoms such as leukemia, lymphoma, Langerhans cell histiocytosis (LCH), and autoimmune or autoinflammatory disorders.[7,8]
HLH results in a multisystem impairment and infiltration of inflammatory cells in relative tissues or organs, such as spleen, liver, bone marrow, and central nervous system (CNS), leading to a series of clinical presentations.[9,10,11] CNS symptoms are one of the clinical manifestations frequently found in HLH patients. It was previously reported that CNS involvement of HLH children in Korea was associated with poor outcome.[12] In a study on patients with HLH in China, it was reported that 46.7% (20/43) of all investigated patients had CNS involvement.[13] However, the incidence and prognosis of CNS involvement in different kinds of HLH in Chinese children have not been investigated.
To explore this question, we retrospectively analyzed the clinical data of 179 Chinese children diagnosed with HLH at Beijing Children's Hospital and compared their different CNS involvement and outcomes in FHL, EBV-sHLH, and other sHLH.
METHODS
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Beijing Children's Hospital (No. 2018-k-47). As a retrospective study, this study was exempt from informed patients' consent.
Patients
A total of 179 children were diagnosed as HLH at our center between January 2010 and December 2015. Diagnosis and treatment were based on the HLH-2004 protocol.[14] The median follow-up time is 3 years (until March 31, 2017, 17 failed to follow-up). Nineteen cases were diagnosed as FHL based on the fact that homozygous or compound heterozygous mutations were found in HLH-related genes. Because EBV-sHLH is regarded as an important subtype of secondary HLH, 97 EBV-sHLH cases were selected as another group. Additional 63 secondary HLH cases were defined as other sHLH. Therefore, we distributed the total cases into three groups: FHL, EBV-sHLH, and other sHLH.
Central nervous system involvement
HLH patients with one or more neurological manifestation of seizure, irritability, somnolence, coma, myasthenia, hypomyotonia, paralysis, and unconsciousness were considered as CNS involvement. Their clinical data were subjected to further analyses.
Cranial magnetic resonance imaging (MRI) was performed on 160 patients of total 179 patients investigated. The imaging evidence of pathological CNS alteration included white matter signal changes, cerebral volume loss, edema, enlargement of ventricles, and intracerebral calcification.
Lumbar punctures were performed in 117 patients to detect the leukocytes and protein content in cerebrospinal fluid (CSF). Leukocyte number above 19 × 109/L or total protein concentration above 500 mg/L in CSF was regarded as abnormal.[15]
Therapeutic regimen
Once patients were diagnosed as HLH, they were immediately received HLH-94/04 protocol. After the causes were determined, they were given the corresponding etiological treatment. For the CNS-HLH patients, intrathecal injections were performed based on the HLH-94/04 protocol.[14]
Statistical analysis
Median and range of the data, which did not conform to a normal distribution, were displayed. GraphPad Prism 5 statistical analysis software (GraphPad Software, Inc., CA, USA) was used. The two-tailed Chi-squared test was applied to compare constituent ratio between different groups. A value of P < 0.05 was considered to denote a significant difference. Kaplan-Meier survival curves were used to analyze the overall survival (OS) of HLH patients.
RESULTS
Patients
We divided the total 179 patients into three groups (FHL, EBV-sHLH, and other sHLH). Detailed information about the patients is shown in Table 1. In total, 19 (10.6%) of 179 patients had familial HLH. The mean age was 2.80 ± 0.67 years (range: 5 months to 9.6 years), and the median age was 1.4 years. Of 179 patients, 97 (54.2%) were diagnosed as EBV-sHLH (mean age of 3.60 ± 0.35 years; range: 39 days to 14.8 years; median: 2.1 years). Of 179 patients, 63 (35.2%) children had other sHLH caused by infection of CMV or HSV or associated with diseases such as leukemia, lymphoma, LCH, and some autoimmune disorders or uncertain reasons. The mean age was 3.60 ± 0.50 years (range: 48 days to 13.8 years) and the median age was 1.8 years.
Table 1.
General information of the 179 children with HLH
| HLH | Total | FHL | EBV-sHLH | Other sHLH |
|---|---|---|---|---|
| Number | 179 | 19 | 97 | 63 |
| Proportion (%) | 100 | 10.6 | 54.2 | 35.2 |
| Male/female | 99/80 | 13/6 | 51/46 | 35/28 |
| Mean age (years) | 3.50 ± 0.27 | 2.80 ± 0.67 | 3.60 ± 0.35 | 3.60 ± 0.50 |
| Median age (years) | 2.0 | 1.4 | 2.1 | 1.8 |
Data are presented as mean ± SD. HLH: Hemophagocytic lymphohistiocytosis; FHL: Familial HLH; EBV-sHLH: Epstein-Barr virus-associated secondary HLH; Other sHLH: Other secondary HLH; SD: Standard deviation.
Neurological symptoms
HLH patients always have involvement of multiple tissues and organs, including hematology system, cardiovascular system, respiratory system, and urology system. In this study, 38 (21.2%) of 179 HLH children had CNS symptoms, regardless of their subgroups of HLH. Among them, nine (23.7%) patients were diagnosed as FHL, 14 (36.8%) as EBV-sHLH, and the rest 15 (39.5%) as other sHLH. Neurological complications included seizures, irritation, somnolence, coma, myasthenia, hypomyotonia, paralysis, and unconsciousness. Irritation was the most common CNS manifestation and found in 23 (50.5%) of 38 children. Seizure of general or focal nature was involved in 15 (39.5%) of 38 patients, and unconsciousness or coma was seen in 11 (28.9%) of 38 children. Other neurological complications included drowsiness (8/38, 21.1%), hypotonia or hypomyotonia (5/38, 13.2%), positively pathological reflex (5/38, 13.2%), cranial nerve palsy (3/38, 7.9%), and meningitis (2/38, 5.3%). A total of 20 (52.6%) patients of the 38 patients with CNS symptoms had more than one abovementioned clinical presentations.
Regarding the percentage of CNS symptoms in different HLH patients, 47.4% (9/19) of children had neurological symptoms in FHL group, higher than that in EBV-sHLH group (14.4%, 14/97, χ2= 10.840, P = 0.001) or in other sHLH group (23.8%, 15/63, χ2= 3.914, P = 0.048) [Table 2]. In summary, neurological symptoms were found more frequently in patients with FHL, rather than those in EBV-sHLH and other sHLH groups. Significant differences could be found among these three groups (χ2= 10.690, P = 0.005) [Table 2].
Table 2.
Central nervous system involvement of children with HLH
| Parameters | CNS symptoms | Imaging findings | CSF results | CNS involvement | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | With symptoms | Proportion (%) | n | Imaging changes | Proportion (%) | n | CSF abnormality | Proportion (%) | n | With involvement | Proportion (%) | |
| Total | 179 | 38 | 21.2 | 160 | 80 | 50.0 | 116 | 17 | 14.7 | 156 | 94 | 60.3 |
| FHL | 19 | 9 | 47.4 | 18 | 13 | 72.2 | 12 | 7 | 58.3 | 17 | 13 | 76.5 |
| EBV-sHLH | 97 | 14 | 14.4 | 88 | 44 | 50.0 | 63 | 3 | 4. | 87 | 49 | 56.3 |
| Other sHLH | 63 | 15 | 23.8 | 54 | 23 | 42.6 | 41 | 7 | 17.1 | 52 | 32 | 61.5 |
| χ2 | 10.690 | 4.741 | 23.430 | 2.464 | ||||||||
| P | 0.005 | 0.093 | <0.0001 | 0.292 | ||||||||
Data are presented as numbers and proportions of different groups. HLH: Hemophagocytic lymphohistiocytosis; FHL: Familial HLH; EBV-sHLH: Epstein-Barr virus-associated secondary HLH; Other sHLH: Other secondary HLH; CNS: Central nervous system; CSF: Cerebrospinal fluid.
Neurological imaging findings
A total of 160 children (18 FHL, 88 EBV-sHLH, and 54 other sHLH) subjected to MRI scanning, and 80 (50.0%) of them had radiological changes. Of 80 patients with CNS imaging abnormality, 13 (16.3%) were in FHL group, 44 (55.0%) were in EBV-sHLH group, and the remaining 23 patients (28.8%) were in other sHLH [Table 2]. The abnormal findings of cranial MRI included white matter signal changes, cerebral volume loss, intracerebral calcification, enlargement of ventricles, and edema. The two most frequent changes were white matter signal abnormalities (67.5%, 54/80) and encephalatrophy (52.5%, 42/80), and 21 (26.3%) of 80 patients had both of the two changes.
For patients with different kinds of HLH, 13 (72.2%) of 18 patients showed imaging abnormalities in FHL group, with 50.0% (44/88) in EBV-sHLH group and 42.6% (23/54) in other sHLH group. Although radiological abnormalities were detected more commonly in FHL children, there were no significant difference among different groups of HLH (χ2= 4.741, P = 0.093) [Table 2]. However, the fact that six out of ten patients with imaging changes in FHL group had extensive and multifocal changes in white matter implied that FHL patients were prone to have severe changes in CNS. To be noted, the proportions of patients with altered radiological findings were higher than those with CNS symptoms in all three groups. This indicates that imaging abnormalities are not equivalent to clinical presentation in HLH children, regardless of FHL, EBV-sHLH, or other sHLH.
Cerebrospinal fluid
Lumbar punctures were performed in 116 HLH patients to detect CSF content, including white blood cell (WBC) number as well as total protein concentration. Of 12 FHL children, 7 (58.3%) presented changes in CSF. By contrast, only three (4.8%) of all 63 EBV-sHLH patients subjected to lumbar punctures exhibited abnormal CSF. In other sHLH group, abnormal CSF results were found in seven (17.1%) of 41 children [Table 2]. These results revealed that CSF abnormality is more closely associated with FHL patients, but not the EBV-sHLH patients, which showed lowest ratio of abnormal CSF. Significant differences could be found in the three groups (χ2= 23.430, P < 0.0001) [Table 2]. In these 17 patients who had abnormal CSF results, 13 (76.5%) only had increased protein level, two patients (11.8%) only had increased WBC number, and the remaining two (11.8%) children had both abnormalities.
Central nervous system involvement
CNS involvement is defined as abnormalities in one or more of CNS symptoms, radiological findings, and CSF. Excluding 23 patients without neurological imaging or CSF data, (among them, 21 without CNS symptoms), 94 (94/156, 60.3%) were identified as having CNS involvement. Among FHL children, 76.5% (13/17) had CNS involvement. There were 56.3% (49/87) of patients who had CNS involvement in EBV-sHLH group, while 61.5% (32/52) in other sHLH. However, no significant difference was found among these groups (χ2= 2.464, P = 0.292) [Table 2].
Among these 94 patients with CNS involvement, 11 (11.7%) of them only had CNS symptoms, 54 (57.4%) only had imaging changes, and three (3.2%) only had abnormal CSF. There were 35 (37.2%) of 94 patients who had two or three evidence of CNS involvement. Among these 35 HLH children, 12 were in FHL group, 12 were in EBV-sHLH group, and 11 were in other sHLH groups. Interestingly, all FHL patients with neurological clinical manifestations showed imaging abnormalities. In EBV-sHLH and other sHLH group, most children (79.5% [35/44] and 69.6% [16/23], respectively) with radiological involvement did not have the CNS symptom.
Prognosis
Except for 17 patients who were failed to follow-up, 162 cases were investigated until March 31, 2017, and 117 of them were alive at that time.
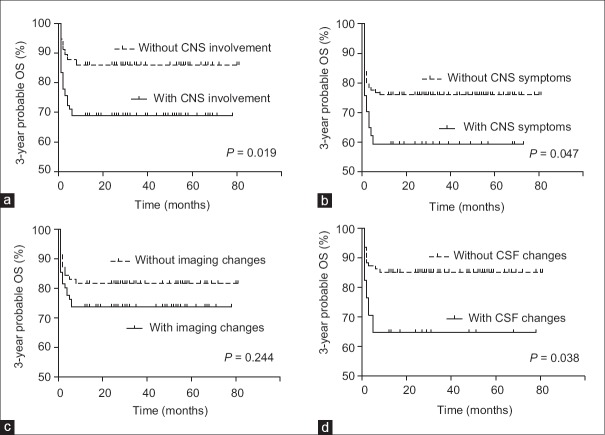
Abnormalities in one or more of CNS symptoms, radiological findings, and CSF were regarded as CNS involvement. Excluding the cases lost to follow-up and with incomplete data, 3-year probable OS was 68.9% ± 4.9% (62/90) in the group of CNS involvement, which was obviously lower than that in patients without CNS involvement (86.0% ± 4.6%, 49/57; hazard ratio [HR] = 2.286, P = 0.019) [Table 3 and Figure 1a]. Thus, we need to figure out which factors in these three factors (CNS symptoms, imaging findings, and CSF) were more important to influence prognosis.
Table 3.
Prognosis of children diagnosed HLH with or without CNS involvement
| HLH | With/without (+/–) | Alive | Dead | OS (%) | HR | P |
|---|---|---|---|---|---|---|
| CNS involvement | + | 62 | 28 | 68.9 ± 4.9 | ||
| – | 49 | 8 | 86.0 ± 4.6 | 2.286 | 0.019 | |
| CNS symptoms | + | 22 | 15 | 59.5 ± 8.1 | ||
| – | 95 | 30 | 76.0 ± 3.8 | 2.147 | 0.047 | |
| Imaging changes | + | 56 | 20 | 73.7 ± 5.1 | ||
| – | 58 | 13 | 81.7 ± 4.6 | 0.657 | 0.244 | |
| Abnormal CSF | + | 11 | 6 | 64.7 ± 11.6 | ||
| – | 80 | 14 | 85.1 ± 3.7 | 0.255 | 0.038 |
Data are presented as mean ± SD. HLH: Hemophagocytic lymphohistiocytosis; CNS: Central nervous system; CSF: Cerebrospinal fluid; HR: Hazard ratio; OS: Overall survival; SD: Standard deviation.
Figure 1.
Kaplan-Meier survival curves of children with hemophagocytic lymphohistiocytosis. (a) Survival curve of HLH patients with or without CNS symptoms. (b) Survival curve of HLH patients with or without imaging changes. (c) Survival curve of HLH patients with or without CSF changes. (d) Survival curve of HLH patients with or without CNS involvement. HLH: Hemophagocytic lymphohistiocytosis; CNS: Central nervous system; CSF: Cerebrospinal fluid; OS: Over-all survival.
Based on the CNS symptoms, 15 children with neurological symptoms were dead, but 22 were alive (one failed to follow-up). On the other hand, 30 of 125 patients without CNS clinical manifestations were dead. The 3-year probable OS of children with neurological symptoms (59.5% ± 8.1%) was lower than those without CNS symptoms (76.0% ± 3.8%; HR = 2.147, P = 0.047) [Table 3 and Figure 1b]. In addition, among the 15 patients with seizure, eight were alive, and the 3-year OS was 53.3%; among the 11 patients with coma, five were alive, and the 3-year OS was 45.5%; there were six patients with both seizure and coma, and the 3-year OS was 50.0% ± 17.2% (three of them were alive).
In the group of 76 children with abnormal imaging findings, 56 children were alive. In the other group of 71 patients without MRI changes, 58 patients were alive. The 3-year probable OS were 73.7% ± 5.1% (with imaging changes) and 81.7% ± 4.6% (without imaging changes), respectively, with no statistical difference (HR = 0.657, P = 0.244) [Table 3 and Figure 1c]. However, patients with extensive and multifocal white matter signal abnormalities in T2-weighted images showed a lower 3-year OS of 50% ± 15.8% (5/10) compared with those who only had punctuate or focal changes or without changes (79.6% ± 3.5%, 109/137, HR = 0.208, P = 0.033).
In a group of 17 patients with abnormal CSF, 11 (64.7% ± 11.6%) were alive. In the normal CSF group, 80/94 (85.1% ± 3.7%) were alive. And, the difference was statistically significant (HR = 0.255, P = 0.038) [Table 3 and Figure 1d].
Among FHL, EBV-HLH, and other-sHLH groups, no significant difference could be found in their OS (P = 0.257). This might be contributed to the limited cases in FHL group, and more patients should be collected to analyze more accurately.
DISCUSSION
The study showed that CNS involvement was an important risk factor for HLH patients, and CNS symptoms might be the most important prognostic factor in CNS involvement. It was reported that neurological symptoms could be seen in the range from 10% to 38% of HLH patients.[12,13,16,17,18] According to our conclusions and other reports, irritability and seizure were the two most frequent CNS symptoms.[13,15,18] Moreover, we noticed that HLH patients with seizure or coma had the worst prognosis. Doctors should pay more attention to these HLH patients who have CNS symptoms, especially those with coma or seizure. Early treatments with focus on CNS involvement and stem cell transplantation are needed to improve their prognosis.
Both previous study and our results showed that white matter signal changes and cerebral volume loss are the two most frequent abnormalities in HLH patients with CNS involvement.[19] However, no changes in MRI could be considered as a specific manifestation of HLH so far.[20,21] These changes can also be found in some other demyelinating diseases, such as multiple sclerosis and acute disseminated encephalomyelitis. If a patient has cerebral white matter signal changes in T2-weighted images accompanied with fever, splenomegaly, cytopenia, or elevated liver enzymes, HLH with CNS involvement should be considered. Serum ferritin, triglyceride, fibrinogen, NK cell activity, sCD25, and bone marrow smear should be tested to confirm or exclude the diagnosis of HLH with CNS involvement. Although there are no specific changes in imaging findings till now, we discovered that extensive and multifocal changes in white matter often indicated worse prognosis in HLH patients. Furthermore, we found that MRI image changes could be partially reversed in 63.8% (51/80) patients after treatment (especially in EBV-sHLH patients). It was not easy for the abnormalities to return to normal (7.5%, 6/80).
We also found that increased CSF protein level was the main abnormality and contributed to a bad prognosis in former study.[18] We have not found any specific change in the CSF results of HLH patients yet. Thus, we must differentiate other diseases carefully, such as CNS infection and autoimmune CNS disorder. We found that once HLH patients had both CNS symptoms (especially coma) and abnormal CSF (especially increased protein level), they would have worse prognosis.
Pediatric FHL patients always show worse outcome compared with those with secondary HLH. Interestingly, in this retrospective study, we found that FHL patients with CNS symptoms all had abnormal imaging findings, whereas 55 of 80 sHLH patients with abnormal cranial imaging performance did not have CNS clinical manifestations. This suggested that abnormal cranial imaging could occur earlier than CNS clinical manifestations. Thus, all HLH patients should take the MRI examination, regardless whether they have CNS symptoms or not.
In regard to treatment, CNS involvement is not an isolated disease itself, but a part of a systemic inflammatory response, predicting a worse outcome. Thus, in this study, we chose HLH-2004 protocol with intrathecal treatment as a standard care or first-line therapy to treat patients with CNS involvement.[14,21] As reports showed that dexamethasone has a longer half-life and better CSF penetration than prednisone, we injected intrathecal with dexamethasone. In the 17 patients with abnormal CSF, 14 of them reexamined CSF after 4 weeks and 85.7% (12/14) returned to normal, which was similar with a latest study.[22] Besides, hematopoietic stem cell transplantation (HSCT) was performed in eight patients and seven (87.5%) of them had long survivals. In this study, five out of seven patients got improvements in cerebral MRI images after HSCT. In all, early HSCT after well control of HLH status is necessary for HLH patients with CNS involvement.[23]
However, there are also some limitations in this study. For example, in the other HLH group, the primary diseases were variables, including infections caused by other agents such as CMV, HSV, or Leishmania major; hematologic malignancies such as leukemia, lymphoma, and LCH; autoimmune or autoinflammatory disorders such as systemic lupus erythematosus, systemic juvenile idiopathic arthritis, and scleroderma; and some uncertain causes. It had been known that different protopathies might lead to different outcomes. In this study, there were five patients with lymphoma, and two of them were dead. There were two acute myeloid leukemia-related HLH patients: one was dead and the other with whom we lost contact. Patients with connective tissue disease seemed to show a better prognosis in another research (unpublished) which need to accumulate more data. In our following study, more cases should be included to analyze the OS according to different primary diseases.
Moreover, HLH always impairs multiple tissues and organs, not only CNS but also digestive system, respiratory system, and immune system. Therefore, many laboratory examinations could be found such as increased liver enzyme, ferritin, inflammatory medium and triglyceride, and decreased fibrinogen. It is well known that HLH is a systematic inflammatory disease, but the pathogenic mechanism of CNS susceptibility is unclear. There is a question that whether there are some correlations between these abnormal laboratory results and CNS involvement. It is difficult to conclude based on the limited data, and improved study should be performed in the future.
In summary, we find that HLH children with CNS involvement always have worse outcomes than those without CNS involvement. CNS symptoms (especially coma) and extensive and multifocal white matter changes in MRI and abnormal CSF results are found having more significance to the prognosis. FHL patients are prone to have CNS involvement and show the poorest outcome, compared with EBV-sHLH and other sHLH groups. Systemic treatment of HLH and intrathecal injection with methotrexate and corticosteroids is still first-line treatment of HLH with CNS involvement. Early HSCT after keeping HLH status in control is strongly recommended for HLH patients with CNS involvement.
Financial support and sponsorship
This work was supported by grants from the Technology Key Projects (No. 2017ZX09304029004), the Beijing Municipal Science and Technology Commission (No. Z171100001017050), the National Natural Science Foundation of China (No. 81700186), the Scientific Research Common Program of Beijing Municipal Commission of Education (No. KM201710025019), the Pediatric Project of Ai You Foundation (No. AYEK201802), and the Talent Training Project-Fostering Fund of National Natural Science Foundation of Beijing Children's Hospital, Capital Medical University (No. GPY201713).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Peng Lyu
REFERENCES
- 1.Chuang HC, Lay JD, Hsieh WC, Su IJ. Pathogenesis and mechanism of disease progression from hemophagocytic lymphohistiocytosis to epstein-barr virus-associated T-cell lymphoma: Nuclear factor-kappa B pathway as a potential therapeutic target. Cancer Sci. 2007;98:1281–7. doi: 10.1111/j.1349-7006.2007.00549.x. doi: 10.1111/j.1349-7006.2007.00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Molleran Lee S, Villanueva J, Sumegi J, Zhang K, Kogawa K, Davis J, et al. Characterisation of diverse PRF1 mutations leading to decreased natural killer cell activity in North American families with haemophagocytic lymphohistiocytosis. J Med Genet. 2004;41:137–44. doi: 10.1136/jmg.2003.011528. doi: 10.1136/jmg.2003.011528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janka GE. Hemophagocytic syndromes. Blood Rev. 2007;21:245–53. doi: 10.1016/j.blre.2007.05.001. doi: 10.1016/j.blre.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Degar B. Familial hemophagocytic lymphohistiocytosis. Hematol Oncol Clin North Am. 2015;29:903–13. doi: 10.1016/j.hoc.2015.06.008. doi: 10.1016/j.hoc.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 5.Jin YK, Xie ZD, Yang S, Lu G, Shen KL. Epstein-barr virus-associated hemophagocytic lymphohistiocytosis: A retrospective study of 78 pediatric cases in mainland of China. Chin Med J. 2010;123:1426–30. doi: 10.3760/cma.j.issn.0366-6999.2010.11.014. [PubMed] [Google Scholar]
- 6.Ishii E, Ohga S, Imashuku S, Yasukawa M, Tsuda H, Miura I, et al. Nationwide survey of hemophagocytic lymphohistiocytosis in Japan. Int J Hematol. 2007;86:58–65. doi: 10.1532/IJH97.07012. doi: 10.1532/IJH97.07012. [DOI] [PubMed] [Google Scholar]
- 7.Filipovich AH. Hemophagocytic lymphohistiocytosis and related disorders. Curr Opin Allergy Clin Immunol. 2006;6:410–5. doi: 10.1097/01.all.0000246626.57118.d9. doi: 10.1097/01.all.0000246626.57118.d9. [DOI] [PubMed] [Google Scholar]
- 8.Janka GE. Familial and acquired hemophagocytic lymphohistiocytosis. Eur J Pediatr. 2007;166:95–109. doi: 10.1007/s00431-006-0258-1. doi: 10.1007/s00431-006-0258-1. [DOI] [PubMed] [Google Scholar]
- 9.Decaminada N, Cappellini M, Mortilla M, Del Giudice E, Sieni E, Caselli D, et al. Familial hemophagocytic lymphohistiocytosis: Clinical and neuroradiological findings and review of the literature. Childs Nerv Syst. 2010;26:121–7. doi: 10.1007/s00381-009-0957-9. doi: 10.1007/s00381-009-0957-9. [DOI] [PubMed] [Google Scholar]
- 10.Gupta S, Weitzman S. Primary and secondary hemophagocytic lymphohistiocytosis: Clinical features, pathogenesis and therapy. Expert Rev Clin Immunol. 2010;6:137–54. doi: 10.1586/eci.09.58. doi: 10.1586/eci.09.58. [DOI] [PubMed] [Google Scholar]
- 11.Koh KN, Im HJ, Chung NG, Cho B, Kang HJ, Shin HY, et al. Clinical features, genetics, and outcome of pediatric patients with hemophagocytic lymphohistiocytosis in Korea: Report of a nationwide survey from Korea histiocytosis working party. Eur J Haematol. 2015;94:51–9. doi: 10.1111/ejh.12399. doi: 10.1111/ejh.12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim MM, Yum MS, Choi HW, Ko TS, Im HJ, Seo JJ, et al. Central nervous system (CNS) involvement is a critical prognostic factor for hemophagocytic lymphohistiocytosis. Korean J Hematol. 2012;47:273–80. doi: 10.5045/kjh.2012.47.4.273. doi: 10.5045/kjh.2012.47.4.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang S, Zhang L, Jia C, Ma H, Henter JI, Shen K, et al. Frequency and development of CNS involvement in Chinese children with hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2010;54:408–15. doi: 10.1002/pbc.22239. doi: 10.1002/pbc.22239. [DOI] [PubMed] [Google Scholar]
- 14.Henter JI, Horne A, Aricó M, Egeler RM, Filipovich AH, Imashuku S, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48:124–31. doi: 10.1002/pbc.21039. doi: 10.1002/pbc.21039. [DOI] [PubMed] [Google Scholar]
- 15.Haddad E, Sulis ML, Jabado N, Blanche S, Fischer A, Tardieu M, et al. Frequency and severity of central nervous system lesions in hemophagocytic lymphohistiocytosis. Blood. 1997;89:794–800. [PubMed] [Google Scholar]
- 16.Janka GE. Familial hemophagocytic lymphohistiocytosis. Eur J Pediatr. 1983;140:221–30. doi: 10.1007/BF00443367. doi: 10.1007/BF00443367. [DOI] [PubMed] [Google Scholar]
- 17.Hirst WJ, Layton DM, Singh S, Mieli-Vergani G, Chessells JM, Strobel S, et al. Haemophagocytic lymphohistiocytosis: Experience at two U.K. centres. Br J Haematol. 1994;88:731–9. doi: 10.1111/j.1365-2141.1994.tb05111.x. doi: 10.1111/j.1365.2141.1994.tb05111.x. [DOI] [PubMed] [Google Scholar]
- 18.Horne A, Trottestam H, Aricò M, Egeler RM, Filipovich AH, Gadner H, et al. Frequency and spectrum of central nervous system involvement in 193 children with haemophagocytic lymphohistiocytosis. Br J Haematol. 2008;140:327–35. doi: 10.1111/j.1365-2141.2007.06922.x. doi: 10.1111/j.1365-2141.2007.06922.x. [DOI] [PubMed] [Google Scholar]
- 19.Fitzgerald NE, MacClain KL. Imaging characteristics of hemophagocytic lymphohistiocytosis. Pediatr Radiol. 2003;33:392–401. doi: 10.1007/s00247-003-0894-9. doi: 10.1007/s00247-003-0894-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson TL, Carr CM, Kaufmann TJ. Central nervous system imaging findings of hemophagocytic syndrome. Clin Imaging. 2015;39:1090–4. doi: 10.1016/j.clinimag.2015.07.013. doi: 10.1016/j.clinimag.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 21.Horne A, Wickström R, Jordan MB, Yeh EA, Naqvi A, Henter JI, et al. How to treat involvement of the central nervous system in hemophagocytic lymphohistiocytosis? Curr Treat Options Neurol. 2017;19:3. doi: 10.1007/s11940-017-0439-4. doi: 10.1007/s11940-017-0439-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song Y, Pei RJ, Wang YN, Zhang J, Wang Z. Central nervous system involvement in hemophagocytic lymphohistiocytosis in adults: A retrospective analysis of 96 patients in a single center. Chin Med J. 2018;131:776–83. doi: 10.4103/0366-6999.228234. doi: 10.4103/0366-6999.228234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jordan MB, Allen CE, Weitzman S, Filipovich AH, McClain KL. How I treat hemophagocytic lymphohistiocytosis. Blood. 2011;118:4041–52. doi: 10.1182/blood-2011-03-278127. doi: 10.1182/blood-2011-03-278127. [DOI] [PMC free article] [PubMed] [Google Scholar]