Abstract
Background:
Technical aspects of the correct placement of medial support locking screws in the locking plate for proximal humerus fractures remain incompletely understood. This study was to evaluate the clinical relationship between the number of medial support screws and the maintenance of fracture reduction after locked plating of proximal humerus fractures.
Methods:
We retrospectively evaluated 181 patients who had been surgically treated for proximal humeral fractures (PHFs) with a locking plate between September 2007 and June 2013. All cases were then subdivided into one of four groups as follows: 75 patients in the medial cortical support (MCS) group, 26 patients in the medial multiscrew support (MMSS) group, 29 patients in the medial single screw support (MSSS) group, and 51 patients in the no medial support (NMS) group. Clinical and radiographic evaluations included the Constant-Murley score (CM), visual analogue scale (VAS), complications, and revision surgeries. The neck-shaft angle (NSA) was measured in a true anteroposterior radiograph immediately postoperation and at final follow-up. One-way analysis of variance or Kruskal-Wallis test was used for statistical analysis of measurement data, and Chi-square test or Fisher's exact test was used for categorical data.
Results:
The mean postoperative NSAs were 133.46° ± 6.01°, 132.39° ± 7.77°, 135.17° ± 10.15°, and 132.41° ± 7.16° in the MCS, MMSS, MSSS, and NMS groups, respectively, and no significant differences were found (F = 1.02, P = 0.387). In the final follow-up, the NSAs were 132.79° ± 6.02°, 130.19° ± 9.25°, 131.28° ± 12.85°, and 127.35° ± 8.50° in the MCS, MMSS, MSSS, and NMS groups, respectively (F = 4.40, P = 0.008). There were marked differences in the NSA at the final follow-up between the MCS and NMS groups (P = 0.004). The median (interquartile range [IQR]) NSA losses were 0.0° (0.0–1.0)°, 1.3° (0.0–3.1)°, 1.5° (1.0–5.2)°, and 4.0° (1.2–7.1)° in the MCS, MMSS, MSSS, and NMS groups, respectively (H = 60.66, P < 0.001). There were marked differences in NSA loss between the MCS and the other three groups (MCS vs. MMSS, Z = 3.16, P = 0.002; MCS vs. MSSS, Z = 4.78, P < 0.001; and MCS vs. NMS, Z = 7.34, P < 0.001). There was also significantly less NSA loss observed in the MMSS group compared to the NMS group (Z = −3.16, P = 0.002). However, there were no significant differences between the MMSS and MSSS groups (Z = −1.65, P = 0.225) or the MSSS and NMS groups (Z = −1.21, P = 0.099). The average CM scores were 81.35 ± 9.79, 78.04 ± 8.97, 72.76 ± 10.98, and 67.33 ± 12.31 points in the MCS, MMSS, MSSS, and NMS groups, respectively (F = 18.68, P < 0.001). The rates of excellent and good CM scores were 86.67%, 80.77%, 65.52%, and 43.14% in the MCS, MMSS, MSSS, and NMS groups, respectively (χ2= 29.25, P < 0.001). The median (IQR) VAS scores were 1 (0–2), 1 (0–2), 2 (1–3), and 3 (1–5) points in the MCS, MMSS, MSSS, and NMS groups, respectively (H = 27.80, P < 0.001). Functional recovery was markedly better and VAS values were lower in the MCS and MMSS groups (for CM scores: MCS vs. MSSS, P < 0.001; MCS vs. NMS, P < 0.001; MMSS vs. MSSS, P = 0.031; and MMSS vs. NMS, P < 0.001 and for VAS values: MCS vs. MSSS, Z = 3.31, P = 0.001; MCS vs. NMS, Z = 4.64, P < 0.001; MMSS vs. MSSS, Z = −2.09, P = 0.037; and MMSS vs. NMS, Z = −3.16, P = 0.003).
Conclusions:
Medial support screws might help enhance mechanical stability and maintain fracture reduction when used to treat PHFs with medial metaphyseal comminution or malreduction.
Keywords: Bone Plates; Bone Screws; Fracture Fixation, Internal; Humeral Fractures, Proximal; Postoperative Complications
摘要
背景:
内侧支撑螺钉在锁定钢板内固定治疗肱骨近端骨折中的临床意义仍有待进一步研究。探讨锁定钢板治疗肱骨近端骨折,内侧柱支撑螺钉数量与其固定稳定性和临床功能结果的关系。
方法:
2007年9月至2013年6月我们采用肱骨近端锁定钢板系统治疗181例肱骨近端骨折患者。根据术后X线片所示肱骨近端内侧柱支撑重建情况分为4组:内侧骨皮质支撑组(MCS,Medial cortical support ,75例)、多枚内侧支撑螺钉组(MMSS ,Medial multi-screws support,26例)、单枚内侧支撑螺钉组(MSSS ,Medial single screw support, 29例)和无重建内侧柱支撑组(NMS ,No medial support,51例)。随访记录并比较四组患者的肩关节功能Constant-Murley评分、视觉模拟评分(VAS, Visual analogue scale)、术后及末次随访时肱骨颈干角、术后肱骨头内翻角度及并发症发生情况。计量资料比较采用采用因素方差分析或Kruskal-Wallis检验,计数资料比较采用卡方检验或Fisher确切概率法。
结果:
181例患者术后获12–45个月(平均19.5个月)随访。MCS组、MMSS组、MSSS组及NMS组患者术后肱骨颈干角分别为133.46 ± 6.01、132.39 ± 7.77、135.17 ± 10.15和132.41 ± 7.16度,比较差异无统计学意义(F = 1.02, P= 0.387)。末次随访时,MCS~NMS四组患者肱骨颈干角分别为132.79 ± 6.02、130.19 ± 9.25、131.28 ± 12.85和127.35 ± 8.50度(F = 4.40, P = 0.008),其中MCS 组与NMS组比较差异有统计学意义(P = 0.004)。四组患者术后肱骨头内翻角度中位数(四分位数间距)分别为0.0°(0.0 –1.0)°、 1.3°(0.0– 3.1)°、1.5°(1.0–5.2)°和4.0°(1.2–7.1)° (H = 60.66, P < 0.001),其中MCS组与余三组比较差异均有统计学意义(P 值均< 0.05),MMSS组与NMS组比较差异有统计学意义(P = 0.002)。四组患者Constant-Murley 评分分别为81.35 ± 9.79、78.04 ± 8.97、72.76 ± 10.98 和67.33 ± 12.31分(F = 18.68, P < 0.001),优良率分别为86.67%、 80.77%、 65.52%和 43.14% (χ2 = 29.25, P < 0.001)。四组患者VAS评分中位数(四分位数间距)分别为1(0–2)、1(0–2)、2(1–3)和3(1–5) 分(H = 27.80, P < 0.001)。MCS 组和MMSS组患者Constant-Murley评分及VAS评分均优于MSSS组及NMS组患者(P值均 < 0.05)。
结论:
采用肱骨近端锁定钢板系统治疗肱骨近端骨折,当内侧皮质粉碎、骨缺损或骨皮质复位欠佳时,内侧支撑螺钉可能有助于增强其固定的稳定性、维持骨折的复位。
INTRODUCTION
Proximal humeral fractures (PHFs) are the second most common fractures of the upper extremity, accounting for approximately 5% of all fractures,[1] and the incidence increases with age.[2,3] The majority of these fractures are minimally displaced and stable and can be treated conservatively with physical therapy.[1,4] However, displaced and unstable fractures might require surgical treatment to achieve fracture stability and allow for early functional exercises. An increasing number of surgeons prefer to fix the unstable PHFs with locking plates due to their mechanical advantage over standard implants.[5] These plates alleviate the risk of malreduction and preserve the blood supply to the bone. Clinically, many studies have shown that locking plates provide high rates of union for displaced PHFs.[6,7,8,9,10] However, there remain a significant number of complications arising from this technically demanding procedure, such as the loss of reduction, screw penetration, and osteonecrosis.[11,12] Further study is needed to determine what technical errors and patient characteristics are risk factors for failure of this now common fixation technique. Some studies found that mechanical support of the medial region is important for maintenance of reduction when PHFs are treated with locking plates.[11,13,14] Gardner et al.[14] proposed that anatomic reduction of the medial cortex and medial support locking screws can provide a stable medial support column to create a load-sharing situation and minimize forces at the screw–bone interface. They found that placing medial support locking screws was an important method, particularly in patients with medial comminution or medial malreduction. Another study by Zhang et al.[15] additionally showed that medial support screws enhance the mechanical stability of locking plate fixation, particularly in complex PHFs, and allow for better maintenance of reduction. However, technical aspects of the correct medial support locking screw placement remain incompletely understood, especially the influence of the number of medial support screws on the mechanical stability of locking plating of PHFs.
The purpose of this study was to determine whether the number of medial support locking screws affects the mechanical stability of fracture fixation. Our hypothesis was that the number of medial support screws would be particularly important in establishing a stable construct, particularly in patients with medial comminution or medial malreduction.
METHODS
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Shanghai Sixth People's Hospital (No. 2013[L]-03). As a retrospective study and data analysis was performed anonymously, this study was exempt from the informed consent from patients.
General materials
Between September 2007 and June 2013, 212 patients were treated for PHFs who met the criteria in our institution. Inclusion criteria included closed, unstable PHFs without neurovascular complications at the time of injury which were subsequently treated by open reduction and internal fixation using PHILOS plates (Proximal Humerus Interlocking System, Synthes, Oberdorf, Switzerland). Exclusion criteria included pseudarthrosis, pathologic fractures and refractures, open fractures, or concomitant fractures of the ipsilateral elbow or distal radius. In addition, patients with existing disorders likely to affect the healing process and function such as rheumatoid arthritis, or relevant neurologic disorders, and patients with polytrauma with an Injury Severity Score >16 were excluded. Thirty-one patients were excluded, including four patients who died, eleven patients who were lost to follow-up or refused to participate, eight patients with a history of shoulder surgery or treatment, five patients with polytrauma, and another three patients with a pathologic fracture. The remaining 181 patients (117 females and 64 males) were included in this study.
All fractures were classified according to the Neer classification.[16] Demographic information, trauma mechanism, surgical approach, and perioperative complications were collected from the medical records.
Surgical technique and rehabilitation
Surgery was performed within 2 weeks after injury in all cases by the same two senior surgeons in this study. All operations were performed in the beach chair position and used a deltopectoral approach. The fracture was exposed and special attention was paid to minimize surgical trauma to the adjacent soft tissue. After surgical reduction, the PHILOS plates were placed at least 5–8 mm inferior to the upper end of the greater tuberosity and 2–4 mm lateral to the bicipital groove. Drilling was begun subchondrally under fluoroscopic control to avoid perforation into the glenohumeral joint. The length of the drilling hole was then measured and screws 4–5 mm shorter were chosen. At least five locking screws were placed in the proximal fragment in all cases. Tension band type sutures were added over the greater tuberosity to provide additional stability for osteoporotic fractures or combine with comminuted greater tuberosity fractures. At the end of surgery, the reduction result and length of screws were checked by fluoroscopy in three different views (anteroposterior [AP] internal, external rotation, and axial).
Postoperatively, the arm was immobilized in a sling and active motions of the elbow, wrist, and hand, as well as pendulum exercises were begun on the 1st postoperative day. Passive shoulder motions were allowed from 1 to 3 weeks postoperatively depending on fracture type, the degree of medial comminution, and intraoperative fixation stability. Active rehabilitation was started 4–6 weeks postoperatively. Stretching and resistive strengthening exercises were allowed when evidence of fracture healing was noted on follow-up radiographic imaging.
Follow-up
The patients underwent regular clinical and radiographic follow-up at 4, 8, and 12 weeks and at 6 and 12 months after surgery, then yearly thereafter. Complications, shoulder function, pain, and routine radiological measurement were recorded at each follow-up by an independent blinded observer. For clinical assessment, the Constant–Murley score (CM) was used.[17] The visual analogue scale (VAS) was used to evaluate the shoulder pain. Fracture healing was assessed in the standard AP view and scapular Y-view radiographs. Neck-shaft angle (NSA) was measured as an indication of displacement of the humeral head [Figure 1].[18] The change of the NSA from immediate postoperative radiographs to final follow-up was calculated.
Figure 1.
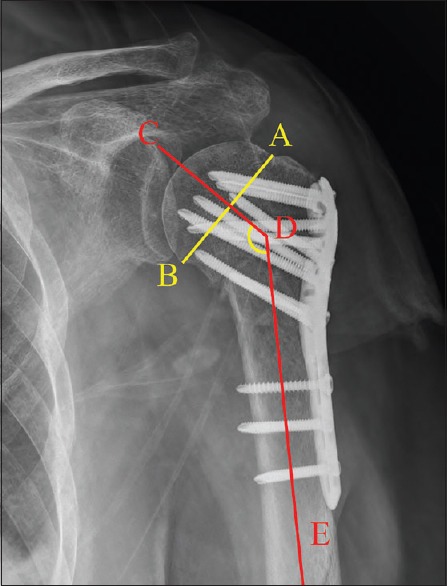
The NSA was measured by drawing a line from the superior to the inferior border of the articular surface (A–B line) and then a perpendicular line to the A–B line through the center of the humeral head (C–D line). The angle between this line and the line bisecting the humeral shaft (D–E line) was measured as the NSA (CDE). NSA: Neck-shaft angle.
Postoperative complications included screw penetration, humeral head avascular necrosis, subacromial impingement, and implant failures and other general complications such as nonunion or wound infection. Screw penetration was defined as protrusion of screws into the glenohumeral joint, which was not seen on the postoperative radiograph. Humeral head avascular necrosis was defined radiographically with irregularity of the outline of the humeral head at further follow-up. Implant failures were either broken plates or pulled out screws. Subacromial impingement was clinically defined in patients with none of the aforementioned complications having a painful arc starting between 60° and 120° of abduction. For infection, at least one microbiological culture had to be positive for bacterial growth. Revision surgery was indicated by the event that most likely led to the intervention because an associated complication might also lead to a reoperation.
All cases were then subdivided into one of four groups according to the presence or absence of medial mechanical support and the number of medial support screws in the proximal humeral head fragment. The fracture was considered to have medial cortical support (MCS) group if the medial pillar of the proximal humerus was not comminuted and anatomically reduced or the shaft was impacted into the head fragment. The fracture was designated as medial multiscrew support (MMSS) group if there were two or three medial support screws, or medial single screw support (MSSS) group if there was only one medial support screw [Figures 2 and 3]. Medial support screws were defined as the screws of D and E holes of the PHILOS plate were placed into the inferomedial quadrant of the proximal humeral head within 5 mm of the subchondral bone [Figure 4].[15] Conversely, fractures that did not fulfill one of these criteria were designated as having inadequate medial support (no medial support [NMS] group).
Figure 2.
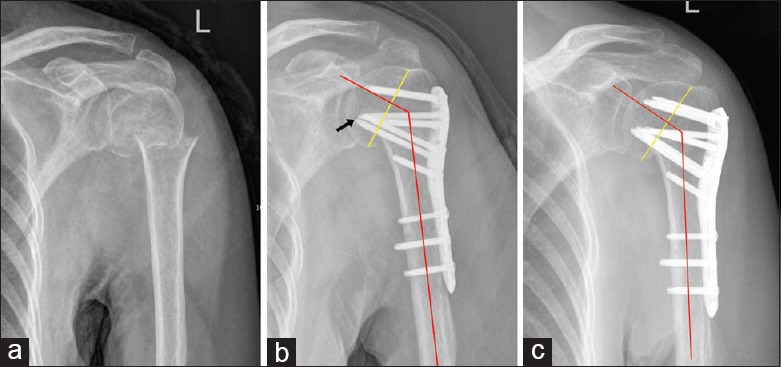
Typical case in the MMSS group. (a) Two-part fracture of the surgical neck in a 59-year-old female (left). (b) Immediate postoperative AP X-ray showed no anatomic reduction of the medial cortex. However, two medial support screws were used in this case (arrow). The NSA was 126°. (c) Six months postoperatively the humeral head alignment was well maintained, and the fracture healed. MMSS: Medial multiscrew support group; AP: Anteroposterior; NSA: Neck-shaft angle.
Figure 3.
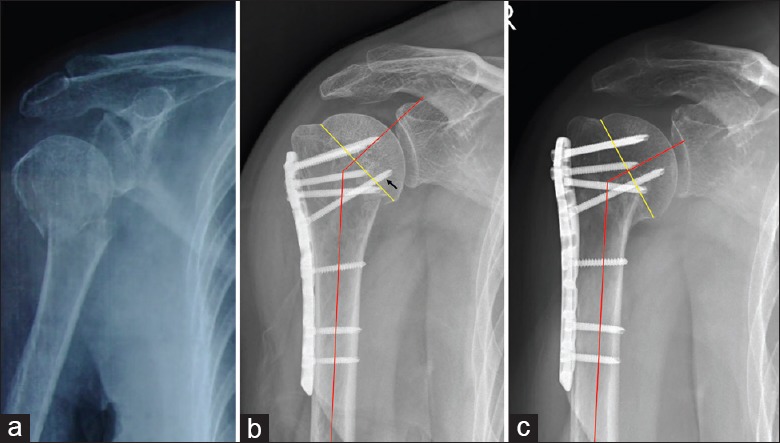
Typical case in the MSSS group. (a) Two-part fracture of the surgical neck in a 60-year-old male (right). (b) Immediate postoperative AP X-ray showed the medial cortex was malreduced, the NSA was 135.1°, and only one medial support screw was used (arrow). (c) At the 6-month follow-up, a radiograph showed complete bone union but the humeral head had failed in varus with an NSA of 121.0°. MSSS: Medial single screw support group; AP: Anteroposterior; NSA: Neck-shaft angle.
Figure 4.
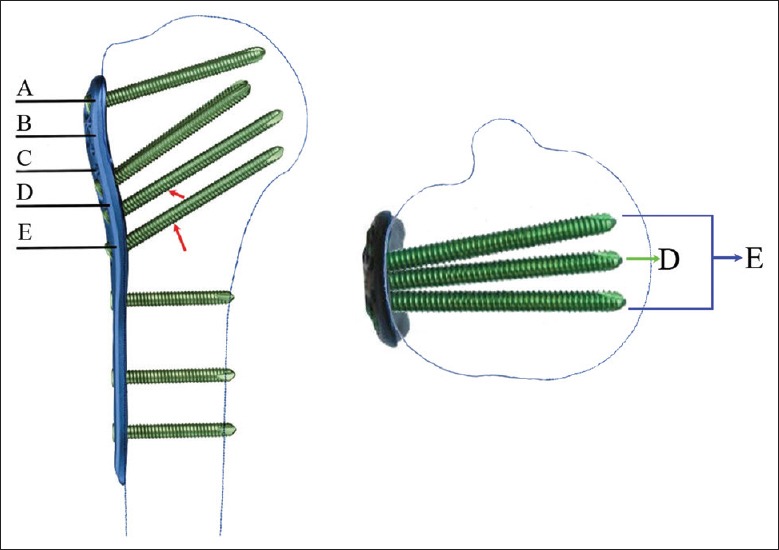
The proximal screw distribution of the PHILOS plate. There were three screws (D and E, E further includes two screws) could be considered as media support screws if the screws were placed into the inferomedial quadrant of the proximal humeral head within 5 mm of the subchondral bone (red arrows). PHILOS: Proximal Humerus Interlocking System; A: The first row screws; B: The second row screws; C: The third row screws; D: The fourth row screw; E: The fifth row screws.
Statistical analysis
For the statistical analysis, SAS 11.0 (SAS Institute Inc., Cary, NC, USA) was used. Statistical analyses were performed by an independent statistician blinded to surgical outcomes. Descriptive statistics were expressed using mean ± standard deviation for normally distributed variables and median (interquartile range [IQR]) for nonnormally distributed variables. One-way analysis of variance was used for measurement data (age, postoperative NSA, NSA at final follow-up, and CM). The Tukey's post hoc test was used to differentiate between groups for significant differences. The Chi-square test or Fisher's exact test was performed to analyze categorical data (sex, fracture side, fracture type, tension band suture application, NSA loss ≥5°, excellent and good rate of CM, complications, and revision surgery). Kruskal-Wallis and Wilcoxon rank sum tests were used to analyze the data of NSA loss and VAS value. Statistical significance was set at P < 0.05.
RESULTS
There were no significant differences in demographics, fracture patterns, and tension band suture application between any of the groups [Table 1]. The mean age was 57.41 years (18–88 years). There were 78 two-part, 75 three-part, and 28 four-part fractures according to the Neer classification. Average follow-up was 19.5 months (range, 12–45 months). All patients were followed up at least until clinical and radiographic healing was documented by the treating surgeon or revision surgery was performed. Seventy-five patients were considered to have medial cortex support and were designated as the MCS group. Fifty-five patients were considered to have medial locking screw support and were designated as the MMSS group (n = 26) and MSSS group (n = 29). The remaining 51 patients were in the NMS group.
Table 1.
Demographic data and fracture type
| Characteristics | MCS (n = 75) | MMSS (n = 26) | MSSS (n = 29) | NMS (n = 51) | Statistics | P |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Males | 28 | 8 | 9 | 19 | 0.69* | 0.877 |
| Females | 47 | 18 | 20 | 32 | ||
| Age (years) | 56.91 ± 11.24 | 59.50 ± 13.09 | 57.00 ± 13.91 | 57.31 ± 12.29 | 0.31† | 0.821 |
| Fracture side | ||||||
| Left | 39 | 17 | 15 | 30 | 1.79* | 0.617 |
| Right | 36 | 9 | 14 | 21 | ||
| Fracture type (Neer) | ||||||
| Two part | 37 | 11 | 10 | 20 | 4.11* | 0.662 |
| Three part | 28 | 9 | 15 | 23 | ||
| Four part | 10 | 6 | 4 | 8 | ||
| Tension band suture application | 45 (60.00) | 15 (57.69) | 17 (58.62) | 33 (64.71) | 0.51* | 0.917 |
Data are presented as mean ± SD or n or n (%). *χ2 value; †F values. MCS: Medial cortical support group; MMSS: Medial multiscrew support group; MSSS: Medial single screw support group; NMS: No medial support; SD: Standard deviation.
There were no significant differences in postoperative NSA between any groups (F = 1.02, P = 0.387) [Table 2]. In the final follow-up, the NSAs were 132.79° ± 6.02°, 130.19° ± 9.25°, 131.28° ± 12.85°, and 127.35° ± 8.50° in the MCS, MMSS, MSSS, and NMS groups, respectively (F = 4.40, P = 0.008). There were marked differences in NSA at final follow-up between the MCS and NMS groups (P = 0.004). No other significant differences were noted. The median (IQR) NSA losses were 0.0° (0.0–1.0)°, 1.3° (0.0–3.1)°, 1.5° (1.0–5.2)°, and 4.0° (1.2–7.1)° in the MCS, MMSS, MSSS, and NMS groups, respectively (H = 60.66, P < 0.001). There were marked differences in NSA loss between the MCS group and the other three groups (MCS vs. MMSS, Z = 3.16, P = 0.002; MCS vs. MSSS, Z = 4.78, P < 0.001; and MCS vs. NMS, Z = 7.34, P < 0.001). A significant difference in NSA loss was also observed between MMSS and NMS groups (Z = −3.16, P = 0.002). However, there were no significant differences between the MSSS and NMS groups (Z = −1.21, P = 0.099) or the MMSS and MSSS groups (Z = −1.65, P = 0.225). The rates of NSA loss >5° were 0 (0/75), 11.54% (3/26), 27.59% (8/29), and 39.22% (20/51), respectively, in the MCS, MMSS, MSSS, and NMS groups (χ2= 35.84, P < 0.001). There were marked differences in the occurrence of NSA loss >5° between the MCS group and the other three groups (MCS vs. MMSS, χ2= 8.92, P = 0.016; MCS vs. MSSS, χ2= 22.41, P < 0.001; and MCS vs. NMS, χ2= 34.96, P < 0.001) and MMSS and NMS groups (χ2= 6.30, P = 0.017). No other occurrence differences were noted [Table 2].
Table 2.
Head-shaft angle loss and postoperative shoulder function
| Characteristics | MCS (n = 75) | MMSS (n = 26) | MSSS (n = 29) | NMS (n = 51) | Statistics | P |
|---|---|---|---|---|---|---|
| Postoperative NSA (°) | 133.46 ± 6.01 | 132.39 ± 7.77 | 135.17 ± 10.15 | 132.41 ± 7.16 | 1.02* | 0.387 |
| NSA at final follow-up (°) | 132.79 ± 6.02§ | 130.19 ± 9.25 | 131.28 ± 12.85 | 127.35 ± 8.50 | 4.40* | 0.008 |
| NSA loss (°) | 0.0 (0.0–1.0)|| | 1.3 (0.0–3.1)¶ | 1.5 (1.0–5.2) | 4.0 (1.2–7.1) | 60.66† | <0.001 |
| NSA loss ≥5° | 0 (0)|| | 3 (11.54)¶ | 8 (27.59) | 20 (39.22) | 35.84‡ | <0.001 |
| CM | 81.35 ± 9.79** | 78.04 ± 8.97†† | 72.76 ± 10.98 | 67.33 ± 12.31 | 18.68* | <0.001 |
| Excellent and good rate of CM | 65 (86.67)** | 21 (80.77)¶ | 19 (65.52) | 22 (43.14) | 29.25‡ | <0.001 |
| VAS value | 1 (0–2)** | 1 (0–2)†† | 2 (1–3) | 3 (1–5) | 27.80† | <0.001 |
Data are presented as mean ± SD, median (IQR) or n (%). *F values; †H values; ‡χ2 values; §Significant differences were found between MCS and NMS groups; ||Significant differences were found between MCS and the other three groups; ¶Significant differences were found between MMSS and NMS groups; **Significant differences were found between MCS and MSSS, NMS groups; ††Significant differences were found between MMSS and MSSS, NMS groups. MCS: Medial cortical support group; MMSS: Medial multiscrew support group; MSSS: Medial single screw support group; NMS: No medial support; NSA: Neck-shaft angle; CM: Constant-Murley score; VAS: Visual Analogue Scale; SD: Standard deviation; IQR: Interquartile range
The average CM scores were 81.35 ± 9.79, 78.04 ± 8.97, 72.76 ± 10.98, and 67.33 ± 12.31 points in the MCS, MMSS, MSSS, and NMS groups, respectively (F = 18.68, P < 0.001), showing marked differences between the MCS and MSSS groups (P < 0.001), MCS and NMS groups (P < 0.001), MMSS and MSSS groups (P = 0.031), and MMSS and NMS groups (P < 0.001). However, there were no significant differences between the MCS and MMSS groups (P = 0.183) or the MSSS and NMS groups (P = 0.055). The excellent and good rates, respectively, were 86.67% (65/75), 80.77% (21/26), 65.52% (19/29), and 43.14% (22/51) in the MCS, MMSS, MSSS, and NMS groups (χ2= 29.25, P < 0.001). There were significant differences between the MCS and MSSS (χ2= 6.02, P = 0.014), the MCS and NMS (χ2= 26.91, P < 0.001), and the MMSS and NMS groups (χ2= 9.89, P = 0.002). No other significant differences were noted [Table 2].
With respect to VAS score, the median (IQR) VAS scores were 1 (0–2), 1 (0–2), 2 (1–3), and 3 (1–5) points, respectively, in the MCS, MMSS, MSSS, and NMS groups (H = 27.80, P < 0.001). MCS was significantly different from both MSSS (Z = 3.31, P = 0.001) and NMS (Z = 4.64, P < 0.001), and MMSS was significantly different from both MSSS (Z = −2.09, P = 0.037) and NMS (Z = −3.16, P = 0.003). However, no significant differences were noted between MCS and MMSS (Z = 1.23, P = 0.219) or between MSSS and NMS (Z = −1.24, P = 0.215) [Table 2].
The overall incidence of complications was 20.44% [n = 37, Table 3]. Ten patients developed asymptomatic osteonecrosis of the humeral head. Screw penetration was observed in seventeen patients, subacromial impingement was observed in ten patients, and implant failure occurred in two patients. Fracture nonunion was observed in two patients, and infection occurred in one patient. Five patients suffered concomitant with humeral head avascular necrosis and screw penetration, including two cases in the MCS group, one case in the MSSS group, and two cases in the NMS group. A considerable difference was observed between the MCS and NMS groups regarding the screw penetration rate (4.00% in the MCS group vs. 21.57% in the NMS group, χ2= 9.50, P = 0.003). Revision surgery was carried out in ten patients with screw penetration, two patients with asymptomatic osteonecrosis of the humeral head, two patients with fracture nonunion, one patient with infection, and one patient with pulled out screws. A considerable difference was observed between the MCS and NMS groups regarding the total complication rate (10.67% in the MCS group vs. 39.22% in the NMS group, χ2= 14.32, P < 0.001) and revision surgery rate (4.00% in the MCS group vs. 19.61% in the NMS group, χ2= 7.99, P = 0.007) [Table 3]. No other significant differences were noted.
Table 3.
Complications and revision surgery
| Characteristics | MCS (n = 75) | MMSS (n = 26) | MSSS (n = 29) | NMS (n = 51) | χ2 | P |
|---|---|---|---|---|---|---|
| Screw penetration | 3 (4.00)* | 1 (3.85) | 2 (6.9) | 11 (21.57)* | 12.60 | 0.010 |
| Humeral head avascular necrosis | 3 (4.00) | 1 (3.85) | 1 (3.45) | 5 (9.80) | 2.50 | 0.608 |
| Subacromial impingement | 4 (5.33) | 1 (3.85) | 1 (3.45) | 4 (7.84) | 0.91 | 0.875 |
| Implant failures | 0 (0.00) | 0 (0.00) | 1 (3.45) | 1 (1.96) | 2.93 | 0.342 |
| Nonunion | 0 (0.00) | 0 (0.00) | 1 (3.45) | 1 (1.96) | 2.93 | 0.342 |
| Deep infection | 0 (0.00) | 1 (3.85) | 0 (0.00) | 0 (0.00) | 5.27 | 0.304 |
| Total number of complications | 8 (10.67)* | 4 (15.38) | 5 (17.24) | 20 (39.22)* | 16.05 | 0.001 |
| Revision surgery | 3 (4.00)* | 1 (3.85) | 2 (6.90) | 10 (19.61)* | 10.46 | 0.021 |
Data are presented as n (%). *Significant differences were found between MCS and NMS groups. MCS: Medial cortical support group; MMSS: Medial multiscrew support group; MSSS: Medial single screw support group; NMS: No medial support group.
DISCUSSION
Anatomic reduction of the medial cortex is preferable for PHF, which can provide a stable medial support column and substantially decrease the risk of postoperative failures in locking plating of PHFs.[14,15,19] A biomechanical experiment by Lescheid et al.[20] showed that anatomica reduction with medial cortical contact provided greater stiffness and stability in a two-part PHF model with locking plates.
Gardner et al.[14] first described the correlation between medial support and subsequent reduction loss after fixation of PHFs. They found that mechanical support of the medial region of the humeral head is important for maintenance of reduction when PHFs are treated with locking plates. The MCS might be obtained through medial cortical contact: the medial cortex of the proximal humerus was not comminuted and anatomically reduced or the medial shaft was impacted on the inferomedial region of the head fragment in the case of minimally impacted fractures. In addition to anatomic or impacted stable reduction, the medial support screw was recognized as a key feature in achieving stable reduction and fixation of the fracture.
The present study affirms the concept of MCS by fixation of PHFs. Medial comminution might prevent cortical contact, and in these cases, the proximal humeral head fragment might be impacted slightly laterally in the distal fragment. However, when both these situations cannot be achieved, fixation with inferomedial screws decreases intrafragmentary motion and better maintains reduction.[21] These locked screws are exposed to compressive forces and therefore provide a buttress against posteromedial instability.
However, few clinical studies have directly investigated the role of medial support locking screws and the clinical outcomes compared to other fixations methods and did not provide guidelines as to how these screws should be used such that the mechanical advantage could be optimized. The clinical results of the study showed a statistically significant association between the number of medial support locking screws and the reduction in the NSA. The median (IQR) loss of NSA in the MMSS group 1.3° (0.0–3.1)° was less than that in the NMS group 4.0° (1.2–7.1)° (Z = −3.16, P = 0.002); however, no significant difference was observed between the MSSS group and NMS group regarding the reduction in the NSA. A high correlation was observed in the study between the number of medial support locking screws and better shoulder function recovery. The mean CM was higher in the MMSS group (78.04 ± 8.97) than those in the MSSS group (72.76 ± 10.98) or the NMS group (67.33 ± 12.31) (P were 0.031 and <0.001, respectively).
These results indicate that, when there is no MCS and no anatomic reduction, medial support screws might help resist varus stress applied to the humeral head and therefore avoid displacement of the fracture to a certain extent. The number of medial support screws might influence their role in maintaining mechanical stability. Two or more medial support screws tend to lead to better recovery of shoulder function. If the surgeon is not able to achieve anatomic reduction and restoration of the MCS intraoperatively, then as many as possible of the medial support screws should be placed to restore the medial mechanical stability. In addition, other strategies for structural augmentation in the locking plate fixation of PHFs include intramedullary fibular grafts,[22,23,24] calcium phosphate or sulfate cement,[25,26] and iliac crest bone autologous grafting.[27]
We are aware of several inherent limitations in this study. First, the number of patients is relatively small, especially in the MMSS and MSSS subgroups. Second, the quality of radiographs might influence the measurement results. The quality might vary from one radiologist to another, introducing a significant uncontrolled variable. In addition, an independent blinded observer subsequently reviewed all X-rays and made the digital calculations, but we acknowledge this as a potential source of error. Finally, because this was a retrospective and single-center study, which included patients of various ages and with different fracture patterns, the results might not be replicable in other centers with different surgical indications. A larger, long-term, multicenter, prospective study would appropriately address these issues.
In conclusion, we found that anatomic reduction of the medial cortex is preferable to reconstructing medial support for locking plate fixation of PHFs. However, the medial support screws inserted into the medioinferior region of the humeral head might contribute to increasing mechanical stability and maintaining fracture reduction in the fractures with no MCS. Therefore, we suggest that restoring medial support with multiple medial support screws is important for improving outcomes in locking plating of PHFs with medial metaphyseal comminution or malreduction.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Peng Lyu
REFERENCES
- 1.Court-Brown CM, Caesar B. Epidemiology of adult fractures: A review. Injury. 2006;37:691–7. doi: 10.1016/j.injury.2006.04.130. doi: 10.1016/j.injury.2006.04.130. [DOI] [PubMed] [Google Scholar]
- 2.Vachtsevanos L, Hayden L, Desai AS, Dramis A. Management of proximal humerus fractures in adults. World J Orthop. 2014;5:685–93. doi: 10.5312/wjo.v5.i5.685. doi: 10.5312/wjo.v5.i5.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Launonen AP, Lepola V, Saranko A, Flinkkilä T, Laitinen M, Mattila VM, et al. Epidemiology of proximal humerus fractures. Arch Osteoporos. 2015;10:209. doi: 10.1007/s11657-015-0209-4. doi: 10.1007/s11657-015-0209-4. [DOI] [PubMed] [Google Scholar]
- 4.Schumaier A, Grawe B. Proximal humerus fractures: Evaluation and management in the elderly patient. Geriatr Orthop Surg Rehabil. 2018;9:2151458517750516. doi: 10.1177/2151458517750516. doi: 10.1177/2151458517750516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friess DM, Attia A. Locking plate fixation for proximal humerus fractures: A comparison with other fixation techniques. Orthopedics. 2008;31 doi: 10.3928/01477447-20081201-07. pii:orthosupersite.com/view.asp?rID=34698. doi: 10.3928/01477447-20081201-07. [DOI] [PubMed] [Google Scholar]
- 6.Brunner F, Sommer C, Bahrs C, Heuwinkel R, Hafner C, Rillmann P, et al. Open reduction and internal fixation of proximal humerus fractures using a proximal humeral locked plate: A prospective multicenter analysis. J Orthop Trauma. 2009;23:163–72. doi: 10.1097/BOT.0b013e3181920e5b. doi: 10.1097/BOT.0b013e3181920e5b. [DOI] [PubMed] [Google Scholar]
- 7.Doshi C, Sharma GM, Naik LG, Badgire KS, Qureshi F. Treatment of proximal humerus fractures using PHILOS plate. J Clin Diagn Res. 2017;11:RC10–RC13. doi: 10.7860/JCDR/2017/26782.10304. doi: 10.7860/jcdr/2017/26782.10304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y, Zhang K, Qiang M, Li H, Dai H. Computer-assisted preoperative planning for proximal humeral fractures by minimally invasive plate osteosynthesis. Chin Med J. 2014;127:3278–85. doi: 10.1002/rcs.1604. [PubMed] [Google Scholar]
- 9.Esenyel CZ, Kalkar I, Adaş M, Dedeoǧlu SS, Büyükkurt CD, Cabuk H, et al. Is medial calcar continuity necessary in plate osteosynthesis for proximal humerus fractures? Niger J Clin Pract. 2018;21:362–6. doi: 10.4103/njcp.njcp_400_16. doi: 10.4103/njcp.njcp_400_16. [DOI] [PubMed] [Google Scholar]
- 10.LaMartina J 2nd, Christmas KN, Simon P, Streit JJ, Allert JW, Clark J, et al. Difficulty in decision making in the treatment of displaced proximal humerus fractures: The effect of uncertainty on surgical outcomes. J Shoulder Elbow Surg. 2018;27:470–7. doi: 10.1016/j.jse.2017.09.033. doi: 10.1016/j.jse.2017.09.033. [DOI] [PubMed] [Google Scholar]
- 11.Sproul RC, Iyengar JJ, Devcic Z, Feeley BT. A systematic review of locking plate fixation of proximal humerus fractures. Injury. 2011;42:408–13. doi: 10.1016/j.injury.2010.11.058. doi: 10.1016/j.injury.2010.11.058. [DOI] [PubMed] [Google Scholar]
- 12.Carbone S, Papalia M. The amount of impaction and loss of reduction in osteoporotic proximal humeral fractures after surgical fixation. Osteoporos Int. 2016;27:627–33. doi: 10.1007/s00198-015-3304-x. doi: 10.1007/s00198-015-3304-x. [DOI] [PubMed] [Google Scholar]
- 13.Laux CJ, Grubhofer F, Werner CM, Simmen HP, Osterhoff G. Current concepts in locking plate fixation of proximal humerus fractures. J Orthop Surg Res. 2017;12:137. doi: 10.1186/s13018-017-0639-3. doi: 10.1186/s13018-017-0639-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gardner MJ, Weil Y, Barker JU, Kelly BT, Helfet DL, Lorich DG, et al. The importance of medial support in locked plating of proximal humerus fractures. J Orthop Trauma. 2007;21:185–91. doi: 10.1097/BOT.0b013e3180333094. doi: 10.1097/BOT.0b013e3180333094. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L, Zheng J, Wang W, Lin G, Huang Y, Zheng J, et al. The clinical benefit of medial support screws in locking plating of proximal humerus fractures: A prospective randomized study. Int Orthop. 2011;35:1655–61. doi: 10.1007/s00264-011-1227-5. doi: 10.1007/s00264-011-1227-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neer CS., 2nd Four-segment classification of proximal humeral fractures: Purpose and reliable use. J Shoulder Elbow Surg. 2002;11:389–400. doi: 10.1067/mse.2002.124346. doi: 10.1067/Mse.124346. [DOI] [PubMed] [Google Scholar]
- 17.Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;214:160–4. doi: 10.1097/00003086-198701000-00023. [PubMed] [Google Scholar]
- 18.Agel J, Jones CB, Sanzone AG, Camuso M, Henley MB. Treatment of proximal humeral fractures with Polarus nail fixation. J Shoulder Elbow Surg. 2004;13:191–5. doi: 10.1016/j.jse.2003.12.005. doi: 10.1016/s1058274603003100. [DOI] [PubMed] [Google Scholar]
- 19.Yang P, Zhang Y, Liu J, Xiao J, Ma LM, Zhu CR, et al. Biomechanical effect of medial cortical support and medial screw support on locking plate fixation in proximal humeral fractures with a medial gap: A finite element analysis. Acta Orthop Traumatol Turc. 2015;49:203–9. doi: 10.3944/AOTT.2015.14.0204. doi: 10.3944/aott.2015.14.0204. [DOI] [PubMed] [Google Scholar]
- 20.Lescheid J, Zdero R, Shah S, Kuzyk PR, Schemitsch EH. The biomechanics of locked plating for repairing proximal humerus fractures with or without medial cortical support. J Trauma. 2010;69:1235–42. doi: 10.1097/TA.0b013e3181beed96. doi: 10.1097/TA.0b013e3181beed96. [DOI] [PubMed] [Google Scholar]
- 21.Zhang W, Zeng L, Liu Y, Pan Y, Zhang W, Zhang C, et al. The mechanical benefit of medial support screws in locking plating of proximal humerus fractures. PLoS One. 2014;9:e103297. doi: 10.1371/journal.pone.0103297. doi: 10.1371/journal.pone.0103297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berkes MB, Little MT, Lazaro LE, Cymerman RM, Pardee NC, Helfet DL, et al. Intramedullary allograft fibula as a reduction and fixation tool for treatment of complex proximal humerus fractures with diaphyseal extension. J Orthop Trauma. 2013;28:e56–64. doi: 10.1097/BOT.0b013e31829a346d. doi: 10.1097/BOT.0b013e31829a346d. [DOI] [PubMed] [Google Scholar]
- 23.Hsiao CK, Tsai YJ, Yen CY, Lee CH, Yang TY, Tu YK, et al. Intramedullary cortical bone strut improves the cyclic stability of osteoporotic proximal humeral fractures. BMC Musculoskelet Disord. 2017;18:64. doi: 10.1186/s12891-017-1421-8. doi: 10.1186/s12891-017-1421-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cha H, Park KB, Oh S, Jeong J. Treatment of comminuted proximal humeral fractures using locking plate with strut allograft. J Shoulder Elbow Surg. 2017;26:781–5. doi: 10.1016/j.jse.2016.09.055. doi: 10.1016/j.jse.2016.09.055. [DOI] [PubMed] [Google Scholar]
- 25.Scola A, Gebhard F, Röderer G. Augmentation technique on the proximal humerus. Unfallchirurg. 2015;118:749–54. doi: 10.1007/s00113-015-0061-4. doi: 10.1007/s00113-015-0061-4. [DOI] [PubMed] [Google Scholar]
- 26.Somasundaram K, Huber CP, Babu V, Zadeh H. Proximal humeral fractures: The role of calcium sulphate augmentation and extended deltoid splitting approach in internal fixation using locking plates. Injury. 2013;44:481–7. doi: 10.1016/j.injury.2012.10.030. doi: 10.1016/j.injury.2012.10.030. [DOI] [PubMed] [Google Scholar]
- 27.Zhu L, Liu Y, Yang Z, Li H, Wang J, Zhao C, et al. Locking plate fixation combined with iliac crest bone autologous graft for proximal humerus comminuted fracture. Chin Med J. 2014;127:1672–6. [PubMed] [Google Scholar]


