Abstract
Background:
Cytokines play an important role in occurrence and recovery of hepatitis B virus (HBV) infection. The aim of this study was to investigate the changes of cytokines concentration and its correlation to alanine aminotransferase (ALT), HBV deoxyribonucleic acid (HBV-DNA), hepatitis B envelope antigen (HBeAg), and HBV surface antigen (HBsAg) in the development of chronic hepatitis B (CHB).
Methods:
Thirteen healthy individuals (HI), 30 chronic HBV-infected patients in immune tolerant (IT) phase, and 55 CHB patients were enrolled between August 2015 and May 2017. The peripheral blood samples were collected from all individuals. The levels of interferon (IFN)-α2, interleukin (IL)-10, transforming growth factor (TGF)-β1, HBV-DNA, HBsAg, and HBeAg and liver function were measured. The quantitative determinations of cytokines levels, including IFN-α2, IL-10, and TGF-β1 were performed using Luminex multiplex technology. The correlation of cytokines to ALT, HBV-DNA, HBsAg, and HBeAg was analyzed by linear regression analysis.
Results:
IFN-α2 levels were similar between HI and IT groups (15.35 [5.70, 67.65] pg/ml vs. 15.24 [4.07, 30.73] pg/ml, Z = −0.610, P = 0.542), while it elevated significantly in CHB group (35.29 [15.94, 70.15] pg/ml vs. 15.24 [4.07, 30.73] pg/ml; Z = −2.522, P = 0.012). Compared with HI group (3.73 [2.98, 11.92] pg/ml), IL-10 concentrations in IT group (5.02 [2.98, 10.11] pg/ml), and CHB group (7.48 [3.10, 18.00] pg/ml) slightly increased (χ2= 2.015, P = 0.365), and there was no significant difference between IT and CHB group (Z = −1.419, P = 0.156). The TGF-β1 levels among HI (3.59 ± 0.20 pg/ml), IT (3.62 ± 0.55 pg/ml), and CHB groups (3.64 ± 0.30 pg/ml) were similar (χ2= 2.739, P = 0.254). In all chronic HBV-infected patients (including patients in IT and CHB groups), the elevation of IFN-α2 level was significantly associated with ALT level (β= 0.389, t = 2.423, P = 0.018), and was also negatively correlated to HBV-DNA load (β = −0.358, t = −2.308, P = 0.024), HBsAg (β = −0.359, t = −2.288, P = 0.025), and HBeAg contents (β = −0.355, t = −2.258, P = 0.027). However, when both ALT level and cytokines were included as independent variable, HBV-DNA load, HBsAg, and HBeAg contents were only correlated to ALT level (β = −0.459, t = −4.225, P = 0.000; β = −0.616, t = −6.334, P = 0.000; and β = −0.290, t = −2.433, P = 0.018; respectively).
Conclusions:
IFN-α2 elevation was associated with ALT level in patients with chronic HBV infection. However, in CHB patients, only ALT level was correlated to HBV-DNA, HBsAg and HBeAg contents.
Keywords: Chronic Hepatitis B, Hepatitis B Virus Deoxyribonucleic Acid, Hepatitis B Virus Surface Antigen, Interferon-α2, Interleukin-10
摘要
背景:
细胞因子在乙型肝炎病毒感染的发生和恢复中起着重要的作用。本研究的目的是研究细胞因子浓度的变化,与丙氨酸氨基转移酶(ALT)、HBV DNA含量及HBsAg、HBeAg水平的相关性。
方法:
在本研究中,2015年8月至2017年5月期间共入组了13例健康成人(HI)、30例免疫耐受期的慢性HBV感染者(IT)、55例慢性乙型肝炎(CHB)患者。采集这些入组的研究对象的外周血样本。检测各组患者的IFN-α2、IL-10、TGFβ1等细胞因子、ALT水平、HBV DNA含量、HBsAg水平、HBeAg含量等。应用Luminex技术检测IFN-α2, IL-10, TGF-β1等细胞因子水平。本研究采用spss11.5进行统计分析。通过线性回归分析,分析了细胞因子与ALT、HBV DNA、HBsAg和HBeAg的相关性。
结果:
IFN-α2水平在HI组和IT组相似(15.35[5.70, 67.65] pg/ml vs. 15.24 [4.07, 30.73] pg/ml, Z = –0.610, P = 0.542),而在慢性乙肝患者中显著升高(35.29 [15.94,70.15] pg/ml; Z=–2.522, P = 0.012);与HI 组IL-10含量(3.73 [2.98,11.92] pg/ml)相比,IL-10含量在IT组(5.02 [2.98,10.11] pg/ml)和CHB组(7.48 [3.10,18.00] pg/ml)略有增加(χ2 = 2.015, P = 0.365),但在IT组和CHB组没有差别(Z = –1.419, P = 0.156); TGF-β1水平在HI组、IT组及CHB组相似(3.59 ± 0.20 pg/ml和3.62 ± 0.55 pg/ml vs. 3.64 ± 0.30 pg/ml, χ2 = 2.739, P = 0.254)。在所有的慢性HBV感染患者中(包括IT组和CHB组), IFN-α2升高与ALT水平显著相关(β = 0.389, t = 2.423, P = 0.018), 也与HBV DNA、HBsAg和HBeAg显著相关(β = –0.358, t = –2.308, P = 0.024 和 β = –0.359, t = –2.288, P = 0.025 比 β = –0.355, t = –2.258, P = 0.027)。然而,当把ALT水平作为独立变量纳入分析时,HBV DNA含量、HBsAg水平、HBeAg含量仅与ALT水平呈显著相关性(β = –0.459, t = –4.225, P = 0.000 和 β = –0.616, t = –6.334, P = 0.000 比 β = –0.290, t = –2.433, P = 0.018)。
结论:
在慢性HBV感染患者IFN-α2升高与ALT水平相关。然而,在慢性乙型肝炎中,患者肝脏炎症水平(ALT水平)仅与HBV DNA含量、HBeAg水平和HBeAg含量相关。
INTRODUCTION
More than 95% of patients who acquired hepatitis B virus (HBV) infection in adult life could recover from the infection while more than 90% of HBV patients who acquired the infection in infant or at birth would develop chronic hepatitis.[1] The natural history of chronic HBV infection can be divided into four phases: immune-tolerance, hepatitis B envelope antigen (HBeAg)-positive immune active or called immune clearance, inactive carrier, and HBeAg-negative reactive phases.[2,3] HBeAg-positive immune active phase is characterized as elevated alanine aminotransferase (ALT) and HBV deoxyribonucleic acid (HBV-DNA) levels in conjunction with liver injury; and immune-tolerant (IT) phase is characterized as persistent normal ALT level and mild liver inflammation although HBeAg positive and high load of HBV-DNA. In HBV infection, virus clearance and hepatitis development involve a complex immune response between interactions of immune cells direct or indirect with cytokines.
Plasmacytoid dendritic cells (pDC) are the most important cells to connect innate and adaptive immune responses, and also the main source of interferon (IFN)-α.[4] IFN-α can suppress the activity of HBV enhancers,[5] control HBV-posttranscriptional replication,[6,7,8] and inhibit HBV nucleocapsid formation in virus replication.[9] It can also limit virus infection by modulating both innate and adaptive immunity, directly activate natural killer (NK) cells to enhance their cytotoxicity to eliminate-infected cells, promote the maturation of DCs, stimulate naive cluster of differentiation antigen 8+ (CD8+) T cells, resulting in clonal expansion and proliferation, and increase expression of chemokines that recruit NK, T and B cells to the site of infection. Yet, in chronic HBV infection, HBV, HBeAg, and HBV surface antigen (HBsAg) can impair the mature and function of DCs.[10,11,12] However, it is not fully understood what cytokines involve in triggering the immune response against HBV during IT phase transition to immune clearance phase, and why chronic hepatitis B (CHB) patients with higher ALT level could obtain more HBeAg seroconversion after pegylated (PEG)-IFN α2 therapy. In the process of immune response to HBV infection, IFN-α2 is an important cytokine with immune stimulation action, and interleukin (IL)-10 is the important cytokine with immune suppressive action. In CHB, clinical outcomes depend on the balance of Th1/Th2 immune response. IFN-α2 is the important cytokine association with virus clearance, IL-10 is very important to down-regulation of other immune cells thus lead infection persistence, and transforming growth factor (TGF)-β is pivotal cytokine to induce tolerance through the regulation of lymphocyte proliferation, differentiation, and survival, and which is associated with hepatic fibrosis.[13,14] This study detected the immune regulation cytokines, including IFN α2, IL-10, and TGF-β1 contents, in individuals with chronic HBV infection of IT phase and patients with CHB to explore whether the increase of IFN-α2 content or decrease of IL-10 and TGF-β1 are correlated to ALT level, HBV-DNA load, and HBsAg level.
METHODS
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethical Review Committee of Beijing Ditan Hospital. Informed written consent was obtained from all participants before their enrollment in this study.
Study population
In order to minimize selection bias, subjects who presented to the Department of Hepatology, Beijing Ditan Hospital, Capital Medical University between August 2015 and May 2017, confirmed to the inclusion criteria, and were willing attend to this study were enrolled. Chronic HBV infection in IT phase was diagnosed as HBsAg positive more than 6 months, HBeAg positive, HBV-DNA >107 U/ml, persistent normal ALT level (<40 U/L), or mild inflammation and fibrosis in histological examination; CHB was diagnosed as HBsAg positive more than 6 months, HBeAg positive, ALT abnormal (>200 U/L) for more than 3 months, or obvious inflammation in liver by histological examination;[15] Healthy individuals were subjects with negative for HBV biomarkers, and ALT normal (<40 U/L). The patients with coinfection with hepatitis C virus, human immunodeficiency virus, hepatitis D virus, other liver diseases (alcoholic liver disease, fatty liver, autoimmune liver disease, metabolic liver disease, and liver tumor), and fibrosis or cirrhosis confirmed by fibroscan were excluded from this study.[16] Thirteen healthy individuals (HIs), 30 chronic HBV-infected patients in IT phase (IT group), and 55 CHB patients (CHB group) were included in this study.
Laboratory assays
Serum HBV-DNA load was quantitated with a Roche CobasAmpliPrep/CobasTaqMan 96 full automatic real-time fluorescence quantitative polymerase chain reaction detection reagent (with a lower limit of 20 U/ml) (Roche, Pleasanton, CA, USA). The levels of HBsAg, anti-HBs, HBeAg, and anti-HBe were measured using an Abbott Architect i2000 detection reagent (Abbott Diagnostics, Abbott Park, IL, USA); the HBsAg dynamic range was 0.05–250.00 U/ml. Samples with HBsAg levels >250 U/ml were automatically re-tested at 1:500 dilution. HBsAg <0.05 U/ml was defined as the disappearance of HBsAg.
The parameters of liver and kidney function, including ALT, aspartate aminotransferase (AST), total bilirubin, albumin, creatinine, and blood urea nitrogen, were measured using a Hitachi 7600 automatic biochemical analyzer (Hitachi 7600-020, Japan).
Cytokine levels detection
Peripheral venous blood was collected, then plasma was separated and stored in an −80°C refrigerator for cytokine detection. The levels of cytokines, including IFN-α2, IL-10, and TGF-β1, in plasma were quantitated by Luminex assay and analyzed using FlexMap 3D analyzer (Austin, TX, USA) according to manufacturer's instructions. Each sample was assayed in duplicate, and cytokine standards supplied by the manufacturer were run on each plate.
Statistical analysis
Statistical analyses were performed using SPSS version 11.5 (SPSS Inc., Chicago, IL, USA). Serum HBV-DNA levels and HBsAg concentrations were logarithmically transformed for analysis. Continuous variables were expressed as mean ± standard deviation (SD) or median (Q1, Q3), and categorical variables as absolute and relative frequencies. Characteristics were compared between groups using Chi-square or Fisher's exact tests for analysis of categorical variables and Mann-Whitney U-test, F-test (analysis of variance), and Kruskal-Wallis test was used for comparisons within groups for analysis of continuous variables, as appropriate. A P < 0.05 was considered statistically significant. Correlation of the detected cytokines to clinical index (ALT level, HBV-DNA load, HBsAg level, and HBeAg content) was analyzed by linear regression analysis.
RESULTS
Clinical characteristics
Compared to IT group, CHB group had significant higher ALT level (median 29.85 U/L vs. 233.75 U/L, P = 0.000), lower HBV-DNA load (8.11 ± 0.48 log U/ml vs. 7.09 ± 1.18 log U/ml, P = 0.000), lower HBsAg level (4.64 ± 0.51 log U/ml vs. 3.81 ± 0.69 log U/ml, P = 0.000), and HBeAg level (median 1606.30 PEU/ml vs. 878.35 PEU/ml, P = 0.000). The basic demographics of all individuals are summarized in Table 1.
Table 1.
Characteristics of Health individuals, chronic HBV-infected patients in Immune tolerance phase and Chronic hepatitis B patients
| Characteristics | HI group (n = 13) | IT group (n = 30) | CHB group (n = 55) | |||
|---|---|---|---|---|---|---|
| Male/female, n | 2/11 | 14/16 | 33/22 | |||
| Age (years) | 26.1 ± 1.9 | 29.9 ± 7.3 | 32.0 ± 7.7 | |||
| ALT (U/L) | 10.10 (8.20, 13.00) | 29.85 (21.83, 39.85) | 233.75 (129.52, 334.48) | |||
| AST (U/L) | 17.20 (13.09, 18.55) | 23.40 (19.45, 26.83) | 127.30 (68.00, 172.70) | |||
| TBil (µmol/L) | 12.12 ± 4.31 | 12.11 ± 6.45 | 15.72 ± 6.88 | |||
| ALB (g/L) | 47.51 ± 2.95 | 45.45 ± 2.42 | 44.48 ± 4.36 | |||
| HBV DNA load (log U/ml) | NA | 8.11 ± 0.48 | 7.09 ± 1.18 | |||
| HBsAg level (log U/ml) | NA | 4.64 ± 0.51 | 3.81 ± 0.69 | |||
| HBeAg content (PEU/ml) | NA | 1606.30 (1556.50, 1679.38) | 878.35 (373.22, 1374.40) | |||
| Characteristics | All group | HI versus IT | IT versus CHB | |||
| Statistics | P | Statistics | P | Statistics | P | |
| Male/female | 8.564* | 0.014 | 3.799* | 0.051 | 1.396* | 0.237 |
| Age | 3.993† | 0.022 | −2.648‡ | 0.012 | −1.285‡ | 0.202 |
| ALT | 22.086† | 0.000 | −7.238‡ | 0.000 | −7.265‡ | 0.000 |
| AST | 19.897† | 0.000 | −2.854‡ | 0.007 | −6.969‡ | 0.000 |
| TBil | 3.668† | 0.029 | 0.005‡ | 0.996 | −2.341‡ | 0.022 |
| ALB | 7.797† | 0.001 | 0.068‡ | 0.946 | −3.972‡ | 0.000 |
| HBV DNA load | NA | NA | NA | NA | 5.439‡ | 0.000 |
| HBsAg level | NA | NA | NA | NA | 6.250‡ | 0.000 |
| HBeAg content | NA | NA | NA | NA | 9.319‡ | 0.000 |
Data were shown as mean ± SD, median (Q1, Q3), or n. *χ2 values; †F values; ‡t values. HIs: Health individuals; IT: Immune tolerance phase; CHB: Chronic hepatitis B; HBsAg: Hepatitis B surface antigen; HBeAg: Hepatitis B envelope antigen; HBV: Hepatitis B virus; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; TBil: Total bilirubin; ALB: Albumin; NA: Not applicable; SD: Standard deviation.
Cytokine contents
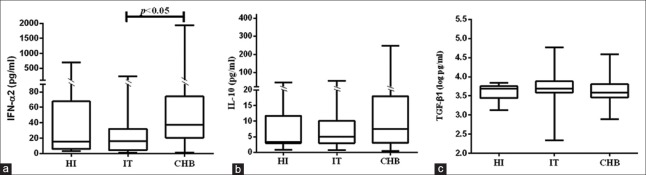
In this study, IFN-α2, IL-10, and TGF-β1 contents in IT group were 15.24 (4.07, 30.73) pg/ml, 5.02 (2.98, 10.11) pg/ml, and 3.62 ± 0.55 pg/ml, which were similar to those in HI group (15.35 [5.70, 67.65] pg/ml, 3.73 [2.98, 11.92] pg/ml, and 3.59 ± 0.20 pg/ml, respectively). However, compared to IT group, CHB group had significantly higher IFN-α2 level (35.29 [15.94, 70.15] pg/ml, Z = −2.522, P = 0.012), but had similar contents of IL-10 and TGF-β1 (7.48 [3.10, 18.00] pg/ml and 3.64 ± 0.30 pg/ml; respectively) [Figure 1].
Figure 1.
Cytokine concentrations in HI (n = 13), IT (n = 30), and CHB (n = 55) groups. IFN-α2 concentrations were similar between HI and IT groups, but elevated significantly in CHB group (a). Compared HI group, IL-10 concentrations in HBV-infected patients increased lightly, however the difference between IT and CHB groups was not significant (b). The TGF-β1 levels among HI, IT, and CHB groups were similar (c). HI: Health individual; IT: Immune tolerance phase; CHB: Chronic hepatitis B; IFN: Interferon; IL: Interleukin; TGF: Transforming growth factor; HBV: Hepatitis B virus.
Correlation of cytokines to alanine aminotransferase level, hepatitis B virus deoxyribonucleic acid load and hepatitis B virus surface antigen level
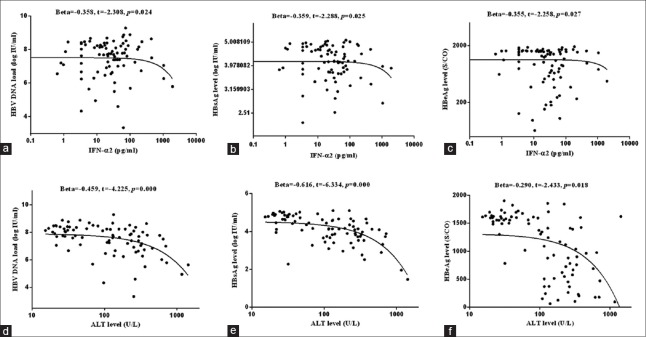
In chronic HBV-infected patients, including patients in IT and CHB groups, the results of regression analysis showed that while the detected cytokines was included as independent variable, ALT level was only correlated to IFN-α2 content [β = 0.389, t = 2.423, P = 0.018; Figure 2]; and IFN-α2 content was negatively associated with serum HBV-DNA load (β = −0.358, t = −2.308, P = 0.024), HBsAg level (β = −0.359, t = −2.288, P = 0.025), and HBeAg concentration (β = −0.355, t = −2.258, P = 0.027). However, when both ALT level and the detected cytokines were included as independent variables, the results of regression analysis showed that only ALT level, but not IFN-α2 level, was correlated to serum HBV-DNA load (β = −0.459, t = −4.225, P = 0.000), HBsAg level (β = −0.616, t = −6.334, P = 0.000), and HBeAg concentration [β = −0.290, t = −2.433, P = 0.018; Figure 3].
Figure 2.
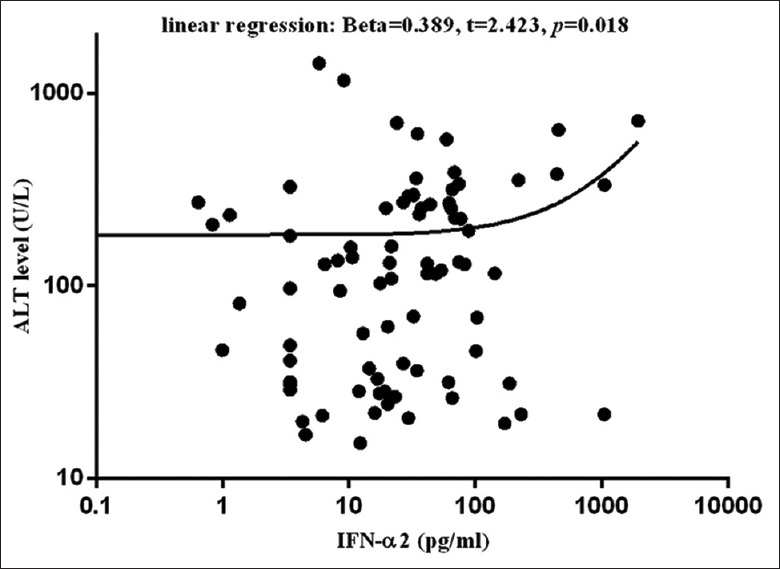
Correlation between IFN-α2 content and ALT level in chronic HBV-infected patients (including 30 IT individuals and 55 CHB patients). ALT elevation was correlated to increase of IFN-α2 content. ALT: Alanine aminotransferase; IFN: Interferon; IT: Immune tolerance phase; CHB: Chronic hepatitis B; HBV: Hepatitis B virus.
Figure 3.
Correlation of cytokines to ALT level, HBV-DNA load and HBsAg level. In chronic HBV-infected patients, including 30 patients in IT group and 55 patients in CHB groups, increase of IFN-α2 levels was correlated to the decline of HBV-DNA load (a), HBsAg level (b), and HBeAg concentration (c); and the decrease of HBV-DNA load (d), HBsAg level (e), and HBeAg concentration (f) were associated with the elevation of ALT level. ALT: Alanine aminotransferase; HBV: Hepatitis B virus; DNA: Deoxyribonucleic acid; HBsAg: HBV surface antigen; IT: Immune tolerance phase; CHB: Chronic hepatitis B; IFN: Interferon; HBeAg: Hepatitis B envelope antigen.
DISCUSSION
IT is clinically described as no significant immune response to the virus, and patients show persistently normal ALT levels without significant liver inflammation or fibrosis.[17] Studies have shown that defects in DC function, caused by high levels of HBeAg and HBsAg, are important factors in the host-specific T-cell IT to viral infection.[13,18,19] DC immunization can break cytotoxic T lymphocyte tolerance in HBV transgenic mice.[20] Results indicated that pDC is important to trigger the immune response to HBV in chronic HBV infection. Secretion of IFN-α is one of the most important functions of pDC to simulate immune response. In this study, we examined IFN-α2 concentration in IT group, CHB group, and HI group. Results showed that IFN-α2 content in IT group was similar to HIs, however, it elevated significantly in CHB group. Furthermore, IFN-α2 content was positively correlated to ALT level in CHB group. It was shown that there was an association of serum IFN-α level increase with the activation of immune response to HBV in chronic HBV-infected patients. In clinical practice, ALT elevation, which is associated with good response to the therapy,[21] commonly exist in patients treated with PEG-IFN-α.[22] Results suggested that the activation of pDC and the elevation IFN-α might contribute to the activation of hepatitis B from IT phase. Results could also explain why the efficacy of IFN therapy is poor in patients in IT phase but good in CHB patients, especially those with high ALT level and severe liver inflammatory in clinical practice.[23]
IL-10 is an important anti-inflammation cytokine. In patients with HBV infection, high levels of HBsAg and HBeAg can induce IL-10 secretion by Tregs,[24,25] Bregs, and monocytes mediate the suppression of HBV-specific CD8 T-cell responses,[25,26] and contribute to chronic HBV infection.[24] Furthermore, TGF-β is pivotal cytokine to induce tolerance through the regulation of lymphocyte proliferation, differentiation, and survival.[14] Theoretically, the decrease of IL-10 and TGF-β levels should reduce the intensity of inhibition of immune response and cause ALT raise, also should be associated with the decrease of HBV-DNA load and HBsAg level. However, in this study, it was shown that, in CHB group, IL-10 and TGF-β1 contents did not decrease, instead even slightly elevated, compared with IT group. Results in this study suggested that not IL-10 nor TGF-β1 decreasing, but IFN-α2 elevation in chronic HBV-infected patients triggers immune response to HBV during IT phase transition to immune active phase, and elevation of IFN-α level was accompany with increasing of IL-10 or TGF-β levels. These results can help to explain why few patients with CHB can recover from infection spontaneous in clinical practice, although they had immune response to virus, and the progressive liver disease with fibrosis should develop during the long-term hepatitis activation.
IFN-α2 can interact with the adaptive and innate immune responses to promote proliferation of memory T-cells activation of NK cells and differentiation of Th1 cell to re-establish a Th1/Th2 population balance,[27] thus lead to killing of the infected hepatic cells and inhibition of HBV replication though cytokines. In this study, the IFN-α level was significantly negative correlation to HBV-DNA load, HBeAg content, and HBsAg level. In the light of our study, the result of regression analysis showed that IFN-α2 content was significantly correlated to HBV DNA load, HBsAg level, and HBeAg content.[23,28,29]
In the antiviral treatment of CHB, IFN-α can inhibit virus replication and degenerate virus mRNA, which can lead to the decline of serum HBV-DNA load, HBsAg levels, and HBeAg content, also it can stimulate the HBV specific or nonspecific immune response to clear the infected hepatic cells expressed as ALT elevation and decrease of virus and antigens levels. However, in our study, when ALT level and cytokines were all included into the analysis as independent variables, it was showed that the decreases of HBV-DNA load, HBsAg level and HBeAg concentration were only correlated to the elevation of ALT level. Results could suggest that, in patients with active hepatitis B, decreases of HBV-DNA, HBeAg, and HBsAg levels were mainly due to killing of the infected hepatic cells mediated by IFN-α2 triggering immune response.
There are several limitations in our study. First, the sample size of this study was relatively small. Second, the occurrence of chronic HBV infection (including IT and CHB) is often sporadic. The participants were consecutively enrolled, thus it was difficult to pair some baseline factors and enroll the large and the same number of participants among three groups during the study period, which might lead to a bias. Therefore, the randomized controlled trials with large samples are required to further resolve these issues in the future.
In conclusion, IFN-α2 elevation was associated with ALT level in patients with chronic HBV infection. However, in CHB patients, only ALT level was correlated to HBV-DNA and, HBsAg and HBeAg contents.
Financial support and sponsorship
The work was supported by grants from the Basic and Clinical Fund of Capital Medical University (No. 17JL88) and National Natural Science Foundation of China (No. 81071344).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Ning-Ning Wang
REFERENCES
- 1.Gish RG, Given BD, Lai CL, Locarnini SA, Lau JY, Lewis DL, et al. Chronic hepatitis B: Virology, natural history, current management and a glimpse at future opportunities. Antiviral Res. 2015;121:47–58. doi: 10.1016/j.antiviral.2015.06.008. doi: 10.1016/j.antiviral.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 2.Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH, et al. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63:261–83. doi: 10.1002/hep.28156. doi: 10.1002/hep.28156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Association for Study of Liver, Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH clinical practice guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63:237–64. doi: 10.1016/j.jhep.2015.04.006. doi: 10.1016/j.jhep.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Lambotin M, Raghuraman S, Stoll-Keller F, Baumert TF, Barth H. A look behind closed doors: Interaction of persistent viruses with dendritic cells. Nat Rev Microbiol. 2010;8:350–60. doi: 10.1038/nrmicro2332. doi: 10.1038/nrmicro2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nawa T, Ishida H, Tatsumi T, Li W, Shimizu S, Kodama T, et al. Interferon-α suppresses hepatitis B virus enhancer II activity via the protein kinase C pathway. Virology. 2012;432:452–9. doi: 10.1016/j.virol.2012.07.002. doi: 10.1016/j.virol.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Li MH, Xie Y, Zhang L, Lu Y, Shen G, Wu SL, et al. Hepatitis B surface antigen clearance in inactive hepatitis B surface antigen carriers treated with peginterferon alfa-2a. World J Hepatol. 2016;8:637–43. doi: 10.4254/wjh.v8.i15.637. doi: 10.4254/wjh.v8.i15.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Viganò M, Invernizzi F, Lampertico P. Optimal therapy of chronic hepatitis B: How do I treat my HBeAg-negative patients? Liver Int. 2015;35(Suppl 1):107–13. doi: 10.1111/liv.12717. doi: 10.1111/liv.12717. [DOI] [PubMed] [Google Scholar]
- 8.Brouwer WP, Xie Q, Sonneveld MJ, Zhang N, Zhang Q, Tabak F, et al. Adding pegylated interferon to entecavir for hepatitis B e antigen-positive chronic hepatitis B: A multicenter randomized trial (ARES study) Hepatology. 2015;61:1512–22. doi: 10.1002/hep.27586. doi: 10.1002/hep.27586. [DOI] [PubMed] [Google Scholar]
- 9.Wieland SF, Eustaquio A, Whitten-Bauer C, Boyd B, Chisari FV. Interferon prevents formation of replication-competent hepatitis B virus RNA-containing nucleocapsids. Proc Natl Acad Sci U S A. 2005;102:9913–7. doi: 10.1073/pnas.0504273102. doi: 10.1073/pnas.0504273102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woltman AM, Op den Brouw ML, Biesta PJ, Shi CC, Janssen HL. Hepatitis B virus lacks immune activating capacity, but actively inhibits plasmacytoid dendritic cell function. PLoS One. 2011;6:e15324. doi: 10.1371/journal.pone.0015324. doi: 10.1371/journal.pone.0015324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hatipoglu I, Ercan D, Acilan C, Basalp A, Durali D, Baykal AT, et al. Hepatitis B virus e antigen (HBeAg) may have a negative effect on dendritic cell generation. Immunobiology. 2014;219:944–9. doi: 10.1016/j.imbio.2014.07.020. doi: 10.1016/j.imbio.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 12.Lan S, Wu L, Wang X, Wu J, Lin X, Wu W, et al. Impact of HBeAg on the maturation and function of dendritic cells. Int J Infect Dis. 2016;46:42–8. doi: 10.1016/j.ijid.2016.03.024. doi: 10.1016/j.ijid.2016.03.024. [DOI] [PubMed] [Google Scholar]
- 13.Li MO, Sanjabi S, Flavell RA. Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and – Independent mechanisms. Immunity. 2006;25:455–71. doi: 10.1016/j.immuni.2006.07.011. doi: 10.1016/j.immuni.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 14.Li MH, Zhang D, Zhang L, Qu XJ, Lu Y, Shen G, et al. Ratios of T-helper 2 cells to T-helper 1 cells and cytokine levels in patients with hepatitis B. Chin Med J. 2017;130:1810–5. doi: 10.4103/0366-6999.211541. doi: 10.4103/03666999.211541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang ML, Liaw YF. Hepatitis B flares in chronic hepatitis B: Pathogenesis, natural course, and management. J Hepatol. 2014;61:1407–17. doi: 10.1016/j.jhep.2014.08.033. doi: 10.1016/j.jhep.2014.08.033. [DOI] [PubMed] [Google Scholar]
- 16.Castera L, Chan HL, Arrese M, Afdhal N, Bedossa P, Friedrich-Rust M, et al. EASL-ALEH clinical practice guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63:237–64. doi: 10.1016/j.jhep.2015.04.006. doi: 10.1016/j.jhep.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Zhang L, Li MH, Cao WH, Qi TL, Lu Y, Wu SL, et al. Negative correlation of serum hepatitis B surface antigen and hepatitis B e antigen levels with the severity of liver in ammation in treatment-naiïve patients with chronic hepatitis B virus infection. Chin Med J. 2017;130:2697–702. doi: 10.4103/0366-6999.218000. doi: 10.4103/0366-6999.218000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Op den Brouw ML, Binda RS, van Roosmalen MH, Protzer U, Janssen HL, van der Molen RG, et al. Hepatitis B virus surface antigen impairs myeloid dendritic cell function: A possible immune escape mechanism of hepatitis B virus. Immunology. 2009;126:280–9. doi: 10.1111/j.1365-2567.2008.02896.x. doi: 10.1111/j.1365-2567.2008.02896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trépo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet. 2014;384:2053–63. doi: 10.1016/S0140-6736(14)60220-8. doi: 10.1016/S0140-6736(14)60220-8. [DOI] [PubMed] [Google Scholar]
- 20.Shimizu Y, Guidotti LG, Fowler P, Chisari FV. Dendritic cell immunization breaks cytotoxic T lymphocyte tolerance in hepatitis B virus transgenic mice. J Immunol. 1998;161:4520–9. [PubMed] [Google Scholar]
- 21.Sonneveld MJ, Hansen BE, Piratvisuth T, Jia JD, Zeuzem S, Gane E, et al. Response-guided peginterferon therapy in hepatitis B e antigen-positive chronic hepatitis B using serum hepatitis B surface antigen levels. Hepatology. 2013;58:872–80. doi: 10.1002/hep.26436. doi: 10.1002/hep.26436. [DOI] [PubMed] [Google Scholar]
- 22.Li MH, Zhang L, Qu XJ, Lu Y, Shen G, Wu SL, et al. Kinetics of hepatitis B surface antigen level in chronic hepatitis B patients who achieved hepatitis B surface antigen loss during pegylated interferon alpha-2a treatment. Chin Med J. 2017;130:559–65. doi: 10.4103/0366-6999.200554. doi: 10.4103/0366-6999.200554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liaw YF, Jia JD, Chan HL, Han KH, Tanwandee T, Chuang WL, et al. Shorter durations and lower doses of peginterferon alfa-2a are associated with inferior hepatitis B e antigen seroconversion rates in hepatitis B virus genotypes B or C. Hepatology. 2011;54:1591–9. doi: 10.1002/hep.24555. doi: 10.1002/hep.24555. [DOI] [PubMed] [Google Scholar]
- 24.Gong Y, Zhao C, Zhao P, Wang M, Zhou G, Han F, et al. Role of IL-10-producing regulatory B cells in chronic hepatitis B virus infection. Dig Dis Sci. 2015;60:1308–14. doi: 10.1007/s10620-014-3358-1. doi: 10.1007/s10620-014-3358-1. [DOI] [PubMed] [Google Scholar]
- 25.Das A, Ellis G, Pallant C, Lopes AR, Khanna P, Peppa D, et al. IL-10-producing regulatory B cells in the pathogenesis of chronic hepatitis B virus infection. J Immunol. 2012;189:3925–35. doi: 10.4049/jimmunol.1103139. doi: 10.4049/jimmunol.1103139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi B, Ren G, Hu Y, Wang S, Zhang Z, Yuan Z, et al. HBsAg inhibits IFN-α production in plasmacytoid dendritic cells through TNF-α and IL-10 induction in monocytes. PLoS One. 2012;7:e44900. doi: 10.1371/journal.pone.0044900. doi: 10.1371/journal.pone.0044900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feld JJ, Hoofnagle JH. Mechanism of action of interferon and ribavirin in treatment of hepatitis C. Nature. 2005;436:967–72. doi: 10.1038/nature04082. doi: 10.1038/nature04082. [DOI] [PubMed] [Google Scholar]
- 28.Li CX, Li RT, Zhang W. Progess in non-invasive detection of liver fibrosis. Cancer Biol Med. 2018;15:124–36. doi: 10.20892/j.issn.2095-3941.2018.0018. doi: 10.20892/j.issn.2095-3941.2018.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li MH, Zhang L, Qu XJ, Lu Y, Shen G, Li ZZ, et al. The predictive value of baseline HBsAg level and early response for HBsAg loss in patients with HBeAg-positive chronic hepatitis B during pegylated interferon alpha-2a treatment. Biomed Environ Sci. 2017;30:177–84. doi: 10.3967/bes2017.025. doi: 10.3967/bes2017.025. [DOI] [PubMed] [Google Scholar]