Abstract
Purpose
Radiation therapy results in severe chronic keratopathy and dry eye disease. We developed a novel mouse model for radiation keratopathy to allow future mechanistic studies.
Methods
Six to 8-week-old BALB/c mice underwent sublethal irradiation to the head only from a Cesium-137 irradiator, 2 × 550 rad, 3-hours apart. Irradiated mice were clinically evaluated by corneal fluorescein staining (CFS) at 1, 2, and 3 months, after which corneas were excised and immunofluorescence histochemistry performed with anti-CD45, anti-MHC class II, and anti-β-tubulin antibodies.
Results
The survival rate after irradiation was 100%. Mice demonstrated significant CFS and hair loss around the eyes. Corneal nerve density decreased in the central and peripheral corneas (P < 0.01) at 2 and 3 months, respectively. CD45+ immune cell densities increased in the central and peripheral corneas (P < 0.005, P < 0.001) at 2 and 3 months, respectively. MHC class II, a sign of antigen presenting cell activation, significantly increased after irradiation in the central and peripheral corneas at 2 and 3 months (P = 0.02). A strong inverse correlation was noted between decreased corneal nerves and increase in CD45+ cells in the central cornea at 2 (P = 0.04, r = −0.89) and 3 months (P = 0.03, r = −0.91) after irradiation.
Conclusions
We present a model of radiation keratopathy and demonstrate significant nerve loss and increase in immune cell influx and activation within months. This model will enable future investigations to understand the effects of radiation therapy on the eye, and to study mechanisms of neuro-immune crosstalk in the cornea.
Keywords: radiation keratopathy, irradiation, mouse model, corneal nerves, inflammation
Cancers of the head and neck, which include cancers of the oral cavity, larynx, pharynx, salivary glands, and nose/nasal passages, are a diverse group of cancers ranging from squamous cell carcinomas (91%), adenocarcinomas, melanomas and other nonspecified tumors (7%), and sarcomas (2%).1 Head and neck cancers account for approximately 3% of all malignancies in the United States2 and 4% of those in Europe.1 Worldwide, 550,000 new cases are diagnosed and 380,000 deaths occur every year3 with men having a four times higher incidence of head and neck cancers than women.1–3 Risk factors for head and neck cancers are primarily tobacco and alcohol use, followed by human papillomavirus (HPV) infection (for oropharyngeal cancer) and Epstein-Barr virus (EBV) infection (for nasopharyngeal cancer).4 Standard treatments for head and neck cancers, which depend on the tumor location, tumor stage, and patient's age and overall health, include radiation therapy, surgery, chemotherapy, targeted therapy, or a combination of treatments.5
Advances in techniques attempt to minimize damage to surrounding healthy tissues when patients undergo radiation therapy. This includes selectively shielding key ocular structures, such as the lacrimal gland, fine focusing of the angle of the radiation field and using beam attenuators to allow the dose to be delivered uniformly.6 Nevertheless, complications from radiation therapy still occur and ocular tissues may be affected, but may not manifest for weeks, months, or even years later.7 Some of these adverse effects include chronic dry eye disease (DED) due to the apparent damaging effects of radiation on the lacrimal or meibomian glands,8 radiation retinopathy,9 or radiation keratopathy.10 Interestingly, even if the lacrimal gland is shielded during the irradiation procedure, radiation keratopathy still can develop, which means that the tear film alone is not a factor in the damage to the cornea.11
Radiation keratopathy can manifest clinically with several presentations. Acute radiation keratopathy can be transient and negligible and present with superficial punctate keratitis, stromal keratitis, stromal scarring, and corneal edema, and typically is treated with topical anti-inflammatory and lubricating eye drops.12 In contrast, chronic radiation keratopathy generally is thought to be permanent and irreversible.12 Early signs are loss of corneal sensation that can result in neurotrophic corneal ulcers, corneal melting, and in severe cases, corneal perforation.11 In these cases, wound healing may occur with medical therapy, but could result in permanent corneal neovascularization and scarring.13 Clinically, chronic corneal inflammation and concurrent decreased corneal sensation have been described with the development of limbal stem cell deficiency in patients with radiation keratopathy.14,15 Unfortunately, most patients are not referred to ophthalmologists for monitoring after receiving radiation therapy for cancers of the head and neck. Therefore, if damage to the cornea does occur, treatment may be started after the disease has advanced and the effects of radiation become irreversible and, thus, treatment options become more limited. This is partially due to the neurotrophic state of the cornea from corneal nerve loss and lack of symptoms in patients with early disease.
The specific mechanisms involved in the development of radiation keratopathy remain elusive. Corneal inflammation has been demonstrated during the early stages of radiation keratopathy. Further, loss of corneal sensation indicates that corneal nerves are being affected by radiation and/or inflammation, potentially due to apoptosis or necrosis. Given the current evidence, we hypothesized that there is a potential interplay between nerve damage and inflammation, resulting in the development and progression of this disease. To test our hypothesis, we developed a novel mouse model for radiation keratopathy without any direct insult to the cornea, mimicking human disease, allowing us to study the pathogenesis and specific mechanisms of this disease.
Materials and Methods
Animals
Six- to 8-week-old male BALB/c mice were obtained through Charles River Laboratories (Wilmington, MA, USA). Experiments were performed in concordance with the ARVO Statement for the Use of Animals in Ophthalmic and Visual Research and were approved by the Harvard Medical Area Standing Committee on Animals and Schepens Institutional Animal Care and Use Committee. For each time point, at least three, but up to six mice were used.
Irradiation Procedure
The initial rationale was to mimic the human radiation treatment dose for head and neck cancers (66–74 Gy given as 2.0 Gy/fraction daily Monday through Friday for 7 weeks; Rad = 0.01 Gy) in two ways—70 Gy split 3 hours apart or 70 Gy split between 5 days once per day. In both instances even with shielding, all animals died. We next assessed if the standard radiation dose to generate bone marrow chimeras,16 a much lower dose than radiation to the head and neck, resulted in corneal changes. Thus, we used a similar protocol that allows sublethal irradiation, which affects the corneas without resulting in death of animals. This resulted in adaptation of the following lower radiation model, which allowed us to study the effect of radiation on the cornea and ocular surface.
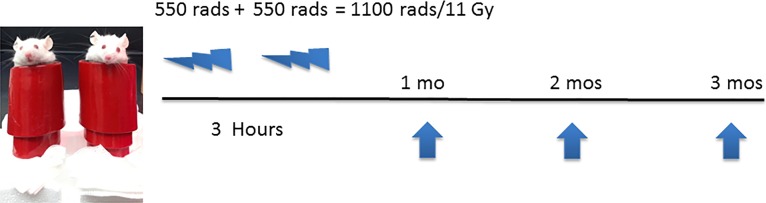
Six- to 8-week-old adult male BALB/c mice were anesthetized with a mixture of ketamine (112.5 mg/kg) and xylazine (22.5 mg/kg) and placed upright in a 50 mL conical tube wrapped in 6.6 mm lead shielding (Electron Microscopy Services, Hatfield, PA, USA) with the heads exposed. The mice underwent sublethal irradiation from a Cesium-137 irradiator (Mark 1 Irradiator; JL Shepherd & Associates, San Fernando, CA, USA), 2 × 550 rad, 3 hours apart. Each radiation exposure lasted 2 minutes 39 seconds. Immediately afterwards, Genteal gel (Alcon, Fort Worth, TX, USA) was added to their corneas, and they recovered in their cages. Animals were kept under viral antibody–free/specific pathogen–free (VAF/SPF) barrier conditions on standard laboratory chow and sterile water with antibiotic-containing water (sulfatrim) ad libitum (Fig. 1).
Figure 1.
Six- to 8-week-old adult BALB/c mice were anesthetized and placed upright in a 50 mL conical tube wrapped in 6.6 mm lead shielding with the heads exposed. The mice underwent sublethal irradiation from a Cesium-137 irradiator, 2 × 550 rad, 3 hours apart.
Clinical Evaluation and Corneal Fluorescein Staining
Irradiated mice were evaluated clinically by corneal fluorescein staining (CFS) at 1, 2, and 3 months, after which normal and irradiated corneas were excised respectively. Briefly, a FUL-GLO Fluorescein Sodium Strip (Akorn, Lake Forest, IL, USA) was placed in 1 mL 1 × PBS (Life Technologies, Carlsbad, CA, USA) in a 5 mL syringe. The diluted fluorescein solution was dropped on the surface of the eye of anesthetized mice as above. Excess fluorescein was removed using a cotton applicator outside the lid margin. Nonspecific fluorescein was washed away with 2 additional drops of 1 × PBS. Pictures were taken under cobalt blue light to document corneal fluorescein staining. Uveitis was assessed by eye redness or pain. Evidence of pain would be if the animals had a ruffled coat, extreme lethargy, or inability to eat/drink.
Immunofluorescence Histochemistry
Upon euthanizing the mice, whole corneas with surrounding bulbar conjunctiva were excised, and fixed in ice cold 100% acetone (Sigma-Aldrich Corp., St. Louis, MO, USA) for 15 minutes. Following three washings with 1 × PBS, tissues were blocked in 3% BSA (Sigma-Aldrich Corp.) containing 1% anti-CD16/CD32 Fc receptor (FcR) mAb (clone 2.4G2; Bio X Cell, West Lebanon, NH, USA) for 1 hour at room temperature (RT). Next, samples were incubated overnight at 4°C with FITC-conjugated CD45 (BioLegend, San Diego, CA, USA) or FITC anti-I-A/I-E (MHC class II; BD Biosciences, San Jose, CA, USA) to determine immune cell alterations and with Northern Lights 557 (NL557)-conjugated anti-neuron-specific β-III tubulin antibody (R&D Systems, Minneapolis, MN, USA) to evaluate corneal nerve changes in central and peripheral corneas. Corneas then were washed with 1 × PBS three times for 10 minutes each, and then were mounted on slides with Vectashield mounting media with 4′,6-diamidino-2-phenylendole (DAPI; Vector Laboratories, Burlingame, CA, USA) and underwent confocal microscopy with a Leica TCS SP5 confocal microscope (Leica Microsystems, Inc., Buffalo Grove, IL, USA). Analysis was performed with Imaris (Bitplane, South Windsor, CT, USA) to calculate cell density of immune cells and with NeuronJ, a plugin for ImageJ (http://imagej.nih.gov/ij/; provided in the public domain by the National Institutes of Health, Bethesda, MD, USA) to calculate nerve density.17
Results
Irradiation Results in Significant Corneal Fluorescein Staining
The survival rate of irradiated mice was 100% with the body lead shielding at 3 months. Compared to previous studies,18,19 this method did not require generation of bone marrow chimeras to maintain survival. Mice demonstrated significant corneal fluorescein staining as well as hair loss around the eyes at 3 months after irradiation (Fig. 2). No mice showed symptoms of uveitis, such as redness or pain.
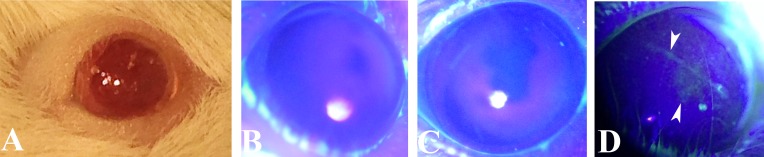
Figure 2.
Clinical findings of irradiated mice at 1, 2, and 3 months. Alopecia areata occurs around the eye beginning at 2 months (A). CFS in 2 representative mice at 1 (B), 2 (C) and 3 (D) months showing diffuse staining of the epithelium (white arrows) indicating damage to the ocular surface.
Irradiation Results in Significant Corneal Nerve Loss Within Months
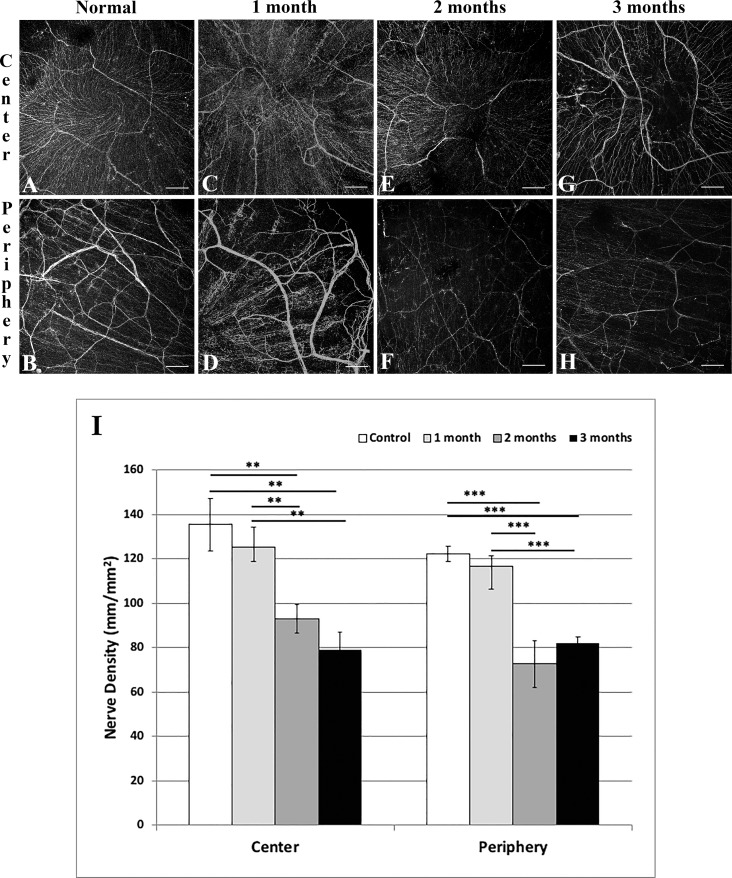
Corneal nerve density (Figs. 3A–I) decreased in the central cornea from 135.29 ± 11.67 mm/mm2 in naïve mice (Fig. 3A) to 92.84 ± 6.42 (P = 0.009) and 78.83 ± 8.17 (P = 0.005) mm/mm2 at 2 (Fig. 3E) and 3 (Fig. 3G) months after irradiation, respectively. Peripheral corneal nerve density decreased from 122.01 ± 3.49 (Fig. 3B) to 72.55 ± 4.93 (P < 0.001; Fig. 3F) and 82.10 ± 2.80 (P < 0.001; Fig. 3H) mm/mm2, respectively. There was no significant difference in corneal nerve density between naïve mice and those at 1 month after irradiation (Figs. 3C, 3D) in either the center or periphery.
Figure 3.
Representative histologic images of central (A, C, E, G) and peripheral (B, D, F, H) corneas stained with neuron-specific anti-β III tubulin NL557-conjugated antibody in normal (A, B) mice, and at 1 (C, D), 2 (E, F), and 3 (G, H) months after irradiation. Graph results shown in (I). Scale bar: 100 μm. Mean ± SEM. **P < 0.01; ***P < 0.001.
Irradiation Results in Significant Immune Cell Influx, as Well as Activation and Maturation of Antigen Presenting Cells in the Cornea Within Months
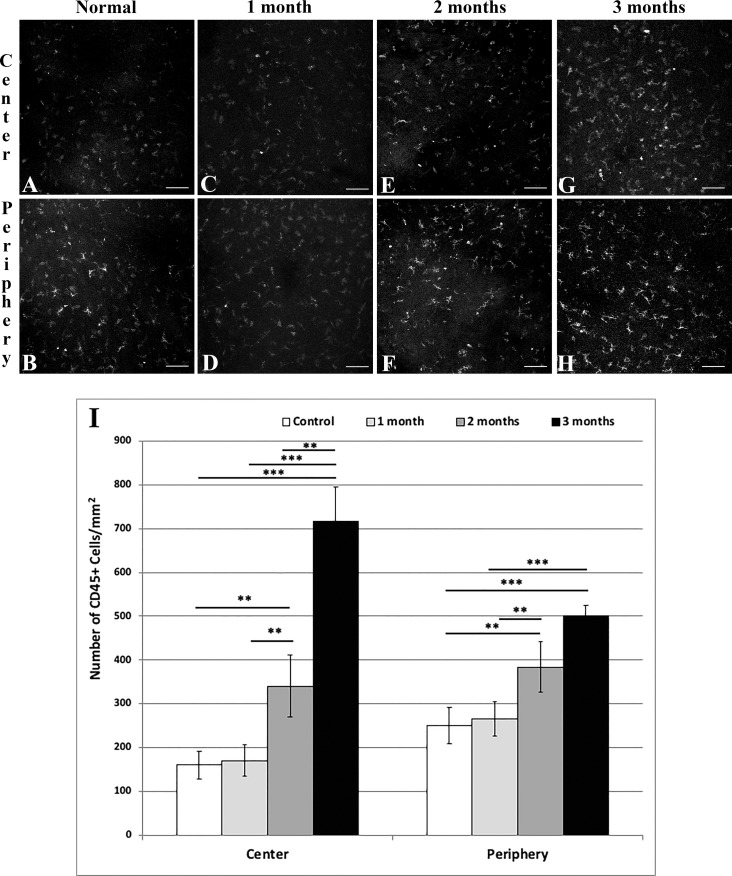
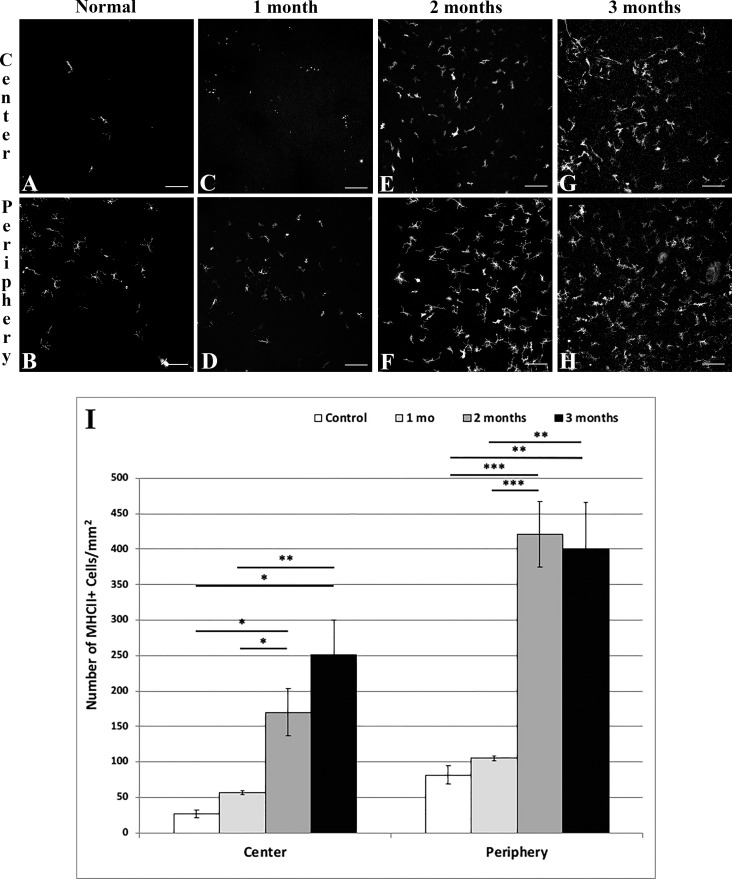
CD45+ immune cell densities (Figs. 4A–I) continuously increased in the central cornea from 66.42 ± 13.27 cells/mm2 in naïve mice (Fig. 4A) to 141.15 ± 29.39 (P = 0.004) and 297.81 ± 32.42 (P < 0.001) at 2 (Fig. 4E) and 3 (Fig. 4G) months after irradiation, respectively. In the peripheral cornea, CD45+ immune cell densities increased from 103.79 ± 17.34 Fig. 4B) to 159.42 ± 23.94 (P = 0.002; Fig. 4F) and 208.13 ± 10.15 (P < 0.001; Fig. 4H) cells/mm2, respectively.
Figure 4.
Representative histologic images of central (A, C, E, G) and peripheral (B, D, F, H) cornea stained with anti-CD45 FITC-conjugated antibody in normal (A, B) mice, and at 1 (C, D), 2 (E, F), and 3 (G, H) months after irradiation. Graph results shown in (I). Scale bar: 100 μm. Mean ± SEM. **P < 0.01; ***P < 0.001.
Further, MHC-II, a sign of antigen presenting cell activation and maturation, significantly increased in the central cornea from 11.07 ± 2.11 cells/mm2 in naïve mice (Fig. 5A) to 70.58 ± 13.96 (P < 0.05) and 104.07 ± 20.69 (P < 0.05) cells/mm2 at 2 (Fig. 5E) and 3 (Fig. 5G) months after irradiation, respectively. In the peripheral cornea, MHC-II+ immune cell densities increased from 33.90 ± 5.47 (Fig. 5B) to 174.78 ± 18.92 (P < 0.001; Fig. 5F) and 166.20 ± 26.98 (P < 0.01; Fig. 5H) cells/mm2. Neither CD45+ nor MHC-II+ cell densities were significantly different between naïve mice and those at 1 month after irradiation (Figs. 4C, 4D, 5C, 5D).
Figure 5.
Representative histologic images of central (A, C, E, G) and peripheral (B, D, F, H) cornea stained with anti-MHC class II FITC-conjugated antibody in normal (A, B) mice, and at 1 (C, D), 2 (E, F), and 3 (G, H) months after irradiation. Graph results shown in (I). Scale bar: 100 μm. Mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
Irradiation Results in a Strong Inverse Correlation Between Corneal Nerves Loss and Increased Density of Corneal CD45+ Cells
A strong inverse correlation was noted between decreased corneal nerves and increased CD45+ cells in the central cornea (r = −0.83; P < 0.001) using the Pearson correlation coefficient (Fig. 6).
Figure 6.
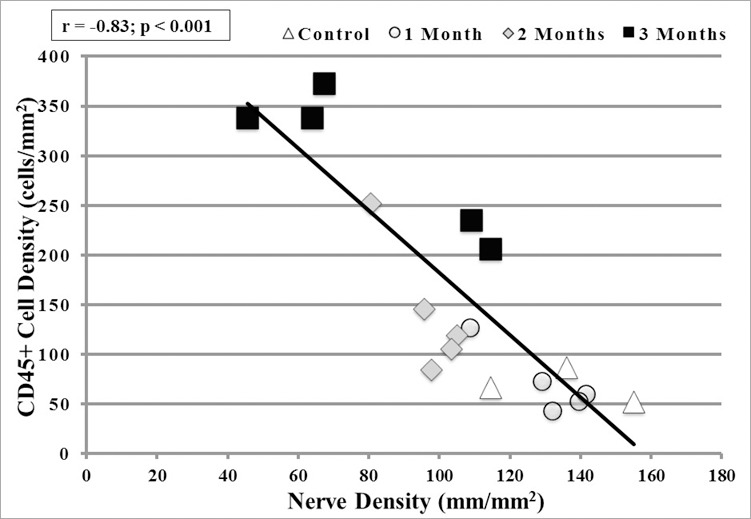
There was a severe significant reverse correlation (r = −0.83) between CD45+ cell density and nerve density in the central cornea of irradiated mice (P < 0.001).
Discussion
Radiation doses to head and neck tumors are typically given between 50 and 74 Gy (2.0 Gy/fraction; daily Monday–Friday for 7 weeks),20 but to our knowledge no data exist on the dose received by surrounding normal tissue. Parsons et al.21 described a subset of 20 of 33 evaluated patients with extracranial head and neck tumors who received irradiation of the entire orbit and who had a higher risk of severe dry eye complications due to the location of tumor and exposure of the lacrimal gland. Specifically, the probability of complications, including edema, ulceration, bacterial infection, vascularization, opacification, and perforation, appeared to increase steeply at doses >30 Gray. Bhandare et al.22 emphasized the chronic problem of severe radiation-induced DED that can significantly affect quality of life due to compromised vision and severe pain caused by radiation injury to one or several of the ocular structures, including major and accessory lacrimal glands, conjunctival goblet cells, and meibomian glands. They suggested to reduce the total dose and fraction size to all components of the lacrimal system to reduce the incidence of delayed severe DED. Further, Fujishima et al.15 described a severe case of radiation keratopathy with temporary corneal stem cell dysfunction that developed after radiation therapy and resulted in ocular pain and loss of vision followed by partial conjunctivalization of the cornea.15 Thus, radiation keratopathy can result in significant ocular surface disease, stem cell deficiency, vision loss, discomfort, and subsequent poor quality of life.
Kwok et al.11 analyzed the incidence and risk factors of severe radiation keratopathy. The incidence increased from 0% after doses <59 Gy to 100% after doses >70 Gy. They proposed that the threshold radiation dose of 45 to 60 Gy to the cornea is the most important factor in determining the outcome of radiation keratopathy and, therefore, an important prognostic factor. After this dose is reached, visual outcome is in serious jeopardy. They also refuted prior literature indicating that protecting only the lacrimal gland would prevent severe radiation keratopathy especially when the corneal dose was >70 Gy. In addition, they emphasized the impact of irradiation on the corneal nerves, resulting in epithelial defects and delaying corneal would healing. They noted the necessity of clinical trials on newer strategies, such as for limbal and conjunctival autographs, to treat ocular surfaces exposed to heavy irradiation and the hope that the treatment to the cornea be started as early as possible before damage occurs to the cornea.
Clinically, the management of radiation keratopathy is extremely difficult, due to lack of effective therapies and poor understanding of the disease.23 The goal of treating a patient with a head or neck cancer is to obtain the best possible outcome of eliminating the growth with as few side effects as possible. However, studies have demonstrated that while patients do not suffer from immediate side effects from the treatment, late stage ocular complications affect their quality of life and vision. Thus, additional mechanistic studies to understand the pathogenesis of this debilitating disease are needed. However, the lack of animal models of radiation keratopathy to date has hampered progress in this field.
We developed a murine model of irradiation keratopathy by utilizing a cesium irradiator. While the human-equivalent dose from the human treatment for head and neck tumors was lethal to mice, even our sublethal lower dose (11 vs. 70 Gy) demonstrated clinical signs of radiation keratopathy, including increased corneal fluorescein staining, indicating that the corneal epithelium is affected, although not as severely as those undergoing high radiation doses for head and neck cancers.15 Further, we demonstrate periorbital hair loss or alopecia due to the sensitivity of the hair follicle to radiation.24 Moreover, these mice did not suffer uveitis or symptoms of pain. Although we did not specifically look for any effects on the posterior segment in our model, studies have shown posterior injury following irradiation, including iris neovascularization, neovascular glaucoma,25 optic neuropathy,26 radiation maculopathy and radiation retinopathy.9,27,28 Furthermore, Müther et al.29 using a similar dose of full-body irradiation as in our model, demonstrated leukocyte infiltration in the retina.
Neuropathy and progressive cognitive impairment are a well-recognized late complications after radiation therapy. Radiation neuropathy may develop and persist long after treatment.30–34 Yet, the mechanisms of radiation-induced neuronal injury are poorly understood. One hypotheses includes the role of glial cells, which are responsible for myelination in the central nervous system (CNS), which might be a prime target of radiation-induced demyelination and necrosis of the white matter of CNS.35 Histology has shown a significant decrease in nerve fiber density, especially affecting large nerve fibers after doses higher than 20 Gy.36 Another group performed electron microscopic analysis and demonstrated an increase in microtubule density and neurofilament accumulation in axons of irradiated nerves.36 These findings suggest radiation-induced hypoxia, resulting in axonal damage and subsequent nerve fiber loss as a mechanism of late radiation injury to the peripheral nerve.36 Other theories of vascular occlusion,37 free radical injury,38 direct damage to cellular DNA,39 and damage to the blood–brain barrier40 have been proposed to explain the pathophysiology. Regardless, irradiation triggers a complex and multifactorial response involving a persistent increase of reactive oxygen species (ROS) and pro-inflammatory cytokines that actively participate in remodeling of the irradiated microenvironment.41–43 However, such changes have been suggested not to be irreversible.43 Acharya et al.33 reported that transplantation of human embryonic stem cells may rescue the radiation-induced cognitive impairment.
Neurogenic inflammation results from damage to the nociceptor sensory neurons, most widely studied with the application of the chemical capsaicin.44 This, then, results in sensation of pain and release of potent neuropeptides, notably calcitonin gene-related peptide (CGRP), substance P (SP), and neurokinin A from activated nerve terminals, which subsequently bind to their respective receptors neurokinin-1 receptor (NK-1R) located on neurons,45 leukocytes,46 and epithelial cells.47 In response to SP stimulation, macrophages, for example, can release inflammatory mediators, such as interleukins,48 chemokines,49 and growth factors.50 The resulting inflammation is due to vasodilation, microvasculature permeability, leukocyte infiltration, and mast cell degranulation.51 This process has been implicated in various human diseases of the nervous system,52 respiratory system,53 gastrointestinal tract,54 skin,55 and more relevant to this study, the ocular surface.35,56,57
Our current animal model showed that the protracted nature of radiation responses contributes to the inhibition of neuronal regeneration or persistent neuronal injury as shown by decreased total nerve density in the central and peripheral corneas seen as early as 2 months after irradiation. Further studies are required to reveal the mechanisms of nerve loss and assess the validity of the existing hypothesis. Our study did not demonstrate changes in total nerve density or an increase in CD45+ or MHC-II+ cells at 1 month after irradiation. Nevertheless, it is possible that changes to individual trunks and branches of the nerves at that point and even earlier may have been present. Alternatively functional alterations may have preceded morphological nerve changes.
Our findings are novel in that the breakdown of immune homeostasis due to corneal nerve damage may be a key pathologic mechanism of radiation keratopathy. On a microscopic level, we showed that irradiation results in an infiltration of CD45+ bone marrow-derived cells and increased level of MHC-II+ cells, indicating infiltration and activation of leukocytes. In addition, there is a strong inverse correlation between nerve loss and increased leukocyte density in the central cornea at 2 and 3 months. MHC-II+ cells also had an inverse correlation with total nerve loss, but it was not significant (P = 0.09). Similarly, our mechanical denervation model of the cornea involving transecting the ciliary nerves using a lateral conjunctival approach17 results in complete loss of sensory nerves and increased expression of vascular adhesion molecules, leading to migration of bone marrow–derived immune cells to infiltrate the cornea as early as 24 hours postoperatively. Increase in proinflammatory cytokines and heme- and lymphangiogenesis follows, disrupting the homeostasis of this immune privileged site solely from loss of nerves to the cornea. Clinically, we also recently reported the interaction between immune and nervous systems in the human cornea.58–60 Collectively, our preclinical and clinical studies suggested a critical role of the peripheral nervous system in maintaining corneal immune privilege.61
In conclusion, we presented a novel and clinically relevant murine model of radiation keratopathy and demonstrated significant nerve loss and increase in leukocyte influx and their activation within months. This model will enable future investigations to understand the mechanisms and effects of radiation therapy on the eye, and as well as the studies of the effects of chronic nerve loss on the corneal immune homeostasis. Given the current results, future studies are necessary to assess the use of corneal shielding for patients undergoing radiation for head and neck tumors, and early monitoring by ophthalmologists for these patients may be warranted.
Acknowledgments
The authors thank Ulrich H. von Andrian, MD, PhD, for his critical consultations and suggestions in experimental design, Harvard Center for Comparative Medicine for assisting in the animal studies, and Donald Pottle at Schepens for his help and expertise in using the confocal microscope.
Supported by National Institutes of Health (Bethesda, MD, USA) Grants R01-EY022695 (PH), K08-EY020575 (PH), and NIH K12-EY016335 (PH); the Falk Medical Research Foundation (PH); Research to Prevent Blindness Career Development Award (PH); Fight for Sight Grant-in-Aid (PH), and Uehara Memorial Foundation Fellowship (TY).
Disclosure: D.L. Harris, None; T. Yamaguchi, None; P. Hamrah, None
References
- 1.Gatta G., Botta L., Sanchez MJ, et al. Prognoses and improvement for head and neck cancers diagnosed in Europe in early 2000s: the EUROCARE-5 population-based study. Eur J Cancer. 2015;51:2130–2143. doi: 10.1016/j.ejca.2015.07.043. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 3.Fitzmaurice C., Allen C., Barber RM, et al. Global Burden of Disease Cancer Collaboration. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3:524–548. doi: 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pezzuto F., Buonaguro L., Caponigro F., et al. Update on head and neck cancer: current knowledge on epidemiology, risk factors, molecular features and novel therapies. Oncology. 2015;89:125–136. doi: 10.1159/000381717. [DOI] [PubMed] [Google Scholar]
- 5.National Cancer Institute. Head and Neck–Patient Version. Available at: https://www.cancer.gov/types/head-and-neck.
- 6.Rubin P., Constantine LS, Marks LB. ALERT – Adverse Late Effects of Cancer Treatment: Volume 2: Normal Tissue Specific Sites and Systems. New York: Springer;; 2013. [Google Scholar]
- 7.Kwan L., Ilsen PF. Ocular complications from radiation: therapy of the head and neck. Clin Refract Optom. 2008;19:336–350. [Google Scholar]
- 8.Parsons JT, Bova FJ, Mendenhall WM, Million RR, Fitzgerald CR. Response of the normal eye to high dose radiotherapy. Oncology. 1996;10:837–852. [PubMed] [Google Scholar]
- 9.Reichstein D. Current treatments and preventive strategies for radiation retinopathy. Curr Opin Ophthalmol. 2015;26:157–166. doi: 10.1097/ICU.0000000000000141. [DOI] [PubMed] [Google Scholar]
- 10.Mendez CA, Singh AD. Radiation therapy: anterior segment complications. Dev Ophthalmol. 2013;52:102–113. doi: 10.1159/000351087. [DOI] [PubMed] [Google Scholar]
- 11.Kwok SK, Ho PC, Leung SF, et al. An analysis of the incidence and risk factors of developing severe keratopathy in eyes after megavoltage external beam irradiation. Ophthalmology. 1998;105:2051–2055. doi: 10.1016/s0161-6420(98)91123-x. [DOI] [PubMed] [Google Scholar]
- 12.Ober M., Servodidio CA, Abramson D. Ocular complications due to cancer treatment. In: Schwartz CL, Hobbie WL, Constine LS, Ruccione KS, editors. Survivors of Childhood and Adolescent Cancer: A Multidisciplinary Approach. Berlin, Heidelberg: Springer Berlin Heidelberg;; 2005. pp. 81–94. [Google Scholar]
- 13.Macfaul PA, Bedford MA. Ocular complications after therapeutic irradiation. Br J Ophthalmol. 1970;54:237–247. doi: 10.1136/bjo.54.4.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le Q., Xu J., Deng SX. The diagnosis of limbal stem cell deficiency. Ocul Surf. 2018;16:58–69. doi: 10.1016/j.jtos.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujishima H., Shimazaki J., Tsubota K. Temporary corneal stem cell dysfunction after radiation therapy. Br J Ophthalmol. 1996;80:911–914. doi: 10.1136/bjo.80.10.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delrez M., Haot J., Betz EH. Effect of normal and irradiated marrow grafts on the haemopoietic recovery after a sublethal irradiation. Experientia. 1971;27:453–454. doi: 10.1007/BF02137308. [DOI] [PubMed] [Google Scholar]
- 17.Yamaguchi T., Turhan A., Harris DL, et al. Bilateral nerve alterations in a unilateral experimental neurotrophic keratopathy model: a lateral conjunctival approach for trigeminal axotomy. PLoS One. 2013;8:e70908. doi: 10.1371/journal.pone.0070908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lorenz E., Uphoff D., Reid TR, Shelton E. Modification of irradiation injury in mice and guinea pigs by bone marrow injections. J Natl Cancer Inst. 1951;12:197–201. [PubMed] [Google Scholar]
- 19.Lorenz E., Congdon CC. Modification of lethal irradiation injury in mice by injection of homologous or heterologous bone. J Natl Cancer Inst. 1954;14:955–965. [PubMed] [Google Scholar]
- 20.Yeh SA. Radiotherapy for head and neck cancer. Semin Plast Surg. 2010;24:127–136. doi: 10.1055/s-0030-1255330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parsons JT, Bova FJ, Fitzgerald CR, Mendenhall WM, Million RR. Severe dry-eye syndrome following external beam irradiation. Int J Radiat Oncol Biol Phys. 1994;30:775–780. doi: 10.1016/0360-3016(94)90348-4. [DOI] [PubMed] [Google Scholar]
- 22.Bhandare N., Moiseenko V., Song WY, Morris CG, Bhatti MT, Mendenhall WM. Severe dry eye syndrome after radiotherapy for head-and-neck tumors. Int J Radiat Oncol Biol Phys. 2012;82:1501–1508. doi: 10.1016/j.ijrobp.2011.05.026. [DOI] [PubMed] [Google Scholar]
- 23.Barabino S., Raghavan A., Loeffler J., Dana R. Radiotherapy-induced ocular surface disease. Cornea. 2005;24:909–914. doi: 10.1097/01.ico.0000154235.64359.d3. [DOI] [PubMed] [Google Scholar]
- 24.Kyoizumi S., Suzuki T., Teraoka S., Seyama T. Radiation sensitivity of human hair follicles in SCID-hu mice. Radiat Res. 1998;149:11–18. [PubMed] [Google Scholar]
- 25.Wen JC, Oliver SC, McCannel TA. Ocular complications following I-125 brachytherapy for choroidal melanoma. Eye (Lond) 2009;23:1254–1268. doi: 10.1038/eye.2009.43. [DOI] [PubMed] [Google Scholar]
- 26.Brown GC, Shields JA, Sanborn G., Augsburger JJ, Savino PJ, Schatz NJ. Radiation optic neuropathy. Ophthalmology. 1982;89:1489–1493. doi: 10.1016/s0161-6420(82)34612-6. [DOI] [PubMed] [Google Scholar]
- 27.Brown GC, Shields JA, Sanborn G., Augsburger JJ, Savino PJ, Schatz NJ. Radiation retinopathy. Ophthalmology. 1982;89:1494–1501. doi: 10.1016/s0161-6420(82)34611-4. [DOI] [PubMed] [Google Scholar]
- 28.Archer DB, Amoaku WM, Gardiner TA. Radiation retinopathy--clinical, histopathological, ultrastructural and experimental correlations. Eye (Lond) 1991;5:239–251. doi: 10.1038/eye.1991.39. [DOI] [PubMed] [Google Scholar]
- 29.Müther PS, Semkova I., Schmidt K., et al. Conditions of retinal glial and inflammatory cell activation after irradiation in a GFP-chimeric mouse model. Invest Ophthalmol Vis Sci. 2010;51:4831–4839. doi: 10.1167/iovs.09-4923. [DOI] [PubMed] [Google Scholar]
- 30.Hutcheson KA, Lewin JS, Barringer DA, et al. Late dysphagia after radiotherapy-based treatment of head and neck cancer. Cancer. 2012;118:5793–5799. doi: 10.1002/cncr.27631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Awan MJ, Mohamed ASR, Lewin JS, et al. Late radiation-associated dysphagia (late-rad) with lower cranial neuropathy after oropharyngeal radiotherapy: a preliminary dosimetric comparison. Oral Oncol. 2014;50:746–752. doi: 10.1016/j.oraloncology.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen AM, Wang PC, Daly ME, et al. Dose--volume modeling of brachial plexus-associated neuropathy after radiation therapy for head-and-neck cancer: findings from a prospective screening protocol. Int J Rad Oncol Biol Phys. 2014;88:771–777. doi: 10.1016/j.ijrobp.2013.11.244. [DOI] [PubMed] [Google Scholar]
- 33.Acharya MM, Christie LA, Lan ML, et al. Rescue of radiation-induced cognitive impairment through cranial transplantation of human embryonic stem cells. Proc Natl Acad Sci U S A. 2009;106:19150–19155. doi: 10.1073/pnas.0909293106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chiang CS, Hong JH, Stalder A., Sun JR, Withers HR, McBride WH. Delayed molecular responses to brain irradiation. Int J Rad Biol. 1997;72:45–53. doi: 10.1080/095530097143527. [DOI] [PubMed] [Google Scholar]
- 35.Bignami F., Rama P., Ferrari G., Substance P. and its inhibition in ocular inflammation. Curr Drug Targets. 2015;17:1265–1274. doi: 10.2174/1389450116666151019100216. [DOI] [PubMed] [Google Scholar]
- 36.Vujaskovic Z. Structural and physiological properties of peripheral nerves after intraoperative irradiation. J Peripher Nerv Syst. 1997;2:343–349. [PubMed] [Google Scholar]
- 37.Stewart FA, Hoving S., Russell NS. Vascular damage as an underlying mechanism of cardiac and cerebral toxicity in irradiated cancer patients. Radiat Res. 2010;174:865–869. doi: 10.1667/RR1862.1. [DOI] [PubMed] [Google Scholar]
- 38.Pollycove M. Nonlinearity of radiation health effects. Environ Health Perspect. 1998;106(suppl 1):363–368. doi: 10.1289/ehp.98106s1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muzalov II, Mikhailenko VM. Peculiarities of DNA damage caused by exogenous nitric oxide combined with fractionated low dose ionizing radiation in normal and tumor cells. Exp Oncol. 2015;37:40–43. [PubMed] [Google Scholar]
- 40.Adair JC, Baldwin N., Kornfeld M., Rosenberg GA. Radiation-induced blood-brain barrier damage in astrocytoma: relation to elevated gelatinase B and urokinase. J Neurooncol. 1999;44:283–289. doi: 10.1023/a:1006337912345. [DOI] [PubMed] [Google Scholar]
- 41.Kim JH, Brown SL, Jenrow KA, Ryu S. Mechanisms of radiation-induced brain toxicity and implications for future clinical trials. J Neuro-Oncol. 2008;87:279–286. doi: 10.1007/s11060-008-9520-x. [DOI] [PubMed] [Google Scholar]
- 42.Fike JR, Rola R., Limoli CL. Radiation response of neural precursor cells. Neurosurg Clin N Am. 2007;18:115–127. doi: 10.1016/j.nec.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 43.Fike JR, Rosi S., Limoli CL. Neural precursor cells and central nervous system radiation sensitivity. Sem Rad Oncol. 2009;19:122–132. doi: 10.1016/j.semradonc.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Szolcsanyi J. Selective responsiveness of polymodal nociceptors of the rabbit ear to capsaicin, bradykinin and ultra-violet irradiation. J Physiol. 1987;388:9–23. doi: 10.1113/jphysiol.1987.sp016598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nagy I., Maggi CA, Dray A., Woolf CJ, Urban L. The role of neurokinin and N-methyl-D-aspartate receptors in synaptic transmission from capsaicin-sensitive primary afferents in the rat spinal cord in vitro. Neuroscience. 1993;52:1029–1037. doi: 10.1016/0306-4522(93)90549-u. [DOI] [PubMed] [Google Scholar]
- 46.Mikami N., Matsushita H., Kato T., et al. Calcitonin gene-related peptide is an important regulator of cutaneous immunity: effect on dendritic cell and T cell functions. J Immunol. 2011;186:6886–6893. doi: 10.4049/jimmunol.1100028. [DOI] [PubMed] [Google Scholar]
- 47.Yang L., Sui W., Li Y., et al. Substance P inhibits hyperosmotic stress-induced apoptosis in corneal epithelial cells through the mechanism of Akt activation and reactive oxygen species scavenging via the neurokinin-1 receptor. PLoS One. 2016;11:e0149865. doi: 10.1371/journal.pone.0149865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blum A., Setiawan T., Hang L., Stoyanoff K., Weinstock JV. Interleukin-12 (IL-12) and IL-23 induction of substance p synthesis in murine T cells and macrophages is subject to IL-10 and transforming growth factor beta regulation. Infect Immun. 2008;76:3651–3656. doi: 10.1128/IAI.00358-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spitsin S., Meshki J., Winters A., Tuluc F., Benton TD, Douglas SD. Substance P-mediated chemokine production promotes monocyte migration. J Leukoc Biol. 2017;101:967–973. doi: 10.1189/jlb.1AB0416-188RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bignami F., Rama P., Ferrari G., Substance P. and its inhibition in ocular inflammation. Curr Drug Targets. 2016;17:1265–1274. doi: 10.2174/1389450116666151019100216. [DOI] [PubMed] [Google Scholar]
- 51.Chiu IM, von Hehn CA, Woolf CJ. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat Neurosci. 2012;15:1063–1067. doi: 10.1038/nn.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Malhotra R. Understanding migraine: potential role of neurogenic inflammation. Ann Indian Acad Neurol. 2016;19:175–182. doi: 10.4103/0972-2327.182302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Butler CA, Heaney LG. Neurogenic inflammation and asthma. Inflamm Allergy Drug Targets. 2007;6:127–132. doi: 10.2174/187152807780832238. [DOI] [PubMed] [Google Scholar]
- 54.Wesselmann U. Neurogenic inflammation and chronic pelvic pain. World J Urol. 2001;19:180–185. doi: 10.1007/s003450100201. [DOI] [PubMed] [Google Scholar]
- 55.Antiga E., Verdelli A., Bonciani D., Bonciolini V., Caproni M., Fabbri P. Acne: a new model of immune-mediated chronic inflammatory skin disease. G Ital Dermatol Venereol. 2015;150:247–254. [PubMed] [Google Scholar]
- 56.Micera A., Lambiase A., Bonini S. The role of neuromediators in ocular allergy. Curr Opin Allergy Clin Immunol. 2008;8:466–471. doi: 10.1097/ACI.0b013e32830e6b17. [DOI] [PubMed] [Google Scholar]
- 57.Beuerman RW, Stern ME. Neurogenic inflammation: a first line of defense for the ocular surface. Ocul Surf. 2005;3:S203–206. doi: 10.1016/s1542-0124(12)70256-2. [DOI] [PubMed] [Google Scholar]
- 58.Müller RT, Abedi F., Cruzat A., et al. Degeneration and regeneration of subbasal corneal nerves after infectious keratitis: a longitudinal in vivo confocal microscopy study. Ophthalmology. 2015;122:2200–2209. doi: 10.1016/j.ophtha.2015.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cruzat A., Hamrah P., Cavalcanti BM, Zheng L., Colby K., Pavan-Langston D. Corneal reinnervation and sensation recovery in patients with herpes zoster ophthalmicus: an in vivo and ex vivo study of corneal nerves. Cornea. 2016;35:619–625. doi: 10.1097/ICO.0000000000000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cruzat A., Schrems WA, Schrems-Hoesl LM, et al. Contralateral clinically unaffected eyes of patients with unilateral infectious keratitis demonstrate a sympathetic immune response. Invest Ophthalmol Vis Sci. 2015;56:6612–6620. doi: 10.1167/iovs.15-16560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hamrah P., Seyed-Razavi Y., Yamaguchi T. Translational immunoimaging and neuroimaging demonstrate corneal neuroimmune crosstalk. Cornea. 2016;35(suppl 1):S20–S24. doi: 10.1097/ICO.0000000000001014. [DOI] [PMC free article] [PubMed] [Google Scholar]