Abstract
Endothelial cell–cell junctions must perform seemingly incompatible tasks during vascular development—providing stable connections that prevent leakage, while allowing dynamic cellular rearrangements during sprouting, anastomosis, lumen formation, and functional remodeling of the vascular network. This review aims to highlight recent insights into the molecular mechanisms governing endothelial cell–cell adhesion in the context of vascular development.
The mature vasculature of vertebrates is characterized by a hierarchically branched network of tubes, with fine adaptations in branching and vessel caliber to optimally distribute nutrients and oxygen to all organs, mediate transport of metabolic waste products, and circulate hormonal messengers. In development, the formation of the first vessels follows an assembly process, termed vasculogenesis. However, the vast majority of blood vessels form by angiogenic sprouting during which endothelial cells (ECs) in preexisting, often perfused, vessels create new branches to extend and shape the network (Fig. 1). ECs face a formidable task in this process. Once activated by environmental cues, they extend dynamic cellular processes toward attractive cytokines usually provided by hypoxic cells in the surrounding tissue. At the same time, they need to maintain stable connections with their neighboring cells to guide and extend a new vascular branch. Nascent sprouts establish apical-basal polarity while forming and expanding the lumen, and form connections through anastomosis with neighboring vessels. Throughout this process cells intercalate, change positions, and proliferate all while maintaining the permeability barrier and vessel integrity through dynamic cell–cell junctions.
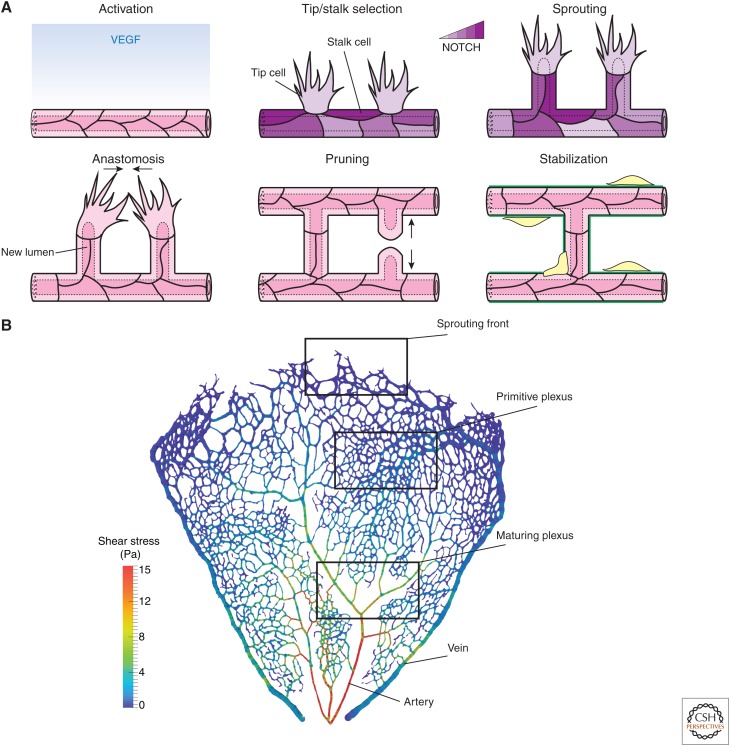
Figure 1.
Vascular morphogenesis. (A) Schematic representation of angiogenesis. On activation with vascular endothelial growth factor (VEGF) endothelial cells (ECs) acquire transient tip and stalk cell phenotypes through NOTCH signaling to form a sprout. Sprouts elongate through collective migration and proliferation. Sprouts fuse by anastomosis to form new vascular loops. During sprouting and anastomosis, the hemodynamic force drives lumen expansion. Nascent networks are remodeled by elimination of unnecessary branches in a process called pruning. Eventually, ECs become quiescent, and the new vasculature is stabilized by recruitment of pericytes and deposition of extracellular matrix. (B) Segmented retinal vasculature of a 6-day mouse color-coded for local wall shear stress levels (image generated using open-source tool Bernabeu et al. 2014). Formation of new vessels, lumenization, and anastomosis take place at the sprouting front. These processes lead to the formation of a nascent network (“primitive plexus”). As remodeling progresses, arteries and veins become specified, vessel caliber is adjusted, and unnecessary branches eliminated to form a hierarchical vascular tree.
Cell–cell adhesion in the endothelium is mediated by vascular endothelial cadherin (VE-cadherin)-based adherens junctions (AJs); tight junctions (TJs), composed of claudins, occludins, junctional adhesion molecules (JAMs), endothelial-cell-selective adhesion molecule (ESAM), and nectins; and a few additional transmembrane proteins including PECAM-1 and CD146 (reviewed in Dejana and Orsenigo 2013; Huveneers and de Rooij 2013; Dorland and Huveneers 2016). Early experiments in mice showed that genetic inactivation of VE-cadherin leads to embryonic lethality, caused by poor endothelial survival and defects in vascular patterning, highlighting the essential role of endothelial junctions in orchestrating vascular morphogenesis (Carmeliet et al. 1999; Gory-Fauré et al. 1999). More recently, with the advent of live imaging of embryonic development, the appreciation of the plasticity of the junctions necessary for the functional rearrangements shaping the vascular network has been developing. In this review, we highlight recent advances in the understanding of vascular morphogenesis, with a focus on the dynamics and regulation of the endothelial cell–cell contacts at the different stages of sprouting angiogenesis.
SPROUTING
Angiogenic sprouting is initiated by the activation of previously quiescent ECs by vascular endothelial growth factor (VEGF)-A (Fig. 1A). Sprouting ECs acquire tip (leader) and stalk (follower) cell phenotypes in a mechanism governed by Dll4-Notch signaling operating in a feedback loop with the VEGF receptor 2 (VEGFR2) (reviewed in Potente et al. 2011; Blanco and Gerhardt 2013). The elongation of the sprout is achieved by collective migration and coordinated cell shape changes and is supported by cell division (Blum et al. 2008; Sauteur et al. 2014).
Junctions in Angiogenic Sprouts Undergo Dynamic Remodeling
Live imaging of the junctional architecture in sprouting vessels of zebrafish embryos has revealed some of the complexity of junctional rearrangements. At the beginning of sprouting, the tip cell is connected to the stalk cells by a single ring-shaped junction (Blum et al. 2008). Subsequently, as the sprout grows, the junctions between the cells in a sprout elongate, increasing the surface area of the shared membrane interface. Additionally, the tip and stalk cell phenotypes are not stable cell fates and cells in the sprout frequently change positions (Jakobsson et al. 2010). Imaging of mosaic EC labeling in vivo and in 3D-sprouting assays showed that while cells shuffle and intercalate they maintain coherent contacts, which necessitates the nucleation of new junctions and resolution of the old ones (Blum et al. 2008; Jakobsson et al. 2010; Lenard et al. 2013). All of these processes require local and dynamic remodeling of cell–cell junctions with simultaneous preservation of the integrity of the sprout to avoid leakage and detachment of cells.
VEGF Acts by Regulation of Local VE-Cadherin Turnover in the Sprout
Several studies illustrate that VEGF-A plays a dual role in sprouting, on one hand activating endothelial tip cell formation and actin dynamics to induce cell migration, on the other hand loosening AJs by the same pathway that triggers the known VEGF-mediated increase in vascular permeability (see Vestweber et al. 2014 for a recent review on permeability regulation).
Downstream from VEGFR2, the adaptor protein TSAd recruits and activates c-SRC. Through the phosphorylation of tyrosine residues in VE-cadherin, c-SRC increases junctional permeability and loosens adhesions sufficiently to allow for effective sprouting (Gordon et al. 2016). The mechanism of VEGF-cSRC signaling to junctions and their involved components have been mapped in greater detail in the context of the permeability response (Matsumoto et al. 2005; Sun et al. 2012; Li et al. 2016; see also Lampugnani et al. 2017), but whether and how they drive junctional dynamics for cellular rearrangements is less clear. A computational model suggested that the intercalation of cells allowing sprout elongation is enabled by local differences in adhesive properties between cells (Bentley et al. 2014). The VEGF-Dll4-Notch feedback mechanism that patterns tip and stalk behavior also appears to control differential adhesion by regulating VE-cadherin turnover. Cells with higher Notch activity show a reduced pool of dynamic VE-cadherin suggesting that Notch signaling influences the tethering of VE-cadherin molecules at the junction. Whether the differences in VE-cadherin turnover are mediated directly by Notch, or indirectly via changes in the VEGFR2 pathway, remains to be studied.
VE-Cadherin Organizes Actin Cytoskeleton to Achieve Sprout Elongation
A recent zebrafish study found that sprout extension is mediated by a combination of two mechanisms: migratory behavior of the tip cells and elongation of the stalk cells (Sauteur et al. 2014). In this context, VE-cadherin is required to maintain sprout cohesion because in VE-cadherin mutants tip cells often detached from the stalk. Furthermore, VE-cadherin organizes the junctional actin cytoskeleton and this activity appears necessary for the increase of the junctional contact. Loss of VE-cadherin or deletion of its cytoplasmic actin-binding tail led to stunted sprouts with clustered ECs that failed to elongate. Inhibiting actin polymerization phenocopied these defects. The investigators suggested that nucleators of branched actin, such as Arp2/3, provide the necessary force to deform the junction and achieve the shape change; another study found that formin-mediated actin nucleation at junctions is also critical for junction elongation (Phng et al. 2015).
During migration of the angiogenic sprout, the contractility of the junctional actomyosin cytoskeleton also has to be tightly controlled. Too little contractility and junctions cannot be stabilized, too much and they will not be plastic enough to allow sprouting. In vitro, sprouting and migration of ECs requires down-regulation of Rho kinase (ROCK) and myosin light chain 2 (MLC2) that is, decrease in contractility (Mavria et al. 2006; Abraham et al. 2009). One mechanism regulating the contractile apparatus in ECs during sprouting involves Rap1-induced formation of a Raf1-VE-cadherin complex, which in turn recruits ROCK to locally potentiate actomyosin (Wimmer et al. 2012). The functional importance of this regulation is underscored by the observation that in an in vitro sprouting assay, Raf1-deficient tip cells often detached from the rest of the sprout, indicating that junctional contacts cannot be properly stabilized. In vivo knockout of Raf1 decreased the speed of sprouting but increased the number of tip cells.
Junctional Proteins Are Involved in Front–Rear Polarity and Coordination of Collective Migration
There is increasing evidence that in angiogenic sprouts, junctions may play a coordinating role by, on one hand, polarizing the activity of the migration machinery and, on the other hand, maintaining sprout cohesion. The junction-associated proteins angiomotin (AMOT) and angiomotin-like-1 (AMOTL1) localize at the leading edge of migrating cells as well as to cell contacts. They have been shown to recruit polarity proteins Patj and Pals1, which in turn localize the Rho GEF Syx and Rac1 GAP Rich1, thus regulating the local activity of Rho GTPases (Zheng et al. 2009). Sprouts in AMOT, AMOTL1 and Syx knockout or morphant animals show impaired migration and defects in the front–rear cell polarity (as marked by the orientation of the Golgi-nuclear axis), with additional problems in sprout cohesion in the case of AMOTL1 (Aase et al. 2007; Garnaas et al. 2008; Ernkvist et al. 2009; Zheng et al. 2009).
Syx recruitment to junctions by Patj-Pals1 was also shown to mediate the junction-stabilizing response to the cytokine angiopoietin 1 (Ang1), which balances the permeability-inducing activity of VEGF (Ngok et al. 2012). It has been suggested that directional migration in the VEGF gradient is mechanistically achieved by trafficking of the Patj-Pals1-RhoA-Syx complex from disassembling junctions to the leading edge of the cell (Wu et al. 2011, reviewed in detail in Lizama and Zovein 2013).
A recent study found that specialized structures, termed engulfed cadherin fingers, coordinate collective cell movements by transmitting polarity and steering signals between leading and following cells in vitro in human umbilical vein endothelial cell (HUVEC) monolayers (Hayer et al. 2016). The asymmetric recruitment of junction-associated proteins is also at the heart of at least some mechanisms of force transmission between ECs (for a recent review on mechanotransduction through endothelial junctions, see Dorland and Huveneers 2016). For example, the F-bar protein pacsin2, which recognizes convex membranes, is recruited specifically to one side of a junction experiencing unbalanced actomyosin-based pulling (Dorland et al. 2016). The presence of pacsin2 slows down internalization of VE-cadherin, protecting the junction from excessive and uncontrolled opening. What force transmission mechanisms are used to coordinate EC behaviors in angiogenic sprouts and mature vessels in vivo is an exciting area of current investigation.
ANASTOMOSIS
New Junctions Are Formed during Sprout Fusion
Live imaging of the vasculature in zebrafish embryos revealed that anastomosis is a highly stereotypical process (Fig. 2A) (Herwig et al. 2011; Lenard et al. 2013; reviewed in Betz et al. 2016). Migrating tip cells project multiple filopodia decorated with junctional proteins such as VE-cadherin and zonula occludens 1 (ZO-1) (Blum et al. 2008). On contact formation, a single spot of VE-cadherin is deposited at the contact site and later elaborated into a ring-shaped junction (Blum et al. 2008; Herwig et al. 2011; Lenard et al. 2013). The additional filopodial projections are retracted and the membrane between the anastomosing cells acquires apical markers such as podocalyxin (Lenard et al. 2013). Subsequently, cells continue to rearrange and convert the sprout into a multicellular tube. This is accompanied by an increase in the surface of junctions. The elaboration of the VE-cadherin spot into a junctional ring is accompanied by increased actin polymerization and, in zebrafish embryos, the partial inhibition of actin polymerization with low-dose latrunculin B resulted in a delay in the formation of the dorsal longitudinal anastomosing vessel (DLAV) (Phng et al. 2013).
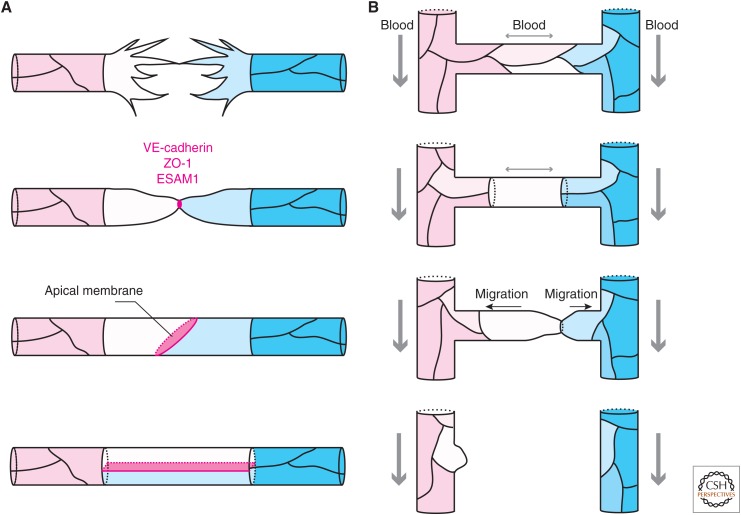
Figure 2.
Junction remodeling during anastomosis and pruning. (A) Vessel fusion. Opposing tip cells project dynamic filopodia. On contact formation, filopodia are retracted and adhesion proteins are deposited at the contact site. The site is further elaborated into a ring-shaped junction and apical membrane is specified. Further junction remodeling results in the formation of a multicellular tube. (B) Vessel pruning. Irregular flow initiates vessel regression. Cells in the regressing branch rearrange their junctions to form a unicellular tube. Cells migrate away from the low-flow-branch resolving the junction between them. Cells from the regressing branch are incorporated into the neighboring vessels.
VE-Cadherin Is Required to Cease Sprouting Activity
VE-cadherin facilitates not only the initial adhesion, but also the coordination of the early steps of anastomosis (Lenard et al. 2013; Sauteur et al. 2017). In zebrafish carrying a VE-cadherin null mutation, the additional filopodia are not retracted on contact formation (Lenard et al. 2013). As a result, several contact sites are formed. A similar effect was observed in VE-cadherin morphant fish (Abraham et al. 2009; Montero-Balaguer et al. 2009) and in the retinas of VE-cadherin conditional knockout mice (Gaengel et al. 2012). This indicates that VE-cadherin is responsible for the recognition of the fusion event and initiates a signal to halt sprouting activity. This signal is not VEGFR2 dependent because dampening of VEGFR2 signaling with a chemical inhibitor in the VE-cadherin mutant did not prevent formation of multiple contact sites (Lenard et al. 2013). At this point, it is not understood how engagement of VE-cadherin at the contact site limits the formation of multiple connections and stops filopodia formation.
Despite the fact that in mutant embryos contacts lacking VE-cadherin were unstable and did not efficiently convert to a ring shape, they contained patches of ZO-1 and ESAM1 (Lenard et al. 2013). Additionally, podocalyxin was recruited to the contact sites showing that at least initial specification of the apical membrane can be acquired even in the absence of VE-cadherin (Herwig et al. 2011; Lenard et al. 2013). A recent follow-up study showed that ESAM1 cooperates with VE-cadherin to establish and reinforce the anastomotic contacts. Additionally, it was shown that ESAM1 is required for the proper specification of the apical membrane (Sauteur et al. 2017).
LUMEN FORMATION
During the assembly of the first vessels by vasculogenesis the process of lumen formation proceeds by the establishment and reorganization of junctional contacts, specification of the apical and basal membrane compartments, and cell shape changes to open the luminal space (Strilić et al. 2009, 2010; reviewed in Zeeb et al. 2010). Importantly, patent lumens are established before the onset of cardiac activity; their formation is therefore blood flow-independent. In contrast, a recent report showed that in angiogenesis lumens are propagated from the existing vessels by hemodynamic force, highlighting important mechanistic differences between the two processes (Gebala et al. 2016).
Lumen Propagation Coincides with Junctional Rearrangements
Live imaging of lumenizing vessels in zebrafish embryos expressing junctional and membrane markers revealed that lumens can form either by membrane invagination into single cells (transcellular lumen) or in the space between multiple cells (extracellular lumen) (Herwig et al. 2011; Lenard et al. 2013). The difference of pressure between a lumenized and nonlumenized compartment causes local membrane deformations, which push the lumen either into the interior of a single cell or into the space between two adjacent cells (Gebala et al. 2016).
Lumen formation is accompanied by dynamic cell rearrangements (Herwig et al. 2011; Lenard et al. 2013; Yu et al. 2015). Following the establishment of the initial anastomotic contact, cells migrate and expand their junctional interfaces. Subsequently, the spaces between the apposed cells become inflated. Alternatively, anastomosing cells first form lumenized seamless tubes and then remodel their junctions in the presence of patent lumens. Since the underlying mechanism of lumen expansion is the same in both cases, the choice between these two scenarios is likely dictated by timing or mechanical constrains in a particular context. In the end, most mature vessels have a multicellular architecture, suggesting that junctional rearrangements are critical for the stabilization of open lumens (Blum et al. 2008; Lenard et al. 2013; Phng et al. 2015).
Endothelial Junctions Orchestrate Apico-Basal Polarity
The establishment of apico-basal polarity is a critical step in lumen formation both de novo in vasculogenesis and in angiogenic sprouts because knockdown of the apical markers moesin and podocalyxin leads to a failure in lumenization (Strilić et al. 2009; Wang et al. 2010). Furthermore, junctions play a central role in the reinforcement of polarity because knockdown of VE-cadherin leads to improper localization of these apical markers (Strilić et al. 2009; Wang et al. 2010).
Cell junctions provide docking sites for the proteins involved in apico-basal polarity acquisition and maintenance and, conversely, the apico-basal polarity program regulates the expression and organization of EC junction components (reviewed in Rodriguez-Boulan and Macara 2014; Worzfeld and Schwaninger 2016). The underlying molecular mechanisms are perhaps best studied in the epithelium and research over the last few years has confirmed that much of the epithelial polarity machinery is expressed and functional also in the endothelium (Worzfeld and Schwaninger 2016). However, mechanistic details of polarity programs can differ between tissues and developmental contexts and indeed some important endothelial-specific features have been identified.
In the epithelium, polarity is established and maintained by conserved sets of proteins: the Par proteins, atypical protein kinase C (aPKC), Cdc42, and Rac1, the Crumbs complex (CRB3, Pals1, and Patj), and the Scribble complex (Scribble, Lgl, and Dlg) (Rodriguez-Boulan and Macara 2014). In a polarized epithelial cell, these proteins are differentially targeted to the apical membrane and TJs, or to the basolateral compartment by a reciprocal exclusion mechanism. The apically located aPKC phosphorylates and excludes proteins of the basolateral compartment, whereas Par1 at the basolateral cortex phosphorylates apically targeted proteins. CRB, the only transmembrane protein in this list, anchors aPKC in the apical membrane through an interaction with Pals1 and Par6. The small GTPases Cdc42 and Rac1 play multiple roles in the polarity program but one of them is to bind Par6 and release its intrinsic ability to activate aPKC at the apical membrane (Mertens et al. 2006; Iden and Collard 2008). Par3 resides at the TJs owing to its interaction with nectins and JAM proteins. Despite its ability to bind aPKC and Par6, Par3 is not a constitutive member of the apical complex but, through a currently unresolved mechanism, is required for the apical delivery of aPKC (Morais-de-Sá et al. 2010). Apart from the differences in protein content, the apical and basolateral compartments maintain functionally important unique lipid signatures through the differential distribution of the PI3K kinase and PTEN phosphatase (Rodriguez-Boulan and Macara 2014).
In the endothelium, in vivo, VE-cadherin, aPKC, PTEN, Par3, and RhoGTPases are required for polarity establishment (Strilić et al. 2009; Lampugnani et al. 2010; Wang et al. 2010; Zovein et al. 2010). Additionally, signaling from the extracellular matrix might provide initial spatial cues for the organization of polarity because endothelial-specific knockout of integrin β1 in mouse embryos led to disorganization of cell contacts, decreased Par3 expression and caused defects in lumen formation (Zovein et al. 2010).
In vitro, VE-cadherin appears to interact directly with polarity proteins Pals1 (Brinkmann et al. 2016), Par6, and Par3 (Iden et al. 2006; Tyler et al. 2010). Mutation of Pals1 and Par3 binding sites on VE-cadherin prevented polarity establishment as observed by ectopic localization of podocalyxin (Brinkmann et al. 2016). In addition, VE-cadherin is required for the recruitment and activation of aPKC to the cell junctions (Lampugnani et al. 2010). Furthermore, the Rac1-specific GEF Tiam1, which has been shown to participate in polarity establishment in other systems (Mertens et al. 2006), is recruited to the cell junctions by interactions with VE-cadherin and Par3 (Lampugnani et al. 2002; Liu et al. 2013). Importantly, knockdown of Tiam1 prevented lumen formation in an in vitro assay (Lampugnani et al. 2010).
Vascular endothelial protein tyrosine phosphatase (VE-PTP), an endothelial-specific phosphatase is another important factor during the acquisition of apico-basal polarity and formation of the lumen. It acts by stabilizing VE-cadherin contacts (Hayashi et al. 2013). VE-PTP dephosphorylates several targets in the endothelium including VE-cadherin, plakoglobin, VEGFR2, and the Ang1 receptor Tie2. In vivo, removal of VE-PTP leads to distorted junctional organization and to defects in apico-basal polarity and lumen formation. This phenotype has been suggested to result from the inability of Ang1 to exert its stabilizing function on endothelial junctions in the absence of VE-PTP.
In the epithelium, cadherins promote the assembly of TJs, reinforcing the apico-basal polarity by providing a barrier to the redistribution of proteins and lipids between the two compartments. However, in the endothelium, there is no clear spatial distinction between TJs and AJs (Dejana et al. 2009). Moreover, much of the lumen formation occurs in the absence of TJs (Carmeliet et al. 1999; Liebner et al. 2008) and animals deficient in TJ components do not show luminal defects (reviewed in Zeeb et al. 2010). All this supports the idea that in the context of lumen formation AJs alone are capable of inducing polarity. VE-cadherin expression and membrane-targeting precedes and triggers TJ organization (Jin et al. 2005; Taddei et al. 2008). However, it is possible that in mature junctions the polarity complexes are relocalized from AJ to TJ to maintain polarity, because in ECs polarity proteins have been found to associate with TJ components (Ebnet et al. 2001, 2003). Of note, while blood flow has a role in reinforcing apico-basal polarity and barrier maturation at later stages of vascular development (reviewed in Worzfeld and Schwaninger 2016), the initial establishment of the apical compartment at the interface between cells during lumen formation is flow-independent (Wang et al. 2010; Herwig et al. 2011).
Endothelial apico-basal polarity establishment during lumen formation is also regulated by CCM1 (Krit1)—a member of a family of three genes identified as defective in patients with cerebral cavernous malformations (CCM) (reviewed in Draheim et al. 2014, see Lampugnani et al. 2017, for an in-depth review on CCM). CCM lesions occur mostly in the nervous system and are characterized by the presence of multiple lumens, disorganized junctions, and hemorrhages. CCM1 is an effector of the small GTPase Rap1, which in its active state releases the CCM1 FERM domain enabling it to interact with β-catenin and afadin (Glading et al. 2007). While at the junction, CCM1 recruits more Rap1, in effect stabilizing and concentrating VE-cadherin, presumably by affecting the organization of the junctional actin (Lampugnani et al. 2010). Importantly, CCM1 and Rap1 activity are required for proper localization of Tiam1 and aPKC during lumen formation and apico-basal polarity is lost in patient samples with familial and sporadic form of the disease as evidenced by podocalyxin and collagen IV staining (Lampugnani et al. 2010). Additionally, CCM1 recruits another CCM protein, CCM2, to the junction where it can act to reduce local RhoA activity and promote junctional stability. Targeting of CCM2 in mice leads to early embryonic death and failure in lumen formation (Whitehead et al. 2009). Another major function of CCM1 is the sequestration of β-catenin at the junction of quiescent ECs to prevent its translocation to the nucleus and induction of the β-catenin transcriptional program (Glading and Ginsberg 2010). These changes might cooperate with transforming growth factor (TGF)-β and bone morphogenetic protein (BMP) signaling to drive endothelial-to-mesenchymal transition and formation of abnormal vascular structures (Maddaluno et al. 2013; Bravi et al. 2016).
Junctional Actin Supports Tube Formation
Dynamic regulation of junctional actin enables cell rearrangements and cell shape changes that are necessary for the formation of stable open lumens. In vivo, perturbing the VE-cadherin–actin link, either by expression of truncated VE-cadherin (Sauteur et al. 2014) or knockout of β-catenin (Cattelino et al. 2003) leads to defects in lumen formation and maintenance. In angiogenic sprouts F-actin becomes enriched at junctions between cells shortly before lumenization (Phng et al. 2015). If junctional actin cables are absent, as in the case when the formin fmnl3 is inhibited, lumens fail to form. Additionally, disorganized actin cables are unable to support already established lumens. Moreover, this function of junctions in lumen formation and maintenance is independent of the role of junctions in organization of apico-basal polarity because podocalyxin localized to the apical compartment normally in fmnl3 morphants. This is in contrast to a recent in vitro report, which showed that fmnl3 is required for the transport of podocalyxin to a newly forming apical compartment (Richards et al. 2015). Importantly, inhibition of fmnl3 in zebrafish affected only small caliber angiogenic vessels and not large vessels such as the dorsal aorta (DA) or the posterior cardinal vein (PCV). This indicates that formin activity at junctions supports cellular rearrangements required specifically for lumen formation in sprouting vessels.
Additionally, junctions may play a role in lumen maintenance by organizing the actin cytoskeleton to promote a certain cell shape. For example, in the mouse and zebrafish DA, VE-cadherin is linked to radial actin stress fibers via a complex of β-catenin, the scaffold protein MAGI1 and angiomotin-like-2 (AMOTL2) (Hultin et al. 2014). The tension exerted by the contractile stress fibers perpendicular to the junctional interface allows the cells of the DA to assume an elongated shape. Interfering with these structures leads to lumen collapse. This observation indicates that cells in the DA exert a tensile force on one another to support a patent lumen. Interestingly, this mechanism does not seem to operate in the PCV, which suggests that there are differences in force transmission between veins and arteries.
The spatio-temporal regulation of junctional integrity is essential to support lumen formation. Rasip1, a Rap1 effector, together with its binding partner Radil cooperate to localize ArhGAP29 to the junction to locally inhibit RhoA and enhance Rac1 activity (reviewed in Wilson and Ye 2014). In vitro, this has been shown to promote cortical actin assembly and stabilization of the junctions (Post et al. 2013, 2015). In vivo, Rasip1 knockout animals present defects in lumen morphology in the DA (Xu et al. 2011; Wilson et al. 2013; Koo et al. 2016). Although the details of the reported phenotypes are somewhat conflicting, the studies agree that lumen maintenance in all growing vessels is affected in the absence of Rasip1. Importantly, Rasip1 knockout does not seem to affect quiescent mature vessels indicating that the molecular requirements of junctional stability change as vascular networks mature.
VASCULAR REMODELING
Newly formed primitive vascular networks undergo profound remodeling through elimination (or pruning) of superfluous connections and reinforcement of others, as well as adapting vessel diameter. Collectively, these processes lead to the establishment of a hierarchical vascular tree, which optimally distributes blood flow (Fig. 1B). Importantly, the endothelium retains its ability to remodel in adults when physiological requirements change in response to, for example, exercise or injury.
Although there are some particular developmental contexts in which paracrine signaling induces regression of entire capillary plexuses independently of flow (Lobov et al. 2005; Rao et al. 2007), vascular remodeling is generally mediated by hemodynamic forces (Lucitti et al. 2007; Chen et al. 2012). Recent data indicate that vascular pruning is driven by EC polarization and migration away from low to high flow regions (Fig. 2B) (Chen et al. 2012; Kochhan et al. 2013; Franco et al. 2015). Significant progress has been made in understanding how blood shear forces are sensed by the endothelium, and cell junctions represent an important part of the mechanism. In turn, signals from flow also orchestrate the migratory behavior of ECs and the remodeling of junctions, although the underlying mechanisms remain elusive.
Junctional Proteins Are Involved in Flow Sensing
The two forces exerted on the endothelium by blood flow are circumferential strain, the stretching force resulting from the pressure on vessel walls, and shear stress, the frictional drag acting on the apical surface of the endothelium (Baeyens and Schwartz 2016). Although circumferential strain has a well-documented role in endothelial homeostasis and morphology in vitro (Jufri et al. 2015), its specific role in the remodeling of vascular networks in vivo has been difficult to address experimentally. This is in part because of the potential role of circumferential strain in lumen maintenance, which might be an indirect factor influencing the selection of pruned branches because collapsed lumens are an early sign of vascular regression (Franco et al. 2015; Lenard et al. 2015). In contrast, strong experimental evidence, including direct manipulation of blood viscosity (Lucitti et al. 2007), established that shear stress is a major determinant of the remodeling process.
The junctional complex of PECAM-1, VE-cadherin and VEGF receptors 2 and 3 is by far the best-characterized element of the endothelial shear-stress mechanosensing apparatus. The current model, based on evidence from a number of biochemical and biophysical experiments (Tzima et al. 2005; Collins et al. 2012; Conway et al. 2013; Coon et al. 2015) postulates that shear stress triggers an as-of-yet-unidentified upstream sensor that initiates rapid association of vimentin filaments with PECAM-1. This leads to PECAM-1 phosphorylation and subsequent activation of Src-family kinases (SFK). VE-cadherin then serves as a scaffold bringing VEGFRs in close proximity to PECAM-1 allowing for their activation by SFK, which in turn triggers downstream responses. These in the short-term include strengthening of cell–matrix adhesion and polarized activation of Rho-GTPases (Tzima 2002; Tzima et al. 2003; Collins et al. 2012) and, in the long-term, reorientation of the Golgi-centrosome-nucleus axis and cell body elongation parallel to the direction of flow, reorganization of the actin cytoskeleton as well as changes in gene expression.
Direct measurements of forces exerted on PECAM-1 and VE-cadherin molecules in response to flow using calibrated fluorescence resonance energy transfer (FRET) tension sensors revealed several unexpected features of this system (Conway et al. 2013). First, the flow response is associated with increased tension on PECAM-1 and a simultaneous release of tension from VE-cadherin. Both of these effects are myosin-dependent and rely on the reorganization of the junction-cytoskeleton link presumably by the recruitment of additional adaptor proteins. Second, no directionality in the tension exerted on the junction with respect to the direction of flow was observed. Finally, the magnitude of forces measured on PECAM-1 molecules is two orders of magnitude higher than the force directly exerted by shear. This strongly argues that shear force is not passively transduced to the points of cell adhesion but involves an active response of the cell. It also indicates that ECs possess a mechanism for amplification of small forces that explains how, despite its relatively small magnitude compared with stretch forces, shear can play a major role in the shaping of the developing vascular network (for a more detailed discussion, see Conway and Schwartz 2015; Baeyens and Schwartz 2016).
Pruning Requires Rearrangement of Junctions
Detailed imaging of junctional markers in zebrafish (Kochhan et al. 2013; Lenard et al. 2015) and mouse retina (Franco et al. 2015) showed that pruning is a highly ordered process mediated by dynamic rearrangement of cell junctions (Fig. 2B). First, a multicellular tube is transformed into a partially unicellular tube connecting to the neighboring vessel by a single ring-shaped junction. In a second step, this junction is resolved while the cells from the disappearing branch integrate into the neighboring vessels, creating new junctions and redistributing their apical membrane markers. Presumably, this sequence of events, resembling anastomosis in reverse, is required for controlled elimination of a branch without rupture or hemorrhage.
How the asymmetry in junctional dynamics is achieved and what drives the changes in the surface area and positions of the apical compartments are both interesting and thus far unresolved questions. Some clues came from understanding how flow influences apico-basal and front–rear cell polarity determinants at the junctions. Recent work identified that Rac1 activation by flow requires PECAM-1, acting through the Rac GEF VAV2; however, Rac1 spatial polarization requires junctional localization of TIAM1 (Liu et al. 2013). Flow induces TIAM1 association with VE-cadherin and the Par3–Par6–aPKC polarity complex at the downstream edge of the cell. Surprisingly, TIAM1 is required not for its GEF activity but rather for coupling of Rac1 with its effector, the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase complex. These data point toward a highly intricate polarization mechanism involving a noncanonical function for TIAM1 in conjunction with membrane receptors and polarity proteins. However, whether this also represents the key to polarized migration against flow during remodeling in vivo remains to be seen. Curiously, the in vitro work showing Rac1 activation observes cell polarity and movement with the direction of flow (Tzima 2002; Tzima et al. 2003; Liu et al. 2013), not against it, as is observed in vivo during remodeling (Franco et al. 2015). The advent of transgenic polarity reporters in zebrafish will aid in elucidating the principle of flow-induced endothelial polarity in vivo (Kwon et al. 2016).
VESSEL MAINTENANCE
Adherens junctions are important for the stabilization of the nascent network to prevent unwanted regression. It has been suggested that AJ send prosurvival signals to the ECs because in some systems depletion of VE-cadherin results in increased apoptosis (Carmeliet et al. 1999). Additionally, mature vascular networks are characterized by endothelial quiescence, that is, reduced motility and proliferation, loss of the activated tip-stalk cell phenotype and reinforcement of the apico-basal polarity. Several reports suggest that VE-cadherin engagement at endothelial junctions actively suppresses sprouting and migration, acting as a quiescence signal (Abraham et al. 2009; Montero-Balaguer et al. 2009). This may be in part mediated through the increase of junctional contractility by RhoC activation and subsequent ROCK-dependent phosphorylation of myosin light chain 2 (Abraham et al. 2009). Increased junctional contractility stabilizes the AJ and suppresses VEGF/VEGFR2-dependent sprouting. Conversely, reduction of contractility results in the activation of Rac1, formation of membrane projections and induction of sprouting. Rac1 is normally activated by VEGFR2 signaling and it is tempting to speculate that engagement of VEGFR2 with VE-cadherin at the junction inhibits the signaling to Rac1 since VE-cadherin has been shown to modify VEGFR2 signaling. At least in vitro, on VEGF stimulation in confluent cell monolayers, VEGFR2 associates with VE-cadherin. VE-cadherin can antagonize VEGFR2 signaling by recruitment of junctional phosphatases to the VEGF-VEGFR2 complex, limiting the rate of the receptor internalization (Lampugnani et al. 2002, 2006). This interaction is further modified by additional signaling pathways, for example sphingosine-1-phosphate, provided by the blood stream and acting on the endothelial receptor S1P1. Loss of S1P1 expression in ECs phenotypically mimics VE-cadherin deficiency (Gaengel et al. 2012). S1P stimulates junctional VE-cadherin localization and adhesion, and limits VEGF induced VE-cadherin internalization, thereby reducing sprouting angiogenesis. Interestingly, S1P1 has also been suggested to function directly as a flow sensor, showing ligand independent activation by flow, thereby contributing to EC polarity and vessel maturation (Jung et al. 2012).
The most prominent signaling pathway associated with vessel maturation and quiescence however is Ang1 signaling through the endothelial tyrosine kinase receptor Tie2. Ang1 is produced by various cell types, notably including pericytes, the mural cells that support and stabilize capillary networks and limit endothelial proliferation. Ectopic stimulation with Ang1 was shown to partially rescue loss of pericytes in the mouse retina model (Uemura et al. 2002). In vitro, Tie2 activation by Ang1 promotes EC survival via Akt-dependent endothelial constitutive nitric oxide synthase (eNOS) phosphorylation only when Tie2 is present at cell–cell contacts and not in cell–matrix contacts (Saharinen et al. 2008). Ang1 limits endothelial permeability through the association and dephosphorylation of Tie2 by interacting with VE-PTP at cell junctions. The association of Tie2 with VE-PTP is mediated by their cytoplasmic domains, and blocking VE-PTP drives Tie2 internalization, hyperactivation, and consequently endothelial proliferation and vessel enlargement (Winderlich et al. 2009). Strikingly, Ang1 stimulation can trigger Tie2 complexes in trans, with evidence that interaction between Tie2 receptors from adjacent cells is required for activation (Saharinen et al. 2008). The effect of Ang1 on reducing VEGF induced vessel permeability has been shown to involve mDia-mediated inhibition of Src, thereby preventing redistribution of VE-cadherin (Gavard et al. 2008).
OUTLOOK
In vascular biology, endothelial cell–cell junctions have moved from the periphery to take center stage, orchestrating molecular and physical signals from tissue environment, the blood stream and between ECs to achieve functional vascular patterning in angiogenesis. Yet, as the picture becomes more complete, it is increasingly apparent that details of the spatio-temporal context of the various molecular complexes in relation to specific EC behaviors that shape the vascular network are still largely unknown. Where and when do cells engage distinct pathways and complexes at endothelial junctions, and in response to what? What is an instructive cellular activity and what is local reaction to forces? Elucidating these questions will require the development of new tools and approaches that allow analyzing molecular interactions and junctional dynamics in the context of collective EC behavior during the distinct stages of vascular morphogenesis, maturation, and adaptation. A deeper understanding of the regulation of endothelial junctions during endothelial reactivation and physiological vessel adaptations will hopefully generate approaches and opportunities to modify their plasticity in vascular disease.
ACKNOWLEDGMENTS
We thank Dr. Anne-Clemence Vion for providing the segmented retina image. We are grateful to Dr. Veronique Gebala and all the members of the Integrative Vascular Biology Laboratory for helpful discussions. This work was supported by the European Molecular Biology Organization (EMBO) Long-Term Fellowship ALTF1625-2014 to A.S. and a European Research Council (ERC) Consolidating Grant RESHAPE #311719 to H.G.
Footnotes
Editors: Carien M. Niessen and Alpha S. Yap
Additional Perspectives on Cell–Cell Junctions available at www.cshperspectives.org
REFERENCES
*Reference is also in this subject collection.
- Aase K, Ernkvist M, Ebarasi L, Jakobsson L, Majumdar A, Yi C, Birot O, Ming Y, Kvanta A, Edholm D, et al. 2007. Angiomotin regulates endothelial cell migration during embryonic angiogenesis. Genes Dev 21: 2055–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham S, Yeo M, Montero-Balaguer M, Paterson H, Dejana E, Marshall CJ, Mavria G. 2009. VE-cadherin-mediated cell–cell interaction suppresses sprouting via signaling to MLC2 phosphorylation. Curr Biol 19: 668–674. [DOI] [PubMed] [Google Scholar]
- Baeyens N, Schwartz MA. 2016. Biomechanics of vascular mechanosensation and remodeling. Mol Biol Cell 27: 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley K, Franco CA, Philippides A, Blanco R, Dierkes M, Gebala V, Stanchi F, Jones M, Aspalter IM, Cagna G, et al. 2014. The role of differential VE-cadherin dynamics in cell rearrangement during angiogenesis. Nat Cell Biol 16: 309–321. [DOI] [PubMed] [Google Scholar]
- Bernabeu MO, Jones ML, Nielsen JH, Krüger T, Nash RW, Groen D, Schmieschek S, Hetherington J, Gerhardt H, Franco CA, et al. 2014. Computer simulations reveal complex distribution of haemodynamic forces in a mouse retina model of angiogenesis. J R Soc Interface R Soc. 11: 20140543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz C, Lenard A, Belting HG, Affolter M. 2016. Cell behaviors and dynamics during angiogenesis. Development 143: 2249–2260. [DOI] [PubMed] [Google Scholar]
- Blanco R, Gerhardt H. 2013. VEGF and Notch in tip and stalk cell selection. Cold Spring Harb Perspect Med. 3: a006569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum Y, Belting HG, Ellertsdottir E, Herwig L, Lüders F, Affolter M. 2008. Complex cell rearrangements during intersegmental vessel sprouting and vessel fusion in the zebrafish embryo. Dev Biol 316: 312–322. [DOI] [PubMed] [Google Scholar]
- Bravi L, Malinverno M, Pisati F, Rudini N, Cuttano R, Pallini R, Martini M, Larocca LM, Locatelli M, Levi V, et al. 2016. Endothelial cells lining sporadic cerebral cavernous malformation cavernomas undergo endothelial-to-mesenchymal transition. Stroke J Cereb Circ 47: 886–890. [DOI] [PubMed] [Google Scholar]
- Brinkmann BF, Steinbacher T, Hartmann C, Kummer D, Pajonczyk D, Mirzapourshafiyi F, Nakayama M, Weide T, Gerke V, Ebnet K. 2016. VE-cadherin interacts with cell polarity protein Pals1 to regulate vascular lumen formation. Mol Biol Cell 27: 2811–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, Lampugnani MG, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oosthuyse B, Dewerchin M, et al. 1999. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell 98: 147–157. [DOI] [PubMed] [Google Scholar]
- Cattelino A, Liebner S, Gallini R, Zanetti A, Balconi G, Corsi A, Bianco P, Wolburg H, Moore R, Oreda B, et al. 2003. The conditional inactivation of the β-catenin gene in endothelial cells causes a defective vascular pattern and increased vascular fragility. J Cell Biol 162: 1111–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Jiang L, Li C, Hu D, Bu J, Cai D, Du J. 2012. Haemodynamics-driven developmental pruning of brain vasculature in zebrafish. PLoS Biol 10: e1001374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins C, Guilluy C, Welch C, O’Brien ET, Hahn K, Superfine R, Burridge K, Tzima E. 2012. Localized tensional forces on PECAM-1 elicit a global mechanotransduction response via the integrin-RhoA pathway. Curr Biol 22: 2087–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway DE, Schwartz MA. 2015. Mechanotransduction of shear stress occurs through changes in VE-cadherin and PECAM-1 tension: Implications for cell migration. Cell Adhes Migr 9: 335–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway DE, Breckenridge MT, Hinde E, Gratton E, Chen CS, Schwartz MA. 2013. Fluid shear stress on endothelial cells modulates mechanical tension across VE-cadherin and PECAM-1. Curr Biol 23: 1024–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coon BG, Baeyens N, Han J, Budatha M, Ross TD, Fang JS, Yun S, Thomas JL, Schwartz MA. 2015. Intramembrane binding of VE-cadherin to VEGFR2 and VEGFR3 assembles the endothelial mechanosensory complex. J Cell Biol 208: 975–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejana E, Orsenigo F. 2013. Endothelial adherens junctions at a glance. J Cell Sci 126: 2545–2549. [DOI] [PubMed] [Google Scholar]
- Dejana E, Tournier-Lasserve E, Weinstein BM. 2009. The control of vascular integrity by endothelial cell junctions: Molecular basis and pathological implications. Dev Cell 16: 209–221. [DOI] [PubMed] [Google Scholar]
- Dorland YL, Huveneers S. 2016. Cell–cell junctional mechanotransduction in endothelial remodeling. Cell Mol Life Sci 74: 279–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorland YL, Malinova TS, van Stalborch AMD, Grieve AG, van Geemen D, Jansen NS, de Kreuk BJ, Nawaz K, Kole J, Geerts D, et al. 2016. The F-BAR protein pacsin2 inhibits asymmetric VE-cadherin internalization from tensile adherens junctions. Nat Commun 7: 12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draheim KM, Fisher OS, Boggon TJ, Calderwood DA. 2014. Cerebral cavernous malformation proteins at a glance. J Cell Sci 127: 701–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebnet K, Suzuki A, Horikoshi Y, Hirose T, Meyer Zu Brickwedde MK, Ohno S, Vestweber D. 2001. The cell polarity protein ASIP/PAR-3 directly associates with junctional adhesion molecule (JAM). EMBO J 20: 3738–3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebnet K, Aurrand-Lions M, Kuhn A, Kiefer F, Butz S, Zander K, Meyer zu Brickwedde MK, Suzuki A, Imhof BA, Vestweber D. 2003. The junctional adhesion molecule (JAM) family members JAM-2 and JAM-3 associate with the cell polarity protein PAR-3: A possible role for JAMs in endothelial cell polarity. J Cell Sci 116: 3879–3891. [DOI] [PubMed] [Google Scholar]
- Ernkvist M, Luna Persson N, Audebert S, Lecine P, Sinha I, Liu M, Schlueter M, Horowitz A, Aase K, Weide T, et al. 2009. The Amot/Patj/Syx signaling complex spatially controls RhoA GTPase activity in migrating endothelial cells. Blood 113: 244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco CA, Jones ML, Bernabeu MO, Geudens I, Mathivet T, Rosa A, Lopes FM, Lima AP, Ragab A, Collins RT, et al. 2015. Dynamic endothelial cell rearrangements drive developmental vessel regression. PLoS Biol 13: e1002125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaengel K, Niaudet C, Hagikura K, Laviña B, Siemsen BL, Muhl L, Hofmann JJ, Ebarasi L, Nyström S, Rymo S, et al. 2012. The sphingosine-1-phosphate receptor S1PR1 restricts sprouting angiogenesis by regulating the interplay between VE-cadherin and VEGFR2. Dev Cell 23: 587–599. [DOI] [PubMed] [Google Scholar]
- Garnaas MK, Moodie KL, Liu M, Samant GV, Li K, Marx R, Baraban JM, Horowitz A, Ramchandran R. 2008. Syx, a RhoA guanine exchange factor, is essential for angiogenesis in vivo. Circ Res 103: 710–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavard J, Patel V, Gutkind JS. 2008. Angiopoietin-1 prevents VEGF-induced endothelial permeability by sequestering Src through mDia. Dev Cell 14: 25–36. [DOI] [PubMed] [Google Scholar]
- Gebala V, Collins R, Geudens I, Phng LK, Gerhardt H. 2016. Blood flow drives lumen formation by inverse membrane blebbing during angiogenesis in vivo. Nat Cell Biol 18: 443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glading AJ, Ginsberg MH. 2010. Rap1 and its effector KRIT1/CCM1 regulate β-catenin signaling. Dis Model Mech 3: 73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glading A, Han J, Stockton RA, Ginsberg MH. 2007. KRIT-1/CCM1 is a Rap1 effector that regulates endothelial cell–cell junctions. J Cell Biol 179: 247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon EJ, Fukuhara D, Westrom S, Padhan N, Sjostrom EO, van Meeteren L, He L, Orsenigo F, Dejana E, Bentley K, et al. 2016. The endothelial adaptor molecule TSAd is required for VEGF-induced angiogenic sprouting through junctional c-Src activation. Sci Signal 9: ra72. [DOI] [PubMed] [Google Scholar]
- Gory-Fauré S, Prandini MH, Pointu H, Roullot V, Pignot-Paintrand I, Vernet M, Huber P. 1999. Role of vascular endothelial-cadherin in vascular morphogenesis. Development 126: 2093–2102. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Majumdar A, Li X, Adler J, Sun Z, Vertuani S, Hellberg C, Mellberg S, Koch S, Dimberg A, et al. 2013. VE-PTP regulates VEGFR2 activity in stalk cells to establish endothelial cell polarity and lumen formation. Nat Commun 4: 1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayer A, Shao L, Chung M, Joubert LM, Yang HW, Tsai FC, Bisaria A, Betzig E, Meyer T. 2016. Engulfed cadherin fingers are polarized junctional structures between collectively migrating endothelial cells. Nat Cell Biol 18: 1311–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herwig L, Blum Y, Krudewig A, Ellertsdottir E, Lenard A, Belting HG, Affolter M. 2011. Distinct cellular mechanisms of blood vessel fusion in the zebrafish embryo. Curr Biol 21: 1942–1948. [DOI] [PubMed] [Google Scholar]
- Hultin S, Zheng Y, Mojallal M, Vertuani S, Gentili C, Balland M, Milloud R, Belting HG, Affolter M, Helker CSM, et al. 2014. AmotL2 links VE-cadherin to contractile actin fibres necessary for aortic lumen expansion. Nat Commun 5: 3743. [DOI] [PubMed] [Google Scholar]
- Huveneers S, de Rooij J. 2013. Mechanosensitive systems at the cadherin–F-actin interface. J Cell Sci 126: 403–413. [DOI] [PubMed] [Google Scholar]
- Iden S, Collard JG. 2008. Crosstalk between small GTPases and polarity proteins in cell polarization. Nat Rev Mol Cell Biol 9: 846–859. [DOI] [PubMed] [Google Scholar]
- Iden S, Rehder D, August B, Suzuki A, Wolburg-Buchholz K, Wolburg H, Ohno S, Behrens J, Vestweber D, Ebnet K. 2006. A distinct PAR complex associates physically with VE-cadherin in vertebrate endothelial cells. EMBO Rep 7: 1239–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson L, Franco CA, Bentley K, Collins RT, Ponsioen B, Aspalter IM, Rosewell I, Busse M, Thurston G, Medvinsky A, et al. 2010. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat Cell Biol 12: 943–953. [DOI] [PubMed] [Google Scholar]
- Jin SW, Beis D, Mitchell T, Chen JN, Stainier DYR. 2005. Cellular and molecular analyses of vascular tube and lumen formation in zebrafish. Development 132: 5199–5209. [DOI] [PubMed] [Google Scholar]
- Jufri NF, Mohamedali A, Avolio A, Baker MS. 2015. Mechanical stretch: Physiological and pathological implications for human vascular endothelial cells. Vasc Cell 7: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung B, Obinata H, Galvani S, Mendelson K, Ding B, Skoura A, Kinzel B, Brinkmann V, Rafii S, Evans T, et al. 2012. Flow-regulated endothelial S1P receptor-1 signaling sustains vascular development. Dev Cell 23: 600–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochhan E, Lenard A, Ellertsdottir E, Herwig L, Affolter M, Belting HG, Siekmann AF. 2013. Blood flow changes coincide with cellular rearrangements during blood vessel pruning in zebrafish embryos. PLoS ONE 8: e75060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo Y, Barry DM, Xu K, Tanigaki K, Davis GE, Mineo C, Cleaver O. 2016. Rasip1 is essential to blood vessel stability and angiogenic blood vessel growth. Angiogenesis 19: 173–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HB, Wang S, Helker CSM, Rasouli SJ, Maischein HM, Offermanns S, Herzog W, Stainier DYR. 2016. In vivo modulation of endothelial polarization by Apelin receptor signalling. Nat Commun 7: 11805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampugnani MG, Zanetti A, Breviario F, Balconi G, Orsenigo F, Corada M, Spagnuolo R, Betson M, Braga V, Dejana E. 2002. VE-cadherin regulates endothelial actin activating Rac and increasing membrane association of Tiam. Mol Biol Cell 13: 1175–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampugnani MG, Orsenigo F, Gagliani MC, Tacchetti C, Dejana E. 2006. Vascular endothelial cadherin controls VEGFR-2 internalization and signaling from intracellular compartments. J Cell Biol 174: 593–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampugnani MG, Orsenigo F, Rudini N, Maddaluno L, Boulday G, Chapon F, Dejana E. 2010. CCM1 regulates vascular-lumen organization by inducing endothelial polarity. J Cell Sci 123: 1073–1080. [DOI] [PubMed] [Google Scholar]
- *.Lampugnani MG, Dejana E, Giampietro C. 2017. VE-cadherin, endothelial adherens junctions, and vascular disease. Cold Spring Harb Perspect Biol. 10.1101/cshperspect.a029322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenard A, Ellertsdottir E, Herwig L, Krudewig A, Sauteur L, Belting HG, Affolter M. 2013. In vivo analysis reveals a highly stereotypic morphogenetic pathway of vascular anastomosis. Dev Cell 25: 492–506. [DOI] [PubMed] [Google Scholar]
- Lenard A, Daetwyler S, Betz C, Ellertsdottir E, Belting HG, Huisken J, Affolter M. 2015. Endothelial cell self-fusion during vascular pruning. PLoS Biol 13: e1002126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Padhan N, Sjöström EO, Roche FP, Testini C, Honkura N, Sáinz-Jaspeado M, Gordon E, Bentley K, Philippides A, et al. 2016. VEGFR2 pY949 signalling regulates adherens junction integrity and metastatic spread. Nat Commun 7: 11017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla CJ, Reis M, Felici A, Wolburg H, Fruttiger M, et al. 2008. Wnt/β-catenin signaling controls development of the blood–brain barrier. J Cell Biol 183: 409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Collins C, Kiosses WB, Murray AM, Joshi M, Shepherd TR, Fuentes EJ, Tzima E. 2013. A novel pathway spatiotemporally activates Rac1 and redox signaling in response to fluid shear stress. J Cell Biol 201: 863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizama CO, Zovein AC. 2013. Polarizing pathways: Balancing endothelial polarity, permeability, and lumen formation. Exp Cell Res 319: 1247–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobov IB, Rao S, Carroll TJ, Vallance JE, Ito M, Ondr JK, Kurup S, Glass DA, Patel MS, Shu W, et al. 2005. WNT7b mediates macrophage-induced programmed cell death in patterning of the vasculature. Nature 437: 417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucitti JL, Jones EAV, Huang C, Chen J, Fraser SE, Dickinson ME. 2007. Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development 134: 3317–3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddaluno L, Rudini N, Cuttano R, Bravi L, Giampietro C, Corada M, Ferrarini L, Orsenigo F, Papa E, Boulday G, et al. 2013. EndMT contributes to the onset and progression of cerebral cavernous malformations. Nature 498: 492–496. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Bohman S, Dixelius J, Berge T, Dimberg A, Magnusson P, Wang L, Wikner C, Qi JH, Wernstedt C, et al. 2005. VEGF receptor-2 Y951 signaling and a role for the adapter molecule TSAd in tumor angiogenesis. EMBO J 24: 2342–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavria G, Vercoulen Y, Yeo M, Paterson H, Karasarides M, Marais R, Bird D, Marshall CJ. 2006. ERK-MAPK signaling opposes Rho-kinase to promote endothelial cell survival and sprouting during angiogenesis. Cancer Cell 9: 33–44. [DOI] [PubMed] [Google Scholar]
- Mertens AEE, Pegtel DM, Collard JG. 2006. Tiam1 takes PARt in cell polarity. Trends Cell Biol 16: 308–316. [DOI] [PubMed] [Google Scholar]
- Montero-Balaguer M, Swirsding K, Orsenigo F, Cotelli F, Mione M, Dejana E. 2009. Stable vascular connections and remodeling require full expression of VE-cadherin in zebrafish embryos. PLoS ONE 4: e5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais-de-Sá E, Mirouse V, St Johnston D. 2010. aPKC phosphorylation of Bazooka defines the apical/lateral border in Drosophila epithelial cells. Cell 141: 509–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngok SP, Geyer R, Liu M, Kourtidis A, Agrawal S, Wu C, Seerapu HR, Lewis-Tuffin LJ, Moodie KL, Huveldt D, et al. 2012. VEGF and angiopoietin-1 exert opposing effects on cell junctions by regulating the Rho GEF Syx. J Cell Biol 199: 1103–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phng LK, Stanchi F, Gerhardt H. 2013. Filopodia are dispensable for endothelial tip cell guidance. Development 140: 4031–4040. [DOI] [PubMed] [Google Scholar]
- Phng LK, Gebala V, Bentley K, Philippides A, Wacker A, Mathivet T, Sauteur L, Stanchi F, Belting HG, Affolter M, et al. 2015. Formin-mediated actin polymerization at endothelial junctions is required for vessel lumen formation and stabilization. Dev Cell 32: 123–132. [DOI] [PubMed] [Google Scholar]
- Post A, Pannekoek WJ, Ross SH, Verlaan I, Brouwer PM, Bos JL. 2013. Rasip1 mediates Rap1 regulation of Rho in endothelial barrier function through ArhGAP29. Proc Natl Acad Sci 110: 11427–11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post A, Pannekoek WJ, Ponsioen B, Vliem MJ, Bos JL. 2015. Rap1 spatially controls ArhGAP29 to inhibit Rho signaling during endothelial barrier regulation. Mol Cell Biol 35: 2495–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potente M, Gerhardt H, Carmeliet P. 2011. Basic and therapeutic aspects of angiogenesis. Cell 146: 873–887. [DOI] [PubMed] [Google Scholar]
- Rao S, Lobov IB, Vallance JE, Tsujikawa K, Shiojima I, Akunuru S, Walsh K, Benjamin LE, Lang RA. 2007. Obligatory participation of macrophages in an angiopoietin 2-mediated cell death switch. Development 134: 4449–4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards M, Hetheridge C, Mellor H. 2015. The formin FMNL3 controls early apical specification in endothelial cells by regulating the polarized trafficking of podocalyxin. Curr Biol 25: 2325–2331. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Macara IG. 2014. Organization and execution of the epithelial polarity programme. Nat Rev Mol Cell Biol 15: 225–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saharinen P, Eklund L, Miettinen J, Wirkkala R, Anisimov A, Winderlich M, Nottebaum A, Vestweber D, Deutsch U, Koh GY, et al. 2008. Angiopoietins assemble distinct Tie2 signalling complexes in endothelial cell–cell and cell–matrix contacts. Nat Cell Biol 10: 527–537. [DOI] [PubMed] [Google Scholar]
- Sauteur L, Krudewig A, Herwig L, Ehrenfeuchter N, Lenard A, Affolter M, Belting HG. 2014. Cdh5/VE-cadherin promotes endothelial cell interface elongation via cortical actin polymerization during angiogenic sprouting. Cell Rep 9: 504–513. [DOI] [PubMed] [Google Scholar]
- Sauteur L, Affolter M, Belting HG. 2017. Distinct and redundant functions of Esama and VE-cadherin during vascular morphogenesis. Development 144: 1554–1565. [DOI] [PubMed] [Google Scholar]
- Strilić B, Kučera T, Eglinger J, Hughes MR, McNagny KM, Tsukita S, Dejana E, Ferrara N, Lammert E. 2009. The molecular basis of vascular lumen formation in the developing mouse aorta. Dev Cell 17: 505–515. [DOI] [PubMed] [Google Scholar]
- Strilić B, Eglinger J, Krieg M, Zeeb M, Axnick J, Babál P, Müller DJ, Lammert E. 2010. Electrostatic cell-surface repulsion initiates lumen formation in developing blood vessels. Curr Biol 20: 2003–2009. [DOI] [PubMed] [Google Scholar]
- Sun Z, Li X, Massena S, Kutschera S, Padhan N, Gualandi L, Sundvold-Gjerstad V, Gustafsson K, Choy WW, Zang G, et al. 2012. VEGFR2 induces c-Src signaling and vascular permeability in vivo via the adaptor protein TSAd. J Exp Med 209: 1363–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei A, Giampietro C, Conti A, Orsenigo F, Breviario F, Pirazzoli V, Potente M, Daly C, Dimmeler S, Dejana E. 2008. Endothelial adherens junctions control tight junctions by VE-cadherin-mediated upregulation of claudin-5. Nat Cell Biol 10: 923–934. [DOI] [PubMed] [Google Scholar]
- Tyler RC, Peterson FC, Volkman BF. 2010. Distal interactions within the par3–VE-cadherin complex. Biochemistry 49: 951–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzima E. 2002. Activation of Rac1 by shear stress in endothelial cells mediates both cytoskeletal reorganization and effects on gene expression. EMBO J 21: 6791–6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzima E, Kiosses WB, del Pozo MA, Schwartz MA. 2003. Localized cdc42 activation, detected using a novel assay, mediates microtubule organizing center positioning in endothelial cells in response to fluid shear stress. J Biol Chem 278: 31020–31023. [DOI] [PubMed] [Google Scholar]
- Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. 2005. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 437: 426–431. [DOI] [PubMed] [Google Scholar]
- Uemura A, Ogawa M, Hirashima M, Fujiwara T, Koyama S, Takagi H, Honda Y, Wiegand SJ, Yancopoulos GD, Nishikawa SI. 2002. Recombinant angiopoietin-1 restores higher-order architecture of growing blood vessels in mice in the absence of mural cells. J Clin Invest 110: 1619–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestweber D, Wessel F, Nottebaum AF. 2014. Similarities and differences in the regulation of leukocyte extravasation and vascular permeability. Semin Immunopathol 36: 177–192. [DOI] [PubMed] [Google Scholar]
- Wang Y, Kaiser MS, Larson JD, Nasevicius A, Clark KJ, Wadman SA, Roberg-Perez SE, Ekker SC, Hackett PB, McGrail M, et al. 2010. Moesin1 and Ve-cadherin are required in endothelial cells during in vivo tubulogenesis. Development 137: 3119–3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead KJ, Chan AC, Navankasattusas S, Koh W, London NR, Ling J, Mayo AH, Drakos SG, Jones CA, Zhu W, et al. 2009. The cerebral cavernous malformation signaling pathway promotes vascular integrity via Rho GTPases. Nat Med 15: 177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CW, Ye W. 2014. Regulation of vascular endothelial junction stability and remodeling through Rap1-Rasip1 signaling. Cell Adhes Migr 8: 76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CW, Parker LH, Hall CJ, Smyczek T, Mak J, Crow A, Posthuma G, De Mazière A, Sagolla M, Chalouni C, et al. 2013. Rasip1 regulates vertebrate vascular endothelial junction stability through Epac1–Rap1 signaling. Blood 122: 3678–3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer R, Cseh B, Maier B, Scherrer K, Baccarini M. 2012. Angiogenic sprouting requires the fine tuning of endothelial cell cohesion by the Raf-1/Rok-α complex. Dev Cell 22: 158–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winderlich M, Keller L, Cagna G, Broermann A, Kamenyeva O, Kiefer F, Deutsch U, Nottebaum AF, Vestweber D. 2009. VE-PTP controls blood vessel development by balancing Tie-2 activity. J Cell Biol 185: 657–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worzfeld T, Schwaninger M. 2016. Apicobasal polarity of brain endothelial cells. J Cereb Blood Flow Metab 36: 340–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Agrawal S, Vasanji A, Drazba J, Sarkaria S, Xie J, Welch CM, Liu M, Anand-Apte B, Horowitz A. 2011. Rab13-dependent trafficking of RhoA is required for directional migration and angiogenesis. J Biol Chem 286: 23511–23520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Sacharidou A, Fu S, Chong DC, Skaug B, Chen ZJ, Davis GE, Cleaver O. 2011. Blood vessel tubulogenesis requires Rasip1 regulation of GTPase signaling. Dev Cell 20: 526–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JA, Castranova D, Pham VN, Weinstein BM. 2015. Single-cell analysis of endothelial morphogenesis in vivo. Development 142: 2951–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeb M, Strilic B, Lammert E. 2010. Resolving cell–cell junctions: Lumen formation in blood vessels. Curr Opin Cell Biol 22: 626–632. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Vertuani S, Nyström S, Audebert S, Meijer I, Tegnebratt T, Borg JP, Uhlén P, Majumdar A, Holmgren L. 2009. Angiomotin-like protein 1 controls endothelial polarity and junction stability during sprouting angiogenesis. Circ Res 105: 260–270. [DOI] [PubMed] [Google Scholar]
- Zovein AC, Luque A, Turlo KA, Hofmann JJ, Yee KM, Becker MS, Fassler R, Mellman I, Lane TF, Iruela-Arispe ML. 2010. β1 integrin establishes endothelial cell polarity and arteriolar lumen formation via a Par3-dependent mechanism. Dev Cell 18: 39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]