Abstract
During the last decade, several high-throughput technologies have been applied to gather deeper understanding on the biological events elicited by vaccination. The main goal of systems biology is to integrate different sources of data and extract biologically meaningful information. This holistic approach has provided new insights on the impact that the innate immune status has on vaccine responsiveness. Other factors like chronic infections, age, microbiome, and metabolism can influence the outcome of vaccination, and systems biology offers unique opportunities to expand our understanding of their role on the immune response. However, a few challenges that still need to be overcome will be discussed.
Great Debates
What are the most interesting topics likely to come up over dinner or drinks with your colleagues? Or, more importantly, what are the topics that don't come up because they are a little too controversial? In Immune Memory and Vaccines: Great Debates, Editors Rafi Ahmed and Shane Crotty have put together a collection of articles on such questions, written by thought leaders in these fields, with the freedom to talk about the issues as they see fit. This short, innovative format aims to bring a fresh perspective by encouraging authors to be opinionated, focus on what is most interesting and current, and avoid restating introductory material covered in many other reviews.
The Editors posed 13 interesting questions critical for our understanding of vaccines and immune memory to a broad group of experts in the field. In each case, several different perspectives are provided. Note that while each author knew that there were additional scientists addressing the same question, they did not know who these authors were, which ensured the independence of the opinions and perspectives expressed in each article. Our hope is that readers enjoy these articles and that they trigger many more conversations on these important topics.
Systems biology in the field of vaccines emerged with the need to integrate large sets of data coming from new high-throughput technologies using mathematical and computational modeling. Initially, most of the data were represented by microarray gene-expression profiling following vaccination. In recent years, however, next-generation sequencing technologies have been replacing microarrays because of their enhanced throughput (Mutz et al. 2013). At the same time, new protocols and novel single-cell sorting technologies allow for the analysis of the complete B-cell repertoire evolution and the profiling of T-cell repertoire in response to vaccination. Novel immune cell–profiling technologies, such as polychromatic flow cytometry or mass cytometry (CyTOF), are enabling the integration of transcriptional signatures with phenotypic information (Fourati et al. 2016). Large datasets are also generated by proteomic analysis of the serum (Wine et al. 2015) and by deep sequencing analysis of microbiomes (Walker 2016). Finally, some investigators developed a “systems serology” approach to dissect the characteristics of the antibody responses using multiple functional and biochemical assays (Chung et al. 2015).
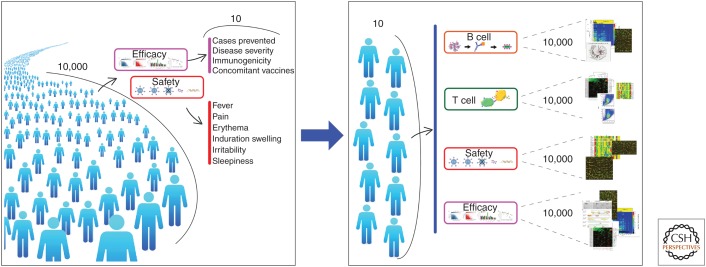
Originally, systems biology was expected to accelerate the development of novel vaccines by providing faster and more accurate responses about safety and efficacy using much smaller clinical samples. As schematically represented in Figure 1, the goal was to change the conventional approach to clinical trials in which thousands of people are used to collect a limited number of information into a new paradigm, in which more information could be gained by collecting thousands of readouts from a limited number of people using high-throughput technologies. Unfortunately, this goal has not been achieved yet. However, thanks to systems biology approaches to vaccines studies, we have learned many new things about adjuvants and innate immunity that are very important to the future of vaccines. In this article, we will review the new information provided by systems biology and the challenges ahead.
Figure 1.
The goal of systems biology. A conventional approach to a clinical trial (left) uses large numbers of people (10,000 in the figure), and each of them is monitored for a few parameters about safety and efficacy. This is an ideal framework for future clinical trials (right), in which more information could be gained by analyzing thousands of readouts collected from a limited number of people (10 in the figure), using high-throughput technologies and systems biology.
PROFILING ADJUVANTS IN ANIMAL STUDIES
Systems biology approaches have been used extensively to distinguish different classes of vaccine adjuvants by studying the expression profiles generated in vivo (Mosca et al. 2008; Didierlaurent et al. 2009; Morel et al. 2011; Knudsen et al. 2016) or in immune cells in vitro (Dupuis et al. 1998; Morefield et al. 2005). One of the first applications in vivo was the analysis of the gene-expression profile induced by the vaccine adjuvants alum, CpG, or the oil-in-water emulsion MF59 in mouse muscle (Mosca et al. 2008). All three adjuvants were shown to activate a core set of transcripts that are indicative of recruitment of neutrophils and antigen-presenting cells to the site of immunization. In addition, each of the three adjuvants was also found to regulate specific sets of genes, and MF59 regulated the largest number of them. Profiling of adjuvant-regulated genes continued with the GlaxoSmithKline adjuvants AS03 and AS04 (Didierlaurent et al. 2009; Morel et al. 2011) and, more recently, with the head-to-head comparison of five different adjuvants, including alum, the emulsion MF59, GLA-SE, IC31 containing agonists for TLR4 and TLR9, respectively, and the liposome-based adjuvant CAF01 (Knudsen et al. 2016). These studies concluded that whereas each adjuvant has an antigen-independent unique profile, overall they can be distinguished in two broad categories, those that favored a TH1 response profile inducing interferon γ (IFN-γ), tumor necrosis factor (TNF), interleukin (IL)-6, and IL-17 and those that favored a T helper (TH)0 or TH2 profile inducing IL-5 and IL-10. The amount, ratio, and timing of gene regulation and cytokine production changed greatly from one adjuvant to the other. Today, systems biology studies in animal models are still very useful in the characterization of vaccine adjuvants and formulations as they can provide insights into the immune-response profile that will be induced. It should be noted, however, that the immune responses observed in animal models may not accurately reflect what happens in humans.
HUMAN STUDIES
The major contribution of systems biology came from studies in humans. Two pioneering studies were conducted on healthy adults vaccinated with the live attenuated yellow fever vaccine 17D (YF-17D) (Gaucher et al. 2008; Querec et al. 2009). These studies independently showed that adaptive responses to YF-17D rely on the early (3–7 days) activation of multiple innate immune components, including the type I IFN, complement, and inflammasome responses. They also identified specific sets of gene-encoding transcripts whose abundance in the peripheral blood, within 1 week following vaccination, was able to predict later CD8+ T-cell and functional antibody responses with >80% prediction accuracy (Querec et al. 2009).
Systems biology studies were subsequently applied to vaccines other than YF-17D, including live attenuated, inactivated, and subunit vaccines against both bacteria and viruses. These studies identified additional blood-derived immune signatures associated with vaccine immunogenicity, including the transcriptional response of genes involved in antigen processing and presentation, plasma-cell response, along with the response of chemoattractant cytokines and a particular family of CD4+ T follicular helper (TFH) cells (Nakaya et al. 2011; Bentebibel et al. 2013). Furthermore, a recent comparative study reported that different vaccines elicit distinct signatures that correlate with the antibody response, suggesting that a universal signature capable of predicting the seroresponse across vaccines is unlikely to exist (Li et al. 2014). The holistic approach enabled by systems biology also allowed an appreciation of emerging rules of noncanonical factors that were never previously associated with vaccine responsiveness. These include the antagonistic effect of preexisting immunity and of sex-related factors (such as testosterone) in the functional antibody responses to influenza vaccination (Furman et al. 2014; Tsang et al. 2014; Fourati et al. 2016). Blood transcriptional biomarkers were also identified for the prediction of vaccine efficacy. Based on the availability of a human challenge model for malaria infection, a systems biology-based study of an experimental RTS,S malaria vaccine found that differential expression of genes in the immunoproteasome pathway, right before the challenge, distinguished protected from nonprotected persons (Vahey et al. 2010).
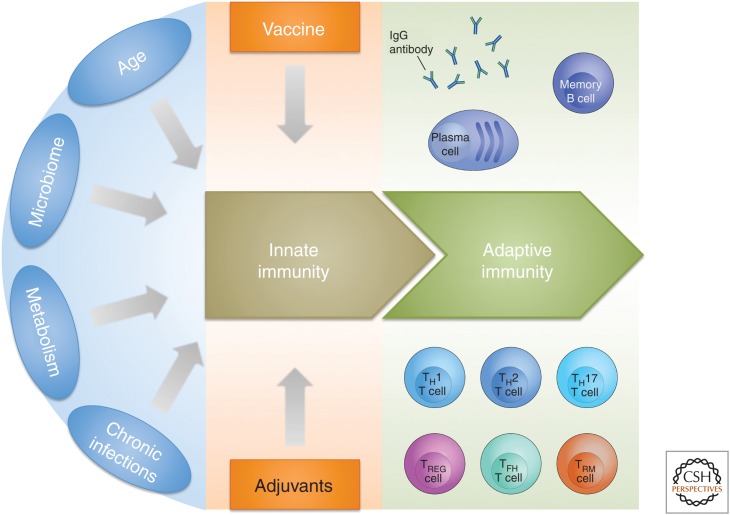
Among the most important contributions of systems biology is the increased appreciation that the immune system is tightly linked to environmental and metabolic factors and, therefore, that age, microbiome, metabolism (diet, hygiene, geographic location), and chronic infections have a profound influence on the response to vaccination (Fig. 2) (Pulendran 2014).
Figure 2.
Schematic representation showing the existence of intrinsic variables (age, microbiome, metabolism, and chronic infections) that can influence innate immunity, which is the basis for the response to vaccination. Adjuvants provided with vaccination are used to modify the innate immune status and to induce the desired vaccine response. IgG, Immunoglobulin G; TH, T helper; TREG, T regulatory; TFH, T follicular helper; TRM, tissue-resident memory T cell.
AGE
It is well known that the immune system in early childhood and in the elderly population provides a suboptimal response to vaccination when compared with adolescents and adults. The molecular mechanisms behind it, which are still poorly understood, have been studied by systems biology approaches in several clinical studies (Vesikari et al. 2011; Nakaya et al. 2016). In one of them, an MF59-adjuvanted influenza vaccine that had been previously shown to provide 86% protection from infection in children was compared with a conventional influenza vaccine that had 43% protection. The study was performed with 14 24-month-old children (Nakaya et al. 2016). Results showed that the transcriptional responses to the conventional vaccine measured at day 1 after vaccination were greatly attenuated and present only in a subset of children when compared with adults. The MF59 adjuvant increased the frequency and robustness of the transcriptional responses characterized by the antiviral IFN signature, dendritic-cell activation, and innate immunity–associated genes. Additionally, the frequency of multi-cytokine-producing CD4+ T cells, antigen-specific IFN-γ-producing CD4+ T cells, and antibody titers were greatly increased, providing a response that mimicked more closely the response in adults. The conclusion was that in early childhood the poor response to vaccination is attributable to a weak innate immune activation and that this weakness can be overcome by adjuvants.
In a similar study, Fourati et al. (2016) compared the transcriptional responses to hepatitis B vaccine in elderly people (>65 years old) with that of young people (25–35 years old). The gene-expression profile of young people was characterized by transcriptional modules (groups of genes sharing the same response pattern and participating in the same biological process) involved in B-cell signaling, T-cell receptor signaling, and antiviral response. In marked contrast, the transcriptome profile of the elderly population was characterized by the activation of modules involved in the inflammatory response, cell motility, and type II IFN. Based on these differences, they established a formula to calculate an integrated score that they called “BioAge.” Interestingly, whereas most of the young people had low BioAge, the elderly could be divided into two groups that they named BioAge young and BioAge old. Remarkably, the BioAge was a much better predictor of the vaccine response than chronological age. Ultimately, the authors claim that the poor response in the elderly is characterized by a signature of inflammation and that transcriptional profiling before vaccination allows for the prediction of those that will have a poor response to vaccination.
MICROBIOME
Studies in germ-free mice had shown already in the 1960s that, in the absence of commensals, mice had a defective development of the lymphoid tissue in the spleen, thymus, and the gut-associated lymphoid structures, including smaller Peyer’s patches and mesenteric lymph nodes (Gensollen et al. 2016). The recent ability to profile the microbiome by 16S RNA and genome sequencing allowed for the establishment of the changes to the human microbiome with regard to age and geography (Yatsunenko et al. 2012), suggesting that the different microbial flora present in newborns and adults, in people born in low- and high-income countries, or the alteration caused by antibiotic use may have profound effects on the outcome of vaccination. Indeed, microbial colonization of the human body begins at birth and does not become stable until ∼2 years of age. During the first 2 years of life, when most vaccinations are performed, the microbiota is highly variable and sensitive to environmental exposures (Palmer et al. 2007). The difference in the gut microbial composition may well explain why, in the regions of the world with poor sanitation where increased fecal–oral bacterial exposure occurs early in life, there is clear evidence of reduced immunogenicity generated via oral vaccines such as polio, rotavirus, and cholera relative to efficacy in developed countries (Ferreira et al. 2010; Jiang et al. 2010; Levine 2010; Lopman et al. 2012). Finally, it was shown that antibiotic-treated mice had a poor response to influenza vaccination and that stimulation of TLR5 by bacterial flagellin was essential for a normal response (Oh et al. 2014). Expanding our understanding of the intimate interplay between the human microbiota and its immune system will likely have a profound impact on the future of vaccines, including a major shift in the current approach to vaccine development.
METABOLISM
One of the first surprising results of systems biology was the identification of a correlation between expression of the GCN2 (mammalian general control nonderepressible 2) gene in the blood and the magnitude of the CD8+ T-cell response to the YF-17D vaccine (Gaucher et al. 2008). It was later shown to have a key role in virus-induced GCN2 activation in programming dendritic cells to initiate autophagy and enhanced antigen presentation to both CD4+ and CD8+ T cells (Ravindran et al. 2014). Recently, the same authors showed, in transgenic mice models, that GCN2 suppresses intestinal inflammation and TH17 responses via a mechanism dependent on autophagy and sequestration of ROS, a trigger for inflammasome activation (Ravindran et al. 2016), revealing a mechanism that couples amino acid starvation with control of intestinal inflammation. GCN2 can therefore impact immune homeostasis in the intestine, consequently modulating the immune response during conditions of amino acid restriction. These unexpected variables open a key question on how immunity to vaccination can be affected by metabolic states (Hotamisligil 2006). The link between the immune system and metabolism was also confirmed in clinical settings where inhibition of the mammalian target of rapamycin (mTOR), a serine/threonine kinase that regulates cellular metabolism, growth, and proliferation, was reported to strengthen the immune response to influenza vaccination to levels usually achieved only with strong adjuvants (Mannick et al. 2014).
CHRONIC INFECTIONS
It has been shown that vaccine response is generally reduced in subjects with chronic infections (Cooper et al. 2001; Nookala et al. 2004; Elias et al. 2008) and that chronic infection with the herpes virus, human cytomegalovirus (HCMV), alters responses to human influenza vaccination (Furman et al. 2015). Moreover, people chronically infected with intestinal helminths have lower responses to vaccination with Calmette–Guérin (BCG) (Elias et al. 2001), cholera (Cooper et al. 2001), and tetanus toxoid (Nookala et al. 2004). Recently, Reese et al. have shown that mice infected with multiple common pathogens (viruses and helminths) induces blood immune gene signatures that distinguish them from noninfected mice, and that such signatures translate into an altered immune response with reduced antibody production after vaccination with YF-17D (Reese et al. 2016). Interestingly, changes in the immune gene expression are not species-specific, as they partially recapitulate differences observed in gene expression of adult versus cord blood in humans.
CORRELATES OF PROTECTION
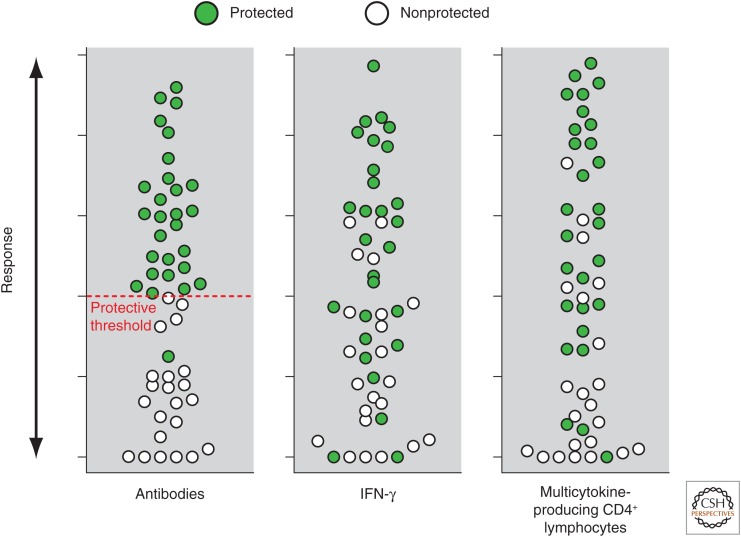
Systems biology has not yet contributed much to the identification of new correlates of protection. Antibody levels and their functionality measured by ELISA, neutralization, bactericidal, and opsonization activity still represent the best correlates of efficacy for many vaccines. For those vaccines such as live attenuated influenza, HIV, malaria, and tuberculosis, in which antibodies are not a good correlate, we have learned that a strong innate signature of IFN-γ-mediated transcriptional response following vaccination and the presence of antigen-specific multicytokine-producing T cells are somewhat correlated with increased protection (Nakaya et al. 2016); however, we have not identified a threshold that predicts protection, and the values in protected and nonprotected people largely overlap (Fig. 3). It may well be that the limitation in sampling human lymphoid organs represents a challenge in properly identifying and profiling some key players of the immune response. T cells do not spend most of their time in the blood, where we routinely analyze immune responses, but in the tissues where they exert their function. In this regard, a study in nonhuman primates, immunized with irradiated sporozoites, showed that antigen-specific CD8+ T cells were present in the liver 100 times more frequently than in the blood, and these were the best predictors of protection (Ishizuka et al. 2016). Thus, immunization regimens designed to enhance the recruitment of effector T cells to tissues in which they can develop locally into long-lived tissue- resident memory T cells (TRM) are desirable (Mueller and Mackay 2015), and the ability to measure them could provide key information about protection. However, because of practical and ethical limitations, probing the immune response in tissues other than peripheral blood remains a major challenge. Nonetheless, a recent step in this direction was made by the Innovative Medicine Initiative funded project BioVacSafe (Lewis and Lythgoe 2015), in which muscle biopsies have been collected from humans following vaccination (see clinicaltrials.gov/show/NCT02368327). A quantitative measure of T-cell immunity is probably what we need the most, and achieving it should not be impossible now that the role of T cells in direct protection from diseases has been beautifully consolidated, especially in cancer using chimeric antigen receptors (CARs) or checkpoint inhibitors (Marcus and Eshhar 2014).
Figure 3.
Schematic representation of the association between serological responses and vaccine-induced protection. While for serum antibodies there is usually a threshold titer that differentiates protected from nonprotected people, in the case of interferon γ (IFN-γ) and multicytokine-producing T cells, increased responses suggest a higher level of protection, but there is a large number of values in which protected and nonprotected people overlap.
Eventually, our ability to understand vaccine-induced immunity will come from the integration of the data on innate immunity and T cells described above with those of B-cell subsets, number of antigen-specific B cells, and their repertoire. Today, new tools available for an in-depth characterization of antibodies allow combining these data, in addition to the traditional functional profile of specific antibodies, with information on their affinity, subclass, glycosylation, and effector function. The availability of human challenge models such as malaria, salmonella, and shigella represent a unique opportunity as these may shed light on the mechanisms underlying immune protection by identifying causal relationships linking the early molecular and cellular signatures provided by systems biology to established immunological correlates of protection. This would ultimately set the path for the development of new improved vaccines.
One final challenge is represented by the capability to process and analyze the data. Whereas recent technologies are capable of producing data with high throughput, the complexity of the datasets generated requires considerable time for their analysis, making the overall process a low-throughput one.
CONCLUSIONS
Systems biology provides new information by profiling adjuvants, people, and immune responses. It also opens new unexplored areas of vaccinology by showing how vaccines act differently on diverse populations, in which the preexisting immune status has an impact on the response to vaccination. However, so far, systems biology failed to find new correlates of protection, especially for those vaccines that do not rely on antibodies. The contribution to vaccine safety is also very limited, even if the European project, BioVacSafe, is still ongoing in this area.
Ultimately, there is no doubt that systems biology, by integrating the information coming from increasingly powerful technologies, will continue to contribute to vaccine development. In doing so, it will need to overcome a few challenges such as sampling tissue-resident immune cells and the timely analysis of the data.
Footnotes
Editors: Shane Crotty and Rafi Ahmed
Additional Perspectives on Immune Memory and Vaccines: Great Debates available at www.cshperspectives.org
REFERENCES
- Bentebibel S, Lopez S, Obermoser G, Schmitt N, Mueller C, Harrod C, Flano E, Mejias A, Albrecht R, Blankenship D, et al. 2013. Induction of ICOS+ CXCR3+ CXCR5+ TH cells correlates with antibody responses to influenza vaccination. Sci Transl Med 5: 176ra32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung AW, Kumar MP, Arnold KB, Yu WH, Schoen MK, Dunphy LJ, Suscovich TJ, Frahm N, Linde C, Mahan AE, et al. 2015. Dissecting polyclonal vaccine-induced humoral immunity against HIV using systems serology. Cell 163: 988–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper PJ, Chico M, Sandoval C, Espinel I, Guevara A, Levine MM, Griffin GE, Nutman TB. 2001. Human infection with Ascaris lumbricoides is associated with suppression of the interleukin-2 response to recombinant cholera toxin B subunit following vaccination with the live oral cholera vaccine CVD 103-HgR. Infect Immun 69: 1574–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didierlaurent AM, Morel S, Lockman L, Giannini SL, Bisteau M, Carlsen H, Kielland A, Vosters O, Vanderheyde N, Schiavetti F, et al. 2009. AS04, an aluminum salt- and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J Immunol 183: 6186–6197. [DOI] [PubMed] [Google Scholar]
- Dupuis M, Murphy TJ, Higgins D, Ugozzoli M, van Nest G, Ott G, McDonald DM. 1998. Dendritic cells internalize vaccine adjuvant after intramuscular injection. Cell Immunol 186: 18–27. [DOI] [PubMed] [Google Scholar]
- Elias D, Wolday D, Akuffo H, Petros B, Bronner U, Britton S. 2001. Effect of deworming on human T cell responses to mycobacterial antigens in helminth-exposed individuals before and after Bacillus Calmette–Guérin (BCG) vaccination. Clin Exp Immunol 123: 219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias D, Britton S, Aseffa A, Engers H, Akuffo H. 2008. Poor immunogenicity of BCG in helminth infected population is associated with increased in vitro TGF-β production. Vaccine 26: 3897–3902. [DOI] [PubMed] [Google Scholar]
- Ferreira RBR, Antunes LCM, Finlay BB. 2010. Should the human microbiome be considered when developing vaccines? PLoS Pathog 6: e1001190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourati S, Cristescu R, Loboda A, Talla A, Filali A, Railkar R, Schaeffer AK, Favre D, Gagnon D, Peretz Y, et al. 2016. Pre-vaccination inflammation and B-cell signalling predict age-related hyporesponse to hepatitis B vaccination. Nat Commun 7: 10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman D, Hejblum BP, Simon N, Jojic V, Dekker CL, Thiébaut R, Tibshirani RJ, Davis MM. 2014. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc Natl Acad Sci 111: 869–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman D, Jojic V, Sharma S, Shen-Orr SS, Angel CJL, Onengut-Gumuscu S, Kidd BA, Maecker HT, Concannon P, Dekker CL, et al. 2015. Cytomegalovirus infection enhances the immune response to influenza. Sci Transl Med 7: 281ra43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaucher D, Therrien R, Kettaf N, Angermann BR, Boucher G, Filali-Mouhim A, Moser JM, Mehta RS, Drake DR III, Castro E, et al. 2008. Yellow fever vaccine induces integrated multilineage and polyfunctional immune responses. J Exp Med 205: 3119–3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensollen T, Iyer SS, Kasper DL, Blumberg RS. 2016. How colonization by microbiota in early life shapes the immune system. Science 352: 539–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS. 2006. Inflammation and metabolic disorders. Nature 444: 860–867. [DOI] [PubMed] [Google Scholar]
- Ishizuka AS, Lyke KE, DeZure A, Berry AA, Richie TL, Mendoza FH, Enama ME, Gordon IJ, Chang LJ, Sarwar UN, et al. 2016. Protection against malaria at 1 year and immune correlates following PfSPZ vaccination. Nat Med. 22: 614–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang V, Jiang B, Tate J, Parashar UD, Patel MM. 2010. Performance of rotavirus vaccines in developed and developing countries. Hum Vaccin 6: 532–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen NPH, Olsen A, Buonsanti C, Follmann F, Zhang Y, Coler RN, Fox CB, Meinke A, D Oro U, Casini D, et al. 2016. Different human vaccine adjuvants promote distinct antigen-independent immunological signatures tailored to different pathogens. Sci Rep 6: 19570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine MM. 2010. Immunogenicity and efficacy of oral vaccines in developing countries: Lessons from a live cholera vaccine. BMC Biol 8: 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DJM, Lythgoe MP. 2015. Application of “systems vaccinology” to evaluate inflammation and reactogenicity of adjuvanted preventative vaccines. J Immunol Res 2015: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Rouphael N, Duraisingham S, Romero-Steiner S, Presnell S, Davis C, Schmidt DS, Johnson SE, Milton A, Rajam G, et al. 2014. Molecular signatures of antibody responses derived from a systems biology study of five human vaccines. Nat Immunol 15: 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopman BA, Pitzer VE, Sarkar R, Gladstone B, Patel M, Glasser J, Gambhir M, Atchison C, Grenfell BT, Edmunds WJ, et al. 2012. Understanding reduced rotavirus vaccine efficacy in low socio-economic settings. PLoS ONE 10.1371/journal.pone.0041720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannick JB, Del Giudice G, Lattanzi M, Valiante NM, Praestgaard J, Huang B, Lonetto MA, Maecker HT, Kovarik J, Carson S, et al. 2014. mTOR inhibition improves immune function in the elderly. Sci Transl Med 6: 268ra179. [DOI] [PubMed] [Google Scholar]
- Marcus A, Eshhar Z. 2014. Allogeneic chimeric antigen receptor-modified cells for adoptive cell therapy of cancer. Expert Opin Biol Ther 14: 947–954. [DOI] [PubMed] [Google Scholar]
- Morefield GL, Sokolovska A, Jiang D, Hogenesch H, Robinson JP, Hem SL. 2005. Role of aluminum-containing adjuvants in antigen internalization by dendritic cells in vitro. Vaccine 23: 1588–1595. [DOI] [PubMed] [Google Scholar]
- Morel S, Didierlaurent A, Bourguignon P, Delhaye S, Baras B, Jacob V, Planty C, Elouahabi A, Harvengt P, Carlsen H, et al. 2011. Adjuvant System AS03 containing α-tocopherol modulates innate immune response and leads to improved adaptive immunity. Vaccine 29: 2461–2473. [DOI] [PubMed] [Google Scholar]
- Mosca F, Tritto E, Muzzi A, Monaci E, Bagnoli F, Iavarone C, O’Hagan D, Rappuoli R, De Gregorio E. 2008. Molecular and cellular signatures of human vaccine adjuvants. Proc Natl Acad Sci 105: 10501–10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SN, Mackay LK. 2015. Tissue-resident memory T cells: Local specialists in immune defence. Nat Rev Immunol 16: 1–11. [DOI] [PubMed] [Google Scholar]
- Mutz K, Heilkenbrinker A, Lo M, Stahl F, Lönne M, Walter JG. 2013. Transcriptome analysis using next-generation sequencing. Curr Opin Biotechnol 24: 22–30. [DOI] [PubMed] [Google Scholar]
- Nakaya HI, Wrammert J, Lee EK, Racioppi L, Marie-Kunze S, Haining WN, Means AR, Kasturi SP, Khan N, Li GM, et al. 2011. Systems biology of vaccination for seasonal influenza in humans. Nat Immunol 12: 786–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaya HI, Clutterbuck E, Kazmin D, Wang L, Cortese M, Bosinger SE, Patel NB, Zak DE, Aderem A, Dong T, et al. 2016. Systems biology of immunity to MF59-adjuvanted versus nonadjuvanted trivalent seasonal influenza vaccines in early childhood. Proc Natl Acad Sci 113: 1853–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nookala S, Srinivasan S, Kaliraj P, Narayanan RB, Nutman TB. 2004. Impairment of tetanus-specific cellular and humoral responses following tetanus vaccination in human lymphatic filariasis. Infect Immun 72: 2598–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh JZ, Ravindran R, Chassaing B, Carvalho FA, Maddur MS, Bower M, Hakimpour P, Gill KP, Nakaya HI, Yarovinsky F, et al. 2014. TLR5-mediated sensing of gut microbiota is necessary for antibody responses to seasonal influenza vaccination. Immunity 41: 478–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. 2007. Development of the human infant intestinal microbiota. PLoS Biol 5: 1556–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulendran B. 2014. Systems vaccinology: Probing humanity’s diverse immune systems with vaccines. Proc Natl Acad Sci 111: 12300–12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querec TD, Akondy RS, Lee EK, Cao W, Nakaya HI, Teuwen D, Pirani A, Gernert K, Deng J, Marzolf B, et al. 2009. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat Immunol 10: 116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindran R, Khan N, Nakaya HI, Li S, Loebbermann J, Maddur MS, Park Y, Jones DP, Chappert P, Davoust J, et al. 2014. Vaccine activation of the nutrient sensor GCN2 in dendritic cells enhances antigen presentation. Science 343: 313–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindran R, Loebbermann J, Nakaya HI, Khan N, Ma H, Gama L, Machiah DK, Lawson B, Hakimpour P, Wang Y, et al. 2016. The amino acid sensor GCN2 controls gut inflammation by inhibiting inflammasome activation. Nature 531: 523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese TA, Bi K, Kambal A, Filali-Mouhim A, Beura LK, Bürger MC, Pulendran B, Sekaly RP, Jameson SC, Masopust D, et al. 2016. Sequential infection with common pathogens promotes human-like immune gene expression and altered vaccine response. Cell Host Microbe 19: 713–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang JS, Schwartzberg PL, Kotliarov Y, Biancotto A, Xie Z, Germain RN, Wang E, Olnes MJ, Narayanan M, Golding H, et al. 2014. Global analyses of human immune variation reveal baseline predictors of postvaccination responses. Cell 157: 499–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahey MT, Wang Z, Kester KE, Cummings J, Heppner DG, Nau ME, Ofori-Anyinam O, Cohen J, Coche T, Ballou WR, et al. 2010. Expression of genes associated with immunoproteasome processing of major histocompatibility complex peptides is indicative of protection with adjuvanted RTS,S malaria vaccine. J Infect Dis 201: 580–589. [DOI] [PubMed] [Google Scholar]
- Vesikari T, Knuf M, Wutzler P, Karvonen A, Kieninger-Baum D, Schmitt HJ, Baehner F, Borkowski A, Tsai TF, Clemens R. 2011. Oil-in-water emulsion adjuvant with influenza vaccine in young children. N Engl J Med 365: 1406–1416. [DOI] [PubMed] [Google Scholar]
- Walker A. 2016. Studying the human microbiota. Adv Exp Med Biol 902: 5–32. [DOI] [PubMed] [Google Scholar]
- Wine Y, Horton AP, Ippolito GC, Georgiou G. 2015. Serology in the 21st century: The molecular-level analysis of the serum antibody repertoire. Curr Opin Immunol 35: 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, et al. 2012. Human gut microbiome viewed across age and geography. Nature 486: 222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]