Abstract
Background
The anti-microbial protein cathelicidin LL-37 plays an important role in the pathogenesis of psoriasis by inducing inflammation. Our previous study showed that the chlamydial plasmid-encoded protein pGP3 forms a stable complex with LL-37 to neutralize its pro-inflammatory activity. Here, we explored whether pGP3 can inhibit the development of lesions in mice with imiquimod-induced psoriasis.
Material/Methods
The protein pGP3 was expressed in bacteria and purified using glutathione-conjugated agarose beads and a precision protease. The ability of the purified pGP3 to block chemotaxis mediated by LL-37 was tested in vitro using bone marrow-derived neutrophils. The ability of the protein to inhibit the development of psoriasis-like lesions was tested by topically or subcutaneously administering pGP3 in doses of 10 or 50 μg to mice previously treated with imiquimod. Mouse skin was evaluated using the psoriasis area and severity index (PASI) score and photography. Skin biopsies were taken on day 8 and analyzed histologically.
Results
Purified pGP3 inhibited LL-37-mediated chemotaxis. Mice treated with 50 μg pGP3 showed clinical improvement with less severe erythema, infiltration, and scales; these mice also showed thinner dermis and less hyperkeratosis, parakeratosis, and inflammatory cell infiltration than mice treated with without 10 μg pGP3.
Conclusions
PGP3 can inhibit the development of psoriasis-like lesions in mice, possibly through its ability to bind LL-37. Future work should examine the mechanisms underlying this therapeutic effect.
MeSH Keywords: Cell Migration Inhibition, Inflammation, Psoriasis
Background
Psoriasis is a common, chronic, systemic, T cell-driven autoimmune inflammatory disease characterized by red and scaly skin plaques[1,2]. Its pathogenesis, still only partially understood, involves aberrant keratinocyte proliferation and differentiation, development of new blood vessels, and infiltration of T lymphocytes, dendritic cells (DCs), neutrophils, and other elements of innate immunity [3,4]. Psoriasis has been associated with several genetic susceptibility loci [5]. It has also been associated with comorbidities such as cardiovascular disease, psoriasis arthritis, and depression [6]. Patients suffering from psoriasis often need lifelong treatment.
The initial trigger of psoriasis onset remains unknown, but environmental factors such as trauma, infection, and stress appear to play an important role [7]. In early stages of the disease, dermal and plasmacytoid DCs are activated and they in turn activate antigen-specific T cells in the draining lymph nodes. Psoriasis-specific autoantigens have not been definitively identified [6], but one trigger of inflammation in psoriasis appears to be cathelicidin LL-37. LL-37 is the only anti-microbial peptide in the human cathelicidin family. The 37-residue cationic peptide is generated when serine proteases cleave the C-terminal end of the human cationic antibacterial protein of 18 kDa (hCAP18) in the extracellular space [8]. Apart from its anti-microbial activity, LL-37 regulates immune responses. It increases cytokine and chemokine release from local cells and leukocytes, and it exerts chemotactic effects on a large number of immune cells [9]. Psoriatic plaques contain high levels of LL-37 [10]. Lande and colleagues [10] showed that self-DNA enters intracellular Toll-like receptor (TLR) compartments of plasmacytoid DCs by lipid raft-mediated endocytosis, where it is bound by endogenous LL-37, triggering high levels of interferon (IFN)-α production via a TLR9/MyD88/IRF7 pathway. IFN-α activates self-reactive T cells, driving immune reactions that result in formation of a psoriatic lesion. This mechanism implies that blocking TLR or activating TLR inhibitors can inhibit LL-37-dependent activation of plasmacytoid DCs in the dermis, ameliorating cutaneous inflammation in psoriasis [6].
Recently, we reported that chlamydial plasmid-encoded protein pGP3 forms a stable complex with LL-37 and neutralizes its anti-chlamydial activity [11]. We hypothesized that the binding of pGP3 to LL-37 may block LL-37-induced pathology in the development of psoriasis. Therefore, we undertook the present study to evaluate the effect of pGP3 on the development of psoriasis-like skin inflammation in mice. Such inflammation was induced through repeated topical application of Aldara™ cream containing 5% imiquimod as described previously [12]. This mouse model, widely used in preclinical studies of psoriasis, recapitulates the erythema and scaling of human psoriasis, as well as the histopathological features of parakeratosis, neoangiogenesis, and infiltration of immune cells into the dermis [13].
Here, we show in vitro that pGP3 blocks the ability of neutrophils to undergo LL-37-induced chemotaxis, and we demonstrate that topically or subcutaneously administering pGP3 to imiquimod-treated mice reduces scaling, hyperkeratosis, parakeratosis, and inflammatory cell infiltration; accelerates healing time; and leads to thinner dermis. Our data suggest that pGP3 has potential for preventing psoriasis development in mice, and that LL-37 may be an effective therapeutic target.
Material and Methods
Expression and purification of pGP3
The expression and purification of pGP3 and CT795 were described previously [14,15]. Briefly, the pGP3 and CT795 genes were cloned into a pGEX-6P2 vector (Amersham Pharmacia Biotech, Piscataway, NJ, USA) and expressed with glutathione S-transferase (GST) fused to its N-terminus. The GST-pGP3 and GST-CT795 fusion proteins were purified in 2 steps. First, bacterial lysates containing GST-pGP3 and GST-CT795 were bound to glutathione-conjugated agarose beads (Pharmacia), then pGP3 and GST-CT795 were cleaved free of the bead-bound GST using a fusion protein of GST and precision protease (Pharmacia). In this way, the cleaved pGP3 and CT795 were released into solution, while the GST-protease was retained on the beads. The cleaved pGP3 and CT795 were collected and concentrated using Centricon units with a molecular weight cut-off of 10 kDa. The concentrations of purified pGP3 and CT795 were quantitated using a Bio-Rad protein assay dye reagent (Cat#500-0006, Bio-Rad, Hercules, CA, USA). Purity was checked in polyacrylamide gels using Coomassie Blue dye and further confirmed by Western blot as described elsewhere [14,15].
Preparation of bone marrow-derived neutrophils for chemotaxis assays
Bone marrow cells were sterilely harvested from tibiae and femurs of C3H/HeJ mice. After lysing red blood cells, bone marrow cells were cultured at a cell density of 2×106 per ml in 10-cm dishes (BD Biosciences, Durham, NC, USA) at 37°C in an atmosphere of 5% CO2. Cells were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum (cat#100-106, Gemini Bio-Products, West Sacramento, CA, USA), 2 mM l-glutamine (cat#G7513, Sigma, St. Louis, MO, USA), 1X MEM non-essential amino acids (cat#11140-050, Gibco, Grand Island, NY, USA), 1 mM sodium pyruvate (cat#25-000-CI, Cellgro, Manassas, VA, USA), 0.5 mM beta-mercaptoethanol (cat#M3148, Sigma), 100 U/ml penicillin G, and 100 μg/ml streptomycin (cat#30-002-CI, Cellgro).
To make neutrophils, bone marrow cells were cultured in growth medium containing 100 ng/ml recombinant G-CSF (cat#250-05, PeproTech). Every 3 days, floating cells were harvested and re-cultured in fresh growth medium. On day 8, cells floating in the supernatants were analyzed by flow cytometry for the presence of Ly-6G or Gr1. When positivity was >80%, the cells were used as neutrophils in chemotaxis assays.
Chemotaxis assay
Chemotaxis was measured in 24-well plates containing a ThinCert™ Multiwell Plate Insert (cat#662631, Greiner Bio-One, Monroe, NC, USA). The inserts had a translucent bottom membrane of polyethylene terephthalate with a pore size of 3 μm. Bone marrow-derived neutrophils in 200 ml of chemotaxis medium (RPMI 1604 with 1% sterile, lipopolysaccharide-free bovine serum albumin) were added to the insert or the upper chamber at a cell density of 2×106 per ml. LL-37 or the positive control chemo-attractant MIP-2 [16] in 800 μl of the same chemotaxis medium was placed at the indicated concentration in the well below or the lower chamber. In some experiments, LL-37 was previously incubated with pGP3 for 30 min. After 2 h of incubation at 37°C, cells in the upper and lower chambers were counted. The percentage of neutrophils that migrated from the upper to lower chamber was calculated from 3 independent experiments.
Mouse model of psoriasis
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the US National Institutes of Health. BALB/c female mice 6–8 weeks old were purchased from Tianjin Oid Laboratory Products (Tianjin, China). Psoriasis-like skin inflammation was induced by applying a daily dose of 45 mg of 5% imiquimod cream (Aldara™ 5%; Meda AB, Solna, Sweden) topically onto the shaved back of the mouse for 7 consecutive days. The mice were divided into 8 groups with 3 mice in each group (Table 1).
Table 1.
Comparison of epidermis thickness of lesioned skin on day 8 of treatment.
| Epidermal thickness (μm) | |
|---|---|
| IMQ-treated | 84.48±14.03 |
| Topical CT795 (50 μg) | 81.07±14.06 |
| Topical pGP3 (50 μg) | 63.68±12.63* |
| Topical pGP3 (10 μg) | 74.85±13.56 |
| Injected CT795 (50 μg) | 81.30±15.10 |
| Injected pGP3 (50 μg) | 61.68±13.16* |
| Injected pGP3 (10 μg) | 69.21±14.60 |
Epidermis was significantly thinner in mice treated with 50 μg pGP3 than in mice treated with 50 μg CT795. Results are mean ±SD. Statistics:
p<0.05 versus mice treated only with IMQ.
Treatment with pGP3
Mice were treated with 10 or 50 μg purified pGP3 by subcutaneous injection or topically. For topical treatment, pGP3 dissolved in 50 μl PBS was mixed with 50 μl gel containing Poloxmaer 407 and hyaluronic acid sodium salt (Fudan-Zhangjiang Pharmaceutical, Shanghai, China). For subcutaneously injection, 10 or 50 μg pGP3 was dissolved in 100 μl PBS. PGP3 was applied to mice for 7 days on the skin area previously treated with Aldara cream (each day, Aldara was topically applied in the morning, and pGP3 was applied in the evening, either topically or subcutaneously). To confirm the specificity of pGP3 function, control mice were treated with the same dose of the chlamydial protein CT795 instead of pGP3 (Table 1).
Clinical assessment
At various time points during treatment, mouse skin was evaluated clinically based on photographs and PASI score [17], which was determined by evaluating the degree of erythema, thickness, and scaling on the affected skin surface. PASI was measured on a 4-point scale (0=none; 1=slight; 2=moderate; 3=marked; 4=very marked). The severity of skin inflammation was measured by the combined scores (erythema plus scaling plus thickening) giving a range of scores of 0–12.
On day 8, mice were sacrificed and 4-mm punch biopsies were taken from the treated mouse skin. Biopsies were fixed in formalin, embedded in paraffin, sectioned into 4-μm slices and stained with hematoxylin and eosin.
The slices were scanned with a slice scanner and the thickness of the epidermis from the basement membrane to the granular Layer was measured using the ruler on the software. Measurements were made every 15 μm, performing 25 measurements for each sample. The results were averaged and analyzed statistically with the Analysis Tool Pack for MS Excel.
Statistical analysis
Inter-group differences in PASI score were assessed for significance using a non-parametric one-way ANOVA. All analyses were performed using SPSS 19.0 (IBM, Chicago, IL, USA) with p<0.05 defined as significant.
Results
Expression and purification of pGP3
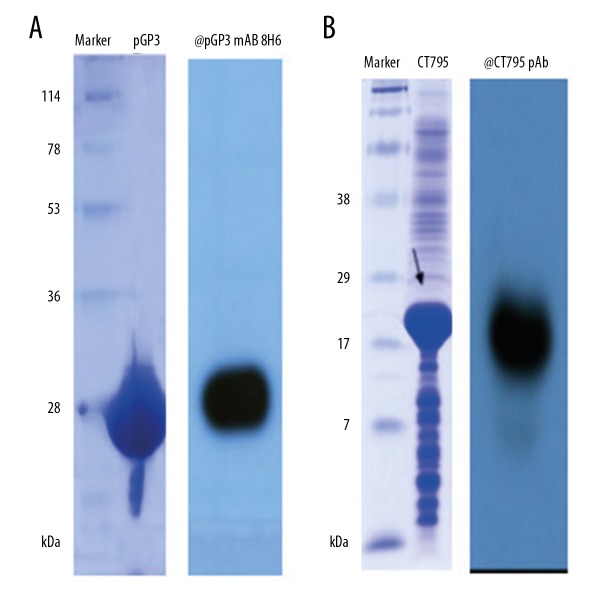
GST-pGP3 and GST-CT795 were expressed in E. coli, cleaved with precision protease, and eluted from GST beads. The purified pGP3 showed an apparent molecular weight of 28 kDa (Figure 1A) and CT795 showed a molecular weight of 18 kDa (Figure 1B). Protein purity were further confirmed by Western blot using a previously described anti-pGP3 monoclonal antibody [14] and an anti-CT795 polyclonal antibody [15], which revealed a single band at the expected molecular weight (Figure 1A, 1B).
Figure 1.
SDS-PAGE analysis of (A) purified GST-free pGP3 protein (A) and (B) CT795 protein. The approximate molecular weight of tag-free pGP3 was 28 kDa and CT795 was 18 kDa. Recombinant purified pGP3 was recognized by anti-pGP3 monoclonal antibody 8H6 (A) and CT795 was recognized by anti-CT795 polyclonal antibody (B).
Recombinant pGP3 inhibits the ability of LL-37 to induce chemotaxis
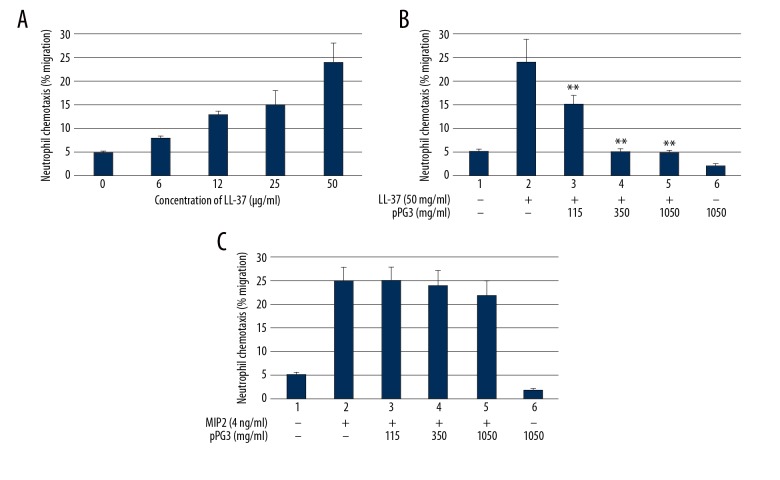
LL-37 is a multifaceted immune molecule; it shows strong, direct anti-microbial activity, and it can rapidly amplify innate immune responses, including enhancing chemotaxis [18,19] and cytokine induction [20–22]. To verify the functionality of recombinant pGP3 and begin to understand how it might affect pathogenesis of psoriasis, we evaluated its ability to prevent LL-37 from acting as a chemo-attractant of immune cells. Bone marrow-derived neutrophils from C3H/Hej mice, which are unable to respond to lipopolysaccharide, were used as reporter cells in the chemotaxis assay. When neutrophils in the upper chamber were exposed to 5 μg/ml LL-37 in the lower chamber, approximately 25% of the cells migrated into the lower chamber (Figure 2A). Significantly smaller percentages of neutrophils migrated into the lower chamber when the LL-37 was previously incubated with pGP3, and this inhibition of chemotaxis was proportional to pGP3 concentration (Figure 2B). In contrast, chemotaxis induced by the well-known chemo-attractant MIP-2 was unaffected by preincubation with pGP3 (Figure 2C), indicating that the observed effects of pGP3 were specific to LL-37.
Figure 2.
Recombinant pGP3 inhibited neutrophil chemotaxis induced by LL-37. (A) LL-37 at 50 mg/ml exhibited the strongest chemo-attractant activity, and this concentration was used in chemotaxis assays. (B) The following chemo-attractant formulations were used: LL-37 alone, LL-37 pre-incubated with various concentrations of pGP3, and pGP3 alone at 1050 mg/ml. (C) MIP-2 at 4 ng/ml was pre-incubated with various concentrations of pGP3 to test whether the inhibitory effect of pGP3 on LL-37 was specific. ** Significantly lower than with LL-37 alone, p<0.01.
The protein pGP3 inhibits development of psoriasis-like lesions in mice
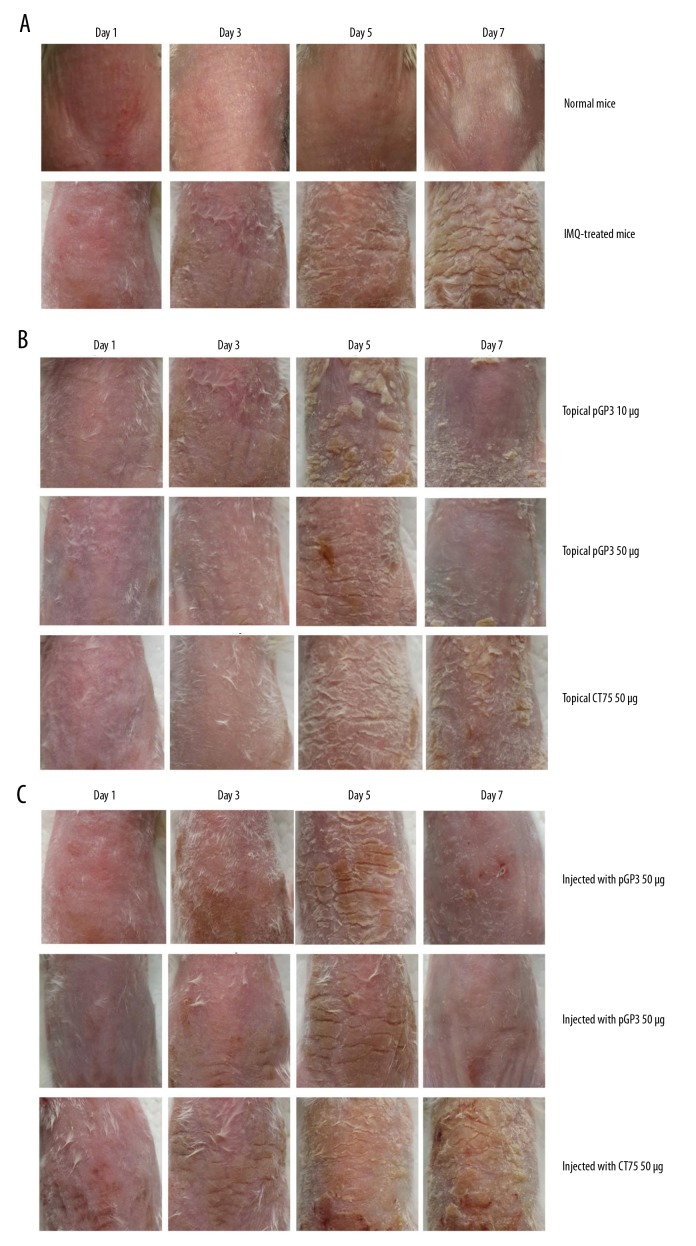
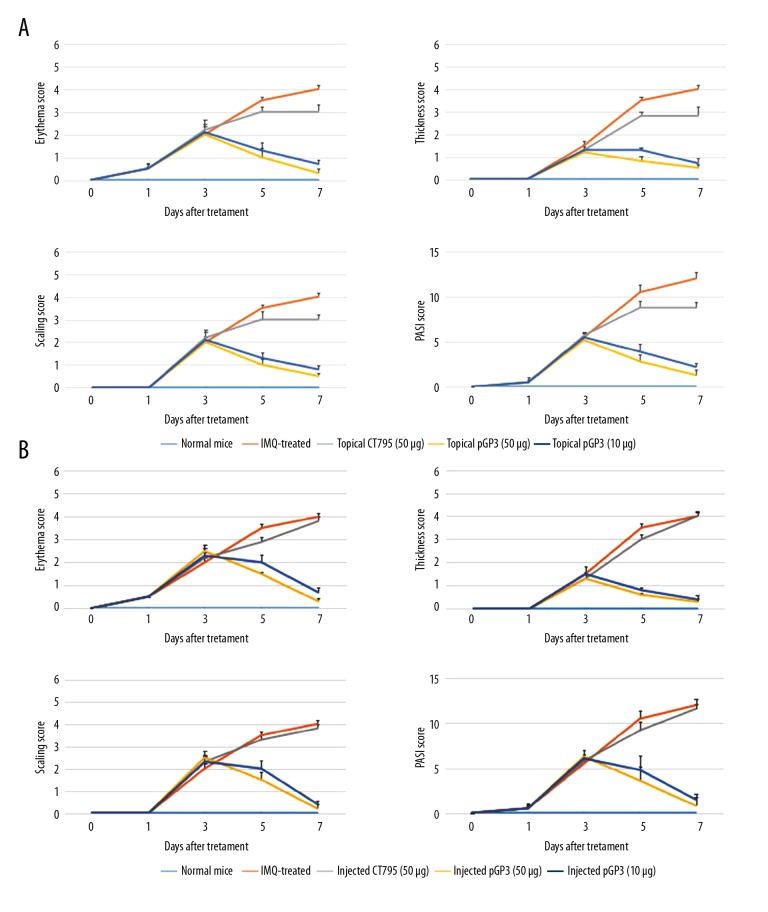
Mice were topically treated or subcutaneously injected with different pGP3 doses on shaved skin of the back during 7 consecutive days of imiquimod treatment. On day 8, punch biopsies of psoriasis-like lesion skin were taken and analyzed by histology. As expected, imiquimod treatment caused the back skin to display signs of erythema, scaling, and thickening, and these signs increased in severity until the end of the experiment (Figure 3A). These effects were much milder in animals treated topically or subcutaneously with pGP3 (Figure 3B, 3C). In control animals, PASI scores increased until reaching the maximum of 4, whereas the scores in animals treated either topically or subcutaneously with pGP3 increased in the first 3 days but decreased quickly thereafter (Figure 4A, 4B). These results suggest that pGP3 can inhibit the development of psoriasis-like lesions induced by imiquimod. The therapeutic effects of pGP3 were greater at a dose of 50 μg than at 10 μg. These effects were not observed when animals were treated with another chlamydial protein, CT795, instead of pGP3, consistent with a specific interaction between pGP3 and LL-37.
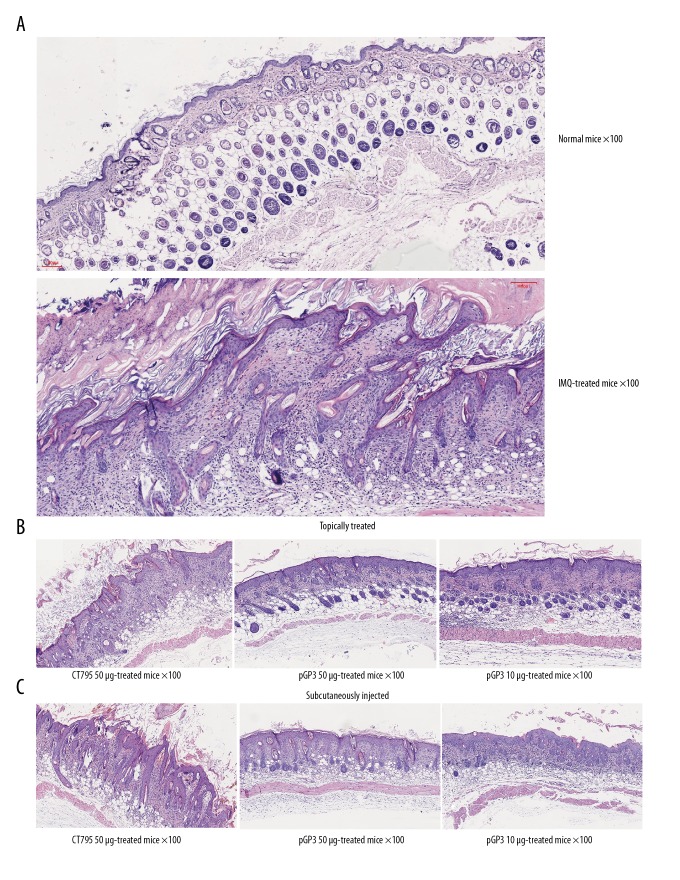
Figure 3.
Recombinant pGP3 alleviated psoriasis-like signs in imiquimod (IMQ)-treated mice. (A) Imiquimod induced psoriasis-like lesions on mouse skin. (B) Topical application of pGP3 alleviated the psoriasis-like lesions in a dose-dependent manner. In contrast, no effects were observed when the chlamydial protein CT795C instead of pGP3 was topically applied. (C) Similar results as in panel (B) were observed when pGP3 or CT795 was injected subcutaneously into the skin area treated with imiquimod.
Figure 4.
Recombinant pGP3 alleviated psoriasis-like signs in imiquimod-treated mice, based on PASI score. Mice were treated (A) topically or (B) by subcutaneous injection with pGP3 or CT795 and assessed in terms of PASI score or the individual subscores assessing erythema, thickness, or scaling.
Histology using hematoxylin-eosin showed that imiquimod induced hyperkeratosis, parakeratosis, and mononuclear cell infiltration (Figure 5). Treatment with pGP3 showed thinner epidermis (Table 1) and less hyperkeratosis, parakeratosis, and mononuclear cell infiltration. Taken together, our analyses indicate that pGP3 can inhibit development of psoriasis-like lesions in mice.
Figure 5.
Recombinant pGP3 alleviated psoriasis-like signs in imiquimod (IMQ)-treated mice, based on histology. Mice were treated with imiquimod for 7 days, then skin biopsies were taken on day 8 and stained with hematoxylin-eosin. (A) Mice treated only with imiquimod showed obvious epidermal thickening, hyperkeratosis, parakeratosis, Munro micro-abscesses and inflammation in dermis. (B) Mice treated with imiquimod and topically treated with pGP3 showed thinner epidermis and less hyperkeratosis, parakeratosis, and inflammation. (C) Similar results were observed in mice treated with imiquimod and injected subcutaneously with pGP3. Regardless of mode of administration, CT795 did not affect histopathology induced by imiquimod.
Discussion
Recent studies suggest that the activity of DCs and altered expression of anti-microbial peptides such as LL-37 play an important role in the pathogenesis of psoriasis. LL-37 forms a complex with self-DNA to activate plasmacytoid DCs in a TLR-dependent manner [10]. This activation increases production of type I IFN, leading to myeloid DC activation and consequently to Th1/Th17 differentiation and keratinocyte activation [23]. Ultimately, this induces the expression of various cytokines [6,24]. These findings imply that LL-37 may be a therapeutic target in treatment of psoriasis.
Here, we demonstrate that pGP3, a chlamydial plasmid-encoded protein that we previously showed to form a stable complex with LL-37, can reduce severity and slow progression of psoriasis-like skin inflammation in mice treated with imiquimod. Mice treated topically or subcutaneously with pGP3 showed less scaling, erythema, and thickness in a dose-dependent manner, based on PASI scores. Consistent with these results, histology showed thinner dermis and less hyperkeratosis, parakeratosis, and inflammatory cell infiltration.
Our results here already provide potential clues about how pGP3 inhibits the activity of LL-37 to reduce psoriasis-like lesions in mice. Infiltration of inflammatory cells such as neutrophils and lymphocytes is a major histopathological feature of psoriasis. LL-37 acts as a chemo-attractant of neutrophils, monocytes, and T lymphocytes. In addition, LL-37 up-regulates expression of interleukin (IL)-8 in macrophages, and neutrophils are stimulated by IL-8 to release LL-37. This further induces inflammatory cell aggregation, ultimately leading to formation of Munro micro-abscesses in psoriatic lesions. Our results suggest that pGP3 prevents LL-37 from chemo-attracting neutrophils, which reduces inflammatory cell infiltration and thereby ameliorates psoriatic lesions.
Further studies are needed to examine the therapeutic mechanism of pGP3. Since pGP3 forms a stable complex with LL-37, it may compete with self-DNA to bind LL-37, thereby reducing levels of LL-37/self-DNA complexes that initiate the IFN-α response. Since the part of pGP3 that binds LL-37 has been identified [11], a future study should examine whether this domain is required for the therapeutic effects of pGP3 against psoriasis. Future studies should also evaluate cytokine production and inflammatory cell distribution in mice treated with imiquimod with or without pGP3.
Conclusions
Here, we demonstrate that exogenous pGP3 significantly reduced the severity of psoriasis-like erythema, scaling, and thickening in mice treated with imiquimod. These effects may be mediated by binding of pGP3 to LL-37, which our in vitro assays suggest can block the ability of LL-37 to chemo-attract neutrophils. Our results confirm the potential of LL-37 as a therapeutic target in psoriasis and justify further studies to elucidate the mechanism of action of pGP3.
Footnotes
Source of support: This work was supported by the National Natural Science Foundation (to SH) through the project “Identification of Chlamydia trachomatis virulence genes and research on the mechanism of their contribution to oviduct pathology” (81301469)
References
- 1.Peric M, Koglin S, Kim SM, et al. IL-17A enhances vitamin D3-induced expression of cathelicidin antimicrobial peptide in human keratinocytes. J Immunol. 2008;181(12):8504–12. doi: 10.4049/jimmunol.181.12.8504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007;445(7130):866–73. doi: 10.1038/nature05663. [DOI] [PubMed] [Google Scholar]
- 3.Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361(5):496–509. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]
- 4.Kahlenberg JM, Kaplan MJ. Little peptide, big effects: The role of LL-37 in inflammation and autoimmune disease. J Immunol. 2013;191(10):4895–901. doi: 10.4049/jimmunol.1302005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nickoloff BJ, Nestle FO. Recent insights into the immunopathogenesis of psoriasis provide new therapeutic opportunities. J Clin Invest. 2004;113(12):1664–75. doi: 10.1172/JCI22147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dombrowski Y, Schauber J. Cathelicidin LL-37: A defense molecule with a potential role in psoriasis pathogenesis. Exp Dermatol. 2012;21(5):327–30. doi: 10.1111/j.1600-0625.2012.01459.x. [DOI] [PubMed] [Google Scholar]
- 7.Tonel G, Conrad C. Interplay between keratinocytes and immune cells – recent insights into psoriasis pathogenesis. Int J Biochem Cell Biol. 2009;41(5):963–68. doi: 10.1016/j.biocel.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 8.Larrick JW, Hirata M, Balint RF, et al. Human CAP18: A novel antimicrobial lipopolysaccharide-binding protein. Infect Immun. 1995;63(4):1291–97. doi: 10.1128/iai.63.4.1291-1297.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schauber J, Gallo RL. Expanding the roles of antimicrobial peptides in skin: Alarming and arming keratinocytes. J Invest Dermatol. 2007;127(3):510–12. doi: 10.1038/sj.jid.5700761. [DOI] [PubMed] [Google Scholar]
- 10.Lande R, Gregorio J, Facchinetti V, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449(7162):564–69. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 11.Hou S, Dong X, Yang Z, et al. Chlamydial plasmid-encoded virulence factor Pgp3 neutralizes the antichlamydial activity of human cathelicidin LL-37. Infect Immun. 2015;83(12):4701–9. doi: 10.1128/IAI.00746-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van der Fits L, Mourits S, et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 2009;182(9):5836–45. doi: 10.4049/jimmunol.0802999. [DOI] [PubMed] [Google Scholar]
- 13.Barlow PG, Li Y, Wilkinson TS, et al. The human cationic host defense peptide LL-37 mediates contrasting effects on apoptotic pathways in different primary cells of the innate immune system. J Leukoc Biol. 2006;80(3):509–20. doi: 10.1189/jlb.1005560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Z, Chen D, Zhong Y, et al. The chlamydial plasmid-encoded protein pgp3 is secreted into the cytosol of Chlamydia-infected cells. Infect Immun. 2008;76(8):3415–28. doi: 10.1128/IAI.01377-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qi M, Lei L, Gong S, et al. Chlamydia trachomatis secretion of an immunodominant hypothetical protein (CT795) into host cell cytoplasm. J Bacteriol. 2011;193(10):2498–509. doi: 10.1128/JB.01301-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neumann B, Emmanuilidis K, Stadler M, Holzmann B. Distinct functions of interferon-gamma for chemokine expression in models of acute lung inflammation. Immunology. 1998;95(4):512–21. doi: 10.1046/j.1365-2567.1998.00643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Na Takuathung M, Wongnoppavich A, Panthong A, et al. Antipsoriatic effects of Wannachawee recipe on imiquimod-induced psoriasis-like dermatitis in BALB/c mice. Evid Based Complement Alternat Med. 2018;2018:7931031. doi: 10.1155/2018/7931031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tjabringa GS, Ninaber DK, Drijfhout JW, et al. Human cathelicidin LL-37 is a chemoattractant for eosinophils and neutrophils that acts via formyl-peptide receptors. Int Arch Allergy Immunol. 2006;140(2):103–12. doi: 10.1159/000092305. [DOI] [PubMed] [Google Scholar]
- 19.Kurosaka K, Chen Q, Yarovinsky F, et al. Mouse cathelin-related antimicrobial peptide chemoattracts leukocytes using formyl peptide receptor-like 1/mouse formyl peptide receptor-like 2 as the receptor and acts as an immune adjuvant. J Immunol. 2005;174(10):6257–65. doi: 10.4049/jimmunol.174.10.6257. [DOI] [PubMed] [Google Scholar]
- 20.Byfield FJ, Kowalski M, Cruz K, et al. Cathelicidin LL-37 increases lung epithelial cell stiffness, decreases transepithelial permeability, and prevents epithelial invasion by Pseudomonas aeruginosa. J Immunol. 2011;187(12):6402–9. doi: 10.4049/jimmunol.1102185. [DOI] [PubMed] [Google Scholar]
- 21.Shaykhiev R, Sierigk J, Herr C, et al. The antimicrobial peptide cathelicidin enhances activation of lung epithelial cells by LPS. FASEB J. 2010;24(12):4756–66. doi: 10.1096/fj.09-151332. [DOI] [PubMed] [Google Scholar]
- 22.Montreekachon P, Chotjumlong P, Bolscher JG, et al. Involvement of P2X(7) purinergic receptor and MEK1/2 in interleukin-8 up-regulation by LL-37 in human gingival fibroblasts. J Periodontal Res. 2011;46(3):327–37. doi: 10.1111/j.1600-0765.2011.01346.x. [DOI] [PubMed] [Google Scholar]
- 23.Morizane S, Gallo RL. Antimicrobial peptides in the pathogenesis of psoriasis. J Dermatol. 2012;39(3):225–30. doi: 10.1111/j.1346-8138.2011.01483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu J, Mookherjee N, Wee K, et al. Host defense peptide LL-37, in synergy with inflammatory mediator IL-1beta, augments immune responses by multiple pathways. J Immunol. 2007;179(11):7684–91. doi: 10.4049/jimmunol.179.11.7684. [DOI] [PubMed] [Google Scholar]