Abstract
Background
lncRNA-ATB plays an oncogenic role in various types of malignancies, but its involvement in papillary thyroid carcinoma (PTC) cells, which is a main type of thyroid cancer, is unknown.
Material/Methods
A total of 76 patients with PTC and 28 people with normal physiological conditions were included in this study. Tumor tissues and adjacent healthy tissues were collected from PTC patients and blood was extracted from both patients and healthy controls. Expression of lncRNA-ATB in those tissues was detected by qRT-PCR. All patients were followed up for 5 years and diagnostic and prognostic values of serum lncRNA-ATB for PTC were investigated by ROC curve analysis and survival curve analysis, respectively. lncRNA-ATB overexpression PTC cell lines were established and effects of lncRNA-ATB overexpression on cell migration and invasion were investigated by Transwell cell migration and invasion assay, respectively. Effects of lncRNA-ATB overexpression on TGF-β1 expression were investigated by Western blot.
Results
lncRNA-ATB expression level was higher in tumor tissues than in adjacent healthy tissues in most PTC patients. Serum level of lncRNA-ATB was higher in cancer patients than in healthy control. Serum lncRNA-ATB can be used to accurately predict PTC and its prognosis. lncRNA-ATB overexpression promoted tumor cell migration and invasion, lncRNA-ATB overexpression showed no significant effects on TGF-β1 expression, and TGF-β1 treatment increased the expression level of lncRNA-ATB.
Conclusion
Upregulation of lncRNA-ATB by TGF-β1 promotes migration and invasion of PTC cells.
MeSH Keywords: Carcinoma, Papillary; Latent TGF-beta Binding Proteins; RNA, Long Noncoding; Transcellular Cell Migration
Background
Thyroid cancer is a group of malignancies that mainly originate from follicular cells, which are the major components of thyroid unicellular epithelium [1]. Although efforts have been made to prevent and treat thyroid cancer, the incidence of this disease has tripled during the last several decades and the age at onset is becoming younger and younger [2]. Papillary thyroid carcinoma (PTC) is the major type of thyroid cancer and accounts for more than 80% of all cases [3]. Although treatment of early stages of PTC has achieved satisfactory outcomes and high overall survival rate [4], many patients are diagnosed at advanced stages and the best time for radical treatment is lost [5]. In addition, once distant metastasis occurs, the recurrence rate of this disease is unacceptably high, even after surgical resection [6]. Therefore, early diagnosis and treatment is still critical for the treatment of PTC.
Besides protein-encoding messenger RNAs (mRNAs), the human genome also transcribes a large set of non-coding RNAs (ncRNAs) with pivotal roles in critical physiological processes and pathological changes [7]. As a subtype of ncRNAs composed of more than 200 nucleotides, long non-coding RNAs, or lncRNAs, are major players in pathogenesis of a variety of different types of human disease, including different types of malignancies [8]. lncRNA-ATB has been proved to play oncogenic roles in several types of cancers, such as breast cancer [9] and colorectal cancer [10]. Its involvement in PTC is known. Therefore, our study aimed to investigate the functionality of lncRNA-ATB in pathogenesis of PTC with an expectation of providing references for the diagnosis and treatment of this disease.
Material and Methods
Subjects
A total of 44 patients with papillary thyroid carcinoma (PTC) were selected in our hospital from October 2010 to October 2012. Those patients included 27 males and 17 females, and age ranged from 25 to 76 years, with an average age of 46.2±8.1 years. PTC was diagnosed according to the standards established by the World Health Organization. Patients with other types of thyroid diseases were excluded. All patients received surgical resection of the primary tumor, and tumor tissues and adjacent healthy tissues within 2 cm around the tumors were collected during the operation. None of them received radiotherapy or chemotherapy before admission. At the same time, 38 healthy people with similar age and sex distribution were also enrolled to serve as a control group. A sample of whole blood was drawn from each participant on the day of admission. Blood was transferred to an anticoagulant tube, followed by centrifugation at 3000 rpm for 20 min to collect plasma. This study was approved by the Ethics Committee of our hospital. All patients signed informed consent.
Cell lines and cell culture
Human PTC cell lines IHH-4 and HTH83 were provided by the American Type Culture Collection (ATCC, USA). Cells were cultured in DMEM containing 10% FBS (Invitrogen, Carlsbad, CA), 100 U/ml streptomycin (Invitrogen, Carlsbad, CA) and 100 mg/ml penicillin G (Invitrogen, Carlsbad, CA) in an incubator (37°C, 5% CO2). Cells were collected during logarithmic growth phase for subsequent experiments. Serum was not added in case of drug treatment.
Construction of lncRNA-ATB expression vector and transfection
lncRNA-ATB expression vector was constructed by inserting lncRNA-ATB cDNA into pEGFPC3 (Clontech, Palo Alto, CA). Cells of each cell line were cultured overnight to reach 70–80% confluence, and then 10 nM vectors were transfected into 5×105 cells using Lipofectamine 2000 reagent (11668-019, Invitrogen, Carlsbad, CA). Empty pEGFPC vector was used as a negative control (NC).
Transwell migration and invasion assay
Briefly, the upper chamber (BD Biosciences, San Jose, USA) was filled with serum-free RPMI-1640 medium containing 5×104 cells, and RPMI-1640 medium (Thermo Fisher Scientific, USA) containing 20% FCS (Sigma-Aldrich, USA) was used to fill the lower chamber. Membranes were collected after incubation for 24 h and stained with 0.5% crystal violet for 20 min. Stained cells were counted under a light microscope (×400, Olympus, Tokyo, Japan). Before cell invasion assay, the upper chamber was pre-coated with Matrigel (356234, Millipore, Billerica, USA).
Real-time quantitative PCR
In vitro cultured cells and plasma were mixed with Trizol reagent (Invitrogen, USA) to extract total RNA. Tumor tissues and adjacent healthy tissues were ground in liquid nitrogen before the addition of Trizol reagent. Reverse transcription was performed using RNA samples as a template to synthesize cDNA. SYBR® Green Real-Time PCR Master Mix (Thermo Fisher Scientific, USA) was used to prepare PCR reaction system. The following primers were used in PCR reactions: 5′-TCTGGCTGAGGCTGGTTGAC-3′ (forward) and 5′-ATCTCTGGGTGCTGGTGAAGG-3′ (reverse) for lncRNA-ATB; 5′-GACCTCTATGCCAACACAGT-3′ (forward) and 5′-AGTACTTGCGCTCAGGAGGA-3′ (reverse) for β-actin. PCR reaction conditions were: 95°C for 50 s, followed by 40 cycles of 95°C for 15 s and 60°C for 45 s. All data were processed using 2−ΔΔCT method. Relative expression level of lncRNA-ATB was normalized to endogenous control β-actin.
Western blot
Total protein extraction was performed using RIPA solution (Thermo Fisher Scientific, USA) and protein quality was tested by BCA assay. Protein samples (20 μg from each sample) were subjected to 12% SDS-PAGE gel electrophoresis to separate different proteins based on molecular weight, followed by transfer to PVDF membranes. Membranes were blocked by incubating with 5% skimmed milk for 1.5 h at room temperature, followed by incubation with rabbit anti-TGF-β1 (1: 2000, ab92486, Abcam), and anti-GAPDH primary antibody (1: 1000, ab8245, Abcam) overnight at 4°C. The next day, membranes were further incubated with anti-rabbit IgG-HRP secondary antibody (1: 1000, MBS435036, MyBioSource) at room temperature for 1.5 h. ECL (Sigma-Aldrich, USA) was then added to develop signals. Signals were detected by use of a MYECL™ Imager (Thermo Fisher Scientific, USA) and relative expression level of TGF-β1 was normalized to endogenous control GAPDH using Image J software.
Statistical analysis
SPSS19.0 (SPSS Inc., USA) was used to process all the data. Count data, such as sex and other basic information, were processed by chi-square test. Measurement data, such as RNA and protein expression levels, are expressed as (x±s). The t test was used for comparisons between 2 groups, and one-way analysis of variance was used for comparisons among multiple groups. p<0.05 indicated a difference with statistical significance.
Results
Expression of lncRNA-ATB in tumor tissues and healthy tissues of patients with PTC
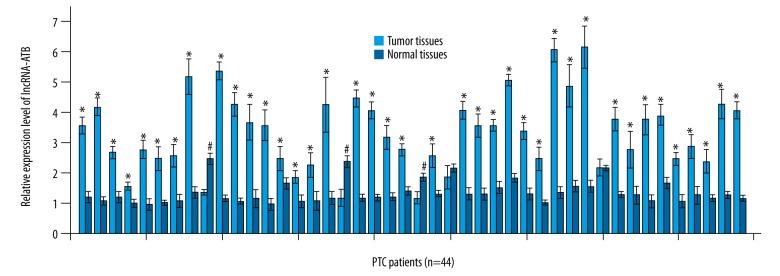
Expression levels of lncRNA-ATB in tumor tissues and healthy tissues of 44 patients with PTC were measured by qRT-PCR and normalized to endogenous control β-actin. As shown in Figure 1, expression level of lncRNA-ATB was significantly higher in tumor tissues than in healthy tissues in 39 out of 44 patients, accounting for 88.6% of all the patients (p<0.05). In contrast, higher expression level of lncRNA-ATB in normal tissues than in tumor tissues was only observed in 3 patients, accounting for 6.8%. No significant difference in expression level of lncRNA-ATB between normal tissues and tumor tissues was found in the other 3 patients. These data suggest that upregulation of lncRNA-ATB is very likely to be involved in the pathogenesis of PTC.
Figure 1.
Expression of lncRNA-ATB in tumor tissues and healthy tissues of patients with PTC. * Compared with adjacent healthy tissues, p<0.05; # compared with tumor tissues, p<0.05.
Comparison of plasma level of circulating lncRNA-ATB between PTC patients and healthy controls and the diagnostic and prognostic values
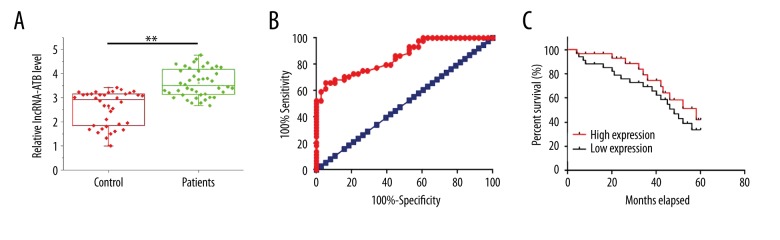
Levels of circulating lncRNA-ATB in plasma of PTC patients and healthy controls were measured by qRT-PCR. As shown in Figure 2A, plasma levels of lncRNA-ATB were significantly higher in PTC patients than in controls (p<0.05). Therefore, ROC curve analysis was further performed to evaluate the diagnostic value of plasma lncRNA-ATB for PTC. As shown in Figure 2B, the area under the curve was 0.8580 with 95% confidence interval of 0.7806 to 0.9353 (p<0.0001). According to the median plasma level of lncRNA-ATB, patients were divided into a high expression group and a low expression group. All patients were followed up for 5 years or until their deaths. Based on the survival data collected during follow-up, survival curves were plotted for low- and high-expression groups using Kaplan-Meier method and were compared by the log rank t test. As shown in Figure 2C, overall survival rate of patients with high plasma level of lncRNA-ATB was significantly lower than that of patients with low plasma level of lncRNA-ATB (p<0.0001).
Figure 2.
Comparison of plasma level of circulating lncRNA-ATB between PTC patients and healthy controls and the diagnostic and prognostic values. (A) Comparison of plasma level of circulating lncRNA-ATB between PTC patients and healthy controls; (B) ROC curve analysis of the diagnostic value of lncRNA-ATB for PTC, red line indicated diagnostic curve and blue line was the line of identity; (C) Comparison of survival curves of high and low serum lncRNA-ATB level groups. * p<0.05.
Correlation between plasma levels of lncRNA-ATB and clinical data of PTC patients
The chi-square test was used to analyze the correlations between plasma levels of lncRNA-ATB and clinical data of PTC patients. As shown in Table 1, plasma levels of lncRNA-ATB were not significantly correlated with sex, age, of patients’ living habits, including smoking and drinking (p>0.05). However, a significant correlation was found between plasma levels of lncRNA-ATB and distant tumor metastasis.
Table 1.
Correlation between plasma levels of lncRNA-ATB and clinical data of PTC patients.
| Items | Groups | Cases | High expression | Low expression | χ2 | p Value |
|---|---|---|---|---|---|---|
| Sex | Male | 27 | 15 | 12 | 0.8627 | 0.353 |
| Female | 17 | 7 | 10 | |||
| Age | >45 (years) | 25 | 14 | 11 | 0.8337 | 0.361 |
| <45 (years) | 19 | 8 | 11 | |||
| Distant metastasis | Yes | 28 | 18 | 10 | 6.2857 | 0.012 |
| No | 16 | 4 | 12 | |||
| Smoking | Yes | 21 | 10 | 11 | 0.0911 | 0.763 |
| No | 23 | 12 | 11 | |||
| Drinking | Yes | 20 | 12 | 8 | 1.4667 | 0.226 |
| No | 24 | 10 | 14 |
Effects of lncRNA-ATB overexpression on migration and invasion of PTC cells
LncRNA-ATB overexpression human PTC cell lines IHH-4 and HTH83 were established and confirmed by measuring the expression level of lncRNA-ATB using qRT-PCR (data not shown). We found that plasma lncRNA-ATB level is closely correlated with occurrence of distant tumor metastasis in PTC patients. Therefore, the effects of lncRNA-ATB overexpression on migration and invasion of PTC cells were investigated. As shown in Figure 3, compared with control cells or negative control cells, lncRNA-ATB overexpression significantly promoted cell migration (p<0.05, Figure 3A) and invasion (p<0.05, Figure 3B) of the 2 PTC cell lines.
Figure 3.
Effects of lncRNA-ATB overexpression on migration and invasion of PTC cells. (A) Effects of lncRNA-ATB overexpression on migration of PTC cells; (B) Effects of lncRNA-ATB overexpression on invasion of PTC cells. * p<0.05.
TGF-β1 is an upstream positive regulator of lncRNA-ATB
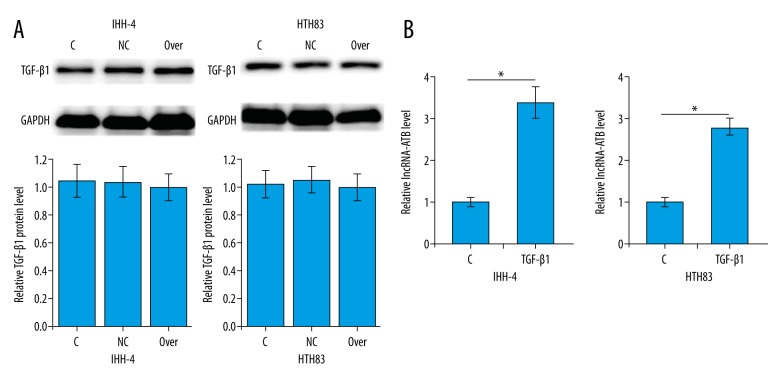
TGF-β1 is involved in the regulation of cancer cell migration and invasion in a variety types of human malignancies, including PTC. Therefore, the possible interactions between TGF-β1 and lncRNA-ATB in PTC cells were explored. As shown in Figure 4A, lncRNA-ATB overexpression showed no significant effect on the expression of TGF-β1 in cells of the 2 PTC cell lines. Treatment with TGF-β1 (10 ng/ml, Sigma-Aldrich) for 1 h significantly promoted lncRNA-ATB expression (p<0.05, Figure 4B). Therefore, TGF-β1 is very likely to be an upstream positive regulator of lncRNA-ATB in PTC.
Figure 4.
TGF-β1 is an upstream positive regulator of lncRNA-ATB. (A) Effects of lncRNA-ATB overexpression on TGF-β1 expression; (B) Effects of TGF-β1 treatment on lncRNA-ATB expression. * p<0.05.
Discussion
Based on recent research progress in the pathogenesis of PTC, the onset, development, and progression of PTC requires the involvement of different lncRNAs. lncRNA HOTAIR is significantly upregulated in PTC tumor tissues compared with adjacent healthy control tissue, and high expression level of HOTAIR predicts the progression of cancer [11]. In contrast, lncRNA GAS8-AS1 showed a downregulated expression pattern during the development of PTC and upregulation of GAS8-AS1 inhibited tumor growth [12]. lncRNA-ATB, as an oncogenic long non-coding RNA, usually shows upregulated expression patterns in human cancers [9,10]. Consistent with previous studies, in our study, higher expression level of lncRNA-ATB in tumor tissues than in normal tissues was detected in about 90% of all patients. These data suggest that upregulation of lncRNA-ATB expression is very likely to be involved in the pathogenesis of PTC.
The development of human disease is usually accompanied by changes of certain substances in the blood, and detection of those substances may provide references for the diagnosis and prognosis of certain diseases [13]. In this study, plasma levels of lncRNA-ATB were found to be significantly higher in PTC patients than in healthy people. In addition, ROC curve analysis showed that lncRNA-ATB can be used to effectively distinguish PTC patients from healthy controls. A high plasma level of lncRNA-ATB also predicted short survival time. These data suggest that lncRNA-ATB can serve as a promising diagnostic and prognostic biomarker for PTC. Various factors such as aging [14], tobacco consumption [15], and alcohol abuse [16] can modify the expression pattern of certain lncRNAs. In our study, plasma levels of lncRNA-ATB showed no significant correlations with age, sex, or living habits, including drinking and smoking, indicating the high reliability of lncRNA-ATB in the diagnosis and prognosis of PTC.
Our study found that plasma levels of lncRNA-ATB were closely correlated with the occurrence of tumor distant metastasis, indicting the potential roles of lncRNA-ATB in the regulation of cancer cell migration and invasion. In a study of renal cell carcinoma, Xiong et al. reported that overexpression of lncRNA-ATB in tumor tissues promoted the migration and invasion of cancer cells [17]. In another study, involvement of lncRNA-ATB in the regulation of migration and invasion of cancer cell was also observed during the development of gastric cancer [18]. In our study, lncRNA-ATB overexpression significantly promoted the cell migration and invasion of the 2 PTC cell lines, indicating the possible enhancing effects of lncRNA-ATB on tumor metastasis in PTC.
The role of TGF-β1 as a key player in the regulation of cancer cell migration and invasion has been well established. TGF-β signaling, which inhibits cell proliferation and promotes cell migration and invasion, has contrasting roles in cancer [19]. A recent study reported that TGF-β signaling induced the expression of lncRNA-ATB to promote invasion-metastasis cascade in hepatocellular carcinoma [20]. In our study, lncRNA-ATB overexpression showed no significant effect on the expression of TGF-β1 in 2 PTC cell lines, while treatment with TGF-β1 significantly promoted the expression of lncRNA-ATB. These data suggest that TGF-β1 is very likely to be an upstream activator of lncRNA-ATB in PTC.
Conclusions
In conclusion, lncRNA-ATB expression was higher in tumor tissues than in adjacent healthy tissues in most PTC patients. Serum level of lncRNA-ATB was higher in cancer patients than in healthy controls. Serum lncRNA-ATB may serve as a promising biomarker for the diagnosis and prognosis of PTC. lncRNA-ATB overexpression promoted tumor cell migration and invasion. lncRNA-ATB overexpression showed no significant effects on TGF-β1 expression, while TGF-β1 treatment increased the expression level of lncRNA-ATB. Therefore, we conclude that lncRNA-ATB can be upregulated by TGF-β1 to promote migration and invasion of PTC cells. Our study is limited by its small sample size. Future studies with larger sample sizes are needed to verify the conclusions of this study.
Footnotes
Source of support: Departmental sources
Conflicts of interests
The authors declare that they have no conflicts of interests.
References
- 1.Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014;159(3):676–90. doi: 10.1016/j.cell.2014.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olaleye O, Ekrikpo U, Moorthy R, et al. Increasing incidence of differentiated thyroid cancer in South East England: 1987–2006. Eur Arch Otorhinolaryngology. 2011;268(6):899–906. doi: 10.1007/s00405-010-1416-7. [DOI] [PubMed] [Google Scholar]
- 3.Londero SC, Krogdahl A, Bastholt L, et al. Papillary thyroid microcarcinoma in Denmark 1996–2008: A national study of epidemiology and clinical significance. Thyroid. 2013;23(9):1159–64. doi: 10.1089/thy.2012.0595. [DOI] [PubMed] [Google Scholar]
- 4.LiVolsi VA. Papillary thyroid carcinoma: An update. Modern Pathology. 2011;24(S2):S1. doi: 10.1038/modpathol.2010.129. [DOI] [PubMed] [Google Scholar]
- 5.Hay ID, Thompson GB, Grant CS, et al. Papillary thyroid carcinoma managed at the Mayo Clinic during six decades (1940–1999): Temporal trends in initial therapy and long-term outcome in 2444 consecutively treated patients. World J Surg. 2002;26(8):879–85. doi: 10.1007/s00268-002-6612-1. [DOI] [PubMed] [Google Scholar]
- 6.Ito Y, Miyauchi A, Kudo T, et al. The effectiveness of prophylactic modified neck dissection for reducing the development of lymph node recurrence of papillary thyroid carcinoma. World J Surg. 2017;41(9):2283–89. doi: 10.1007/s00268-017-4023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mattick JS, Makunin IV. Non-coding RNA. Hum Mol Genet. 2006;15(Suppl 1):R17–29. doi: 10.1093/hmg/ddl046. [DOI] [PubMed] [Google Scholar]
- 8.Cui Z, Ren S, Lu J, et al. The prostate cancer-up-regulated long noncoding RNA PlncRNA-1 modulates apoptosis and proliferation through reciprocal regulation of androgen receptor[C] Urol Oncol. 2013;31(7):1117–23. doi: 10.1016/j.urolonc.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 9.Shi SJ, Wang LJ, Yu B, et al. LncRNA-ATB promotes trastuzumab resistance and invasion-metastasis cascade in breast cancer. Oncotarget. 2015;6(13):11652. doi: 10.18632/oncotarget.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iguchi T, Uchi R, Nambara S, et al. A long noncoding RNA, lncRNA-ATB, is involved in the progression and prognosis of colorectal cancer. Anticancer Res. 2015;35(3):1385–88. [PubMed] [Google Scholar]
- 11.Zhu H, Lv Z, An C, et al. Onco-lncRNA HOTAIR and its functional genetic variants in papillary thyroid carcinoma. Sci Rep. 2016;6:31969. doi: 10.1038/srep31969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin Y, Sun W, Zhang H, et al. LncRNA GAS8-AS1 inhibits cell proliferation through ATG5-mediated autophagy in papillary thyroid cancer. Endocrine. 2018;59(3):555–64. doi: 10.1007/s12020-017-1520-1. [DOI] [PubMed] [Google Scholar]
- 13.Barbieri CE, Chinnaiyan AM, Lerner SP, et al. The emergence of precision urologic oncology: A collaborative review on biomarker-driven therapeutics. Eur Urol. 2017;71(2):237–46. doi: 10.1016/j.eururo.2016.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grammatikakis I, Panda AC, Abdelmohsen K, et al. Long noncoding RNAs (lncRNAs) and the molecular hallmarks of aging. Aging (Albany NY) 2014;6(12):992. doi: 10.18632/aging.100710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maccani MA, Knopik VS. Cigarette smoke exposure-associated alterations to non-coding RNA. Front Genet. 2012;3:53. doi: 10.3389/fgene.2012.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warden AS, Mayfield RD. Gene expression profiling in the human alcoholic brain. Neuropharmacology. 2017;122:161–74. doi: 10.1016/j.neuropharm.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiong J, Liu Y, Jiang L, et al. High expression of long non-coding RNA lncRNA-ATB is correlated with metastases and promotes cell migration and invasion in renal cell carcinoma. Jpn J Clin Oncol. 2016;46(4):378–84. doi: 10.1093/jjco/hyv214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Y, Wei G, Xia H, et al. Long noncoding RNA-ATB promotes cell proliferation, migration and invasion in gastric cancer. Mol Med Rep. 2018;17(1):1940–46. doi: 10.3892/mmr.2017.8077. [DOI] [PubMed] [Google Scholar]
- 19.Akhurst RJ, Derynck R. TGF-β signaling in cancer – a double-edged sword. Trends Cell Biol. 2001;11(11):S44–51. doi: 10.1016/s0962-8924(01)02130-4. [DOI] [PubMed] [Google Scholar]
- 20.Yuan J, Yang F, Wang F, et al. A long noncoding RNA activated by TGF-β promotes the invasion-metastasis cascade in hepatocellular carcinoma. Cancer Cell. 2014;25(5):666–81. doi: 10.1016/j.ccr.2014.03.010. [DOI] [PubMed] [Google Scholar]