Abstract
Prostate cancer remains a lethal disease. Preclinical cancer models that accurately represent the tumors of the patients they are intended to help are necessary to test potential therapeutic approaches and to better translate research discoveries. However, research in the prostate cancer field is hampered by the limited number of human cell lines and xenograft models, most of which do not recapitulate the human disease seen in the clinic today. This work reviews the recent advances in human patient-derived xenograft, organoid, and other explant models to address this need. In contrast to other tumor streams, the prostate cancer field is challenged by this approach, yet despite the limitations, patient-derived models remain an integral component of the preclinical testing pathway leading to better treatments for men with prostate cancer.
There has been a global effort to develop and use preclinical models for research discovery and drug testing that more closely reflect the patients that are seen in the clinic today. Although a limited number of cell and xenograft models have served the field well, especially LNCaP, which has more than 7000 publications referenced in PubMed, more recent and rapid progress in the clinic has prompted the imperative to update our preclinical models. The central role of the androgen receptor (AR) pathway has been established without doubt—AR overexpression is a main driver of progression for most patients to castration-resistant prostate cancer (CRPC) (Knudsen and Penning 2010). Agents more effective than first-line androgen deprivation agents are now used to block this pathway, resulting in widespread use of abiraterone and enzalutamide in the clinic, yet few models incorporate patient lines or tissues after these treatments alone or in combination. This work aims to review the patient-derived models of human prostate cancer that we commonly use, especially contemporary ones, and identify the needs for the field and how we might meet these challenges.
WHAT ARE THE CHALLENGES FOR THE PROSTATE CANCER RESEARCH FIELD?
Profiling the genomes, transcriptomes, and methylomes of prostatic tumors has resulted in substantive sets of data that have advanced our understanding of the molecular features of prostate cancer (Taylor et al. 2010; Lalonde et al. 2014; Robinson et al. 2015; The Cancer Genome Atlas Research Network 2015; Beltran et al. 2016). For example, new pathways to target were identified, new mechanisms of resistance were uncovered, and the overall complexity of the disease, attributable to tumor heterogeneity, was realized (Lalonde et al. 2014; Beltran et al. 2016). Each and all of these discoveries influence patient diagnosis, management, and treatment, but the development of preclinical models has largely failed to keep pace with these changes. Identifying actionable targets is one part of the process, but models must enable proof-of-concept testing to generate new knowledge that can be translated toward a better outcome for the patients.
For example, current models do not comprehensively span the course of disease from localized castrate-sensitive to advanced incurable disease and, overall, there is a paucity of disease stage–specific models for clinical investigation. Thus, models of human cell lines from metastatic lesions are inadequate tools to investigate up-front therapies from men with high-risk localized disease. This example shows one of the reasons for the high failure rate of new candidate therapies once they enter clinical trials because their utility has been guided by inappropriate preclinical models.
The acquisition of specimens for our models is therefore essential to the development of preclinical models and their utility as tools for discovery and validation. Technologies such as bioengineered platforms and tissue scaffolds have also revolutionized our biological models per se, enabling the transition from 2D to 3D cultures and development of advances such as “organ-on-a-chip.”
Altogether it is an exciting time to develop new models of human prostate cancer. Prostatic tumors are notoriously difficult to culture, engraft, and/or serially transplant, but in the following sections we review predominantly how the field has responded to the challenges of developing contemporary models using human patient specimens (see Fig. 1). The purpose and focus of this review is confined to patient-derived models, and references to mouse models are made only in the absence of any human model equivalent; however, a comprehensive review on mouse models of prostate cancer can be found in Arriaga and Abate-Shen (2017).
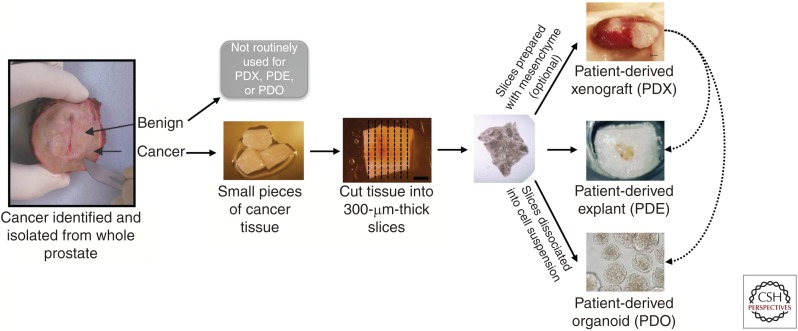
Figure 1.
Common patient-derived prostate cancer models for research discovery and translation. Patient-derived models are established from human prostate cancer tissue, either from the prostate or metastatic sites. From radical prostatectomy specimens, benign tissue can also be obtained, but these samples are not routinely used for patient-derived models. Cancer tissues are cut into small pieces either manually or by precision cutting. Tissue pieces or slices are then prepared for patient-derived xenografts (PDXs; often including supportive mesenchyme), patient-derived explants (PDEs; grown on gelatin sponges), or patient-derived organoids (PDOs; grown in 3D culture with dissociated cells). In addition to fresh primary specimens, PDE and PDO experiments can also be performed using PDX-derived tissues, expanding the capability of the models by allowing long-term propagated tumors to be studied in a high-throughput manner. Alternatively, PDOs can be transplanted back into mice to be grown as PDXs, allowing long-term growth in vivo.
PATIENT-DERIVED PROSTATE CANCER MODELS
There are only a few successful approaches to using patient-derived specimens for preclinical research and discovery sections. Once specimens are obtained from the patient they can be analyzed fresh or preserved for subsequent analysis—for example, by freezing or fixation. This source of material has yielded a great deal of genomic data by providing snapshots of information about prostatic tumors at the time when the samples were taken. Over the often-lengthy course of disease, this has involved systematic sampling from men with localized through to advanced disease and at death (Gundem et al. 2015; Beltran et al. 2016). Many samples of localized prostate cancer are routinely obtained at surgery from cohorts of men who have a radical prostatectomy. Over the last decade, we have seen a subtle shift in the range of these specimens resulting in fewer low-grade tumors because of the uptake of active surveillance (Bruinsma et al. 2016). Previously, metastatic specimens were difficult to obtain and were predominantly from the bone, but with the advent of palliative surgery and rapid autopsy programs, more soft tissue metastases have become available for analyses.
However, with such studies the measurements are static. Therefore, programs were devised to collect specimens from single patients to track the course of their disease and they have been insightful, especially for identifying mechanisms of treatment resistance. The static versus dynamic aspect of models is an important consideration and in the sections below, we address the use of common models that attempt to provide functional information to complement the omics data (xenografts, explants, organoid, and 3D cultures, as summarized in Fig. 1). Finally, it is prescient to remember that the specimens are most likely to be useful when applied to a question relevant to the time of disease from which the specimen was obtained (see Fig. 2). Therefore, if the research question focuses on localized tumors from men with castrate-sensitive and hormone-naïve disease, it would be inappropriate and uninformative to use a metastatic human cell line model, such as LNCaP.
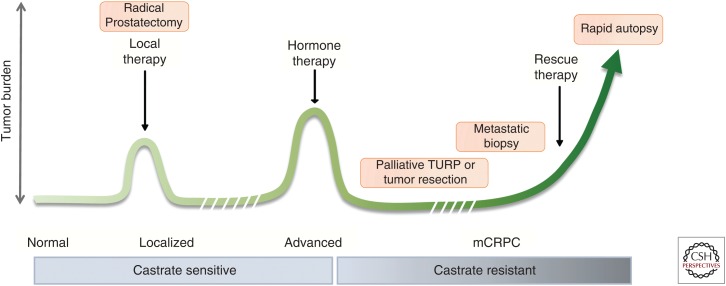
Figure 2.
Human specimens for patient-derived models are acquired at specific stages of progression and can include localized or advanced metastatic disease. Localized, castrate-sensitive prostate cancer is most commonly obtained from radical prostatectomy specimens. Advanced prostate cancer tissues can be obtained from palliative resection for locally recurrent disease or at metastatic sites either by biopsy or tissue collection at autopsy; the latter samples represent metastatic castration-resistant prostate cancer (mCRPC). For studies in which the patient-derived specimens are taken at one time only, it is important to frame the question around this stage and not another one subsequent or prior. Although it is more challenging, the use of longitudinal specimens for modeling is even more desirable and can provide unique patient-based (personalized) information. TURP, Transurethral resection of the prostate.
PATIENT-DERIVED XENOGRAFTS (PDXs)
PDXs are high throughput for many other tumor streams (Gao et al. 2015), but for prostate cancer, they are time consuming and laborious. The take rates are low, owing to the reasons we have previously summarized that include low tumor proliferation, lack of stroma, and site of xenograft (Risbridger and Taylor 2016). Nevertheless, PDXs are considered to be a gold standard preclinical test and provide significant advantage because of their inherent ability to accurately recapitulate the tumor of origin, allowing them to be preserved for subsequent dynamic drug response testing. As a result, worldwide banks and consortia have built collections of these PDX models of human cancer for preclinical studies (Gao et al. 2014; Garralda et al. 2014; Hidalgo et al. 2014; see the www.europdx.eu project website). However, there are relatively few prostate cancer PDXs available in biobanks. For example, the entire Jackson Laboratory for Genomic Medicine (JAX Laboratories) PDX catalog contains only six prostatic PDXs out of a total of 463, equaling ∼1% of the total. The notable exception is the Living Tumor Laboratory in Vancouver where there are approximately 45 patient-derived prostatic tumor lines available for translational cancer research. At both Vancouver and Monash, the cancer tissue xenografts are commonly engrafted to the subrenal capsule, whereas the subcutaneous site is frequently favored for other tumor types, particularly when high-throughput techniques are used (Lawrence et al. 2013; Gao et al. 2015). In general, using the subrenal site for engraftment leads to a relatively lower throughput, which prompted other groups to refine orthotopic models of PDX in which there is the added advantage of the PDX being located in a prostatic microenvironment (Saar et al. 2015; and see Risbridger 2015).
The need for disease stage-specific PDX may appear to be obvious, but the reality of generating PDXs from localized, treatment-naïve specimens through to relapsing, metastatic, and CRPC specimens has proved challenging. For localized specimens, there are only a few PDX lines, probably owing to the low rate of cell proliferation in the tissues of a localized tumor. Radical prostatectomy is a common source of specimen acquisition for localized PDX and may include latent tumors from men at low-to-moderate risk of disease progression who might also have been candidates for active surveillance. In this case, the latency of the tumor is likely to impede development of a transplantable tumor. Interestingly, Lin et al. (2014) reported that the mean time for engraftment is 22 months and this is an experience similar to our own at Monash. Nevertheless, localized specimens and radical prostatectomy in particular are a rich source of material and have led to further refinements such as the use of tissue slices (Zhao et al. 2010) and orthotopic engraftment (Saar et al. 2015).
The serial transplantability of the localized specimens has not been a subject of systematic review, but it would be timely to investigate if there is a difference in engraftment of PDX of tumors from men with low versus high D’Amico scores. At Monash, we have generated many (hundreds) of first-generation PDXs, but only a handful of serially transplantable ones, and a review and correlation to patient outcome could be insightful. In another study, the long-term propagation of a prostate adenocarcinoma (LTL331) in castrate host mice was particularly interesting because of the subsequent emergence of a neuroendocrine subline (LTL331R). This is the first demonstration of progression from adenocarcinoma to neuroendocrine prostate cancer in a PDX model (Lin et al. 2015), and it may mimic a clinical progression in some patients with advanced disease in which there is divergent clonal evolution of castration-resistant neuroendocrine prostate cancer (CRPC-NE) tumors that have neuroendocrine features and are treatment resistant (Beltran et al. 2016). Nevertheless, first-generation PDXs from localized specimens can provide new research discoveries with an impact for patients as we reviewed (Risbridger and Taylor 2016). For example, through the use of first-generation PDXs from BRCA2 carrier men who are at high risk of progressing, we showed that the presence of intraductal carcinoma of the prostate (IDCP) pathology predicted a worse outcome versus IDCP-negative patients (Risbridger et al. 2015).
PDX establishment from men whose disease has progressed and is advanced and metastatic is more common and successful, partly because the growth rate is not as limiting. Xenograft studies spanning decades of research have used the LNCaP line derived from a left supraclavicular lymph node metastasis from a 50-year-old man (LNCaP clone FGC ATCC CRL-1740) and the VCaP line derived from vertebral metastasis (ATCC CRL-2876). Both lines were deposited in 1977 with American Type Culture Collection (ATCC) and are mostly used for first-generation xenograft studies. Vessella and colleagues were one of the first groups to establish serially transplantable PDX models from prostate cancer metastases, known as the LuCaP series (Laitinen et al. 2002; True et al. 2002; Saramaki et al. 2006). LuCaP 23.1 and LuCaP 35 were lymph node metastases established as subcutaneous xenografts, with tumor doubling times of 11 and 18 days and take rates of 100% and 87% (Ellis et al. 1996; Buhler 1997). When injected into the tibia of severe combined immunodeficiency (SCID) mice, LuCaP 23.1 produces osteoblastic reactions, whereas LuCaP 35 produces osteolytic responses (Corey et al. 2002). LuCaP PDXs have also passed through spheroid cultures and back into mice and faithfully retain the original phenotype (Saar et al. 2014; Valta et al. 2016). Bone metastases offer another potential source of tissue for prostate cancer PDXs. Two particular lines, MDA PCa 118a and MDA PCa 118b, were established as subcutaneous PDXs, providing a model of androgen-independent prostate cancer that induces a robust osteoblastic reaction in bonelike matrix and soft tissue (Li et al. 2008). Improvements to bone metastatic PDXs include recent modifications using scaffold/matrices (Talukdar et al. 2011; Holzapfel et al. 2013, 2014; Berner et al. 2015) coupled with better imaging modalities to investigate the tumor biology (Tse et al. 2015).
The sources of tissue vary considerably and subsequently affect the quality of the specimens used for engraftment. Nevertheless, poor quality specimens such as those obtained by transurethral resection of the prostate (TURP) can be grafted subrenally with moderate success (Lawrence et al. 2015). Higher quality specimens from rapid autopsy programs are becoming available and these are an excellent source of PDX material, but the take rates are surprisingly low at <25%–30% (Kohli et al. 2015; Alsop et al. 2016). However, the rapid autopsy specimens now offer the added advantage of being derived from patients after super androgen blockade with contemporary agents such as abiraterone and enzalutamide. In this clinical setting, soft tissue metastases have become more common and are now used for PDX establishment.
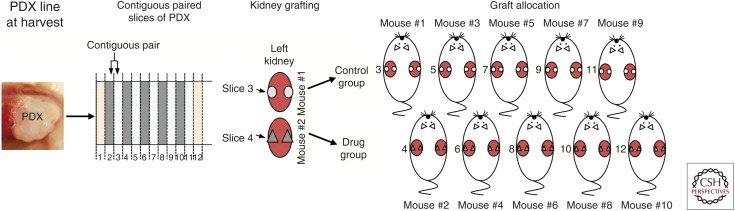
Overall, in 2016, there are disease stage–specific PDX models. Some are more common than others, but many are available and it may be anticipated that PDXs become a gold standard for preclinical research programs. For other tumor streams PDXs are commonly used for PDX clinical trials/tests (PCTs) (Gao et al. 2015). We recently modified the protocols for PCTs as described in Figure 3. To prepare a PCT, precision-cut (300-µm) slices are obtained from a PDX, and contiguous paired slices are regrafted into mice that are assigned to control or drug-treated groups and the tumors reestablished over 3–4 weeks. Host mice are then treated with drug or vehicle and, after treatment, the tumors are analyzed determining significance of paired samples (control vs. drug-treated groups).
Figure 3.
Patient-derived xenograft (PDX)-clinical trial (PCT) design showing how the PDX-derived tumor is sliced, engrafted, and divided among mouse treatment groups. The technique involves precision slicing of PDX tissue and regrafting of contiguous slices across all recipient mice, noting the numerical order of each slice and its position. For each drug treatment, a minimum of 10 mice is required (five vehicle + five controls). Tumors are regrown and mice are treated with vehicle/drug and harvested for analyses.
However, a major disadvantage of the PDX (and the subsequent PCT) is the suppressed immune component in host mice. Although only denoted as an emerging hallmark of cancer (Hanahan and Weinberg 2011), the mechanisms by which cancer cells evade immunological destruction are critical to our understanding of PDX tumor biology and drug response. The absence of a full immune component in PDX is yet another challenge for the field to resolve and is particularly limiting when testing immune therapies for which there is a resurgence of interest.
PATIENT-DERIVED EXPLANTS (PDEs)
Although PDX models offer a system in which human tissues can be grown in vivo over an extended period of time, there are limitations/difficulties in terms of throughput, cost, access, and quantitative endpoints (Centenera et al. 2013). Other in vitro approaches can be used to complement PDX models with similar contemporary patient samples. PDEs are a good example of this.
Ex vivo culture of prostatic tissues originated in the mid-1950s in studies conducted over decades by Lasnitzki (1951, 1954, 1955) who developed organ cultures of murine prostate glands to explore hormone and drug sensitivity and the development of cancerous lesions. This method was subsequently adapted to allow the growth of human prostate tissues. Most commonly, PDEs are derived from fresh patient tissues obtained from radical prostatectomy because access to biopsy or metastasis material is limited and the amount of tissue is limited and often insufficient. Although varied techniques have been reported that include full submersion of the tissue, the use of various scaffolds or supports is more effective where tissues are kept in contact with the media on agar or other metal grids made of titanium (McMahon et al. 1972) or stainless steel (Nevalainen et al. 1993). The grid approach was used to yield novel information such as the role of Jak2/Stat5a signaling to induce epithelial-to-mesenchymal transition and stemlike properties in prostate cancer (Gu et al. 2013; Liao et al. 2015; Talati et al. 2015). With the development of bioengineered scaffolds to support and seed human cells for tissue regeneration, it is likely that these grid supports will lead to further advances as discussed below.
A recent and more widely adopted technique involves the culture of small pieces of fresh tissue that are grown on a gelatin sponge and half submerged in media. Similar to the grid method, this system allows primary tissue to be grown for up to 1 week, although the standard time for most assays is 48 hours. This platform facilitates the culture of human prostate cancer tissues while retaining the native tissue architecture and cell-to-cell signaling of the tumor microenvironment (Centenera et al. 2012).
Importantly for prostate cancer tissues, ex vivo cultures are hormone responsive. AR expression is maintained in the cultured tissue and evidence of AR signaling is evident by the gene expression of prostate-specific antigen (PSA) and detection of PSA protein in the secreted media (Centenera et al. 2013). Of interest, many other proteins, circulating tumor DNA (ctDNA), and exosome products can be detected in the media, indicating active secretion of prostatic products that may be used for biomarker discovery in the future.
The selection of tissue pieces is an important consideration for optimal explant culture techniques. As for other patient-derived models, the stage at which the prostate cancer specimen is obtained is highly dependent on tissue availability, but also the question to be addressed. Thus, there is a need for both castrate-sensitive (i.e., from radical prostatectomy specimens) and castrate-resistant (i.e., from metastatic specimens) tumors. The establishment of explant cultures from PDX-derived tissues has facilitated the study of rare and precious tissues that have been propagated in vivo, allowing short-term, high-throughput preclinical testing to be performed.
In addition, the quality of the specimen must be optimal, including a high proportion of cancer, fast tissue processing to preserve viability, and minimal tissue handling. The use of tissue slices of 200–300 µm in thickness maximizes tissue consistency and perfusion. The advances in precision equipment for tissue slicing over manual dissection has increased the consistency of explant sizes and allows for systematic sampling through tissues, also increasing the efficacy of the explant approach.
When incorporated into preclinical studies, ex vivo cultured tissues enable robust quantitative evaluation of clinically relevant endpoints. This was exemplified by a study using Hsp90 inhibitors in which differential efficacy of novel compounds was identified in the ex vivo cultures that had not been appreciated in cell line or animal studies. The endpoints used for drug efficacy were biomarker expression (Hsp70 in this study) and quantitation of proliferation and apoptosis. This approach, therefore, has the potential to enable more rational selection of therapeutic agents and biomarkers of response for clinical trials (Centenera et al. 2012).
For ex vivo cultures of localized specimens, the proliferation index is ∼20%–30%, which is generally higher than the original tissue specimen, but offers the opportunity to show a reduction in proliferation with drug treatment (Centenera et al. 2012). Schiewer and colleagues (Schiewer et al. 2012) used this approach to test the efficacy of a poly(ADP-ribose) polymerase (PARP) inhibitor (ABT-888) and showed that in control explants the proliferation was higher at 4 days compared with 2 days, indicating long-term proliferation of the tissue slices. The ABT-888 compound strikingly reduced proliferation at both 2- and 4-day time points to as low as 5% (Schiewer et al. 2012). Gene expression changes in androgen-regulated genes (KLK3/PSA, TMPRSS2, and FKBP5) were also significantly reduced after ABT-888 treatment (Schiewer et al. 2012), which is another effective readout of efficacy in this ex vivo culture system. Likewise, apoptosis (measured by cleaved caspase-3 expression) is between 5% and 10%, which also allows for an induction of apoptosis to be detected, such as the Hsp90 inhibitors, which induced 25% apoptosis (Centenera et al. 2012).
PATIENT-DERIVED SPHEROIDS, PROSTASPHERES, AND ORGANOIDS
The ability to establish cell lines from primary human prostate tumors provides additional advantages over PDX and PDE methods, such as rapid expansion of small quantities of primary tissue, greater feasibility for large-scale studies such as drug screening, and the ability to genetically manipulate culture cells using lentiviral or clustered regularly interspaced short palindromic repeat (CRISPR) strategies. However, prostate luminal tumor cells are highly susceptible to anoikis following tissue dissociation, resulting in great difficulty in attempts to establish cell lines from patient specimens (Peehl 2005; Kwon et al. 2014). This has prompted efforts to establish culture conditions that enable the survival and propagation of prostatic luminal cells.
In recent years, the transition from monolayer to 3D culture methods has led to increased efficiency of establishing patient-derived prostate lines, in which incorporation of an extracellular matrix (ECM) scaffold has been integral to increased survival of epithelial cells and maintenance of cellular composition and differentiation. In particular, 3D culture methods for prostate tissue commonly use Matrigel for the ECM component (Kleinman and Martin 2005) with a liquid medium overlay to embed prostate epithelial cells. This general approach has been used for the development of spheroid/prostasphere assays and, more recently, organoid methodologies (Hu et al. 2011; Prins et al. 2014; Ho et al. 2015; Vela and Chen 2015).
The spheroid/prostasphere culture method involves dissociated primary epithelial populations embedded in Matrigel combined with commercially available media such as prostate epithelial cell growth medium (PrEGM). However, similar to adherent cell culture, spheroid culture conditions favor benign basal cell growth (Goldstein et al. 2008; Garraway et al. 2010), whereas the prostatic luminal cells, including tumor cells, do not survive or form spheroids (Garraway et al. 2010). Second, there is limited differentiation of basal cells in spheroid culture to cells with luminal characteristics, but the resulting cells fail to show key hallmarks of prostate epithelium such as nuclear AR expression and response to androgen deprivation (Xin et al. 2007; Hu et al. 2011; Yamamoto et al. 2012). Therefore, 3D methodologies required further adaptation to allow the study of prostate cancer progression.
Subsequently, the development of organoid culture conditions for patient-derived tissues has resulted in a significant improvement in our ability to establish lines from prostate luminal populations. Organoid culture is another 3D method for the growth of primary cells or stem cell lines (embryonic stem cells [ESCs] or induced pluripotent stem cells [iPSCs]) and has similar constituents to spheroid culture, such as the use of Matrigel. However, organoid culture media is typically more defined when compared with spheroid media, with carefully selected growth factors tailored to the tissue of interest. This results in more faithful recapitulation of the in vivo phenotype, including maintenance of several cell types, accurate spatial organization, expression patterns, and biology (Fatehullah et al. 2016). This system was initially adapted for the study of murine intestinal stem cells (Sato et al. 2009) and has been extended to the growth of human tissues and disease states for the intestine and several other organs such as the colon (Sato et al. 2011), stomach (Bartfeld et al. 2015; Schlaermann et al. 2016), and pancreas (Boj et al. 2015; Huang et al. 2015). Adaptation of organoid culture for growth of prostatic epithelium is a recent development and needs further optimization to study the full spectrum of prostate cancer progression. Nonetheless two separate organoid culture methodologies have been reported that allow propagation of isolated mouse and human luminal cells (Chua et al. 2014; Karthaus et al. 2014), which was previously not possible using other culture methods, and provides the premise for organoid culture to be used to propagate human prostate cancer specimens.
Further adaptation of the organoid culture conditions has resulted in limited success in establishing organoid lines from advanced prostate cancer specimens. Gao and colleagues (2014) were able to establish seven organoid lines from metastatic prostate cancer biopsies, from a range of organ sites, as well as from circulating tumor cells (CTCs). These organoids faithfully recapitulated the primary tumor phenotype and their molecular profile, evident by whole genome sequencing and RNA sequencing after 3 months in culture. However, unlike PDX and PDE approaches, the reliability of this system for translational research applications, such as the study of precision medicine, is unknown. Although drug treatment of mouse prostate tumor-derived organoids shows similar therapeutic response to that observed in vivo (Chua et al. 2014), more proof-of-principle treatment studies will need to be performed on prostate organoids to determine their suitability for translational research. Additionally, despite the ability to propagate advanced tumor specimens, disease stage–specific organoid lines from the full spectrum of prostate cancer progression have yet to be achieved. Organoids from patients with localized disease would be highly desirable, but the current methods may not allow this; possibly the current protocols do not include other cells of the tumor microenvironment such as stroma, immune, and neuronal cell populations. Although the current systems focus on epithelial biology in the absence of cofounding microenvironmental factors, it is well known that the tumor microenvironment is an important feature of cancer progression (Taylor and Risbridger 2008). It is possible that localized prostate tumors, in particular, cannot survive without the appropriate support of niche-derived factors. However, in parallel to the development of pure epithelial culture systems, other model systems have been developed that incorporate aspects of the prostate cancer niche, which will be described below.
Organoid cultures have added another important capability. Recent lentiviral infection of primary human benign prostate epithelium in organoid culture has confirmed the role of N-Myc in neuroendocrine prostate cancer development and allowed the study of the cell of origin of human prostate cancer (Lee et al. 2016; Park et al. 2016). Furthermore, these lines can be combined with mouse urogenital sinus mesenchyme (UGM) and established as xenografts in immune-deficient host mice. This allows the ability to study many more aspects of prostate cancer progression in vitro, such as validation of drivers of different aspects of disease progression.
MODELING MICROENVIRONMENT INFLUENCES IN VITRO
Although organoid culture is a significant advance for the prostate cancer field and will ultimately provide a high-throughput approach from limited amounts of patient-derived material, it remains limited by the absence of any cellular components of the tumor microenvironment. Recognizing the importance of the tumor microenvironment, the updated version of cancer hallmarks included cancer-associated fibroblasts (CAFs) and immune and vascular cells as contributors to epithelial cell progression (Hanahan and Weinberg 2011). Patient-derived CAFs in coculture with prostatic epithelia change epithelial cell features and, by conferring tumorigenic properties, confirm an important role of the prostate cancer niche in disease progression (Clark et al. 2013). One of the ways in which CAFs versus nonmalignant matched patient fibroblasts modulate epithelia involves changes to the ECM (Ellem et al. 2014), and this is entirely consistent with the need to use Matrigel for organoid and spheroid culture, although it is not specific to the prostate ECM. Further development of 3D models using bioengineered scaffolds and hydrogels shows great promise and marks the end of the era of “flat biology” for cell culture. The use of scaffolds or similar provides another approach to identify and interrogate the mechanism of multicellular interactions in the tumor microenvironment. It is likely that bioengineering will result in more sophisticated models as there are attempts to generate a “prostate tumor on a scaffold” with its tumor microenvironment. Also, looking forward, one might expect developments in which ECM components or patient-derived CAF or immune cell components are combined with organoid culture methodology to create a more complete in vitro prostate cancer model in which individual components can be independently manipulated and studied (Loessner et al. 2014; Fitzgerald et al. 2015; Katti et al. 2016).
CONCLUDING REMARKS
Each of the models has its own advantages and disadvantages and, in our opinion, there is no single adequate or perfect model; rather it is the combination of models that provides insight to particular research questions (Table 1). In all of these models, the quality of the specimens from the patients is paramount. Poor specimens make the models difficult to replicate both within and between research laboratories. The selection of disease stage–specific specimens is also critical because the selection of inappropriate specimens to answer the question under investigation is likely to be misleading or irrelevant.
Table 1.
Advantages and disadvantages of patient-derived models of prostate cancer
| Model system | Advantages | Disadvantages |
|---|---|---|
| PDX | Tissue architecture remains intact. Endocrine system (including androgen signaling) is intact. Have been established from a wide spectrum of disease progression. Functional assays can be conducted over an extended period (up to 1 year) to provide a realistic measure of response including pathology. |
Quantitative measures are complex and limited to the harvest time point. Mouse model has incomplete immune system. |
| PDE | Tissue architecture remains intact. Androgen signaling is maintained. Functional assays can be conducted in a short time frame, so throughput is moderate. |
Immune system is limited to the cells that were present in the original specimen. Tissue is only viable in culture for 7 days, so long-term functional studies cannot be performed. |
| Organoids | Cells can be expanded indefinitely. Drug treatment can be high throughput. Quantitative measures are simple and accurate. Organoids can be reestablished as PDX grafts for long-term functional studies. |
No stromal or immune influence. Currently only aggressive prostate cancer samples have been established as organoid lines. |
| 3D scaffolds | System allows for complex cell–cell interactions to be established, including stroma–epithelial or immune interactions. Scaffolds can be produced from biological relevant materials. Confocal imaging allows for 3D analysis and examination of cellular interactions. |
Requires expertise from bioengineers, and access to specialized resources that can be limited and expensive. |
PDX, patient-derived xenograft; PDE, patient-derived explant.
Although human preclinical prostate cancer models are challenging, the rewards can be measured in translation of outcomes for patient benefit, although this is mainly realized for a patient population, rather than a specific patient himself. As we strive to achieve more rapid throughput and turnaround, it will be possible to use a patient’s own PDX/organoid/cell line for discovery of, and response to, actionable targets and thereby contribute to tailored treatments that are so desirable and eventually will be more efficient and cost effective.
ACKNOWLEDGMENTS
Each of the authors acknowledge salary support, including from the National Health and Medical Research Council (NHMRC) Senior Principal Research Fellowship (G.P.R.; ID102752), NHMRC Early Career Fellowship (R.T.; ID 1090204), and Victorian Cancer Agency Fellowship (R.A.T.; MCRF15023).
Footnotes
Editors: Michael M. Shen and Mark A. Rubin
Additional Perspectives on Prostate Cancer available at www.perspectivesinmedicine.org
REFERENCES
*Reference is also in this collection.
- Alsop K, Thorne H, Sandhu S, Hamilton A, Mintoff C, Christie E, Spruyt O, Williams S, McNally O, Mileshkin L, et al. 2016. An effective community based model of rapid autopsy in end-stage cancer patients. Nat Biotechnol 34: 1010–1014. [DOI] [PubMed] [Google Scholar]
- *.Arriaga JM, Abate-Shen C. 2017. Genetically engineered mouse models of prostate cancer in the post-genomic era. Cold Spring Harb Perspect Med 10.1101/cshperspect.a030528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartfeld S, Bayram T, van de Wetering M, Huch M, Begthel H, Kujala P, Vries R, Peters PJ, Clevers H. 2015. In vitro expansion of human gastric epithelial stem cells and their responses to bacterial infection. Gastroenterology 148: 126–136.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltran H, Prandi D, Mosquera JM, Benelli M, Puca L, Cyrta J, Marotz C, Giannopoulou E, Chakravarthi BV, Varambally S, et al. 2016. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat Med 22: 298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berner A, Henkel J, Woodruff MA, Saifzadeh S, Kirby G, Zaiss S, Gohlke J, Reichert JC, Nerlich M, Schuetz MA, et al. 2015. Scaffold-cell bone engineering in a validated preclinical animal model: Precursors vs differentiated cell source. J Tissue Eng Regen Med 11: 2081–2089. [DOI] [PubMed] [Google Scholar]
- Boj SF, Hwang CI, Baker LA, Chio, II, Engle DD, Corbo V, Jager M, Ponz-Sarvise M, Tiriac H, Spector MS, et al. 2015. Organoid models of human and mouse ductal pancreatic cancer. Cell 160: 324–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruinsma SM, Bangma CH, Carroll PR, Leapman MS, Rannikko A, Petrides N, Weerakoon M, Bokhorst LP, Roobol MJ; Movember GAP3 consortium. 2016. Active surveillance for prostate cancer: A narrative review of clinical guidelines. Nat Rev Urol 13: 151–167. [DOI] [PubMed] [Google Scholar]
- Buhler KR, QJ, Liu AY, Wang H, Ellis WJ, Vessella RL. 1997. LuCaP 35: An androgen inducible, prostate-specific antigen producing human prostate cancer xenograft. Proc Am Assoc Cancer Res 38: 1600. [Google Scholar]
- Centenera MM, Gillis JL, Hanson AR, Jindal S, Taylor RA, Risbridger GP, Sutherland PD, Scher HI, Raj GV, Knudsen KE, et al. 2012. Evidence for efficacy of new Hsp90 inhibitors revealed by ex vivo culture of human prostate tumors. Clin Cancer Res 18: 3562–3570. [DOI] [PubMed] [Google Scholar]
- Centenera MM, Raj GV, Knudsen KE, Tilley WD, Butler LM. 2013. Ex vivo culture of human prostate tissue and drug development. Nat Rev Urol 10: 483–487. [DOI] [PubMed] [Google Scholar]
- Chua CW, Shibata M, Lei M, Toivanen R, Barlow LJ, Bergren SK, Badani KK, McKiernan JM, Benson MC, Hibshoosh H, et al. 2014. Single luminal epithelial progenitors can generate prostate organoids in culture. Nat Cell Biol 16: 951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AK, Taubenberger AV, Taylor RA, Niranjan B, Chea ZY, Zotenko E, Sieh S, Pedersen JS, Norden S, Frydenberg M, et al. 2013. A bioengineered microenvironment to quantitatively measure the tumorigenic properties of cancer-associated fibroblasts in human prostate cancer. Biomaterials 34: 4777–4785. [DOI] [PubMed] [Google Scholar]
- Corey E, Quinn JE, Bladou F, Brown LG, Roudier MP, Brown JM, Buhler KR, Vessella RL. 2002. Establishment and characterization of osseous prostate cancer models: Intra-tibial injection of human prostate cancer cells. Prostate 52: 20–33. [DOI] [PubMed] [Google Scholar]
- Ellem SJ, De-Juan-Pardo EM, Risbridger GP. 2014. In vitro modeling of the prostate cancer microenvironment. Adv Drug Deliv Rev 79–80: 214–221. [DOI] [PubMed] [Google Scholar]
- Ellis WJ, Vessella RL, Buhler KR, Bladou F, True LD, Bigler SA, Curtis D, Lange PH. 1996. Characterization of a novel androgen-sensitive, prostate-specific antigen-producing prostatic carcinoma xenograft: LuCaP 23. Clin Cancer Res 2: 1039–1048. [PubMed] [Google Scholar]
- Fatehullah A, Tan SH, Barker N. 2016. Organoids as an in vitro model of human development and disease. Nat Cell Biol 18: 246–254. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KA, Guo J, Tierney EG, Curtin CM, Malhotra M, Darcy R, O’Brien FJ, O’Driscoll CM. 2015. The use of collagen-based scaffolds to simulate prostate cancer bone metastases with potential for evaluating delivery of nanoparticulate gene therapeutics. Biomaterials 66: 53–66. [DOI] [PubMed] [Google Scholar]
- Gao D, Vela I, Sboner A, Iaquinta PJ, Karthaus WR, Gopalan A, Dowling C, Wanjala JN, Undvall EA, Arora VK, et al. 2014. Organoid cultures derived from patients with advanced prostate cancer. Cell 159: 176–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Korn JM, Ferretti S, Monahan JE, Wang Y, Singh M, Zhang C, Schnell C, Yang G, Zhang Y, et al. 2015. High-throughput screening using patient-derived tumor xenografts to predict clinical trial drug response. Nat Med 21: 1318–1325. [DOI] [PubMed] [Google Scholar]
- Garralda E, Paz K, Lopez-Casas PP, Jones S, Katz A, Kann LM, Lopez-Rios F, Sarno F, Al-Shahrour F, Vasquez D, et al. 2014. Integrated next-generation sequencing and avatar mouse models for personalized cancer treatment. Clin Cancer Res 20: 2476–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garraway IP, Sun W, Tran CP, Perner S, Zhang B, Goldstein AS, Hahm SA, Haider M, Head CS, Reiter RE, et al. 2010. Human prostate sphere-forming cells represent a subset of basal epithelial cells capable of glandular regeneration in vivo. Prostate 70: 491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein AS, Lawson DA, Cheng D, Sun W, Garraway IP, Witte ON. 2008. Trop2 identifies a subpopulation of murine and human prostate basal cells with stem cell characteristics. Proc Natl Acad Sci 105: 20882–20887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu L, Liao Z, Hoang DT, Dagvadorj A, Gupta S, Blackmon S, Ellsworth E, Talati P, Leiby B, Zinda M, et al. 2013. Pharmacologic inhibition of Jak2-Stat5 signaling by Jak2 inhibitor AZD1480 potently suppresses growth of both primary and castrate-resistant prostate cancer. Clin Cancer Res 19: 5658–5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundem G, Van Loo P, Kremeyer B, Alexandrov LB, Tubio JM, Papaemmanuil E, Brewer DS, Kallio HM, Hognas G, Annala M, et al. 2015. The evolutionary history of lethal metastatic prostate cancer. Nature 520: 353–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. 2011. Hallmarks of cancer: The next generation. Cell 144: 646–674. [DOI] [PubMed] [Google Scholar]
- Hidalgo M, Amant F, Biankin AV, Budinska E, Byrne AT, Caldas C, Clarke RB, de Jong S, Jonkers J, Maelandsmo GM, et al. 2014. Patient-derived xenograft models: An emerging platform for translational cancer research. Cancer Discov 4: 998–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SM, Cheong A, Lam HM, Hu WY, Shi GB, Zhu X, Chen J, Zhang X, Medvedovic M, Leung YK, et al. 2015. Exposure of human prostaspheres to bisphenol A epigenetically regulates SNORD family noncoding RNAs via histone modification. Endocrinology 156: 3984–3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzapfel BM, Thibaudeau L, Hesami P, Taubenberger A, Holzapfel NP, Mayer-Wagner S, Power C, Clements J, Russell P, Hutmacher DW. 2013. Humanised xenograft models of bone metastasis revisited: Novel insights into species-specific mechanisms of cancer cell osteotropism. Cancer Metastasis Rev 32: 129–145. [DOI] [PubMed] [Google Scholar]
- Holzapfel BM, Wagner F, Loessner D, Holzapfel NP, Thibaudeau L, Crawford R, Ling MT, Clements JA, Russell PJ, Hutmacher DW. 2014. Species-specific homing mechanisms of human prostate cancer metastasis in tissue engineered bone. Biomaterials 35: 4108–4115. [DOI] [PubMed] [Google Scholar]
- Hu WY, Shi GB, Lam HM, Hu DP, Ho SM, Madueke IC, Kajdacsy-Balla A, Prins GS. 2011. Estrogen-initiated transformation of prostate epithelium derived from normal human prostate stem-progenitor cells. Endocrinology 152: 2150–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Holtzinger A, Jagan I, BeGora M, Lohse I, Ngai N, Nostro C, Wang R, Muthuswamy LB, Crawford HC, et al. 2015. Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell- and patient-derived tumor organoids. Nat Med 21: 1364–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karthaus WR, Iaquinta PJ, Drost J, Gracanin A, van Boxtel R, Wongvipat J, Dowling CM, Gao D, Begthel H, Sachs N, et al. 2014. Identification of multipotent luminal progenitor cells in human prostate organoid cultures. Cell 159: 163–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katti KS, Molla MS, Karandish F, Haldar MK, Mallik S, Katti DR. 2016. Sequential culture on biomimetic nanoclay scaffolds forms three-dimensional tumoroids. J Biomed Mater Res A 104: 1591–1602. [DOI] [PubMed] [Google Scholar]
- Kleinman HK, Martin GR. 2005. Matrigel: Basement membrane matrix with biological activity. Semin Cancer Biol 15: 378–386. [DOI] [PubMed] [Google Scholar]
- Knudsen KE, Penning TM. 2010. Partners in crime: Deregulation of AR activity and androgen synthesis in prostate cancer. Trends Endocrinol Metab 21: 315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli M, Wang L, Xie F, Sicotte H, Yin P, Dehm SM, Hart SN, Vedell PT, Barman P, Qin R, et al. 2015. Mutational landscapes of sequential prostate metastases and matched patient derived xenografts during enzalutamide therapy. PLoS ONE 10: e0145176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon OJ, Valdez JM, Zhang L, Zhang B, Wei X, Su Q, Ittmann MM, Creighton CJ, Xin L. 2014. Increased Notch signalling inhibits anoikis and stimulates proliferation of prostate luminal epithelial cells. Nat Commun 5: 4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laitinen S, Karhu R, Sawyers CL, Vessella RL, Visakorpi T. 2002. Chromosomal aberrations in prostate cancer xenografts detected by comparative genomic hybridization. Genes Chromosomes Cancer 35: 66–73. [DOI] [PubMed] [Google Scholar]
- Lalonde E, Ishkanian AS, Sykes J, Fraser M, Ross-Adams H, Erho N, Dunning MJ, Halim S, Lamb AD, Moon NC, et al. 2014. Tumour genomic and microenvironmental heterogeneity for integrated prediction of 5-year biochemical recurrence of prostate cancer: A retrospective cohort study. Lancet Oncol 15: 1521–1532. [DOI] [PubMed] [Google Scholar]
- Lasnitzki I. 1951. Precancerous changes induced by 20-methylcholanthrene in mouse prostates grown in vitro. Br J Cancer 5: 345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasnitzki I. 1954. The effect of estrone alone and combined with 20-methylcholanthrene on mouse prostate glands grown in vitro. Cancer Res 14: 632–639. [PubMed] [Google Scholar]
- Lasnitzki I. 1955. The effect of testosterone propionate on organ cultures of the mouse prostate. J Endocrinol 12: 236–240. [DOI] [PubMed] [Google Scholar]
- Lawrence MG, Taylor RA, Toivanen R, Pedersen J, Norden S, Pook DW, Frydenberg M, Papargiris MM, Niranjan B, Richards MG, et al. 2013. A preclinical xenograft model of prostate cancer using human tumors. Nat Protoc 8: 836–848. [DOI] [PubMed] [Google Scholar]
- Lawrence MG, Pook DW, Wang H, Porter LH, Frydenberg M, Kourambas J, Appu S, Poole C, Beardsley EK, Ryan A, et al. 2015. Establishment of primary patient-derived xenografts of palliative TURP specimens to study castrate-resistant prostate cancer. Prostate 75: 1475–1483. [DOI] [PubMed] [Google Scholar]
- Lee JK, Phillips JW, Smith BA, Park JW, Stoyanova T, McCaffrey EF, Baertsch R, Sokolov A, Meyerowitz JG, Mathis C, et al. 2016. N-Myc drives neuroendocrine prostate cancer initiated from human prostate epithelial cells. Cancer Cell 29: 536–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZG, Mathew P, Yang J, Starbuck MW, Zurita AJ, Liu J, Sikes C, Multani AS, Efstathiou E, Lopez A, et al. 2008. Androgen receptor-negative human prostate cancer cells induce osteogenesis in mice through FGF9-mediated mechanisms. J Clin Invest 118: 2697–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Z, Gu L, Vergalli J, Mariani SA, De Dominici M, Lokareddy RK, Dagvadorj A, Purushottamachar P, McCue PA, Trabulsi E, et al. 2015. Structure-based screen identifies a potent small molecule inhibitor of Stat5a/b with therapeutic potential for prostate cancer and chronic myeloid leukemia. Mol Cancer Therapeut 14: 1777–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D, Wyatt AW, Xue H, Wang Y, Dong X, Haegert A, Wu R, Brahmbhatt S, Mo F, Jong L, et al. 2014. High fidelity patient-derived xenografts for accelerating prostate cancer discovery and drug development. Cancer Res 74: 1272–1283. [DOI] [PubMed] [Google Scholar]
- Lin D, Dong X, Wang K, Wyatt AW, Crea F, Xue H, Wang Y, Wu R, Bell RH, Haegert A, et al. 2015. Identification of DEK as a potential therapeutic target for neuroendocrine prostate cancer. Oncotarget 6: 1806–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loessner D, Holzapfel BM, Clements JA. 2014. Engineered microenvironments provide new insights into ovarian and prostate cancer progression and drug responses. Adv Drug Deliv Rev 79–80: 193–213. [DOI] [PubMed] [Google Scholar]
- McMahon MJ, Butler AV, Thomas GH. 1972. Morphological responses of prostatic carcinoma to testosterone in organ culture. Br J Cancer 26: 388–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevalainen MT, Harkonen PL, Valve EM, Ping W, Nurmi M, Martikainen PM. 1993. Hormone regulation of human prostate in organ culture. Cancer Res 53: 5199–5207. [PubMed] [Google Scholar]
- Park JW, Lee JK, Phillips JW, Huang P, Cheng D, Huang J, Witte ON. 2016. Prostate epithelial cell of origin determines cancer differentiation state in an organoid transformation assay. Proc Natl Acad Sci 113: 4482–4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peehl DM. 2005. Primary cell cultures as models of prostate cancer development. Endocr Relat Cancer 12: 19–47. [DOI] [PubMed] [Google Scholar]
- Prins GS, Hu WY, Shi GB, Hu DP, Majumdar S, Li G, Huang K, Nelles JL, Ho SM, Walker CL, et al. 2014. Bisphenol A promotes human prostate stem-progenitor cell self-renewal and increases in vivo carcinogenesis in human prostate epithelium. Endocrinology 155: 805–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbridger GP. 2015. Prostate cancer: Novel xenografts in mice—A new wave of preclinical models. Nat Rev Urol 12: 540–541. [DOI] [PubMed] [Google Scholar]
- Risbridger GP, Taylor RA. 2016. Patient-derived prostate cancer: From basic science to the clinic. Horm Cancer 7: 236–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbridger GP, Taylor RA, Clouston D, Sliwinski A, Thorne H, Hunter S, Li J, Mitchell G, Murphy D, Frydenberg M, et al. 2015. Patient-derived xenografts reveal that intraductal carcinoma of the prostate is a prominent pathology in BRCA2 mutation carriers with prostate cancer and correlates with poor prognosis. Eur Urol 67: 496–503. [DOI] [PubMed] [Google Scholar]
- Robinson D, Van Allen EM, Wu YM, Schultz N, Lonigro RJ, Mosquera JM, Montgomery B, Taplin ME, Pritchard CC, Attard G, et al. 2015. Integrative clinical genomics of advanced prostate cancer. Cell 161: 1215–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saar M, Zhao H, Nolley R, Young SR, Coleman I, Nelson PS, Vessella RL, Peehl DM. 2014. Spheroid culture of LuCaP 147 as an authentic preclinical model of prostate cancer subtype with SPOP mutation and hypermutator phenotype. Cancer Lett 351: 272–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saar M, Korbel C, Linxweiler J, Jung V, Kamradt J, Hasenfus A, Stockle M, Unteregger G, Menger MD. 2015. Orthotopic tumorgrafts in nude mice: A new method to study human prostate cancer. Prostate 75: 1526–1537. [DOI] [PubMed] [Google Scholar]
- Saramaki OR, Porkka KP, Vessella RL, Visakorpi T. 2006. Genetic aberrations in prostate cancer by microarray analysis. Int J Cancer 119: 1322–1329. [DOI] [PubMed] [Google Scholar]
- Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, Van Es JH, Abo A, Kujala P, Peters PJ, et al. 2009. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459: 262–265. [DOI] [PubMed] [Google Scholar]
- Sato T, Stange DE, Ferrante M, Vries RG, Van Es JH, Van den Brink S, Van Houdt WJ, Pronk A, Van Gorp J, Siersema PD, et al. 2011. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 141: 1762–1772. [DOI] [PubMed] [Google Scholar]
- Schiewer MJ, Goodwin JF, Han S, Brenner JC, Augello MA, Dean JL, Liu F, Planck JL, Ravindranathan P, Chinnaiyan AM, et al. 2012. Dual roles of PARP-1 promote cancer growth and progression. Cancer Discov 2: 1134–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaermann P, Toelle B, Berger H, Schmidt SC, Glanemann M, Ordemann J, Bartfeld S, Mollenkopf HJ, Meyer TF. 2016. A novel human gastric primary cell culture system for modelling Helicobacter pylori infection in vitro. Gut 65: 202–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talati PG, Gu L, Ellsworth EM, Girondo MA, Trerotola M, Hoang DT, Leiby B, Dagvadorj A, McCue PA, Lallas CD, et al. 2015. Jak2-Stat5a/b signaling induces epithelial-to-mesenchymal transition and stem-like cell properties in prostate cancer. Am J Pathol 185: 2505–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talukdar S, Mandal M, Hutmacher DW, Russell PJ, Soekmadji C, Kundu SC. 2011. Engineered silk fibroin protein 3D matrices for in vitro tumor model. Biomaterials 32: 2149–2159. [DOI] [PubMed] [Google Scholar]
- Taylor RA, Risbridger GP. 2008. Prostatic tumor stroma: A key player in cancer progression. Curr Cancer Drug Targets 8: 490–497. [DOI] [PubMed] [Google Scholar]
- Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, Arora VK, Kaushik P, Cerami E, Reva B, et al. 2010. Integrative genomic profiling of human prostate cancer. Cancer Cell 18: 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Cancer Genome Atlas Research Network. 2015. The molecular taxonomy of primary prostate cancer. Cell 163: 1011–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- True LD, Buhler K, Quinn J, Williams E, Nelson PS, Clegg N, Macoska JA, Norwood T, Liu A, Ellis W, et al. 2002. A neuroendocrine/small cell prostate carcinoma xenograft-LuCaP 49. Am J Pathol 161: 705–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse BW, Cowin GJ, Soekmadji C, Jovanovic L, Vasireddy RS, Ling MT, Khatri A, Liu T, Thierry B, Russell PJ. 2015. PSMA-targeting iron oxide magnetic nanoparticles enhance MRI of preclinical prostate cancer. Nanomedicine (Lond) 10: 375–386. [DOI] [PubMed] [Google Scholar]
- Valta MP, Zhao H, Saar M, Tuomela J, Nolley R, Linxweiler J, Sandholm J, Lehtimaki J, Harkonen P, Coleman I, et al. 2016. Spheroid culture of LuCaP 136 patient-derived xenograft enables versatile preclinical models of prostate cancer. Clin Exp Metastasis 33: 325–337. [DOI] [PubMed] [Google Scholar]
- Vela I, Chen Y. 2015. Prostate cancer organoids: A potential new tool for testing drug sensitivity. Expert Rev Anticancer Ther 15: 261–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin L, Lukacs RU, Lawson DA, Cheng D, Witte ON. 2007. Self-renewal and multilineage differentiation in vitro from murine prostate stem cells. Stem Cells 25: 2760–2769. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Masters JR, Dasgupta P, Chandra A, Popert R, Freeman A, Ahmed A. 2012. CD49f is an efficient marker of monolayer- and spheroid colony-forming cells of the benign and malignant human prostate. PloS ONE 7: e46979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Nolley R, Chen Z, Peehl DM. 2010. Tissue slice grafts: An in vivo model of human prostate androgen signaling. Am J Pathol 177: 229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]