Abstract
Ras controls a multitude of cellular signaling processes, including cell proliferation, differentiation, and apoptosis. Deregulation of Ras cycling often promotes tumorigenesis and various other developmental disorders, termed RASopothies. Although the structure of Ras has been known for many decades, it is still one of the most highly sought-after drug targets today, and is often referred to as “undruggable.” At the center of this paradoxical protein is a lack of understanding of fundamental differences in the G domains between the highly similar Ras isoforms and common oncogenic mutations, despite the immense wealth of knowledge accumulated about this protein to date. A shift in the field during the past few years toward a high-resolution understanding of the structure confirms the hypothesis that each isoform and oncogenic mutation must be considered individually, and that not all Ras mutations are created equal. For the first time in Ras history, we have the ability to directly compare the structures of each wild-type isoform to construct a “base-line” understanding, which can then be used as a springboard for analyzing the effects of oncogenic mutations on the structure–function relationship in Ras. This is a fundamental and large step toward the goal of developing personalized therapies for patients with Ras-driven cancers and diseases.
The small GTPase Ras was discovered more than 30 years ago because of its oncogenic potential, and is frequently mutated in ∼20% of all human cancers. Classically, Ras is referred to as a monomeric switch protein that is signaling active when bound to guanosine triphosphate (GTP) and inactive when bound to guanosine diphosphate (GDP). This active/inactive cycle is tightly controlled by regulatory proteins knows as guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs). In the context of cellular signaling, Ras requires the aid of these regulatory proteins to overcome its high affinity for the bound nucleotide and intrinsically slow hydrolysis rate (Ahmadian et al. 1997; Boriack-Sjodin et al. 1998; Bos et al. 2007). Changes in the nucleotide bound in the active site are accompanied by dynamic conformational changes in the switch I (residues 30–40) and switch II (residues 60–76) motifs of Ras. Mutations at residues G12, G13, and Q61 lead to GAP-insensitivity and disruption of this tightly controlled cycling, in which constitutively active Ras promotes tumorigenesis.
The structures of Ras proteins contain a common central 6-stranded β-sheet flanked by five α-helices in a Rossmann-type fold (Vetter 2014). The G domain of the proteins (residues 1–166) is generally divided into two halves, the effector lobe (residues 1–86) and the allosteric lobe (87–166), based on function (Buhrman et al. 2011a). All GTPases contain conserved sequence motifs throughout the protein that impart nucleotide-binding specificity and serve specific roles central to the function of these molecular switches (Bourne et al. 1991). Residues in the NKxD (116NKCD119) and ExSAK (143ETSAK147) motifs, as well as F28, are imperative for recognizing and positioning the guanine nucleotide within the active site, while the P-loop (GxxxxGK[S/T]; 10GAGGVGKS17 in Ras) provides the appropriate charge and solvent environment for the phosphate groups and Mg2+ (Valencia et al. 1991). The neutron crystal structure of H-Ras (Protein Data Bank [PDB] ID 4RSG) shows that the γ-phosphate of the bound GTP analog is protonated (overall charge of –3) in the crystal, providing an active site that is amenable to both intrinsic and GAP-catalyzed hydrolysis of GTP through a dissociative-like mechanism (Knihtila et al. 2015). Residues in the switch I, specifically T35, and DxxG (57DTAG60) motifs recognize and stabilize the Mg2+-bound GTP in the active site when Ras is signaling active. The Mg2+ ion is a crucial cofactor in GTPases and is involved in all aspects of GTPase function, including binding to effector and regulatory protein partners and in the hydrolysis of GTP to GDP (Pan and Wessling-Resnick 1998; Rudack et al. 2012). When Ras is bound to GTP, the active site Mg2+ is coordinated by the β- and γ-phosphates and the side chains of switch I residues S17 and T35, while two water molecules complete the octahedral coordination sphere. This switch I conformation is referred to as state 2, in which T35 interacts with the Mg2+ and Y32 is often located over the nucleotide to create a “closed” active site (Shima et al. 2010). When T35 is not interacting with the Mg2+, switch I samples a range of more “open” switch I conformations, leading to greater solvent accessibility of the active site (Araki et al. 2011). The state 2 switch I conformation is observed in structures of Ras bound to effectors, whereas the state 1 conformation results in Ras that is “effector-binding-deficient” and more susceptible to nucleotide exchange (Spoerner et al. 2001).
The three isoforms of Ras, H-, N-, and K-Ras, share 90% identity in their G domain, with conserved structural and biochemical properties. There are two alternative splice variants of K-Ras: K-Ras4A and K-Ras4B. In most tissues, the K-Ras4A isoform is less predominantly expressed, although recent evidence suggests a role in some cancers (Tsai et al. 2015). Throughout this review, K-Ras will refer to the 4B splice variant, as it has been the most studied of the two. Importantly, the Ras isoforms have unique carboxy-terminal hypervariable regions (HVRs) that contain sites for posttranslational modification (PTM) imperative for correct function and localization of each isoform (Henis et al. 2009). All Ras proteins are farnesylated at C186 (C185 in K-Ras4B) to promote binding to the plasma membrane, where Ras interacts with regulatory and effector protein partners. Although the plasma membrane is the major site of Ras functional output, signaling from endomembranes has also been observed, particularly for N-Ras (Fehrenbacher et al. 2009). This may be a result, in part, of the presence of a singular palmitoyl group at C181 for this isoform, whereas H-Ras is doubly palmitoylated at C181 and C184 (Ahearn et al. 2012). K-Ras4B contains a hexalysine polybasic region (residues 175–180), which uniquely targets this isoform to areas of the plasma membrane enriched in acidic phospholipids (Hancock et al. 1990). Proteins such as PDEδ and calmodulin (CaM) interact specifically with the carboxy-terminal HVR on Ras to regulate trafficking and function on the membrane (Abraham et al. 2009; Dharmaiah et al. 2016). Isoform-specific localization patterns have been proposed as a mechanism for organizing Ras signaling clusters within the crowded cellular membrane environment (Hancock and Parton 2005). Nanoclusters containing around five to eight Ras proteins are essential for high-fidelity signal output, although the exact protein and lipid composition of these nanoclusters has been debated (Abankwa et al. 2007; Tian et al. 2007; Zhou et al. 2014). Recently, Ras dimerization and higher order oligomerization has become an area of intense research (Thompson 2013). However, the details of Ras dimerization are highly contested and unclear, such as the residues involved in the dimerization interface, the importance of isoform-specific homo- and heterodimerization, the mechanism of dimer formation, the role of the membrane, and the signaling relevance of dimers (Santos 2014; Holderfield and Morrison 2017). It is becoming increasingly clear that targeting Ras dimerization could abrogate nanocluster formation and signaling output (Chen et al. 2016; Spencer-Smith et al. 2016), although there is still confusion in the literature as to the location of the dimer interface (Jang et al. 2016; Prakash et al. 2017).
Although the majority of the G domain is highly conserved across the three Ras isoforms, residue differences in the allosteric lobe are hypothesized to play a role in isoform-specific membrane localization and orientation, which in turn has been linked to regulation of Ras–effector interactions and downstream signaling (Abankwa et al. 2010; Parker and Mattos 2015). Importantly, these allosteric lobe residues can influence intraprotein communication, connecting the membrane-interacting allosteric lobe residues to those in the active site of the effector lobe (Kearney et al. 2014). The predominant assumption in the field has been that the 100% sequence conserved effector lobe, where Ras interacts with a wide array of effector proteins such as Raf, PI3K, and RalGEF, promoted identical functions in the isoforms. However, recent evidence suggests that the structure, biochemistry, and dynamics of the effector lobe of each isoform are fundamentally different and require individual attention (Johnson et al. 2017; JA Parker, AY Volmar, S Pavlopoulos, and C Mattos, in prep.). Importantly, the balance between the more “open” state 1 and “closed” state 2 conformation can potentially be exploited in the design of isoform- and mutant-specific Ras inhibitors (Kauke et al. 2017). For the first time, both the structures and biochemical properties of the GTP-bound form of wild-type (WT) Ras isoforms are available, allowing for unprecedented comparison and novel insights into the important differences that contribute to the complicated field of Ras biology and cancer. Insights gleaned from the careful study of the WT isoforms can then be applied to oncogenic mutants, which are linked to isoform- and mutant-specific cancer and tissue-type frequencies (Prior et al. 2012; Lu et al. 2016a).
GLOBAL INTRAMOLECULAR COMMUNICATION NETWORKS IN Ras
The signaling active, GTP-bound form of Ras relies on intricate interplay between the effector and allosteric lobes. A highly conserved interswitch region (β2–β3 loop, L3) connects the switch I and II regions of the effector lobe with the allosteric lobe. Specifically, D47 and E49 engage in stable salt bridge interactions with helix 5 residues R161 and R164, respectively. These residues have been dubbed “nucleotide-sensing residues” because of their ability to influence orientation of Ras with respect to the plasma membrane to ensure correct interactions with effector or regulatory proteins based on whether GDP or GTP is bound to Ras (Abankwa et al. 2008). In addition to the interswitch, water-mediated hydrogen bond networks connect membrane-interacting residues in the allosteric lobe to the effector lobe, whereas information based on the nucleotide bound in the active site is also relayed to the allosteric lobe (Kearney et al. 2014). One such network, termed the “helix 5 network,” spans nearly 15 Å across Ras to connect R161 and R164 with active site residue Y32 via the catalytically important bridging water molecule (Kearney et al. 2014). This network, which is abolished in inactive GDP-bound Ras, can therefore directly sense the presence of the γ-phosphate and relay this information to membrane-interacting residues in the allosteric lobe. Molecular dynamics (MD) simulations support these nucleotide-dependent shifts in the conformational ensemble of Ras (Grant et al. 2009; Raimondi et al. 2011). Oncogenic mutations, such as Q61L and G12V, result in a severed connection between the active site and the helix 5 network because of a direct H-bond between Y32 and the γ-phosphate in the absence of the active site water molecule that bridges Y32 to the γ-phosphate (the bridging water molecule) (Marcus and Mattos 2015). It is important to point out that this communication pathway uses residues in the allosteric lobe, particularly in helix 5, helix 4, and loop 8 that are highly sequence divergent among the isoforms. Therefore, it is possible that these isoform-specific residues differentially regulate communication in the isoforms, contributing to the fact that each isoform can promote overlapping yet distinct signaling outputs (Parker and Mattos 2015). The contribution of the G domain to functional specificity in the isoforms has been largely overlooked. This review focuses on recent advances linking biochemical and dynamics features of the G domain to isoform- specific differences in the allosteric lobe.
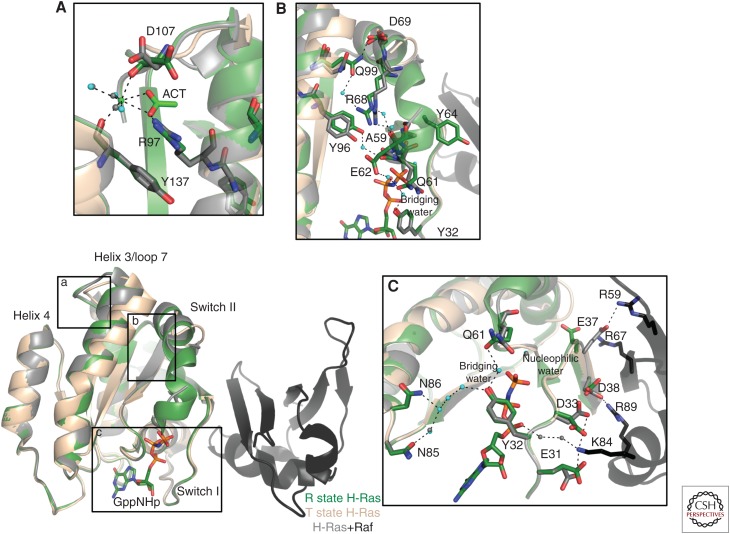
A ligand-binding site in the allosteric lobe has been identified as an important modulator of switch II conformations in the effector lobe (Buhrman et al. 2010). Calcium and acetate, which could be acting as a surrogate for a negatively charged membrane component in H-Ras crystals, bind in the allosteric site of Ras and promote a shift in the helix 3/loop 7 motif toward the allosteric lobe to allow ordering of switch II in a fully helical conformation, as seen in the crystal structure with PDB ID 3K8Y. A water-mediated hydrogen bond network from the allosteric site through helix 3 to switch II leads to the placement of the catalytic Q61 residue in the active site, where it interacts with the bridging water molecule (Fig. 1A–C) (Buhrman et al. 2010). This conformation is referred to as the R state, whereas an empty allosteric site and consequently disordered switch II is referred to as the T state. When switch I is present in the state 2 conformation and Y32 closed over the nucleotide, similar to that seen in the H-Ras/Raf complex (PDB ID 4G0N), the active site achieves a conformation to promote intrinsic hydrolysis. While Raf orders switch I, stabilizing the catalytically competent state 2 conformation, switch II, with catalytic residue Q61, remains disordered in the T state unless ligand binds in the allosteric site to promote the R state in which switch II is ordered and Ras catalytically competent. This promotes the allosteric network associated with the R state Ras, prompting the hypothesis that these networks could regulate signaling output for the Ras/Raf/Mek/Erk, through a GAP-independent hydrolysis mechanism, by promoting intrinsic hydrolysis of GTP (Buhrman et al. 2011b). Specifically, this level of regulation may be important in the context of Raf binding, which can outcompete GAP binding because of its nanomolar affinity for Ras-GTP (Smith and Ikura 2014). Evidence of the importance of Ras regulation via the allosteric network is seen in oncogenic G12D structures of Ras, where the connection to the allosteric site is cut off because of steric hindrance from the aspartate side chain in the active site, in effect hindering both GAP-assisted and intrinsic hydrolysis (Marcus and Mattos 2015).
Figure 1.
Allosteric network communication pathway in H-Ras. The T state of H-Ras, exemplified by the Protein Data Bank (PDB) ID 2RGE (wheat), shows a disordered switch II motif. On binding of an allosteric ligand as shown by PDB ID 3K8Y (green), the helix 3/loop 7 motif shifts toward helix 4, promoting formation of a water-mediated hydrogen bond network from the allosteric site (A) through an ordered switch II motif (B) to place Q61 in the active site (C). This allosteric mechanism is important in signaling through Raf, which binds at switch I as shown by the H-Ras/Raf complex (PDB ID 4G0N, H-Ras in gray and Raf in black).
Unlike all other known Ras effector proteins, Raf binds solely to switch I of Ras, leaving switch II free to attain multiple conformations. This point is crucial to the intrinsic hydrolysis mechanism and has driven research to focus intensely on the Ras/Raf/Mek/Erk pathway, a major regulator of cell proliferation and, consequently, tumorigenesis (McCubrey et al. 2007). We have previously shown in H-Ras that Q61 is critical to signaling through Raf, in which oncogenic mutants such as Q61L promote an anticatalytic conformation associated with the T state and prevent hydrolysis in the presence of Raf (Buhrman et al. 2007). On the other hand, the oncogenic G12V mutant promotes a relatively minor increase in mitogen-activated protein kinase (MAPK) phosphorylation in cells (Buhrman et al. 2011b), further confirming the growing realization that oncogenic mutants have differential effects on the signaling output of Ras. A combination of crystallographic and computational results revealed previously unrealized characteristics of the Ras/Raf complex. Raf binding to WT H-Ras induces an increase in conformational flexibility of switch II, while rigidifying residues in the allosteric site that directly bind Ca2+, consistent with the intrinsic hydrolysis mechanism (Fetics et al. 2015). Most interestingly, a similar study of the H-RasQ61L/Raf complex (PDB ID 4G3X) shows how the Q61L mutation rigidifies switch II while increasing the flexibility of the Ca2+-binding residues in the allosteric site (Fetics et al. 2015). Unlike previous assumptions that oncogenic mutants perturb only their local environment and binding with GAP, these results show that a single mutation can have global effects on the communication pathways between Ras and effector proteins, and that single-residue mutations are able to alter the balance of conformational states. These changes in global communication pathways throughout Ras raise the possibility of allosteric effects on Ras dimerization and the Ras–membrane interaction as a result of oncogenic mutations.
DIFFERENCES IN THE G DOMAIN OF THE Ras ISOFORMS
Until recently, only crystal structures of WT H-Ras bound to GTP-analogs were available in the PDB, and these structures were assumed to be good models for the K-Ras and N-Ras isoforms because of their 95% conserved G domains. Despite the fact that the isoforms share 100% sequence identity in areas where they interact with effector proteins, the networks linking the two G domain lobes led us to expect significant differences in the kinetics, biochemistry, and signaling output for H-, K-, and N-Ras. Our recent structures of both WT N-Ras and K-Ras bound to GTP analogs gives us the unprecedented ability to directly compare the three isoforms (Johnson et al. 2017; JA Parker, AY Volmar, S Pavlopoulos, and C Mattos, in prep.). The observed structural differences can be linked to functional differences associated with distinct balance in the conformational states and dynamics in each of the isoforms (Gorfe et al. 2008; Harrison et al. 2016).
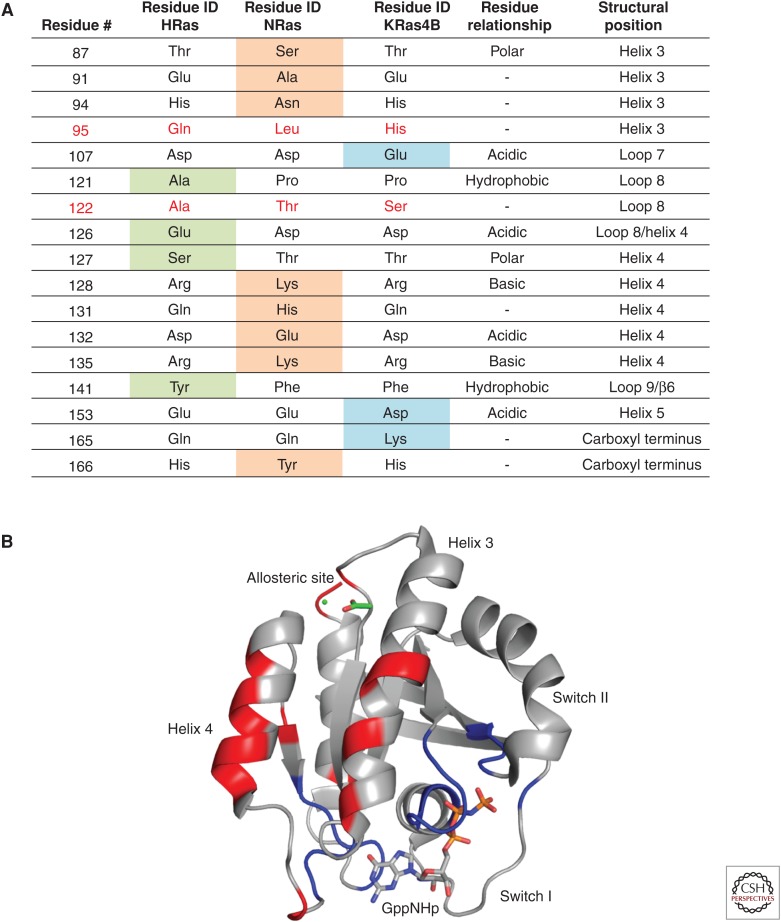
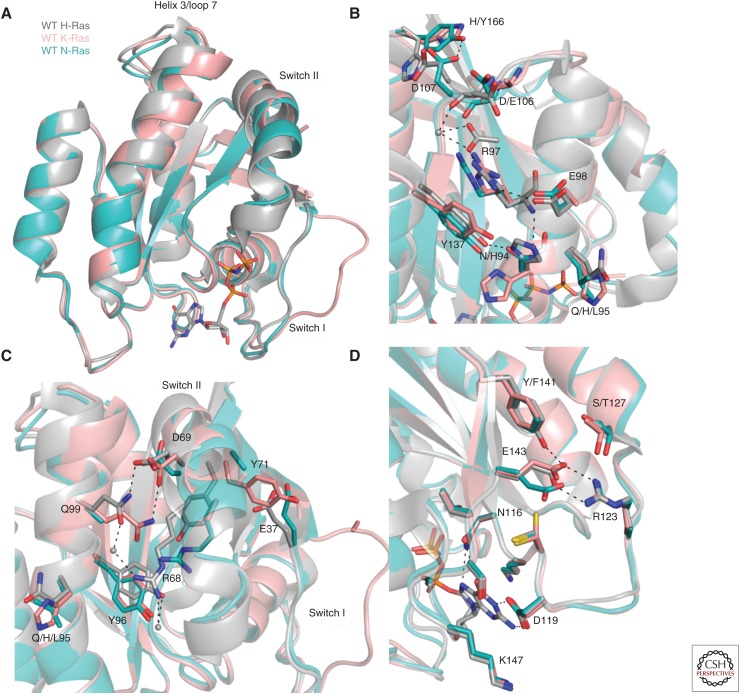
In terms of the G domain, there are 17 residue differences across the isoforms (Fig. 2A). When mapped onto the structure, these isoform-specific residues cluster in the helix 3, loop 8, and helix 4 areas of Ras, sandwiching highly conserved residues in the 116NKCD119 and 143ETSAK147 motifs, as well as the allosteric site (Fig. 2B). The superposed structures of WT H-Ras, K-Ras, and N-Ras bound to GTP analogs show differences in the state 1 versus state 2 conformations in switch I and R versus T states associated with switch II (Fig. 3A). It is interesting to note that, among the numerous WT H-Ras structures in the GTP-bound state found in the PDB, a single one shows a state 1 conformation and that was obtained only by seeding crystals with the constitutive state 1 mutant H-RasT35S-GppNHp (Araki et al. 2011). The predominance of state 2 in WT H-Ras found both in the crystal structures and by 31P nuclear magnetic resonance (NMR) (Spoerner et al. 2010) contrasts with the WT K-Ras crystal structure with an open switch I conformation consistent with a more exposed P-loop as determined by recently published hydrogen/deuterium exchange mass spectrometry (HDX-MS) experiments on both isoforms. As discussed above, the positioning of the helix 3/loop 7 motif is crucial in mediating the T-to-R state transition and formation of the allosteric network that connects membrane-interacting residues in Ras to the active site. In the R state H-Ras structure (PDB ID 3K8Y), a hydrogen bond between helix 4 residue Y137 and helix 3 residue H94 stabilizes the R-state helix 3 conformation (Fig. 3B). Both residues 94 and 95 are sites of isoform sequence diversity, in which position 95 is one of two where the residue identity is completely different across the isoforms (L95 in N-Ras, H95 in K-Ras, and Q95 in H-Ras). Differences in the hydrogen-bonding network of the allosteric site propagate across helix 3 toward switch II, where an altered set of interactions in K-Ras promotes a Y71 conformation that pushes switch I away from the active site (Fig. 3C). Although the T-state conformation is preferred in both N-Ras and K-Ras, this conformation is more extreme in the WT K-Ras structure (PDB ID 5UK9) (JA Parker, AY Volmar, S Pavlopoulos, and C Mattos, in prep.) and more intermediate in the WT N-Ras structure (PDB ID 5UHV) (Fig. 3A) (Johnson et al. 2017). Single-turnover hydrolysis experiments show that the hydrolysis rate constants for WT K- and N-Ras are indeed lower than that of H-Ras, consistent with a more prominent state 1 and/or T state conformation in solution (Johnson et al. 2017). Given that the binding affinity of K-Ras and H-Ras with Raf is essentially the same, we suspect that the energy barrier between state 1 and state 2 is lower in K-Ras than in N-Ras, which shows about a twofold reduction in binding affinity for Raf (Johnson et al. 2017). Because the effector lobe is 100% identical in terms of sequence among the three isoforms, the differences in biochemical properties of the isoforms can be attributed to differences in the allosteric lobe and differences in the balance of conformational states in solution.
Figure 2.
Residue differences in the three Ras isoforms. (A) H-Ras contains four unique residues (green), K-Ras contains three (blue), and N-Ras contains eight (orange). Positions 95 and 122 are different among the three isoforms (red). (B) Residue differences (red) mapped onto H-Ras (Protein Data Bank [PDB] ID 3K8Y, gray) cluster around 100% conserved motifs in Ras (blue).
Figure 3.
Isoform-specific residues promote structural differences in Ras isoforms. (A) Alignment of N-Ras(GppNHp) (Protein Data Bank [PDB] IL 5UHV, cyan), K-Ras (GppCH2p) (PDB ID 5UK9, salmon), and H-Ras(GppNHp) (PDB ID 3K8Y, gray). Isoform-specific residues in the allosteric site (B) promote differences in switch II conformations in the isoforms (C). The altered salt bridge interaction between E123 and R143 in N-Ras is shown in D.
Isoform-specific residues in the allosteric lobe tend to cluster around structurally important regions of Ras, such as motifs crucial for interacting with the nucleotide in the active site or the membrane. One example is the R123-E143 salt bridge, which connects the conserved 116NKCD119 and 143ETSAK147 motifs to position these residues for optimal interactions with the nucleotide. While the salt bridge is a head-on interaction in both H- and K-Ras, it is offset in N-Ras such that one nitrogen atom from R123 and one oxygen atom from E143 can interact (Fig. 3D) (Johnson et al. 2017). Isoform-specific residues A/P121, S/T127, and Y/F141 (H-/K-Ras, respectively) surround this important salt bridge, providing unique interactions in H-Ras compared with K-Ras in their GTP-bound forms. In addition to this salt bridge, the conserved motifs are in close proximity to residue pockets on Ras that are proposed to modulate Ras–membrane interactions in an isoform-specific manner. The helix 4 R128 and R135 hot spots and a pocket formed by H94, L133, S126, and Y137 in H-Ras, may have evolved to accommodate an isoform-specific set of lipid head groups, depending on where each isoform microlocalizes within the plasma membrane (Parker and Mattos 2015). Importantly, these isoform-specific allosteric residues are positioned along the helix 5 water-mediated hydrogen bond network that connects the active site to the membrane-interacting regions of Ras (Kearney et al. 2014). Therefore, residue differences in H-, N-, and K-Ras could lead to altered mechanisms of intraprotein communication, contributing to isoform-specific structure, biochemistry, and functional output.
K-Ras AND DYNAMICS PROVIDE INSIGHT INTO THE MOST ONCOGENIC ISOFORM
Despite their similarities in global structure and the effector lobe where they interact with a similar set of effector proteins, H-, N-, and K-Ras each promote distinct signaling outputs (Ehrhardt et al. 2002; Abankwa et al. 2010). While the HVR is implicated as the major driving force for these differences in the membrane environment (Henis et al. 2009), differences in the G domain of the isoforms could also drive isoform-specific functions. Unique structural and dynamical features of the Ras isoforms have thus become an area of intense research toward understanding the fundamental divergence among the isoforms, especially in the search for isoform-specific Ras therapies. In fact, MD simulations of the three isoforms find that K-Ras is significantly more flexible than N- or H-Ras (Gorfe et al. 2008). Importantly, both switch I and allosteric lobe regions, including helix 4, helix 5, and nucleotide bind loops, of K-Ras display the most conformational flexibility, indicating that this isoform has both unique nucleotide-binding characteristics and effector-allosteric lobe communication pathways (Gorfe et al. 2008; Kapoor and Travesset 2015). Over the course of simulations, K-Ras samples more “open” state 1 conformations, while N-Ras samples more intermediate conformations between state 2 (“closed”) and state 1 (Gorfe et al. 2008). It is interesting to note that in the inactive GDP-bound structures switch I is the most rigid in K-Ras when compared with the other two isoforms (Kapoor and Travesset 2015), suggesting a stable interaction mostly mediated by the canonical Y32-Y40 hydrogen bond observed in all GDP-bound Ras structures. These dynamical observations correlate directly to the open state 1 conformation of switch I observed in our recent crystal structure of WT K-Ras-GppCH2p (PDB ID 5UK9) (JA Parker, AY Volmar, S Pavlopoulos, and C Mattos, in prep.). 1H NMR experiments of WT K-Ras bound to nucleotide analogs indicate that the state 1 conformation is sampled in solution much more prominently than is the case for H-Ras, consistent with the crystal structures of the two isoforms (JA Parker, AY Volmar, S Pavlopoulos, and C Mattos, in prep.). The highly dynamic nature of K-Ras may pose additional challenges for targeting this isoform, which displays the highest mutation profile in human cancers (Hobbs et al. 2016).
Unlike the structure of activated WT K-Ras, the crystal structure of K-RasG12D-GppNHp (PDB ID 4DSN) shows a state 2 conformation for switch I, with T35 interacting with the Mg2+-nucleotide complex in the active site and Y32 placed over the nucleotide (Maurer et al. 2012). In this structure, the bridging water molecule is also present, interacting with both Y32 and the mutated D12 side chains. The negatively charged D12 side chain would clash with both the arginine finger and catalytically important residue Q61 and thus interfere with both GAP-mediated and intrinsic hydrolysis, respectively, of GTP to GDP on Ras (Franken et al. 1993; Scheffzek et al. 1997). Consistently, the 1H NMR signatures of K-RasG12D are markedly different from those of WT K-Ras, in which K- RasG12D appears to favor a state 2 conformation that is not sensitive to temperature changes (JA Parker, AY Volmar, S Pavlopoulos, and C Mattos, in prep.). These data indicate that oncogenic K-RasG12D obtains a more stable effector-competent binding conformation to propagate signaling through interactions with effector proteins while simultaneously resisting hydrolysis to lock the protein in this active conformation. The addition of solution NMR data provides novel insight into how specific oncogenic mutants can alter the balance of conformational states to increase their oncogenic propensity. These data can be used to direct more informed targeting of oncogenic Ras, especially in moving toward the goal of targeting the most prevalent K-Ras oncogenic amino acid substitution at codon 12 (Pylayeva-Gupta et al. 2011; Prior et al. 2012). The recent findings for WT K-Ras and K-RasG12D, driven by structural and biophysical characterization, advance the goal of obtaining an atomic level understanding of each isoform and oncogenic mutant, especially given the mounting evidence for isoform and mutant-specific functions and signaling outcomes (Cammarata et al. 2016; Hobbs et al. 2016).
CHALLENGES, SUCCESSES, AND FUTURE DIRECTIONS FOR TARGETING Ras
Since its discovery more than three decades ago, Ras has been one of the most highly sought-after drug targets in the fight against cancer. The failure of the blockbuster farnesyltransferase inhibitors (FTIs) was disappointing, and placed a spotlight on how little Ras structure, function, and biology was understood (Whyte et al. 1997). As discussed, when H-Ras is considered the model for the other isoforms, many key details of each proteins’ individual structure, including the balance of conformational states, biochemistry, and function, are overlooked. Today, although Ras is still considered “undruggable” in the traditional sense, the field has made major strides forward.
The picomolar affinity of Ras for its nucleotide, along with the millimolar cellular concentrations of GDP and GTP, make designing a small molecule that can directly compete with nucleotide binding a daunting task (John et al. 1990). Yet, there has been moderate success for small molecules designed to covalently target the G12C oncogenic mutation in Ras, especially important for the goal of selectively targeting prominent mutations linked to specific cancer types, in this case lung adenocarcinoma (Prior et al. 2012). These molecules bind near the active site, either directly in place of the GDP nucleotide or extending into the space between switch II and helix 3 (Ostrem et al. 2013; Lim et al. 2014). One drawback to these molecules is their limitation of targeting the reactive G12C side chain, a technique that will not work on other more prominent oncogenic side chains at residues 12, 13, and 61 (Hobbs et al. 2016). Other small molecules have been shown to bind to the outer switch II region of GDP-bound Ras, in which they interfere with the GEF-mediated nucleotide exchange to keep Ras in its signaling-inactive conformation (Maurer et al. 2012; Sun et al. 2012). Although blocking the GEF-mediated exchange may be a successful approach to abrogating signaling through WT Ras, many of the oncogenic mutants that maintain Ras in the GTP-bound state would be unaffected by this type of intervention.
Aside from the active site, the surface of Ras is relatively flat and lacks well-defined pockets that can be exploited for drug design. Targeting the allosteric site between helix 3, loop 7, and helix 4, which is key in the T-to-R state transition to form an active site poised for intrinsic hydrolysis, could be a useful tactic for targeting Ras with oncogenic mutations that impair GAP-mediated hydrolysis (Buhrman et al. 2010). Additionally, because intrinsic hydrolysis is proposed to be a major regulatory mechanism in the Ras/Raf/Mek/Erk signaling cascade, this approach could target Ras in a pathway-selective manner (Buhrman et al. 2011b). To date, no allosteric site inhibitor has been identified, possibly because of the small volume of the allosteric site, although design of inhibitors that mimic the size, shape, and chemical functionality of membrane lipid head group molecules should be investigated. A zinc cyclen, the only molecule that has been shown by crystallography to bind at the carboxyl terminus and loop 7 close to the allosteric site, appears to promote the effector-binding-deficient state 1 conformation of switch I (Rosnizeck et al. 2010). This is one example of an inhibitor that takes advantage of stabilizing a conformational state known to impair Ras-effector binding. The allosteric lobe contains other hot spots that have been identified experimentally and computationally as sites of protein–ligand interactions (Buhrman et al. 2011a). These sites have been largely unexplored, yet could provide opportunities to target Ras in an isoform-specific manner (Marcus and Mattos 2015). For example, many of these sites include membrane-binding residues that could be targeted to modulate the Ras–membrane interaction in a way that disrupts the functionally critical intramolecular communication networks between the allosteric and effector lobes (Parker and Mattos 2015). Another exciting area of interest is targeting Ras dimerization at the membrane, which may be a crucial mediator of correct Ras nanocluster formation and signaling conformations at the membrane (Holderfield and Morrison 2017).
Blocking the interaction between Ras and its multitude of effector proteins, including Raf, RalGDS, and PI3K, is an area of intense interest given the potential for one therapeutic to block multiple Ras–effector interactions and shut down signaling through Ras. However, targeting the protein–protein interaction (PPI) interface on Ras, which includes both dynamic switch I and switch II regions and is large and relatively flat, presents challenges for traditional small molecules (Lu et al. 2016b). Larger cyclic peptides and stapled SOS1 peptides have shown moderate success in binding Ras with mid-nanomolar affinities to block signaling through inhibition of the Ras–Raf interaction (Upadhyaya et al. 2014; Leshchiner et al. 2015). Other attempts at designing larger molecules to target the effector-binding sites of Ras include single-antibody fragments and monobodies (Tanaka et al. 2007; Spencer-Smith et al. 2016; Keeton et al. 2017).
With the recent advances in understanding the structural and conformational ensemble differences among the Ras isoforms, we are beginning to see that these isoform-specific properties could be exploited in design of potent and specific inhibitors. The first example of an inhibitor that takes advantage of differing conformational states between WT and mutant-activated K-Ras has been illustrated by the crystallization of a small engineered binding protein identified by yeast surface display in complex with K-RasG12D-GppNHp and WT K-Ras-GppNHp (PDB ID 5UFQ and 5UFE, respectively) (Kauke et al. 2017). This small protein binds Ras at switch II, in which a high number of hydrophobic residues selected during an affinity maturation process intercalate with hydrophobic residues on Ras. Although this highly complementary interaction contributes to the single-digit nanomolar affinity to K-Ras, moderate selectivity for the G12D mutant is achieved by taking advantage of the differences in conformational states in the switch I region observed in K-Ras. Although the binder protein does not directly interact with the oncogenic G12D mutation in the active site, the state 2 conformation of switch I in this mutant leads to a unique “lysine claw” interaction between residues in switch I and II and binder residues K32 and K40. This interaction is weakened in the WT K-Ras complex, indicating that the residue selection process directed the formation of a binder protein that can take advantage of the differences in conformational states in switch I, despite binding at switch II. Excitingly, the low nanomolar affinity and larger size of this binder can directly compete with Ras/Raf complex formation and has been shown to directly interfere with signaling through the Ras/Raf/Mek/Erk pathway (Kauke et al. 2017).
The future of cancer therapies, including those directed toward Ras-driven cancers, needs to focus on patient-specific treatments. Given that mutations in Ras differentially affect clinical outcomes, lengths of treatment, and treatment effectiveness, personalized medicine will be key in the fight against Ras (Asati et al. 2017). Studies have shown that a cancer with a G12D mutation responds to anti-Ras therapies, but other G12 mutations are unaffected (Mao et al. 2013). Additionally, screening for K-Ras biomarkers in patients to determine the most effective treatment has shown promise in improving individual clinical outcomes (Smith et al. 2010; Miller and Miller 2011). Given the hints from recent structural and biochemical studies, the hypothesis that each mutation can uniquely alter the balance of conformational states, especially in the most oncogenic isoform K-Ras, should be considered in future efforts to target these proteins. Overall, the idea that H-Ras can serve as an accurate model for the other isoforms should be reevaluated, with more focus on the details of the unique characteristics of each Ras isoform and how these can be exploited for design of potent and specific inhibitors.
Footnotes
Editors: Linda VanAelst, Julian Downward, and Frank McCormick
Additional Perspectives on Ras and Cancer in the 21st Century available at www.perspectivesinmedicine.org
REFERENCES
- Abankwa D, Gorfe AA, Hancock JF. 2007. Ras nanoclusters: Molecular structure and assembly. Semin Cell Dev Biol 18: 599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abankwa D, Gorfe AA, Hancock JF. 2008. Mechanisms of Ras membrane organization and signalling: Ras on a rocker. Cell Cycle 7: 2667–2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abankwa D, Gorfe AA, Inder K, Hancock JF. 2010. Ras membrane orientation and nanodomain localization generate isoform diversity. Proc Natl Acad Sci 107: 1130–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham SJ, Nolet RP, Calvert RJ, Anderson LM, Gaponenko V. 2009. The hypervariable region of K-Ras4B is responsible for its specific interactions with calmodulin. Biochemistry 48: 7575–7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahearn IM, Haigis K, Bar-Sagi D, Philips MR. 2012. Regulating the regulator: Post-translational modification of RAS. Nat Rev Mol Cell Biol 13: 39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadian MR, Stege P, Scheffzek K, Wittinghofer A. 1997. Confirmation of the arginine-finger hypothesis for the GAP-stimulated GTP-hydrolysis reaction of Ras. Nature 4: 686–689. [DOI] [PubMed] [Google Scholar]
- Araki M, Shima F, Yoshikawa Y, Muraoka S, Ijiri Y, Nagahara Y, Shirono T, Kataoka T, Tamura A. 2011. Solution structure of the state 1 conformer of GTP-bound H-Ras protein and distinct dynamic properties between the state 1 and state 2 conformers. J Biol Chem 286: 39644–39653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asati V, Mahapatra DK, Bharti SK. 2017. K-Ras and its inhibitors towards personalized cancer treatment: Pharmacological and structural perspectives. Eur J Med Chem 125: 299–314. [DOI] [PubMed] [Google Scholar]
- Boriack-Sjodin PA, Margarit SM, Bar-Sagi D, Kuriyan J. 1998. The structural basis of the activation of Ras by Sos. Nature 394: 337–343. [DOI] [PubMed] [Google Scholar]
- Bos JL, Rehmann H, Wittinghofer A. 2007. GEFs and GAPs: Critical elements in the control of small G proteins. Cell 129: 865–877. [DOI] [PubMed] [Google Scholar]
- Bourne HR, Sanders DA, McCormick F. 1991. The GTPase superfamily: Conserved structure and molecular mechanism. Nature 349: 117–127. [DOI] [PubMed] [Google Scholar]
- Buhrman G, Wink G, Mattos C. 2007. Transformation efficiency of RasQ61 mutants linked to structural features of the switch regions in the presence of Raf. Structure 15: 1618–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhrman G, Holzapfel G, Fetics S, Mattos C. 2010. Allosteric modulation of Ras positions Q61 for a direct role in catalysis. Proc Natl Acad Sci 107: 4931–4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhrman G, O’Connor C, Zerbe B, Kearney BM, Napoleon R, Kovrigina EA, Vajda S, Kozakov D, Kovrigin EL, Mattos C. 2011a. Analysis of binding site hot spots on the surface of Ras GTPase. J Mol Biol 413: 773–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhrman G, Kumar VS, Cirit M, Haugh JM, Mattos C. 2011b. Allosteric modulation of Ras-GTP is linked to signal transduction through RAF kinase. J Biol Chem 286: 3323–3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammarata MB, Schardon CL, Mehaffey MR, Rosenberg J, Singleton J, Fast W, Brodbelt JS. 2016. Impact of G12 mutations on the structure of K-Ras probed by ultraviolet photodissociation mass spectrometry. J Am Chem Soc 10.1021/jacs.6b04474. [DOI] [PubMed] [Google Scholar]
- Chen M, Peters A, Huang T, Nan X. 2016. Ras dimer formation as a new signaling mechanism and potential cancer therapeutic target. Mini Rev Med Chem 16: 391–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmaiah S, Bindu L, Tran TH, Gillette WK, Frank PH, Ghirlando R, Nissley DV, Esposito D, McCormick F, Stephen AG, et al. 2016. Structural basis of recognition of farnesylated and methylated KRas4b by PDEd. Proc Natl Acad Sci 13: E6766–E6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhardt A, Ehrhardt GR, Guo X, Schrader JW. 2002. Ras and relatives—Job sharing and networking keep an old family together. Exp Hematol 30: 1089–1106. [DOI] [PubMed] [Google Scholar]
- Fehrenbacher N, Bar-Sagi D, Philips M. 2009. Ras/MAPK signaling from endomembranes. Mol Oncol 3: 297–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetics SK, Guterres H, Kearney BM, Buhrman G, Ma B, Nussinov R, Mattos C. 2015. Allosteric effects of the oncogenic RasQ61L mutant on Raf-RBD. Structure 23: 505–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken SM, Scheidig AJ, Krengel U, Rensland H, Lautwein A, Geyer M, Scheffzek K, Goody RS, Kalbitzer HR, Pai EF, et al. 1993. Three-dimensional structures and properties of a transforming and a nontransforming glycine-12 mutant of p21H-Ras. Biochemistry 32: 8411–8420. [DOI] [PubMed] [Google Scholar]
- Gorfe AA, Grant BJ, McCammon JA. 2008. Mapping the nucleotide and isoform-dependent structural and dynamical features of Ras proteins. Structure 16: 885–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BJ, Gorfe AA, McCammon JA. 2009. Ras conformational switching: Simulating nucleotide-dependent conformational transitions with accelerated molecular dynamics. PLoS Comput Biol 5: e1000325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock JF, Parton RG. 2005. Ras plasma membrane signalling platforms. Biochem J 389: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock JF, Peterson H, Marshall CJ. 1990. A polybasic domain or palmitoylation is required in addition to the CAAX motif to localize p21Ras to the plasma membrane. Cell 63: 133–139. [DOI] [PubMed] [Google Scholar]
- Harrison RA, Lu J, Carrasco M, Hunter J, Manandhar A, Gondi S, Westover KD, Engen JR. 2016. Structural dynamics in Ras and related proteins upon nucleotide switching. J Mol Biol 428: 4723–4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henis YI, Hancock JF, Prior IA. 2009. Ras acylation, compartmentalization and signaling nanoclusters. Mol Membr Biol 26: 80–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs GA, Der CJ, Rossman KL. 2016. RAS isoforms and mutations in cancer at a glance. J Cell Sci 129: 287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holderfield M, Morrison DK. 2017. RAS signaling: Divide and conquer. Nat Chem Biol 13: 7–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang H, Muratcioglu S, Gursoy A, Keskin O, Nussinov R. 2016. Membrane-associated Ras dimers are isoform-specific: K-Ras dimers differ from H-Ras dimers. Biochem J 473: 1719–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John J, Sohmen R, Feuerstein J, Linke R, Wittinghofer A, Goody RS. 1990. Kinetics of interaction of nucleotides with nucleotide-free H-ras p21. Biochemistry 29: 6058–6065. [DOI] [PubMed] [Google Scholar]
- Johnson CW, Reid D, Parker JA, Salter S, Knihtila R, Kuzmic P, Mattos C. 2017. The small GTPases K-Ras, N-Ras and H-Ras have distinct biochemical properties determined by allosteric effects. J Biol Chem 292: 12981–12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor A, Travesset A. 2015. Differential dynamics of RAS isoforms in GDP- and GTP-bound states. Proteins 83: 1091–1106. [DOI] [PubMed] [Google Scholar]
- Kauke MJ, Traxlmayr MW, Parker JA, Kiefer JK, Knihtila R, McGee J, Verdine G, Mattos C, Wittrup KD. 2017. An engineered protein antagonist of K-Ras/B-Raf interaction. Sci Rep 7: 5831–5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney BM, Johnson CW, Roberts DM, Swartz P, Mattos C. 2014. DRoP: A water analysis program identifies Ras-GTP-specific pathway of communication between membrane-interacting regions and the active site. J Mol Biol 426: 611–629. [DOI] [PubMed] [Google Scholar]
- Keeton AB, Salter EA, Piazza GA. 2017. The RAS-effector interaction as a drug target. Cancer Res 77: 221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knihtila R, Holzapfel G, Weiss K, Meilleur F, Mattos C. 2015. Neutron crystal structure of RAS GTPase puts in question the protonation state of the GTP γ-phosphate. J Biol Chem 290: 31025–31036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshchiner ES, Parkhitko A, Bird GH, Luccarelli J, Bellairs JA, Escudero S, Opoku-Nsiah K, Godes M, Perrimon N, Walensky LD. 2015. Direct inhibition of oncogenic KRAS by hydrocarbon-stapled SOS1 helices. Proc Natl Acad Sci 112: 1761–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SM, Westover KD, Ficarro SB, Harrison RA, Choi HG, Pacold ME, Carrasco M, Hunter J, Kim ND, Xie T, et al. 2014. Therapeutic targeting of oncogenic K-Ras by a covalent catalytic site inhibitor. Angew Chem Int Ed Engl 53: 199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S, Jang H, Nussinov R, Zhang J. 2016a. The structural basis of oncogenic mutations G12, G13 and Q61 in small GTPase K-Ras4B. Sci Rep 6: 21949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S, Jang H, Gu S, Zhang J, Nussinov R. 2016b. Drugging Ras GTPase: A comprehensive mechanistic and signaling structural view. Chem Soc Rev 45: 4929–4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao C, Huang YF, Yang ZY, Zheng DY, Chen JZ, Tang JL. 2013. KRAS p.G13D mutation and codon 12 mutations are not created equal in predicting clinical outcomes of cetuximab in metastatic colorectal cancer: A systematic review and meta-analysis. Cancer 119: 714–721. [DOI] [PubMed] [Google Scholar]
- Marcus K, Mattos C. 2015. Direct attack on RAS: Intramolecular communication and mutation-specific effects. Clin Cancer Res 21: 1810–1818. [DOI] [PubMed] [Google Scholar]
- Maurer T, Garrenton LS, Oh A, Pitts K, Anderson DJ, Skelton NJ, Fauber BP, Pan B, Malek S, Stokoe D, et al. 2012. Small-molecule ligands bind to a distinct pocket in Ras and inhibit SOS-mediated nucelotide exchange activity. Proc Natl Acad Sci 109: 5299–5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M, Tafuri A, et al. 2007. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta 1773: 1263–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MS, Miller LD. 2011. RAS mutations and oncogenesis: Not all RAS mutations are created equally. Front Genet 2: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrem JM, Peters U, Sos ML, Wells JA, Shokat KM. 2013. K-RasG12C inhibitors allosterically control GTP affinity and effector interactions. Nature 503: 548–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan JY, Wessling-Resnick M. 1998. GEF-mediated GDP/GTP exchange by monomeric GTPases: A regulatory role for Mg2+? BioEssays 20: 516–521. [DOI] [PubMed] [Google Scholar]
- Parker JA, Mattos C. 2015. The Ras-membrane interface: Isoform-specific differences in the catalytic domain. Mol Cancer Res 13: 595–603. [DOI] [PubMed] [Google Scholar]
- Prakash P, Sayyed-Ahmad A, Cho KJ, Dolino DM, Chen W, Li H, Grant BJ, Hancock JF, Gorfe AA. 2017. Computational and biochemical characterization of two partially overlapping interfaces and multiple weak-affinity K-Ras dimers. Sci Rep 7: 40109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior IA, Lewis PD, Mattos C. 2012. A comprehensive survey of Ras mutations in cancer. Cancer Res 72: 2457–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pylayeva-Gupta Y, Grabocka E, Bar-Sagi D. 2011. RAS oncogenes: Weaving a tumorigenic web. Nat Rev Cancer 11: 761–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimondi F, Portella G, Orozco M, Fanelli F. 2011. Nucleotide binding switches the information flow in Ras GTPases. PLoS Comput Biol 7: e1001098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosnizeck IC, Graf T, Spoerner M, Tränkle J, Filchtinski D, Herrmann C, Gremer L, Vetter IR, Wittinghofer A, König B, et al. 2010. Stabilizing a weak binding state for effectors in the human Ras protein by cyclen complexes. Angew Chem Int Ed Engl 49: 3830–3833. [DOI] [PubMed] [Google Scholar]
- Rudack T, Xia F, Schlitter J, Kötting C, Gerwert K. 2012. The role of magnesium for geometry and charge in GTP hydrolysis, revealed by quantum mechanics/molecular mechanics simulations. Biophys J 103: 293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos E. 2014. Dimerization opens new avenues into Ras signaling research. Sci Signal 7: e12. [DOI] [PubMed] [Google Scholar]
- Scheffzek K, Ahmadian MR, Kabsch W, Wiesmüller L, Lautwein A, Schmitz F, Wittinghofer A. 1997. The Ras-RasGAP complex: Structural basis for GTPase activation and its loss in oncogenic Ras mutants. Science 277: 333–338. [DOI] [PubMed] [Google Scholar]
- Shima F, Ijiri Y, Muraoka S, Liao J, Ye M, Araki M, Matsumoto K, Yamamoto N, Sugimoto T, Yoshikawa Y, et al. 2010. Structural basis for conformational dynamics of GTP-bound Ras protein. J Biol Chem 285: 22696–22705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MJ, Ikura M. 2014. Integrated RAS signaling defined by parallel NMR detection of effectors and regulators. Nature Chem Biol 10: 223–230. [DOI] [PubMed] [Google Scholar]
- Smith G, Bounds R, Wolf H, Steele RJ, Carey FA, Wolf CR. 2010. Activating K-Ras mutations outwith “hotspot” codons in sporadic colorectal tumours—Implications for personalised cancer medicine. Br J Cancer 102: 693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer-Smith R, Koide A, Zhou Y, Eguchi RR, Sha F, Gajwani P, Santana D, Gupta A, Jacobs M, Herrero-Garcia E, et al. 2016. Inhibition of Ras function through targeting an allosteric regulatory site. Nature Chem Biol 13: 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoerner M, Herrmann C, Vetter IR, Kalbitzer HR, Wittinghofer A. 2001. Dynamic properties of the Ras switch I region and its importance for binding to effectors. Proc Natl Acad Sci 98: 4944–4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoerner M, Hozsa C, Poetzl A, Reiss K, Ganser P, Geyer M, Kalbitzer HR. 2010. Conformational states of human rat sarcoma (Ras) protein complexed with its natural ligand GTP and their role for effector interaction and GTP hydrolysis. J Biol Chem 285: 39768–39778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Burke JP, Phan J, Burns MC, Olejniczak ET, Waterson AG, Lee T, Rossanese OW, Fesik SW. 2012. Discovery of small molecules that bind to K-Ras and inhibit Sos-mediated activation. Agnew Chem Int Ed 51: 6140–6143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Williams RL, Rabbitts TH. 2007. Tumor prevention by a single antibody domain targeting the interaction of signal transduction proteins with RAS. EMBO J 26: 3250–3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson H. 2013. US National Cancer Institute’s new Ras project targets an old foe. Nat Med 19: 949–950. [DOI] [PubMed] [Google Scholar]
- Tian T, Harding A, Inder K, Plowman S, Parton RG, Hancock JF. 2007. Plasma membrane nanoswitches generate high-fidelity Ras signal transduction. Nat Cell Biol 9: 905–914. [DOI] [PubMed] [Google Scholar]
- Tsai FD, Lopes MS, Zhou M, Court H, Ponce O, Fiordalisi JJ, Gierut JJ, Cox AD, Haigis KM, Philips MR. 2015. K-Ras4A splice variant is widely expressed in cancer and uses a hybrid membrane-targeting motif. Proc Natl Acad Sci 112: 779–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyaya P, Qian Z, Habir NA, Pei D. 2014. Direct Ras inhibitors identified from a structurally rigidified bicyclic peptide library. Tetrahedron 70: 7714–7720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencia A, Chardin P, Wittinghofer A, Sander C. 1991. The Ras protein family: Evolutionary tree and the role of conserved amino acids. Biochemistry 30: 4637–4648. [DOI] [PubMed] [Google Scholar]
- Vetter IR. 2014. The structure of the G domain of the Ras superfamily. In Ras superfamily small G proteins: Biology and mechanisms 1: General (ed. Wittinghofer A), pp. 25–50. Springer, Vienna. [Google Scholar]
- Whyte DB, Kirschmeier P, Hockenberry TN, Nunez-Oliva I, James L, Catino JJ, Bishop WR, Pai JK. 1997. K- and N-Ras are geranylgeranylated in cells treated with farnesyl protein transferase inhibitors. J Biol Chem 272: 14459–14464. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Liang H, Rodkey T, Ariotti N, Parton RG, Hancock JF. 2014. Signal integration by lipid-mediated spatial cross talk between Ras nanoclusters. Mol Cell Biol 34: 862–876. [DOI] [PMC free article] [PubMed] [Google Scholar]