Abstract
Most truncating cadherin 1 (CDH1) pathogenic alterations confer an elevated lifetime risk of diffuse gastric cancer (DGC) and lobular breast cancer (LBC). However, transcripts containing carboxy-terminal premature stop codons have been demonstrated to escape the nonsense-mediated mRNA decay pathway, and gastric and breast cancer risks associated with these truncations should be carefully evaluated. A female patient underwent multigene panel testing because of a personal history of invasive LBC diagnosed at age 54, which identified the germline CDH1 nonsense alteration, c.2506G>T (p.Glu836*), in the last exon of the gene. Subsequent parental testing for the alteration was negative and additional short tandem repeat analysis confirmed the familial relationships and the de novo occurrence in the proband. Based on the de novo occurrence, clinical history, and rarity in general population databases, this alteration was classified as a likely pathogenic variant. This is the most carboxy-terminal pathogenic alteration reported to date. Additionally, this alteration contributed to the classification of six other upstream CDH1 carboxy-terminal truncating variants as pathogenic or likely pathogenic. Identifying the most distal pathogenic alteration provides evidence to classify other carboxy-terminal truncating variants as either pathogenic or benign, a fundamental step to offering presymptomatic screening and prophylactic procedures to the appropriate patients.
Keywords: neoplasm of the breast, neoplasm of the gastrointestinal tract
CASE PRESENTATION
The cadherin 1 (CDH1) gene (NM_004360.3) encodes E-cadherin, a cellular adhesion protein that acts as a tumor suppressor by inhibiting cell invasion (Kourtidis et al. 2017). Germline mutations in CDH1 cause hereditary diffuse gastric cancer (HDGC) and lobular breast cancer (LBC) syndrome (OMIM 137215). For carriers of pathogenic alterations, the estimated lifetime risk of developing diffuse gastric cancer (DGC) is up to 70% for men and up to 56% for women (Hansford et al. 2015). Women also have an approximate 42% lifetime risk of LBC (Hansford et al. 2015). A clinical diagnosis of HDGC is established in a proband with confirmed DGC and meeting one of the following criteria: (1) at least one first- or second-degree relative with gastric cancer at any age; (2) a diagnosis of DGC before the age of 40; (3) family history of both DGC and LBC (one diagnosis before the age of 50). Confirmatory genetic testing is suggested. Genetic testing should also be considered for patients with other HDGC-associated phenotypes, including history of bilateral LBC or family history of at least two LBC cases before the age of 50, patients with history of DGC and cleft lip/palate, and those identified with signet ring cell (SRC) carcinoma precursor lesions by gastric endoscopy (van der Post et al. 2015a).
The majority of germline CDH1 pathogenic alterations result in premature stop codons (e.g., nonsense, frameshift, canonical ±1 or 2 splice sites), most of which are predicted to elicit nonsense-mediated decay (NMD), a mRNA quality control pathway that destabilizes abnormal transcripts containing premature stop codons (Karam et al. 2008). NMD is triggered by the translation machinery detecting an exon junction protein complex downstream from a premature stop codon; however, premature stop codons located in close proximity to, or beyond, the last exon–exon junction are predicted to escape NMD (Holbrook et al. 2004; Rivas et al. 2015). This “NMD boundary” is located in the penultimate exon of any transcript, ∼55 nt upstream of the last exon–exon junction. Premature stop codons located downstream from the boundary are predicted to escape NMD because there is no exon–exon junction complex downstream to be detected by the translation machinery (Holbrook et al. 2004; Karam et al. 2013). This is particularly relevant for variant interpretation, because transcripts containing truncating variants located downstream from the boundary cannot be assumed to undergo degradation by the NMD pathway. For this reason, the American College of Medical Genetics and Genomics and the Association for Molecular Pathology (ACMG/AMP) recommends caution and consideration of additional lines of evidence, such as clinical evidence, RNA analysis, and/or structural analysis, when classifying truncating variants predicted to escape NMD (Richards et al. 2015).
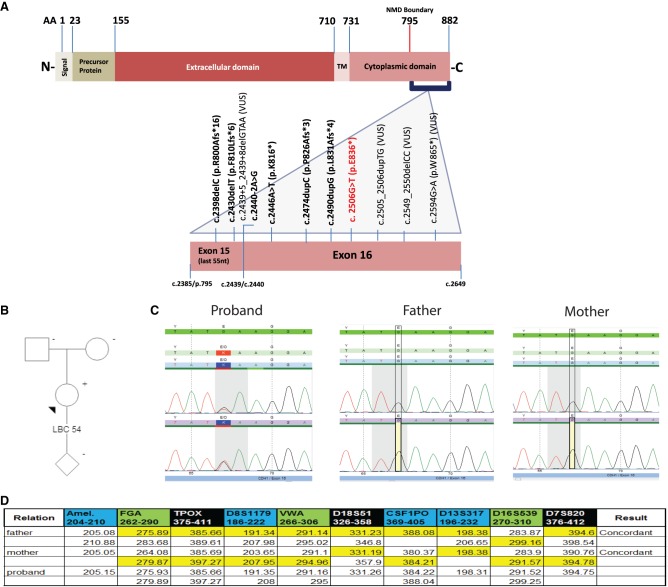
With this recommendation in mind, a retrospective review of a diagnostic laboratory cohort of approximately 490,000 sequenced CDH1 alleles was performed to identify all CDH1 alterations that resulted in premature stop codons past the NMD boundary (∼c.2385/p.795). A total of 11 carboxy-terminal alterations were identified (Fig. 1A), the most carboxy-terminal pathogenic truncation being CDH1 c.2506G>T (p.Glu836*). This alteration was identified in an Asian female individual that underwent multigene panel testing (MGPT) because of a personal history of an ER+/PR−/HER2− LBC diagnosed at age 54 (Fig. 1B). Of note, the MGPT also included and was negative for alterations in the following genes: ATM, BARD1, BRCA1, BRCA2, BRIP1, CHEK2, EPCAM, MLH1, MRE11A, MSH2, MSH6, MUTYH, NBN, NF1, PALB2, PMS2, PTEN, RAD50, RAD51C, RAD51D, SMARCA4, STK11, and TP53. Family history was not suspicious for HDGC. Subsequent parental testing revealed both parents were negative for the alteration (Fig. 1C), and additional short tandem repeat analysis confirmed the familial relationships and the de novo occurrence in the proband (Fig. 1D). In addition, seven alterations were identified downstream from the CDH1 NMD boundary but upstream of c.2506G>T (p.Glu836*), as well as three other truncations past amino acid position 836 (Fig. 1A).
Figure 1.
Characterization of CDH1 carboxy-terminal variants. (A) Schematic representation of the E-cadherin protein highlighting the germline alterations identified in the gene's NMD resistant zone. (B) Proband with a personal history of LBC diagnosed at age 54, tested positive for CDH1 c.2506G>T (p.Glu836*) on MGPT. (C) Sanger sequencing of the proband and her parents confirmed the alteration in the proband and revealed parents were negative. (D) Short tandem repeat analysis confirmed paternity and de novo occurrence of CDH1 c.2506G>T (p.Glu836*) in the proband.
TECHNICAL ANALYSIS AND METHODS
A retrospective review of the Ambry Genetics database was performed to identify all CDH1 variants that resulted in premature stop codons past the NMD boundary. Demographic, clinical history, and cancer family history were collected from test requisition forms, clinic notes, pathology reports, and pedigrees provided by ordering clinicians at the time of testing. All variants underwent assessment and review of available evidence (e.g., population frequency information, published case reports, case/control and functional studies, internal cooccurrence and cosegregation data, evolutionary conservation, and in silico predictions). Variants were further classified following a five-tier variant classification protocol (pathogenic mutation; likely pathogenic [LP]; variant of uncertain significance [VUS]; likely benign [LB]; and benign) which is based on published guidelines by the American College of Medical Genetics and Genomics and the Association for Molecular Pathology (ACMG/AMP) (Richards et al. 2015; Pesaran et al. 2016). All variants classified by Ambry Genetics are submitted to the ClinVar public database.
All patients were tested between May 2013 and January 2018, and underwent analysis of the CDH1 gene via multigene panel testing, single-gene analysis, or targeted testing for a familial alteration. All testing at Ambry Genetics was performed by next-generation sequencing analysis or Sanger sequencing, depending on the clinical test ordered. Targeted next-generation sequencing was performed on samples received for multigene panels (Shendure and Ji 2008; Mamanova et al. 2010). Briefly, genomic deoxyribonucleic acid (gDNA) was isolated from the patient's specimen using standardized methodology and quantified. Sequence enrichment was carried out by a bait-capture methodology using long biotinylated oligonucleotide probes followed by polymerase chain reaction (PCR) and next-generation sequencing on HiSeq2500 or NextSeq500 instruments (Illumina). Additional Sanger sequencing was performed for any regions missing or with insufficient read depth coverage for reliable heterozygous variant detection. Reportable small insertions and deletions, potentially homozygous variants, variants in regions complicated by pseudogene interference, and single-nucleotide variant calls not satisfying 100× depth of coverage and 40% het ratio thresholds were verified by Sanger sequencing (Mu et al. 2016). Sanger sequencing was performed on samples received for single-site analysis or full-gene analysis (Sanger et al. 1977; Smith et al. 1986). Briefly, gDNA was isolated from the patient's specimen using standardized methodology, quantified, and amplified with gene-specific primers and bidirectionally sequenced using Big Dye Terminator version 3.1 on an ABI3730xl DNA analyzer (Applied Biosystems). Chromatogram analysis was conducted using Sequence Pilot version 4.2.1 (JSI Medical Systems).
VARIANT INTERPRETATION
Although most CDH1 variants resulting in truncations upstream of the NMD boundary can be confidently classified as pathogenic, classification of carboxy-terminal truncations that escape NMD are less straightforward, as these transcripts may result in partially functional proteins (Sasaki et al. 2000). In this scenario, the ACMG/AMP guidelines recommend that other lines of evidence be considered, such as the existence of other well-characterized downstream carboxy-terminal truncating pathogenic variants (Richards et al. 2015). To our knowledge, the most carboxy-terminal pathogenic truncation previously described in the literature is c.2430delT (Hansford et al. 2015). Therefore CDH1 c.2506G>T (p.Glu836*), being downstream from this alteration, could not be assumed to result in loss of function, and interpretation depended on additional evidence. In this specific case, the de novo occurrence proved fundamental to its classification, in addition to the proband's clinical history and the variant's rarity in general population databases (Table 1). Altogether this evidence was sufficient to classify CDH1 c.2506G>T (p.Glu836*) as a likely pathogenic clinically actionable alteration. According to the National Comprehensive Cancer Network (NCCN) and others (van der Post et al. 2015a), medical management for carriers of clinically actionable CDH1 alterations (pathogenic/likely pathogenic) includes presymptomatic screening and preventive measures, such as prophylactic gastrectomy and consideration of risk-reducing mastectomy. Because of the high cancer risks and potential for morbidity associated with pathogenic CDH1 alterations, it is important to correctly identify these alterations and manage at-risk individuals appropriately.
Table 1.
Summary of evidence for CDH1 carboxy-terminal variants identified in a diagnostic laboratory cohort of approximately 490,000 sequenced CDH1 alleles
| Genomic location | HGVS cDNA | HGVS protein | Zygosity | Proband tumor (age) | Family criteria Met (A-F)a | Ambry frequency (X/490,000 CDH1 alleles) | Population frequency (gnomAD) | Literature | ACMG/AMP criteria Metb | Variant interpretation |
|---|---|---|---|---|---|---|---|---|---|---|
| Chr 16:68863659 (GRCh37) | NM_004360.3: c.2398delC | p.Arg800Alafs*16 | Heterozygous | NP | NP | 1/490,000 | NA | Kaurah et al. 2007 (Newfoundland founder mutation; four Newfoundland families with DGC/LBC), Petridis et al. 2014 (patient with bilat LCIS and unilateral LBC in 30s), Muir et al. 2016 (two gastric/breast cancer families) | PVS1 PM2 PP4 |
Pathogenic |
| Chr 16:68863691 (GRCh37) | NM_004360.3: c.2430delT | p.Phe810Leufs*6 | Heterozygous | 1) LCIS 39 2) DGC 68 3) LBC 37 4) IDC 40s |
1) D 2) A 3) D 4) None |
4/490,000 | NA | Hansford et al. 2015 (seen in a patient with DGC at 45 and three family members with gastric cancer) | PVS1 PM2 PP4 |
Pathogenic |
| Chr 16:68863705-68863708 (GRCh37) | NM_004360.3: c.2439+5_2439+8delGTAA | NA | Heterozygous | 1) Breast NOS 76 2) Endometrial 64 |
1) None 2) None |
2/490,000 | NA | van der Post et al. 2015b (patient with DGC, LBC in 50s) | PM2 PP3 PP4 |
Variant of uncertain significance |
| Chr 16:68867191 (GRCh37) | NM_004360.3: c.2440-2A>G | NA | Heterozygous | LBC 40s | None | 1/490,000 | NA | NA | PVS1 PM2 PP3 PP4 |
Likely pathogenic |
| Chr 16:68867199 (GRCh37) | NM_004360.3: c.2446A>T | p.Lys816* | Heterozygous | DGC 32 | B | 1/490,000 | NA | NA | PVS1 PM2 PP4 |
Likely pathogenic |
| Chr 16:68867227 (GRCh37) | NM_004360.3: c.2474dupC | p.Pro826Alafs*3 | Heterozygous | LBC 52 | None | 1/490,000 | NA | NA | PVS1 PM2 PP4 |
Likely pathogenic |
| Chr 16:68867243 (GRCh37) | NM_004360.3: c.2490dupG | p.Leu831Alafs*4 | Heterozygous | DGC 31 | B | 1/490,000 | NA | NA | PVS1 PM2 PP4 |
Likely pathogenic |
| Chr 16:68867259 (GRCh37) | NM_004360.3: c.2506G>T | p.Glu836* | Heterozygous | LBC 54 confirmed de novo | None | 1/490,000 | NA | NA | PVS1 PS2 PM2 PP4 |
Likely pathogenic |
| Chr 16:68867258-68867259 (GRCh37) | NM_004360.3: c.2505_2506dupTG | p.Gln836Valfs*11 | Heterozygous | IDC 45 | None | 1/490,000 | NA | NA | PM2 | Variant of uncertain significance |
| Chr 16:68867302-68867303 (GRCh37) | NM_004360.3: c.2549_2550delCC | p.Ser850Phefs*10 | Heterozygous | Bilat breast 43 (right: IDC/LBC, left: DCIS) | None | 1/490,000 | NA | NA | PM2 | Variant of uncertain significance |
| Chr 16:68867347 (GRCh37) | NM_004360.3: c.2594G>A | p.Trp865* | Heterozygous | None | None | 1/490,000 | NA | NA | PM2 | Variant of uncertain significance |
Bilat, bilateral; DCIS, ductal carcinoma in situ; DGC, diffuse gastric cancer; GC, gastric cancer; IDC, invasive ductal carcinoma; LBC, lobular breast cancer; LCIS, lobular carcinoma in situ; NOS, not otherwise specified; NP, not provided; NA, not available.
aCDH1 testing criteria adapted from van der Post et al. (2015a):
A) Two or more GC cases regardless of age, at least one confirmed DGC, in first-degree and second-degree relatives
B) One case of DGC <40
C) Personal or family history of DGC and LBC, one diagnosed <50
D) Bilateral LBC or family history of 2 or more cases of LBC <50
E) A personal or family history of cleft lip/palate in a patient with DGC
F) In situ signet ring cells and/or pagetoid spread of signet ring cells
bACMG/AMP criteria described by Richards et al. 2015:
PVS1 (pathogenic, very strong evidence): null variant (nonsense, frameshift, canonical ±1 or 2 splice sites, initiation codon, single, or multiexon deletion) in a gene where LOF is a known mechanism of disease.
PS2 pathogenic, strong evidence): de novo (both maternity and paternity confirmed) in a patient with the disease and no family history.
PM2 (pathogenic, moderate evidence): absent from controls (or at extremely low frequency if recessive) in Exome Sequencing Project, 1000 Genomes Project, or Exome Aggregation Consortium.
PP3 (pathogenic, supporting evidence): multiple lines of computational evidence support a deleterious effect on the gene or gene product (splicing impact).
PP4 (pathogenic, supporting evidence): patient's phenotype or family history is highly specific for a disease with a single genetic etiology.
The five carboxy-terminal truncations located upstream of CDH1 c.2506G>T (p.Glu836*) were c.2398delC (p.Arg800Alafs*16), c.2430delT (p.Phe810Leufs*6), c.2446A>T (p.Lys816*), c.2474dupC (p.Pro826Alafs*3), and c.2490dupG (p.Leu831Alafs*4). They all met the predicted loss of function, phenotype, and rarity ACMG/AMP criteria and were classified as pathogenic or likely pathogenic variants (Table 1). These variants were associated with DGC and/or LBC in our cohort or the literature and were absent in population databases (Table 1). Because these alterations are predicted to result in transcripts that escape NMD, the proposed mechanism for pathogenicity is loss of function due to disruption of the cytoplasmic domain, which includes the catenin-binding domain (Fig. 1A). The catenin-binding domain promotes protein clustering at the adherens junction and stabilizes cell adhesion (Kourtidis et al. 2017). Therefore, truncation of this region of the protein may impair cellular adhesion and promote cell invasion.
Of the two splicing variants identified, c.2439+5_2439+8delGTAA is described in the literature in a family meeting clinical diagnostic criteria for CDH1 testing (van der Post et al. 2015b). However, its functional effect is uncertain, and, as such, it is classified as a variant of uncertain significance (VUS) at this time. The other splicing variant, c.2440-2A>G, is predicted to affect splicing by in silico tools and was seen in a patient with LBC. It was classified as a likely pathogenic variant because of its position at the canonical splice acceptor site, in silico predictions, and rarity (Table 1).
With regards to the three truncations identified past CDH1 c.2506G>T (p.Glu836*)—c.2505_2506dupTG (p.Gln836Valfs*11), c.2549_2550delCC (p.Ser850Phefs*10), and c.2594G>A (p.Trp865*)—predicted loss of function was not used in their classification, because of the uncertainty surrounding the clinical relevance of the amino acids located downstream from amino acid position 836. Additionally, none of the reported families met clinical diagnostic criteria for CDH1 testing. Therefore, the three truncations located downstream from CDH1 c.2506G>T (p.Glu836*) were classified as VUS (Table 1).
SUMMARY
Carboxy-terminal truncating alterations in CDH1 may escape the NMD pathway and result in proteins retaining partial function. Because of the uncertainty surrounding the functional impact of these alterations, multiple lines of evidence are essential in determining pathogenicity. Here we describe the most carboxy-terminal CDH1 clinically actionable alteration in our cohort, in addition to other carboxy-terminal truncations in this gene. Identifying the most distal pathogenic alteration provides evidence to correctly classify other carboxy-terminal truncating variants. This is a critical component to proper patient management that highlights the importance of continued data sharing efforts.
ADDITIONAL INFORMATION
Database Deposition and Access
All interpreted variants are deposited in ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/) and can be found under accession numbers SCV000700322.1, SCV000218032.4, SCV000187463.4, SCV000700319.1, SCV000700320.1, SCV000665400.1, SCV000580713.2, SCV000661653.1, SCV000665390.1, SCV000185568.4, and SCV000700321.1.
Ethics Statement
This study was approved and carried out in accordance with the recommendations of the Western Institutional Review Board (WIRB), as part of the protocol titled “Sharing the results of genetic functional assessments performed for subjects previously submitted for clinical genomic testing.” Appropriate written informed consent was obtained prior to the collection of study data and the use of this data in analysis and the resulting publication.
Acknowledgments
We would like to thank the patients and medical providers that contributed with samples and clinical data.
Author Contributions
K.K. and R.K. drove the development of the intellectual concepts, performed analyses, interpreted data, and wrote the manuscript.
Funding
This study was funded entirely by Ambry Genetics.
Competing Interest Statement
All authors were employees of Ambry Genetics when they were engaged with this project.
Referees
Raymond Kim
Anonymous
REFERENCES
- Hansford S, Kaurah P, Li-Chang H, Woo M, Senz J, Pinheiro H, Schrader KA, Schaeffer DF, Shumansky K, Zogopoulos G, et al. 2015. Hereditary diffuse gastric cancer syndrome: CDH1 mutations and beyond. JAMA Oncol 1: 23–32. [DOI] [PubMed] [Google Scholar]
- Holbrook JA, Neu-Yilik G, Hentze MW, Kulozik AE. 2004. Nonsense-mediated decay approaches the clinic. Nat Genet 36: 801–808. [DOI] [PubMed] [Google Scholar]
- Karam R, Carvalho J, Bruno I, Graziadio C, Senz J, Huntsman D, Carneiro F, Seruca R, Wilkinson MF, Oliveira C. 2008. The NMD mRNA surveillance pathway downregulates aberrant E-cadherin transcripts in gastric cancer cells and in CDH1 mutation carriers. Oncogene 27: 4255–4260. [DOI] [PubMed] [Google Scholar]
- Karam R, Wengrod J, Gardner LB, Wilkinson MF. 2013. Regulation of nonsense-mediated mRNA decay: implications for physiology and disease. Biochim Biophys Acta 1829: 624–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaurah P, MacMillan A, Boyd N, Senz J, De Luca A, Chun N, Suriano G, Zaor S, Van Manen L, Gilpin C, et al. 2007. Founder and recurrent CDH1 mutations in families with hereditary diffuse gastric cancer. JAMA 297: 2360–2372. [DOI] [PubMed] [Google Scholar]
- Kourtidis A, Lu R, Anastasiadis PZ. 2017. A central role for cadherin signaling in cancer. Exp Cell Res 358: 78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamanova L, Coffey AJ, Scott CE, Kozarewa I, Turner EH, Kumar A, Howard E, Shendure J, Turner DJ. 2010. Target-enrichment strategies for next-generation sequencing. Nat Methods 7: 111–118. [DOI] [PubMed] [Google Scholar]
- Mu W, Lu HM, Chen J, Li S, Elliott AM. 2016. Sanger confirmation is required to achieve optimal sensitivity and specificity in next-generation sequencing panel testing. J Mol Diagn 18: 923–932. [DOI] [PubMed] [Google Scholar]
- Muir J, Aronson M, Esplen MJ, Pollett A, Swallow CJ. 2016. Prophylactic total gastrectomy: a prospective cohort study of long-term impact on quality of life. J Gastrointest Surg 20: 1950–1958. [DOI] [PubMed] [Google Scholar]
- Pesaran T, Karam R, Huether R, Li S, Farber-Katz S, Chamberlin A, Chong H, LaDuca H, Elliott A. 2016. Beyond DNA: an integrated and functional approach for classifying germline variants in breast cancer genes. Int J Breast Cancer 2016: 2469523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petridis C, Shinomiya I, Kohut K, Gorman P, Caneppele M, Shah V, Troy M, Pinder SE, Hanby A, Tomlinson I, et al. 2014. Germline CDH1 mutations in bilateral lobular carcinoma in situ. Br J Cancer 110: 1053–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, et al. 2015. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17: 405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas MA, Pirinen M, Conrad DF, Lek M, Tsang EK, Karczewski KJ, Maller JB, Kukurba KR, DeLuca DS, Fromer M, et al. 2015. Human genomics. Effect of predicted protein-truncating genetic variants on the human transcriptome. Science 348: 666–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F, Nicklen S, Coulson AR. 1977. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci 74: 5463–5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki CY, Lin H, Morin PJ, Longo DL. 2000. Truncation of the extracellular region abrogrates cell contact but retains the growth-suppressive activity of E-cadherin. Cancer Res 60: 7057–7065. [PubMed] [Google Scholar]
- Shendure J, Ji H. 2008. Next-generation DNA sequencing. Nat Biotechnol 26: 1135–1145. [DOI] [PubMed] [Google Scholar]
- Smith LM, Sanders JZ, Kaiser RJ, Hughes P, Dodd C, Connell CR, Heiner C, Kent SB, Hood LE. 1986. Fluorescence detection in automated DNA sequence analysis. Nature 321: 674–679. [DOI] [PubMed] [Google Scholar]
- van der Post RS, Vogelaar IP, Carneiro F, Guilford P, Huntsman D, Hoogerbrugge N, Caldas C, Schreiber KE, Hardwick RH, Ausems MG, et al. 2015a. Hereditary diffuse gastric cancer: updated clinical guidelines with an emphasis on germline CDH1 mutation carriers. J Med Genet 52: 361–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Post RS, Vogelaar IP, Manders P, van der Kolk LE, Cats A, van Hest LP, Sijmons R, Aalfs CM, Ausems MG, Gómez García EB, et al. 2015b. Accuracy of hereditary diffuse gastric cancer testing criteria and outcomes in patients with a germline mutation in CDH1. Gastroenterology 149: 897–906.e19. [DOI] [PubMed] [Google Scholar]