Abstract
Objectives
The varying mechanical properties of human bone have influence on the study results. Pullout and shear forces of human bone were compared to different substitutes to evaluate their suitability for biomechanical studies.
Methods
After bone mineral density (BMD) determination, axial pullout tests were performed with cortical 3.5 mm nonlocking (NL) and 2.7 mm head locking (HL) screws on human, porcine and polyurethane composite bones. Porcine and human constructs were additionally loaded in shear direction.
Results
Apparent BMD was significantly lower in osteoporotic (159 mgHA/ccm ± 56) and nonosteoporotic (229 mgHA/ccm ± 25) human bone than that in porcine bone (325 mgHA/ccm ± 42; p < 0.01). Axial construct stiffness and ultimate pullout force of porcine bone (NL: 666N/mm ± 226, 910N ± 140; HL: 309N/mm ± 88, 744N ± 185) was significantly different from composite bone (NL: 1284N/mm ± 161; 1175N ± 116; HL: 1241N/mm ± 172, 1185N ± 225) and osteoporotic human bone (NL: 204N/mm ± 121, 185N ± 113; HL: 201N/mm ± 65; 189N ± 58) but not from nonosteoporotic human bone (NL: 620N/mm ± 205, 852N ± 281; HL: 399N/mm ± 224; 567N ± 242). Porcine bone exhibited an ultimate shear force (NL: 278N ± 99; HL: 431N ± 155) comparable to nonosteoporotic human bone (NL: 207 ± 68: HL: 374N ± 137).
Conclusion
Screw pullout and shear forces of porcine bone are close to nonosteoporotic human bone.
The translational potential of this article
Human bone specimens used in biomechanical studies are predominantly of osteoporotic bone quality. Conclusions on nonosteoporotic human bone behaviour are difficult. Alternatives such as porcine bone and composite bone were investigated, and it could be shown that screw pullout and screw shear forces of porcine bone are close to nonosteoporotic human bone.
Keywords: Biomechanical testing, Bone substitute, Bone quality, Composite bone, Human bone, Porcine bone
Introduction
Fresh frozen human bone specimens are considered as golden standard for biomechanical testing, reflecting most appropriately the in vivo situation. However, they have several disadvantages such as ethical concerns, difficult and complicated acquisition, preparation, storage and handling which have to follow certain laboratory requirements [1]. The interindividual variance in mechanical properties and bone geometry of human samples directly influences biomechanical study results [2], sometimes hiding existing differences in-between bone-implant constructs.
Bone quality is reduced or even osteoporotic in most of the human bone specimens, especially the female ones, since donor age is almost always advanced. A valid alternative such as synthetic surrogate or animal bones is of high interest. Bones from several animals, especially porcine, bovine and ovine bones, have been used as human substitutes in biomechanical testing [3], [4], [5], [6], [7], [8]. Because fixation techniques for young human nonosteoporotic bone could not be investigated in specimens with osteoporotic bone quality without influencing the results [3], porcine and bovine bones are often used as substitute for biomechanical studies on sports medical topics [3], [5], [6], [8] and was partially compared to human bone specimens [9], [10]. Porcine bone available in the slaughterhouse is collected mainly from relatively young animals, not older than 0.5–2 years, having the potential to mimic human bone from young athletic individuals.
This study investigated the suitability of porcine bone and synthetic composite bone as human bone substitutes for biomechanical studies on fore and midfoot fixation techniques. Their mechanical properties and the bone mineral density (BMD) of porcine bone are compared to nonosteoporotic and osteoporotic human bone.
It is hypothesised that pullout and shear properties of porcine bone are closer to that of nonosteoporotic human bone than the pullout and shear properties of osteoporotic human bone specimens.
Materials and methods
Six surrogate large left first metatarsal fourth generation composite bones, made from specially formulated polyurethane foam and designed for biomechanical testing (Sawbones Europe, Malmö, Sweden, reference number 3422), six porcine cuboids (mean donor age 8 month, acquired from local slaughtery), six human first metatarsals and cuboids of nonosteoporotic bone quality (mean donor age 32 years range, 12; 5 male, 1 female; 1 right, 5 left) and six human cuboids of osteoporotic bone quality (mean donor age 81 years range, 6; 4 male, 2 female; 4 right, 2 left) were used in this study, divided into five study groups with six specimens each. The intact cuboids, harvested from human and porcine feet, were scanned with a peripheral quantitative computed tomography scanner (Xtreme-CT, Scanco Medical AG, Brüttisellen, Switzerland) with a slice thickness of 123 μm and 854 evaluated slices per specimen for (BMD) evaluation before instrumentation.
A 3.5-mm, self-tapping stainless steel cortex screw (DePuy Synthes GmbH, Zuchwil, Switzerland), was inserted bicortically into each specimen after predrilling with a 2.5 mm drill bit. Axial pullout tests were performed after the instrumentation on a material testing machine (Instron 4302, Instron Inc., Canton MA, USA) with a 10 kN load cell, operated in displacement control mode at a cross-head speed of 5 mm/min. The screw head was inserted unlocked in the upper part of a testing jig, which was attached to the load cell. The midpoint of the screw head was aligned in the machine axis to ensure pure axial pullout force during the test. The bone specimens were fixed in the lower part of the jig, rigidly connected to the test frame, but restricting the specimen movement solely in the direction of the applied load (Figure 1A and B). Further, same instrumentation procedure, followed by pullout test, was repeated with 2.7 mm self-tapping stainless steel head locking (HL) screws (DePuy Synthes GmbH, Zuchwil, Switzerland) with predrilled Ø2.0 mm hole, inserted into each one of the specimens.
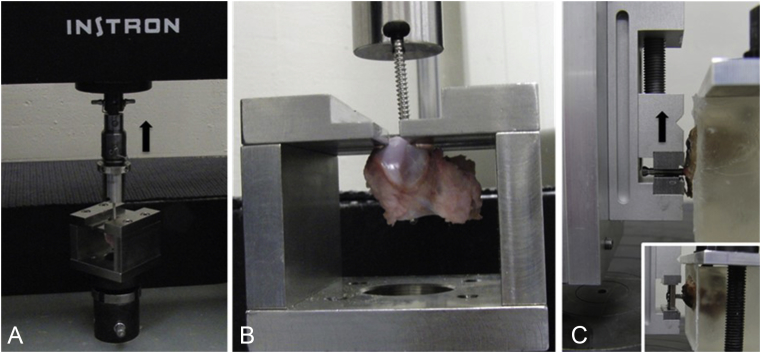
Figure 1.
Test setup with fixed specimen. (A) Axial pullout test. The screw head is connected to the load cell of the test system. The black arrow indicates the direction of the applied load. (B) To fix the bone, the screw shaft was inserted in the slot of the lower fixation block. (C) Shear test. The 2.7 mm head locking screw is locked in a vise, being connected to the test system. The embedded bone is fixed to the ground plate of the system. The insert shows a 3.5 mm nonlocking screw, being attached to the test system via a conventional nonlocking plate hole.
For shear load tests, the porcine and human cuboids were instrumented with bicortically placed 3.5 mm nonlocking (NL) screws previously inserted in a NL plate hole to mimic the loading pattern of a NL screw in combination with a NL plate, where tilting of the screw in the plate hole during loosening in the bony screw hole is possible. Additionally, 2.7 mm HL screws were locked in the test fixture to mimic the loading pattern of a locking screw with locked screw head in the plate, according to the principle of internal fixator, where no tilting of the screw in the plate hole during loosening in the bony screw hole is possible. Predrilling was performed in the same manner as for pullout tests. Bones were embedded in a polymethylmethacrylate block. Screw tips were covered with plasticine before embedding to allow free movement of the screw in the bone during the test. The polymethylmethacrylate block was rigidly connected to the base plate of the test frame to fix the bone with the screw shaft axis oriented in the horizontal plane, orthogonal to the load axis of the testing machine. The plate was rigidly connected to a linear slide via a vise. The plate was moved orthogonally to the screw shaft axis to apply shear load to the screw. The screw head of the locking screw was fixed in a vise being rigidly connected to a linear slide to simulate HL. The vise was moved orthogonally to the screw shaft axis to apply shear load to the screw. Both, nonlocking and locking screws were loaded orthogonal to their shaft axis. Testing machine and load protocol for shear load tests were identical to the axial pullout tests (Figure 1C). Test setup for pullout and shear tests was similar to that described by Seebeck et al. [11].
Axial load and axial displacement were recorded from the test system's transducers at a frequency of 10 Hz. Axial pullout stiffness and shear stiffness were determined from the linear part of the load–displacement curves, below the yield point. The ultimate axial pullout force and ultimate shear force was derived from the maximum value of the corresponding load–displacement curve, corresponding to Seebeck et al. [11].
Statistical analysis was performed using SPSS (IBM SPSS Statistics 19.0, SPSS Inc., Chicago, IL). The significance level was set to 0.05. Normal distribution within each group was evaluated by the Shapiro–Wilk Test. For the detection of significant differences between the groups, the one way analysis of variance and the unpaired t test and the Kruskal–Wallis test were used, both with Bonferroni post hoc correction. Correlation was analysed with Spearman's correlation test.
Results
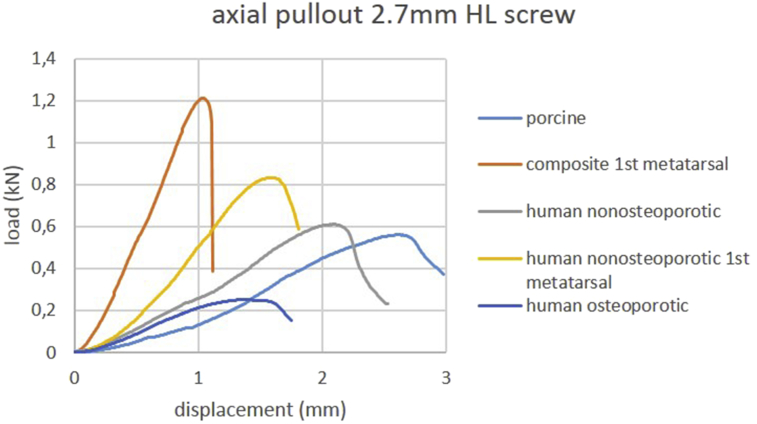
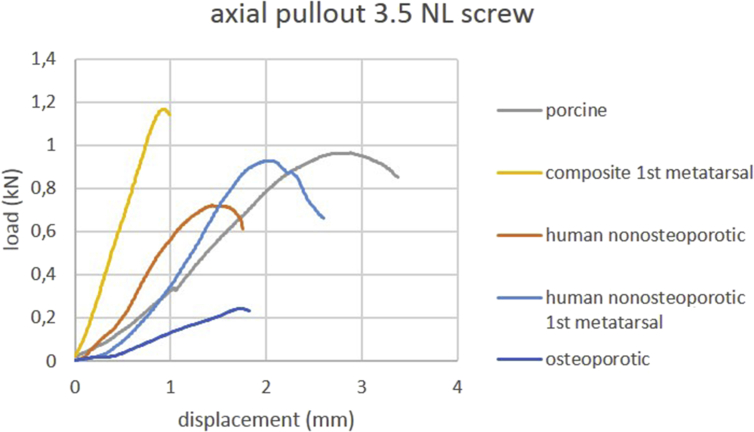
Mean stiffness under axial pullout force and mean ultimate axial pullout force values are provided in Table 1. Load-displacement curves of axial pullout testing of one representative specimen per group are shown in Figure 2, Figure 3. Osteoporotic human cuboid bone specimens exhibited the lowest stiffness values, whereas in composite bone specimens, the highest stiffness values were observed. The p-values of the respective comparisons are listed in Table 2 for 2.7 mm locking screws and in Table 3 for 3.5 mm NL screws. Porcine and nonosteoporotic human cuboids exhibited a comparable mean ultimate axial pullout force. Stiffness values were significantly higher for composite first metatarsal bones within both screw types compared to all other groups. Stiffness values were significantly lower for osteoporotic human cuboids within both screw types compared to all other groups except for nonosteoporotic cuboids using 2.7 mm locking screws.
Table 1.
Ultimate axial pullout force and axial construct stiffness of 2.7 mm head locking (HL) and 3.5 mm nonlocking (NL) screws inserted into composite first metatarsal, human nonosteoporotic first metatarsal, porcine and human cuboid bone. Mean values and standard deviation (SD) are given.
| 2.7 HL screw | 3.5 NL screw | |
|---|---|---|
| Ultimate axial pullout load (N) mean ± SD | ||
| Composite first metatarsal | 1185 ± 225 | 1175 ± 116 |
| Human nonosteoporotic first metatarsal | 791 ± 130 | 943 ± 304 |
| Porcine cuboid | 744 ± 185 | 910 ± 140 |
| Human nonosteoporotic cuboid | 567 ± 242 | 852 ± 281 |
| Human osteoporotic cuboid | 167 ± 78 | 185 ± 113 |
| Axial stiffness (N/mm) mean ± SD | ||
| Composite first metatarsal | 1241 ± 172 | 1284 ± 161 |
| Human nonosteoporotic first metatarsal | 679 ± 122 | 807 ± 323 |
| Porcine cuboid | 309 ± 88 | 666 ± 226 |
| Human nonosteoporotic cuboid | 399 ± 224 | 620 ± 205 |
| Human osteoporotic cuboid | 177 ± 88 | 204 ± 121 |
Figure 2.
Load-displacement curves of axial pullout testing using 2.7 mm head locking screws. Curves of one representative specimen per group are shown.
HL = head locking.
Figure 3.
Load-displacement curves of axial pullout testing using 3.5 mm nonlocking screws. Curves of one representative specimen per group are shown.
NL = nonlocking.
Table 2.
The p values of the respective comparisons of ultimate axial pullout force and axial construct stiffness of 2.7 mm head locking screws.
|
p values stiffness (2.7 mm screws axial pullout) |
||||||
|---|---|---|---|---|---|---|
| MT I composite | MT I nonosteoporotic | Cuboid porcine | Cuboid nonosteoporotic | Cuboid osteoporotic | ||
| p values maximum load (2.7 mm screws axial pullout) | MT I composite | <0.01 | <0.01 | <0.01 | <0.01 | |
| MT I nonosteoporotic | <0.01 | <0.01 | 0.02 | <0.01 | ||
| Cuboid porcine | <0.01 | n.s. | n.s. | <0.01 | ||
| Cuboid nonosteoporotic | <0.01 | n.s. | n.s. | n.s. | ||
| Cuboid osteoporotic | <0.01 | <0.01 | <0.01 | 0.047 | ||
Table 3.
The p values of the respective comparisons of ultimate axial pullout force and axial construct stiffness of 3.5 mm nonlocking screws.
|
p values stiffness (3.5 mm screws axial pullout) |
||||||
|---|---|---|---|---|---|---|
| MT I composite | MT I nonosteoporotic | Cuboid porcine | Cuboid nonosteoporotic | Cuboid osteoporotic | ||
| p values maximum load (3.5 mm screws axial pullout) | MT I composite | 0.01 | <0.01 | <0.01 | <0.01 | |
| MT I nonosteoporotic | n.s. | n.s. | n.s. | <0.01 | ||
| Cuboid porcine | n.s. | n.s. | n.s. | 0.01 | ||
| Cuboid nonosteoporotic | n.s. | n.s. | n.s. | 0.03 | ||
| Cuboid osteoporotic | <0.01 | <0.01 | <0.01 | <0.01 | ||
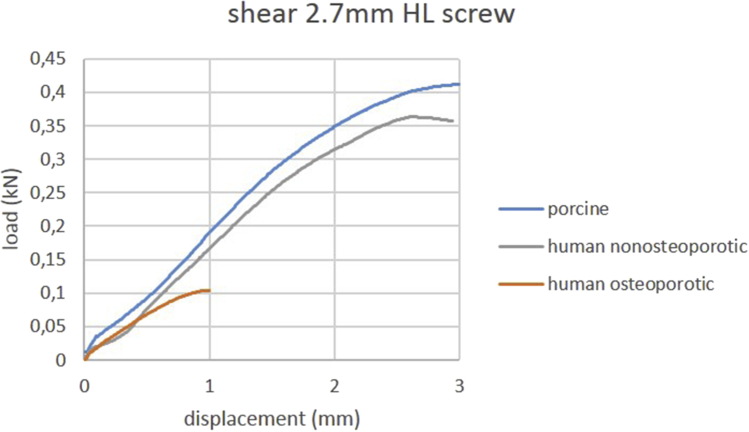
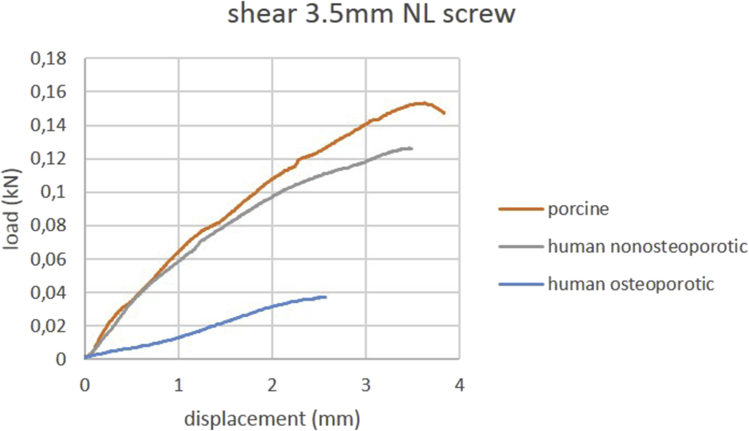
Mean ultimate shear pullout force and mean shear stiffness of locked and nonlocked screws are summarized in Table 4. Load-displacement curves of shear testing of one representative specimen per group are shown in Figure 4, Figure 5. Porcine bone exhibited a mean ultimate shear force comparable to nonosteoporotic human bone. Locking screws exhibited a significantly higher shear stiffness in all groups (all p < 0.01) than nonlocking screws.
Table 4.
Ultimate (maximal) shear force and construct stiffness in shear direction of 2.7 mm head locking screws (HL) and 3.5 mm nonlocking screws inserted into porcine and human cuboid bone. Mean values and standard deviation (SD) are given.
| 2.7 HL screw | 3.5 NL screw | |
|---|---|---|
| Ultimate shear load (N) mean ± SD | ||
| Porcine cuboid | 431 ± 155 | 278 ± 99 |
| Nonosteoporotic cuboid | 374 ± 137 | 207 ± 68 |
| Osteoporotic cuboid | 169 ± 72 | 49 ± 26 |
| Shear stiffness (N/mm) mean ± SD | ||
| Porcine cuboid | 305 ± 83 | 77 ± 27 |
| Nonosteoporotic cuboid | 215 ± 46 | 71 ± 38 |
| Osteoporotic cuboid | 285 ± 79 | 28 ± 19 |
Figure 4.
Load-displacement curves of shear testing using 2.7 mm head locking screws. Curves of one representative specimen per group are shown.
HL = head locking.
Figure 5.
Load-displacement curves of shear testing using 3.5 mm nonlocking screws. Curves of one representative specimen per group are shown.
NL = nonlocking
Porcine bone specimens provided a significantly higher mean apparent BMD (325 mgHA/ccm ± 42) than osteoporotic (159 mgHA/ccm ± 56; p < 0.01) and nonosteoporotic (229 mgHA/ccm ± 25; p < 0.01) human bone specimens. Comparing the apparent BMD of osteoporotic and nonosteoporotic human cuboid bones, the difference was not statistically significant. The apparent BMD and the axial pullout load of 3.5 mm cortical screws showed a rank correlation with a Spearman's correlation coefficient of rs = −0.829 for porcine bone and rs = 0.886 for osteoporotic human bone.
Discussion
This study compared mechanical properties of porcine and synthetic composite foot bone to nonosteoporotic and osteoporotic human foot bone to evaluate the suitability of these substitutes for biomechanical studies.
Porcine bone exhibited a mechanical strength in axial pullout direction as well as in shear direction comparable to nonosteoporotic human bone. Porcine bone represents an adequate substitute for young human nonosteoporotic bone, which is difficult to acquire. Unlike humans, pigs have a plexiform bone with osteonal banding. On the other hand, the annual skeletal remodeling rate is similar to that in humans (20–50%). Size and shape of the porcine skeleton might restrict the use for human implant testing, especially with regard to long bones. Porcine and bovine bones are frequently used in biomechanical studies on cruciate ligament reconstruction [6], [9], anchor fixation [5], [10] and acromioclavicular ligament repair with porcine metatarsal bones as substitute [8] and a good comparability towards the clinical situation. Pullout force in nonosteoporotic human bone and porcine bone was comparable in our study but is significantly higher as in osteoporotic human bone. To provide an adequate osteoporotic bone model with cancellous bone structure and thin cortex, the human cuboid was chosen, which is the central structure in the so-called “nutcracker fracture,” and because of its almost completely cancellous architecture, osteosynthesis is often difficult. Using the cuboid, we were able to point out differences in screw fixation in osteoporotic and nonosteoporotic bone. Since composite bones of the cuboid for biomechanical testing are not available and composite bones from the same production line provide similar mechanical characteristics, only first metatarsal bones were investigated and additionally compared to nonosteoporotic human first metatarsals. The low mechanical strength of osteoporotic human bone could not be mimicked by any of the substitutes tested. Animal bone is not suited as osteoporotic bone substitute, since osteoporosis is unknown in other vertebrates than humans. Although the bones were categorised by age, apparent BMD did not differ significantly between the nonosteoporotic and osteoporotic human cuboid group.
The nonsignificant difference in apparent BMD between nonosteoporotic and osteoporotic human bone is attributed to the higher standard deviation of these values in the osteoporotic human bone group compared to the nonosteoporotic human and porcine bone group, reflecting the reality in biomechanical tests using fresh frozen human bone samples [3], [6].
The inhomogeneity of bone density between the donors, especially in advanced donor age, may additionally influence the study results [6]. Using porcine bone as substitute, a relatively homogeneous BMD could be expected, as shown by the correspondent lower standard deviation. As known from previous studies [12], porcine bone exhibited a significantly higher apparent BMD compared to nonosteoporotic and osteoporotic human bone which is not necessarily reflected in higher stress resistance for porcine bone in the mechanical tests. The BMD provides a rough estimation but not adequately characterises the mechanical bone strength [12].
To allow a realistic comparability of the synthetic composite metatarsal bones to nonosteoporotic human bone, the nonosteoporotic human first metatarsal bone group was added. In both groups, screws are anchored bicortically in the shaft cortices. However, screw pullout force was highest in synthetic composite metatarsal bone with significance for 2.7 mm screws, making this substitute not recommendable without restrictions. Owing to their increased fixation capacity, composite bones will shift the failure site from the bone-implant interface to the implant itself, rendering a clinical interpretation of the results difficult.
Conclusion
Respecting the limited availability of nonosteoporotic human bone specimens, porcine bone exhibits screw pullout and screw shear forces close to nonosteoporotic human bone, mimicking these two mechanical parameters more appropriate than synthetic composite bone.
Conflicts of interest statement
None.
Acknowledgement
The authors are not compensated and there are no other institutional subsidies, corporate affiliations, or funding sources supporting this work unless clearly documented and disclosed. This investigation was performed with the assistance of the AO Foundation via the AOTK System.
Footnotes
This work was performed at the AO Research Institute Davos, Switzerland.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jot.2018.06.001.
Contributor Information
Mark Lenz, Email: lenzmar@web.de.
Boyko Gueorguiev, Email: boyko.gueorguiev@aofoundation.org.
Juan B. Gerstner Garces, Email: jbgerstner@me.com.
Michael P. Swords, Email: foot.trauma@gmail.com.
Stefan Rammelt, Email: Stefan.Rammelt@uniklinikum-dresden.de.
Gunther O. Hofmann, Email: gunther.hofmann@med.uni-jena.de.
Ivan Zderic, Email: ivan.zderic@aofoundation.org.
Manuela Ernst, Email: manuela.ernst@aofoundation.org.
Robert Geoff Richards, Email: geoff.richards@aofoundation.org.
Andrew K. Sands, Email: aksands@gmail.com.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Bredbenner T.L., Haug R.H. Substitutes for human cadaveric bone in maxillofacial rigid fixation research. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90(5):574–580. doi: 10.1067/moe.2000.111025. [DOI] [PubMed] [Google Scholar]
- 2.Pena F., Grontvedt T., Brown G.A., Aune A.K., Engebretsen L. Comparison of failure strength between metallic and absorbable interference screws. Influence of insertion torque, tunnel-bone block gap, bone mineral density, and interference. Am J Sports Med. 1996;24(3):329–334. doi: 10.1177/036354659602400314. [DOI] [PubMed] [Google Scholar]
- 3.Brown G.A., Pena F., Grontvedt T., Labadie D., Engebretsen L. Fixation strength of interference screw fixation in bovine, young human, and elderly human cadaver knees: influence of insertion torque, tunnel-bone block gap, and interference. Knee Surg Sports Traumatol Arthrosc. 1996;3(4):238–244. doi: 10.1007/BF01466626. [DOI] [PubMed] [Google Scholar]
- 4.Mayo A.E., Labrom R.D., Askin G.N., Adam C.J. A biomechanical study of top screw pullout in anterior scoliosis correction constructs. Spine (Phila Pa 1976) 2010;35(13):E587–E595. doi: 10.1097/BRS.0b013e3181cd389d. [DOI] [PubMed] [Google Scholar]
- 5.Rupp S., Georg T., Gauss C., Kohn D., Seil R. Fatigue testing of suture anchors. Am J Sports Med. 2002;30(2):239–247. doi: 10.1177/03635465020300021601. [DOI] [PubMed] [Google Scholar]
- 6.Seil R., Rupp S., Krauss P.W., Benz A., Kohn D.M. Comparison of initial fixation strength between biodegradable and metallic interference screws and a press-fit fixation technique in a porcine model. Am J Sports Med. 1998;26(6):815–819. doi: 10.1177/03635465980260061301. [DOI] [PubMed] [Google Scholar]
- 7.Wähnert D., Windolf M., Brianza S., Rothstock S., Radtke R., Brighenti V. A comparison of parallel and diverging screw angles in the stability of locked plate constructs. J Bone Joint Surg Br. 2011;93(9):1259–1264. doi: 10.1302/0301-620X.93B9.26721. [DOI] [PubMed] [Google Scholar]
- 8.Wellmann M., Zantop T., Petersen W. Minimally invasive coracoclavicular ligament augmentation with a flip button/polydioxanone repair for treatment of total acromioclavicular joint dislocation. Arthroscopy. 2007;23(10) doi: 10.1016/j.arthro.2006.12.015. 1132 e1131-1135. [DOI] [PubMed] [Google Scholar]
- 9.Ayzenberg M., Arango D., Gershkovich G.E., Samuel P.S., Saing M. Pullout strength of a novel hybrid fixation technique (Tape Locking Screw) in soft-tissue ACL reconstruction: a biomechanical study in human and porcine bone. Orthop Traumatol Surg Res. 2017;103(4):591–595. doi: 10.1016/j.otsr.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Christensen J., Fischer B., Nute M., Rizza R. Fixation strength of polyetheretherketone sheath-and-bullet device for soft tissue repair in the foot and ankle. J Foot Ankle Surg. 2018;57(1):60–64. doi: 10.1053/j.jfas.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Seebeck J., Goldhahn J., Stadele H., Messmer P., Morlock M.M., Schneider E. Effect of cortical thickness and cancellous bone density on the holding strength of internal fixator screws. J Orthop Res. 2004;22(6):1237–1242. doi: 10.1016/j.orthres.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Aerssens J., Boonen S., Lowet G., Dequeker J. Interspecies differences in bone composition, density, and quality: potential implications for in vivo bone research. Endocrinology. 1998;139(2):663–670. doi: 10.1210/endo.139.2.5751. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.