Abstract
The role of Notch signaling in cell-fate decisions has been studied extensively; however, this pathway is also active in adult tissues, including the nervous system. Notch signaling modulates a wide range of behaviors and processes of the nervous system in the nematode Caenorhabditis elegans, but there is no evidence for Notch signaling directly altering synaptic strength. Here, we demonstrate Notch-mediated regulation of synaptic activity at the C. elegans neuromuscular junction (NMJ). For this, we used aldicarb, an inhibitor of the enzyme acetylcholinesterase, and assessed paralysis rates of animals with altered Notch signaling. Notch receptors LIN-12 and GLP-1 are required for normal NMJ function; they regulate NMJ activity in an opposing fashion. Complete loss of LIN-12 skews the excitation/inhibition balance at the NMJ toward increased activity, whereas partial loss of GLP-1 has the opposite effect. Specific Notch ligands and co-ligands are also required for proper NMJ function. The role of LIN-12 is independent of cell-fate decisions; manipulation of LIN-12 signaling through RNAi knockdown or overexpression of the co-ligand OSM-11 after development alters NMJ activity. We demonstrate that LIN-12 modulates GABA signaling in this paradigm, as loss of GABA signaling suppresses LIN-12 gain-of-function defects. Further analysis, in vivo and in silico, suggests that LIN-12 may modulate transcription of the GABAB receptor GBB-2. Our findings confirm a non-developmental role for the LIN-12/Notch receptor in regulating synaptic signaling and identify the GABAB receptor GBB-2 as a potential Notch transcriptional target in the C. elegans nervous system.
Keywords: LIN-12, Notch, GBB-2, C. elegans, neuromuscular junction
Conserved roles for the Notch signaling pathway in development have been extensively studied across metazoan model organisms (Artavanis-Tsakonas et al. 1999; Fortini 2009). Recent studies reveal roles for Notch signaling in non-embryonic tissues, including neurons (Marathe and Alberi 2015). Among the roles of Notch signaling in the nervous system, regulation of synaptic strength, and synaptic activity-dependent behaviors such as learning and memory are prominent. In Drosophila, Notch pathway proteins have been implicated in long-term memory (Ge et al. 2004; Presente et al. 2004; Song et al. 2009), alcohol memory (Kaun et al. 2011) and olfactory adaptation (Lieber et al. 2011). Studies in mice show that the role of Notch in modulating synaptic strength is conserved across species (Costa et al. 2003; Wang et al. 2004; Conboy et al. 2007; Dahlhaus et al. 2008; Alberi et al. 2011; Brai et al. 2014) and the effect of Notch signaling differs from one tissue to another. For instance, increased Notch signaling in the primary visual cortex impairs long-term potentiation (LTP) (Dahlhaus et al. 2008), whereas in the hippocampus it favors LTP (Costa et al. 2003; Alberi et al. 2011). Liu et al. recently reported that the transcription factor RBP-Jκ, which is the downstream activator in Notch signaling, regulates γ-aminobutyric acid (GABA) signaling in the adult hippocampus (Liu et al. 2014). Despite this, the transcriptional targets of the Notch pathway have not been elucidated in behavioral contexts.
In the nematode Caenorhabditis elegans, there are two Notch receptors and they were initially identified for their roles in development. LIN-12 was identified in screens for specification of vulval fates (Greenwald et al. 1983; Ferguson and Horvitz 1985); whereas GLP-1 was first characterized as a regulator of mitosis in the germline (Austin and Kimble 1987). In canonical Notch signaling, Notch receptor undergoes a series of cleavages upon activation by ligands. This results in the release of the intracellular domain of Notch that subsequently translocates into the nucleus and associates with the transcription factor LAG-1 and other co-factors to initiate expression of target genes (reviewed in (Kopan and Ilagan 2009)). Studies in C. elegans identified a total of ten DSL (Delta/Serrate/LAG-2) proteins as canonical Notch ligands (Lambie and Kimble 1991; Henderson et al. 1994; Mango et al. 1994; Chen and Greenwald 2004) and five DOS (Delta/OSM-11) motif proteins as possible co-ligands (Komatsu et al. 2008). Other than roles in development, canonical Notch signaling in C. elegans regulates many behaviors, such as spontaneous reversals in adults (Chao et al. 2005), chemotaxis, and sleep (Singh et al. 2011).
Here, we assess the role of Notch signaling at the C. elegans neuromuscular junction (NMJ), the most studied synapse in this organism. The NMJ consists of three main elements: the muscle, cholinergic motor neurons that excite the muscles (reviewed in (Rand 2007)) and GABAergic motor neurons that inhibit them (reviewed in (Schuske et al. 2004)). Acetylcholine (ACh) in the synaptic cleft is broken down by the enzyme acetylcholinesterase, terminating the excitation. An inhibitor of this enzyme, aldicarb, has been extensively used by the C. elegans community to identify genes important for proper synaptic signaling at the NMJ (Nonet et al. 1993; Rand 2007). Inhibition of the NMJ activity by GABA signaling is not mediated only through ionotropic GABAA receptors expressed in the muscle. Metabotropic GABAB receptors, GBB-1 and GBB-2 are expressed in cholinergic neurons. They are thought to sense spillover GABA in periods of high activity and act through inhibitory G-protein Gαo to decrease ACh release. Since GABA release is dependent on ACh signaling, inhibition via GABAB receptors creates a negative feedback loop on cholinergic neurons (Dittman and Kaplan 2008; Schultheis et al. 2011).
In this study, we report that Notch signaling alters response to aldicarb. We identify components of the Notch signaling pathway necessary for proper synaptic transmission. Using genetic and pharmacological manipulations, we elucidate a role for LIN-12 in the regulation of GABAergic signaling at the NMJ via the GBB-2 GABAB receptor, a likely Notch transcriptional target.
Materials and Methods
C. elegans strains
Strains used for this study are listed in Table S1. Unless the allele was temperature sensitive all strains were reared at 25° under standard conditions, except for levamisole assays for which the animals were reared at 20°. For all experiments young adult animals were used. All animals carrying the lin-12(n137) mutation were kept as heterozygotes over the unc-32(e189) variant, singled to homozygose the n137 mutation, and the progeny of homozygous animals were tested for all assays. lin-12(n941) variant was kept on the homozygous lethal qC1[dpy-19(e1259) glp-1(q339) qIs26] balancer chromosome; homozygous lin-12(n941) mutants were used for experiments. lag-1(om13) animals were tested within one month of thawing from frozen stock. All experiments were performed at room temperature (22°), unless the alleles were temperature sensitive. Temperature sensitive mutants and their respective controls were reared at the permissive temperature, switched to the restrictive temperature in the first larval stage and assayed as young adults. These animals were kept at the restrictive temperature in between aldicarb time points.
Pharmacological assays
Aldicarb (Sigma 33386) and levamisole (Sigma L9756) assays were performed as previously described (Sorkaç et al. 2016). Nematode growth medium (NGM) plates with 1mM aldicarb or 0.4mM levamisole were prepared the day before the assay. On the day of the assay, plates were seeded with 30μL E. coli strain OP50 and left to dry for 30min. Young adult animals were transferred onto plates and scored for paralysis every hour for 6-8 hr. Paralysis was defined as absence of both movement and pharyngeal pumping upon prodding with a platinum wire. For RNAi experiments, 1mM aldicarb plates were prepared with 1mM ampicillin, 1mM tetracycline and 1mM isopropyl β-D-1-thiogalactopyranoside (IPTG). These plates were seeded with 30μL E. coli strain Ht115 carrying either the empty RNAi vector or the RNAi vector of the corresponding gene, according to the RNAi feeding strain on which the animals were kept before the aldicarb assay. Log-rank p-value, pairwise over strata, was calculated for significance using the Kaplan-Meier estimator from IBM SPSS Statistics 22.
Muscimol (Sigma G019) assays were performed similarly to what was done in (Han et al. 2015). 1mM muscimol plates were prepared the same way as aldicarb plates. One control and one experimental young adult were transferred onto 1mM muscimol plates. The first photo was taken at t = 0min. The second photo was taken at t = 60min at the same magnification level. Images were analyzed on ImageJ. The midline of each animal was traced with the segmented line tool, and the length of the midline trace was calculated in pixels. The lengths at t = 0min and t = 60min per single animal were used to calculate percent elongation after 1h of 1mM muscimol treatment. Student’s t-test was performed to calculate significance.
Heat-shock experiments
Heat-shock (hs) animals were reared at 15°, switched to 20° the night before the assay. The day of the assay, a 75 min heat-shock treatment was applied at 35° to young adults, followed by 1h of recovery at 20°. Mock heat-shock (no hs) controls were treated the same way without the 75 min heat-shock at 35°.
RNAi experiments
RNAi plates were prepared by adding ampicillin, tetracycline and IPTG to 1mM final concentrations into the standard NGM mixture. Plates were seeded with 10X concentrated E. coli strain HT115 carrying corresponding RNAi vectors or the control vector. After drying, gravid adults were egg prepped onto the seeded plates using a 2.5% bleach+1N NaOH solution. For osm-11 RNAi aldicarb assays, hatched wild type animals were transferred to corresponding RNAi feeding bacteria in the late second larval stage. Other RNAi experiments were performed using a neuronal RNAi sensitive strain KP3948 eri-1(mg366) IV; lin-15B(n744) X.
For apx-1, animals were assayed on aldicarb as first generation, RNAi-treated young adults. For dsl-7 and dos-3 aldicarb trials, second generation, RNAi-treated young adults were used. osm-11 and apx-1 RNAi clone was obtained from the Vidal library and dos-3 clone from the Ahringer library. dsl-7 RNAi clone was made using dsl-7 genomic sequence flanked by primers 5′-ATGCTGACTTTATGGTCTTTACTGTTG-3′ and 5′-TTACGACTGTGAATTTAGTCTAACAGG-3′.
Puncta analysis
Young adult animals carrying the juIs1[unc-25p::snb-1::GFP + lin-15(+)] IV transgene in a wild type or lin-12(n137) background were paralyzed in 30mg.mL-1 2,3-Butanedione monoxime (BDM, Sigma B0753) in M9 and mounted on 2% agar pads. Z-stack (distance between images: 0.2μm) images of SNB-1::GFP puncta were taken from the ventral nerve cord between motor neurons VD10 and VD11, using the Zeiss AxioImager ApoTome microscope at 100X magnification. Images formed by merging 3 layers were analyzed using the ‘punctaanalyser” MATLAB program from (Kim et al. 2008). Results from control and experimental animals were tested for significance using Student’s t-test.
Data availability
For wild type responses in Table 1 the raw data are available at GSA FigShare. All data necessary for confirming conclusions of this article are represented in figures and tables. All the strains used in this study, and raw data for all experiments are available upon request. Supplemental material available at Figshare: https://doi.org/10.25387/g3.6480419.
Table 1. Aldicarb response of ligand or co-ligand mutant animals. Loss of OSM-7, OSM-11 or DSL-3 phenocopies LIN-12 loss, whereas decreased LAG-2, DOS-1 or DSL-6 results in resistance to aldicarb similarly to decreased glp-1 function. n/a indicates “not available”. At least 10 animals tested for dos-2, arg-1, dsl-2, dsl-4 and dsl-5. For RNAi experiments, three independent trials were conducted using the neuronal RNAi-sensitive strain eri-1(mg366); lin-15B(n744), with a total of at least 30 animals for each knocked-down gene. For non-wild type responses, aldicarb-induced paralysis rates are in Figure S2. For wild type responses the raw data are available in supplements.
| Gene | Function | Allele | Effect on function | Aldicarb Response |
|---|---|---|---|---|
| osm-7 | DOS co-ligand | tm2256 | null | hypersensitive |
| osm-11 | DOS co-ligand | rt142 | null | hypersensitive |
| dos-1 | DOS co-ligand | ok2398 | null | resistant |
| dos-2 | DOS co-ligand | tm4515 | putative null | wild type |
| dos-3 | DOS co-ligand | n/a | RNAi knockdown | wild type |
| lag-2 | DSL ligand | q420 | loss of function | resistant |
| arg-1 | DSL ligand | ok3127 | putative null | wild type |
| apx-1 | DSL ligand | n/a | RNAi knockdown | wild type |
| dsl-1 | DSL ligand | ok810 | null | wild type |
| dsl-2 | DSL ligand | tm1805 | putative null | wild type |
| dsl-3 | DSL ligand | ok3411 | putative null | hypersensitive |
| dsl-4 | DSL ligand | ok1020 | putative null | wild type |
| dsl-5 | DSL ligand | ok588 | putative null | wild type |
| dsl-6 | DSL ligand | ok2265 | putative null | resistant |
| dsl-7 | DSL ligand | n/a | RNAi knockdown | wild type |
Results and Discussion
LIN-12 and GLP-1 Notch receptors regulate signaling at the C. elegans neuromuscular junction
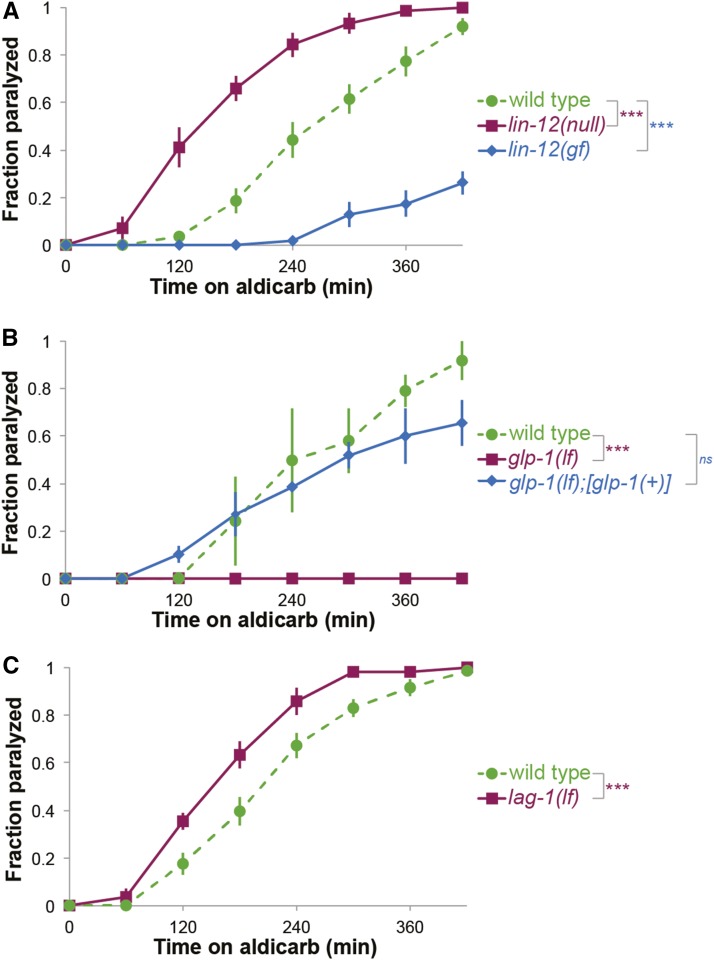
Aldicarb causes paralysis of wild type animals at a specific rate and significant deviations from this rate indicate altered signaling at the NMJ. To investigate the regulation of NMJ function by Notch signaling, we examined paralysis rates of Notch receptor loss-of-function (lf) and gain-of-function (gf) mutant animals on 1mM aldicarb. Animals that completely lack LIN-12/Notch signaling, lin-12(n941null), paralyzed faster than wild type control animals. Consistent with this result, lin-12(n137) strong gf mutants were resistant to aldicarb, paralyzing more slowly (Figure 1A). The paralysis rate of animals carrying the weaker, cold sensitive gf mutation lin-12(n137n460) did not differ from control wild type animals (Figure S1A). And, animals carrying the weaker lf mutation lin-12(q269) trended toward mild aldicarb hypersensitivity, but not significantly (Figure S1B). Taken together, these results indicate that large changes in LIN-12 receptor signaling impact NMJ activity.
Figure 1.
Notch signaling is required for proper activity at the NMJ. (A) Time course of paralysis for lin-12(n941null) and lin-12(n137gf) mutants in response to aldicarb. Decreased LIN-12 signaling accelerates paralysis upon aldicarb treatment whereas (B) decreased GLP-1 signaling results in strong resistance to aldicarb induced paralysis. glp-1(q231lf) is rescued by transgenic glp-1 expression. (C) Partial loss of the CSL transcription factor LAG-1, lag-1(om13lf), confers hypersensitivity to 1mM aldicarb. (at least three independent trials for each experiment, n > 30 for all genotypes, error bars represent SEM *: log-rank p-value < 0.05, **: log-rank p-value < 0.01, ***: log-rank p-value < 0.001, ns: no significant difference. For (B) and (C) all animals were reared at 15°C and switched to the restrictive temperature of 25°C in the first larval stage.)
Decreased function of GLP-1, the other C. elegans Notch receptor, had the opposite impact on NMJ function. glp-1(q231) temperature sensitive lf mutant animals reared at the restrictive temperature failed to paralyze after 7 hr on 1mM aldicarb (Figure 1B). Another lf mutation, glp-1(q224), caused a less dramatic resistance to aldicarb (Figure S1C). The resistance of glp-1(q231) animals was rescued by the introduction of an integrated multi-copy transgene that expresses normal GLP-1 protein under the control of the glp-1 promoter (tnIs39, (Sallee et al. 2015)) (Figure 1B). Moderate changes in GLP-1 signaling, in glp-1(bn18) lf or glp-1(ar202) gf animals, did not alter aldicarb response (Figure S1D). We conclude that activity of both LIN-12 and GLP-1 Notch receptors impacts response to aldicarb and NMJ function.
In canonical Notch signaling, the intracellular domain of the activated Notch receptor associates with the CSL (mammalian CBF1/RBP-Jκ, Drosophila Suppressor of Hairless, C. elegans LAG-1) transcription factor. Having observed opposing aldicarb response defects after perturbation of the two C. elegans Notch receptor genes, we next examined the aldicarb response of animals with impaired LAG-1 activity to determine if this canonical downstream target is involved. If lag-1(lf) animals exhibit an increased rate of paralysis, then LIN-12 signaling is likely functions through LAG-1 in this paradigm. Alternatively, if lag-1(lf) animals are resistant, then GLP-1 likely acts via the canonical LAG-1 pathway. Since complete loss of LAG-1 function results in lethality, we used lag-1(om13) temperature-sensitive, partial lf animals. They were hypersensitive to aldicarb when reared at the restrictive temperature; this defect was reminiscent of defects seen when LIN-12 function was lost (Figure 1C). This result supports the hypothesis that LIN-12 signals through the canonical pathway, but we cannot draw any conclusions about downstream targets of GLP-1 signaling.
Identification of pertinent ligands
C. elegans Notch receptors are activated by DSL ligands and DOS-motif proteins are thought to act as co-ligands. Given the altered responses of receptor mutant animals to aldicarb, we investigated whether loss of various ligands or co-ligands altered aldicarb paralysis rates (Figure S2). Complete or partial loss-of-function alleles were available for eight out of ten DSL ligands and for four out of five DOS co-ligands. apx-1 loss is lethal and no alleles were available for two other genes; their impact on NMJ function was examined by RNA interference (RNAi). For this we knocked-down gene function by feeding animals bacteria expressing double-stranded RNA for the corresponding gene. For RNAi studies, we used a C. elegans strain whose neurons are hypersensitive to RNAi by feeding. The results are summarized in Table 1.
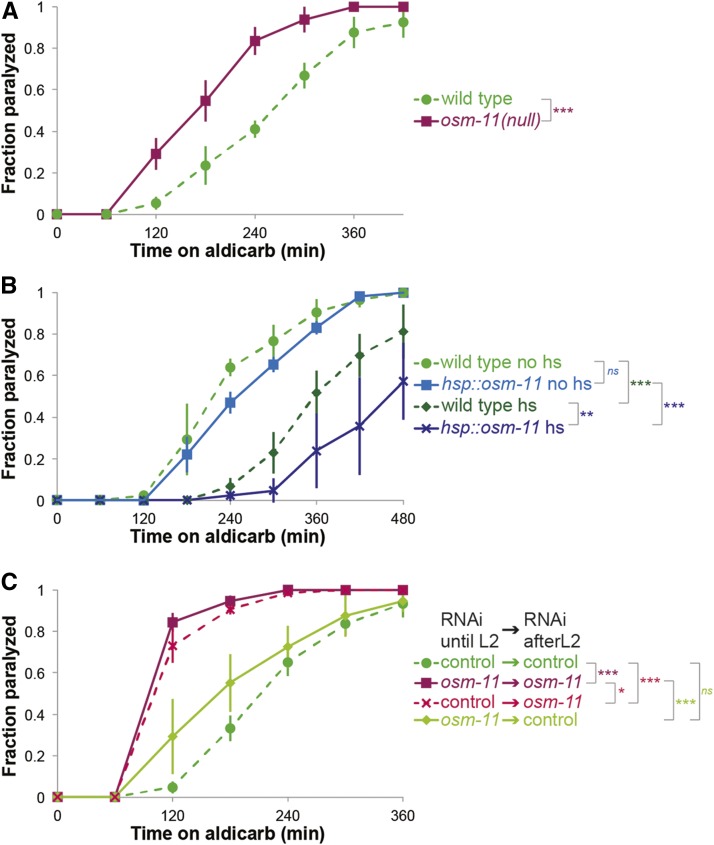
OSM-11 is necessary and sufficient for modulation of the NMJ
For the remainder of this study, we focused on the LIN-12 Notch receptor. The secreted DOS co-ligand osm-11 is epistatic to lin-12 in determination of vulval cell fates (Komatsu et al. 2008). Loss of the secreted DOS co-ligand OSM-11 leads to aldicarb hypersensitivity, as does LIN-12 loss (Figure 2A). It seemed likely that OSM-11 activates the LIN-12 Notch receptor in this paradigm. If so, then increased OSM-11 might induce aldicarb resistance, reminiscent of lin-12(n137gf) mutant animals. We used a transgenic line in which expression of osm-11 is under the control of a heat-shock promoter (Singh et al. 2011) to determine if increasing OSM-11 in adult animals would slow aldicarb paralysis rates in otherwise wild type animals. We noted that, even without the transgene, heat-shock slightly increased the aldicarb resistance of wild type animals. But, the impact of OSM-11 over-expression was much more dramatic; elevating OSM-11 in adult animals resulted in even slower paralysis (Figure 2B). These results suggest that increasing the OSM-11 activity in adult animals was sufficient to alter the aldicarb response and that the role of Notch signaling in this paradigm is independent of cell-fate decisions. To confirm these results, we examined the impact of osm-11 knockdown by feeding RNAi on aldicarb response.
Figure 2.
Altering OSM-11 DOS co-ligand levels affect the paralysis rate on aldicarb. (A) Response of osm-11(rt142null) animals to aldicarb. Animals that lack the DOS co-ligand OSM-11 are hypersensitive to aldicarb, similar to lin-12(null) animals. (B) Overexpression of OSM-11 in adults by heat-shock causes resistance to aldicarb compared to heat-shocked wild type controls. (C) osm-11 knockdown by feeding RNAi recapitulates aldicarb hypersensitivity seen in osm-11(rt142null) animals. Knockdown of osm-11 starting from late L2 is enough to cause increased hypersensitivity to aldicarb in adults and restoring osm-11 expression after late L2 stage reverses this hypersensitivity. (hs: heat-shock, no hs: mock heat-shock, at least three independent trials for each experiment, n > 30 for all conditions, error bars represent SEM *: log-rank p-value < 0.05, **: log-rank p-value < 0.01, ***: log-rank p-value < 0.001, ns: no significant difference.)
Reminiscent of osm-11(null) animals, aldicarb hypersensitivity is seen in wild type animals fed bacteria expressing double stranded osm-11 RNA (osm-11→osm-11) For these studies, aldicarb response is compared to control animals reared on empty RNAi vector carrying bacteria (control→control) (Figure 2C). However, given the various roles of Notch signaling in nervous system development, we wanted to rule out cell-fate decisions as a possible cause of the aldicarb phenotypes we observe. Since the identities of body wall muscles and almost all C. elegans nervous system including cholinergic and GABAergic neurons at the NMJ are determined by the end of the second larval stage (Sulston 1976; Sulston and Horvitz 1977), we examined the impact of knocking-down osm-11 from late second larval (L2) stage onwards. If these animals become hypersensitive to aldicarb, cell-fate decisions in the nervous system are likely irrelevant in this paradigm. We found that L2 animals switched from control RNAi to osm-11 RNAi (control→osm-11) exhibited faster paralysis on aldicarb as young adults, recapitulating osm-11 lf defects (Figure 2C). Conversely, animals reared on osm-11 RNAi and switched to control RNAi plates as late L2s (osm-11→control) exhibited paralysis rates comparable to animals fed with control RNAi throughout larval development (Figure 2C). We conclude that restoring OSM-11 expression after neuronal cell-fate decisions have been made is sufficient to rescue the aldicarb response to wild type levels. Next, we aimed to identify which neurotransmitter signaling system is modulated by LIN-12 signaling in the developed nervous system.
LIN-12 Notch receptor modulates GABA signaling
Aldicarb directly impairs acetylcholinesterase and alters cholinergic signaling at the NMJ. But, the C. elegans NMJ is also directly influenced by GABA neurotransmission. GABA mutant animals have accelerated paralysis on aldicarb (Loria et al. 2004) and Notch pathway signaling alters GABA levels in mouse hippocampus (Liu et al. 2014). Hence, we investigated whether the aldicarb defects observed in lin-12 mutants were due to altered GABA signaling.
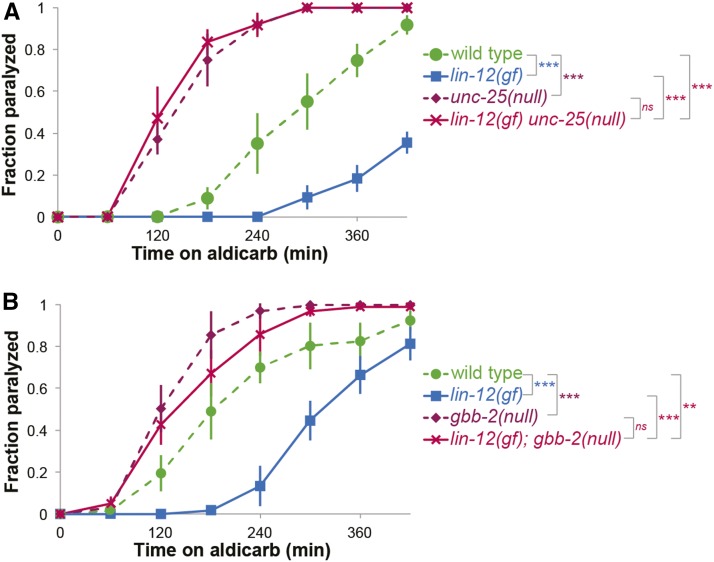
Glutamic acid decarboxylase (GAD) converts glutamic acid to GABA at the NMJ. The C. elegans GAD ortholog is encoded by the gene unc-25 and animals that completely lack UNC-25 activity are hypersensitive to aldicarb, due to the decreased muscle inhibition (Loria et al. 2004). If the decreased rate of paralysis observed in lin-12(n137gf) animals is due to changes in GABA, then loss of lin-12 likely causes increased GABA signaling. And, completely abolishing GABA production in lin-12(n137gf) animals should result in paralysis on aldicarb at a rate comparable to that of unc-25(e156null) animals. Consistent with this hypothesis, we found that complete loss of GABA production in lin-12(n137gf) unc-25(null) double mutant animals suppressed the lin-12 gf aldicarb resistance defect; these animals were indistinguishable from unc-25(null) single mutants in their aldicarb sensitivity (Figure 3A). It is, therefore, likely that changes in LIN-12 signaling result in altered GABA signaling.
Figure 3.
LIN-12 regulates GABA signaling. (A) Paralysis rates of lin-12(n137gf);unc-25(null) double mutants on 1mM aldicarb. Loss of GABA production completely suppresses the aldicarb resistance of lin-12(n137gf) animals. (B) Paralysis rates of lin-12(n137gf);gbb-2(null) double mutants on 1mM aldicarb. Lack of GBB-2 GABAB receptor subunit completely suppresses the aldicarb resistance observed in lin-12(n137gf) animals. (at least three independent trials for each experiment, n > 30 for all genotypes, error bars represent SEM *: log-rank p-value < 0.05, **: log-rank p-value < 0.01, ***: log-rank p-value < 0.001, ns: no significant difference.)
Do mutations in the LIN-12 pathway alter pre- or post-synaptic function? In order to assess this, we tested osm-11(null) animals on the acetylcholine agonist levamisole since resistance to levamisole indicated muscle defects. Loss of osm-11 resulted in hypersensitivity to this drug (Figure S3) Mutants hypersensitive to aldicarb and levamisole can exhibit ACh- or GABA-related pre- and/or post-synaptic defects (Loria et al. 2004; Vashlishan et al. 2008; Jospin et al. 2009). Given the aldicarb hypersensitivity of osm-11(null) animals we could not draw conclusions as to where the synaptic function is disrupted in LIN-12 pathway mutants. We, therefore, turned to directly testing GABA release and reception.
Post-synaptically, an increase in number or activity of GABAA receptors could account for aldicarb resistance in lin-12 gf animals, just as loss of GABAA receptors leads to hypersensitivity (Vashlishan et al. 2008). Alternatively, lin-12(gf) animals may have presynaptic defects, with increased presynaptic input onto the muscles from GABAergic motor neurons. To discriminate between these scenarios, we examined the response of animals to a GABAA receptor agonist and investigated presynaptic synaptobrevin localization.
The drug muscimol is a GABAA receptor agonist that can cause muscle relaxation in C. elegans, which is easily assessed as increased body length (McIntire et al. 1993). Animals lacking the postsynaptic GABAA receptor subunit encoded by unc-49 are resistant to muscimol in this assay, while animals with presynaptic GABA defects are sensitive to muscimol exhibit increased body length when exposed to the drug (McIntire et al. 1993). If muscles of lin-12(n137gf) animals are more responsive to GABA, muscimol exposure should elongate these animals, compared to wild type animals. However, we found that the muscimol-induced elongation of lin-12(n137gf) animals was not significantly different than elongation of the wild type controls (Figure S4A), ruling out a postsynaptic defect due to overactive or more numerous GABAA receptors. Given these results, it is possible that the aldicarb resistance of lin-12(n137gf) animals is due to presynaptic defects.
To visualize presynaptic terminals, we examined animals that express C. elegans synaptobrevin, SNB-1 tagged with GFP under the control of a GABAergic promoter (Jorgensen et al. 1995). Examination of the ventral cord, between motor neurons VD10 and VD11, (Figure S4B) revealed no difference in puncta fluorescence intensity (Figure S4C), volume (Figure S4D) or density (Figure S4E). This result suggests that increased LIN-12 activity does not lead to synaptic vesicle accumulation in GABA motor neurons nor does it increase the size or number of GABAergic synapses.
GBB-2 is downstream of the LIN-12 Notch receptor in modulating the NMJ function
To define the mechanism through which LIN-12 impacts NMJ function, we looked for potential LIN-12/LAG-1 transcriptional targets. Specifically, we looked for genes 1) that are known to modulate or play an intrinsic role in GABA signaling, 2) whose loss of function results in hypersensitivity to aldicarb and 3) with conserved consensus LAG-1 binding sites (either 5′-YRTGRGAA-3′ or 5′-YGTGDGAA-3′ (Greenwald and Kovall 2013) in putative transcriptional regulatory sequences, based on comparison across four Caenorhabditis species: C. elegans, C. briggsae, C. remanei and C. brenneri. Only one gene satisfied all of these criteria: gbb-2, which encodes a metabotropic glutamate receptor.
At the NMJ, muscles and neurons respond independently to GABA. GABA directly opposes muscle contraction via GABAA receptors expressed by body wall muscle cells. But, GABA also activates GABAB receptors expressed in cholinergic motor neurons and decreases synaptic release via the Go protein alpha subunit GOA-1, thereby indirectly opposing muscle contraction. Loss of either C. elegans GABAB receptor, gbb-1 or gbb-2, or loss of goa-1 results in hypersensitivity to aldicarb, due to increased acetylcholine release by cholinergic motor neurons (Dittman and Kaplan 2008). Genomic sequences near the C. elegans gbb-2 gene translation initiation site contain two consensus LAG-1 binding sites: one in the third intron and one in the fourth intron. A consensus LAG-1 binding site in the fourth intron is also found in C. briggsae, C. remanei and C. brenneri (Fig S5). Since gbb-2 is a potential LIN-12/LAG-1 target, we determined if loss of gbb-2 would suppress the aldicarb resistance of lin-12(n137gf) animals. As predicted, the aldicarb response of lin-12(n137); gbb-2(tm1165null) double mutant animals was statistically indistinguishable from that of gbb-2(null) single mutants (Figure 3B), indicating that gbb-2 acts downstream of lin-12 signaling in this paradigm.
The data presented in this article suggests that Notch signaling is required for proper neuromuscular junction signaling in C. elegans. Although the role of Notch signaling as a regulator of cell-fate specification has been studied extensively in the field, Notch also alters adult nervous system functioning. In addition to numerous other behaviors and the role Notch plays in muscle arm development in vulval NMJ (Li et al. 2013) we present, for the first time in C. elegans, that Notch signaling regulates synaptic function.
This is not the first time that the two Notch receptor paralogs have been shown to regulate the same process in the nervous system of C. elegans. Loss of LIN-12 or GLP-1 results in an increase in the amount of sleep animals exhibit during the last larval molt, possibly due to the low quality of sleep (Singh et al. 2011). The two Notch receptors synergistically affect the response of animals to aversive chemicals (Singh et al. 2011). LIN-12 and GLP-1 affect the reversal rate of adult animals in a complex manner ((Chao et al. 2005), Hart Lab unpublished results). However, Notch receptors do not always affect the same processes in the nervous system. In 2012, El Bejjani and Hammarlund showed that loss of LIN-12 increased regeneration rates in injured GABA motor neuron processes, whereas decreased GLP-1 signaling had no effect on regeneration (El Bejjani and Hammarlund 2012). In our paradigm, LIN-12 and GLP-1 affect NMJ signaling in opposing manners.
This is particularly interesting since the intracellular domains of GLP-1 and LIN-12, important for canonical Notch signaling, have been shown to be highly homologous (Yochem and Greenwald 1989) and GLP-1 can fully substitute for LIN-12 in vulval cell-fate decisions (Fitzgerald et al. 1993). Differential modulation of the NMJ by the two Notch receptors can be explained by these two receptors acting in different tissues and/or at different times. The two receptors might also be altering different communication mechanisms between neurons, namely neurotransmitters and neuropeptides, since neuropeptides have also been shown to alter aldicarb responses (Vashlishan et al. 2008; Zhang and Kubiseski 2010; Hu et al. 2011; Choi et al. 2015). It can further be speculated that one of the receptors act through a non-canonical signaling pathway. Although there is currently no evidence for C. elegans Notch receptors to engage in a non-canonical cascade of protein interactions, previous Drosophila axon pathfinding studies suggest such an interaction (Crowner et al. 2003; Le Gall et al. 2008).
In C. elegans canonical Notch signaling, intracellular domains of activated Notch receptors associate with the CSL transcription factor LAG-1 to initiate transcription of target genes. Given the fact that partial loss LAG-1 accelerates the paralysis of animals on aldicarb, it is very likely that the LIN-12 Notch receptor acts through the canonical pathway to alter the signaling at the NMJ. The same cannot be said for the GLP-1 receptor. We tried to monitor for genetic interactions between GLP-1 and C. elegans orthologs of proteins from the Drosophila non-canonical signaling pathway. However, these proteins did not seem to modulate GLP-1 mediated regulation of the NMJ (data not shown).
From our analysis using ligand or co-ligand loss-of-functions, we speculate as to which ligands are acting on which receptors in our paradigm. Since loss of DSL-3, OSM-7 or OSM-11 leads to hypersensitivity to aldicarb, they are likely acting on LIN-12 Notch receptor. LAG-2, DSL-6 and DOS-1 likely activate GLP-1 given that their losses phenocopy glp-1(lf) animals. Further studies are required to provide more specific conclusions about the genetic interactions between the ligands and the corresponding receptors.
We chose to manipulate levels of the DOS co-ligand OSM-11 to exclude developmental defects as a basis for the aldicarb sensitivity we observed in lin-12(null) animals, as complete loss or overexpression of OSM-11 does not lead to infertility or lethality (Komatsu et al. 2008; Singh et al. 2011). Overexpression of this secreted co-ligand in adults slowed down paralysis rates on aldicarb. This result has two implications. First of all, it shows that increasing levels of a Notch co-ligand is sufficient to induce resistance to aldicarb. Furthermore, it corroborates the idea that the changes we observed in LIN-12 mutants are not due to developmental defects caused by altered cell-fate decisions. However, overexpression of a secreted protein can have ectopic effects. To overcome this problem, we knocked-down osm-11 starting at the end of the second larval stage. By this time, animals have mostly completed cell-fate specification in their nervous system. Decreasing levels of OSM-11 in late L2 animals caused hypersensitivity to aldicarb, suggesting that the phenotype is not due to improper cell-fate decisions. Moreover, this phenotype is easily reversible by restoring OSM-11 synthesis. This suggests that absence of the Notch co-ligand OSM-11 during development does not interfere with the regulation of the adult NMJ by Notch signaling.
Further genetic analysis of lin-12(gf) animals showed that their resistance to aldicarb was dependent on increased GABAergic signaling; the phenotype is completely suppressed by abolishment of GABA production through loss of UNC-25. The mechanism through which LIN-12 increases GABA signaling involves the GBB-2 GABAB receptor subunit that detects spillover GABA and modulate acetylcholine release from cholinergic motor neurons (Dittman and Kaplan 2008; Schultheis et al. 2011). Although our analysis lacks the direct evidence for transcriptional control of gbb-2 under the control of LIN-12 Notch receptor, the presence of conserved LAG-1 binding sites in the gbb-2 gene supports regulation. Future studies should focus on the context in which the Notch pathway regulates GABA signaling and the role of the GBB-2 receptor.
Acknowledgments
We would like to thank the Greenwald, Kimble and Hammarlund Laboratories for C. elegans strains, Schedl Laboratory for tnIs39, and the Zhen Laboratory for the punctaanalyser program. Other C. elegans strains were supplied by the Japanese Knockout Consortium and Caenorhabditis Genetics Center (CGC), which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). We are grateful to Komudi Singh and other Hart Laboratory members for helpful discussions. This work was supported by National Institutes of Health (NIH) grants R01 GM078171 and NS055813 (ACH), Brown Institute for Brain Science Suna Kiraç Fund for Brain Science (AS), Brown University Karen T. Romer Undergraduate Teaching and Research Awards (MAD and HKG), and National Institute of Neurological Disorders and Stroke, F31NS089201-01A1 Predoctoral Ruth L. Kirschstein National Research Service Award (NRSA) (PJO).
Footnotes
Supplemental material available at Figshare: https://doi.org/10.25387/g3.6480419.
Communicating editor: S. Lee
Literature Cited
- Alberi L., Liu S., Wang Y., Badie R., Smith-Hicks C., et al. , 2011. Activity-induced Notch signaling in neurons requires Arc/Arg3.1 and is essential for synaptic plasticity in hippocampal networks. Neuron 69: 437–444. 10.1016/j.neuron.2011.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S., Rand M. D., Lake R. J., 1999. Notch signaling: cell fate control and signal integration in development. Science 284: 770–776. 10.1126/science.284.5415.770 [DOI] [PubMed] [Google Scholar]
- Austin J., Kimble J., 1987. glp-1 is required in the germ line for regulation of the decision between mitosis and meiosis in C. elegans. Cell 51: 589–599. 10.1016/0092-8674(87)90128-0 [DOI] [PubMed] [Google Scholar]
- Brai E., Marathe S., Zentilin L., Giacca M., Nimpf J., et al. , 2014. Notch1 activity in the olfactory bulb is odour-dependent and contributes to olfactory behaviour. Eur. J. Neurosci. 40: 3436–3449. 10.1111/ejn.12719 [DOI] [PubMed] [Google Scholar]
- Chao M. Y., Larkins-Ford J., Tucey T. M., Hart A. C., 2005. lin-12 Notch functions in the adult nervous system of C. elegans. BMC Neurosci. 6: 45 10.1186/1471-2202-6-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Greenwald I., 2004. The lateral signal for LIN-12/Notch in C. elegans vulval development comprises redundant secreted and transmembrane DSL proteins. Dev. Cell 6: 183–192. 10.1016/S1534-5807(04)00021-8 [DOI] [PubMed] [Google Scholar]
- Choi S., Taylor K. P., Chatzigeorgiou M., Hu Z., Schafer W. R., et al. , 2015. Sensory Neurons Arouse C. elegans Locomotion via Both Glutamate and Neuropeptide Release. PLoS Genet. 11: e1005359 10.1371/journal.pgen.1005359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy L., Seymour C. M., Monopoli M. P., O’Sullivan N. C., Murphy K. J., et al. , 2007. Notch signalling becomes transiently attenuated during long-term memory consolidation in adult Wistar rats. Neurobiol. Learn. Mem. 88: 342–351. 10.1016/j.nlm.2007.04.006 [DOI] [PubMed] [Google Scholar]
- Costa R. M., Honjo T., Silva A. J., 2003. Learning and memory deficits in Notch mutant mice. Curr. Biol. 13: 1348–1354. 10.1016/S0960-9822(03)00492-5 [DOI] [PubMed] [Google Scholar]
- Crowner D., Le Gall M., Gates M. A., Giniger E., 2003. Notch steers Drosophila ISNb motor axons by regulating the Abl signaling pathway. Curr. Biol. 13: 967–972. 10.1016/S0960-9822(03)00325-7 [DOI] [PubMed] [Google Scholar]
- Dahlhaus M., Hermans J. M., Van Woerden L. H., Saiepour M. H., Nakazawa K., et al. , 2008. Notch1 signaling in pyramidal neurons regulates synaptic connectivity and experience-dependent modifications of acuity in the visual cortex. J. Neurosci. 28: 10794–10802. 10.1523/JNEUROSCI.1348-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittman J. S., Kaplan J. M., 2008. Behavioral impact of neurotransmitter-activated G-protein-coupled receptors: muscarinic and GABAB receptors regulate Caenorhabditis elegans locomotion. J. Neurosci. 28: 7104–7112. 10.1523/JNEUROSCI.0378-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Bejjani R., Hammarlund M., 2012. Notch signaling inhibits axon regeneration. Neuron 73: 268–278. 10.1016/j.neuron.2011.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson E. L., Horvitz H. R., 1985. Identification and characterization of 22 genes that affect the vulval cell lineages of the nematode Caenorhabditis elegans. Genetics 110: 17–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald K., Wilkinson H. A., Greenwald I., 1993. glp-1 can substitute for lin-12 in specifying cell fate decisions in Caenorhabditis elegans. Development 119: 1019–1027. [DOI] [PubMed] [Google Scholar]
- Fortini M. E., 2009. Notch signaling: the core pathway and its posttranslational regulation. Dev. Cell 16: 633–647. 10.1016/j.devcel.2009.03.010 [DOI] [PubMed] [Google Scholar]
- Ge X., Hannan F., Xie Z., Feng C., Tully T., et al. , 2004. Notch signaling in Drosophila long-term memory formation. Proc. Natl. Acad. Sci. USA 101: 10172–10176. 10.1073/pnas.0403497101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald I., Kovall R., 2013. Notch signaling: genetics and structure. WormBook, ed. The C. elegans Research Community, WormBook 1–28. 10.1895/wormbook.1.10.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald I. S., Sternberg P. W., Horvitz H. R., 1983. The lin-12 locus specifies cell fates in Caenorhabditis elegans. Cell 34: 435–444. 10.1016/0092-8674(83)90377-X [DOI] [PubMed] [Google Scholar]
- Han B., Bellemer A., Koelle M. R., 2015. An Evolutionarily-Conserved Switch in Response to GABA Affects Development and Behavior of the Locomotor Circuit of Caenorhabditis elegans. Genetics 199: 1159–1172. 10.1534/genetics.114.173963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson S. T., Gao D., Lambie E. J., Kimble J., 1994. lag-2 may encode a signaling ligand for the GLP-1 and LIN-12 receptors of C. elegans. Development 120: 2913–2924. [DOI] [PubMed] [Google Scholar]
- Hu Z., Pym E. C., Babu K., Vashlishan Murray A. B., Kaplan J. M., 2011. A neuropeptide-mediated stretch response links muscle contraction to changes in neurotransmitter release. Neuron 71: 92–102. 10.1016/j.neuron.2011.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen E. M., Hartwieg E., Schuske K., Nonet M. L., Jin Y., et al. , 1995. Defective recycling of synaptic vesicles in synaptotagmin mutants of Caenorhabditis elegans. Nature 378: 196–199. 10.1038/378196a0 [DOI] [PubMed] [Google Scholar]
- Jospin M., Qi Y. B., Stawicki T. M., Boulin T., Schuske K. R., et al. , 2009. A neuronal acetylcholine receptor regulates the balance of muscle excitation and inhibition in Caenorhabditis elegans. PLoS Biol. 7: e1000265 10.1371/journal.pbio.1000265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaun K. R., Azanchi R., Maung Z., Hirsh J., Heberlein U., 2011. A Drosophila model for alcohol reward. Nat. Neurosci. 14: 612–619. 10.1038/nn.2805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. S., Lilley B. N., Zhang C., Shokat K. M., Sanes J. R., et al. , 2008. A chemical-genetic strategy reveals distinct temporal requirements for SAD-1 kinase in neuronal polarization and synapse formation. Neural Dev. 3: 23 10.1186/1749-8104-3-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu H., Chao M. Y., Larkins-Ford J., Corkins M. E., Somers G. A., et al. , 2008. OSM-11 facilitates LIN-12 Notch signaling during Caenorhabditis elegans vulval development. PLoS Biol. 6: e196 10.1371/journal.pbio.0060196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopan R., Ilagan M. X., 2009. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 137: 216–233. 10.1016/j.cell.2009.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambie E. J., Kimble J., 1991. Two homologous regulatory genes, lin-12 and glp-1, have overlapping functions. Development 112: 231–240. [DOI] [PubMed] [Google Scholar]
- Le Gall M., De Mattei C., Giniger E., 2008. Molecular separation of two signaling pathways for the receptor, Notch. Dev. Biol. 313: 556–567. 10.1016/j.ydbio.2007.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Collins K. M., Koelle M. R., Shen K., 2013. LIN-12/Notch signaling instructs postsynaptic muscle arm development by regulating UNC-40/DCC and MADD-2 in Caenorhabditis elegans. eLife 2: e00378 10.7554/eLife.00378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber T., Kidd S., Struhl G., 2011. DSL-Notch signaling in the Drosophila brain in response to olfactory stimulation. Neuron 69: 468–481. 10.1016/j.neuron.2010.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Wang Y., Worley P. F., Mattson M. P., Gaiano N., 2014. The canonical Notch pathway effector RBP-J regulates neuronal plasticity and expression of GABA transporters in hippocampal networks. Hippocampus 25: 670–678. 10.1002/hipo.22402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loria P. M., Hodgkin J., Hobert O., 2004. A conserved postsynaptic transmembrane protein affecting neuromuscular signaling in Caenorhabditis elegans. J. Neurosci. 24: 2191–2201. 10.1523/JNEUROSCI.5462-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mango S. E., Thorpe C. J., Martin P. R., Chamberlain S. H., Bowerman B., 1994. Two maternal genes, apx-1 and pie-1, are required to distinguish the fates of equivalent blastomeres in the early Caenorhabditis elegans embryo. Development 120: 2305–2315. [DOI] [PubMed] [Google Scholar]
- Marathe S., Alberi L., 2015. Notch in memories: Points to remember. Hippocampus 25: 1481–1488. 10.1002/hipo.22426 [DOI] [PubMed] [Google Scholar]
- McIntire S. L., Jorgensen E., Horvitz H. R., 1993. Genes required for GABA function in Caenorhabditis elegans. Nature 364: 334–337. 10.1038/364334a0 [DOI] [PubMed] [Google Scholar]
- Nonet M. L., Grundahl K., Meyer B. J., Rand J. B., 1993. Synaptic function is impaired but not eliminated in C. elegans mutants lacking synaptotagmin. Cell 73: 1291–1305. 10.1016/0092-8674(93)90357-V [DOI] [PubMed] [Google Scholar]
- Presente A., Boyles R. S., Serway C. N., de Belle J. S., Andres A. J., 2004. Notch is required for long-term memory in Drosophila. Proc. Natl. Acad. Sci. USA 101: 1764–1768. 10.1073/pnas.0308259100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand, J.B. (2007). Acetylcholine. WormBook, 1–21. [DOI] [PMC free article] [PubMed]
- Sallee M. D., Aydin T., Greenwald I., 2015. Influences of LIN-12/Notch and POP-1/TCF on the Robustness of Ventral Uterine Cell Fate Specification in Caenorhabditis elegans Gonadogenesis. G3 (Bethesda) 5: 2775–2782. 10.1534/g3.115.022608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultheis C., Brauner M., Liewald J. F., Gottschalk A., 2011. Optogenetic analysis of GABAB receptor signaling in Caenorhabditis elegans motor neurons. J. Neurophysiol. 106: 817–827. 10.1152/jn.00578.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuske K., Beg A. A., Jorgensen E. M., 2004. The GABA nervous system in C. elegans. Trends Neurosci. 27: 407–414. 10.1016/j.tins.2004.05.005 [DOI] [PubMed] [Google Scholar]
- Singh K., Chao M. Y., Somers G. A., Komatsu H., Corkins M. E., et al. , 2011. C. elegans Notch signaling regulates adult chemosensory response and larval molting quiescence. Curr. Biol. 21: 825–834. 10.1016/j.cub.2011.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Q., Sun K., Shuai Y., Lin R., You W., et al. , 2009. Suppressor of Hairless is required for long-term memory formation in Drosophila. J. Neurogenet. 23: 405–411. 10.3109/01677060903096133 [DOI] [PubMed] [Google Scholar]
- Sorkaç A., Alcantara I. C., Hart A. C., 2016. In Vivo Modelling of ATP1A3 G316S-Induced Ataxia in C. elegans Using CRISPR/Cas9-Mediated Homologous Recombination Reveals Dominant Loss of Function Defects. PLoS One 11: e0167963 10.1371/journal.pone.0167963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston J. E., 1976. Post-embryonic development in the ventral cord of Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 275: 287–297. 10.1098/rstb.1976.0084 [DOI] [PubMed] [Google Scholar]
- Sulston J. E., Horvitz H. R., 1977. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev. Biol. 56: 110–156. 10.1016/0012-1606(77)90158-0 [DOI] [PubMed] [Google Scholar]
- Vashlishan A. B., Madison J. M., Dybbs M., Bai J., Sieburth D., et al. , 2008. An RNAi screen identifies genes that regulate GABA synapses. Neuron 58: 346–361. 10.1016/j.neuron.2008.02.019 [DOI] [PubMed] [Google Scholar]
- Wang Y., Chan S. L., Miele L., Yao P. J., Mackes J., et al. , 2004. Involvement of Notch signaling in hippocampal synaptic plasticity. Proc. Natl. Acad. Sci. USA 101: 9458–9462. 10.1073/pnas.0308126101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yochem J., Greenwald I., 1989. glp-1 and lin-12, genes implicated in distinct cell-cell interactions in C. elegans, encode similar transmembrane proteins. Cell 58: 553–563. 10.1016/0092-8674(89)90436-4 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Kubiseski T. J., 2010. Caenorhabditis elegans wsp-1 regulation of synaptic function at the neuromuscular junction. J. Biol. Chem. 285: 23040–23046. 10.1074/jbc.M109.096164 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
For wild type responses in Table 1 the raw data are available at GSA FigShare. All data necessary for confirming conclusions of this article are represented in figures and tables. All the strains used in this study, and raw data for all experiments are available upon request. Supplemental material available at Figshare: https://doi.org/10.25387/g3.6480419.
Table 1. Aldicarb response of ligand or co-ligand mutant animals. Loss of OSM-7, OSM-11 or DSL-3 phenocopies LIN-12 loss, whereas decreased LAG-2, DOS-1 or DSL-6 results in resistance to aldicarb similarly to decreased glp-1 function. n/a indicates “not available”. At least 10 animals tested for dos-2, arg-1, dsl-2, dsl-4 and dsl-5. For RNAi experiments, three independent trials were conducted using the neuronal RNAi-sensitive strain eri-1(mg366); lin-15B(n744), with a total of at least 30 animals for each knocked-down gene. For non-wild type responses, aldicarb-induced paralysis rates are in Figure S2. For wild type responses the raw data are available in supplements.
| Gene | Function | Allele | Effect on function | Aldicarb Response |
|---|---|---|---|---|
| osm-7 | DOS co-ligand | tm2256 | null | hypersensitive |
| osm-11 | DOS co-ligand | rt142 | null | hypersensitive |
| dos-1 | DOS co-ligand | ok2398 | null | resistant |
| dos-2 | DOS co-ligand | tm4515 | putative null | wild type |
| dos-3 | DOS co-ligand | n/a | RNAi knockdown | wild type |
| lag-2 | DSL ligand | q420 | loss of function | resistant |
| arg-1 | DSL ligand | ok3127 | putative null | wild type |
| apx-1 | DSL ligand | n/a | RNAi knockdown | wild type |
| dsl-1 | DSL ligand | ok810 | null | wild type |
| dsl-2 | DSL ligand | tm1805 | putative null | wild type |
| dsl-3 | DSL ligand | ok3411 | putative null | hypersensitive |
| dsl-4 | DSL ligand | ok1020 | putative null | wild type |
| dsl-5 | DSL ligand | ok588 | putative null | wild type |
| dsl-6 | DSL ligand | ok2265 | putative null | resistant |
| dsl-7 | DSL ligand | n/a | RNAi knockdown | wild type |