Abstract
Background
In the current study, we pooled imaging data in newly diagnosed glioblastoma (GBM) patients from international multicenter clinical trials, single institution databases, and multicenter clinical trial consortiums to identify the relationship between postoperative residual enhancing tumor volume and overall survival (OS).
Methods
Data from 1511 newly diagnosed GBM patients from 5 data sources were included in the current study: (i) a single institution database from UCLA (N = 398; Discovery); (ii) patients from the Ben and Cathy Ivy Foundation for Early Phase Clinical Trials Network Radiogenomics Database (N = 262 from 8 centers; Confirmation); (iii) the chemoradiation placebo arm from an international phase III trial (AVAglio; N = 394 from 120 locations in 23 countries; Validation); (iv) the experimental arm from AVAglio examining chemoradiation plus bevacizumab (N = 404 from 120 locations in 23 countries; Exploratory Set 1); and (v) an Alliance (N0874) phase I/II trial of vorinostat plus chemoradiation (N = 53; Exploratory Set 2). Postsurgical, residual enhancing disease was quantified using T1 subtraction maps. Multivariate Cox regression models were used to determine influence of clinical variables, O6-methylguanine-DNA methyltransferase (MGMT) status, and residual tumor volume on OS.
Results
A log-linear relationship was observed between postoperative, residual enhancing tumor volume and OS in newly diagnosed GBM treated with standard chemoradiation. Postoperative tumor volume is a prognostic factor for OS (P < 0.01), regardless of therapy, age, and MGMT promoter methylation status.
Conclusion
Postsurgical, residual contrast-enhancing disease significantly negatively influences survival in patients with newly diagnosed GBM treated with chemoradiation with or without concomitant experimental therapy.
Keywords: bevacizumab, clinical trials, contrast-enhancing tumor volume, GBM, new glioblastoma, prognosis, T1 subtraction
Importance of the study
While there is overwhelming evidence suggesting extent of surgical resection is a significant prognostic factor for OS in newly diagnosed GBM, distinction between investigator-defined extent of resection for use in clinical trials remains subjective and highly variable across institutions and investigators. Further, estimates are subject to errors associated with postoperative blood products, and most clinical trials do not collect preoperative images for independent verification. In the current study, we examined a dataset of newly diagnosed GBM patients from single institutions, academic consortia, and clinical trials and demonstrate that postoperative, pretreatment, baseline enhancing tumor volume quantified using T1 digital subtraction is a significant prognostic factor for OS in newly diagnosed GBM independently of clinical covariates and the type of therapy employed. Results have important implications in clinical trial design, suggesting steps should be taken to ensure balance among treatment arms in terms of distribution of tumor size and effects of postoperative tumor size considered when interpreting therapeutic efficacy.
Contrast-enhanced T1-weighted magnetic resonance (MR) imaging has been the standard for glioblastoma (GBM) detection, diagnosis, and clinical monitoring for nearly 30 years. There is a well-documented association between contrast enhancement and histological1–4 and genetic5–7 features of malignant gliomas. There is overwhelming evidence to suggest that the extent of surgical resection, partitioned into gross total resection, subtotal resection, or biopsy, is a significant prognostic factor for overall survival (OS) in newly diagnosed GBM as evidenced by a French study (N = 952),8 a Chinese study (N = 816),9 US study (N = 1672),10 a meta-analysis summarizing 200 publications from phase II and III trials (N = 17213),11 and 2 large studies examining data from the Surveillance, Epidemiology, and End Results registry between 1973–200712 and 2000–2009 (N = 21783 and 14675).13 Studies that have suggested resections anywhere beyond 70%–80%14–16 provide an OS survival advantage for patients with GBM; however, the distinction between investigator-defined gross total resection and subtotal resection for use in clinical trials remains highly subjective and variable across both institutions and investigators.
Precise quantification of residual enhancing tumor can be particularly challenging in the presence of postsurgical blood products. Contrast-enhanced T1-weighted digital subtraction maps, or “T1 subtraction maps,” may overcome these issues.17–20 T1 subtraction maps allow for discrimination between regions of true contrast enhancement and blood products through digital subtraction of precontrast from postcontrast T1-weighted images.18–20 Thus, we hypothesize that postoperative, residual contrast-enhancing tumor volume quantified using T1 subtraction maps is a significant prognostic factor for OS in newly diagnosed GBM.
In the current study, we examined a dataset of newly diagnosed GBM patients from single institutions, academic consortia, and clinical trials to test the hypothesis that postoperative, pretreatment, baseline enhancing tumor volume is a significant prognostic factor for OS in newly diagnosed GBM. We hypothesize that large enhancing tumor volume after surgery is associated with shortened OS regardless of the type of therapy employed. To test this hypothesis, we used a discovery cohort of patients from a single institution (N = 398) treated with standard chemoradiation, a confirmation cohort of patients from a multicenter academic consortium (N = 262) treated with standard chemoradiation, and a validation cohort from the placebo arm in a multicenter phase III clinical trial (N = 394) treated with standard chemoradiation. Next, we explored whether postoperative residual enhancing tumor volume was a significant prognostic factor in an exploratory cohort of patients treated with chemoradiation in addition to bevacizumab as part of a multicenter phase III trial (N = 404) as well as an exploratory cohort of patients treated with chemoradiation plus vorinostat as part of a multicenter phase I/II trial (N = 53).
Methods
Patients
A total of 1511 patients with pathologically confirmed newly diagnosed GBM from 5 data sources were included in this retrospective study. A dataset from a single center (UCLA) with 398 newly diagnosed GBM treated with standard chemoradiation was used for initial discovery; a multicenter dataset from the Ben and Catherine Ivy Foundation Clinical Trials Network including 262 newly diagnosed GBM patients treated with standard chemoradiation was used for confirmation; and the placebo arm from a multicenter phase III trial (AVAglio, ClinicalTrials.gov #NCT00943826) consisting of 394 newly diagnosed GBM patients treated with chemoradiation was used for validation. All patients in these 3 cohorts received concurrent radiation therapy and temozolomide (TMZ) followed by adjuvant TMZ, per Stupp et al,21 until first recurrence.
In addition to standard chemotherapy, we explored whether postoperative residual enhancing tumor volume was predictive of OS in patients treated with chemoradiation plus bevacizumab using 404 patients with newly diagnosed GBM from the experimental arm of AVAglio. Additionally, we explored whether postoperative residual enhancing tumor was predictive of OS in a phase I/II trial in 53 patients treated with chemoradiation plus vorinostat (ClinicalTrials.gov #NCT00731731). Data acquisition was performed in compliance with all applicable regulations of the Health Insurance Portability and Accountability Act.
UCLA Neuro-Oncology Single Center Database
A cohort of 398 patients from UCLA who met the following criteria were examined: (i) histologically confirmation of isocitrate dehydrogenase (IDH) wild-type GBM; (ii) postoperative, preradiation anatomic MR images available for analysis; and (iii) uniform treatment with standard chemoradiation per Stupp et al,21 consisting of concurrent radiation therapy in daily fractions of 2 Gy, given 5 days per week for 6 weeks and concomitant TMZ (Temodar, Merck) at 75 mg/m2 daily during radiation, followed by a 4-week treatment break and then maintenance TMZ consisting of 150 to 200 mg/m2 daily for the first 5 days in a 28-day cycle for up to 6 cycles or until disease progression. Only 13% (52 of 398) of UCLA patients had O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation status available using the Sanger sequence and methylation-specific PCR as previously described.22,23 UCLA patients in this study signed institutional review board–approved informed consent to have their data included in our research database for subsequent studies.
Ben and Catherine Ivy Foundation Clinical Trials Network Radiogenomic Database
A group of 262 patients from 7 centers (Dana-Farber Cancer Institute, N = 67; MD Anderson Cancer Center, N = 13; Massachusetts General Hospital, N = 51; Memorial Sloan Kettering Cancer Center, N = 8; UCLA, N = 25; UCSF, N = 47; and University of Utah, N = 51) were combined as part of the Ben and Catherine Ivy Foundation Clinical Trials Network Radiogenomic Database. All patients had: (i) histologically confirmed GBM; (ii) postoperative, preradiation anatomic MR images available for analysis; and (iii) uniform treatment with standard chemoradiation per Stupp et al,21 as outlined above. At the time of this analysis, MGMT promoter methylation status was not yet available. A total of 10 of the 282 patients (3.5%) were IDH mutants and were kept in the resulting analysis. All patients in this cohort provided written consent as part of an institutional review board–approved multicenter research database.
A Phase III Study Comparing Chemoradiation Plus Bevacizumab or Placebo in Newly Diagnosed GBM (AVAglio)
Included in the current study (ClinicalTrials.gov #NCT00943826) were a total of 798 patients with histologically confirmed GBM from up to 120 institutions from 23 countries enrolled in AVAglio, a phase III study comparing upfront chemoradiation plus bevacizumab or placebo, with adequate postoperative, preradiation MR images. Specific inclusion and exclusion criteria for this trial can be found at https://clinicaltrials.gov/ct2/show/NCT00943826). Any patients with IDH mutation were also excluded from analyses (349 patients had IDH status available, for which 10 were IDH mutants, as reported by Sandmann et al24). For validation, the placebo arm of the trial consisting of 394 histologically confirmed newly diagnosed GBM patients was used. These patients were treated according to Stupp et al,21 or concurrent radiation therapy and TMZ plus placebo followed by maintenance TMZ for up to 6 cycles, similar to the above cohorts. The experimental arm of this trial was then used to determine whether baseline tumor volume was predictive of OS in patients treated with chemoradiation plus bevacizumab. This experimental arm consisted of 404 patients with newly diagnosed GBM treated with concurrent radiation therapy in daily fractions of 2 Gy, given 5 days per week for 6 weeks, concomitant TMZ at 75 mg/m2 daily and bevacizumab (Avastin, Hoffman La-Roche) 10 mg/kg i.v. every 2 weeks during radiation, followed by a 4-week treatment break, and then maintenance bevacizumab 10 mg/kg i.v. every 2 weeks and TMZ 150 to 200 mg/m2 daily for the first 5 days in a 28-day cycle for up to 6 cycles or until disease progression. A total of 76% of patients with imaging data available for analysis also had MGMT promoter methylation status available (610 of 798), with 78% of patients in the placebo arm (307 of 394) and 75% (303 of 404) of patients in the bevacizumab arm having MGMT status information available.
A Phase I/II Study of Vorinostat, Temozolomide, and Radiation Therapy in Newly Diagnosed GBM (Alliance N0874/ABTC-0902)
Lastly, we explored whether postoperative residual enhancing tumor volume was predictive of OS in a phase I/II trial of chemoradiation plus vorinostat in 53 patients with newly diagnosed GBM with adequate postoperative, preradiation MR images available as part of the phase II portion of the study (ClinicalTrials.gov #NCT00731731). Specific inclusion and exclusion criteria for this trial can be found at https://clinicaltrials.gov/ct2/show/NCT00731731). All patients included received radiation therapy in daily fractions of 2 Gy, given 5 days per week for 6 weeks, concomitant TMZ at 75 mg/m2 daily, and oral vorinostat (Zolinza, Merck) 300 mg/day on days 1–5, 8–12, 15–19, 22–26, 29–33, and 36–40 during radiation, followed by a 4–6 week treatment break. During the maintenance phase, TMZ was given at 150 to 200 mg/m2 daily for the first 5 days and vorinostat 400 mg/day on days 1–7 and 15–21 in a 28-day cycle for up to 12 cycles or until disease progression or unacceptable toxicity. MGMT promoter methylation status was available for 19 of 53 patients (36%) and IDH mutation status was not available at the time of analyses.
Magnetic Resonance Imaging
Anatomic MR images were acquired for all patients in the current study using a 1.5T or 3T clinical MR scanner using pulse sequences supplied by their respective manufacturers and according to their local standard of care protocols. Precontrast anatomic axial T1-weighted fast spin-echo or 3D gradient echo sequences were acquired along with T2-weighted fast spin-echo and fluid-attenuated inversion recovery (FLAIR) sequences. In addition, parameter matched T1-weighted images enhanced with gadolinium chelates (eg, gadopentetate dimeglumine [Magnevist, Berlex], 0.1 mmol/kg) were acquired after contrast agent injection.
Contrast-Enhanced T1-Weighted Digital Subtraction Maps
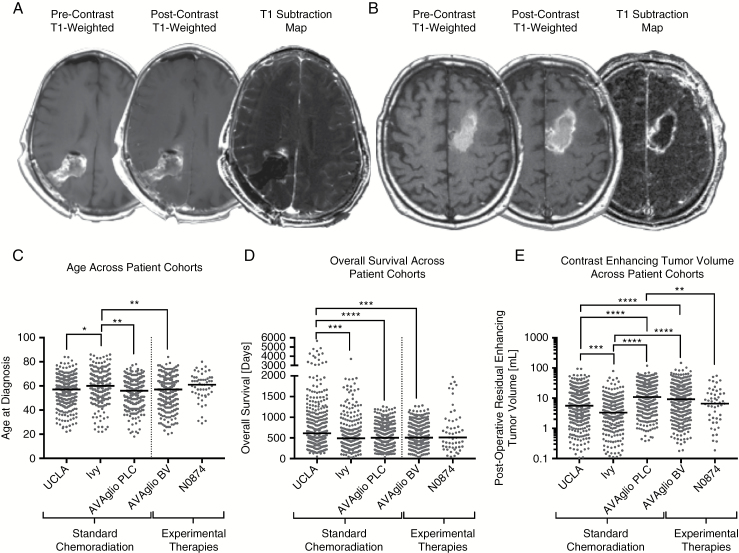
Contrast-enhanced T1-weighted subtraction maps (Fig. 1A, B) were created using previously described methods.25–27 Briefly, linear registration was performed between T2-weighted FLAIR and non-enhanced and contrast-enhanced T1-weighted images. Next, normalization of image intensity for both non-enhanced and contrast-enhanced T1-weighted images was performed. Lastly, voxel-by-voxel subtraction between the normalized non-enhanced and contrast-enhanced T1-weighted images was performed to create T1 subtraction maps. Image voxels with a positive (greater than zero) value after subtraction of pre- and postcontrast images (ie, voxels increasing in MR signal after contrast agent administration) within T2-weighted FLAIR hyperintense regions were isolated as volumes of interest (VOIs). Final VOIs included areas of contrast enhancement on T1 subtraction maps, excluding any areas of residual necrotic (T1 hypointense) tissue. A team of trained lab technologists created initial VOIs and all final VOIs were reviewed by a single investigator (B.M.E.) who was blinded to other relevant metrics until study completion.
Fig. 1.
Contrast-enhanced T1-weighted digital subtraction maps and comparison of age, overall survival, and postoperative enhancing tumor volumes across patient cohorts. In order to increase lesion conspicuity in the presence of postsurgical changes, pre- and postcontrast T1-weighted images were intensity normalized, co-registered, and subtracted voxel-by-voxel, highlighting only areas of increased signal intensity following contrast administration. The resulting “T1 subtraction maps” were then used to quantify enhancing tumor volume by excluding blood products and areas of necrosis. (A) Precontrast T1-weighted images, postcontrast T1-weighted images, and T1 subtraction maps for a 58-year-old male patient with newly diagnosed GBM treated at the Massachusetts General Hospital submitted as part of the Ben and Catherine Ivy Foundation Clinical Trials Network Radiogenomic Database. (B) Precontrast T1-weighted images, postcontrast T1-weighted images, and T1 subtraction maps for a 67-year-old male patient with newly diagnosed GBM treated with chemoradiation plus vorinostat at the Mayo Clinic as part of the Alliance N0874 trial. Note extensive precontrast T1 shortening due to postsurgical changes and increased enhancing tumor conspicuity on T1 subtraction maps after these postsurgical changes were removed. (C) Distribution of patient age across different study cohorts. (D) Distribution of overall survival (OS) in patients across different study cohorts. (E) Distribution of postoperative, residual contrast-enhancing tumor volume in patients across the different study cohorts. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 for adjusted P-values from Dunn’s test for multiple comparisons. N0874 = chemoradiation plus vorinostat. AVAglio PLC = placebo arm from AVAglio trial (standard chemoradiation). AVAglio BV = bevacizumab arm from AVAglio trial (chemoradiation plus bevacizumab).
Statistical Analysis
A one-way nonparametric Kruskal–Wallis test with adjusted P-values from Dunn’s test for multiple comparisons was used to compare age, OS, and postoperative enhancing tumor volumes across patient cohorts. Log-rank analysis on Kaplan–Meier data and Cox proportional hazards regression models were used to understand the relationship between postoperative contrast-enhancing tumor volume and OS, independent of other factors, including age. Log-linear regression (model: OS = a·log10(Volume) + b) and log-rank test for trends were used to explore trends between postoperative contrast-enhancing tumor volume and OS. Covariates available for multivariable Cox regression analyses included age and treatment type. MGMT promoter methylation status was not available for the majority of patients in the current study. No adjustments for multiple comparisons were performed. All statistical tests were performed using GraphPad Prism v6.0h or Stata v12.
Results
T1 subtraction maps were able to isolate contrast-enhancing tumor in the presence of postoperative changes, including blood products in all trial patients included in the current study (Fig. 1A, B). In general, age, OS, and contrast-enhancing volume were significantly different across all patient cohorts (Table 1). Specifically, patients within the Ivy radiogenomics database had a significantly higher (60 y old) median age (Fig. 1C; Kruskal–Wallis, P = 0.0002) compared with the UCLA cohort (median = 57; Dunn’s test, Adj P = 0.0129) and both AVAglio treatment arms (median = 56 for placebo arm and 57 for bevacizumab arm; Adj P = 0.0013 and 0.0039, respectively). Examination of OS across patient cohorts showed a significantly longer median OS in the UCLA cohort (median OS = 613 days) (Fig. 1D; P < 0.0001) compared with the Ivy radiogenomics database (median OS = 490 days, Adj P = 0.0004) and both AVAglio treatment arms (median OS = 502 and 505.5 days for placebo and bevacizumab arms; Adj P < 0.0001 and 0.0003, respectively). Postoperative contrast-enhancing tumor volume also varied significantly across patient groups (Fig. 1E; P < 0.0001). In particular, the placebo arm of AVAglio demonstrated a significantly higher postoperative tumor volume (median = 10.9 mL, mean = 17.2 mL) compared with UCLA (median = 5.6 mL, mean = 10.1 mL; Adj P = 0.0004), the Ivy radiogenomics database (median = 3.3 mL, mean = 6.2 mL; Adj P < 0.0001), and the vorinostat trial (median = 6.6 mL, mean = 10.9 mL; Adj P = 0.0028). Patients within the bevacizumab treatment arm of AVAglio also had higher postoperative tumor volumes (median = 9.2 mL, mean = 15.7 mL) compared with UCLA (Adj P < 0.0001) and the Ivy radiogenomics database (Adj P < 0.0001). UCLA patients had significantly higher volumes compared with the Ivy radiogenomics database (Adj P = 0.0004). No significant difference in tumor volumes was detected between treatment arms in AVAglio (Adj P = 0.1158).
Table 1.
Summary data from patient cohorts used in the current study
| Treatment | Dataset | Age, y | Volume, mL | Overall Survival, days |
|---|---|---|---|---|
| Chemoradiation | Discovery—UCLA (N = 398) | 56.1 ± 0.6 SEM | 10.2 ± 0.6 SEM | 878 ± 42 SEM |
| MGMT Methylation Status Available (N = 52; 16 = M, 36 = U) | Median = 5.6 | Median = 613 | ||
| Chemoradiation | Confirmation—Ivy Radiogenomics (N = 262) | 59.1 ± 0.8 SEM | 6.2 ± 0.5 SEM | 630 ± 30 SEM |
| MGMT Methylation Status Available (N = 0) | Median = 3.3 | Median = 490 | ||
| Chemoradiation | Validation—AVAglio PLC Arm (N = 394) | 55.5 ± 0.5 SEM | 17.2 ± 0.8 SEM | 546 ± 14 SEM |
| MGMT Methylation Status Available (N = 307; 108 = M, 199 = U) | Median = 10.9 | Median = 502 | ||
| Chemoradiation | AVAglio BV Arm (N = 404) | 55.6 ± 0.6 SEM | 15.7 ± 0.9 SEM | 570 ± 14 SEM |
| +Bevacizumab | MGMT Methylation Status Available (N = 303; 102 = M, 201 = U) | Median = 9.2 | Median = 506 | |
| Chemoradiation | Alliance N0874 (N = 53) | 59.2 ± 1.6 SEM | 10.9 ± 1.8 SEM | 676 ± 72 SEM |
| +Vorinostat | MGMT Methylation Status Available (N = 19; 11 = M, 8 = U) | Median = 6.6 | Median = 511 |
PLC = placebo; BV = bevacizumab; M = methylated; U = unmethylated; SEM = standard error about the mean
Discovery—UCLA Neuro-Oncology Single Center Database
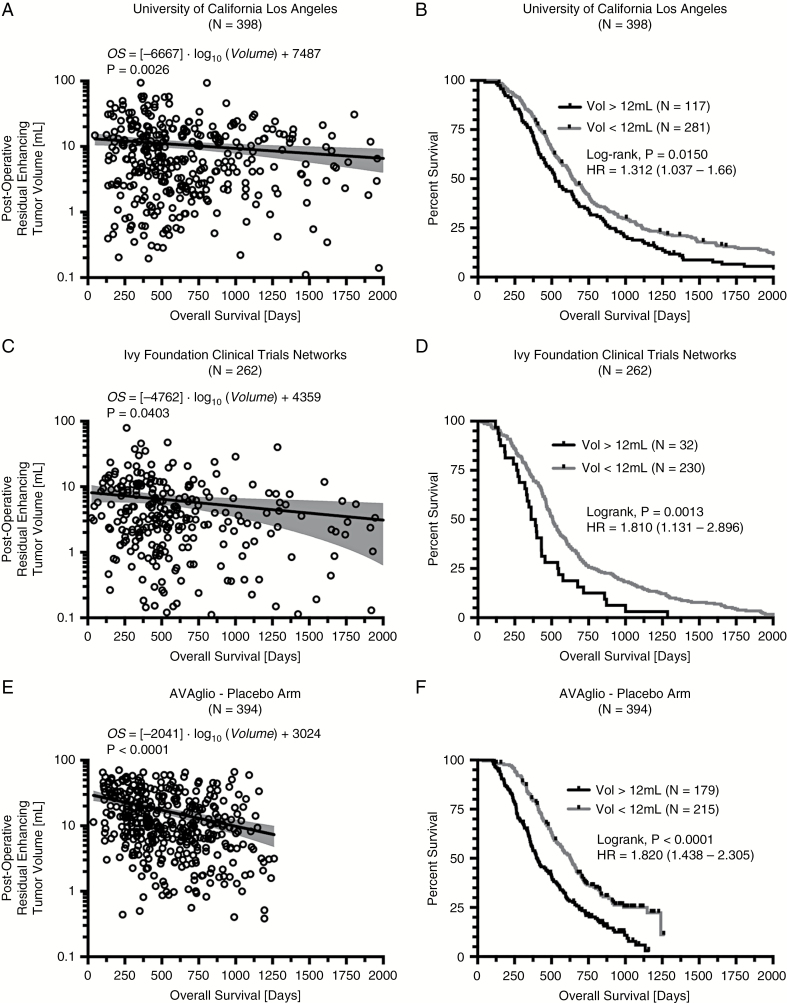
Results demonstrated a statistically significant log-linear relationship between postoperative enhancing tumor volume and OS (Fig. 2A; P = 0.0026; OS = [−6667]·log10(Volume) + 7487 days). Univariate log-rank analysis of postoperative enhancing tumor volume stratified by 12 mL, the average volume of the entire chemoradiation cohort from all 1054 patients in all cohorts, indicated tumors smaller than 12 mL had a significantly longer OS compared with those in patients with residual enhancing tumor volumes larger than 12 mL (Fig. 2B; median OS = 643 vs 525 days; P = 0.0150; hazard ratio [HR] = 1.312). The Cox multivariable proportional hazards test determined that both continuous volume (P = 0.0007, HR = 1.0124) and age (P < 0.0001, HR = 1.0246) were independently predictive of OS in newly diagnosed GBM treated with standard chemoradiation (Table 2).
Fig. 2.
Log-linear correlation and survival analysis results for discovery, confirmation, and validation cohorts of newly diagnosed GBM treated with standard chemoradiation. (A) Log-linear correlation between postoperative tumor volume and OS in a single center cohort from the UCLA Neuro-Oncology Database (N = 398). (B) Kaplan–Meier survival plots demonstrating a survival advantage for patients with tumor volumes less than 12 mL, the mean volume of residual tumor from all chemoradiation only trials. (C) Log-linear correlation between postoperative tumor volume and OS in the Ivy Foundation Clinical Trials Network Radiogenomic Database (N = 262). (D) Kaplan–Meier survival plots confirming a survival advantage in patients with a small (<12 mL) residual enhancing tumor remaining following surgical resection. (E) Log-linear correlation between postoperative tumor volume and OS in the AVAglio placebo arm (N = 384). (F) Kaplan–Meier survival plots validating the survival advantage in patients with small (<12 mL) enhancing tumors. (G) Log-linear correlation in all patients treated with standard chemoradiation (N = 1054). (H) Kaplan–Meier survival plots showing increasingly longer OS with smaller tumors. (I) Plot of average enhancing tumor volume versus median OS for tumor volumes from 0 to 20 mL in increments of 5 mL.
Table 2.
Multivariate Cox regression model results including age and continuous tumor volume (excluding necrosis) for newly diagnosed GBM patients treated with standard chemoradiation with and without experimental therapy
| Treatment | Dataset | Variable | Coefficient | Hazard Ratio | 95% CI | P-value |
|---|---|---|---|---|---|---|
| Chemoradiation | Discovery—UCLA (N = 398) | Age | 0.0243 ± 0.0047 | 1.0246 | (1.0153–1.0340) | <0.0001 |
| Volume | 0.0123 ± 0.0036 | 1.0124 | (1.0052–1.0196) | 0.0007 | ||
| Chemoradiation | Confirmation—Ivy Radiogenomics (N = 262) | Age | 0.0220 ± 0.0052 | 1.0222 | (1.0118–1.0327) | <0.0001 |
| Volume | 0.0199 ± 0.0069 | 1.0201 | (1.0064–1.0340) | 0.0038 | ||
| Chemoradiation | Validation—AVAglio PLC Arm (N = 394) | Age | 0.0264 ± 0.0060 | 1.0267 | (1.0147–1.0390) | <0.0001 |
| Volume | 0.0196 ± 0.0033 | 1.0198 | (1.0131–1.0264) | <0.0001 | ||
| Chemoradiation+Bevacizumab | AVAglio BV Arm (N = 404) | Age | 0.0132 ± 0.0053 | 1.0133 | (1.0029–1.0238) | 0.0122 |
| Volume | 0.0166 ± 0.0027 | 1.0167 | (1.0114–1.0221) | <0.0001 | ||
| Chemoradiation | Alliance N0874 (N = 53) | Age | 0.0181 ± 0.0150 | 1.0183 | (0.9888–1.03486) | 0.2271 |
| +Vorinostat | Volume | 0.0287 ± 0.0089 | 1.0291 | (1.0113–1.0473) | 0.0013 |
PLC = placebo. BV = bevacizumab.
Confirmation—Multicenter Ivy Foundation Radiogenomics Database
Multicenter data collected as part of the Ivy Foundation Clinical Trial Network Radiogenomics Database confirmed a statistical log-linear relationship between postoperative enhancing tumor volume and OS (Fig. 2C; P = 0.0403; OS = [−4762]·log10(Volume) + 4359 days), with smaller tumors demonstrating a longer OS. Univariate log-rank analysis of postoperative enhancing tumor volume stratified by 12 mL, the average volume for all chemoradiation patients from all cohorts included in this study, also confirmed a significant survival advantage for patients with smaller residual tumor burden (Fig. 2D; median OS = 508 vs 375 days; P = 0.0013, HR = 1.81). The Cox multivariable proportional hazards test confirmed that both continuous volume (P = 0.0038, HR = 1.0201) and age (P < 0.0001, HR = 1.0222) were independently predictive of OS in newly diagnosed GBM treated with standard chemoradiation when evaluated across multiple institutions (Table 2).
Validation—International Multicenter Phase III AVAglio Trial
Results from the placebo arm in AVAglio validated the log-linear relationship between postoperative residual enhancing tumor volume and OS in newly diagnosed GBM treated with standard chemoradiation (Fig. 2E; P < 0.0001; OS = [−2041]·log10(Volume) + 3024 days). Univariate log-rank analysis of postoperative enhancing tumor volume in the AVAglio placebo arm verified that patients with smaller residual enhancing tumor volume (<12 mL) have a significantly longer OS compared with larger tumors (>12 mL) in patients treated with standard chemoradiation (Fig. 2F; median OS = 508 vs 375 days; P < 0.0001, HR = 1.820). The Cox multivariable proportional hazards test further verified the previous data, confirming that both continuous volume (P < 0.0001, HR = 1.0198) and age (P < 0.0001, HR = 1.0267) were independently predictive of OS (Table 2). In patients with MGMT promoter methylation status available, a separate Cox multivariable proportional hazards model confirmed that continuous measures of postoperative enhancing tumor volume (P < 0.0001, HR = 1.0224), age (P < 0.0001, HR = 1.0346), and MGMT promoter methylation status (P < 0.0001, HR = 0.3267) were all independent predictive factors for OS (Table 3).
Table 3.
Multivariate Cox regression model results including age, MGMT promoter methylation status, and continuous tumor volume (excluding necrosis) for a subset of newly diagnosed GBM patients treated with standard chemoradiation with or without bevacizumab and MGMT status available
| Treatment | Dataset | Variable | Coefficient | Hazard Ratio | 95% CI | P-value |
|---|---|---|---|---|---|---|
| Chemoradiation | Validation—AVAglio PLC Arm w/ MGMT (N = 307) | Age | 0.0340 ± 0.0073 | 1.0346 | (1.0199–1.0495) | <0.0001 |
| MGMT Status* | -1.1188 ± 0.1537 | 0.3267 | (0.2417–0.4415) | <0.0001 | ||
| Volume | 0.0222 ± 0.0037 | 1.0224 | (1.0150–1.0299) | <0.0001 | ||
| Chemoradiation +Bevacizumab | AVAglio BV Arm w/ MGMT (N = 303) | Age | 0.0188 ± 0.0062 | 1.0189 | (1.0066–1.0314) | 0.0025 |
| MGMT Status* | -0.9420 ± 0.1643 | 0.3899 | (0.2825–0.5380) | <0.0001 | ||
| Volume | 0.0152 ± 0.0028 | 1.0153 | (1.0097–1.0209) | <0.0001 |
*MGMT status, 0 = unmethylated, 1 = methylated. PLC = placebo. BV = bevacizumab.
Summary of Standard Chemoradiation Results
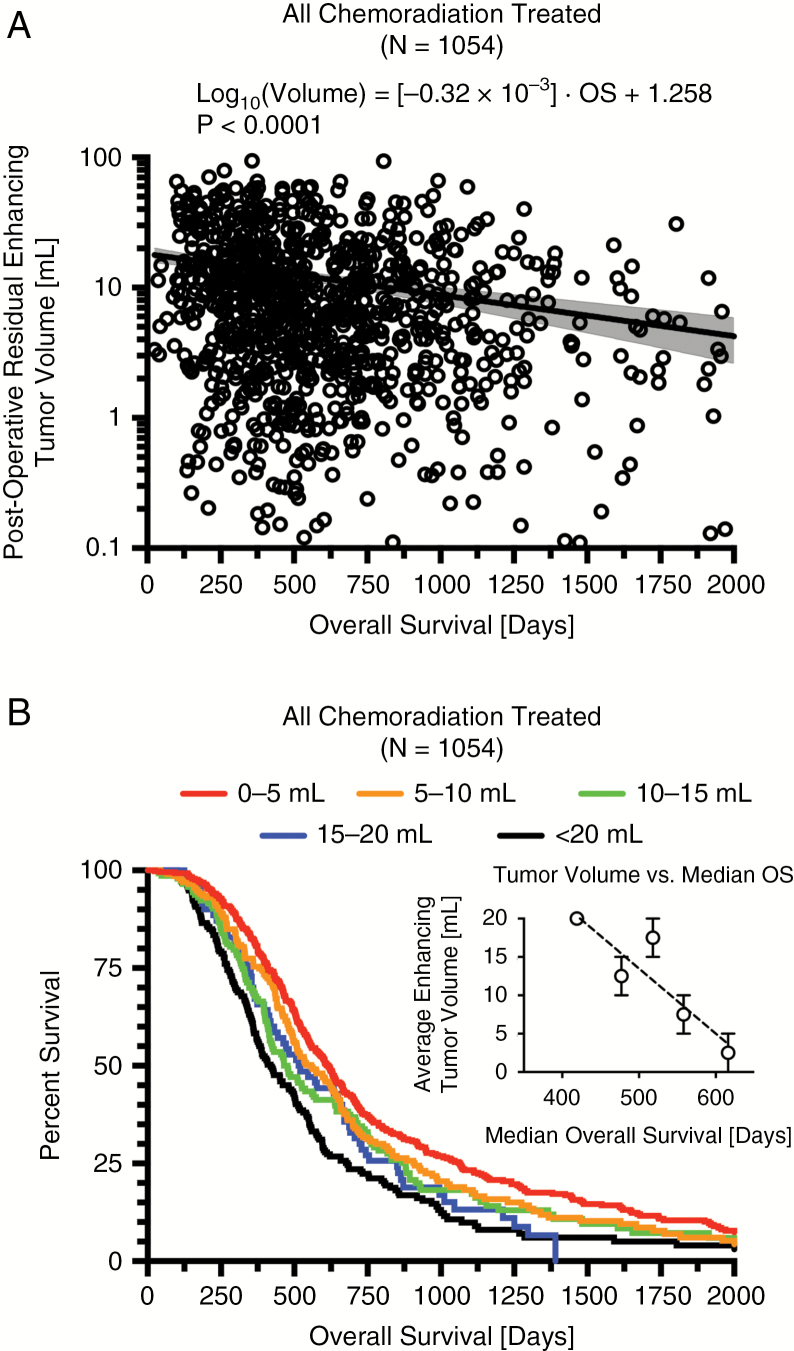
Combined results from all available newly diagnosed GBM in patients treated with standard chemoradiation (N = 1054) demonstrated a strong log-linear relationship between enhancing tumor volume and OS (Fig. 3A; P < 0.0001; OS = [−3125]·log10(Volume) + 3931 days). Log-rank analysis suggested a significant trend between residual volume categories ranging from 0 to 20 mL in increments of 5 mL (Fig. 3B, C; P < 0.0001; pairwise log-rank comparisons in Table 4) and a Cox multivariable proportional hazards model confirmed that continuous volume (P < 0.0001, HR = 1.0153) and age (P < 0.0001, HR = 1.0249) were significant prognostic factors for OS when all patients treated with chemoradiation were pooled. Additionally, no statistically significant differences were observed between Cox regression coefficients from the Discovery, Confirmation, and Validation datasets (ANOVA, P = 0.3597), suggesting that postsurgical contrast-enhancing tumor volume may provide similar prognostic value across the different datasets explored.
Fig. 3.
Log-linear correlation and survival analysis results for combined cohort of newly diagnosed GBM patients treated with standard chemoradiation. (A) Log-linear correlation in all patients treated with standard chemoradiation (N = 1054). (B) Kaplan–Meier survival plots showing increasingly longer OS with smaller tumors. (C) Plot of average enhancing tumor volume versus median OS for tumor volumes from 0 to 20 mL in increments of 5 mL.
Table 4.
Pairwise log-rank comparisons between residual volume categorizations shown in Fig. 3
| Residual Volume | 0–5 mL | 5–10 mL | 10–15 mL | 15–20 mL | >20 mL |
|---|---|---|---|---|---|
| 0–5 mL | – | ||||
| 5–10 mL | 0.1298 | – | |||
| 10–15 mL | 0.0077 | 0.3454 | – | ||
| 15–20 mL | 0.0110 | 0.2620 | 0.6023 | – | |
| >20 mL | <0.0001 | 0.0016 | 0.0559 | 0.1726 | – |
Chemoradiation Plus Bevacizumab
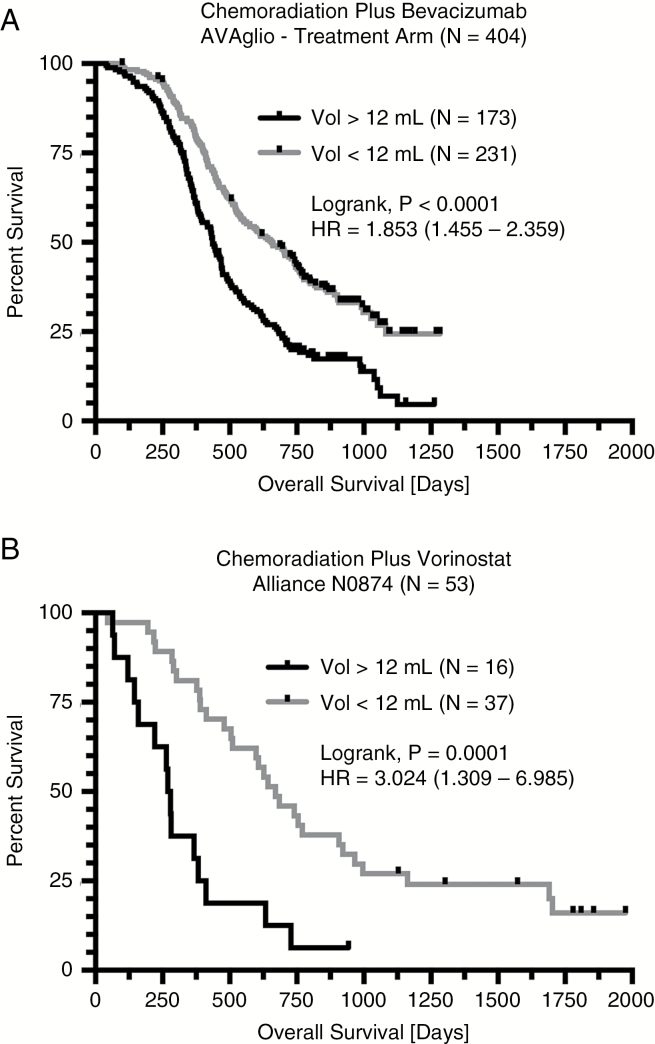
To explore whether postoperative residual enhancing tumor volume was also prognostic for OS in patients treated with standard chemoradiation plus experimental therapies, we first examined data from the bevacizumab experimental arm from AVAglio. Univariate results suggested that smaller tumors (<12 mL) had significantly longer OS compared with large (>12 mL) tumors (Fig. 4A; median OS = 656 vs 436 days; P < 0.0001; HR = 1.853). The Cox multivariable proportional hazards analysis again demonstrated that both continuous volume (P < 0.0001, HR = 1.0167) and age (P = 0.0122, HR = 1.0133) were independently predictive of OS in patients treated with upfront chemoradiation and bevacizumab (Table 2). An additional Cox regression model in 303 of 404 available patients with MGMT status information available further confirmed that continuous enhancing tumor volume (P < 0.0001, HR = 1.0153), age (P = 0.0025, HR = 1.0190), and MGMT promoter methylation status (P < 0.0001, HR = 0.3899) were independent prognostic factors for OS (Table 3).
Fig. 4.
Survival analysis results for newly diagnosed GBM patients treated with standard chemoradiation plus experimental therapy. (A) Kaplan–Meier survival plots demonstrating a longer OS in patients with small postoperative enhancing tumors (<12 mL) prior to chemoradiation plus bevacizumab in the experimental treatment arm of AVAglio. (B) Similarly, Kaplan–Meier survival plots demonstrating a longer OS in patients with small enhancing tumors (<12 mL) prior to treatment with chemoradiation plus vorinostat in Alliance N0874.
Standard Chemoradiation Plus Vorinostat
Lastly, we examined data from the Alliance N0874 trial involving newly diagnosed GBM treated with chemoradiation plus vorinostat. Univariate results suggested that smaller tumors (<12 mL) had significantly longer OS compared with large (>12 mL) tumors (Fig. 4B; median OS = 670 vs 274 days; P < 0.0001; HR = 3.024). A Cox multivariable proportional hazards model indicated that continuous residual enhancing tumor volume (P = 0.0013, HR = 1.0291), but not age (P = 0.2271), was a significant predictor of OS (Table 2).
Discussion
Results from the current study validate the hypothesis that postoperative, baseline residual contrast-enhancing tumor is a significant prognostic factor for OS in newly diagnosed GBM treated with standard chemoradiation plus experimental therapies including bevacizumab and vorinostat. This conclusion is supported through careful analysis of multiple datasets, including a single institution database, a multicenter database of US academic institutions, a phase I/II multicenter clinical trial, and an international phase III multicenter randomized trial. This represents the largest and most comprehensive study validating the hypothesis that postoperative, residual enhancing tumor volume quantified through use of T1 digital subtraction is prognostic for OS under a variety of therapeutic scenarios commonly employed in newly diagnosed GBM, including both standard chemoradiation as well as experimental therapies including anti-angiogenic and radiosensitizing (eg, histone deacetylase inhibitor) agents.
The observation that postsurgical residual tumor burden is prognostic for OS in newly diagnosed GBM in standard chemoradiation with or without experimental treatment has important implications for clinical trial design as well as interpretation. Randomized trials with two or more arms may need to balance tumor volumes evenly over different treatment arms, particularly for trials with smaller sample sizes. At a minimum, appropriate statistical accountability for postsurgical residual enhancing tumor volume in the evaluation of therapeutic efficacy in clinical trials is warranted.
Study Limitations
A limitation to the current study was lack of uniform clinical information on all patients pooled into the composite cohort. Lack of information including sex, racial demographics, subsequent treatments, MGMT promoter methylation status, IDH status, performance status, steroid dose, and other factors may have significantly influenced our results. Another limitation was the lack of uniform imaging acquisition and the timing of image acquisition after surgery, which may have led to inaccuracies when segmenting the enhancing lesion and potential contamination from postsurgical reactive changes, respectively. To account for differences in image quality and contrast, we performed intensity normalization, digital subtraction, and manual inspection of all cases to increase consistencies in quantitation. Additionally, it is conceivable that inherent preoperative bias to be more or less conservative with resection based on tumor location, age, or general frailty may have skewed poor performing patients into the group of patients with more residual tumor. We contend our significant findings speak to the robustness of the results and the strength of the effects demonstrated in the current study despite these potential limitations.
Conclusion
Postsurgical, residual contrast-enhancing disease quantified using T1 subtraction significantly influences survival in patients with newly diagnosed GBM treated with chemoradiation with or without concomitant experimental therapy.
Funding
This work was supported by the Ben and Catherine Ivy Foundation Clinical Trials Network (B.M.E., S.J.N., N.B., T.F.C., K.L.L., E.R.G., H.C., J.d.G., S.C., I.M., J.W.T., I.A-R., D.R., M.P., P.Y.W.); National Brain Tumor Society Research Grant (B.M.E., T.F.C.); American Cancer Society (ACS) Research Scholar Grant (RSG-15-003-01-CCE) (B.M.E.); Roche/Genentech Research Grant (B.M.E., T.F.C.); Art of the Brain (T.F.C.); Ziering Family Foundation in memory of Sigi Ziering (T.F.C.); Singleton Family Foundation (T.F.C.); UCLA SPORE in Brain Cancer (NIH/NCI 1P50CA211015-01A1) (B.M.E., W.B.P., T.F.C.)
Conflict of interest statement. B.M.E—Advisory board: Hoffman La-Roche, Siemens, Nativis, Medicenna, MedQIA, Bristol Meyers Squibb, Imaging Endpoints, Agios; paid consultant: Nativis, MedQIA, Siemens, Hoffman La-Roche, Imaging Endpoints, Medicenna, Agios; grant funding: Hoffman La-Roche, Siemens, Agios, Janssen.
H.C.—Advisory board: Roche, Genentech, Novocure, Insys, Abbvie; grant funding: Newlink Genetics, Plexxikon, Kadmon, Orbus, merck, DNATrix.
W.P.M.—Consultant: Roche, Merck, Abbvie, Celgene, Triphase.
N.B.—Advisory board: Medicenna, Genentech, Omniox, Five Prime; research funding: Medicenna, BMS, Beigene, Delmar, EnGenIC, Abbvie, Orbus, Ipsen, Five Prime, EpicentRx.
J.W.T.—Advisory board: Novocure.
P.Y.W.—Advisory board: Abbvie, Alexion, AstraZeneca, Genentech/Roche, GW pharmaceutical, Insys, Kadmon, Monteris, Vascular Biogenic, Ziopharm; speaker: Merck; data and safety monitoring board: Monteris, Tocagen; research support: Acerta, Agios, Astra Zeneca, Celgene, Ely Lilly, Genentech/Roche, Karyopharm, Merck, Novartis, Oncoceutics, Sanofi-Aventis, Vascular Biogenics.
T.F.C.—Advisory board: Roche/Genentech, Amgen, Tocagen, NewGen, LPath, Proximagen, Celgene, Vascular Biogenics Ltd, Insys, Agios, Cortice Bioscience, Pfizer, Human Longevity, BMS, Merck, Notable Lab, MedQIA.
Acknowledgments
All authors contributed equally to data collection, editing and manuscript review, and data interpretation. B.M.E. contributed figures, study design, data analysis, writing.
References
- 1. Kelly PJ, Daumas-Duport C, Scheithauer BW, Kall BA, Kispert DB. Stereotactic histologic correlations of computed tomography- and magnetic resonance imaging-defined abnormalities in patients with glial neoplasms. Mayo Clin Proc. 1987;62(6):450–459. [DOI] [PubMed] [Google Scholar]
- 2. Kelly PJ, Daumas-Duport C, Kispert DB, Kall BA, Scheithauer BW, Illig JJ. Imaging-based stereotaxic serial biopsies in untreated intracranial glial neoplasms. J Neurosurg. 1987;66(6):865–874. [DOI] [PubMed] [Google Scholar]
- 3. Barajas RF Jr, Phillips JJ, Parvataneni R, et al. . Regional variation in histopathologic features of tumor specimens from treatment-naive glioblastoma correlates with anatomic and physiologic MR imaging. Neuro Oncol. 2012;14(7):942–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Earnest F 4th, Kelly PJ, Scheithauer BW, et al. . Cerebral astrocytomas: histopathologic correlation of MR and CT contrast enhancement with stereotactic biopsy. Radiology. 1988;166(3):823–827. [DOI] [PubMed] [Google Scholar]
- 5. Pope WB, Chen JH, Dong J, et al. . Relationship between gene expression and enhancement in glioblastoma multiforme: exploratory DNA microarray analysis. Radiology. 2008;249(1):268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barajas RF Jr, Hodgson JG, Chang JS, et al. . Glioblastoma multiforme regional genetic and cellular expression patterns: influence on anatomic and physiologic MR imaging. Radiology. 2010;254(2):564–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ellingson BM. Radiogenomics and imaging phenotypes in glioblastoma: novel observations and correlation with molecular characteristics. Curr Neurol Neurosci Rep. 2015;15(1):506. [DOI] [PubMed] [Google Scholar]
- 8. Bauchet L, Mathieu-Daude H, Fabbro-Peray P, et al. . Oncological patterns of care and outcome for 952 patients with newly diagnosed glioblastoma in 2004. Neuro Oncol. 2010;12(7):725–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Qin JJ, Liu ZX, Wang JM, et al. . Prognostic factors influencing clinical outcomes of malignant glioblastoma multiforme: clinical, immunophenotypic, and fluorescence in situ hybridization findings for 1p19q in 816 chinese cases. Asian Pac J Cancer Prev. 2015;16(3):971–977. [DOI] [PubMed] [Google Scholar]
- 10. Li J, Wang M, Won M, et al. . Validation and simplification of the Radiation Therapy Oncology Group recursive partitioning analysis classification for glioblastoma. Int J Radiat Oncol Biol Phys. 2011;81(3):623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hauch H, Sajedi M, Wolff JE. Treatment arms summarizing analysis of 220 high-grade glioma studies. Anticancer Res. 2005;25(5):3585–3590. [PubMed] [Google Scholar]
- 12. Zinn PO, Colen RR, Kasper EM, Burkhardt JK. Extent of resection and radiotherapy in GBM: a 1973 to 2007 surveillance, epidemiology and end results analysis of 21,783 patients. Int J Oncol. 2013;42(3):929–934. [DOI] [PubMed] [Google Scholar]
- 13. Pan IW, Ferguson SD, Lam S. Patient and treatment factors associated with survival among adult glioblastoma patients: a USA population-based study from 2000–2010. J Clin Neurosci. 2015;22(10):1575–1581. [DOI] [PubMed] [Google Scholar]
- 14. Chaichana KL, Jusue-Torres I, Navarro-Ramirez R, et al. . Establishing percent resection and residual volume thresholds affecting survival and recurrence for patients with newly diagnosed intracranial glioblastoma. Neuro Oncol. 2014;16(1):113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Oppenlander ME, Wolf AB, Snyder LA, et al. . An extent of resection threshold for recurrent glioblastoma and its risk for neurological morbidity. J Neurosurg. 2014;120(4):846–853. [DOI] [PubMed] [Google Scholar]
- 16. Sanai N, Berger MS. Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2008;62(4):753–764; discussion 264–266. [DOI] [PubMed] [Google Scholar]
- 17. Suto Y, Caner BE, Tamagawa Y, et al. . Subtracted synthetic images in Gd-DTPA enhanced MR. J Comput Assist Tomogr. 1989;13(5):925–928. [DOI] [PubMed] [Google Scholar]
- 18. Lloyd GA, Barker PG, Phelps PD. Subtraction gadolinium enhanced magnetic resonance for head and neck imaging. Br J Radiol. 1993;66(781):12–16. [DOI] [PubMed] [Google Scholar]
- 19. Lee VS, Flyer MA, Weinreb JC, Krinsky GA, Rofsky NM. Image subtraction in gadolinium-enhanced MR imaging. AJR Am J Roentgenol. 1996;167(6):1427–1432. [DOI] [PubMed] [Google Scholar]
- 20. Gaul HP, Wallace CJ, Crawley AP. Reverse enhancement of hemorrhagic brain lesions on postcontrast MR: detection with digital image subtraction. AJNR Am J Neuroradiol. 1996;17(9):1675–1680. [PMC free article] [PubMed] [Google Scholar]
- 21. Stupp R, Hegi ME, Mason WP, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 22. Lalezari S, Chou AP, Tran A, et al. . Combined analysis of O6-methylguanine-DNA methyltransferase protein expression and promoter methylation provides optimized prognostication of glioblastoma outcome. Neuro Oncol. 2013;15(3):370–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lai A, Kharbanda S, Pope WB, et al. . Evidence for sequenced molecular evolution of IDH1 mutant glioblastoma from a distinct cell of origin. J Clin Oncol. 2011;29(34):4482–4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sandmann T, Bourgon R, Garcia J, et al. . Patients with proneural glioblastoma may derive overall survival benefit from the addition of bevacizumab to first-line radiotherapy and temozolomide: retrospective analysis of the AVAglio trial. J Clin Oncol. 2015;33(25):2735–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ellingson BM, Kim HJ, Woodworth DC, et al. . Recurrent glioblastoma treated with bevacizumab: contrast-enhanced T1-weighted subtraction maps improve tumor delineation and aid prediction of survival in a multicenter clinical trial. Radiology. 2014;271(1):200–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ellingson BM, Gerstner E, Smits M, et al. . Diffusion MRI phenotypes predict overall survival benefitfrom anti-VEGF monotherapy in recurrent glioblastoma: converging evidence from phase II trials. Clin Cancer Res. 2017;23(19):5745–5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ellingson BM, Harris RJ, Woodworth DC, et al. . Baseline pretreatment contrast enhancing tumor volume including central necrosis is a prognostic factor in recurrent glioblastoma: evidence from single and multicenter trials. Neuro Oncol. 2017;19(1):89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]