Abstract
Background
Anti-angiogenic therapy is known to induce a greater degree of hypoxia, including in glioblastoma (GBM). Evofosfamide (Evo) is a hypoxia-activated prodrug which is reduced, leading to the release of the alkylating agent bromo-isophosphoramide mustard. We assessed the safety, tolerability, preliminary efficacy, and biomarkers of Evo plus bevacizumab (Bev) in Bev-refractory GBM.
Methods
Twenty-eight patients with Bev-refractory GBM were enrolled in a dose escalation study receiving from 240 mg/m2 (cohort 1) to 670 mg/m2 (cohort 4) of Evo every 2 weeks in combination with Bev. Patients deemed surgical candidates underwent a single dose of Evo or placebo with pimonidazole immediately prior to surgery for biomarker evaluation, followed by dose escalation upon recovery. Assessments included adverse events, response, and survival.
Results
Evo plus Bev was well tolerated up to and including the maximum dose of 670 mg/m2, which was determined to be the recommended phase II dose. Overall response rate was 17.4%, with disease control (complete response, partial response, and stable disease) observed in 14 (60.9%) of the 23 patients. The ratio of enhancement to non-enhancement was significant on log-rank analysis with time to progression (P = 0.023), with patients having a ratio of less than 0.37 showing a median progression-free survival of 98 days versus 56 days for those with more enhancement.
Conclusions
Evo plus Bev was well tolerated in patients with Bev-refractory GBM, with preliminary evidence of activity that merits further investigation.
Keywords: bevacizumab, evofosfamide, glioblastoma, hypoxia, recurrence
Importance of the study
Survival of patients after failing bevacizumab remains dismal even if treated on a second bevacizumab-containing regimen, and new therapeutic options are needed. Hypoxia, which is exacerbated by anti-angiogenic therapy, is known to drive resistance and a more infiltrative phenotype. Evofosfamide is a hypoxia-activated prodrug, and this is the first study describing its use in combination with bevacizumab or in GBM. This study shows that Evo can be safely given at 670 mg/m2 in combination with Bev 10 mg/kg following Bev failure, and provides preliminary evidence of activity. Importantly, given that Bev failure is not homogeneous and can include both de novo and acquired resistance patterns, preliminary biomarker data are provided which may assist in defining subgroups that benefit. These findings additionally extend existing knowledge on radiographic and cellular features of bevacizumab-refractory glioblastoma.
Glioblastoma (GBM; grade IV astrocytoma) is the most common and most aggressive of the primary malignant brain tumors in adults. Approximately 13000 cases of GBM will be diagnosed each year, and it remains incurable, with a median survival below 2 years.1 Two drugs (temozolomide2 and bevacizumab3) and one device have been FDA approved in the last 2 decades for treatment of patients with GBM, resulting in only incremental improvement in survival without enduring benefit. Additional treatment options are needed.
Tumor growth requires the presence of a local vascular network supplying both oxygen and nutrients to the tumor cells. A highly proliferative tumor mass, such as GBM, grows faster than the vasculature, leading to an environmental deficiency of oxygen. Increased hypoxia ultimately leads to the formation of necrotic areas, which is one of the pathognomonic histologic features of GBM.4 Based on measurements of tissue hypoxia using the 2-nitroimidazole agent EF5, the majority of GBMs show severe hypoxia (pO2 ≤ 0.1%).5 Similarly, more definitive assessment by Rampling et al6 using polarographic electrodes directly in tumors from 10 patients with GBM revealed a median 39.5% of pO2 measurements at multiple sites with values <2.5 mm Hg. In comparison, models of other vascular tumor types such as renal cell carcinoma and melanoma show levels of oxygen tension at 38 and 25 mm Hg, respectively.7 By assessing hypoxia non-invasively with [18F]fluoromisonidazole PET, Spence et al showed that the volume and intensity of hypoxia in GBM before radiotherapy are strongly associated with a shorter time to progression (TTP) and poorer survival.8 Thus, whether by imaging or immunohistochemical markers, tumor hypoxia is present in GBM and portends poor survival.
While angiogenesis inhibitors have become a standard part of therapy for multiple malignancies, they generally do not prolong survival of cancer patients for more than months, and survival benefit in patients with GBM has not been demonstrated despite multiple randomized studies. Increasing evidence points to the root cause of angiogenesis, hypoxia, as a driving force for resistance to anti-angiogenics.9 This can be exemplified by the phase II study of bevacizumab (Bev) and irinotecan,10 during which biomarkers were assessed with the finding of hypoxia-induced carbonic anhydrase 9 (CAIX) as the most significant negative predictor of both response to therapy and overall survival (OS) (P = 0.020, χ2; hazard ratio, 2.72; 95% CI = 1.17‒6.36), followed by hypoxia-inducible factor 2α (HIF-2α). Hypoxia as measured by CAIX and HIF-1α has also been found increased at the time of progression for patients who initially responded to Bev,11 supporting its role in acquired resistance. Batchelor et al have shown in a prospective clinical study of the pan‒vascular endothelial growth factor receptor (VEGFR) inhibitor cediranib for GBM12 that the most predictive blood biomarker of tumor progression is stromal derived factor 1α (SDF1α, P = 0.058). As SDF1α is strongly induced by HIF-1α under conditions of hypoxia,13 this suggests that the correlation seen between progression on cediranib and SDF1α may be driven by hypoxia. Metabolomic profiling of animals treated with Bev results in an accumulation of metabolic products, including lactate, choline, and creatine, a combination that is associated with increased hypoxia in human brain tumor spectra on magnetic resonance spectroscopy (MRS), HIF-1α, and an invasive phenotype.14–16
Evofosfamide (Evo), previously known as TH-302, is a nitroimidazole prodrug of the cytotoxin bromo-isophosphoramide mustard (Br-IPM). When exposed to hypoxic conditions, Evo is reduced at the nitroimadazole site of the prodrug by intracellular reductases, leading to the release of the alkylating agent Br-IPM, which can then act as a DNA crosslinking agent. It may also diffuse to adjacent cells in normoxic regions and thus act as a cytotoxic agent outside of the hypoxic activation zone. In vitro cytotoxicity and clonogenic assays indicate that Evo has little activity under normoxic conditions but is highly cytotoxic under hypoxic conditions. Correlation has been found between antitumor activity and tumor hypoxic fraction across the 12 xenograft models profiled. Tumor-bearing animals breathing 95% O2 during dosing periods exhibited attenuated Evo efficacy, while those breathing 10% O2 exhibited enhanced Evo efficacy, both compared with normoxic breathing (21% O2). Evo treatment results in a reduction in the volume of the hypoxic fraction 48 h after dosing and a corresponding increase in the necrotic fraction. Evo-induced DNA damage as measured by including phosphorylation of the histone variant γH2AX was initially only present in the hypoxic regions and then radiated to the entire tumor in a time-dependent manner. Given the known hypoxia associated with GBM, these data suggest Evo as a potential therapeutic agent to target and reduce the hypoxia-induced resistant population in Bev-refractory GBM.
The safety and preliminary efficacy of Evo has been evaluated as monotherapy17 as well as in combination with multiple conventional agents.18–21 In the phase I trial by Weiss et al, the most common adverse events due to Evo when used as monotherapy were nausea, skin rash, fatigue, and vomiting, and severity was dose dependent. 17 Hematologic toxicity was usually mild and not dose limiting. Grade 3 skin and mucosal toxicities were dose limiting at 670 mg/m2. The safety of Evo in patients with GBM or in combination with Bev had not previously been evaluated; therefore, we embarked upon an investigator-initiated study of Evo with Bev in patients with GBM, with a surgical component to allow for tissue analysis.
Methods
Study Design
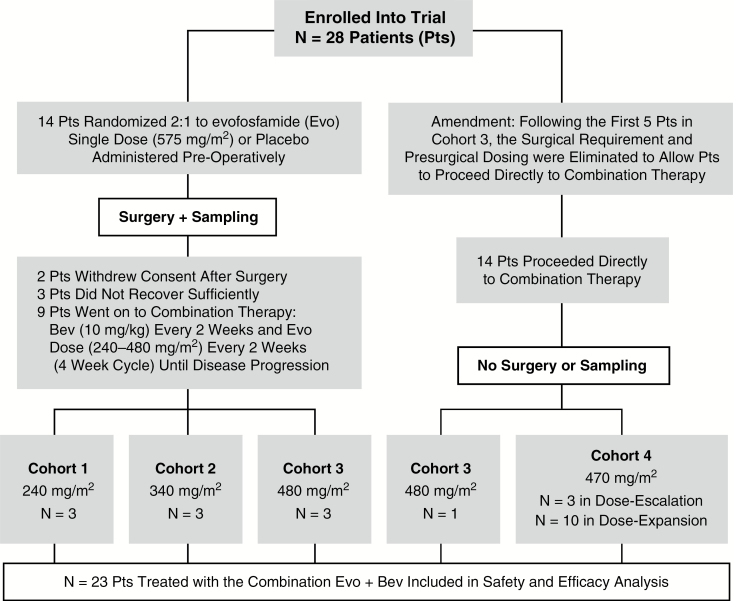
Patients were eligible if they had previously failed prior conventional therapy, with the most recent being Bev, and if they were reasonable candidates for surgical debulking. One line of prior Bev therapy was allowed. Study allowed patients with an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2 to be enrolled. A 4-week wash-out period from Bev was required prior to surgical intervention. MRI demonstrating progression prior to study consideration was defined by Response Assessment in Neuro-Oncology (RANO) criteria. Study schema is detailed in Fig. 1. At surgical day −1, patients received a single dose of Evo (575 mg/m2) or placebo, followed by pimonidazole hydrochloride 500 mg/m2. An exogenous hypoxia marker, pimonidazole is a 2-nitroimidazole compound which forms protein adducts in the presence of reductases that can be detected via immunohistochemistry and provide a reliable estimate of biologically relevant hypoxia.22,23 Excised tumor tissue and serum were collected for further analysis. Tissue was collected intraoperatively, and following a brief agitated saline rinse, separate specimens were flash frozen and formalin fixed within 5 minutes of provision at the bedside. Direct level of drug penetration could not be analyzed from the tumor samples due to the rapid formation of adducts once exposed to tissue. Rather, a blinded comparison in the levels of hypoxia markers (pimonidazole, CAIX) and Evo-induced DNA damage as measured by γH2AX between patients treated with Evo and placebo preoperatively was made at the time of tissue analysis. Following a 4-week period of postsurgical recovery, patients were started on combination therapy of Bev at the standard presurgical dose of 10 mg/kg with Evo every 2 weeks. A classic 3+3 dose escalation design was used, with the initial dose of Evo at 240 mg/m2 and escalation continuing to 340 mg/m2, 480 mg/m2, and 670 mg/m2. In the final cohort of 670 mg/m2, the surgical requirement was eliminated and patients were allowed to go directly to combination therapy with Bev. The protocol was approved by our institutional review board, and all patients provided informed consent.
Fig. 1.
Study schema indicating patient allotment and procedures.
Dose Modifications and Toxicity Management
Dose modifications for toxicity, particularly hematologic and skin toxicity, were independently assessed at each visit. Dose reductions were not required for toxicity less than grade 3, with the exception of grade 2 skin toxicity, which required a dose reduction of 25% upon resolution to grade 1. For nonhematologic toxicity of grade 3 (other than alanine aminotransferase/aspartate aminotransferase elevation or nausea/vomiting), a 25% dose reduction was required. Treatment was discontinued for any nonhematologic grade 4 adverse event. Reduced absolute neutrophil count (ANC) of 1000–1499, and platelet counts of 50000–75000 were managed with a 25% dose reduction. Lower ANC or platelets required doses to be held until recovery to 1500 and 100000, respectively. Hemoglobin was required to be ≥9 g/dL at cycle 1/day 1 and ≥8 g/dL for all subsequent doses. All patients were advised to use pramoxine/phenylephrine/glycerin/petrolatum (Preparation H cream) immediately prior to infusions to prevent perineal rash and anal mucositis. If rash or anal mucositis reached grade 1, Silvadene 1% cream and triamcinolone 0.1% cream both applied twice daily were added to the skin care regimen. Patients were provided skin care medications and instruction prior to starting treatment.
Immunohistochemistry
Representative sections were subjected to immunohistochemistry for CAIX and pimonidazole, as previously described.24 Prior to image processing, representative areas of tumor which was devoid of treatment or surgical artifact were identified by a neuropathologist blinded to presurgical randomization. Morphometric analysis utilizing ImagePro Plus software was performed with inclusion of only the representative areas identified by the neuropathologist. The hypoxic fraction was calculated as the ratio of area of the region of interest (ROI) identified with morphometric analysis to total area of the representative area analyzed. Statistical analysis was used in all instances with P < 0.05 considered significant. Correlation was calculated by linear regression using GraphPad Prism 4 software.
Radiographic Imaging, Response, and Volumetric Assessment
MRI was performed within 3 days of surgery, first postsurgical treatment in those undergoing surgery, or first cycle in those not receiving surgery. Imaging was repeated within 5 days of beginning odd cycles thereafter. For those patients undergoing surgery, only the postsurgical MRI was used as baseline for determining response. Assessment of response was done using RANO criteria by the treating investigator. Volumetric assessment was performed using OsiriX open source software with the IB Neuro plugin (Imaging Biometrics). The co-registered T1 pre- and postcontrast images were standardized and a delta T1 map was obtained by subtraction. An empirically determined threshold of 3500 was applied to the delta T1 maps to obtain an enhancing tumor ROI using the IB Neuro plugin. Standardization was applied to fluid attenuated inversion recovery (FLAIR) images and an empirically determined threshold of 2000 was used to obtain the non-enhancing tumor volume. All ROIs were manually reviewed by at least 2 investigators for accuracy. Noisy T1 voxels around the skull and eye areas were manually removed, as were nonspecific T2 FLAIR regions. Volume ratio was determined as T1 ROI divided by T2 ROI volume.
Metabolomic Profiling
The serum metabolic profile of 11 patients was analyzed before (for all 11 patients) and at progression, several months after treatment administration (for 4 patients) using high-resolution MRS. Samples were prepared as previously described.25 Briefly, the blood serum samples were filtered on Nanosep Omega centrifugal devices (3 kDa cutoff; Pall Life Biosciences). The sera filtrate (polar fraction) was mixed with phosphate buffer (100 mM final concentration, pH 7.0 ± 0.1) containing sodium 3-(trimethylsilyl)propionate-2,2,3,3-d4 (TMSP; Cambridge Isotope Laboratories; 0.5 mM final concentration) as internal reference and with D2O (10% v/v final). Acquisition of 1-dimensional 1H MRS metabolic profiles was performed using Bruker Avance 700 MHz MR spectrometers. Metabolite identification/quantification was performed using Chenomx 8.2, our public database of MR spectra,26 and other public databases.27 Unsupervised multivariate statistical data analysis (principal components analysis [PCA]) was performed using PLS Toolbox (Eigenvector Research). The Euclidean distance between samples in the scores plots were calculated based on the scores along the first and second principal components (PC1 and PC2).
Results
Patient Characteristics
A total of 28 patients were enrolled; 23 of 28 initiated combination therapy, with 3 patients each in the first 2 cohorts, 4 in the third cohort, and 13 in the final cohort (Fig. 1). Of the 23 patients, the median age was 56, and 14 (61%) were male. ECOG performance status was 0 or 1 in 87% of patients; patients had received a median of 3 prior therapies, and the median TTP on the initial chemoradiation was 7.8 months (Table 1). In addition, the median TTP on Bev was only 3.9 months. The median time from Bev progression to first dose of combination Evo/Bev was 73 days in the surgical group and 25 days in the nonsurgical group. Given that Evo activity is not dependent on O6-methylguanine-DNA methyltransferase, MGMT status was not required and was available on only a subset of patients.
Table 1.
Patient characteristics by cohort and overall
| Evofosfamide Dose (mg/m2) | |||||
|---|---|---|---|---|---|
| 240 | 340 | 480 | 670 | Total | |
| (n = 3) | (n = 3) | (n = 4) | (n = 13) | (n = 23) | |
| Age, median (range) | 56 | 47 | 50 | 61 | 56 |
| (43–70) | (44–51) | (35–58) | (42–74) | (35–74) | |
| Male, n | 2 (67%) | 1 (33%) | 1 (25%) | 10 (77%) | 14 (61%) |
| ECOG status | |||||
| 0 | 0 | 0 | 1 (25%) | 3 (23%) | 4 (17%) |
| 1 | 3 (100%) | 3 (100%) | 3 (75%) | 7 (54%) | 16 (70%) |
| 2 | 0 | 0 | 0 | 3 (23%) | 3 (13%) |
| Prior therapies, median | 2 | 3 | 3 | 2 | 3 |
|
Months to progression on
chemo/radiation, median (range) |
11.3 | 9 | 15.6 | 4.5 | 6.6 |
| (2.7–14) | (7.8–14) | (4.5–20) | (1.8–14) | (1.8–20) | |
|
Months to progression on prior
Bev, median (range) |
3.5 | 1.4 | 2.3 | 4.1 | 3.7 |
| (2.5–6.5) | (1.1–4.9) | (1.3–5.5) | (1.0–9.0) | (1.0–9.0) | |
| Median months from progression on Bev to first dose Evo | 1.2 | 3.3 | 1.2 | 0.6 | 0.8 |
| Steroids at study entry | 1 (33%) | 2 (67%) | 0 (0%) | 9 (69%) | 12 (52%) |
Safety
Evo was well tolerated overall, with no grade 4 drug-associated adverse events. Three grade 3 adverse events occurred, including oral mucositis in the first cycle, skin ulceration at the site of infusion in a patient with plegia in the same arm during the second cycle, and thrombocytopenia in the third cycle (Table 2). Patients were instructed to use prophylactic pramoxine/phenylephrine/glycerin/petrolatum cream to prevent skin complications. The most common toxicity was rash, which occurred in 11 patients. Anal inflammation and proctitis was common and dose escalation was not continued beyond 670 mg/m2, as the mucositis was felt to be limiting at 670 mg/m2 for long-term tolerance. Four patients experienced grade 2 anorectal mucositis and 1 patient developed grade 3 oral mucositis, all within the 670 mg/m2 cohort.
Table 2.
Adverse events* for patients treated with Evo/Bev
| Adverse Event | Total | Grade 3/4 |
|---|---|---|
| Rash | 11 (47.8%) | 1 (4.3%) |
| Anal inflammation | 4 (17.4%) | 2 (8.7%) |
| Fatigue | 4 (17.4%) | 2 (8.7%) |
| Proctitis | 4 (17.4%) | 2 (8.7%) |
| Nausea | 4 (17.4%) | 1 (4.3%) |
| Stomatitis | 4 (17.4%) | 0 (0.0%) |
| Thrombocytopenia | 3 (13.0%) | 1 (4.3%) |
*By Common Terminology Criteria for Adverse Events 4.0.
Overall Survival, Time to Progression, and Tumor Response
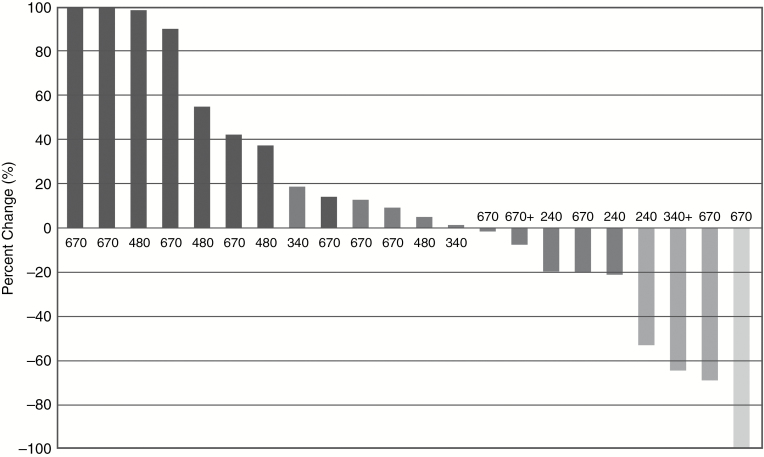
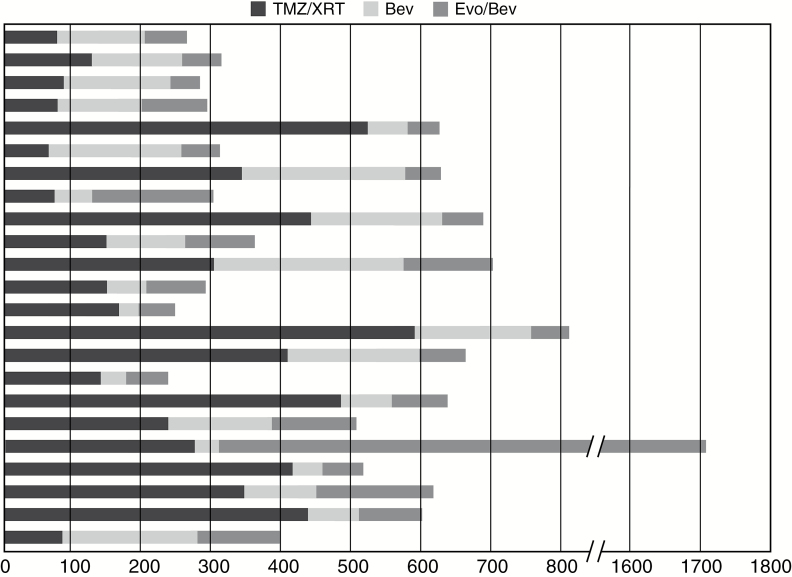
Objective best response (complete response plus partial response as per RANO criteria) was observed in 4 (17.4%) of 23 patients treated overall with Evo. Disease control (complete response, partial response, and stable disease) was observed in 14 (60.9%) of the 23 patients (Fig. 2). Subgroup analysis showed that disease control was achieved in all 3 patients treated in both cohorts with 240 mg/m2 and 340 mg/m2. There was one complete response (670 mg/m2 cohort). Objective best response rate in the largest cohort treated at a dose of 670 mg/m2 was 15.4% (2 of 13 patients) and disease control rate was 53.8% (7 of 13 patients; Fig. 3). Median duration of those who responded was 4.41 months. Time to progression was 2.2 months when all groups were combined. The longest TTP was 46.0 months in 1 patient in the 340 mg/m2 cohort. In 39% (9 of 23) of patients the TTP for the Evo/Bev combination was longer than the TTP for the same patients preceding Bev regimen (Fig. 4). Median OS was 4.6 months in all groups.
Fig. 2.
Best response for target lesions by patient, based on maximal percentage of tumor reduction using RANO criteria.
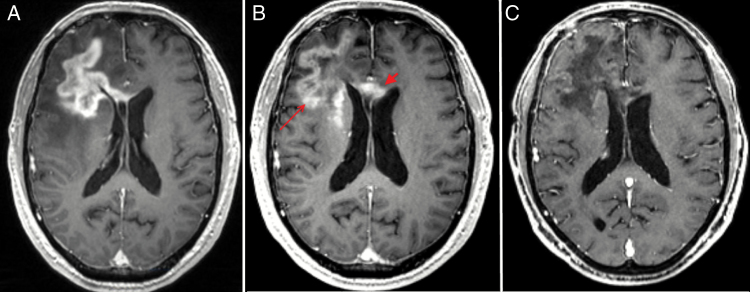
Fig. 3.
T1 MRI following administration of gadolinium enhancement in a responder. (A) Day −61 imaging showing tumor features while on Avastin. (B) Progression on Avastin at day −3 with extension within the frontal lobe (thin arrow) and corpus collosum (thick arrow). (C) Response with decreased enhancement at day 43 (right). Patient had no interruption in Bev therapy prior to initiating Evo.
Fig. 4.
Time to progression for Evo/Bev compared with prior regimens by patient. Black = external beam radiotherapy/temozolomide; dark gray = Bev; light gray = Evo/Bev.
Baseline Tumor Hypoxia and Volumetric Features
Volumetric imaging data were available at baseline for 25 of 27 subjects. The median volume of enhancement and FLAIR signal for the entire cohort was 35 cc and 101 cc, respectively. When volumes were separated into high or low about the median, neither volume of T1 enhancement (P = 0.69) nor the volume of T2 FLAIR signal (P = 0.49) was associated with the TTP on log-rank analysis, while the ratio of enhancing to non-enhancing disease was associated with it (P = 0.023). Patients with a ratio of enhancement less than 0.37 did best with a progression-free survival (PFS) of 98 days versus 56 days for those with more enhancement. Interestingly, a greater non-enhancing volume was the only statistically significant radiographic factor for survival, with the patients having T2 FLAIR volume greater than 104.2 cc having a median OS of 199 days versus 105 days for those having less (P = 0.004). The volume of enhancement was not correlative with OS.
Tissue hypoxia by both CAIX (endogenous hypoxia marker) and pimonidazole (Pimo, exogenous hypoxia marker) was available for 11 subjects, with serum CAIX available for 28 subjects. A high level of concordance was seen between CAIX and pimonidazole (r2 = 0.70, P = 0.001), suggesting that both are suitable for quantification of hypoxic fraction. Samples tended to cluster into low or high hypoxic fraction with a median value of 10% (mean 10%, range 0.7%–23.1%). Tissue hypoxia showed a trend toward correlation with PFS (r2 = 0.42, P = 0.06) and OS (r2 = 0.29, P = 0.13), with patients having higher tumoral hypoxia tending to remain on study longer and living longer. Imaging characteristics at baseline were also correlated with tissue markers of hypoxia. In particular, tissue hypoxic burden by CAIX showed a good fit with FLAIR volume (r2 = 0.7, P = 0.005), while the enhancing volume (r2 = 0.06, P = 0.5) and proportion of enhancement within the tumor (r2 = 0.2, P = 0.2) did not. Soluble CAIX from serum, as well as circulating angiogenic factors (VEGFR1, VEGFR2, SDF1α, and endostatin) were also assessed, but were not significant.
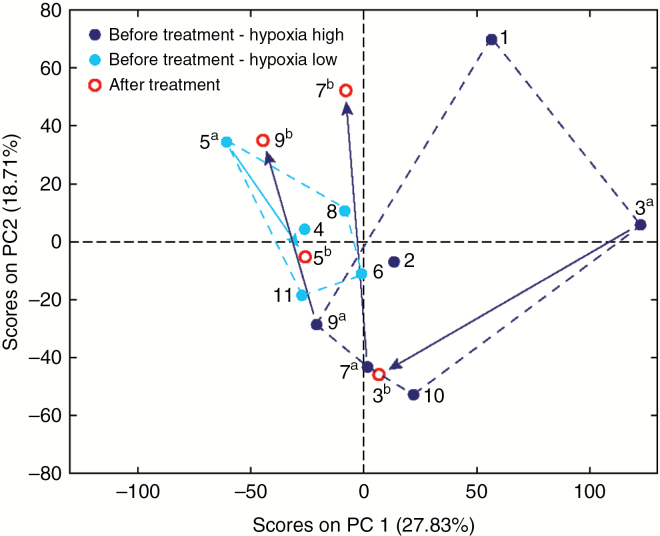
The serum metabolomic profiles in patients before treatment and after progression were analyzed using 1H MRS. In order to determine if the serum metabolic profile is affected by the brain hypoxic fraction and the extent of the serum metabolic changes in patients before Evo treatment and at progression following treatment, the MR spectra were analyzed using PCA. Although the number of patients used for the unsupervised multivariate statistical analysis is limited, the separation observed in the scores plot between sera from patients with low versus high (Fig. 5, light and dark blue circles, respectively) brain tissue hypoxic fraction suggests that the patient serum metabolic profiles can help to discriminate patients who have low levels of brain tissue hypoxia from those with higher levels. In addition, in order to inspect the extent of the differences between the metabolic profile in serum before treatment and at progression, we calculated the distance between the pre- and posttreatment samples (Fig. 4, blue and red circles, respectively) from the same patient in the scores plot. In the 3 patients with high tissue hypoxia levels (18.7%, 23.1%, and 11.9% hypoxic fraction), the distance was found to be larger (240%, 182%, and 129%, respectively) compared with the patient with low (7% tissue hypoxic fraction) tissue hypoxia levels. Moreover, in all patients starting with a high tissue hypoxic fraction, the profile at progression appears to cluster closer to the low level tissue hypoxia group relative to the starting profile before treatment.
Fig. 5.
PCA of serum metabolomics profiles from patients before and after Evo treatment. MR spectra acquired on serum samples from 11 patients were analyzed using unsupervised multivariate statistics (PCA). In the PCA scores plot, samples are color-coded as follows: the 11 pretreatment patients are shown as blue circles; 6 patients with high tissue hypoxic fraction (≥10%) are shown as dark blue solid circles, 5 with low hypoxic fraction (<10%) as light blue solid circles; posttreatment samples (at relapse, several months after treatment), available for 4 patients, are shown as red circles. All samples in the scores plot are labeled with the patient number; for patients with data pre- and posttreatment, samples are labeled as “a” and “b,” respectively. The dashed lines indicate the outline of patients in the same subgroup, based on hypoxic fractions, for pretreatment samples. The arrows indicate the direction and extent of the metabolic change in the PC1–PC2 space for data pre- and posttreatment. The PCA scores plot indicates good grouping and separation between the low (light blue circles) and high (dark blue circles) tissue hypoxic fraction before treatment subgroups, mostly along the PC1. The distance between the pre- (“a” samples in light or dark blue circles) and the corresponding posttreatment (“b” in red circles) samples is larger for samples with high tissue hypoxic fraction, indicating more extensive metabolic changes.
Discussion
We report the results from a first-in-human study combining Bev with the hypoxia-activated prodrug evofosfamide in 23 patients with Bev-refractory GBM. These findings show that Bev and Evo can be safely administered at the full single agent recommended phase II dose of 670 mg/m2 with Bev at 10 mg/kg, both administered every 2 weeks intravenously. Despite the morbidity experienced from disease in the GBM patient population, toxicity was quite manageable when close attention was paid to the skin and patients were instructed on prophylactic use of pramoxine/phenylephrine/glycerin/petrolatum cream. The toxicity profile of Evo and Bev compares favorably to common combination regimens typically used for GBM such as Bev with irinotecan3 or lomustine.28 Evo plus Bev adverse events did not lead to treatment discontinuation in any of our patients and only 3 patients experienced grade 3 adverse events.
Survival of patients after failing Bev remains dismal, even if treated on a second Bev-containing regimen at approximately 3 months29 to about 5 months based on retrospective data. Radiographic responses are rare. In our study, the overall response rate of 17% was higher than any reported in the literature to date for Bev-refractory disease11,29–33 and suggests that further study of Evo in this setting is warranted. While patients were allowed to undergo surgery prior to receiving combined modality therapy, only the postsurgical MRI was used as baseline for response assessment, and prior studies have shown that the PFS and OS results for patients with and without surgical intervention at the TTP are similar, allowing data from these patients to be combined in assessing the benefit of new treatments without the need for stratification or other statistical adjustment.34 Considering this, as well as the number of patients who showed rapid progression on prior regimens included in this study, the OS of patients receiving Evo/Bev was favorable at 4.6 months and higher than the survival of approximately 3 months reported for patients receiving a second Bev-containing regimen after Bev failure.29,31 Given the limited patient population and phase I design, one should not overinterpret these clinical outcomes, which will need validation.
Importantly, subgroup analysis within our study suggests some biomarkers may be able to identify a subpopulation of Bev-refractory patients with increased benefit. Radiographically, patients with primarily non-enhancing disease had the best PFS and OS on study, with PFS being associated only with a low degree of enhancement and not the volume of disease. Patients with a ratio of enhancement of 0.37 or less showed a PFS of 98 days, which is approximately 3-fold what has been seen in Bev-refractory patients receiving a second Bev-containing regimen. Additionally, tissue hypoxia by CAIX showed a trend toward positive correlation with PFS and was highly correlative with the volume of non-enhancing tumor but not enhancing tumor volume. Of course, this raises the question of whether these biomarkers simply predict the biology or behavior of the tumor rather than response to therapy, since there is some retrospective data suggesting that non-enhancing tumor progresses radiographically at a slower rate than enhancing tumor after treatment with Bev.35 Others have shown that the radiographic pattern of progression on Bev correlates with molecular profiles as well, with the enhancing Bev-resistant GBMs closely resembling their matched tumor before Bev, while those that are primarily non-enhancing have a shift in their profile with an increase in hypoxia biomarkers, less vascularity, unchanged proliferation, and higher invasiveness.36 This suggests that the enhancing tumors are de novo resistant, whereas the non-enhancing tumors have acquired resistance with the ability to adapt to the hypoxia induced by loss of vascularity from Bev. Given the mechanism of action of Evo, it follows that the latter group of Bev-refractory tumors would be more responsive and have a longer PFS. Additionally, the impact of hypoxia on outcomes in GBM has been looked at by a number of groups, and consistently has shown reduced survival37 and more rapid progression8 in those patients with higher tumoral hypoxia. Therefore, our finding of improved PFS and OS in patients with non-enhancing tumors, a trend toward improved PFS and OS in patients with higher tumor tissue hypoxia, and the strong correlation between non-enhancing volume and tissue hypoxia suggests that this population, which should do worse from higher tumoral hypoxia, does better with Evo and it is the therapeutic agent that is conferring this difference. This will need to be confirmed in prospective studies.
Funding
This study was supported in part by the UTHSCSA Cancer Therapy and Research Center through the National Institutes of Health, National Cancer Institute P30 award CA054174, an FDA Orphan Products Research Project Grant FD004400-01A2, as well as through a clinical research agreement with Threshold Pharmaceuticals.
Acknowledgments
Conflict of interest statement. J.S., C.H., and S.K. were employed by Threshold Pharmaceuticals.
References
- 1. Ostrom QT, Gittleman H, Liao P, et al. CBTRUS Statistical Report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 2014;16(Supp 4):iv1–iv63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 3. Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. [DOI] [PubMed] [Google Scholar]
- 4. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Evans SM, Judy KD, Dunphy I, et al. Hypoxia is important in the biology and aggression of human glial brain tumors. Clin Cancer Res. 2004;10(24):8177–8184. [DOI] [PubMed] [Google Scholar]
- 6. Rampling R, Cruickshank G, Lewis AD, Fitzsimmons SA, Workman P. Direct measurement of pO2 distribution and bioreductive enzymes in human malignant brain tumors. Int J Radiat Oncol Biol Phys. 1994;29(3):427–431. [DOI] [PubMed] [Google Scholar]
- 7. Ziemer LS, Lee WM, Vinogradov SA, Sehgal C, Wilson DF. Oxygen distribution in murine tumors: characterization using oxygen-dependent quenching of phosphorescence. J Appl Physiol (1985). 2005;98(4):1503–1510. [DOI] [PubMed] [Google Scholar]
- 8. Spence AM, Muzi M, Swanson KR, et al. Regional hypoxia in glioblastoma multiforme quantified with [18F]fluoromisonidazole positron emission tomography before radiotherapy: correlation with time to progression and survival. Clin Cancer Res. 2008;14(9):2623–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cavazos DA, Brenner AJ. Hypoxia in astrocytic tumors and implications for therapy. Neurobiol Dis. 2016;85:227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sathornsumetee S, Cao Y, Marcello JE, et al. Tumor angiogenic and hypoxic profiles predict radiographic response and survival in malignant astrocytoma patients treated with bevacizumab and irinotecan. J Clin Oncol. 2008;26(2):271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Iwamoto FM, Abrey LE, Beal K, et al. Patterns of relapse and prognosis after bevacizumab failure in recurrent glioblastoma. Neurology. 2009;73(15):1200–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Batchelor TT, Sorensen AG, di Tomaso E, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11(1):83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10(8):858–864. [DOI] [PubMed] [Google Scholar]
- 14. Keunen O, Johansson M, Oudin A, et al. Anti-VEGF treatment reduces blood supply and increases tumor cell invasion in glioblastoma. Proc Natl Acad Sci U S A. 2011;108(9):3749–3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lucio-Eterovic AK, Piao Y, de Groot JF. Mediators of glioblastoma resistance and invasion during antivascular endothelial growth factor therapy. Clin Cancer Res. 2009;15(14):4589–4599. [DOI] [PubMed] [Google Scholar]
- 16. Kunkel P, Ulbricht U, Bohlen P, et al. Inhibition of glioma angiogenesis and growth in vivo by systemic treatment with a monoclonal antibody against vascular endothelial growth factor receptor-2. Cancer Res. 2001;61(18):6624–6628. [PubMed] [Google Scholar]
- 17. Weiss GJ, Infante JR, Chiorean EG, et al. Phase 1 study of the safety, tolerability, and pharmacokinetics of TH-302, a hypoxia-activated prodrug, in patients with advanced solid malignancies. Clin Cancer Res. 2011;17(9):2997–3004. [DOI] [PubMed] [Google Scholar]
- 18. Ganjoo KN, Cranmer LD, Butrynski JE, et al. A phase I study of the safety and pharmacokinetics of the hypoxia-activated prodrug TH-302 in combination with doxorubicin in patients with advanced soft tissue sarcoma. Oncology. 2011;80(1-2):50–56. [DOI] [PubMed] [Google Scholar]
- 19. Sankhala KK, Chiorean EG, Armstrong AJ, et al. Phase I/II study of TH-302 in combination with docetaxel in patients with solid tumors including NSCLC and castrate-resistant prostate cancer (CRPC). Paper presented at: 2010 ASCO Annual Meeting 2010. [Google Scholar]
- 20. Vlahovic G, Infante JR, Mita AC, et al. Phase I/II study of TH-302 in combination with pemetrexed in patients with solid tumors including NSCLC. Paper presented at: 2010 ASCO Annual Meeting 2010. [Google Scholar]
- 21. Schelman WR. Phase I/II study of TH-302 in combination with gemcitabine in patients with solid tumors including advanced pancreatic cancer. Paper presented at: 2010 ASCO Annual Meeting 2010. [Google Scholar]
- 22. Raleigh JA, Koch CJ. Importance of thiols in the reductive binding of 2-nitroimidazoles to macromolecules. Biochem Pharmacol. 1990;40(11):2457–2464. [DOI] [PubMed] [Google Scholar]
- 23. Raleigh JA, Chou SC, Bono EL, Thrall DE, Varia MA. Semiquantitative immunohistochemical analysis for hypoxia in human tumors. Int J Radiat Oncol Biol Phys. 2001;49(2):569–574. [DOI] [PubMed] [Google Scholar]
- 24. Sun JD, Liu Q, Wang J, et al. Selective tumor hypoxia targeting by hypoxia-activated prodrug TH-302 inhibits tumor growth in preclinical models of cancer. Clin Cancer Res. 2012;18(3):758–770. [DOI] [PubMed] [Google Scholar]
- 25. Tiziani S, Emwas AH, Lodi A, et al. Optimized metabolite extraction from blood serum for 1H nuclear magnetic resonance spectroscopy. Anal Biochem. 2008;377(1):16–23. [DOI] [PubMed] [Google Scholar]
- 26. Ludwig C, Easton JM, Lodi A, et al. Birmingham metabolite library: a publicly accessible database of 1-D 1H and 2-D 1H J-resolved NMR spectra of authentic metabolite standards (BML-NMR). Metabolomics. 2012;8(1):8–18. [Google Scholar]
- 27. Wishart DS, Tzur D, Knox C, et al. HMDB: the human metabolome database. Nucleic Acids Res. 2007;35:D521–D526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wick W, Brandes AA, Gorlia T, et al. EORTC 26101 phase III trial exploring the combination of bevacizumab and lomustine in patients with first progression of a glioblastoma. J Clin Oncol. 2016;34(15 suppl):2001. [Google Scholar]
- 29. Quant EC, Norden AD, Drappatz J, et al. Role of a second chemotherapy in recurrent malignant glioma patients who progress on bevacizumab. Neuro Oncol. 2009;11(5):550–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Reardon DA, Desjardins A, Peters KB, et al. Phase 2 study of carboplatin, irinotecan, and bevacizumab for recurrent glioblastoma after progression on bevacizumab therapy. Cancer. 2011;117(23):5351–5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Norden AD, Young GS, Setayesh K, et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70(10):779–787. [DOI] [PubMed] [Google Scholar]
- 32. Reardon DA, Desjardins A, Peters K, et al. Phase II study of metronomic chemotherapy with bevacizumab for recurrent glioblastoma after progression on bevacizumab therapy. J Neurooncol. 2011;103(2):371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chamberlain MC, Grimm S, Phuphanich S, et al. ; Brain Tumor Investigational Consortium A phase 2 trial of verubulin for recurrent glioblastoma: a prospective study by the Brain Tumor Investigational Consortium (BTIC). J Neurooncol. 2014;118(2):335–343. [DOI] [PubMed] [Google Scholar]
- 34. Clarke JL, Ennis MM, Yung WK, et al. ; North American Brain Tumor Consortium Is surgery at progression a prognostic marker for improved 6-month progression-free survival or overall survival for patients with recurrent glioblastoma?Neuro Oncol. 2011;13(10):1118–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nowosielski M, Wiestler B, Goebel G, et al. Progression types after antiangiogenic therapy are related to outcome in recurrent glioblastoma. Neurology. 2014;82(19):1684–1692. [DOI] [PubMed] [Google Scholar]
- 36. DeLay M, Jahangiri A, Carbonell WS, et al. Microarray analysis verifies two distinct phenotypes of glioblastomas resistant to antiangiogenic therapy. Clin Cancer Res. 2012;18(10):2930–2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gerstner ER, Zhang Z, Fink JR, et al. ; ACRIN 6684 Trial Group ACRIN 6684: assessment of tumor hypoxia in newly diagnosed glioblastoma using 18F-FMISO PET and MRI. Clin Cancer Res. 2016;22(20):5079–5086. [DOI] [PMC free article] [PubMed] [Google Scholar]