Abstract
Background
Meningiomas are the most common primary brain tumor in adults, and somatic loss of the neurofibromatosis 2 (NF2) tumor suppressor gene is a frequent genetic event. There is no effective treatment for tumors that recur or continue to grow despite surgery and/or radiation. Therefore, targeted therapies that either delay tumor progression or cause tumor shrinkage are much needed. Our earlier work established mammalian target of rapamycin complex mTORC1/mTORC2 activation in NF2-deficient meningiomas.
Methods
High-throughput kinome analyses were performed in NF2-null human arachnoidal and meningioma cell lines to identify functional kinome changes upon NF2 loss. Immunoblotting confirmed the activation of kinases and demonstrated effectiveness of drugs to block the activation. Drugs, singly and in combination, were screened in cells for their growth inhibitory activity. Antitumor drug efficacy was tested in an orthotopic meningioma model.
Results
Erythropoietin-producing hepatocellular receptor tyrosine kinases (EPH RTKs), c-KIT, and Src family kinase (SFK) members, which are biological targets of dasatinib, were among the top candidates activated in NF2-null cells. Dasatinib significantly inhibited phospho-EPH receptor A2 (pEPHA2), pEPHB1, c-KIT, and Src/SFK in NF2-null cells, showing no cross-talk with mTORC1/2 signaling. Posttreatment kinome analyses showed minimal adaptive changes. While dasatinib treatment showed some activity, dual mTORC1/2 inhibitor and its combination with dasatinib elicited stronger growth inhibition in meningiomas.
Conclusion
Co-targeting mTORC1/2 and EPH RTK/SFK pathways could be a novel effective treatment strategy for NF2-deficient meningiomas.
Keywords: EPH receptors, kinome, meningioma, mTORC1/mTORC2, NF2
Neurofibromatosis 2 (NF2) is characterized by bilateral vestibular schwannomas and meningiomas and is caused by mutations in the NF2 gene.1–3 NF2-associated meningiomas cause severe neurologic morbidity and mortality from compression of adjacent brain or spinal cord. Moreover, sporadic meningiomas are the most common primary intracranial tumors in adults, with ~50% having biallelic, somatic inactivation of NF2. Meningiomas that progress despite surgery and radiation are associated with high morbidity due to ineffective chemotherapies for these tumors.1 Therefore, noninvasive medical therapies are greatly needed for NF2-associated and sporadic meningiomas.
NF2 encodes the tumor suppressor protein merlin, which has been implicated in a wide range of mitogenic signaling pathways, including receptor tyrosine kinases (RTKs),4 Rac/p-21 activated kinase (PAK),5,6 mammalian/mechanistic target of rapamycin (mTOR),7,8 and the Hippo9,10 pathways. A nuclear function of merlin through regulation of E3 ubiquitin ligase CRL4DCAF1 is also reported.11 Thus, merlin likely regulates these various implicated pathways in a cell context–dependent manner. We reported that loss of NF2/merlin abnormally activates mTOR complex 1 (mTORC1) signaling in meningiomas and schwannomas, which is blocked by rapamycin.7,12 Subsequent studies in Nf2-null mouse models treated with rapamycin and clinical trials with the mTORC1 inhibitor RAD001 for NF2 patients showed cytostatic effects of delayed growth or stabilization of schwannomas and meningiomas without tumor shrinkage.13–15
As a conserved Ser/Thr kinase, mTOR regulates cell growth, proliferation, and survival through 2 independent functional complexes, mTORC1 and mTORC2, which signal to distinct downstream targets.16,17 Our recent studies employing NF2-null human arachnoidal cells (AC‒clustered regularly interspaced short palindromic repeat [CRISPR]) along with human meningioma (MN) cells established that merlin loss also activates mTORC2-dependent serum/glucocorticoid-regulated kinase 1 (SGK1)/N-Myc downstream-regulated gene 1 (NDRG1) signaling. Treatment of primary NF2-deficient MN cells with the dual mTORC1/mTORC2 inhibitor AZD2014 decreased proliferation with greater efficacy than rapamycin.18 These results led to ongoing clinical trials with AZD2014 for NF2-associated and sporadic MN (NCT02831257, NCT03071874).
In addition to mTORC1/2 signaling, discovering other kinases dysregulated upon NF2 loss, particularly those druggable in disease-relevant human cells, could lead to novel therapeutic strategies. Therefore, to define dynamic changes in kinase activity on a proteomic scale and to identify adaptive kinome response of NF2-null cells to AZD2014 treatment, we employed multiplexed inhibitor beads coupled with mass spectrometry (MIB/MS).19 Here we report the identification of several kinases, including erythropoietin-producing hepatocellular (EPH) RTK family members that are expressed and activated in NF2-null AC-CRISPR lines and primary MN cells. Many of these activated kinases are targets of the FDA-approved kinase inhibitor dasatinib. We show that combination treatment with an mTORC1/mTORC2 inhibitor and dasatinib could be potentially effective for NF2-deficient MN.
Materials and Methods
Cell Culture and Treatment
Human MN used to generate primary cell cultures were collected following Massachusetts General Hospital Human Subjects protocols for tumor acquisition after informed consent. For details of cell lines, growth conditions, immunoblotting, and quantitative reverse-transcriptase (RT)-PCR, see the Supplementary Methods.
MIB Chromatography, Mass Spectrometry, and Analysis
Custom-synthesized kinase inhibitors (CTx-0294885, VI-16832, PP58, Purvalanol-B, UNC-21474, and UNC-8088A) were covalently linked to sepharose beads as described.19,20 Cells were treated for 24 h with AZD2014 (300 nM) and dasatinib (100 nM), singly or combined, or dimethyl sulfoxide (DMSO), and then lysed. Total protein lysate (2–5 mg) was gravity-flowed over inhibitor-linked bead mixture, followed by high and low salt washes. Bound proteins were eluted and trypsinized overnight and, in multiplex experiments, labeled with iTRAQ 4-plex (ABSciex) according to manufacturer instructions. Peptides were extracted and cleaned with C-18 spin columns (Pierce). For liquid chromatography–mass spectrometry (LC-MS), in multiplexed experiments, each sample was equalized for total peptide content, and peptide suspension was then injected onto Easy nLC-1000 or nLC-1200 and analyzed using a Q-Exactive or Q-Exactive HF mass spectrometer. Spectra were searched against the Uniprot/Swiss-Prot database with Sequest HT using Proteome Discoverer software. Data for each treated sample were processed as fold-change relative to DMSO controls (average ratio ± SD). Label-free quantification (LFQ) was performed using MaxLFQ21 with default parameters, except only unique peptides were used. Principal component analysis was performed using Perseus software. For drug response LFQ, missing log2(LFQ) values were imputed across the matrix if at least 3 valid values were identified in at least 1 treatment group. Two-sample tests for treatment-DMSO comparisons were performed in Perseus using permutation-based false discovery rate (FDR). Heatmaps were generated using GENE-E software (The Broad Institute). See the Supplementary Methods for further details.
Cell Viability and Drug Combination Screening
All assays were carried out using the CellTiter-Glo viability kit (Promega). Briefly, cells were seeded into 384-well plates (400 cells/well) 24 h before treatment. For viability assays, treatments using AZD2014, INK128, and dasatinib were performed in growth conditions, and treatment details are outlined in figure legends. Dose response curves were generated using GraphPad Prism 7. For dose-matrix testing of AZD2014, INK128, and/or dasatinib, singly and combined, cells were seeded as above. Viability assays were performed at 24 h post-seeding (D0), and at 96 h post-seeding (D3, 72 h posttreatment). Proliferation rate was determined by change in viability at D3 versus D0. For drug synergy, percent inhibition was determined by calculating the change in viability at D3 (drug-treated vs DMSO, normalized to 0). Isobolograms were generated using Chalice Analyzer (Horizon CombinatoRx; http://chalice. horizondiscovery.com/analyzer-server/cwr/index.jsp)
Animal Model, Bioluminescence Imaging, and In Vivo Dosing
The skull-base MN model was generated as described22 and performed according to the protocol approved by the Institutional Animal Care and Use Committee at Nationwide Children’s Hospital. Briefly, ~106 luciferase-expressing Ben-Men-1-LucB cells were stereotactically injected into the skull bases of 8- to10-week-old NSG mice (NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ; The Jackson Laboratory). Tumor establishment was monitored by bioluminescence imaging (BLI) using a Xenogen IVIS Spectrum (Caliper). Mice bearing tumors were randomized into 4 groups (n = 10/group) and treated by oral gavage with dasatinib (determined maximum tolerated dose [MTD], 20 mg/kg/day in 80 mM citrate buffer, pH 2.1), INK128 (determined MTD, 0.75 mg/kg/day in 5% 1-methyl-2-pyrrolidinone [NMP], 15% polyvinylpyrrolidone K30 [PVP], and 80% water), combination of dasatinib and INK128 (same dosing as above) or vehicle (mixture of citrate buffer and NMP-PVP-water), respectively. Tumor growth was monitored by BLI biweekly. Quantified luminescence for each mouse was normalized to pretreatment signal designated as 100%22 and expressed as the mean normalized luminescence ± SD.
Results
High-Throughput Kinome Analyses in NF2-null Human Arachnoidal Cell Lines
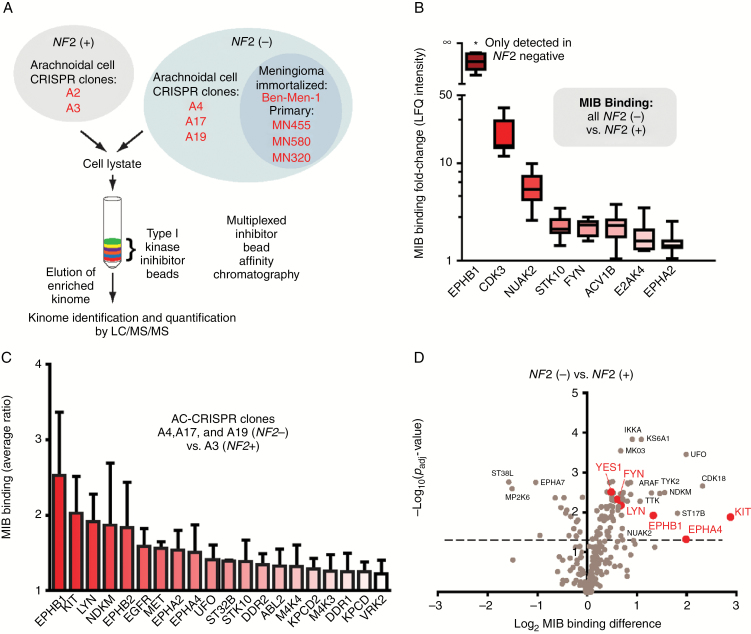
To discover new therapeutic targets for NF2, we undertook a large-scale unbiased kinome screen using MIB/MS analysis.19,20 MIB affinity chromatography employs an array of type I kinase inhibitors to selectively enrich the “functional kinome” based on kinase activity, expression level, and relative affinity for the inhibitor beads followed by MS-based quantification (Fig. 1A). Initial studies were carried out on clonally derived CRISPR/Cas9-modified human AC lines, either merlin-expressing (A2+ and A3+ clones) or merlin-null (A4−, A17−, and A19− clones),18 the immortalized NF2-null MN cell line Ben-Men-122, and 3 primary MN lines (Fig. 1A). Lysate from each cell line was analyzed by MIB/MS, and LFQ intensity (MIB binding) was determined using MaxLFQ (Fig. 1B). Eight kinases displayed increased MIB binding in all NF2-null cell lines, highlighted by EPHB1, an EPH RTK. Strikingly, EPHB1 peptides were detected in only NF2-null lines and not in the 2 NF2-expressing lines. Thus, a ratio of MIB binding could not be calculated. To directly address this concern and take advantage of isogenic AC-CRISPR lines to identify NF2-specific alterations, we next performed MIB/MS on a multiplexed MS run consisting of the merlin-expressing A3+ line and each of the 3 NF2-null AC lines (Fig. 1C). Although multiplexed MS runs can underestimate the true ratio,23 the identification of each kinase produces a valid ratio. MIB/MS identified several members of the EPH RTK family—EPHB1, EPHB2, EPHA2, and EPHA4—as well as c-KIT, Src family kinase LYN, and other RTKs such as epidermal growth factor receptor, MET, UFO/AXL, and discoidin domain receptor 1 and 2 to be enriched at >1.2-fold in NF2-null versus NF2-expressing cells (Fig. 1C). Many of these kinases were constitutively enriched in the NF2-null lines in the absence of serum (Supplementary Fig. S1A). Decreased MIB binding was observed in the NF2-null lines for selected cell cycle kinases such as cyclin-dependent kinase 2, aurora kinase B, and WEE1 (Supplementary Fig. S1B). To permit statistical analyses, biological triplicate lysates from NF2+ and NF2− AC lines were utilized for label-free MIB/MS, and LFQ intensities were determined. Volcano plots were generated to visualize the significance and magnitude of the differences in MIB binding (Fig. 1D) when comparing the NF2-null with the NF2-expressing line. Consistent with our prior observations, kinases such as UFO/AXL and NUAK2 exhibited increased binding in the NF2-null line. Notably, the MIB binding of the EPH RTKs EPHB1 and EPHA4 and Src family kinases LYN, FYN, and YES1 were all significantly increased in the NF2-null line. Taken together, our kinome analysis indicated that NF2 status was consistently and reproducibly associated with alterations in the EPH RTK family and Src family.
Fig. 1.
Kinome profiling identifies elevated EPH RTKs in NF2-null context. (A) Schematic of MIB/MS in NF2-expressing(+) and NF2-null(−) lines. (B) MIB-bound kinases detected in NF2− lines in (A) underwent LFQ with intensity expressed as fold-change versus average LFQ for 2 NF2+ lines. (C) Multiplexed MIB/MS was performed using indicated AC-CRISPR lines, and MIB binding ratio is plotted as average ± SD of each NF2− versus NF2+ line (for kinases with binding ratio >1.2). (D) Volcano plot showing log2-fold change MIB binding (LFQ intensity) for A17− versus A2+ cell line plotted against the −log10 Padj value. Dotted line, P = 0.05.
EPH Receptor Expression and Activation in NF2-null AC and MN Cells
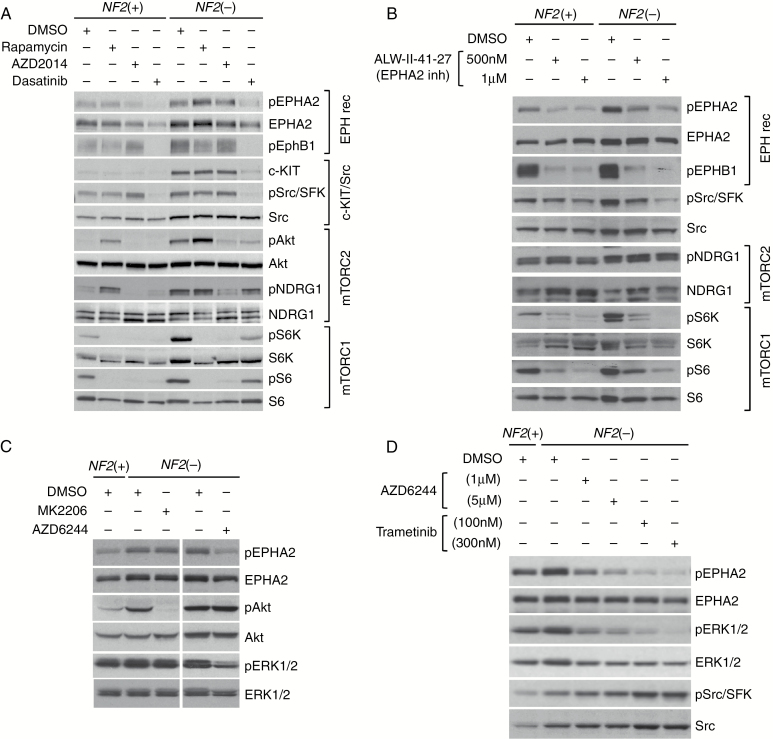
For follow-up studies in AC and MN cells, we chose a subset of EPH receptor family members identified by MIB/MS as well as downstream effector Src/Src family kinase (SFK). We examined the expression/phosphorylation of candidate kinases in a pair of isogenic AC-CRISPR lines with or without merlin expression, Ben-Men-1, and 6 primary NF2-null MN lines (Supplementary Fig. S2). Clinical information including World Health Organization grades of the tumors from which primary cells were derived and the copy number variations in MN lines wherever possible, are shown in Supplementary Table S1. We observed increased expression of EPHA2 as well as activation of EPHA2 and EPHB1 (detected by phosphorylation) in NF2-null ACs and Ben-Men-1 when compared with NF2+ ACs. Poor antibody quality precluded examining total EPHB1 protein levels. Consistent with activation of EPHA2 and EPHB1, an increased phosphorylation of the common Src/SFK(Y416) residue was also observed in NF2-null cells compared with NF2-expressing ACs (Fig. 2A). Dasatinib is a multitarget kinase inhibitor that, in addition to breakpoint cluster region–Abelson murine leukemia (BCR-Abl), effectively blocks EPHA4, EPHA2, EPHB1, EPHB2, SFKs, and c-KIT.24 It is FDA approved for treatment of resistant chronic myelogenous leukemia and is currently in clinical trials for additional cancers. Dasatinib treatment for 2 h led to significant reduction of pEPHA2(S897), pEPHB1(Y594), and pSrc/SFK(Y416), but did not inhibit mTORC1 activation (Fig. 2A). At the transcriptional level, we also observed increased expression of EPHB1 and EPHA4 by quantitative RT-PCR in 3 NF2-null AC-CRISPR lines (Fig. 2B). We observed variable levels of pEPHA2, pEPHB1, and pSrc/SFKs in primary MN cells (Fig. 2C), suggesting heterogeneity among individual tumors. Together, these results confirm activation of the EPH RTK/SFK pathways in NF2-null cells identified by the MIB/MS kinome screen.
Fig. 2.
Expression and activation of EPH RTKs, Src/SFK, and c-KIT in NF2-null AC and MN cells. (A) NF2-null ACs and Ben-Men-1 show increased pEPHA2(S897), pEPHB1(Y594), pSrc/SFK(Y416), c-KIT, and mTORC1 signaling (readouts: pS6K/pS6) compared with NF2(+) ACs. Dasatinib (100 nM, 2 h) inhibits pEPHA2, pEPHB1, and pSFK with no effects on mTORC1 and c-KIT. (B) All NF2− AC-CRISPR lines (A4, A17, A19) show increased transcription of EPHB1 and EPHA4 by quantitative RT-PCR compared with NF2+ A2. *P < 0.06; **P < 0.005. (C) NF2-deficient primary MN lines show heterogeneous activation.
No Cross-talk between the mTOR and EPH Receptor Pathways
We next examined effects of treatment with mTORC1-specific inhibitor rapamycin, dual mTORC1/2 inhibitor AZD2014, or the multikinase inhibitor dasatinib to understand potential cross-talk between mTOR and EPH receptor pathways. After 24 h treatment, both NF2-expressing and NF2-null ACs showed attenuation of mTORC1 signaling [readouts: pS6K(T389)/pS6(S240/244)], using rapamycin or AZD2014, and inhibition of mTORC2 signaling [readouts: pAkt(S473)/pNDRG1(T346)] with AZD2014. Rapamycin treatment is known to activate phosphatidylinositol-3 kinase (PI3K)–dependent prosurvival pathways, which explains activation of Akt and NDRG1 after rapamycin treatment (Fig. 3A). Neither rapamycin nor AZD2014 showed any significant effect on pEPHA2, pEPHB1, c-KIT, or pSrc/SFK. Conversely, dasatinib showed inhibition of pEPHA2, pEPHB1, c-KIT, pSrc/SFK, and pAkt, but had no effect on mTORC2-SGK1 readout pNDRG1, and a modest effect on mTORC1 readouts pS6K and pS6 (Fig. 3A). In addition to dasatinib, we tested ALW-II-41-27 (ALW; provided by Dr Nathanael Gray), which was recently reported to inhibit EPHA2 potently.25 Similar to dasatinib at 100 nM, ALW treatment (500 nM and 1 µM) showed marked attenuation of pEPHB1, pEPHA2, and pSrc/SFK with no effect on mTORC2-SGK1 readout pNDRG1. However, ALW caused a significant reduction of mTORC1 signaling at 1 µM (Fig. 3B). It should be noted that ALW, while a potent inhibitor of EPHA2, has also displayed inhibition toward additional kinases25,26; therefore, it is likely that the attenuation of pS6 may be through a mechanism independent of EPHA2. We also examined the SFK-specific inhibitor saracatinib (AZD0530), and treatment of ACs for 24 h with increasing doses (up to 5 µM) revealed moderate attenuation of pSrc/SFK in NF2-null cells, but only at the dose of 5 µM. Also, saracatinib treatment was able to inhibit pEPHB1 and the mTORC1 and mTORC2 pathways at 500 nM or higher (Supplementary Fig. S3). Collectively, our results show independent activation of the mTOR and EPH receptor pathways upon NF2 loss and dasatinib being superior to ALW and saracatinib for targeting EPH RTKs, c-KIT, and SFKs in NF2-null AC and MN cells.
Fig. 3.
Independent activation of the mTOR and EPH receptor pathways in NF2-null ACs and inhibition of EPHA2(S897) phosphorylation by MEK inhibitors. (A) AC-CRISPR lines (NF2+ and NF2−) treated for 24 h with rapamycin (20 nM) or AZD2014 (300 nM) show inhibition of mTORC1 (readouts: pS6K/pS6) and mTORC2 (readouts: pAkt/pNDRG1) signaling with no effect on EPHA2, EPHB1, c-KIT, or Src/SFK. Dasatinib (100 nM, 24 h) attenuates pEPHA2, pEPHB1, c-KIT, and pSrc/SFK with modest effects on mTORC1/2 signaling. (B) EPHA2 inhibitor ALW-II-41-27 (24 h) blocks pEPHA2, pEPHB1, and pSrc/SFK with no effect on mTORC2 signaling, while attenuating mTORC1 signaling. (C) Treatment of NF2− ACs with AZD6244 (1 µM, 2 h) inhibits pEPHA2(S897), while MK2206 (1 µM) did not. (D) Treatment of NF2− ACs for 24 h with AZD6244 or trametinib inhibits pEPHA2(S897) and pERK1/2 while increasing phospho- and total Src/SFK.
MEK Inhibition Leads to Attenuation of EPHA2(S897) Phosphorylation
EPH RTK family members, including EPHA2 and EPHA4, are often overexpressed/upregulated in the development and progression of various types of human cancers. Targeting EPH receptors, such as EPHA2, has been suggested as a promising therapy for these tumor types.24,27 Activation of PI3K-Akt signaling and phosphorylation of EPHA2 at S897 by Akt is shown to provide a positive oncogenic signal.28 Recent reports have also demonstrated that 90 kDa ribosomal protein S6 kinase 1 (RSK1) can phosphorylate EPHA2(S897), thus placing it downstream of MEK‒extracellular signal-regulated kinase (ERK)‒RSK signaling.29,30 To understand the mechanism of EPHA2(S897) phosphorylation, we treated NF2-null ACs with the selective Akt inhibitor MK-2206 or the MEK inhibitor AZD6244. Our results showed that AZD6244 significantly decreased pEPHA2(S897) (Fig. 3C). We also tested another potent MEK inhibitor trametinib and found that it elicited a stronger reduction of pEPHA2(S897) compared with AZD6244 (Fig. 3D). Interestingly, an increase in pSFK and total Src was seen with both MEK inhibitors, implying an adaptive regulation. Taken together, our results suggest that EPHA2(S897) is phosphorylated in NF2-null cells in a MEK-dependent manner, potentially through activation of MEK-ERK-RSK signaling.
Combination of AZD2014 and Dasatinib Reveals Synergistic Effects on Cell Viability in NF2-null Cells
We examined the effects of AZD2014 and dasatinib, alone and combined, on cell viability. Standard dose-matrix screenings of 2 NF2-deficient primary MN lines were done to assess (i) changes in proliferation rate, comparing cell viability at 0 h versus 72 h timepoints, and (ii) synergistic effects of AZD2014/dasatinib combination after 72 h treatment. While AZD2014 and dasatinib, as single agents, reduced the proliferation rate, combined AZD2014/dasatinib treatment yielded a superior dose-dependent reduction in proliferation rates (Fig. 4A, B), as well as synergistic growth inhibitions in NF2-null primary MN lines (Fig. 4C). As an example, single agent treatment of primary MN lines with 1 µM AZD2014 or dasatinib was required to achieve ~70% growth inhibition compared with DMSO alone, whereas with the AZD2014/dasatinib combination, a 3-fold dose reduction (~333 nM of each drug) led to ~80% growth inhibition (Supplementary Fig. S4). These data suggest that co-targeting mTORC1/2 and EPH RTK/SFK pathways using the AZD2014/dasatinib combination may be a more effective treatment for NF2-deficient MN.
Fig. 4.
Decreased proliferation rates and synergistic effects upon combination treatment with a dual mTORC1/2 inhibitor and dasatinib. (A, B) Proliferation assays of primary MN lines MN309 (A) and MN548 (B) treated with AZD2014 and dasatinib, singly and combined, show relative change in percent proliferation rate (%PR) at day 3 (data normalized to day 0 [%PR = 0]). Each group represents DMSO (d) or the indicated dasatinib doses (Das: 0–1 µM, bracketed at bottom) in combination with increasing doses of AZD2014 (see plot key). Error bars, mean ± SD; *P < 0.05 (compared with DMSO). (C) 6×6 dose matrix screening (same dosing as A–C, 4 replicates) was carried out, and isobolograms generated show synergism of combined AZD2014/dasatinib treatment, defined as combination index (CI)<1 (left shifted) compared with Loewe additivity model (CI = 1).
Kinome Analysis of AZD2014/Dasatinib Combination Treatment Demonstrates Effective Target Inhibition
To extend our findings of synergism between AZD2014 and dasatinib in NF2-null cells, we interrogated the kinome response to these compounds, alone and in combination. In biological triplicate, AC-CRISPR lines A2+ and A17− along with NF2-null Ben-Men-1 cells were treated for 24 h with DMSO, AZD2014, dasatinib, or AZD2014/dasatinib combination. MIB/MS was performed on cell lysates and MIB binding determined by MaxLFQ.21 Hierarchical clustering was performed to identify dominant drug response patterns (Fig. 5A, Supplementary Fig. S5). Although the kinome profile of Ben-Men-1 was clearly separable from the paired AC-CRISPR lines, a strong cluster (“Dasatinib-Combination”) could be identified that was dependent on the inclusion of dasatinib (Fig. 5A, dasatinib and combination treatments compared with DMSO and AZD2014). These potently inhibited kinases included numerous EPH RTKs, c-KIT, SRC/SFKs, and other kinases upregulated specific to NF2-null cells. To further substantiate the dasatinib dependence of the observed response, we performed principal components analysis on the kinome log2 LFQ intensities (Fig. 5B). Although replicate and sample variability could be observed, the most significant alterations were indeed dependent on dasatinib treatment. Since dasatinib is known to target EPH receptors, c-KIT, and many other RTKs in addition to BCR-Abl, to address the potential significance of these changes, we generated volcano plots based upon the log2 difference in MIB binding for each treatment versus DMSO control as well as the associated adjusted P-value (FDR cutoff <0.05). Dasatinib or combination treatment resulted in significant and large-scale changes in all cell lines for numerous dasatinib targets (light blue oval; eg, KIT, ABL1/2, EPH receptors, SFKs) (Fig. 5C–E). Modest mTOR inhibition was also observed in the NF2− lines but not the NF2+ line. Importantly, few kinases were seen to be activated/induced significantly by these drugs, alone or in combination. Akt1 was very slightly increased in the context of AZD2014 alone in A17− cells, and the pseudokinase STRAB (Sterile 20–related kinase adapter protein beta) in the context of dasatinib treatment (Fig. 5C–E). AZD2014 treatment of A17− cells for 24 h effectively inhibits mTORC2-dependent pAkt(S473) while inducing pAkt(T308) (Fig. 5F), which may explain the slight increase in Akt1 observed in MIB/MS (Fig. 5D).
Fig. 5.
Combined AZD2014/dasatinib treatment elicits target inhibition in NF2-null lines. (A) Indicated cell lines were treated 24 h with DMSO, AZD2014, dasatinib, or AZD2014/dasatinib (Combination) followed by MIB/MS. LFQ intensities were log2 transformed and quantile normalized, and hierarchical clustering was performed. Values were mean-centered, and row z-score color key is shown. Strongly responsive “Dasatinib-Combination” cluster is shown. Cell lines and treatments are identified by color key. (B) Principal components analysis of LFQ intensities as in (A). Cell line is indicated by shape and treatment by color. (C–E) Volcano plots show log2 fold-change for each treatment (color-coded) versus DMSO plotted against the −10g10 Padj value. Dotted lines P = 0.05. (F) Immunoblotting shows induction of pAkt(T308) in NF2-null ACs after 24 h AZD2014 treatment.
The Antitumor Efficacy of Dasatinib and Its Combination with the Highly Potent mTORC1/2 Inhibitor INK128 in an Orthotopic NF2-Deficient Meningioma Model
We next tested another potent mTORC1/2 inhibitor, INK128, and examined its antiproliferative effects compared with AZD2014. In Ben-Men-1 and primary MN cells, we observed that INK128 was more potent with half-maximal inhibitory concentration (IC50) values ~10-fold less than AZD2014 (Fig. 6A–C). Further, in Ben-Men-1 cells, dasatinib alone was less effective than AZD2014 or INK128 alone (Fig. 6B), whereas in the primary MN line, AZD2014, INK128, and dasatinib all showed similar inhibition (Fig. 6C). However, combination treatment using either mTORC1/2 inhibitor with dasatinib showed enhanced inhibition compared with single agents alone, with the INK128/dasatinib combination being superior in both lines (Fig. 6B, C). Since the antiproliferative effect of INK128 was more potent compared with AZD2014, we next chose to evaluate the antitumor efficacy of INK128, either alone or combined with dasatinib, using the skull-base Ben-Men-1-LucB MN model.22 The MTD for the INK128 and dasatinib combination was determined to be 0.75 mg/kg/day of INK128 and 20 mg/kg/day of dasatinib by oral gavage. NSG mice bearing established Ben-Men-1-LucB tumors were divided into 4 treatment groups and treated with INK128, dasatinib, INK128/dasatinib combination, or vehicle, respectively, for 14 weeks. As expected, Ben-Men-1-LucB MN in mice receiving vehicle grew steadily over time (Fig. 6D, E). In mice treated with dasatinib, tumors initially grew at a similar rate as vehicle controls; however, after 4 weeks of treatment, tumors began to exhibit slowed growth (Fig. 6E). At 14 weeks, dasatinib inhibited tumor growth by ~65% relative to the vehicle control group. In contrast, INK128, as a single agent, exhibited antitumor effects immediately following treatment and suppressed tumor growth by ~85% at 14 weeks. Furthermore, combined INK128/dasatinib treatment also demonstrated immediate suppressive effects on MN growth, similar to INK128 alone in this model (Fig. 6D, E). These results suggest that the dual mTORC1/2 inhibitor INK128 and its combination with dasatinib possess potent antitumor effects in NF2-deficient MN.
Fig. 6.
INK128 is superior to AZD2014 showing in vivo efficacy alone and combined with dasatinib. (A) Dose response curves for AZD2014 (left) and INK128 (right) were determined for NF2-null Ben-Men-1 and primary MN lines (MN621 and MN626) treated for 72 h with 0–10 µM of indicated drugs (9 dilution points, 3-fold serial dilution). Absolute IC50 (Abs IC50) is shown. Results were plotted as % viability relative to DMSO controls and are presented as ± SEM (3 replicates/dose). (B, C) Treatment of MN lines Ben-Men-1 (B) and MN621 (C) using dasatinib, singly and combined with AZD2014 (left) or INK128 (right), show relative changes in percent proliferation rate (%PR) at D3 where data are normalized to D0 (%PR = 0). Each group represents DMSO (d) or single dasatinib doses (Das: 0–1.1 µM, bracketed at bottom) in combination with increasing doses of AZD2014 or INK128 (see plot key). Error bars, mean ± SD; *P < 0.05 (compared with DMSO). (D, E) Antitumor activities of INK128, dasatinib, and their combination were determined using an orthotopic, quantifiable meningioma model.21 Shown are representative bioluminescence images of groups of Ben-Men-1-LucB meningioma-bearing mice at pretreatment or after 14 weeks (wks) posttreatment with vehicle, dasatinib, INK128, or INK128/dasatinib (D). Tumor-emitted bioluminescence signals at each indicated timepoint following treatment were quantified and mean normalized bioluminescence signals from each treatment group were calculated and shown with standard deviations (E).
Discussion
Meningiomas, which arise from the arachnoidal layer of the meninges, are unresponsive to standard chemotherapies, and therefore identification of an effective targeted therapy is urgently needed.31 To discover additional novel targets in NF2-deficient MN that are druggable, we performed large-scale MIB/MS kinome screening in paired NF2-null and NF2-expressing AC and NF2-deficient MN cell lines. We identified several EPH RTKs and SFKs to be activated in NF2-null cells compared with NF2-expressing cells. Also, we validated the expression and activation of EPH receptors and downstream effector SFK activation in NF2-null tumor cells. Interestingly, dasatinib, an FDA-approved drug that inhibits multiple kinases including EPH receptors, effectively blocked activation of several of the EPH RTKs, c-KIT, and SFKs in NF2-null cells.
EPHA2 overexpression and S897 phosphorylation has been reported in several types of cancers,25,26 and therefore we explored the mechanism by which EPHA2 is activated in NF2-null cells. Mechanistically, we show EPHA2(S897) phosphorylation is MEK dependent, and this finding is consistent with activation of mitogen-activated protein kinase/ERK signaling upon merlin loss reported previously.7,32–34 Moreover, an increase in Src/SFK that we observed upon treatment of NF2-deficient cells with MEK inhibitors (Fig. 3C, D) was effectively suppressed by dasatinib, further strengthening our rationale for using dasatinib.
Emerging studies indicate that with targeted inhibition of specific growth-promoting signaling pathways, tumor cells can circumvent these blockades with adaptive mechanisms that upregulate alternate kinases or reactivate the original targeted pathways. This “adaptive kinome reprogramming” is often a major reason for response failure in patients treated with a kinase inhibitor as a monotherapy.20,35 Our follow-up MIB/MS analyses in NF2-expressing and NF2-null ACs after treatment with AZD2014, dasatinib, and AZD2014/dasatinib combination revealed significant inhibition of many dasatinib targets while exhibiting a modest suppression of mTOR signaling (Fig. 5). We also observed induction of pAkt(T308) as an adaptive response after AZD2014 treatment, which is consistent with an earlier report showing that inhibiting mTORC2 results in release of feedback inhibition of RTK signaling, leading to PI3K activation and an increased Akt(T308) phosphorylation in breast cancer cells.36 Dasatinib, however, did not induce pAkt(T308) (Fig. 5F). Overall, kinases that were increased in the context of NF2 loss can be dramatically inhibited upon treatment with the synergistic combination of AZD2014/dasatinib.
The combination treatment of MN cells with dual mTORC1/mTORC2 inhibitor and dasatinib (i) potently diminished cell proliferation rate and (ii) revealed enhanced effects in growth inhibition (Figures 4 and 6 and Supplementary Fig. S4). Reduced cell viability could result from cell death; however, we did not observe any evidence of apoptosis/necrosis by cleaved caspase-3 or poly(ADP-ribose) polymerase by immunoblotting or flow cytometry analysis of annexin V staining (data not shown). In cell viability assays, we also saw no significant differences in NF2-expressing versus NF2-null ACs with single or combination treatments (data not shown), which could be due to relatively slow growth rates for this cell type.
In the orthotopic, quantifiable Ben-Men-1-LucB MN model, while dasatinib alone was active, it was less potent than INK128. This result is consistent with our in vitro observation of Ben-Men-1 cells treated with these drugs. Also, INK128 inhibits both mTORC1 and mTORC2, which are activated upon NF2 loss.18 While combined treatment with INK128 and dasatinib exhibited growth-inhibitory effects in NF2-deficient MN cells in vitro, the combination treatment only gave rise to tumor suppressive effects similar to INK128 alone in our orthotopic meningioma model.
The differences between our in vitro and in vivo results could be largely explained by the use of different cell lines, ie, Ben-Men-1 and MN621. Ben-Men-1 is a telomerase reverse transcriptase immortalized line, which reveals more copy number variations when compared with the primary MN621 line (Table S1). Primary MN cells do not grow as patient-derived xenografts or xenografts, and Ben-Men-1 is the only in vivo model currently available, and therefore we employed this model. Recent studies in other cancer models have shown that combining either mTORC1 inhibitor or dual mTORC1/2 inhibitor with dasatinib enhances the efficacy of dasatinib.37,38 We believe that our results, taken together, showing the combination of dual mTOR inhibitor with dasatinib to be more effective in primary human MN lines, could provide a promising and new therapeutic opportunity to treat NF2-associated and sporadic MN.
Supplementary Material
Supplementary material is available at Neuro-Oncology online.
Funding
This work was supported by Children’s Tumor Foundation (CTF) Synodos for NF2, NF Northeast, the Manth family, S. Sydney De Young Foundation and the U.S. Army Medical Research and Material Command contract W81XWH-15-1-0414.
Conflict of interest statement. The authors declare that no conflicts of interest exist.
List of Consortia Members
Investigators of the Children’s Tumor Foundation Synodos for NF2 Consortium: Annette Bakker and Salvatore La Rosa, Children’s Tumor Foundation; Wade Clapp, Indiana University; Jaishri Blakeley, Johns Hopkins Medical School; Helen Morrison, Leibniz Institute on Aging – Fritz Lipmann Institute; Bradley Welling, Massachusetts Eye and Ear Infirmary; James Gusella, Stephen Haggarty, Scott Plotkin, Vijaya Ramesh and Anat Stemmer-Rachamimov, Massachusetts General Hospital; Long-Sheng Chang, Nationwide Children’s Hospital; Robert Allaway, Abhishek Pratap and Justin Guinney, Sage Bionetworks; Cristina Fernandez-Valle, University of Central Florida; Gary Johnson, University of North Carolina School of Medicine (Full member list: https://www.synapse.org/-!Synapse:syn2343195/wiki/62126)
Acknowledgments
We thank Dr Nathanael Gray of Dana-Faber Cancer Institute for ALW-II-41-27 inhibitor, Wen-Ning Zhao for technical help, Laura Herring and the UNC Proteomics Core Facility for instrument usage, and the Nationwide Children’s Hospital Tumor Core for animal dosing.
Contributor Information
Children’s Tumor Foundation Synodos for NF2 Consortium:
Annette Bakker, Salvatore La Rosa, Wade Clapp, Jaishri Blakeley, Helen Morrison, Bradley Welling, James Gusella, Stephen Haggarty, Scott Plotkin, Vijaya Ramesh, Anat Stemmer-Rachamimov, Long-Sheng Chang, Robert Allaway, Abhishek Pratap, Justin Guinney, Sage Bionetworks, Cristina Fernandez-Valle, and Gary Johnson
References
- 1. Blakeley JO, Evans DG, Adler J, et al. Consensus recommendations for current treatments and accelerating clinical trials for patients with neurofibromatosis type 2. Am J Med Genet A. 2012;158A(1):24–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Evans DG. Neurofibromatosis 2 [Bilateral acoustic neurofibromatosis, central neurofibromatosis, NF2, neurofibromatosis type II]. Genet Med. 2009;11(9):599–610. [DOI] [PubMed] [Google Scholar]
- 3. Ruggieri M, Praticò AD, Evans DG. Diagnosis, management, and new therapeutic options in childhood neurofibromatosis type 2 and related forms. Semin Pediatr Neurol. 2015;22(4):240–258. [DOI] [PubMed] [Google Scholar]
- 4. McClatchey AI, Fehon RG. Merlin and the ERM proteins–regulators of receptor distribution and signaling at the cell cortex. Trends Cell Biol. 2009;19(5):198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shaw RJ, Paez JG, Curto M, et al. The Nf2 tumor suppressor, merlin, functions in Rac-dependent signaling. Dev Cell. 2001;1(1):63–72. [DOI] [PubMed] [Google Scholar]
- 6. Yi C, Wilker EW, Yaffe MB, Stemmer-Rachamimov A, Kissil JL. Validation of the p21-activated kinases as targets for inhibition in neurofibromatosis type 2. Cancer Res. 2008;68(19):7932–7937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. James MF, Han S, Polizzano C, et al. NF2/merlin is a novel negative regulator of mTOR complex 1, and activation of mTORC1 is associated with meningioma and schwannoma growth. Mol Cell Biol. 2009;29(15):4250–4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. López-Lago MA, Okada T, Murillo MM, Socci N, Giancotti FG. Loss of the tumor suppressor gene NF2, encoding merlin, constitutively activates integrin-dependent mTORC1 signaling. Mol Cell Biol. 2009;29(15):4235–4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hamaratoglu F, Willecke M, Kango-Singh M, et al. The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat Cell Biol. 2006;8(1):27–36. [DOI] [PubMed] [Google Scholar]
- 10. Yin F, Yu J, Zheng Y, Chen Q, Zhang N, Pan D. Spatial organization of Hippo signaling at the plasma membrane mediated by the tumor suppressor Merlin/NF2. Cell. 2013;154(6):1342–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li W, You L, Cooper J, et al. Merlin/NF2 suppresses tumorigenesis by inhibiting the E3 ubiquitin ligase CRL4(DCAF1) in the nucleus. Cell. 2010;140(4):477–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. James MF, Lelke JM, Maccollin M, et al. Modeling NF2 with human arachnoidal and meningioma cell culture systems: NF2 silencing reflects the benign character of tumor growth. Neurobiol Dis. 2008;29(2):278–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Giovannini M, Bonne NX, Vitte J, et al. mTORC1 inhibition delays growth of neurofibromatosis type 2 schwannoma. Neuro Oncol. 2014;16(4):493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goutagny S, Raymond E, Esposito-Farese M, et al. Phase II study of mTORC1 inhibition by everolimus in neurofibromatosis type 2 patients with growing vestibular schwannomas. J Neurooncol. 2015;122(2):313–320. [DOI] [PubMed] [Google Scholar]
- 15. Goutagny S, Giovannini M, Kalamarides M. A 4-year phase II study of everolimus in NF2 patients with growing vestibular schwannomas. J Neurooncol. 2017;133(2):443–445. [DOI] [PubMed] [Google Scholar]
- 16. Betz C, Hall MN. Where is mTOR and what is it doing there?J Cell Biol. 2013;203(4):563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12(1):9–22. [DOI] [PubMed] [Google Scholar]
- 18. Beauchamp RL, James MF, DeSouza PA, et al. A high-throughput kinome screen reveals serum/glucocorticoid-regulated kinase 1 as a therapeutic target for NF2-deficient meningiomas. Oncotarget. 2015;6(19):16981–16997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Duncan JS, Whittle MC, Nakamura K, et al. Dynamic reprogramming of the kinome in response to targeted MEK inhibition in triple-negative breast cancer. Cell. 2012;149(2):307–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stuhlmiller TJ, Miller SM, Zawistowski JS, et al. Inhibition of lapatinib-induced kinome reprogramming in ERBB2-positive breast cancer by targeting BET family bromodomains. Cell Rep. 2015;11(3):390–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cox J, Hein MY, Luber CA, Paron I, Nagaraj N, Mann M. Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol Cell Proteomics. 2014;13(9):2513–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Burns SS, Akhmametyeva EM, Oblinger JL, et al. Histone deacetylase inhibitor AR-42 differentially affects cell-cycle transit in meningeal and meningioma cells, potently inhibiting NF2-deficient meningioma growth. Cancer Res. 2013;73(2):792–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Karp NA, Huber W, Sadowski PG, Charles PD, Hester SV, Lilley KS. Addressing accuracy and precision issues in iTRAQ quantitation. Mol Cell Proteomics. 2010;9(9):1885–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boyd AW, Bartlett PF, Lackmann M. Therapeutic targeting of EPH receptors and their ligands. Nat Rev Drug Discov. 2014;13(1):39–62. [DOI] [PubMed] [Google Scholar]
- 25. Miao B, Ji Z, Tan L, et al. EPHA2 is a mediator of vemurafenib resistance and a novel therapeutic target in melanoma. Cancer Discov. 2015;5(3):274–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Amato KR, Wang S, Hastings AK, et al. Genetic and pharmacologic inhibition of EphA2 promotes apoptosis in NSCLC. J Clin Invest. 2014;124(5):2037–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xi HQ, Wu XS, Wei B, Chen L. Eph receptors and ephrins as targets for cancer therapy. J Cell Mol Med. 2012;16(12):2894–2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Koshikawa N, Hoshino D, Taniguchi H, et al. Proteolysis of EphA2 converts it from a tumor suppressor to an oncoprotein. Cancer Res. 2015;75(16):3327–3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hamaoka Y, Negishi M, Katoh H. EphA2 is a key effector of the MEK/ERK/RSK pathway regulating glioblastoma cell proliferation. Cell Signal. 2016;28(8):937–945. [DOI] [PubMed] [Google Scholar]
- 30. Zhou Y, Yamada N, Tanaka T, et al. Crucial roles of RSK in cell motility by catalysing serine phosphorylation of EphA2. Nat Commun. 2015;6:7679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Blakeley JO, Plotkin SR. Therapeutic advances for the tumors associated with neurofibromatosis type 1, type 2, and schwannomatosis. Neuro Oncol. 2016;18(5):624–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ammoun S, Flaiz C, Ristic N, Schuldt J, Hanemann CO. Dissecting and targeting the growth factor-dependent and growth factor-independent extracellular signal-regulated kinase pathway in human schwannoma. Cancer Res. 2008;68(13):5236–5245. [DOI] [PubMed] [Google Scholar]
- 33. James MF, Stivison E, Beauchamp R, et al. Regulation of mTOR complex 2 signaling in neurofibromatosis 2-deficient target cell types. Mol Cancer Res. 2012;10(5):649–659. [DOI] [PubMed] [Google Scholar]
- 34. Yi C, Troutman S, Fera D, et al. A tight junction-associated Merlin-angiomotin complex mediates Merlin’s regulation of mitogenic signaling and tumor suppressive functions. Cancer Cell. 2011;19(4):527–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Johnson GL, Stuhlmiller TJ, Angus SP, Zawistowski JS, Graves LM. Molecular pathways: adaptive kinome reprogramming in response to targeted inhibition of the BRAF-MEK-ERK pathway in cancer. Clin Cancer Res. 2014;20(10):2516–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rodrik-Outmezguine VS, Chandarlapaty S, Pagano NC, et al. mTOR kinase inhibition causes feedback-dependent biphasic regulation of AKT signaling. Cancer Discov. 2011;1(3):248–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen B, Xu X, Luo J, Wang H, Zhou S. Rapamycin enhances the anti-cancer effect of dasatinib by suppressing Src/PI3K/mTOR pathway in NSCLC cells. PLoS One. 2015;10(6):e0129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Janes MR, Vu C, Mallya S, et al. Efficacy of the investigational mTOR kinase inhibitor MLN0128/INK128 in models of B-cell acute lymphoblastic leukemia. Leukemia. 2013;27(3):586–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.