Abstract
Purpose
To determine the association between Prostate Imaging Reporting and Data System (PI-RADS) version 2 scores and prostate cancer (PCa) in a cohort of patients undergoing biopsy of transition zone (TZ) lesions.
Materials and Methods
A total of 634 TZ lesions in 457 patients were identified from a prospectively maintained database of consecutive patients undergoing prostate magnetic resonance imaging. Prostate lesions were retrospectively categorized with the PI-RADS version 2 system by two readers in consensus who were blinded to histopathologic findings. The proportion of cancer detection for all PCa and for clinically important PCa (Gleason score ≥3+4) for each PI-RADS version 2 category was determined. The performance of PI-RADS version 2 in cancer detection was evaluated.
Results
For PI-RADS category 2 lesions, the overall proportion of cancers was 4% (one of 25), without any clinically important cancer. For PI-RADS category 3, 4, and 5 lesions, the overall proportion of cancers was 22.2% (78 of 352), 39.1% (43 of 110), and 87.8% (129 of 147), respectively, and the proportion of clinically important cancers was 11.1% (39 of 352), 29.1% (32 of 110), and 77.6% (114 of 147), respectively. Higher PI-RADS version 2 scores were associated with increasing likelihood of the presence of clinically important PCa (P < .001). Differences were found in the percentage of cancers in the PI-RADS category between PI-RADS 3 and those upgraded to PI-RADS 4 based on diffusion-weighted imaging for clinically important cancers (proportion for clinically important cancers for PI-RADS 3 and PI-RADS 3+1 were 11.1% [39 of 352] and 30.8% [28 of 91], respectively; P < .001).
Conclusion
Higher PI-RADS version 2 scores are associated with a higher proportion of clinically important cancers in the TZ. PI-RADS category 2 lesions rarely yield PCa, and their presence does not justify targeted biopsy.
© RSNA, 2018
See also the editorial by Weinreb.
Introduction
Multiparametric (MP) magnetic resonance (MR) imaging of the prostate has emerged as a valuable imaging modality in prostate cancer (PCa) detection, staging, and active surveillance (1–4). The main indications for MP MR imaging include further evaluation of patients clinically suspected of having PCa (elevated prostate specific antigen [PSA] level, abnormal digital rectal examination findings, or both) and those with negative standard 12-core systematic biopsy findings in whom suspicion for PCa persists (5). MP MR imaging is highly sensitive in the localization of clinically important cancer in addition to its role in the staging of PCa, thereby providing guidance for clinical management and prognostic assessment. The ability to identify suspicious areas within the gland has facilitated targeted biopsy, while the additional information gleaned from individual sequences has improved risk stratification of PCa aggressiveness on the basis of imaging features and Gleason score (low, intermediate, or high risk) (6,7). It is advantageous to obtain prostate MP MR images to localize tumors prior to biopsy, as this enables accurate targeted biopsy to be performed. Fusion MR imaging and transrectal (TR) ultrasonography (US)-guided biopsy has been shown to improve the detection of clinically important PCa over that with systematic biopsy alone (8–11). Prostate Imaging Reporting and Data System (PI-RADS) version 2 was recently introduced to improve standardization of MR image acquisition, interpretation, and reporting, with early studies validating its scoring for the diagnosis of PCa (12–15). It details specific morphologic criteria when assessing individual sequences and recommends weighting sequences based on zonal anatomy. Identification of transition zone (TZ) lesions remains a challenge, as interpretation of findings is nuanced, balancing the heterogeneous findings of benign prostatic hyperplasia with the likelihood of malignancy. In this revised version, lesions seen at prostate MP MR imaging are assessed with a five-point scale, which can suggest the likelihood of clinically important malignancy. It uses the concept of pulse sequence dominancy in different zones (diffusion-weighted MR imaging and T2-weighted MR imaging for peripheral and transition zones, respectively) to improve performance of the PI-RADS reporting system. Empirical data and validation studies of PI-RADS version 2 are sparse, and this scarcity becomes even more pronounced with regard to literature focusing on TZ lesions because of the inherent limited number of PCas in the TZ. Thus, our study aims to determine the relative association of different PI-RADS version 2 categories with PCa in a patient cohort undergoing fusion MR imaging and TR US–guided biopsy of TZ lesions.
Materials and Methods
Study Design and Patient Population
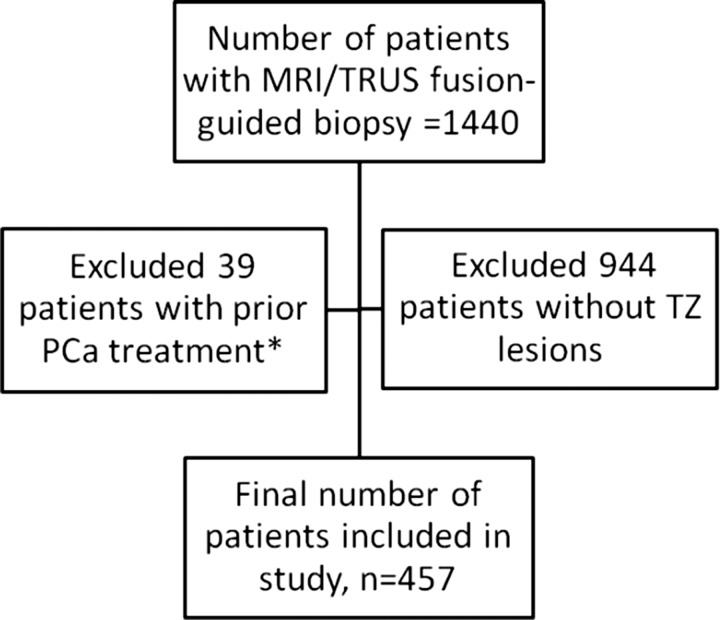
This Health Insurance Portability Accountability Act–compliant institutional review board–approved retrospective study was performed at one institution with a large referral base for prostate malignancy. Data from a prospectively maintained database were collected in consecutive patients who screened positive for PCa and who were referred to our department for prostate MP MR imaging. All patients subsequently underwent fusion MR imaging and TR US–guided biopsy of TZ lesions detected at preprocedural MP MR imaging between January 2012 and February 2015 (n = 1440). The inclusion criteria for the final study were undergoing MP MR imaging and one or more reported TZ lesions that underwent subsequent fusion MR imaging and TR US–guided biopsy. Exclusion criteria for this study were as follows: (a) previous history of PCa treatment (surgery, focal laser ablation, brachytherapy, or external beam radiation therapy) (n = 39), (b) absence of TZ lesion at MP MR imaging (n = 944), and (c) suboptimal or nondiagnostic MP MR imaging findings (due to hip prosthesis–related artifacts) (n = 0). Part of this population was reported in a prior publication, which aimed to evaluate targeted versus standard biopsy methods (11). Of the 457 patients included in the current study, 149 were included in the prior study. The current study differs from the prior study because it includes only the TZ lesions with corresponding PI-RADS version 2 scores.
A total of 634 TZ lesions in 457 patients (225 biopsy naïve patients, 127 patients with prior tumor-negative TR US-guided biopsy findings, 105 patients with prior tumor-positive TR US-guided biopsy findings) (Fig 1) were analyzed by a genitourinary radiologist (B.T., 9 years experience in prostate MR imaging [>1000 prostate MR images read per year]) using an in-house scoring system (16). Detected lesions were not prospectively categorized by using PI-RADS version 2 since the guidelines were not available at the time of the studies. Detected lesions were sampled for biopsy prospectively by using the fusion MR imaging and TR US–guided biopsy platform (UroNav; Philips Medical, Invivo, Gainesville, Fla), as previously described (17). In addition to standard 12-core systematic TR US biopsy, all lesions identified at MP MR imaging were targeted for fusion MR imaging and TR US–guided biopsy. Two targeted cores were obtained from each lesion in the axial and sagittal planes (two biopsy cores per lesion). Biopsies were performed by an experienced team of a urologist (P.A.P.) and an interventional radiologist (B.J.W.), each of whom had performed more than 1000 targeted prostate biopsies in the 5 years before study initiation (January 2012). Histopathologic evaluation of biopsy specimens was performed by a genitourinary pathologist (M.J.M.) with more than 25 years of experience in PCa pathology using the International Society of Urological Pathology–modified Gleason score classification.
Figure 1:
Study population flowchart. * Prior treatment included surgery, focal laser ablation, brachytherapy, and external-beam radiation therapy.
Image Acquisition Protocol and Data Analysis
Multiparametric MR image acquisition.—Preprocedural prostate MP MR images were acquired by using a 3-T imager (Achieva; Philips Healthcare, Best, the Netherlands) with an endorectal coil (BPX-30; Medrad, Pittsburgh, Pa) and a 16-channel phased-array surface coil (SENSE; Philips Healthcare), without prior bowel preparation. The balloon surrounding the endorectal coil was distended with 3 mol/L perfluorocarbon (Fluorinert; 3M, St Paul, Minn) to a volume of approximately 45 mL. MR imaging pulse sequences used included axial T1-weighted imaging, triplanar T2-weighted imaging, and diffusion-weighted (DW) imaging with a b value of 2000 sec/mm2. Apparent diffusion coefficient mapping was performed, and maps were derived from a separate DW MR imaging examination that used five evenly spaced b values ranging from 0 to 750 sec/mm2. In addition, axial three-dimensional fast field-echo dynamic contrast material–enhanced (DCE) images were acquired. DCE images were obtained before, during, and after one dose of gadopentetate dimeglumine (0.1 mmol per kilogram of body weight, Magnevist; Berlex, Wayne, NJ) was administered through a peripheral vein at a rate 3 mL/sec by using a mechanical injector (Spectris MR Injection System; Medrad, Pittsburgh, Pa). MR imaging protocol details with pulse sequence parameters are presented in Table 1.
Table 1:
Multiparametric MR Imaging Parameters
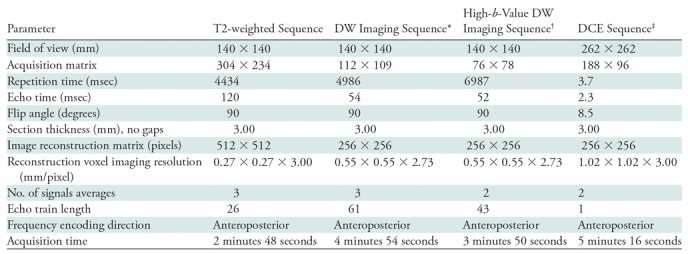
Note.—DCE = dynamic contrast material enhancement, DW = diffusion weighted.
*Five evenly spaced b values (0–750 sec/mm2) were used to calculate the apparent diffusion coefficient map.
†The b value was 2000 sec/mm2.
‡Temporal resolution for DCE MR imaging was 5.6 seconds.
Data analysis.—Over 1 year, two genitourinary radiology fellows (J.N.T., H.A.N.) were trained simultaneously in PI-RADS version 2 scoring, with independent readouts supervised by a senior genitourinary radiologist (B.T., 9 years of experience interpreting prostate MP MR images). All prospectively detected and biopsied TZ lesions were evaluated retrospectively by both readers using PI-RADS version 2 scoring in the same setting. All lesions were identified for the readers, and they were aware that the lesions had been biopsied. Interreader agreement was evaluated with κ analysis. Both readers were blinded to clinical and histopathologic information. Each lesion was scored by strictly following the PI-RADS version 2 criteria for TZ lesions and by using the dominant T2-weighted sequence. For indeterminate lesions that were scored as PI-RADS category 3, the DW sequence was used as a secondary sequence to determine the final PI-RADS category. A DW imaging score of 4 or less would result in a final similar score of PI-RADS category 3, while a DW imaging score of 5 would upgrade the score from PI-RADS category 3 to PI-RADS category 4. The discriminating factor between lesions with a DW imaging score of 4 and those with a score of 5 was lesion size (>15 mm) (18). Any discordance between readers was resolved by consensus. Any continued disagreement in scoring was resolved by the genitourinary radiologist, who made a final determination of PI-RADS version 2 score.
Proportion of cancer detection with each PI-RADS category was reported at per-lesion analysis by using the Gleason scoring system. For malignant lesions, clinically important PCa was defined as a Gleason score of 3+4 or greater. Benign lesions included those classified at histopathology as benign prostatic tissue, benign prostatic hyperplasia, fibromuscular tissue, atypical glandular tissue, or chronic prostatitis.
Further evaluation was performed in PI-RADS category 4 lesions that were initially scored as PI-RADS category 3 but were upgraded to category 4 based on DW imaging size criteria (termed PI-RADS category 3+1), as well as in lesions that were categorized as PI-RADS category 4 based on morphology alone (termed true PI-RADS category 4).
Statistical Analyses
Comparisons were made between detection of all PCas and clinically important PCas (treated as nominal) and PI-RADS version 2 score (ordinal) by using the χ2 test. When appropriate, χ2 analysis was used. In cases where cell counts were small in the contingency table, the Fisher exact test was used. All tests were performed by using two-tailed analysis. P ≤ .05 indicated a significant difference. The proportion of cancer detected was calculated by dividing the number of cancer diagnoses of interest (overall or clinically important cancer) by the number of lesions in the category being described.
In patients with multiple lesions, the pathology specific to the lesion was used; thus, the lesions were treated as independent entities. Calculations of sensitivity, specificity, positive and negative predictive value, and accuracy for MR imaging–visible PCa were obtained. SPSS software (version 21; IBM, Armonk, NY) was used for statistical analysis.
Results
A total of 457 patients with 634 lesions were included in this study; there was a mean of 1.45 TZ lesions per patient (286 patients had one lesion; 120, two lesions; 33, three lesions; 13, four lesions; 4, five lesions; one, eight lesions). The median age was 67 years (interquartile range, 62–71 years), and the median PSA level was 11.6 ng/mL (interquartile range, 5.1–11.5 ng/mL). Additional patient characteristics are presented in Table 2. Analysis of interobserver agreement showed the number of lesions with discordant scoring was small (21 of 634), and this was taken into account when calculating the κ statistics. The scoring of these 21 lesions was resolved either by consensus or with final assignment by the genitourinary radiologist, as described previously. Our analysis showed interobserver agreement with a κ value of 0.95 (95% confidence interval [CI]: 0.93, 0.97). Among the 634 lesions, 251 (39.6%) were found to be PCa, and 185 (29.2%) had clinically important PCa. Among the 25 PI-RADS category 2 lesions, there was one cancer (4.0%) (95% CI: 0.001, 0.2) (Fig E1 [online]). Among the 352 PI-RADS category 3 lesions, 78 (22.2%) were malignant, and clinically important PCa was present in 39 (11.1%) (Fig 2). Among the 110 PI-RADS category 4 lesions, 43 (39.1%) were malignant, and clinically important PCa was present in 32 (29.1%) (Fig 3). Among the 147 PI-RADS category 5 lesions, 129 (87.8%) were malignant, and clinically important cancer was present in 114 (77.6%) (Fig E2 [online]). Higher PI-RADS version 2 scores were associated with increasing likelihood of the presence of clinically important PCa (P = .001). A trend is shown in Table 3 in the overall cancer detection and clinically important cancer detection columns. For overall cancer detection, the trend shown from PI-RADS version 2 score of 2 through 5 was significant (P < .001). For clinically important cancer detection, the trend also was significant (P < .001). By using the PI-RADS version 2 cutoff of PI-RADS category 3 as the threshold of cancer, MR imaging–visible lesion sensitivity in the detection of PCa was 68.5% (95% CI: 0.62, 0.74) and specificity was 77.8% (95% CI: 0.73, 0.82). For clinically important PCa, MR imaging–visible lesion sensitivity was 78.9% (95% CI: 0.72, 0.84) and specificity was 75.3% (95% CI: 0.71, 0.79) (Table 4). Of note, these calculations pertain to MR imaging–visible lesions only, as there could have been PCa that was not visible on MP MR images and was therefore not included in this study. Since PI-RADS category 4 lesions include those that were initially scored as PI-RADS category 3 lesions based on their morphology but were upgraded to PI-RADS category 4 because they were 15 mm or larger according to DW imaging criteria, we further evaluated these lesions (termed PI-RADS 3+1). There were 91 PI-RADS category 4 lesions that were initially scored as PI-RADS category 3 but were upgraded to category 4 on the basis of DW imaging size criteria. Thirty-five of these lesions were found to be malignant, with overall and clinically important cancer detection rates of 38.5% (35 of 91) and 30.8% (28 of 91), respectively (Fig E3 [online]). In comparison, 19 lesions categorized as PI-RADS category 4 based on morphology alone (termed true PI-RADS category 4) had overall and clinically important proportions of cancer detection of 42.1% (eight of 19) and 21.1% (four of 19), respectively (Table 3). When PI-RADS 3+1 lesions were compared with PI-RADS category 3 lesions, differences in the proportion of cancer detection were significant (38.5% [35 of 91] and 22.2% [78 of 352], respectively, for overall cancer [P = .002]; 30.8% [28 of 91] and 11.1% [39 of 352], respectively, for clinically important cancer [P < .001]).
Table 2:
Patient Characteristics
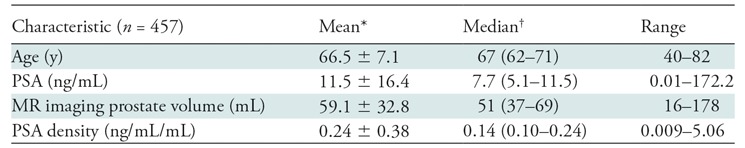
Note.—PSA = prostate-specific antigen.
*Data are mean ± standard deviation
†Data in parentheses are the interquartile range.
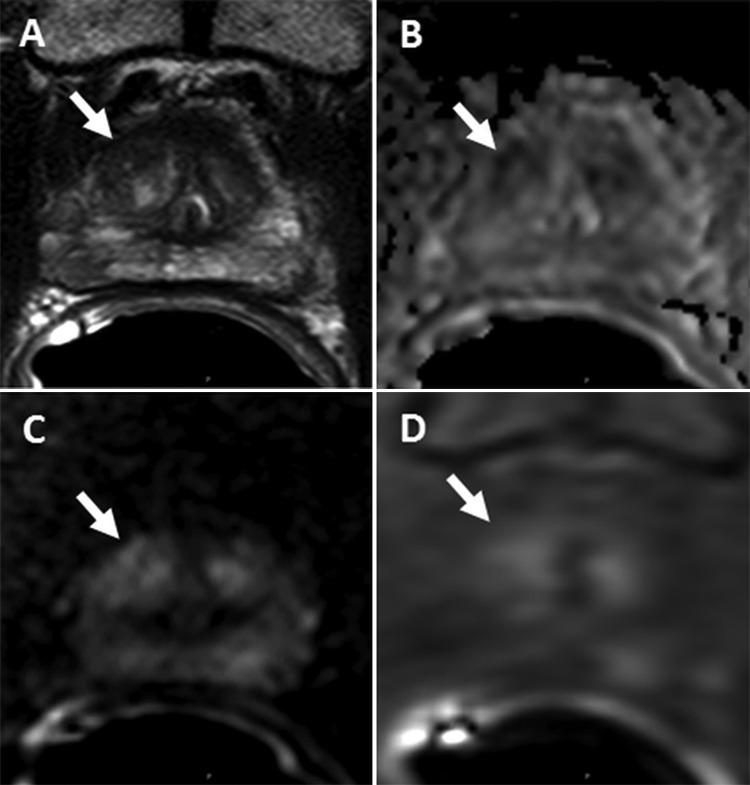
Figure 2:
Images of a lesion (arrow) in a 69-year-old man with a serum prostate-specific antigen level of 2.73 ng/mL. A, Axial T2-weighted MR image shows the 1-cm lesion in the right apical anterior transition zone. B, Apparent diffusion coefficient map shows slight diffusion restriction in this same lesion. C, Diffusion-weighted (DW) MR image (b = 2000 sec/mm2). D, Dynamic contrast-enhanced (DCE) MR image shows slight early enhancement. The lesion was assigned a Prostate Imaging and Reporting Data System (PI-RADS) score of 3 with T2-weighted, DCE, and DW MR imaging and had an overall PI-RADS score of 3. Fusion MR imaging and transrectal US–guided biopsy revealed Gleason 3+4 prostate cancer within this lesion.
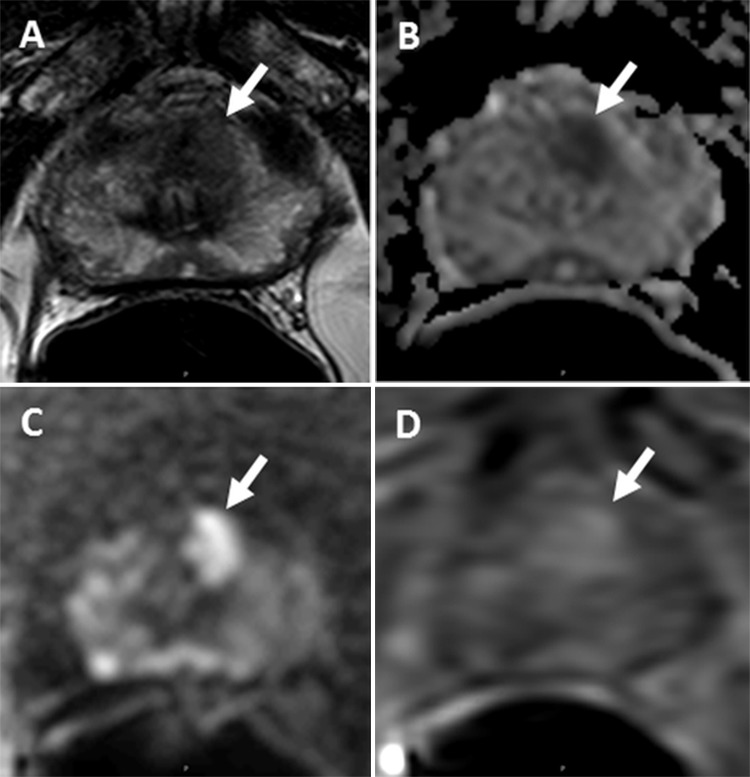
Figure 3:
Images of a lesion (arrow) in a 73-year-old man with a serum prostate-specific antigen level level of 4.08 ng/mL. A, Axial T2-weighted MR image shows the 1.1-cm lesion in the left apical anterior transition zone. B, Apparent diffusion coefficient map shows marked diffusion restriction in this same lesion. C, Diffusion-weighted (DW) MR image (b = 2000 sec/mm2). D, Dynamic contrast-enhanced (DCE) MR image shows early enhancement. The lesion was assigned a Prostate Imaging and Reporting Data System (PI-RADS) score of 4 with T2-weighted and DW MR imaging and had an overall PI-RADS score of 4. Fusion MR imaging and transrectal US–guided biopsy revealed Gleason 3+3 prostate cancer within this lesion.
Table 3:
PI-RADS Version 2 Scoring of Transition Zone Lesions and Proportion of Cancer Detection
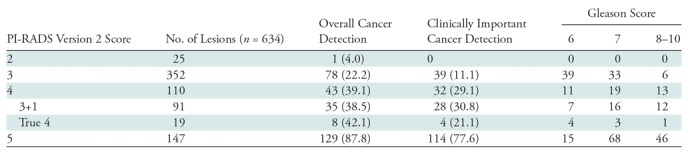
Note.—Data are the number of lesions, and data in parentheses are percentages. PI-RADS = Prostate Imaging and Reporting Data System.
Table 4:
Diagnostic Performance of PI-RADS Version 2 for MR Imaging–visible Prostate Cancer Detection in the Transition Zone
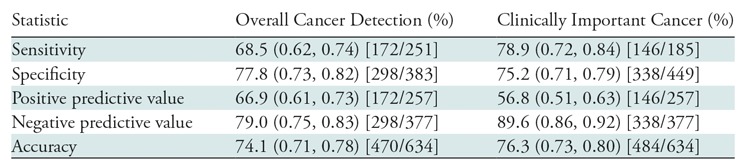
Note.—Data in parentheses are the 95% confidence interval. Data in brackets are the raw data used to calculate the percentages. PI-RADS = Prostate Imaging and Reporting Data System.
Discussion
PI-RADS version 2 introduced guidelines and a new scoring system with the goal of improving detection and characterization of clinically important cancers. In our study, we found higher PI-RADS version 2 scores were associated with increasing likelihood of the presence of clinically important PCa. Specifically, for PI-RADS category 2 lesions, we identified no clinically important cancer at targeted biopsy. For PI-RADS category 3, 4, and 5 lesions, the proportion of clinically important cancer was 11.1% (39 of 352), 29.1% (32 of 110), and 77.6% (114 of 147), respectively. An interesting finding is that specificity and positive predictive value are higher for overall cancer detection than for detection of clinically important cancer. This occurs because clinically important cancer is a subset of overall cancer; thus, there will be some positive test results for low-grade cancer in the overall cancer category that will contribute to the predictive value of overall cancer but not to the predictive value of clinically important cancer.
We also found that the sensitivity of MP MR imaging in the detection of clinically important PCa (78.9%) was higher than that in the detection of overall PCa (68.5%). Sensitivity is a measure of the rate at which a test is positive in a patient with the condition. Thus, for overall cancer detection rate, sensitivity is the rate at which the findings were positive in all patients with cancer. For clinically important PCa, the measure is among men with clinically important PCa only. The fact that sensitivity was higher in this cohort reflects that among men with clinically important PCa, the test results are more consistently correct. In the larger cohort of men with any cancer diagnosis, the test is not correct as consistently as it is in the subgroup of men with clinically important PCa. Additionally, when PI-RADS 3+1 lesions were compared with PI-RADS category 3 lesions, the differences were significant (P = .002 for overall cancer detection rate, P < .001 for clinically important cancer detection rate). This finding suggests that a lesion upgrade from PI-RADS category 3 to PI-RADS category 4 on the basis of DW imaging size criteria is appropriate in TZ lesions.
Current literature on the diagnostic performance of PI-RADS version 2 is sparse, especially with regard to TZ lesions, mainly because of the low prevalence of PCa in this zonal area. In comparison with our results, Muller et al (19) reported PI-RADS version 2 had higher sensitivity and lower specificity (85% and 55%, respectively) in the detection of PCa in the TZ, with a threshold score of PI-RADS category 3, in a study with fusion US and MR imaging–guided biopsy as a reference standard for 20 TZ PCa lesions. In a study with cognitively targeted MR–guided biopsy as the reference standard, Feng et al (20) determined the sensitivity and specificity of PCa in the TZ to be 96% and 90%, respectively, with a cutoff score of 4. In a similar study with MR imaging–guided in-bore biopsy as the reference standard, Tewes et al (21) showed that with PI-RADS version 2, the best diagnostic accuracy in the TZ was reached with a cutoff score of 4 at a sensitivity of 80% and a specificity 100% for all PCa. Polanec et al (22) evaluated PI-RADS version 2 scoring by two readers in a patient population that underwent MR imaging–guided prostate biopsy. They demonstrated sensitivity was 87.5%–100% and specificity was 50%–56.3% for all TZ PCas. With increasing experience with prostate MP MR imaging, general morphologic features associated with malignant and benign pathologic findings have been refined. However, the specific criteria recommended for TZ lesion evaluation have not been separately validated. Our study provides an early validation of the PI-RADS version 2 scoring system with pathologic correlation to confirm benignity of PI-RADS category 2 TZ lesions, which are essentially benign prostatic hyperplasia. The PI-RADS version 2 system evaluated in our study was an accurate scoring system used to diagnose clinically important PCa.
The limitations of this study include a retrospective design. Our validation method was fusion MR imaging and TR US–guided biopsy but not radical prostatectomy specimens. As such, only lesions visible on MR images were biopsied. There could potentially be PCas that were not visualized on MR images. Thus, these possibly missed cancers were not included in our analysis. While this stands as a limitation, having a targeted biopsy population has allowed us to have a less high-risk–biased and a more diverse tumor-bearing patient population. The limitations of fusion MR imaging and TR US–guided targeted needle core biopsy as compared with collection of whole-mount prostatectomy specimens include inherent biopsy sampling error, undersampling, and pathologic undergrading of disease due to tumor heterogeneity, especially in larger lesions where only two cores were obtained per biopsy and there was a small amount of needle biopsy tissue with minimal prostatic carcinoma. Our κ analysis yielded a κ value of 0.95, which differed from that of prior publications. This can be possibly explained by the retrospective nature of PI-RADS categorization. All lesions were identified for the readers, and they were aware that the lesions were biopsied. An additional limitation was the number of true PI-RADS category 4 lesions included in the analysis was comparatively low. This study had a relatively small patient population, since the prevalence of PCa in the TZ was limited. Finally, all lesions were treated as if they were independent with regard to PI-RADS version 2 score and ultimate cancer detection. This assumption may not account for the possibility that lesions from the same MR imaging examination may not be independent and that some unmeasured relationship between lesions measured in the same MR imaging examination would ultimately have affected the overall outcome and generalizability of this study. However, we are not aware of any such proven issue with the assumption of lesion independence, and we chose this analysis as we felt it provided the greatest insight into the relationship of lesion suspicion score with pathology findings.
In conclusion, higher PI-RADS version 2 scores are associated with a higher proportion of clinically important cancers in the TZ. PI-RADS category 2 TZ lesions rarely yield PCa, and their presence does not justify biopsy. Our findings reassure us as to the validity of the PI-RADS version 2 system in the TZ. Further improvement and refinement can be made with large-scale prospective analyses.
Summary
PI-RADS 2 transition zone lesions rarely yield prostate cancer, and their presence does not justify biopsy.
Implications for Patient Care
■ The Prostate Imaging and Reporting Data System (PI-RADS) version 2 scoring system can be used to identify clinically important prostate cancers and benign lesions in the transition zone.
■ With PI-RADS version 2 scoring, prostate multiparametric MR imaging can be used to reliably detect clinically important cancers in the transition zone with high diagnostic accuracy.
SUPPLEMENTAL FIGURES
Received February 20, 2017; revision requested April 17; revision received February 6, 2018; accepted February 9, 2018.
Supported by the Intramural Research Program of the National Institutes of Health, the NIH Clinical Center (ZID BC011242-08), and the National Cancer Institute.
Disclosures of Conflicts of Interest: J.N.T. disclosed no relevant relationships. H.A.N. disclosed no relevant relationships. A.K.G. disclosed no relevant relationships. M.M.S. disclosed no relevant relationships. P.S. disclosed no relevant relationships. F.V.M. disclosed no relevant relationships. M.J.M. disclosed no relevant relationships. P.A.P. disclosed no relevant relationships. P.L.C. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: holds patents for the UroNav biopsy system and for computer-aided detection systems; receives royalties for the UroNav and computer-aided detection systems. Other relationships: disclosed no relevant relationships. B.J.W. Activities related to the present article: has a Cooperative Research and Development Agreement with Philips Healthcare and Philips Invivo. Activities not related to the present article: disclosed no relevant relationships. Other relationships: disclosed no relevant relationships. B.T. disclosed no relevant relationships.
Abbreviations:
- CI
- confidence interval
- DCE
- dynamic contrast material enhanced
- DW
- diffusion weighted
- MP
- multiparametric
- PCa
- prostate cancer
- PI-RADS
- Prostate Imaging and Reporting Data System
- PSA
- prostate-specific antigen
- TR
- transrectal
- TZ
- transition zone
References
- 1.Murphy G, Haider M, Ghai S, Sreeharsha B. The expanding role of MRI in prostate cancer. AJR Am J Roentgenol 2013;201(6):1229–1238. [DOI] [PubMed] [Google Scholar]
- 2.Turkbey B, Pinto PA, Mani H, et al. Prostate cancer: value of multiparametric MR imaging at 3 T for detection—histopathologic correlation. Radiology 2010;255(1):89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frye TP, George AK, Kilchevsky A, et al. Magnetic resonance imaging: transrectal ultrasound guided fusion biopsy to detect progression in patients with existing lesions on active surveillance for low and intermediate risk prostate cancer. J Urol 2017;197(3 Pt 1):640–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walton Diaz A, Shakir NA, George AK, et al. Use of serial multiparametric magnetic resonance imaging in the management of patients with prostate cancer on active surveillance. Urol Oncol 2015;33(5):202.e1–202.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carroll PR, Parsons JK, Andriole G, et al. NCCN guidelines insights: prostate cancer early detection, version 2. 2016. J Natl Compr Canc Netw 2016;14(5):509–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watson MJ, George AK, Maruf M, et al. Risk stratification of prostate cancer: integrating multiparametric MRI, nomograms and biomarkers. Future Oncol 2016;12(21):2417–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kongnyuy M, Sidana A, George AK, et al. Tumor contact with prostate capsule on magnetic resonance imaging: a potential biomarker for staging and prognosis. Urol Oncol 2017;35(1):30.e1–30.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinto PA, Chung PH, Rastinehad AR, et al. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J Urol 2011;186(4):1281–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siddiqui MM, Rais-Bahrami S, Truong H, et al. Magnetic resonance imaging/ultrasound-fusion biopsy significantly upgrades prostate cancer versus systematic 12-core transrectal ultrasound biopsy. Eur Urol 2013;64(5):713–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Filson CP, Natarajan S, Margolis DJ, et al. Prostate cancer detection with magnetic resonance-ultrasound fusion biopsy: the role of systematic and targeted biopsies. Cancer 2016;122(6):884–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siddiqui MM, Rais-Bahrami S, Turkbey B, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA 2015;313(4):390–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinreb JC, Barentsz JO, Choyke PL, et al. PI-RADS prostate imaging: reporting and data system—2015, version 2. Eur Urol 2016;69(1):16–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greer MD, Brown AM, Shih JH, et al. Accuracy and agreement of PIRADSv2 for prostate cancer mpMRI: a multireader study. J Magn Reson Imaging 2017;45(2):579–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mertan FV, Greer MD, Shih JH, et al. Prospective evaluation of the prostate imaging reporting and data system version 2 for prostate cancer detection. J Urol 2016;196(3):690–696. [DOI] [PubMed] [Google Scholar]
- 15.Vargas HA, Hötker AM, Goldman DA, et al. Updated prostate imaging reporting and data system (PIRADS v2) recommendations for the detection of clinically significant prostate cancer using multiparametric MRI: critical evaluation using whole-mount pathology as standard of reference. Eur Radiol 2016;26(6):1606–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rais-Bahrami S, Siddiqui MM, Turkbey B, et al. Utility of multiparametric magnetic resonance imaging suspicion levels for detecting prostate cancer. J Urol 2013;190(5):1721–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muthigi A, George AK, Sidana A, et al. Missing the mark: prostate cancer upgrading by systematic biopsy over magnetic resonance imaging/transrectal ultrasound fusion biopsy. J Urol 2017;197(2):327–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barentsz JO, Weinreb JC, Verma S, et al. Synopsis of the PI-RADS v2 guidelines for multiparametric prostate magnetic resonance imaging and recommendations for use. Eur Urol 2016;69(1):41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muller BG, Shih JH, Sankineni S, et al. Prostate cancer: interobserver agreement and accuracy with the revised prostate imaging reporting and data system at multiparametric MR imaging. Radiology 2015;277(3):741–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng ZY, Wang L, Min XD, Wang SG, Wang GP, Cai J. Prostate cancer detection with multiparametric magnetic resonance imaging: prostate imaging reporting and data system version 1 versus version 2. Chin Med J (Engl) 2016;129(20):2451–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tewes S, Mokov N, Hartung D, et al. Standardized reporting of prostate MRI: comparison of the prostate imaging reporting and data system (PI-RADS) version 1 and version 2. PLoS One 2016;11(9):e0162879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Polanec S, Helbich TH, Bickel H, et al. Head-to-head comparison of PI-RADS v2 and PI-RADS v1. Eur J Radiol 2016;85(6):1125–1131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.