Abstract
Background:
The role of tobacco smoke exposure in the development and persistence of asthma and rhinoconjunctivitis through childhood into adolescence is unclear.
Objectives:
We assessed the associations of parental smoking from fetal life through adolescence with asthma and rhinoconjunctivitis during childhood and adolescence.
Methods:
We analyzed data for 10,860 participants of five European birth cohort studies from the Mechanisms of the Development of Allergy (MeDALL) consortium. Parental smoking habits and health outcomes (early transient, persistent, and adolescent-onset asthma and rhinoconjunctivitis) were based on questionnaires covering the period from pregnancy to 14–16 y of age. Data were combined and analyzed using a one-stage and two-stage individual participant data meta-analysis.
Results:
Overall, any maternal smoking during pregnancy tended to be associated with an increased odds of prevalent asthma [adjusted (95% CI: 0.98, 1.43)], but not prevalent rhinoconjunctivitis [ (95% CI: 0.90, 1.22)], during childhood and adolescence. In analyses with phenotypes related to age of onset and persistence of disease, any maternal smoking during pregnancy was associated with early transient asthma [ (95% CI: 1.14, 2.83)]. Maternal smoking of during pregnancy was associated with persistent asthma [ (95% CI: 1.29, 2.15)] and persistent rhinoconjunctivitis [ (95% CI, 1.09, 2.20)]. Tobacco smoke exposure during fetal life, infancy, childhood, and adolescence was not associated with adolescent-onset asthma or rhinoconjunctivitis.
Conclusions:
Findings from this combined analysis of five European birth cohorts strengthen evidence linking early exposure to tobacco smoke with asthma during childhood and adolescence. Children with high early-life exposure were more likely than unexposed children to have early transient and persistent asthma and persistent rhinoconjunctivitis. https://doi.org/10.1289/EHP2738
Introduction
The prevalence of asthma, rhinoconjunctivitis, and other allergic diseases has increased markedly in the past decades (Asher et al. 2006; Patil et al. 2015). Globally, the prevalence of maternal smoking during pregnancy varies from to (Cheraghi and Salvi 2009). The role of tobacco smoke exposure and other lifestyle factors in the epidemic remain unclear (Frazer et al. 2016). Prenatal and perinatal events are fundamental in health, development of diseases, and aging (Bousquet et al. 2015). In high-income countries, the number of pregnant women who smoke is waning, but in low-income countries, this number is on the rise (Murin et al. 2011). Neonates and children have little control over their environment and are vulnerable to the harmful effects of tobacco smoke. Exposure early in life, starting from exposure to maternal smoking during pregnancy, often continues into early childhood (Murin et al. 2011). Maternal smoking during pregnancy and secondhand tobacco smoke (SHS) exposure in infancy have been associated with an increased risk of developing asthma and rhinitis in young children, and a dose–response relationship has been suggested (Burke et al. 2012; Grabenhenrich et al. 2014; Neuman et al. 2012; Thacher et al. 2014; Vardavas et al. 2016). However, few prospective studies have investigated these risks among children of age (Grabenhenrich et al. 2014), and even less is known about the association between SHS and other allergic diseases such as rhinoconjunctivitis (Civelek et al. 2010; Thacher et al. 2014). Moreover, it is unclear if tobacco smoke exposure during the fetal period, in infancy, or in childhood influences the onset, progression, or persistence of allergic disease up to adolescence. Asthma and rhinoconjunctivitis are both heterogeneous diseases with many phenotypes suggested to have different etiologies (Ballardini et al. 2015; Bousquet et al. 2011). Rhinoconjunctivitis tends to debut somewhat later than asthma, and it is unclear if exposure to maternal smoking during pregnancy or SHS during early life influences age at onset or the persistence of asthma or rhinoconjunctivitis up to adolescence. The associations between tobacco smoke exposure and characterized phenotypes of asthma and rhinoconjunctivitis, which extend into adolescence, have yet to be elucidated.
Long-term birth cohort studies are essential to understanding the life course and childhood predictors of allergy-related diseases (Bousquet et al. 2013). However, single cohorts are often underpowered to provide robust effect estimates. Therefore, within the MeDALL (Mechanisms of the Development of Allergy) project, we attempted to better understand the complex associations between tobacco smoke exposure and asthma as well as rhinoconjunctivitis by pooling ongoing European birth cohorts (Bousquet et al. 2011, 2016).
As part of MeDALL, our aim was to examine the association of exposure to parental smoking during fetal life, infancy, childhood, and adolescence with the onset, progression, and persistence of asthma and rhinoconjunctivitis during childhood and adolescence using data from five ongoing European birth cohorts.
Methods
Study Design and Participants
This study is based on data from five ongoing European birth cohorts in three different countries participating in MeDALL. Of the cohorts included, we selected those with a) relevant information on maternal smoking during pregnancy or SHS exposure during the first year of life; b) follow-up between 14 and 16 y of age; and c) suitable outcome assessments from three predefined age intervals (4–6, 8–10, and 14–16 y).
The following five cohorts were included: Children, Allergy, Milieu, Stockholm, Epidemiology (BAMSE) (single-center, Sweden) (Wickman et al. 2002), German Infant Nutritional Intervention (GINIplus) (multicenter, Germany) (Berg et al. 2010), Influences of Lifestyle-Related Factors on the Immune System and the Development of Allergies in Childhood (LISAplus) (multicenter, Germany) (Zutavern et al. 2008), Multicentre Allergy Study (MAS) (multicenter, Germany) (Bergmann et al. 1994), and Prevention and Incidence of Asthma and Mite Allergy (PIAMA) (multicenter, the Netherlands) (Wijga et al. 2014). BAMSE and MAS were performed in metropolitan areas, and GINIplus, LISAplus, and PIAMA included children from both urban and mixed urban–rural areas. PIAMA and GINIplus included children from both intervention and observational study arms, whereas all other cohorts were purely observational. By design, MAS was risk-enriched, meaning that the cohort purposely included more children with higher allergy risk. Additionally, in GINIplus, LISAplus, and MAS, preterm children ( gestation) were excluded by study design. The cohorts recruited newborns between 1990 (MAS) and 1999 (LISAplus), with sample sizes varying from 1,314 (MAS) to 5,991 (GINIplus) participants (Table 1). Further details on study design, recruitment, and data collection for each cohort are provided in the supplemental material and elsewhere (Berg et al. 2010; Bergmann et al. 1994; Wickman et al. 2002; Wijga et al. 2014; Zutavern et al. 2008). In all cohorts, the information on participants’ health and exposure used in this analysis was assessed through repeated questionnaires completed by the parents and children (at 14–16 y of age). Data and variables were harmonized according to predefined exposure and outcome definitions (Hohmann et al. 2014).
Table 1.
Participant characteristics from five European birth cohorts.
| Characteristic | BAMSE | GINIplus | LISAplus | MAS | PIAMA |
|---|---|---|---|---|---|
| Country | Sweden | Germany | Germany | Germany | The Netherlands |
| Years of recruitment | 1994–1996 | 1995–1998 | 1997–1999 | 1990 | 1996–1997 |
| Number of children at recruitment | 4,089 | 5,991 | 3,094 | 1,314 | 3,963 |
| Number of children included in final study population, n (%) | 3,112 (76.1) | 2,956 (49.3) | 1,456 (47.1) | 560 (42.6) | 2,519 (63.6) |
| Number of children with complete information on all selected covariatesa, n (%) | 2,928 (71.6) | 2,254 (37.6) | 733 (23.7) | 427 (32.5) | 2,423 (61.1) |
| Maternal smoking during pregnancy, n (%) | |||||
| Yes | 379 (12.2) | 352 (12.0) | 183 (13.0) | 106 (20.1) | 362 (14.5) |
| No | 2,732 (87.8) | 2,589 (88.0) | 1,229 (87.0) | 421 (79.9) | 2,140 (85.5) |
| Missing (n) | 1 | 15 | 44 | 33 | 17 |
| SHS during infancy, n (%) | |||||
| Yes | 631 (20.4) | 289 (17.2) | 215 (15.6) | 224 (42.9) | 536 (21.4) |
| No | 2,463 (79.6) | 1,396 (82.8) | 1,161 (84.4) | 298 (57.1) | 1974 (78.6) |
| Missing (n) | 18 | 1,271 | 80 | 38 | 9 |
| Male sex, n (%) | |||||
| Male | 1,530 (49.2) | 1,468 (49.7) | 744 (51.1) | 292 (52.1) | 1,267 (50.3) |
| Female | 1,582 (50.8) | 1,488 (50.3) | 712 (48.9) | 268 (47.9) | 1,252 (49.7) |
| Missing (n) | 0 | 0 | 0 | 0 | 0 |
| Mean birth weight (g), | |||||
| Missing (n) | 29 | 96 | 0 | 3 | 8 |
| Mean gestation age (wk), | |||||
| Missing (n) | 0 | 366 | 24 | 13 | 5 |
| Parental education, n (%) | |||||
| Low | 514 (16.5) | 231 (8.2) | 49 (3.4) | 38 (7.5) | 263 (10.5) |
| Medium | 884 (28.4) | 793 (28.1) | 352 (24.4) | 174 (34.7) | 866 (34.5) |
| High | 1,710 (55.1) | 1,802 (63.7) | 1,044 (72.2) | 290 (57.8) | 1,384 (55.0) |
| Missing (n) | 4 | 130 | 11 | 58 | 6 |
| Parental allergyb, n (%) | |||||
| Yes | 944 (30.6) | 1,303 (46.2) | 713 (52.3) | 270 (50.3) | 1,064 (42.7) |
| No | 2,138 (69.4) | 1,520 (53.8) | 651 (47.7) | 267 (49.7) | 1,427 (57.3) |
| Missing (n) | 30 | 133 | 92 | 23 | 28 |
| Older siblings, n (%) | |||||
| Yes | 1,472 (47.3) | 1469 (50.3) | 652 (44.8) | 219 (39.1) | 1234 (49.0) |
| No | 1640 (52.7) | 1450 (49.7) | 803 (55.2) | 341 (60.9) | 1285 (51.0) |
| Missing (n) | 0 | 37 | 1 | 0 | 0 |
| Breastfeeding, n (%) | |||||
| 2442 (80.3) | 2031 (70.9) | 1138 (82.7) | 338 (60.4) | 990 (39.6) | |
| 600 (19.7) | 835 (29.1) | 238 (17.3) | 222 (39.6) | 1508 (60.4) | |
| Missing (n) | 70 | 90 | 80 | 0 | 21 |
| Early mold or dampness in dwelling (0–2 y), n (%) | |||||
| Yes | 785 (25.3) | 447 (26.1) | 559 (39.0) | 74 (14.2) | 1049 (45.8) |
| No | 2319 (74.7) | 1264 (73.9) | 875 (61.0) | 444 (85.8) | 1242 (54.2) |
| Missing (n) | 8 | 17 | 22 | 42 | 228 |
| Early day care (0–2 y), n (%) | |||||
| Yes | 2548 (84.3) | 126 (4.7) | 384 (44.6) | 145 (29.0) | 1,472 (59.2) |
| No | 476 (15.7) | 2,586 (95.3) | 478 (55.4) | 355 (71.0) | 1,013 (40.8) |
| Missing (n) | 88 | 244 | 594 | 60 | 34 |
Note: Based on children with information about smoke exposure during pregnancy or infancy and at least one health outcome at 14–16 y of age. BAMSE, Children, Allergy, Milieu, Stockholm, Epidemiology; GINIplus, German Infant Nutritional Intervention; LISAplus, Influences of Lifestyle-Related Factors on the Immune System and the Development of Allergies in Childhood; MAS, Multicentre Allergy Study; PIAMA, Prevention and Incidence of Asthma and Mite Allergy; SD, standard deviation; SHS, secondhand smoke.
Participants in the final study population with complete information on the following covariates: maternal smoking during pregnancy, SHS exposure during infancy, sex, birth weight, gestational age, parental education, parental allergy, older siblings, breastfeeding, early mold or dampness in dwelling, and early day-care attendance.
Mother and/or father with asthma and/or hay fever.
In each cohort, parents or legal guardians gave written informed consent, and ethical approval was obtained from local review boards.
Procedures
Maternal smoking during pregnancy was defined as the mother smoking during any trimester of pregnancy.
SHS exposure during infancy was defined as the mother, father, and/or other persons in the home smoking at any time during the infant’s first year of life. For GINIplus, only maternal smoking habits were queried for this time period, and only for participants of the intervention study arm (see Table S1). In BAMSE, parental smoking was queried regardless of location, and in the other cohorts (GINIplus, LISAplus, MAS, and PIAMA), parental smoking in the dwelling was queried.
SHS exposure during childhood and adolescence was defined as the mother, father, and/or other persons in the home smoking. This exposure was divided into four time intervals: 1–2, 4–6, 8–10, and 14–16 y of age.
Health outcomes were determined from symptom-based questionnaires. Available data were pooled for three age intervals: 4–6 y, 8–10 y, and 14–16 y. These age ranges were chosen to include data from as many cohorts as possible. Parental responses were used to assess disease outcomes at 4–6 and 8–10 y of age, and at 14–16 y of age, a combination of parental and children’s responses (symptom-related) were incorporated for PIAMA, GINIplus, LISAplus, and BAMSE; for MAS, only parental responses were used because children’s answers were unavailable (see Table S2; see Table S3 for an outline of the follow-ups and corresponding outcome assessment intervals in the different cohorts). In cohorts with multiple follow-ups for a specific age period, the age with the most complete information was used.
Asthma was defined as fulfilling at least two of the following three criteria: a) doctor-diagnosed asthma ever; b) asthma medication in the past 12 mo; and c) wheezing in the past 12 mo (see Table S2) (Gehring et al. 2015).
Rhinoconjunctivitis was defined as positive if the following criteria were fulfilled based on reports for the previous 12 mo: a) problems with sneezing, or a runny, or blocked nose when the child did not have a cold or flu; and b) nose problem accompanied by itchy-watery eyes (see Table S2) (Gehring et al. 2015).
The definitions of asthma and rhinoconjunctivitis were agreed upon by a panel of experts within the MeDALL collaboration (Gehring et al. 2015).
We categorized asthma and rhinoconjunctivitis into three distinct phenotypes: early transient, persistent, and adolescent-onset disease. Early transient disease was defined as having the disease only during the 4–6 y age interval, but not thereafter (age intervals 8–10 and 14–16 y). Persistent disease was defined as having the disease of interest at the first age interval (4–6 y) and still having the disease at the latest age interval (14–16 y). Finally, the adolescent-onset phenotype was defined as being disease-free at the first two age intervals (4–6 y and 8–10 y) and only having the disease at the latest age interval (14–16 y).
Potential confounders and modifiers of the associations of asthma and rhinoconjunctivitis with tobacco smoke exposure were considered. Based on a priori knowledge, we adjusted all models for child’s sex (male, female); parental allergic history (any maternal or paternal history of asthma or hay fever, yes or no); parental education level, based on the highest level of education for either parent (low: primary school, lower vocational, or lower secondary education; intermediate: intermediate vocational or intermediate/higher secondary education; high: higher vocational education or university degree); older siblings ( older sibling at birth, yes or no); study center for multicenter studies (GINIplus: Munich, Bavaria, Wesel, or North–Rhine–Westphalia; LISAplus: Munich, Leipzig, Wesel, or Bad Honnef; and PIAMA: North—Groningen, Friesland, Drenthe; Central—Utrecht, Gelderland; West—Rotterdam and surrounding municipalities), and intervention versus observational study arms (for GINIplus and PIAMA) (Filipiak et al. 2007; Gehring et al. 2012). We also considered additional covariates, including cohort, birth weight (g), gestational age at birth (wk), breastfeeding (exclusive breastfeeding for , or any breastfeeding for PIAMA participants, yes or no), mold or dampness in the dwelling (signs of mold or dampness in the home at 0, 1, or 2 y of age, yes or no), and day-care attendance (any nursery or day care with other children at 0, 1, or 2 y of age, yes or no). Final models included the a priori covariates plus breastfeeding and early day-care attendance, which were selected based on a change in the odds ratio (OR) with adjustment. In addition, some models of maternal smoking during pregnancy were adjusted for SHS throughout childhood (exposure to SHS at any interval between 1 and 2, 4 and 6, 8 and 10, or 14 and 16 y of age), and some models of SHS during childhood were adjusted for maternal smoking during pregnancy.
Statistical analysis
Data were combined and analyzed using a one-stage and two-stage individual participant data meta-analysis (IPD-MA) (Debray et al. 2013). In these analyses, the overall associations of maternal smoking during pregnancy and SHS during infancy with asthma and rhinoconjunctivitis prevalence until 16 y of age were assessed in each cohort separately using generalized estimating equation (GEE) models, taking into account correlation between repeated observations within subjects. Adjusted cohort-specific odds ratios (ORs) and 95% confidence intervals (CIs) were then combined using inverse variance weighted random effects models, taking into account possible nonrandom variability within and between cohorts. Forest plots were produced with sizes of squares representing the weights of the individual cohort estimates. The statistic was used to evaluate heterogeneity between the cohorts (Higgins and Thompson 2002).
Because we had access to primary data and little heterogeneity of associations was observed between the cohorts, subsequent analyses were conducted using a one-stage IPD-MA model with indicator variables for cohort/study center, which provided additional power to assess asthma and rhinoconjunctivitis phenotypes. Multinomial logistic regression was used to examine associations between exposure to maternal smoking during pregnancy (yes/no and three categories as described above) and early transient, persistent, and adolescent-onset phenotypes, adjusting for the same confounders as in cohort-specific analyses. To assess dose–response relationships between the number of cigarettes smoked and health outcomes, the number of cigarettes smoked during pregnancy (mother) was categorized as follows: a) no smoking during pregnancy (reference category); b) 1–9 cigarettes/day; and c) during any trimester. Potential effect modification by parental allergy and sex were tested using stratified and interaction models. We tested for multiplicative interaction by including product terms for tobacco smoke exposures and sex or parental allergy in multivariable regression analyses. Because asthma and rhinoconjunctivitis often coexist, we also examined the association of tobacco smoke exposure with asthma only, rhinoconjunctivitis only, and concurrent asthma and rhinoconjunctivitis. Each analysis was limited to participants with complete data on the exposure, outcome, and covariates included in that analysis.
All statistical analyses were performed with STATA statistical software (release 12; StataCorp LLC).
Results
Study Participants
The final study population consisted of 10,603 participants for whom information was available on tobacco smoke exposure during pregnancy, infancy, or both, and on at least one health outcome in the age interval 14–16 y (57% of the 18,454 children at recruitment). The distributions of environmental exposures and potential confounders in the study population are given in Table 1 [see Table S4 for the distributions for the entire (baseline) cohort].
The prevalence of maternal smoking during pregnancy ranged from 12–20%, and SHS exposure during infancy ranged from 16–43% (see Figure S1). The prevalence of parental smoking was relatively similar across the cohorts, with the exception of the MAS cohort, which had the highest prevalence.
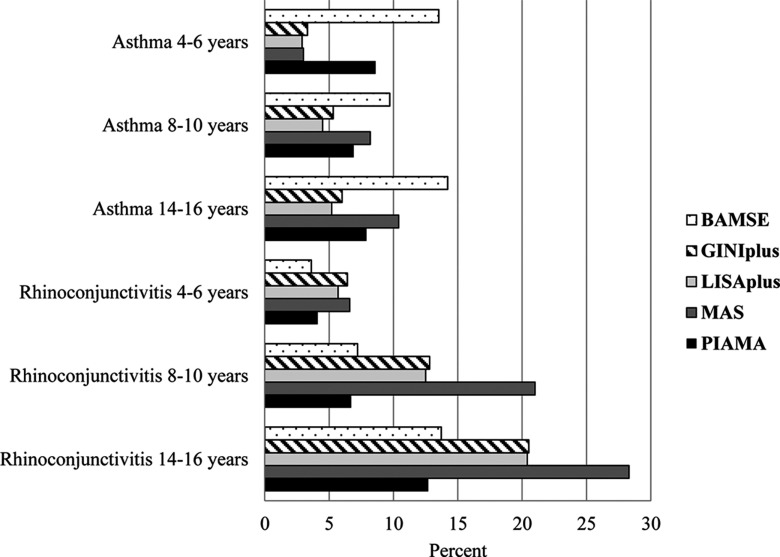
The prevalence of asthma varied across cohorts (Figure 1). At all ages, the prevalence was highest in BAMSE. In GINIplus, LISAplus, and MAS the prevalence nearly doubled from preschool age to primary school age. The prevalence of rhinoconjunctivitis increased with age in all cohorts, and higher prevalences were observed in GINIplus, LISAplus, and MAS than in BAMSE and PIAMA (Figure 1).
Figure 1.
Prevalence of asthma and rhinoconjunctivitis in five European birth cohorts. Note: BAMSE, Children, Allergy, Milieu, Stockholm, Epidemiology; GINIplus, German Infant Nutritional Intervention; LISAplus, Influences of Lifestyle-Related Factors on the Immune System and the Development of Allergies in Childhood; MAS, Multicentre Allergy Study; PIAMA, Prevention and Incidence of Asthma and Mite Allergy.
The prevalences of different asthma phenotypes were comparable (3.1% for early transient, 4.0% for persistent, and 4.3% for adolescent-onset asthma), whereas the prevalence of rhinoconjunctivitis was highest for adolescent-onset (12.2%) compared with early transient (1.6%) and persistent rhinoconjunctivitis (3.5%) (see Figure S2).
Tobacco Smoke Exposure and Prevalence of Asthma and Rhinoconjunctivitis
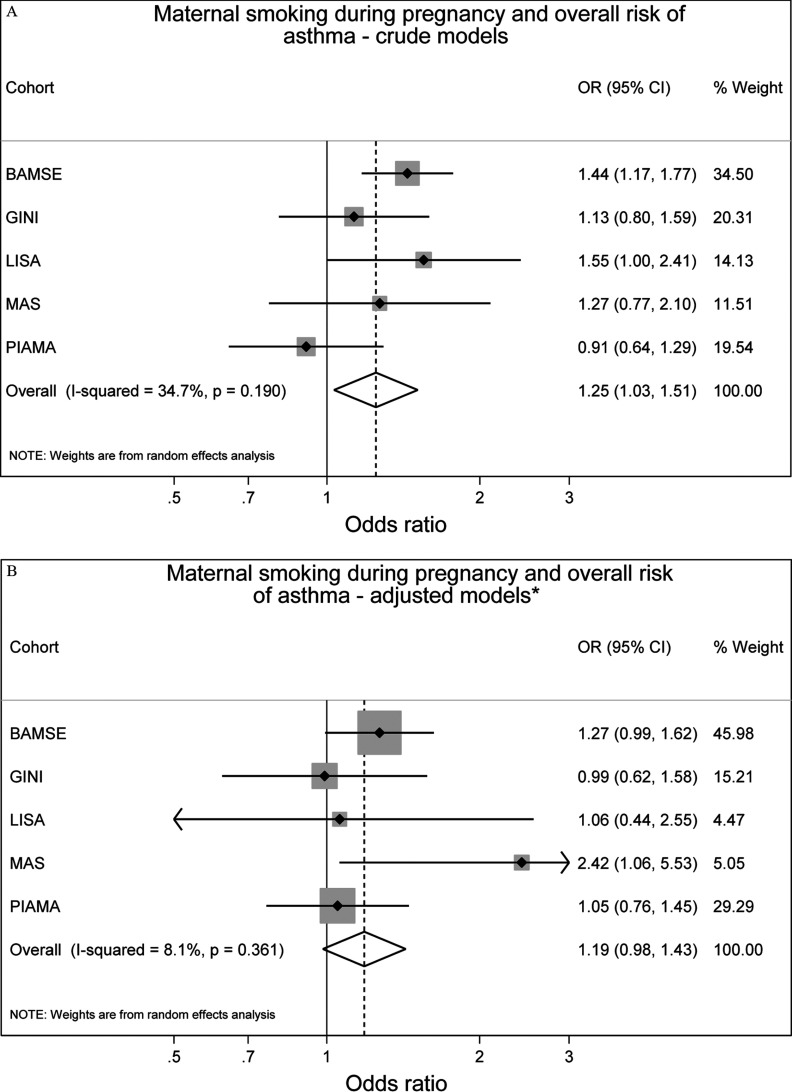
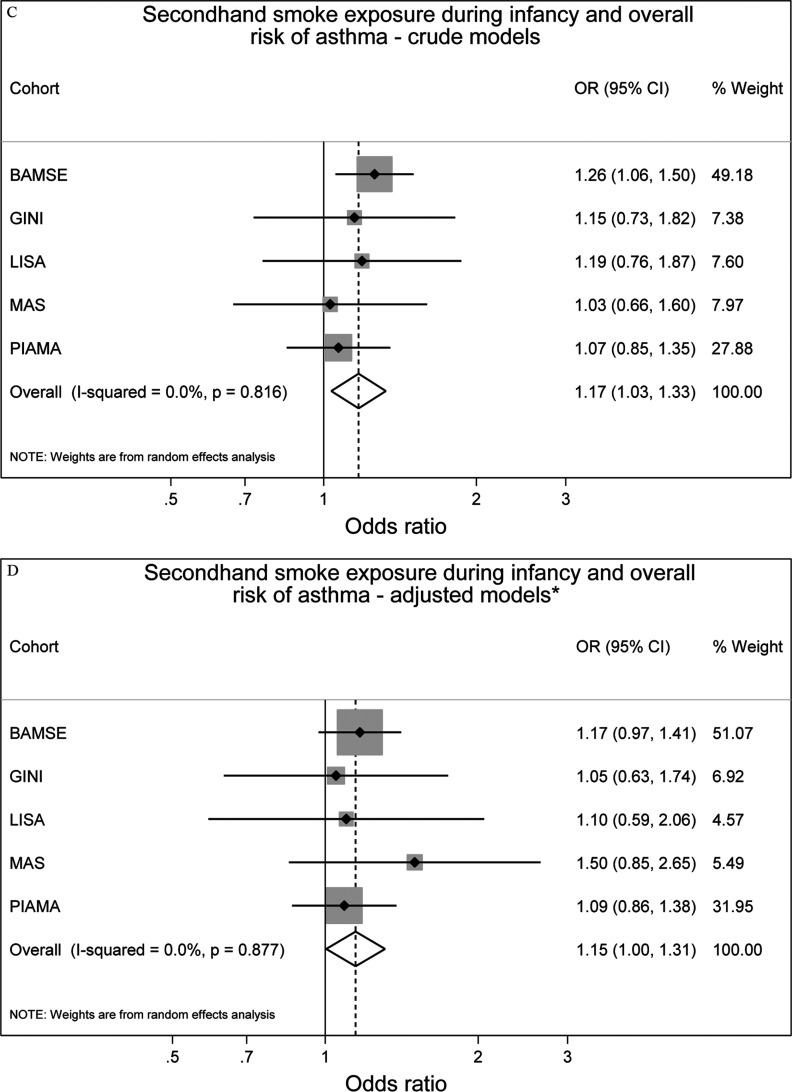
Overall, after adjustment for confounders, we observed increased but not statistically significant odds of prevalent asthma up to 14–16 y of age with any maternal smoking during pregnancy [ (95% CI: 0.98, 1.43)] and SHS exposure during infancy [ (95% CI: 1.00, 1.31)] (Figure 2). Heterogeneity of cohort-specific estimates was low for both maternal smoking during pregnancy and SHS during infancy ( and , respectively).
Figure 2.
Associations between maternal smoking during pregnancy () or any secondhand smoke (SHS) during infancy () and prevalence of asthma up to 14–16 y of age in five European birth cohorts. Cohort-specific odds ratios (ORs) and 95% confidence intervals (CIs) were obtained by generalized estimating equation models. Adjusted for sex, parental education level, parental allergy, older siblings, breastfeeding, study center, intervention arm, and early day-care attendance. Combined OR and 95% CI were derived from cohort-specific OR and 95% CI using a random effects model. Note: BAMSE, Children, Allergy, Milieu, Stockholm, Epidemiology; GINIplus, German Infant Nutritional Intervention; LISA, Influences of Lifestyle-Related Factors on the Immune System and the Development of Allergies in Childhood; MAS, Multicentre Allergy Study; PIAMA, Prevention and Incidence of Asthma and Mite Allergy.
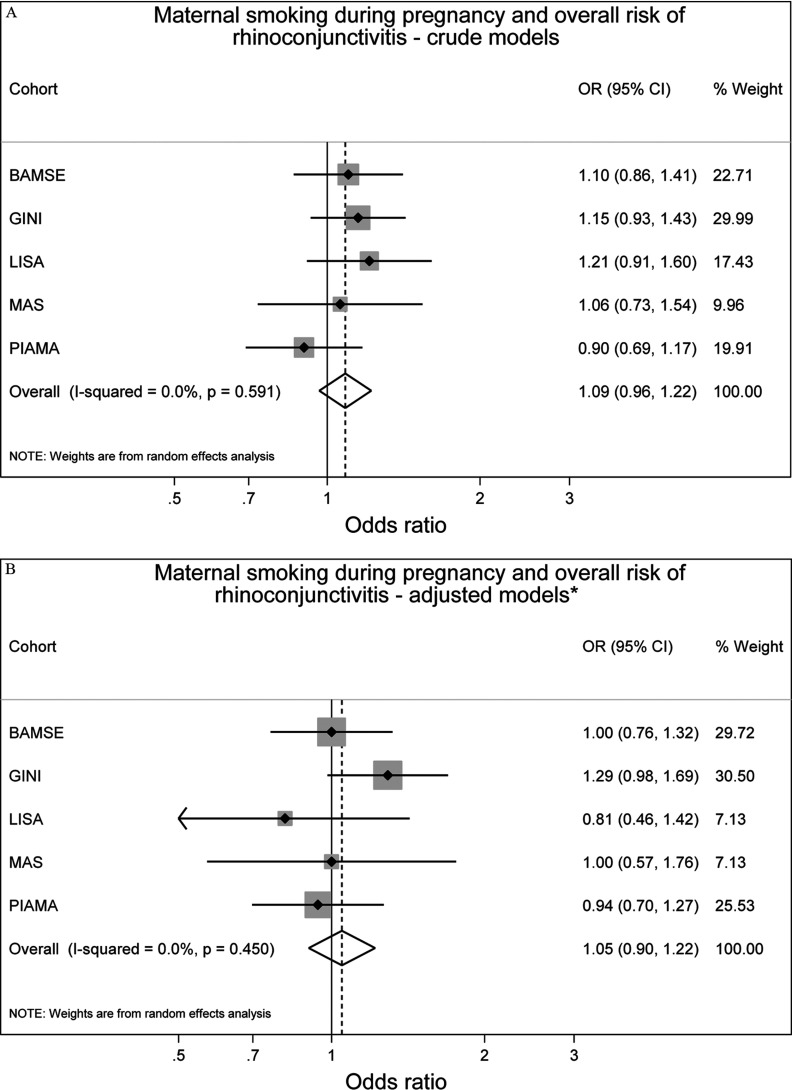
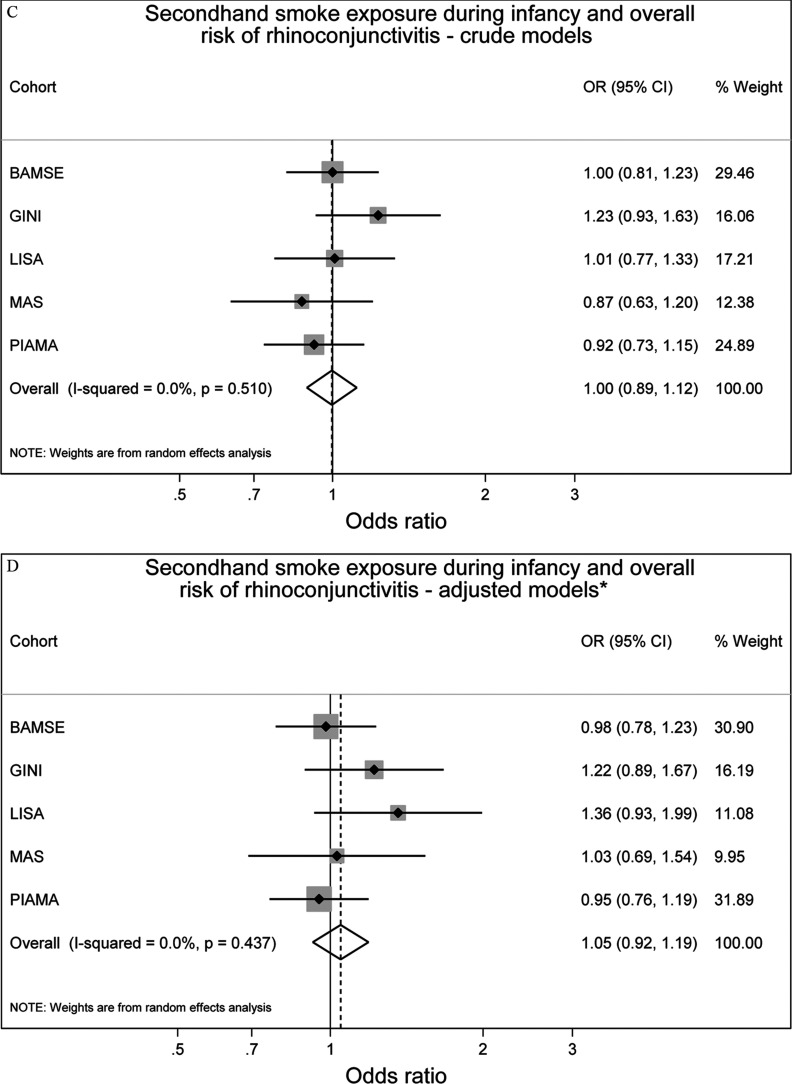
Neither maternal smoking during pregnancy nor SHS exposure during infancy was associated with prevalence of rhinoconjunctivitis up to 14–16 y of age, (95% CI: 0.90, 1.22) and (95% CI: 0.92, 1.19), respectively (Figure 3). There was no significant heterogeneity between the studies ( and , respectively).
Figure 3.
Associations between maternal smoking during pregnancy () or any secondhand smoke (SHS) during infancy () and prevalence of rhinoconjunctivitis up to 14 y of age in five European birth cohorts. Cohort-specific odds ratios (ORs) and 95% confidence intervals (CIs) were obtained by generalized estimating equation models. Adjusted for sex, parental education level, parental allergy, older siblings, breastfeeding, study center, intervention arm, and early day-care attendance. Combined OR and 95% CI were derived from cohort-specific OR and 95% CI using a random effects model. Note: BAMSE, Children, Allergy, Milieu, Stockholm, Epidemiology; GINIplus, German Infant Nutritional Intervention; LISA, Influences of Lifestyle-Related Factors on the Immune System and the Development of Allergies in Childhood; MAS, Multicentre Allergy Study; PIAMA, Prevention and Incidence of Asthma and Mite Allergy.
For asthma and rhinoconjunctivitis, the differences between crude and adjusted effect estimates were generally small (Figures 2 and 3), and slightly reduced but comparable results were obtained from one-stage IPD-MA (see Table S5).
Tobacco Smoke Exposure and Phenotypes of Asthma and Rhinoconjunctivitis
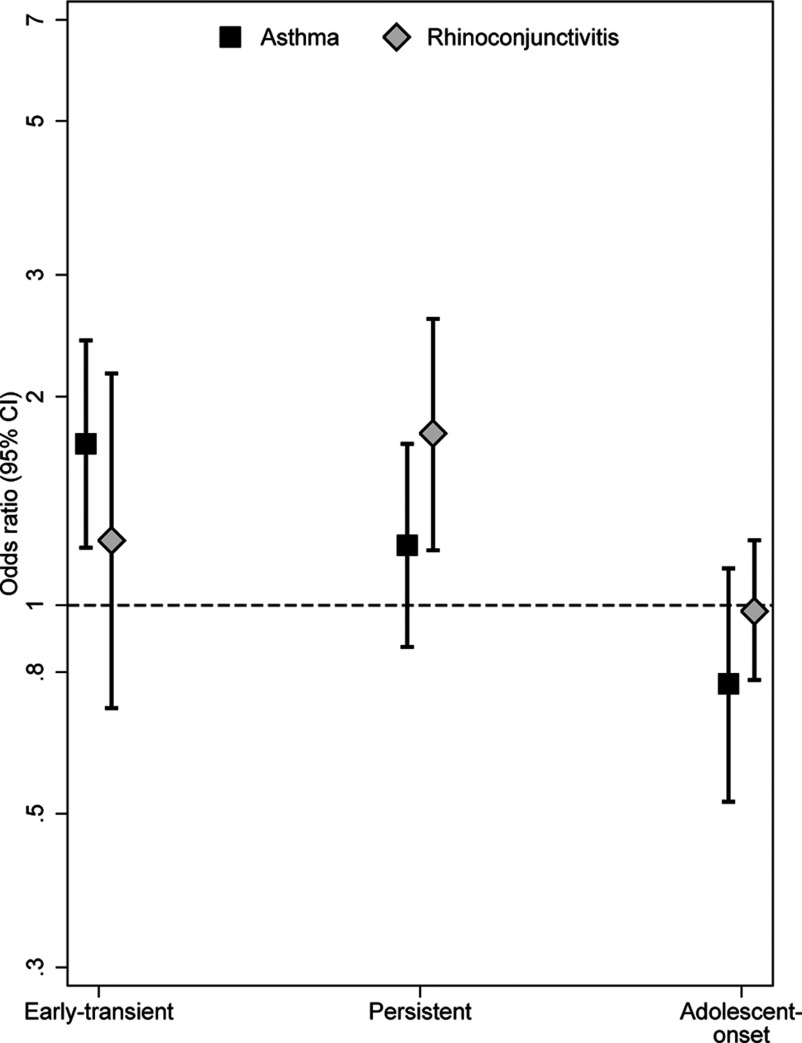
Associations between maternal smoking during pregnancy and early transient, persistent, and adolescent-onset phenotypes are presented in Figure 4. Compared with children who were unexposed during pregnancy, children with any exposure to maternal smoking during pregnancy were more likely to have early transient asthma [ (95% CI: 1.21, 2.41)], and a nonsignificantly increased odds ratio was observed for persistent asthma [ (95% CI: 0.87, 1.71)]. Maternal smoking during pregnancy was also associated with persistent rhinoconjunctivitis [ (95% CI: 1.20, 2.59)]. After additional adjustment for SHS exposure throughout childhood, associations remained similar; however, the association with persistent rhinoconjunctivitis was no longer statistically significant [ (95% CI: 0.92, 3.48)]. In dose–response analyses, children exposed to during pregnancy had significantly increased odds of early transient and persistent asthma as well as persistent rhinoconjunctivitis (Table 2). These results persisted after additional adjustment for SHS exposure through childhood. Maternal smoking during pregnancy was not associated with increased odds of adolescent-onset asthma [ (95% CI: 0.52, 1.13)] or rhinoconjunctivitis [ (95% CI: 0.78, 1.24)], and there were no evident dose–response relationships.
Figure 4.
Maternal smoking during pregnancy and the development of early transient, persistent, and adolescent-onset disease phenotypes (asthma and rhinoconjunctivitis ). Odds ratios (ORs) and 95% confidence intervals (CIs) were obtained by logistic regression and were adjusted for sex, parental education, parental allergy, secondhand smoke (SHS) during infancy, older siblings, breastfeeding, study center, intervention arm, and early day-care attendance.
Table 2.
The development of early-onset and persistent phenotypes of asthma and rhinoconjunctivitis in relation to intensity of maternal smoking during pregnancy
| Exposure | Maternal smoking during pregnancy | |||
|---|---|---|---|---|
| Na | na | Crude OR (95% CI) | Adjusted OR (95% CI)b | |
| Early transient asthma | ||||
| No smoking | 6,172 | 193 | Reference | Reference |
| Total of 1–9 cigarettes/day | 446 | 20 | 1.39 (0.88, 2.17) | 1.53 (1.16, 2.02) |
| Total of cigarettes/day | 294 | 26 | 2.54 (1.68, 3.85) | 2.07 (1.60, 2.68) |
| Persistent asthma | ||||
| No smoking | 6,172 | 253 | Reference | Reference |
| Total of 1–9 cigarettes/day | 446 | 19 | 1.08 (0.70, 1.67) | 1.03 (0.78, 1.37) |
| Total of cigarettes/day | 294 | 26 | 2.25 (1.54, 3.29) | 1.66 (1.29, 2.15) |
| Early transient rhinoconjunctivitis | ||||
| No smoking | 5,489 | 85 | Reference | Reference |
| Total of 1–9 cigarettes/day | 401 | 5 | 0.92 (0.43, 2.00) | 0.74 (0.44, 1.26) |
| Total of cigarettes/day | 283 | 10 | 2.27 (1.24, 4.18) | 1.94 (1.30, 2.90) |
| Persistent rhinoconjunctivitis | ||||
| No smoking | 5,489 | 164 | Reference | Reference |
| Total of 1–9 cigarettes/day | 401 | 21 | 1.72 (1.14, 2.59) | 1.75 (1.32, 2.31) |
| Total of cigarettes/day | 283 | 13 | 1.83 (1.14, 2.93) | 1.55 (1.09, 2.20) |
Note: CI, confidence interval; OR, odds ratio.
N = total number of exposed children; n = number of exposed cases.
ORs and 95% CIs obtained from multinomial logistic regression analyses adjusted for sex, parental education level, parental allergy, older siblings, breastfeeding, study center, intervention arm, and early day-care attendance.
Analyses of SHS exposure during infancy and phenotypes of asthma and rhinoconjunctivitis showed no significant associations (see Figure S3), and there were no apparent dose–response relationships for any of the outcomes studied (see Table S6).
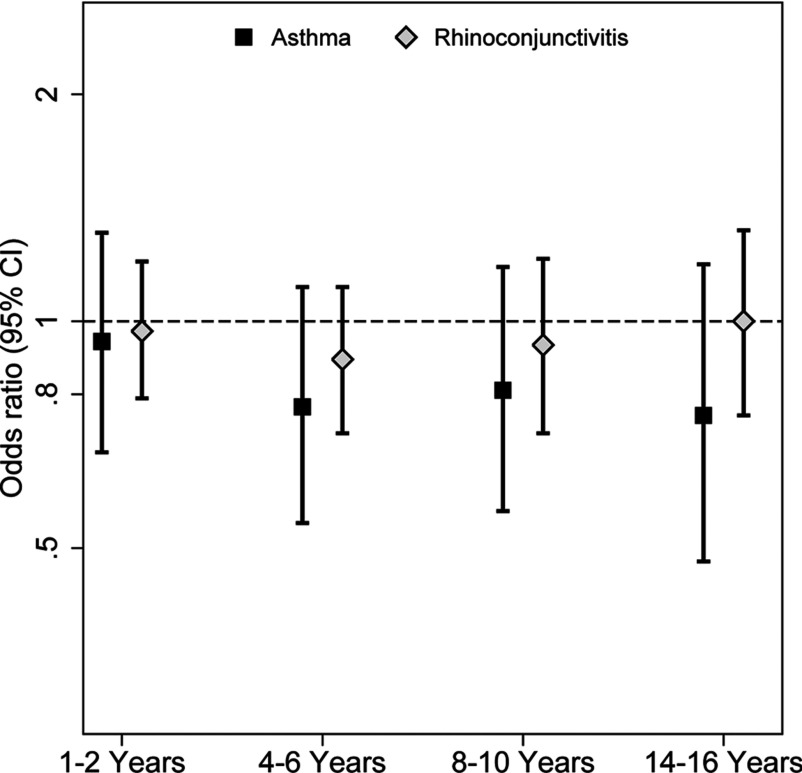
We examined whether exposure to SHS during different periods of childhood (infancy, 1–2, 4–6, 8–10, or 14–16 y of age) was associated with adolescent-onset asthma and rhinoconjunctivitis, but after adjustment for maternal smoking during pregnancy, no significant associations were apparent (Figure 5).
Figure 5.
Secondhand smoke (SHS) exposure in childhood in relation to adolescent-onset asthma and rhinoconjunctivitis. Odds ratios (ORs) and 95% confidence intervals (CIs) were obtained by logistic regression and were adjusted for sex, parental education, parental allergy, older siblings, breastfeeding, study center, intervention arm, day-care attendance, and maternal smoking during pregnancy.
Sensitivity Analyses
Because the BAMSE cohort had the highest weight in the meta-analyses, we performed a sensitivity analysis excluding this cohort from the analyses. Following this exclusion, the point estimate of the overall association between maternal smoking during pregnancy and prevalent asthma up to adolescence remained comparable [ (95% CI: 0.85, 1.53)].
We also performed analyses of the associations with tobacco smoke exposure during pregnancy and during infancy stratified by parental allergy. Associations of maternal smoking during pregnancy with asthma and rhinoconjunctivitis appeared slightly stronger among children without allergic parents than among those with allergic parents, but interactions were not statistically significant: for asthma, (95% CI: 0.88, 1.42) and (95% CI: 0.80, 1.35), respectively (interaction ); for rhinoconjunctivitis (95% CI: 0.93, 1.45) and (95% CI: 0.87, 1.31), respectively (interaction ) (see Table S7). Associations between SHS during infancy and both outcomes were positive among children without parental allergy and null among children with parental allergy, but interactions were not statistically significant (interaction and 0.12 for asthma and rhinoconjunctivitis, respectively) (see Table S8).
Additionally, we performed analyses stratified by sex. Maternal smoking and SHS during infancy were positively associated with asthma in males but not in females (interaction , respectively), but ORs for rhinoconjunctivitis were similar for males and females for both exposures (interaction , respectively) (see Tables S9 and S10).
To assess the impact of the inclusion of preterm infants in the BAMSE and PIAMA cohorts, we repeated analyses excluding preterm infants (see Table S11). The results remained largely unchanged.
In analyses of comorbidity of asthma and rhinoconjunctivitis, we observed no clear associations for asthma only, rhinoconjunctivitis only, or concurrent asthma and rhinoconjunctivitis in relation to exposure to maternal smoking during pregnancy or SHS exposure during infancy (see Figures S4 and S5). We also conducted analyses of different windows of exposure: specifically, maternal smoking during pregnancy only, parental smoking during infancy only, and exposure to both in relation to asthma and rhinoconjunctivitis (see Figure S6). We found evidence suggesting that maternal smoking during pregnancy alone was associated with asthma up to adolescence [ (95% CI: 1.10, 1.87)], but no significant associations were observed for rhinoconjunctivitis. Lastly, we examined associations for any tobacco smoke exposure during infancy or pregnancy compared with never-smoker mothers (defined as mothers who did not smoke during pregnancy and during infancy), but the results were quite similar (see Figure S7).
Discussion
Based on data from five European birth cohorts, exposure to tobacco smoking during fetal life or infancy appears to increase the odds of asthma up to 14–16 y of age, specifically early transient asthma. High exposure to cigarette smoking, during pregnancy, was associated with persistent asthma and persistent rhinoconjunctivitis. In contrast, there were no associations between tobacco smoke exposure (in utero, during infancy, or later childhood) and the onset of asthma or rhinoconjunctivitis in adolescence.
Our finding of an association between fetal and early postnatal tobacco smoke exposure and asthma is in line with the current literature in young children (Mitchell et al. 2012; Neuman et al. 2012). Prospective studies and systematic reviews have also found associations for maternal smoking during pregnancy and childhood asthma (Burke et al. 2012; Silvestri et al. 2015), but few studies have followed children into adolescence. Congruent with our results, an Australian cohort showed an association between maternal smoking during pregnancy and the risk of current asthma at 14 y of age (Hollams et al. 2014). Additionally, BAMSE, MAS, and PIAMA independently reported excess risk of asthma up to adolescence in children exposed to SHS during intrauterine life (Grabenhenrich et al. 2014; Milanzi et al. 2017; Thacher et al. 2014). However, combining multiple cohorts provided power to adequately assess the timing of onset, progression, and persistence through distinct phenotypes. To our knowledge, this is the first study to investigate the effects of tobacco smoke exposure and phenotypes of asthma and rhinoconjunctivitis development in children up to adolescence. Moreover, SHS exposure in infancy or childhood was not associated with onset of asthma in adolescence, and this finding is in line with those of the few other studies that assessed adolescent-onset asthma (Genuneit et al. 2006; Gilliland et al. 2006). Taken together, these results suggest that tobacco smoke exposure during pregnancy primarily increases the risk of early-childhood asthma and that high exposure may increase the risk of persistent asthma.
The evidence for an association between tobacco smoke exposure and rhinoconjunctivitis up to adolescence is mixed (Grabenhenrich et al. 2015; Mitchell et al. 2012; Thacher et al. 2014). We observed no statistically significant association between tobacco smoke exposure and prevalent rhinoconjunctivitis during childhood and adolescence, but a statistically significant dose–response relationship appeared for persistent rhinoconjunctivitis in relation to the number of cigarettes smoked by the mother during pregnancy. Previous studies have provided mixed results ranging from reduced to excess risks in pediatric populations (Civelek et al. 2010; Magnusson et al. 2005; Mitchell et al. 2012). Independent analyses in the BAMSE birth cohort indicated that the risk of rhinitis associated with tobacco smoke exposure was mainly confined to preschool-age children (Thacher et al. 2014).
Suggested mechanisms of how tobacco smoke influences the development of asthma include increased airway responsiveness and impaired immune response to viral pathogens (Macaubas et al. 2003). Exposure to maternal smoking during pregnancy has been shown to cause persistent epigenetic modifications, particularly in enhancer regions regulating the activity of genes involved in airway inflammatory processes, thus contributing to the development of asthma symptoms among young children (Bauer et al. 2016; Joubert et al. 2016). These symptoms may persist into adolescence, particularly in highly exposed individuals. Furthermore, it is possible that altered lung growth and airway remodeling could contribute to persistent asthma among subjects heavily exposed to maternal smoking during pregnancy.
The underlying mechanisms by which exposure to tobacco smoke may affect the development of rhinoconjunctivitis are unclear. Studies indicate that maternal smoking during pregnancy alters the adaptive and innate immune systems of newborns and is associated with nasal obstruction, modifications in mucociliary clearance in the airways, and modifications of the number and function of T lymphocytes (Noakes et al. 2003).
Strengths of this study include the use of several European birth cohorts, resulting in a large sample size and in harmonized definitions of exposure and outcomes. The prospective cohort design and long-term follow-up into adolescence are particularly well suited to study the effects of maternal smoking during pregnancy and SHS during infancy on outcomes in adolescence. A large sample size facilitated robust analyses investigating early transient, persistent, and adolescent-onset phenotypes as well as the assessment of dose–response effects.
Misclassification of exposure or outcome is generally unavoidable in epidemiologic studies. With regard to maternal smoking during pregnancy, all cohorts assessed this information shortly after birth; therefore, differential misclassification of exposure is reduced. The potential exists for parents to underreport smoking habits because of social stigma. Validation studies have reported agreement between questionnaire-reported tobacco smoking and cord blood, plasma, or urinary cotinine levels in some studies (Bauer et al. 2016; Brunekreef et al. 2000; Carlsten et al. 2012; Gehring et al. 2006) but not in all (Britton et al. 2004). If underreporting is present in the included cohorts, our findings are likely to underestimate the true association.
Outcome misclassification is also a potential concern because parents may not correctly recall symptoms or medical diagnoses. We observed minor regional differences in asthma prevalence, which may reflect differing diagnostic criteria because doctor-diagnosed asthma was one of three criteria used to define asthma. The somewhat lower prevalence of asthma in the German cohorts (GINIplus, LISAplus, and MAS) might be explained by different diagnostic conventions between countries (Gehring et al. 2015). Respiratory symptoms are more common among children exposed to parental smoking, and studies indicate that smoking parents may underreport symptoms of wheeze or underutilize health care for mild respiratory symptoms in their children (Crombie et al. 2001). This underreporting could contribute to an underestimation of the true effect. Additionally, exposure to SHS later in childhood is not always independent of symptoms in the child because some parents may quit smoking if their child is diagnosed with asthma (Wijga et al. 2017).
Nonrandom retention of study participants may have influenced our findings and should be acknowledged. We cannot rule out some selection bias due to missing data. Additionally, varying degrees of missing data across cohorts and exposure windows could also have biased our results. Nevertheless, cohort retention was good, and the baseline characteristics of our study population did not differ greatly from those of the initial cohorts. Thus, it is unlikely that our findings are explained by selection bias or by missing data.
In MAS, atopic parents were overrepresented by design. Therefore, we adjusted our analyses for parental allergy, and our results comparing crude and adjusted models were similar. Additionally, cohort-specific estimates in MAS tended to be larger for asthma than those in the other cohorts. This difference may be due to the recruitment period because MAS was established some years before the other cohorts and had the highest smoking rates.
It is possible that the is not sensitive enough to capture heterogeneity between studies if the number of studies is small, and all but one study show similar associations. Similarly, when analyzing pooled data, we included a term for cohort; however, this may not fully account for unmeasured effects in each cohort and should be acknowledged (Rosa et al. 2017). Therefore, we also adjusted for important demographic variables such as education level to capture differences in other social factors between the cohorts. Nevertheless, these five cohorts are from high-income European countries and are likely to be similar in many aspects.
Conclusion
Taken together, our findings strengthen and extend results from previous studies reporting that any form of tobacco smoke exposure during fetal life or infancy increases the risk of asthma in childhood and adolescence, particularly the risk of early transient asthma. The risks of persistent asthma and persistent rhinoconjunctivitis were increased in highly exposed subjects. We found no evidence of an association between tobacco smoke exposure during fetal life, infancy, or any time during childhood and the onset of asthma or rhinoconjunctivitis in adolescence.
Supplemental Material
Acknowledgments
We would like to acknowledge and thank the participating children and parents as well as all staff involved in the birth cohorts. This work was supported by Mechanisms of the Development of Allergy (MeDALL), a collaborative project done within the European Union under the Health Cooperation Work Program of the seventh Framework program (grant no. 261357).
Funding: The research leading to these results has received funding from the European Community’s Seventh Framework Program [grant no. 261357 (Mechanisms of the Development of Allergy; MeDALL)].
The Children, Allergy, Milieu, Stockholm, Epidemiology (BAMSE) study was supported by The Swedish Research Council, The Swedish Heart and Lung Foundation, The Swedish Research Council for Working Life and Social Welfare, the Swedish Asthma and Allergy Association Research Foundation, The Swedish Research Council Formas, and Stockholm County Council (ALF).
The German Infant Nutritional Intervention (GINIplus) study was mainly supported for the first 3 years by the Federal Ministry for Education, Science, Research and Technology (interventional arm) and Helmholtz Zentrum Munich [former Gesellschaft für Strahlenforschung (GSF)] (observational arm). The 4-y, 6-y, 10-y, and 15-y follow-up examinations of the GINIplus study were covered by the budgets of the five study centers [Helmholtz Zentrum Munich [former Gesellschaft für Strahlenforschung (GSF)], Research Institute at Marien-Hospital Wesel, University hospital of Munich (LMU), Technical University (TU) of Munich, and from 6 y onwards also from Leibniz Research Institute (IUF) for Environmental Medicine at the University of Düsseldorf] and by a grant from the Federal Ministry for Environment (IUF Düsseldorf; FKZ 20462296). Further, the 15-y follow-up examination in the GINIplus study was supported by the Commission of the European Communities, the 7th Framework Program: MeDALL project, and by the Mead Johnson and Nestlé companies.
The Influences of Lifestyle-Related Factors on the Immune System and the Development of Allergies in Childhood (LISAplus) study was mainly supported by grants from the Federal Ministry for Education, Science, Research and Technology and from Helmholtz Zentrum Munich (former GSF); Helmholtz Centre for Environmental Research - UFZ, Leipzig; Research Institute at Marien-Hospital Wesel; and Pediatric Practice, Bad Honnef for the first 2 y. The 4-y, 6-y, 10-y, and 15-y follow-up examinations of the LISAplus study were covered by the budgets of the involved partners [Helmholtz Zentrum Munich (former GSF); Helmholtz Centre for Environmental Research - UFZ, Leipzig; Research Institute at Marien-Hospital Wesel; Pediatric Practice, Bad Honnef; and IUF – Leibniz Research Institute for Environmental Medicine at the University of Düsseldorf) and in addition by a grant from the Federal Ministry for Environment (IUF Düsseldorf; FKZ 20462296). Further, the 15-y follow-up examination of the LISAplus study was supported by the Commission of the European Communities, the 7th Framework Program: MeDALL project.
The Prevention and Incidence of Asthma and Mite Allergy (PIAMA) study is supported by The Netherlands Organization for Health Research and Development; The Netherlands Organization for Scientific Research; The Netherlands Asthma Fund (grant no. 4.1.14.001); The Netherlands Ministry of Housing, Spatial Planning and the Environment; and The Netherlands Ministry of Health, Welfare and Sport.
The Multicentre Allergy Study (MAS) was funded by grants from the German Federal Ministry of Education and Research (BMBF; grant nos. 07015633, 07 ALE 27, 01EE9405/5, 01EE9406) and the German Research Foundation (DFG; grant no. KE 1462/2-1).
References
- Asher MI, Montefort S, Björksten B, Lai CK, Strachan DP, Weiland SK, et al. 2006. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet 368(9537):733–743, PMID: 16935684, 10.1016/S0140-6736(06)69283-0. [DOI] [PubMed] [Google Scholar]
- Ballardini N, Bergström A, Wahlgren CF, van Hage M, Hallner E, Kull I, et al. 2015. IgE-antibodies in relation to prevalence and multimorbidity of eczema, asthma and rhinitis from birth to adolescence. Allergy 71(3):342–349, PMID: 26505741, 10.1111/all.12798. [DOI] [PubMed] [Google Scholar]
- Bauer T, Trump S, Ishaque N, Thürmann L, Gu L, Bauer M, et al. 2016. Environment-induced epigenetic reprogramming in genomic regulatory elements in smoking mothers and their children. Mol Syst Biol 12(3):861, PMID: 27013061, 10.15252/msb.20156520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg A, Krämer U, Link E, Bollrath C, Heinrich J, Brockow I, et al. 2010. Impact of early feeding on childhood eczema: development after nutritional intervention compared with the natural course - the GINIplus study up to the age of 6 years. Clin Exp Allergy 40(4):627–636, PMID: 20082618, 10.1111/j.1365-2222.2009.03444.x. [DOI] [PubMed] [Google Scholar]
- Bergmann RL, Bergmann KE, Lau-Schadensdorf S, Luck W, Dannemann A, Bauer CP, et al. 1994. Atopic diseases in infancy. The German multicenter atopy study (MAS-90). Pediatr Allergy Immunol 5(6 Suppl):19–25, PMID: 7728224, 10.1111/j.1399-3038.1994.tb00343.x. [DOI] [PubMed] [Google Scholar]
- Bousquet J, Anto J, Auffray C, Akdis M, Cambon-Thomsen A, Keil T, et al. 2011. MeDALL (Mechanisms of the Development of ALLergy): an integrated approach from phenotypes to systems medicine. Allergy 66(5):596–604, PMID: 21261657, 10.1111/j.1398-9995.2010.02534.x. [DOI] [PubMed] [Google Scholar]
- Bousquet J, Anto J, Sunyer J, Nieuwenhuijsen M, Vrijheid M, Keil T. 2013. Pooling birth cohorts in allergy and asthma: European Union-funded initiatives - a MeDALL, CHICOS, ENRIECO, and GA2LEN joint paper. Int Arch Allergy Immunol 161(1):1–10, PMID: 23258290, 10.1159/000343018. [DOI] [PubMed] [Google Scholar]
- Bousquet J, Anto JM, Akdis M, Auffray C, Keil T, Momas I, et al. 2016. Paving the way of systems biology and precision medicine in allergic diseases: the MeDALL success story: Mechanisms of the Development of ALLergy; EU FP7-CP-IP; Project No: 261357; 2010–2015. Allergy 71(11):1513–1525, PMID: 26970340, 10.1111/all.12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousquet J, Anto JM, Berkouk K, Gergen P, Antunes JP, Augé P, et al. 2015. Developmental determinants in non-communicable chronic diseases and ageing. Thorax 70(6):595–597, PMID: 25616486, 10.1136/thoraxjnl-2014-206304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton GR, Brinthaupt J, Stehle JM, James GD. 2004. Comparison of self-reported smoking and urinary cotinine levels in a rural pregnant population. J Obstet Gynecol Neonatal Nurs 33(3):306–311, PMID: 15180193, 10.1177/0884217504264866. [DOI] [PubMed] [Google Scholar]
- Brunekreef B, Leaderer BP, van Strien R, Oldenwening M, Smit HA, Koopman L, et al. 2000. Using nicotine measurements and parental reports to assess indoor air: The PIAMA birth cohort study. Prevention and Incidence of Asthma and Mite Allergy. Epidemiology 11(3):350–352, PMID: 10784258. [DOI] [PubMed] [Google Scholar]
- Burke H, Leonardi-Bee J, Hashim A, Pine-Abata H, Chen Y, Cook DG, et al. 2012. Prenatal and passive smoke exposure and incidence of asthma and wheeze: systematic review and meta-analysis. Pediatrics 129(4):735–744, PMID: 22430451, 10.1542/peds.2011-2196. [DOI] [PubMed] [Google Scholar]
- Carlsten C, Dimich-Ward H, DyBuncio A, Becker AB, Chan-Yeung M. 2012. Cotinine versus questionnaire: early-life environmental tobacco smoke exposure and incident asthma. BMC Pediatr 12:187, PMID: 23216797, 10.1186/1471-2431-12-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheraghi M, Salvi S. 2009. Environmental tobacco smoke (ETS) and respiratory health in children. Eur J Pediatr 168(8):897–905, PMID: 19301035, 10.1007/s00431-009-0967-3. [DOI] [PubMed] [Google Scholar]
- Civelek E, Yavuz ST, Boz AB, Orhan F, Yuksel H, Uner A, et al. 2010. Epidemiology and burden of rhinitis and rhinoconjunctivitis in 9- to 11-year-old children. Am J Rhinol Allergy 24(5):364–370, PMID: 20579411, 10.2500/ajra.2010.24.3484. [DOI] [PubMed] [Google Scholar]
- Crombie IK, Wright A, Irvine L, Clark RA, Slane PW. 2001. Does passive smoking increase the frequency of health service contacts in children with asthma? Thorax 56(1):9–12, PMID: 11120897, 10.1136/thorax.56.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debray TP, Moons KG, Abo-Zaid GM, Koffijberg H, Riley RD. 2013. Individual participant data meta-analysis for a binary outcome: one-stage or two-stage? PLoS One 8(4):e60650, PMID: 23585842, 10.1371/journal.pone.0060650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipiak B, Zutavern A, Koletzko S, von Berg A, Brockow I, Grübl A, et al. 2007. Solid food introduction in relation to eczema: results from a four-year prospective birth cohort study. J Pediatr 151(4):352–358, PMID: 17889067, 10.1016/j.jpeds.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Frazer K, Callinan JE, McHugh J, van Baarsel S, Clarke A, Doherty K, et al. 2016. Legislative smoking bans for reducing harms from secondhand smoke exposure, smoking prevalence and tobacco consumption. Cochrane Database Syst Rev 2:CD005992, PMID: 26842828, 10.1002/14651858.CD005992.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring U, de Jongste JC, Kerkhof M, Oldewening M, Postma D, van Strien RT, et al. 2012. The 8-year follow-up of the PIAMA intervention study assessing the effect of mite-impermeable mattress covers. Allergy 67(2):248–256, PMID: 22023655, 10.1111/j.1398-9995.2011.02739.x. [DOI] [PubMed] [Google Scholar]
- Gehring U, Leaderer BP, Heinrich J, Oldenwening M, Giovannangelo ME, Nordling E, et al. 2006. Comparison of parental reports of smoking and residential air nicotine concentrations in children. Occup Environ Med 63(11):766–772, PMID: 16912089, 10.1136/oem.2006.027151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring U, Wijga AH, Hoek G, Bellander T, Berdel D, Brüske I, et al. 2015. Exposure to air pollution and development of asthma and rhinoconjunctivitis throughout childhood and adolescence: a population-based birth cohort study. Lancet Respir Med 3(12):933–942, PMID: 27057569, 10.1016/S2213-2600(15)00426-9. [DOI] [PubMed] [Google Scholar]
- Genuneit J, Weinmayr G, Radon K, Dressel H, Windstetter D, Rzehak P, et al. 2006. Smoking and the incidence of asthma during adolescence: results of a large cohort study in Germany. Thorax 61(7):572–578, PMID: 16537668, 10.1136/thx.2005.051227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliland FD, Islam T, Berhane K, Gauderman WJ, McConnell R, Avol E, et al. 2006. Regular smoking and asthma incidence in adolescents. Am J Respir Crit Care Med 174(10):1094–1100, PMID: 16973983, 10.1164/rccm.200605-722OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabenhenrich LB, Gough H, Reich A, Eckers N, Zepp F, Nitsche O, et al. 2014. Early-life determinants of asthma from birth to age 20 years: a German birth cohort study. J Allergy Clin Immunol 133(4):979–988, PMID: 24461583, 10.1016/j.jaci.2013.11.035. [DOI] [PubMed] [Google Scholar]
- Grabenhenrich LB, Keil T, Reich A, Gough H, Beschorner J, Hoffmann U, et al. 2015. Prediction and prevention of allergic rhinitis: a birth cohort study of 20 years. J Allergy Clin Immunol 136(4):932–940.e12, PMID: 25976706, 10.1016/j.jaci.2015.03.040. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG. 2002. Quantifying heterogeneity in a meta-analysis. Stat Med 21(11):1539–1558, PMID: 12111919, 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Hohmann C, Pinart M, Tischer C, Gehring U, Heinrich J, Kull I, et al. 2014. The development of the MeDALL Core Questionnaires for a harmonized follow-up assessment of eleven European birth cohorts on asthma and allergies. Int Arch Allergy Immunol 163(3):215–224, PMID: 24642608, 10.1159/000357732. [DOI] [PubMed] [Google Scholar]
- Hollams EM, de Klerk NH, Holt PG, Sly PD. 2014. Persistent effects of maternal smoking during pregnancy on lung function and asthma in adolescents. Am J Respir Crit Care Med 189(4):401–407, PMID: 24251622, 10.1164/rccm.201302-0323OC. [DOI] [PubMed] [Google Scholar]
- Joubert BR, Felix JF, Yousefi P, Bakulski KM, Just AC, Breton C, et al. 2016. DNA methylation in newborns and maternal smoking in pregnancy: genome-wide consortium meta-analysis. Am J Hum Genet 98(4):680–696, PMID: 27040690, 10.1016/j.ajhg.2016.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaubas C, de Klerk NH, Holt BJ, Wee C, Kendall G, Firth M, et al. 2003. Association between antenatal cytokine production and the development of atopy and asthma at age 6 years. Lancet 362(9391):1192–1197, PMID: 14568741, 10.1016/S0140-6736(03)14542-4. [DOI] [PubMed] [Google Scholar]
- Magnusson LL, Olesen AB, Wennborg H, Olsen J. 2005. Wheezing, asthma, hayfever, and atopic eczema in childhood following exposure to tobacco smoke in fetal life. Clin Exp Allergy 35(12):1550–1556, PMID: 16393320, 10.1111/j.1365-2222.2005.02374.x. [DOI] [PubMed] [Google Scholar]
- Milanzi EB, Brunekreef B, Koppelman GH, Wijga AH, van Rossem L, Vonk JM, et al. 2017. Lifetime secondhand smoke exposure and childhood and adolescent asthma: findings from the PIAMA cohort. Environ Health 16:14, PMID: 28231798, 10.1186/s12940-017-0223-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell EA, Beasley R, Keil U, Montefort S, Odhiambo J, ISAAC Phase Three Study Group. 2012. The association between tobacco and the risk of asthma, rhinoconjunctivitis and eczema in children and adolescents: analyses from Phase Three of the ISAAC programme. Thorax 67(11):941–949, PMID: 22693180, 10.1136/thoraxjnl-2011-200901. [DOI] [PubMed] [Google Scholar]
- Murin S, Rafii R, Bilello K. 2011. Smoking and smoking cessation in pregnancy. Clin Chest Med 32(1):75–91, viii, PMID: 21277451, 10.1016/j.ccm.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Neuman Å, Hohmann C, Orsini N, Pershagen G, Eller E, Kjaer HF, et al. 2012. Maternal smoking in pregnancy and asthma in preschool children: a pooled analysis of eight birth cohorts. Am J Respir Crit Care Med 186(10):1037–1043, PMID: 22952297, 10.1164/rccm.201203-0501OC. [DOI] [PubMed] [Google Scholar]
- Noakes PS, Holt PG, Prescott SL. 2003. Maternal smoking in pregnancy alters neonatal cytokine responses. Allergy 58(10):1053–1058, PMID: 14510725, 10.1034/j.1398-9995.2003.00290.x. [DOI] [PubMed] [Google Scholar]
- Patil VK, Kurukulaaratchy RJ, Venter C, Grundy J, Roberts G, Dean T, et al. 2015. Changing prevalence of wheeze, rhinitis and allergic sensitisation in late childhood: findings from 2 Isle of Wight birth cohorts 12 years apart. Clin Exp Allergy 45(9):1430–1438, PMID: 25809555, 10.1111/cea.12534. [DOI] [PubMed] [Google Scholar]
- Rosa MJ, Pajak A, Just AC, Sheffield PE, Kloog I, Schwartz J, et al. 2017. Prenatal exposure to PM2.5 and birth weight: a pooled analysis from three North American longitudinal pregnancy cohort studies. Environ Int 107:173–180, PMID: 28738263, 10.1016/j.envint.2017.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestri M, Franchi S, Pistorio A, Petecchia L, Rusconi F. 2015. Smoke exposure, wheezing, and asthma development: a systematic review and meta-analysis in unselected birth cohorts. Pediatr Pulmonol 50(4):353–362, PMID: 24648197, 10.1002/ppul.23037. [DOI] [PubMed] [Google Scholar]
- Thacher JD, Gruzieva O, Pershagen G, Neuman A, Wickman M, Kull I, et al. 2014. Pre- and postnatal exposure to parental smoking and allergic disease through adolescence. Pediatrics 134(3):428–434, PMID: 25136039, 10.1542/peds.2014-0427. [DOI] [PubMed] [Google Scholar]
- Vardavas CI, Hohmann C, Patelarou E, Martinez D, Henderson AJ, Granell R, et al. 2016. The independent role of prenatal and postnatal exposure to active and passive smoking on the development of early wheeze in children. Eur Respir J 48(1):115–124, PMID: 26965294, 10.1183/13993003.01016-2015. [DOI] [PubMed] [Google Scholar]
- Wickman M, Kull I, Pershagen G, Nordvall SL. 2002. The BAMSE project: presentation of a prospective longitudinal birth cohort study. Pediatr Allergy Immunol 13(Suppl 15):11–13, PMID: 12688617, 10.1034/j.1399-3038.13.s.15.10.x. [DOI] [PubMed] [Google Scholar]
- Wijga AH, Kerkhof M, Gehring U, de Jongste JC, Postma DS, Aalberse RC, et al. 2014. Cohort profile: the Prevention and Incidence of Asthma and Mite Allergy (PIAMA) birth cohort. Int J Epidemiol 43(2):527–535, PMID: 23315435, 10.1093/ije/dys231. [DOI] [PubMed] [Google Scholar]
- Wijga AH, Schipper M, Brunekreef B, Koppelman GH, Gehring U. 2017. Asthma diagnosis in a child and cessation of smoking in the child's home: the PIAMA birth cohort. J Expo Sci Environ Epidemiol 27(5):521–525, PMID: 27966669, 10.1038/jes.2016.75. [DOI] [PubMed] [Google Scholar]
- Zutavern A, Brockow I, Schaaf B, von Berg A, Diez U, Borte M, et al. 2008. Timing of solid food introduction in relation to eczema, asthma, allergic rhinitis, and food and inhalant sensitization at the age of 6 years: results from the prospective birth cohort study LISA. Pediatrics 121(1):e44–e52, PMID: 18166543, 10.1542/peds.2006-3553. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.