 Schiff-base transition metal complexes display amazing antimicrobial and anticancer activities.
Schiff-base transition metal complexes display amazing antimicrobial and anticancer activities.
Abstract
In recent years, the number of people suffering from cancer and multidrug-resistant infections has sharply increased, leaving humanity without any choice but to search for new treatment options and strategies. Although cancer is considered the leading cause of death worldwide, it also paves the way many microbial infections and thus increases this burden manifold. Development of small molecules as anticancer and anti-microbial agents has great potential and a plethora of drugs are already available to combat these diseases. However, the wide occurrence of multidrug resistance in both cancer and microbial infections necessitates the development of new and potential molecules with desired properties that could circumvent the multidrug resistance problem. A successful strategy in anticancer chemotherapy has been the use of metallo-drugs and this strategy has the potential to be used for treating multidrug-resistant infections more efficiently. As a class of molecules, Schiff bases have been the topic of considerable interest, owing to their versatile metal chelating properties, inherent biological activities and flexibility to modify the structure to fine-tune it for a particular biological application. Schiff base-based metallo-drugs are being researched to develop new anticancer and anti-microbial chemotherapies and because both anticancer and anti-microbial targets are different, heterocyclic Schiff bases can be structurally modified to achieve the desired molecule, targeting a particular disease. In this review, we collect the most recent and relevant literature concerning the synthesis of heterocyclic Schiff base metal complexes as anticancer and anti-microbial agents and discuss the potential and future of this class of metallo-drugs as either anticancer or anti-microbial agents.
1. Introduction
Microbial infections have been haunting human civilization since pre-historic times, resulting in a large proportion of deaths worldwide, and cancer is a fatal and dreadful disease without any appropriate cure, thus threatening humanity in both the developing and developed world. As per report from the Infectious Diseases Society of America, different microbial species like Enterococcus, Staphylococcus, Enterobacter, Klebsiella pneumoniae, Acinetobacter and Pseudomonas aeruginosa are the pathogens of utmost concern1 and according to the World Cancer Report 2015, nearly 14.1 million new cases of cancer occurred globally resulting in 8.8 million deaths.2 Therefore, there is a critical requirement for the design and synthesis of new classes of compounds to circumvent these diseases. In antimicrobial chemotherapy particularly in the development of antibacterial drugs, several compounds like linezolid, GAR-936, daptomycin, and oritavancin have been brought to market3,4 and the exploration of the cytotoxic properties of cisplatin has given tremendous impetus to research the utilization of new metal complexes as spectacular anticancer agents.5
Intensive research efforts all over the world are ongoing, in order to explore benign and effective metal-based biologically active compounds as potential antimicrobial drugs. Among the different therapeutic approaches to wipe out these microbial menaces, the assessment of using transition metal complexes as metallo-drugs has shown them to have great potential.6,7 Metal complexes are supposed to exert their effect by inhibition of enzymes, interaction with intracellular biomolecules, enhanced lipophilicity, alteration of cell membrane functions, arrest of the cell cycle, etc.8 Due to a wide variety of coordination spheres, ligand designs, oxidation states and redox potentials, metal complexes are supposed to alter the kinetic and thermodynamic properties of the complexes towards biological receptors. Thus, chelation causes drastic changes in the biological properties of ligands as well as the metal moiety. Many metal complexes of the quinoline group of antibiotics, viz. ciprofloxacin, norfloxacin and tetracycline, were evaluated and reported to possess enhanced activity compared to antibiotics alone.9–11 Recently, a bismuth–norfloxacin complex was reported to possess enhanced antimicrobial activity compared to norfloxacin alone.12 It is believed that the enhanced activity is due to increased bioavailability of the complex. Hence, transportation of organic ligands into bacterial cells can be facilitated by the formation of metal complexes. The Pd(ii) complex of doxycycline is two times more potent than doxycycline alone against the resistant strain E. coli HB101/pBR322.13
Many reports on Schiff base (SB) transition metal complexes explicate their efficient bioactivity against a range of bacterial and fungal species. In particular, heterocyclic Schiff base (HSB) metal complexes have dominated medicinal chemistry due to their wide range of properties.14,15 SB complexes tethered with heterocyclic moieties like 4-aminoantipyrine, pyrazole, 1,2,4-triazoles, benzoxazole, triazines, and coumarins have received remarkable interest as broad-spectrum antibacterial, antifungal and antiviral agents.16–19 The huge enthusiasm for the preparation of HSB compounds is associated with the extraordinary properties that the heterocyclic system imparts to such ligands and to their metal complexes. Investigations on the synthetic approaches toward SB ligands and their metal complexes have been reported recurrently due to their prospective applications.20–22 In addition to their biological potential, they also find applications as catalysts in several reactions such as the polymerization reaction, reduction of thionyl chloride, oxidation of organic compounds, reduction reaction of ketones, aldol reaction, Henry reaction, epoxidation of alkenes, hydrosilylation of ketones, synthesis of bis(indolyl)methanes and Diels–Alder reaction.23–27
Besides antimicrobial infections, medicinal chemists are also facing great challenges in pacifying the inconvenience caused by cancer. The widespread success of cisplatin against several types of cancers has placed metal-based drugs in the frontline in the fight against cancer.28,29 Although highly effective in treating a variety of cancers, cisplatin shows dose-limiting side effects30 and drug resistance, which is only partially amended by employment of new platinum drugs.31–34 These problems have stimulated an extensive search and prompted chemists to develop alternative strategies, based on different ligands and metals, with improved pharmacological properties and aimed at different targets.35
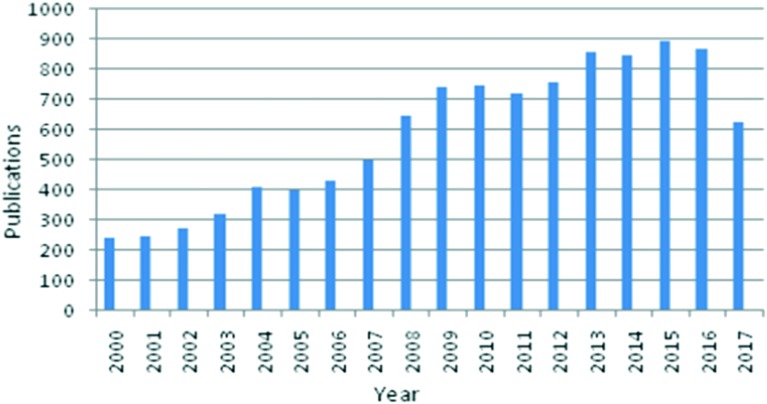
This review describes advances in the synthesis, design, and development of Schiff-base metal complexes as antimicrobial and anticancer agents. Interest in this field has rapidly grown in recent years, as illustrated by the increasing number of publications reported since 2000 (see Fig. 1). Although many reviews related to Schiff bases and their metal complexes have already been published,36–41 there is no comprehensive review discussing the potential of transition metal-based Schiff-base complexes as antimicrobial and anticancer agents and their future prospects. We are of the conviction that this review article will turn out to be a helpful reference material for specialists effectively involved in the design and synthesis of HSBs and their transition metal complexes.
Fig. 1. Number of articles in Web of Science on the topic “Schiff-base metal complexes” from 2000 to 2017.
2. Chemistry of Schiff bases
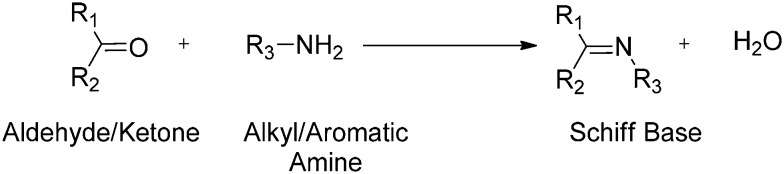
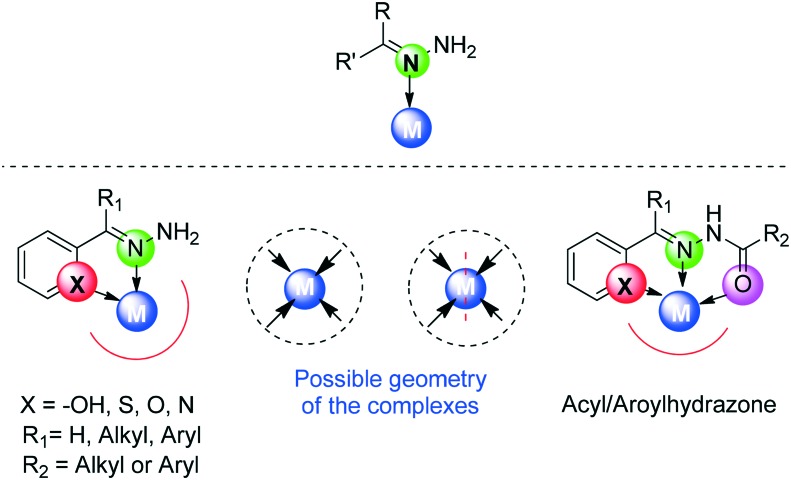
A Schiff base (SB), considered as a condensation product, is typically prepared by the most familiar and classical method discovered by Hugo Schiff, involving the condensation of an aldehyde or a ketone (carbonyl compound) with an amine under diverse reaction conditions and in different solvents.42–46 A SB is a nitrogen cognate of an aldehyde or ketone in which the carbonyl group (CO) has been supplanted by an imine or azomethine (–C N–) group (Fig. 2). The carbonyl group of the aldehydes gives aldimines while ketones give ketoimines. The general formula of a SB is R1R2C N–R3, where R1, R2 and R3 may be an alkyl, aryl or any heteroaryl group.47–49 The –C N– imine bond is responsible for endowing a wide spectrum of biological actions to these azomethinic compounds.50 Several SBs and their transition metal complexes have attracted considerable interest from medicinal chemists and biologists due to their advanced antibacterial,51 antifungal,52 antitumour,53 anticancer,54 antiradical,55 anti-tubercular,56 antimalarial,57 ROS scavenging58 and antiviral activities.59 The enhanced biological activity of these compounds is due to their excellent chelating ability with metals.60 These compounds are considered as versatile ligands due to their synthetic flexibility and structural stability61,62 and are thus being studied/examined deeply in view of their brilliant pharmacological activities.63–65 Additionally, these moieties show dominance in the development of organometallic chemistry.66
Fig. 2. A typical reaction scheme showing the formation of SBs.
Today, we are provided with adequate literature about the variety of methods involved in the synthesis of azomethines/imines.67 In addition to the known classical and other (microwave irradiation, ultrasonic irradiation, ionic solvent, solvent-free, etc.) methods, SBs are prepared by a more efficient, benign and green approach which involves the use of universal solvent (water) in the preparation of these compounds.68,69 SB reactions involve the formation of water molecules which impede the reaction rate by favoring a reversible reaction.70 Dehydrating agents (MgSO4, Na2SO4 or molecular sieves) or Dean–Stark apparatus can be employed to remove the water and favor the formation of SBs.71 Among the solvents, ethanol is the best choice and an imperative solvent for the preparation of SBs at room temperature or under refluxing conditions. Usually, a SB reaction is accelerated by performing the reaction under acidic or basic conditions.72
3. Types of Schiff bases and their metal complexes
As per the literature, presently we are familiar with diverse types of SBs which include salen-type, salophen-type, hydrazone-type and semicarbazone/thiosemicarbazone-type SBs. All these types of SBs have caught the attention of researchers owing to their unique and incogitable role in medicine and coordination chemistry.
3.1. Salen-type ligands
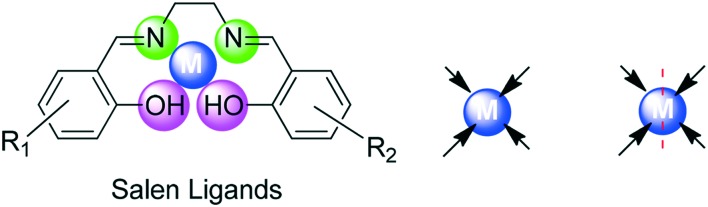
Salen-type ligands are named so because the preliminary materials involved in their preparation are salicylaldehyde, ethylenediamine and their derivatives. As a special SB, the salen ligand and its derivatives are condensation products from salicylaldehyde and ethylenediamine.73,74 Salen-type ligands and their transition metal complexes have been recognized since 1933 and they represent a distinctive system in coordination chemistry, which have received substantial attention because of their versatility and wide range of complexing abilities, and are additionally the key focuses in the advancement of bioinorganic chemistry, catalysis, magnetism, medical imaging, etc.75 In salens, the ligand backbone and the coordinated metal ion can be effortlessly varied which makes these ligand systems extensively useful in various industrial and biological applications.76,77 A wide range of salen-type compounds are present and, depending on the bonding modes, they feature two covalent and two coordinate covalent bonds occupying equatorial positions, thus behaving as [O, N, N, O] tetradentate ligands with two axial sites occupied by ancillary ligands as shown in Fig. 3.
Fig. 3. Salen as an [O, N, N, O] tetradentate ligand.
3.2. Salophen-type ligands
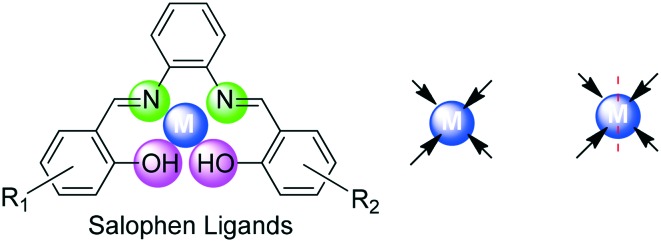
On the other hand, salophen-type ligands are those in which o-phenylenediamine is employed in place of ethylenediamine. These types of ligands are prepared by a typical condensation reaction which involves a reaction between two equivalents of salicylaldehyde or its derivatives and one equivalent of 1,2-phenylenediamine.78 The metal complexes of such ligands have engrossed the attention of researchers to utilize these compounds which are endowed with a huge number of industrial and biomedical applications. Salophen ligands are among the oldest and most widespread class of compounds in coordination chemistry and are very much like salen ligands having four coordinating sites (O, N, N, O) which lie in a plane and hence behave as tetradentate ligands as shown in Fig. 4.
Fig. 4. Salophen as an [O, N, N, O] tetradentate ligand.
3.3. Hydrazone-type ligands
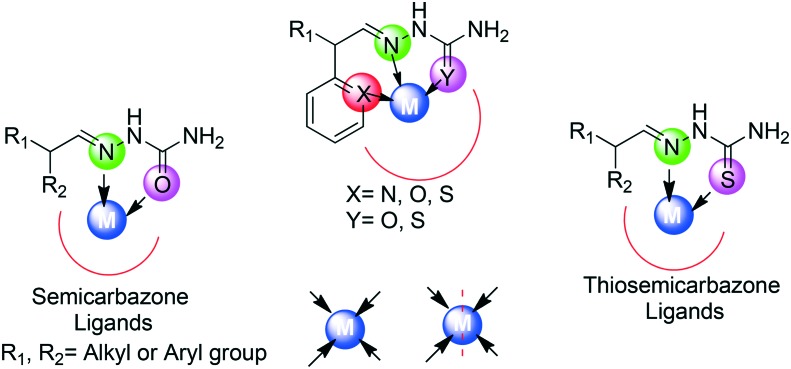
Another important class of SB ligands comprises hydrazone-type (R1R2C NNHR3) ligands which are the condensation products of carbonyl compounds with hydrazine/hydrazide and their derivatives. Hydrazones play a vital role in enhancing the selectivity and lethality profile of certain drug candidates. A vast content of the literature is present on hydrazone-type ligands unfolding their fascinating properties.79–82 A variety of multimetallic complexes of these ligands have been reported to possess different coordination behaviors with different metal atoms. Hydrazones may further include acyl hydrazone or aroylhydrazone SB ligands which are obtained by the condensation of an acylhydrazide or an aroylhydrazide with any carbonyl compound. An acyl or arylhydrazone SB is provided with an additional donor site in the form of a C O group which gives it more flexibility and versatility.83 Actually, hydrazone-type ligands possess a single donating atom (iminic nitrogen, N) and hence should be unidentate but their subgroups (acyl and aroylhydrazones) can behave as bidentate (N, O) or tridentate (N, O, X O, N, S) ligands as can be seen in Fig. 5.
Fig. 5. Hydrazone as a mono-(N), bi-(N, S/N/O) or tri-(N/S/O, N, O) dentate ligand.
3.4. Thiosemicarbazone/semicarbazone ligands
It was in 1800 that thiosemicarbazones/semicarbazones made their first appearance in the literature and proved themselves as excellent chelating agents.84 Thiosemicarbazone/semicarbazone SBs represent a family of compounds that are derived via the condensation reaction of a carbonyl compound (aldehyde or ketone) with thiosemicarbazide or semicarbazide. Their superior chelating ability and extensive lipophilic nature are of remarkable importance.85–89 Extensive literature studies supported the verity that a wide range of transition metal complexes of these typical SB ligands have been synthesized and explored to be endowed with immense applications. Thiosemicarbazones/semicarbazones exhibit a variety of bonding modes due to the presence of different donating sites. Such ligands generally have two donating sites (N, O/S) and hence behave as bidentate, but they can act as tridentate (N, S, X N, S, O) ligands also. A typical representation of bonding modes in such types of ligands is shown in Fig. 6.
Fig. 6. Thiosemicarbazone/semicarbazone as a bi-(N, O) or tri-(N/S/O, N, O) dentate ligand.
4. Biological importance of HSB transition metal complexes
With the advancement in various fields of chemistry, researchers are trying to explore new and versatile metal-based compounds to treat human diseases. Toxicity and multidrug resistance have forced scientists to devote their efforts to synthesizing novel and efficient antimicrobial and anticancer drugs. Among these compounds, HSBs are of prominent importance owing to their unique chelating properties due to the presence of important N, O, and S donor atoms.90 Among the galaxy of compounds, SBs are the most widely used organic compounds and used as synthons for the preparation of many industrial and biologically active molecules. HSBs and their metal complexes are gaining prominent importance because of their diverse applications in several fields.90–96 It has been acknowledged that a large number of these complexes could potentially behave as models for various biologically important species and thus may improve the interest of researchers in the design and synthesis of HSB complexes. A detailed overview of some biologically important HSBs is summarized in Table 1.
Table 1. The chemical structures of some noteworthy heterocyclic Schiff base ligands, metal atoms involved in chelation and their biological proclivity.
| S. no | HSB ligand | Metal atoms | Biological activity | Ref. |
| 1 |
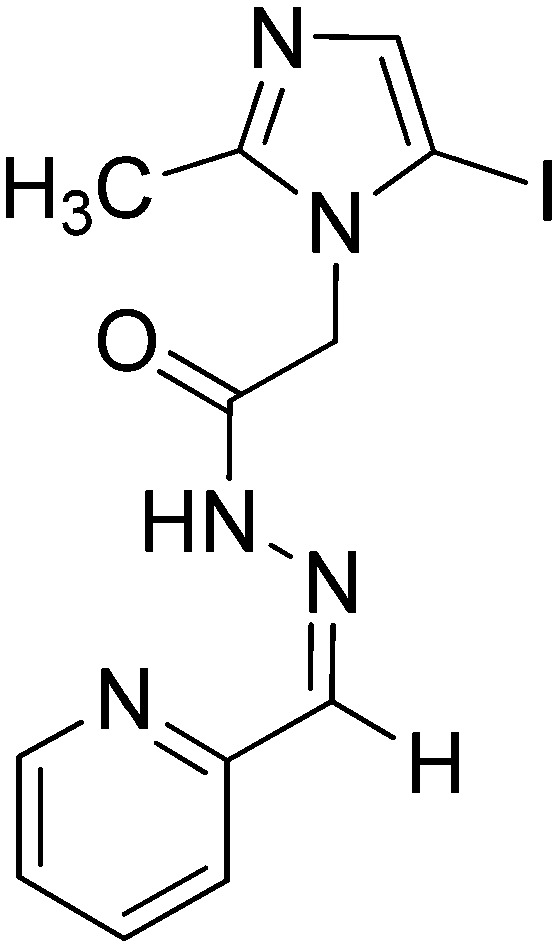
|
Co(ii) | Antifungal | 97 |
| Ni(ii) | ||||
| Cu(ii) | ||||
| 2 |
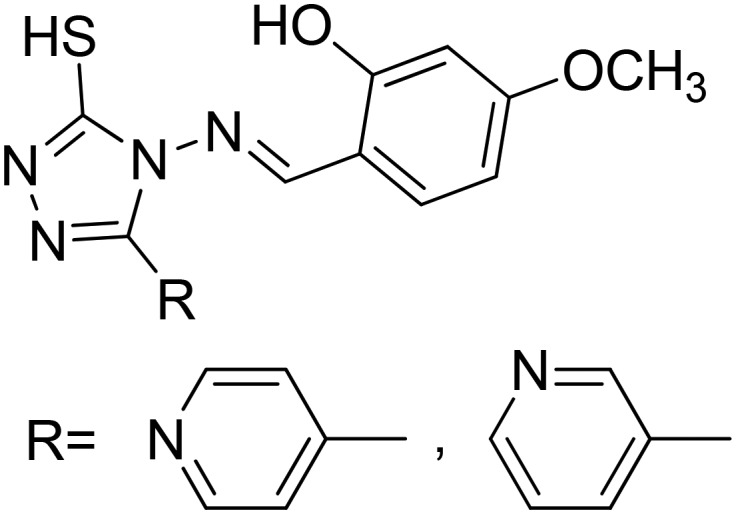
|
Co(ii) | Anticancer | 98 |
| Ni(ii) | ||||
| Cu(ii) | ||||
| Zn(ii) | ||||
| 3 |
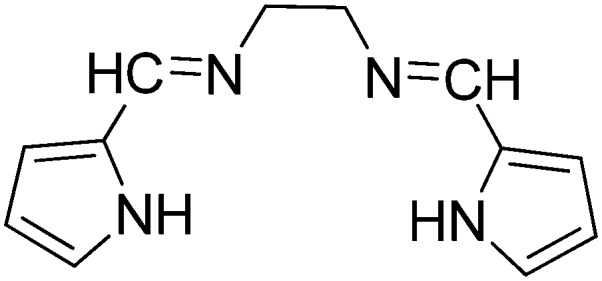
|
Mn(ii) | Antibacterial | 99 |
| Co(ii) | ||||
| Ni(ii) | ||||
| Cu(ii) | ||||
| 4 |
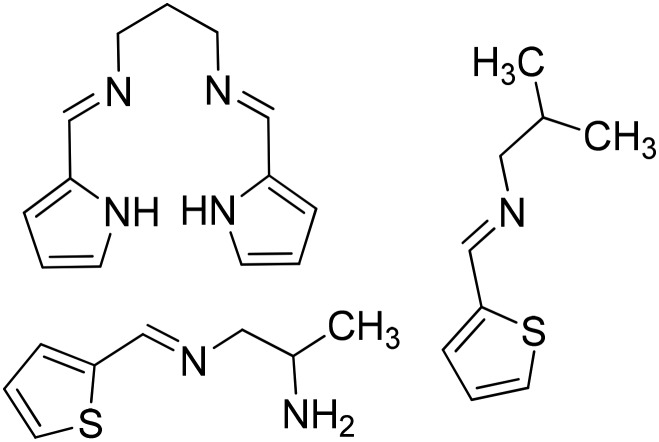
|
Co(iii) | Antibacterial | 100 |
| 5 |
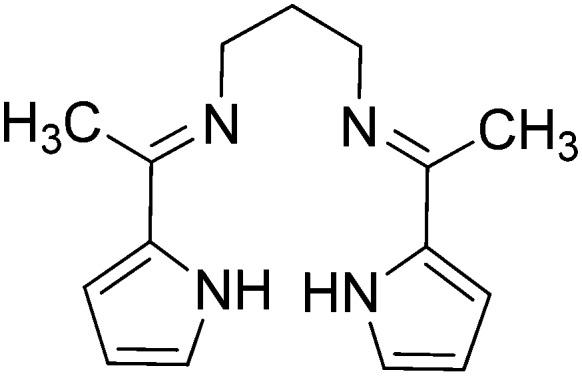
|
Fe(ii) | Antibacterial | 101 |
| Co(ii) | ||||
| Ni(ii) | ||||
| Cu(ii) | ||||
| Zn(ii) | ||||
| 6 |
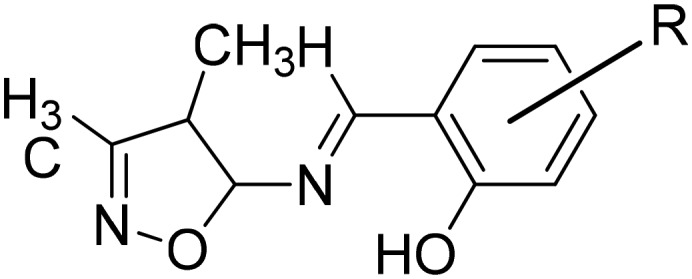 R
H, OH, Br, Cl, OCH3, NO2 R
H, OH, Br, Cl, OCH3, NO2
|
Cu(ii) | Antibacterial | 102 |
| Antitumor | ||||
| 7 |
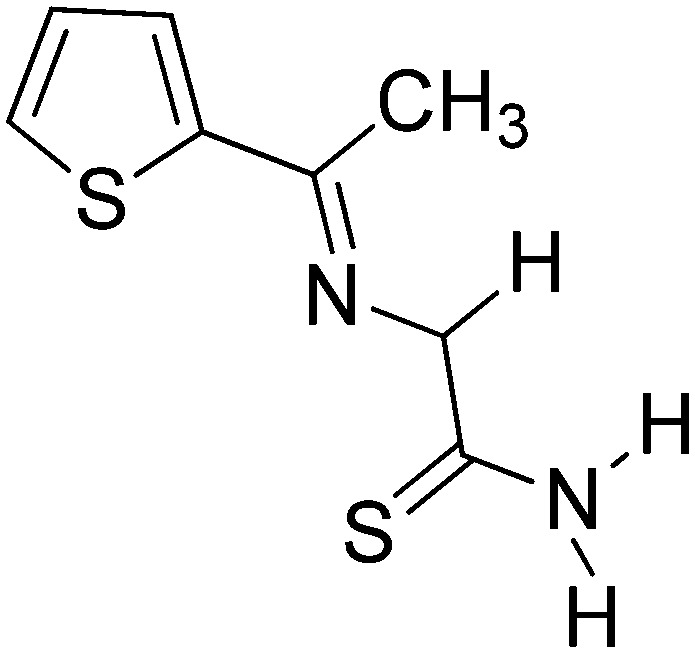
|
Pt(ii) | Antitumor | 103 |
| 8 |
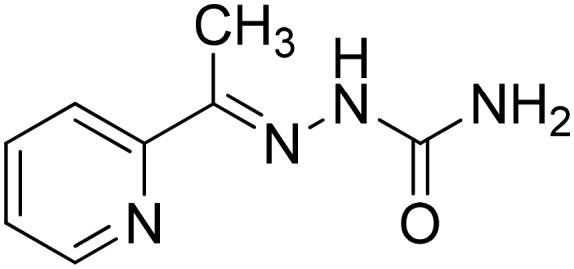
|
Cu(ii) | Antibacterial | 104 |
| Antifungal | ||||
| 9 |
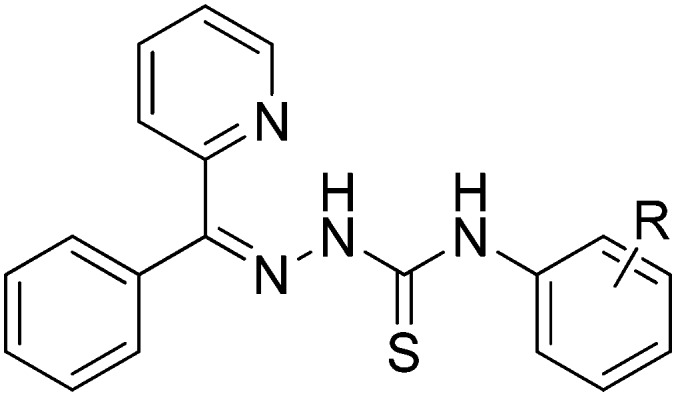 R
p-Br, p-Cl, 2,4-Me, 3,4-OMe 3,5-OMe, p-OMe, m-OMe, o-OMe, p-Me, m-Me R
p-Br, p-Cl, 2,4-Me, 3,4-OMe 3,5-OMe, p-OMe, m-OMe, o-OMe, p-Me, m-Me |
— | Antitumor | 105 |
| Anticancer | ||||
| 10 |
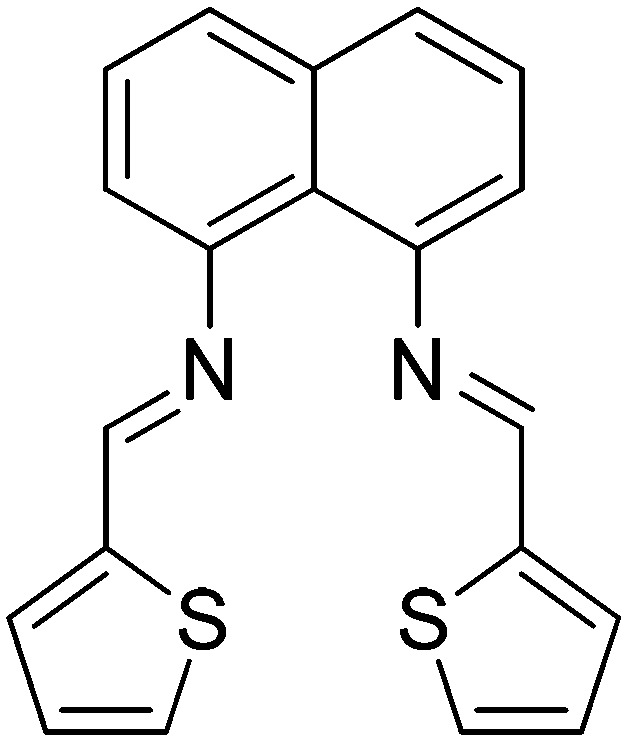
|
Cu(ii) | Anticancer | 106 |
| 11 |
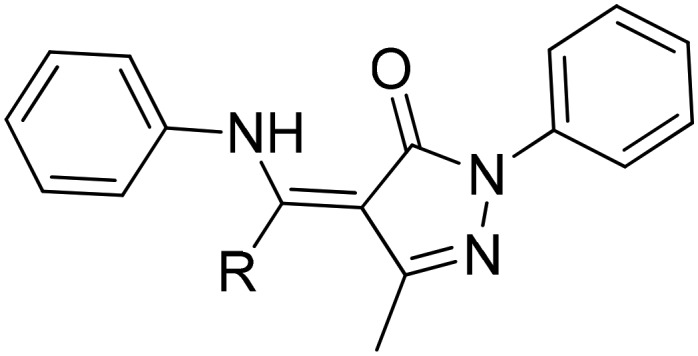 R
ph, naph, et R
ph, naph, et |
Ru(ii) | Anticancer | 107 |
5. Antimicrobial activity of HSB transition metal complexes
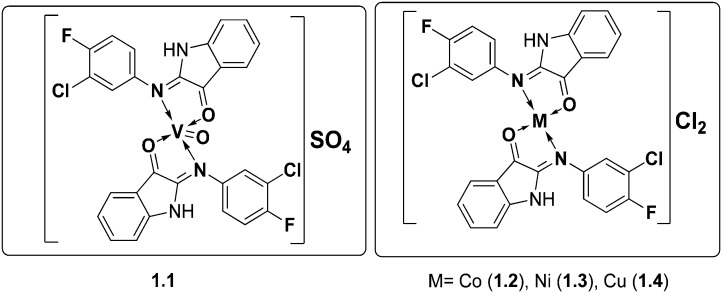
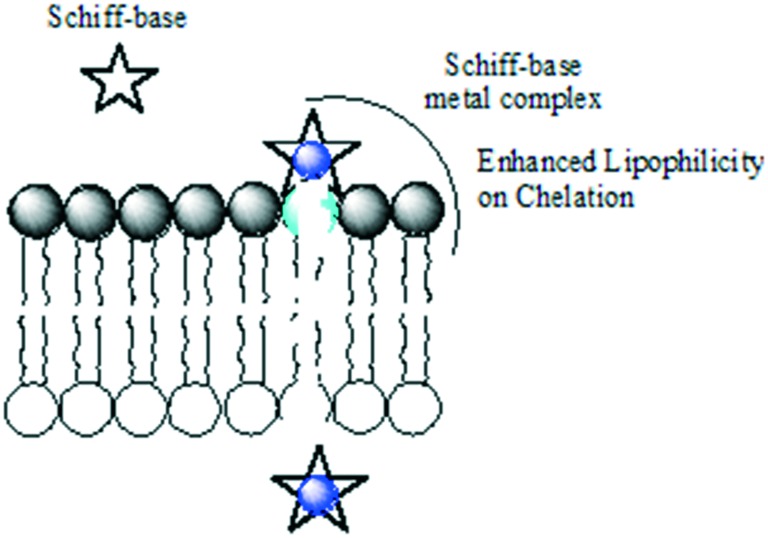
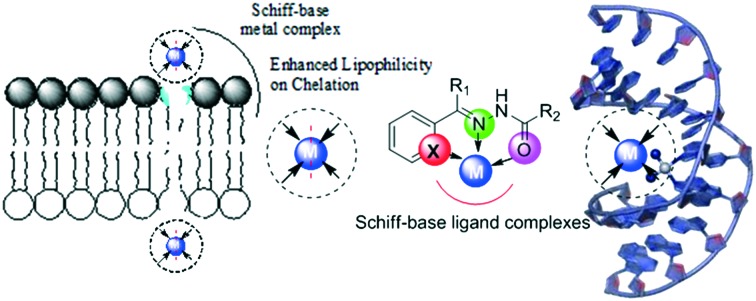
To explore some potential antimicrobial Schiff-base metal complexes, a series of metal complexes of V(ii), Co(ii), Ni(ii) and Cu(ii) were synthesized by conventional and microwave methods and screened for their biological potential. The synthesized SBs and their metal complexes (Fig. 7) exhibited excellent inhibitory action against a panel of bacteria (S. aureus, E. coli and S. fecalis) and fungi (A. niger, T. polysporum, C. albicans and A. flavus). The metal complexes were found to be better antimicrobial candidates compared with their parent SBs with the MIC values of the complexes in the range 10–40 μg mL–1. The enhanced efficiency of the metal complexes was explained by taking Overtone's concept and chelation theory into consideration. Chelation increases the delocalization of sigma electrons in the entire chelate ring and reduces the polarity of the metal ion to a larger extent, thereby favoring easy penetration of the complexes into the lipid membrane (a diagrammatic representation is provided in Fig. 8) and blocking metal binding sites in enzymes of microorganisms.108
Fig. 7. The above metal chelates displayed potential antifungal and antibacterial activities against a range of fungal and bacterial species.
Fig. 8. Schematic showing enhanced lipophilicity of metal complexes on chelation favoring easy penetration of the complexes into the bacterial lipid membrane.
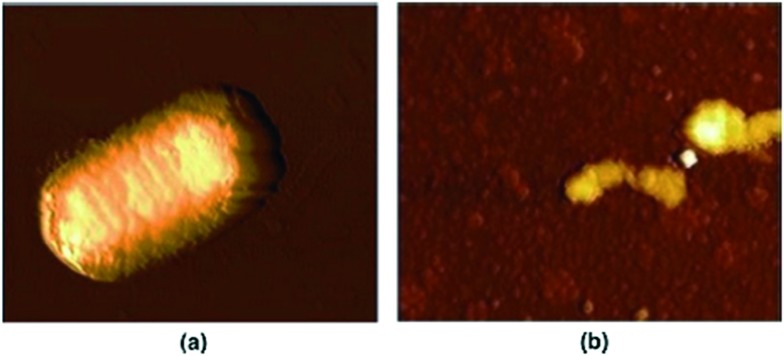
To assess the biological profile, another series of chemotherapeutic agents based on metals Cu(ii), Co(ii), Ni(ii) and Zn(ii) with a 2,4-diiodo-6-((pyridin-2-ylmethylimino)methyl)phenol SB possessing a N, N, O donor system respectively was synthesized and thoroughly analyzed (Fig. 9). The metal chelates showed non-intercalative DNA binding with herring sperm (HS-DNA), probably through grooves and/or on the surface, and displayed potential antimicrobial potency. Although all the metal complexes showed good antimicrobial activity, Co(ii) metal complex 1.6 showed the maximum zone of inhibition (21 mm) compared to the positive control streptomycin (19 mm). The Cu(ii) 1.5, Ni(ii) 1.7 and Zn(ii) 1.8 complexes also showed significant activity towards MRSA, K. pneumoniae, S. typhi and P. mirabilis compared to the positive control streptomycin. This higher antimicrobial activity of the metal complexes compared to Schiff bases and the disparity in activity arise from the different nature of the metal ions and their coordination modes. Chelation reduces the polarity of the metal ion and improves the lipophilicity of the metal complex and, among all the tested complexes, Co(ii) metal complex 1.6 completely inhibited the growth of E. coli cells as shown by the AFM images in Fig. 10. The complete rupture of the cells depicted that the complex bound to the cell wall and ruptured it due to oxidative stress on E. coli, entering into the nucleus, thereby degrading the chromosomal DNA by preventing protein synthesis which is essential for all cellular metabolism.109
Fig. 9. Structure of Cu(ii), Co(ii), Ni(ii) and Zn(ii) metal complexes 1.5–1.8 with 2,4-diiodo-6-((pyridin-2-ylmethylimino)methyl)phenol SB.
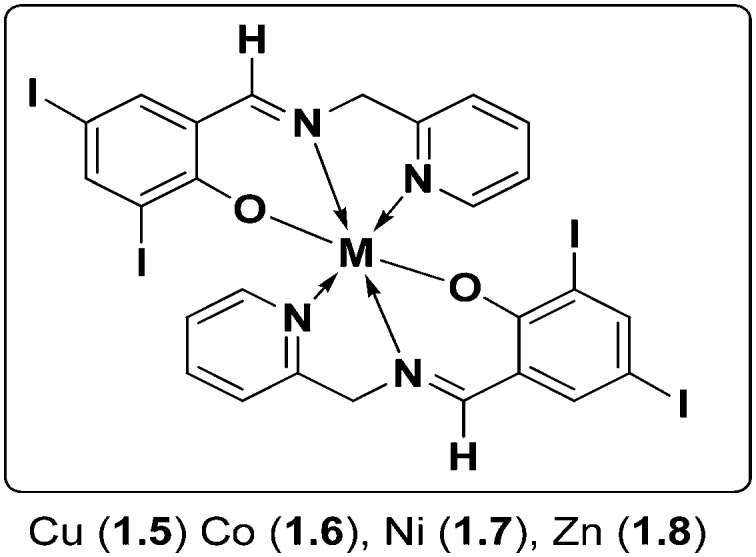
Fig. 10. AFM images of (a) control: rod shape of E. coli and (b) treated sample: Co(ii) complex 1.6 induces cell disruption of E. coli. Copyright permission obtained from Elsevier.109.
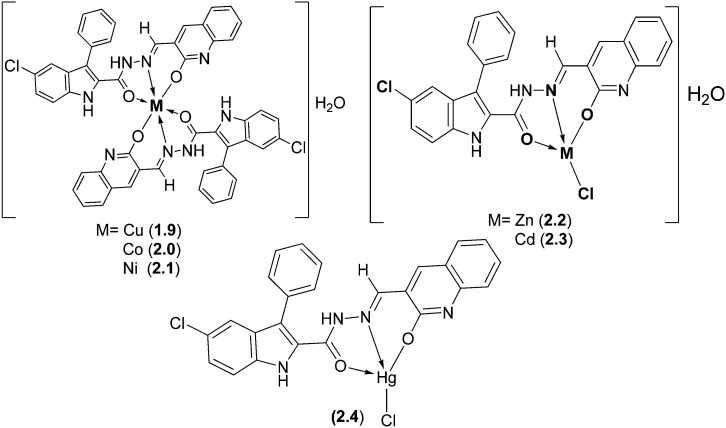
With the specific goal of assessing the impact of metal atoms upon chelation, some biologically active SB ligands obtained from 5-chloro-3-phenyl-1H-indole-2-carboxyhydrazide and 3-formyl-2-hydroxy-1H-quinoline and their Cu(ii), Co(ii), Ni(ii), Zn(ii), Cd(ii), and Hg(ii) complexes were thoroughly studied and characterized by various sophisticated spectral studies (Fig. 11). Antibacterial and antifungal studies were performed by checking the minimum inhibitory concentration (MIC), which revealed that the activity of the ligands against E. coli sharply increased upon complexation. The MIC values were in the range of 12.50–75 μg mL–1 and 12.50–50 μg mL–1 for the bacterial and fungal species, respectively.110
Fig. 11. Structures of metal complexes containing hydrazone-type ligands. Such bioactive metal complexes on screening were found to possess enhanced antimicrobial activities especially against E. coli.
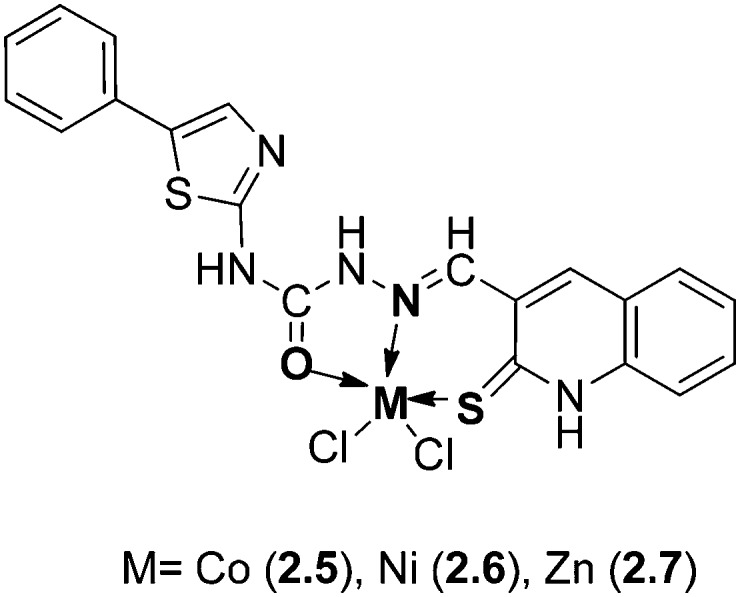
In another study, some novel Co(ii), Ni(ii), and Zn(ii) metal complexes (Fig. 12) of a HSB obtained from N-(4-phenylthiazol-2-yl)hydrazinecarboxamide and 2-thioxo-1,2-dihydroquinoline-3-carbaldehyde exhibited good antibacterial and antifungal activities against E. aerogenes and P. aeruginosa bacteria and A. niger and A. flavus fungal strains, respectively.111 The MIC values of the metal complexes were found to be in the range 0.78–25 μg mL–1. The biological results obtained showed that the metal complexes were more active compared with the ligand, which was explained on the basis of chelation theory, as explained above.108
Fig. 12. Structure of Co, Ni and Zn metal complexes containing heterocyclic moieties.
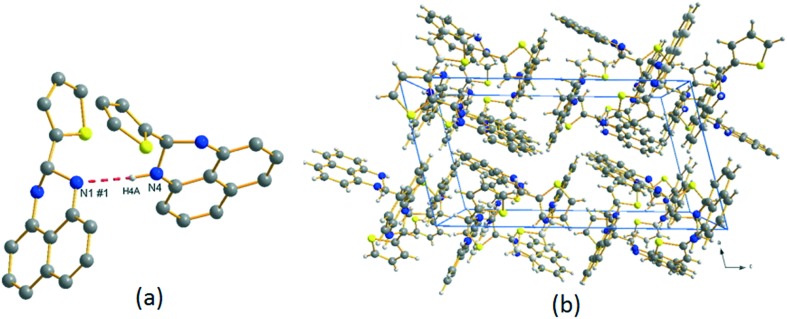
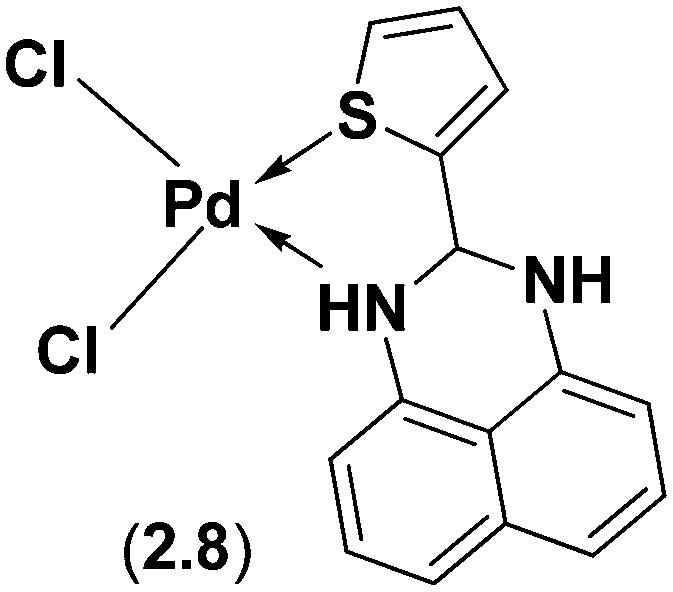
Perimidine and its derivatives display diverse biological properties due to the presence of a pyrimidine ring system in nucleic acids, vitamins, co-enzymes and antibiotics. A novel SB ligand, 2-(2-thienyl)-2,3-dihydro-1H-perimidine, has been synthesized from 2-thiophenecarboxaldehyde and 1,8-diaminonaphthalene and analyzed using various sophisticated spectral techniques.112 X-ray crystallographic structure analysis revealed that the ligand crystallizes in the centrosymmetric monoclinic P21/c space group with all atoms located in general positions and two crystallographically independent 2-(2-thienyl)-2,3-dihydro-1H-perimidine ligands in its asymmetric unit, with four such units in the unit cell (see Fig. 13). The integrated ligand and its Pd(ii) complex 2.8 (Fig. 14) were investigated for their capacity to control the in vitro development of organisms like E. coli, S. aureus, P. aeruginosa, Citrobacter sp., B. subtilis and S. acidaminiphila. The outcomes uncovered that the Pd(ii) complex 2.8 displayed noteworthy antimicrobial activity with an MIC of 50 μg mL–1 as compared to the positive control, amoxicillin, which exhibited an MIC of up to 30 μg mL–1.112
Fig. 13. (a) 2-(2-Thienyl)-2,3-dihydro-1H-perimidine ligand showing intermolecular hydrogen bonding; (b) crystal packing of 2-(2-thienyl)-2,3-dihydro-1H-perimidine viewed (perspective) along the crystallographic b direction. Copyright permission obtained from Elsevier.112.
Fig. 14. Molecular structure of the perimidine-based metal complex. Palladium complex 2.8 exhibits enhanced bioactivity compared to its corresponding ligand.
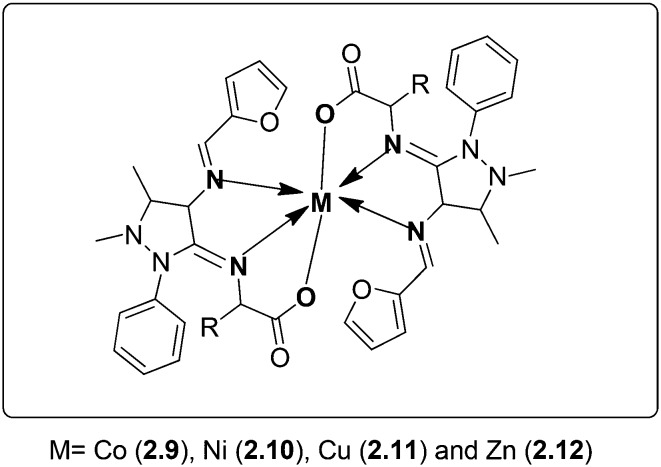
Microbial resistance has necessitated reiterating the search for new and effective drug molecules. A series of novel biologically active metal [Co(ii), Cu(ii), Ni(ii) and Zn(ii)] complexes were synthesized (Fig. 15). The SB was obtained by the condensation of 4-aminoantipyrine with furfural and amino acids (glycine, alanine, valine). The synthesized ligand and its metal complexes were analyzed using various analytical and spectral methods. The metal chelates were evaluated for their expected antibacterial and antifungal actions. Disc diffusion and MIC studies revealed that the metal chelates 2.9–2.12 possessed enhanced antibacterial activity against S. aureus, P. aeruginosa, E. coli, S. epidermidis and K. pneumoniae with MIC values in the range of 2.2–26.4 μg mL–1. In vitro antifungal tests reveal that these complexes are good antifungal substitutes against A. niger, A. flavus, C. lunata, R. bataticola and C. albicans with MIC values in the range of 3.1–26.4 μg mL–1.113
Fig. 15. Proposed structure of metal complexes 2.9–2.12. These complexes have great potential to mitigate the chances of bacterial and fungal infections which has been confirmed from their biological screening.
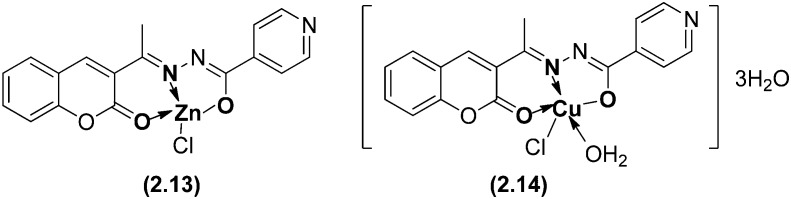
Similar Schiff base ligands from 4-aminoantipyrine, phenyl-3-methyl-5-pyrazolone and their transition metal complexes have been exposed to show interesting antimicrobial activities although not higher than those of standard drugs used as positive controls.114–120 Metal complexes (Fig. 16) derived from 3-acetylcoumarin and isonicotinoyl hydrazide ligand systems have been subjected to antimicrobial screening. The Zn(ii) complex (2.13) showed the highest antifungal activity with an MIC value of 6.25 μg mL–1 while the Cu(ii) complex (2.14) showed the highest antibacterial activity at the same concentration.121
Fig. 16. Molecular structures of Zn(ii) 2.13 and Cu(ii) 2.14 complexes displaying promising antimicrobial activity.
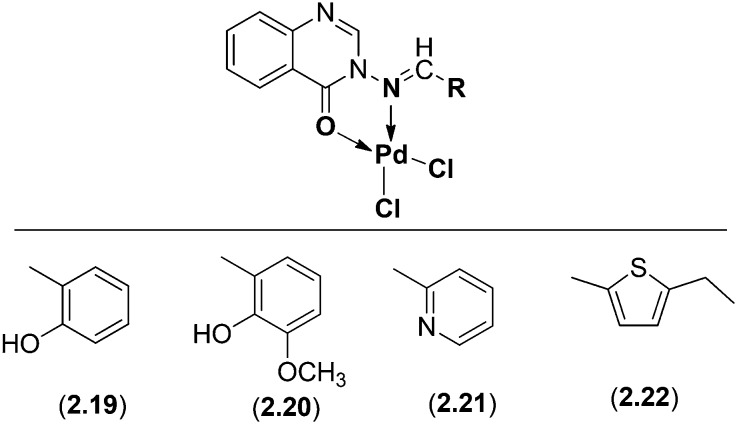
A series of novel mononuclear Pd(ii) complexes (Fig. 17) derived from 3-amino-2-methyl-4(3H)-quinazolinone and substituted aldehydes were analyzed and screened for their biological activities. The structural elucidation of the complexes was confirmed by several spectroscopic studies like IR, 1H NMR, elemental analysis, molar conductance, mass and UV-vis spectrometric and thermal studies. All the ligands (2.15–2.18) and their Pd(ii) complexes (2.19–2.22) were screened against three bacterial species (B. subtilis, S. aureus and E. coli) and showed promising activity against these microorganisms. The biological profile of the Schiff base ligands and their Pd(ii) complexes is summarized below in Table 2.122
Fig. 17. Proposed structure of Pd(ii)complexes 2.19–2.22. These novel mononuclear complexes exhibited proficient antimicrobial activities.
Table 2. Antibacterial activity (zone of inhibition) of ligands (2.15–2.18) and their Pd(ii) complexes (2.19–2.22).
| Compound | Concentration (μg mL–1) | Antibacterial activity (zone of inhibition in %) |
||
| B. subtilis | E. coli | S. aureus | ||
| 2.15 | 100 | 45 | 48 | 44 |
| 2.16 | 100 | 33 | 39 | 37 |
| 2.17 | 100 | 54 | 52 | 51 |
| 2.18 | 100 | 35 | 40 | 38 |
| 2.19 | 100 | 78 | 81 | 78 |
| 2.20 | 100 | 67 | 72 | 77 |
| 2.21 | 100 | 80 | 83 | 81 |
| 2.22 | 100 | 67 | 66 | 71 |
| Gentamicin | 100 | 100 | 100 | 100 |
A series of Cu(ii) SB ligand complexes (Fig. 18) derived from furfurylidene-4-aminoantipyrine and aniline, p-nitroaniline, and p-hydroxyaniline, respectively, were synthesized. The ligands 2.23–2.25 and their metal complexes 2.26–2.28 were characterized using analytical and spectral techniques confirming the square planar geometry of the complexes. The biological profile of these complexes was checked against the bacterial species S. aureus, E. coli, K. pneumoniae, P. vulgaris and P. aeruginosa and contagious species A. niger, R. stolonifer, A. flavus, R. bataticola and C. albicans by the serial dilution method. A related study of the inhibition values of the SB ligands 2.23–2.25 and their complexes 2.26–2.28 reveals that the complexes display superior antimicrobial activity to the SB ligands with their MIC values given in Tables 3 and 4, respectively.123
Fig. 18. Proposed structure of the Cu(ii) complexes 2.26–2.28.
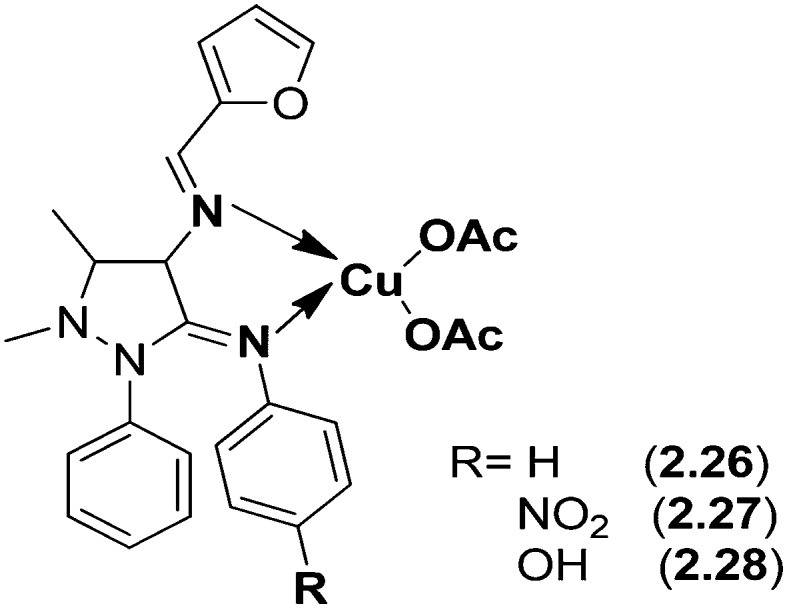
Table 3. Minimum inhibitory concentration of the synthesized compounds against growth of bacteria (μg mL–1).
| Compound | E. coli | K. pneumoniae | P. vulgaris | P. aeruginosa | S. aureus |
| 2.23 | 60 | 64 | 66 | 66 | 72 |
| 2.24 | 24 | 26 | 20 | 16 | 28 |
| 2.25 | 28 | 36 | 26 | 32 | 30 |
| 2.26 | 34 | 38 | 32 | 28 | 42 |
| 2.27 | 26 | 28 | 30 | 26 | 48 |
| 2.28 | 52 | 54 | 58 | 60 | 63 |
| Penicillin G | 10 | 15 | 6 | 12 | 4 |
| Ampicillin | 12 | 10 | 8 | 4 | 6 |
| Vancomycin | 6 | 14 | 12 | 10 | 8 |
| Ofloxacin | 8 | 10 | 4 | 6 | 14 |
Table 4. Minimum inhibitory concentration of the synthesized compounds against growth of fungi (μg mL–1).
| Compound | A. niger | R. stolonifer | A. flavus | R. bataticola | C. albicans |
| 2.23 | 60 | 66 | 72 | 80 | 50 |
| 2.24 | 19 | 20 | 20 | 25 | 22 |
| 2.25 | 24 | 28 | 28 | 34 | 30 |
| 2.26 | 28 | 30 | 34 | 38 | 22 |
| 2.27 | 32 | 26 | 46 | 36 | 6 |
| 2.28 | 52 | 55 | 68 | 80 | 50 |
| Nystatin | 10 | 16 | 8 | 14 | 12 |
| Ketoconazole | 12 | 8 | 16 | 6 | 12 |
| Clotrimazole | 8 | 6 | 12 | 10 | 4 |
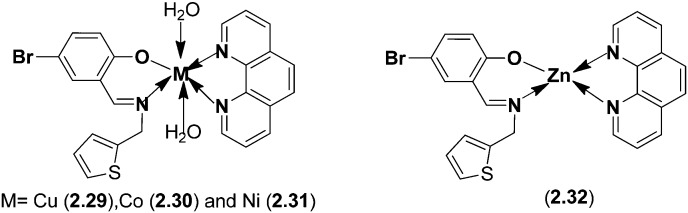
Mixed ligand complexes of Cu(ii), Ni(ii), Co(ii) and Zn(ii) with 1,10-phenanthroline and SB 2-aminomethylthiophenyl-4-bromosalicylaldehyde (Fig. 19) have been synthesized, characterized and explored for their biological activities using the disc diffusion method.124 The biological studies of the ligands and their metal complexes 2.29–2.32 against different selected types of bacteria (S. flexneri, P. vulgaris, S. aureus and B. subtilis) and fungi (A. fumigatus and C. albicans) revealed that they possessed better antimicrobial action than those of standard antibiotics. Among the metal complexes, Zn(ii) complex 2.32 displayed MIC values of 0.06–9.9 μg mL–1 on bacterial species and 1.95–7.81 μg mL–1 on fungal species.
Fig. 19. Structures of SB metal (Cu, Co, Ni and Zn) complexes. Zn(ii) complex 2.32 displayed better antimicrobial activity compared to the other metal complexes 2.29–2.31.
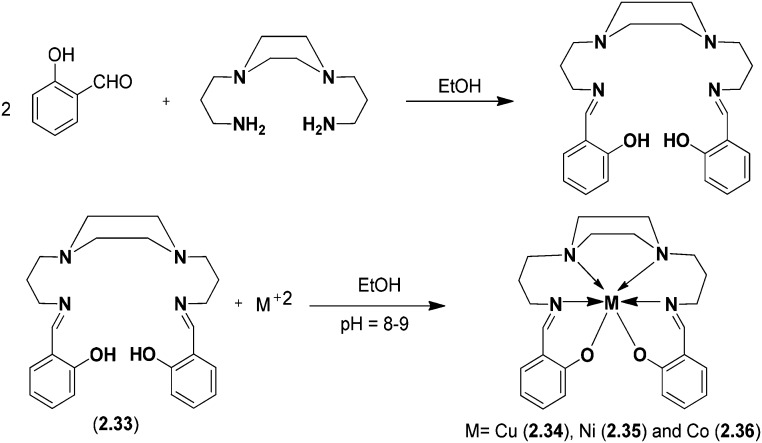
Cu(ii), Co(ii), and Ni(ii) metal complexes of a SB ligand, 1,4-bis[(2-hydroxybenzaldehyde)propyl]piperazine (BHPP), were synthesized and characterized using various spectroscopic techniques. The synthesized ligand exhibited hexavalent coordination involving the two azomethine nitrogen atoms, two nitrogen atoms of the piperazine ring and two deprotonated phenolic OH groups. Spectral and magnetic studies have revealed an octahedral geometry of the metal(ii) complexes (Fig. 20). The synthesized ligand 2.33 and its metal complexes 2.34–2.36 were tested for their antimicrobial actions against various Gram-positive and Gram-negative bacterial strains and fungal strains using a modified Kirby–Bauer disc diffusion method. The bioactive nature of the ligand 2.33 is due to the presence of piperazine-N, the hydroxyl group and the two azomethinic groups. On accessing the biological potential of the ligand and its metal chelates, it was established that the metal chelates were more toxic than the parent SB ligand. It was established that the antimicrobial activity of the synthesized compounds was due to their ability to inhibit the synthesis of peptidoglycans necessary for the formation of the cell wall.125
Fig. 20. Scheme for synthesis of the piperazine-based SB ligand and its metal(ii) complexes. The above metal chelates inhibit cell wall synthesis in microbes and hence behave as potent antimicrobial agents.
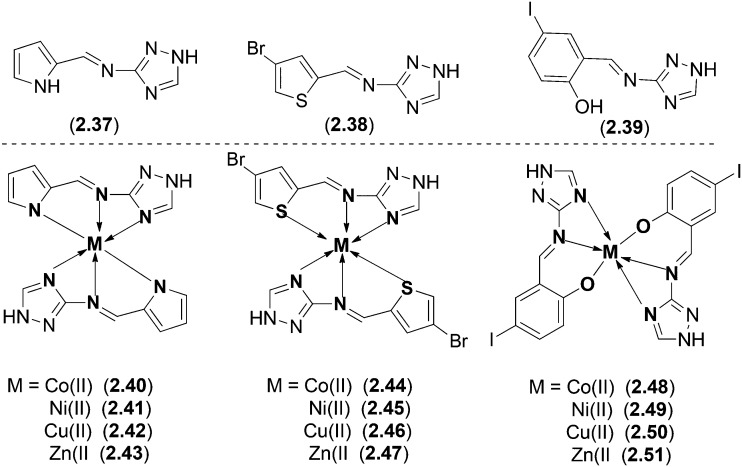
Co(ii), Ni(ii), Cu(ii) and Zn(ii) complexes with three different Schiff base ligands were synthesized (Fig. 21) and characterized using various spectroscopic techniques to affirm the structure of all the complexes.126 The ligands 2.37–2.39 were obtained by the condensation reaction of 3-amino-1H-1,2,4-triazole with pyrrole-2-carboxaldehyde, 4-bromothiophene-2-carboxaldehyde, and 5-iodo-2-hydroxybenzaldehyde, respectively. On biological screening, the ligands displayed varying degrees of inhibitory effects with 2.37 showing significant activity (66% inhibition as determined by MIC). All the metal complexes (2.40–2.51) were tested for their antimicrobial actions using the disc diffusion method against four different Gram-negative, i.e., E. coli, S. sonnei, P. aeruginosa and S. typhi, and two Gram-positive, i.e., B. subtilis and S. aureus, bacterial species. Metal complexes 2.41, 2.45, 2.47, 2.49, 2.50 and 2.51 displayed efficient activity (54–82%) against all the tested bacterial strains. The agar-well diffusion method was employed to check the antifungal activity against fungal species, i.e., T. longifusus, C. albicans, A. flavus, M. canis, F. solani and C. glabrata. The metal complexes displayed significant activity as shown by the higher zone of inhibition compared to their parent ligands. Metal complex 2.41 displayed efficient activity with 73% inhibition against C. glabrata.
Fig. 21. Molecular structures of Co(ii), Ni(ii), Cu(ii) and Zn(ii) complexes with potent biological actions against a range of bacterial and fungal strains.
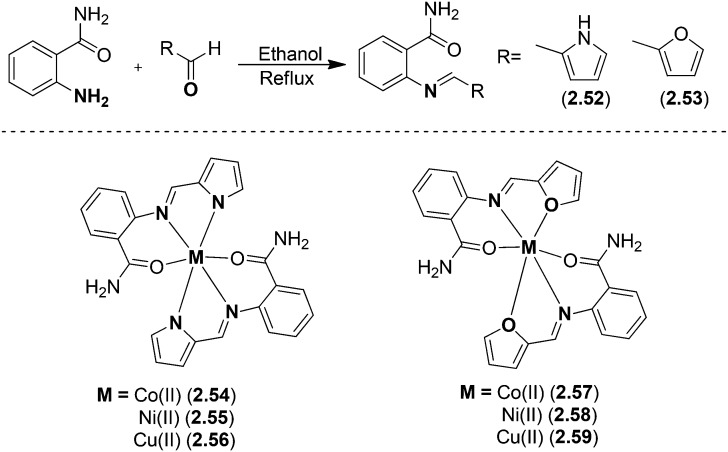
Metal chelates of Cu(ii), Ni(ii) and Co(ii) (Fig. 22) with SB ligands prepared via the condensation of 2-aminobenzamide with pyrrole-2-carboxaldehyde and furan-2-carboxaldehyde were screened for their in vitro antimicrobial activities.127 Biological studies revealed that the metal complexes possessed significant antimicrobial potential against four strains of bacteria and two fungal strains. The ligands 2.52 and 2.53 showed MIC values in the range of 6.250–12.250 μg mL–1 while the complexes 2.54–2.59 exhibited enhanced activity with MIC values of 0.125–12.250 μg mL–1.
Fig. 22. Scheme for synthesis of biologically active ligands 2.52 and 2.53 and their Co, Ni and Zn metal complexes 2.54–2.59.
6. Anticancer activity of HSB transition metal complexes
Normally, our body is incessantly in the process of generating new cells in order to reinstate the dysfunctional and old dead cells. Sometimes, this normal process is disrupted and this results in the formation of new cells even when our body does not need them. This undesired and uncontrolled growth of cells is termed cancer. There seems an unending battle among medicinal chemists about the design and synthesis of potential drugs to treat this deadly and notorious disease. However, efforts are ongoing to develop some new metallo-drugs with improved properties compared to the currently available metal-based drugs available in the market.
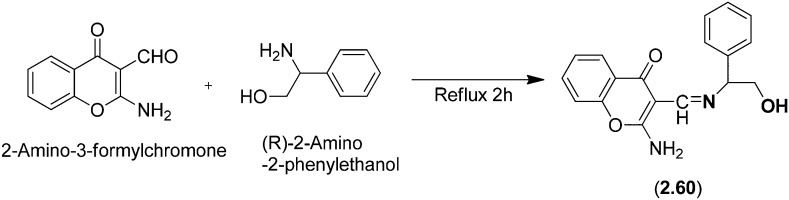
A new chiral HSB ligand (2.60) was obtained by a simple condensation reaction of 2-amino-3-formylchromone with (R)-2-amino-2-phenylethanol involving the combination of the amine functionality and a naturally occurring heterocyclic chromone, 4H-benzopyran-4-one (Fig. 23).
Fig. 23. Synthesis of ligand 2.60 from 2-amino-3-formylchromone and (R)-2-amino-2-phenylethanol via a simple condensation reaction.
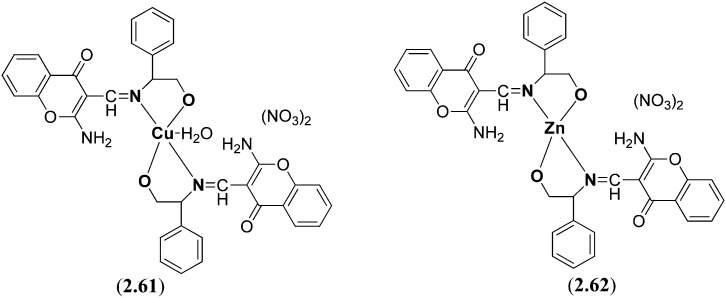
Subsequently, its complexes 2.61 and 2.62 with Cu(NO3)2 and Zn(NO3)2, respectively, were prepared (Fig. 24). The DNA binding studies of the ligand 2.60 and metal chelates 2.61 and 2.62 with CT-DNA were compared with the established anticancer medication cisplatin by utilizing different optical strategies, viz., UV-visible, fluorescence, circular dichroism and viscosity estimations. Besides, 1H and 31P absorption studies with mononucleotides were likewise conducted to examine the base-specific interactions of the transition metal complexes which revealed a higher inclination of copper(ii) complex 2.61 for 50-GMP while for zinc(ii) complex 2.62 towards 50-TMP involving a groove binding mechanism of the complexes towards DNA. The copper(ii) complex 2.61 exhibits an extraordinary DNA cleavage activity with pBR322 DNA in the presence of different activators and the cleavage reaction involves various oxygen species signifying the involvement of active oxygen species in DNA scission.128
Fig. 24. Structures of synthesized Cu (2.61) and Zn (2.62) complexes containing a naturally occurring heterocyclic chromone (4H-benzopyran-4-one) moiety. These heterocyclic chiral metal complexes possess significant DNA binding properties and hence display interesting anticancer activity.
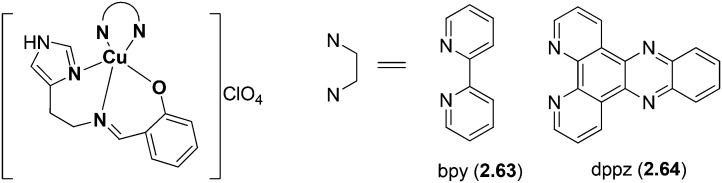
Copper(ii) complexes (2.63, 2.64) of a HSB resulting from the condensation of salicylaldehyde, histamine and pyridyl ligands were prepared, analyzed and studied for their photo-activated DNA cleavage activity, DNA binding and photocytotoxicity.121 The distorted square-pyramidal geometry of the complexes (Fig. 25) showed intercalative CT-DNA binding with a binding constant (Kb) of ∼105 M–1. Unlike other copper(ii) complexes of phenanthroline bases that are known to show undesirable high chemical nuclease activity, the dppz copper(ii) complex 2.64 with moderate chemical nuclease activity is suitable for PDT applications. The SB produced from salicylaldehyde and histamine has been used due to its inherent anti-tumorigenesis activity. The planar polypyridyl ligands are well known for their intercalative DNA binding, photoactivity and preferential nuclear uptake. Significant results of this study include remarkable DNA photocleavage activity in red light involving hydroxyl radicals (–OH) and visible light-induced photocytotoxicity of 2.64 in HeLa cells with low cytotoxicity in the dark and an IC50 value of 1.6 μM. It is found that cell death occurs through an apoptotic pathway involving photo-induced production of intracellular reactive oxygen species (ROS).129
Fig. 25. Structure of mixed ligand (dipyridophenazine, bipyridyl) Cu(ii) complexes. These mixed ligand complexes obtained by simple condensation of salicylaldehyde, histamine and pyridyl possessed prominent anticancer activity.
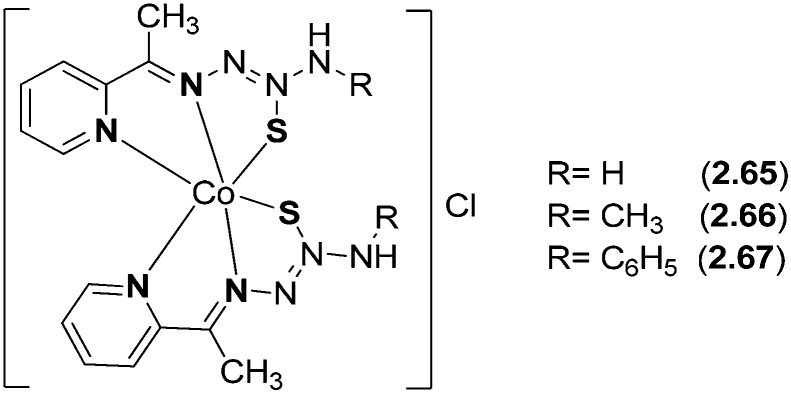
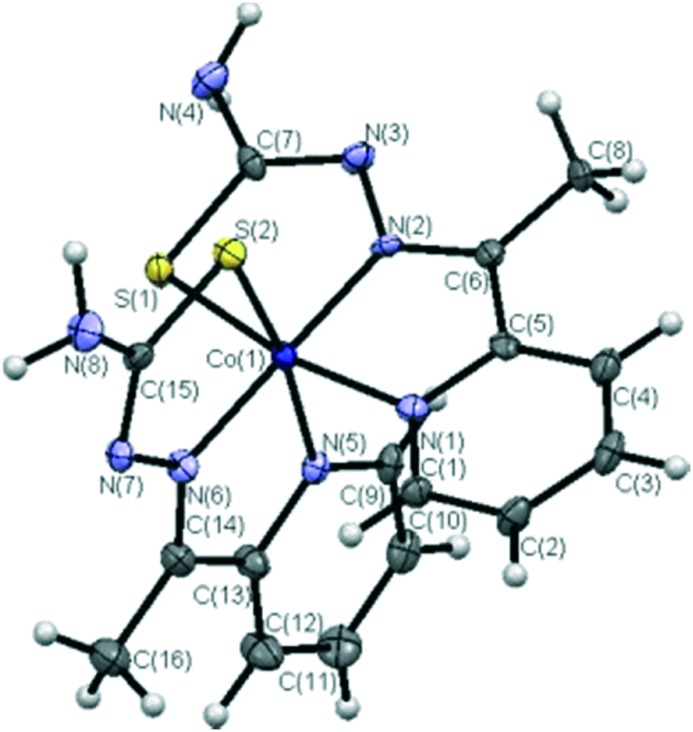
Co(iii) complexes (2.65–2.67) from 2-acetylpyridine N-substituted thiosemicarbazone ligands were prepared and analyzed by various sophisticated spectral studies (Fig. 26). Single crystal X-ray diffraction studies were carried out to determine the molecular structure of the complexes. The coordination geometry around the Co(iii) metal ion was described as a distorted octahedron, where the cobalt atom is bonded to two tridentate NNS donor ligand molecules in such a way that four 5-membered chelate rings are formed. The azomethinic nitrogen atoms [N(2) and N(6)] are placed in a trans-position and the chlorine atom acts as a counterion (Fig. 27). In vitro DNA binding studies of the metal chelates carried out by fluorescence studies showed that the complexes have greater binding constant (Kb) values and confirmed the binding of the complexes to DNA via intercalation. It has also been confirmed through gel electrophoresis assay that the complexes proved to be good DNA cleavage agents. The synthesized metal complexes exhibited enhanced antioxidant activities as compared to the standards BHT and vitamin C. Furthermore, the in vitro cytotoxicity of the metal chelates against human skin carcinoma (A431) and human breast cancer (MCF-7) cell lines was evaluated, which showed prominent action and competently removed the cancer cells even at low concentrations, showing that the complexes possess higher activity than cisplatin [IC50 value = 3.19 μM (MCF-7) and 12.33 μM (A431)].130 Complex 2.67 is more active than the other complexes 2.65 and 2.66 in the cell lines tested, and it exhibited much better activity than cisplatin in the MCF-7 cell line as shown in Table 5. The cytotoxicity of the tested complexes against both cancer cell lines follows the order 2.67 > 2.66 > 2.65. The higher cytotoxic activity of complex 2.67 may be due to the terminal phenyl substitution in the coordinated HL3 ligand.
Fig. 26. Structure of bioactive Co(ii) complexes 2.65–2.67. The metal chelates have been proved to be potential candidates to fight several types of cancers.
Fig. 27. ORTEP view of complex 2.65. Copyright permission obtained from Elsevier.130.
Table 5. IC50 (μM) values of cobalt(iii) complexes 2.65–2.67 and cisplatin against human breast cancer (MCF-7) and skin carcinoma (A431) cell lines.
| Complex | IC50 (μM) |
|
| MCF-7 | A431 | |
| 2.65 | 22.99 ± 0.08 | 32.12 ± 1.03 |
| 2.66 | 7.24 ± 0.09 | 16.85 ± 2.09 |
| 2.67 | 1.99 ± 0.13 | 9.30 ± 0.03 |
| Cisplatin | 3.19 ± 0.02 | 12.33 ± 0.07 |
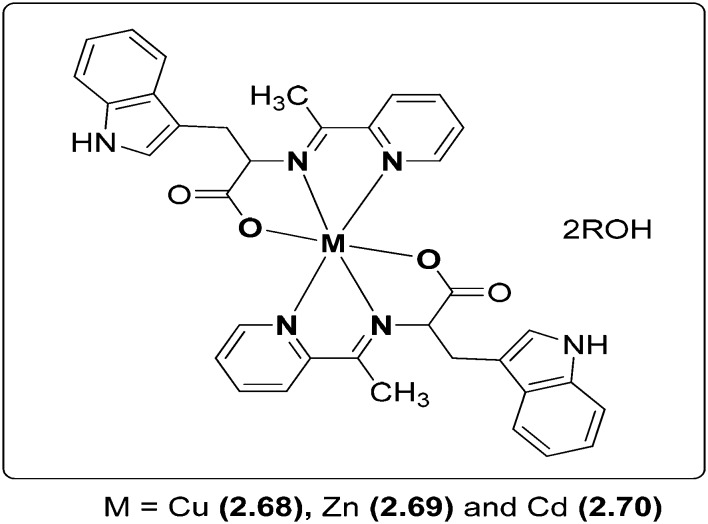
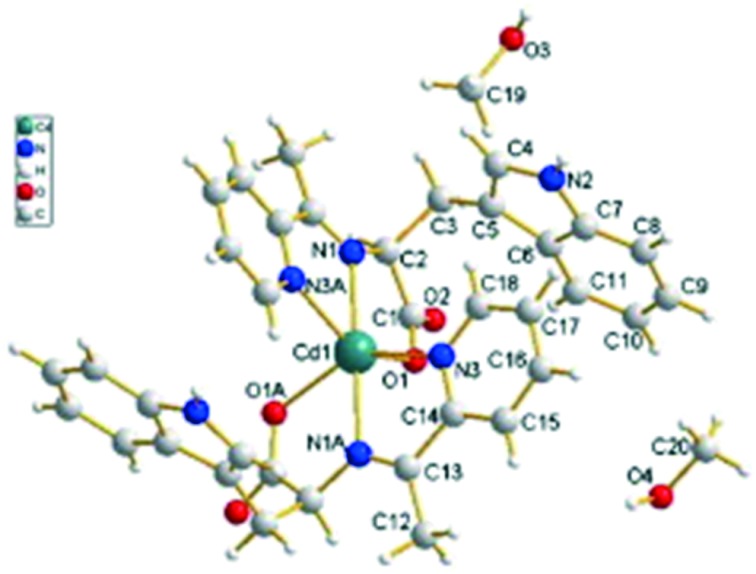
Novel Cu, Zn and Cd transition metal coordination complexes 2.68–2.70 (Fig. 28) have been synthesized and analyzed thoroughly by IR, UV, 1H NMR, single crystal X-ray diffraction and elemental analyses. The ligand involved in complexation was derived by the condensation reaction of 2-acetylpyridine and l-tryptophan. Crystallographic structural analysis revealed that the crystals crystallize in the tetragonal crystal system in which each metal coordinates through six atoms, namely, two nitrogen atoms from C N, two nitrogen atoms from pyridine rings and two carboxylic oxygen atoms from different ligands, forming a type of the 4N + 2O neutrality complex. The molecular structure of the most active Cd complex (2.70) is shown in Fig. 29. The synthesized metal chelates were investigated for their anticancer action on breast cancer cells (MDA-MB-231) which resulted in the inhibition of cellular proliferation. It was well established that the metal complexes have immense potential to be used as proteasome inhibitors and anticancer agents and, among all the synthesized complexes, Cd complex 2.70 (IC50 = 27 μM) had an inhibitory activity higher than that of cisplatin (IC50 = 82 μM) on human breast cancer.131
Fig. 28. Structure of amino acid-based SB metal complexes 2.68–2.70 having potent breast cancer activity.
Fig. 29. The molecular structure of Cd complex 2.70. Copyright permission obtained from Elsevier.131.
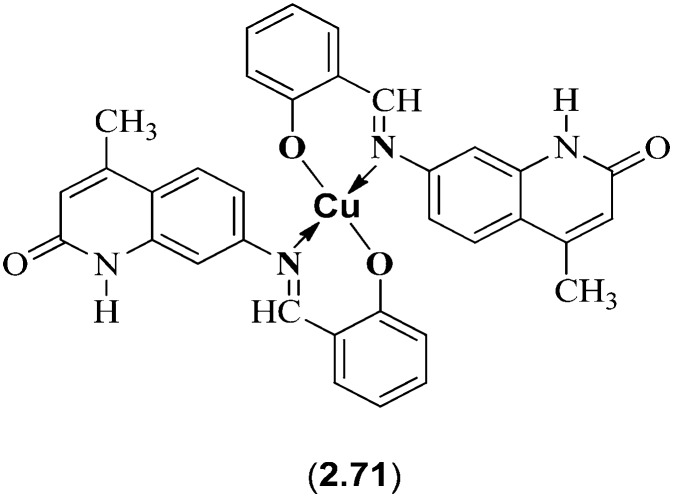
SBs derived from quinolinone heterocycles have received tremendous interest in several fields due to their ability to behave as potential drugs in eliminating dreadful diseases like tuberculosis, cancer and heart failure. Creaven et al. reported a novel series of metal chelates of Cu(ii) with SB ligands derived from condensation of substituted aldehydes with 7-amino-4-methyl-quinolin-2(1H)-one which showed promising biological applications (Fig. 30). Besides showing antifungal activity, these metal complexes showed interesting anticancer activity on in vitro evaluation, using a human hepatic carcinoma cell line (Hep-G2). Among all the ligands and metal complexes screened, Cu(ii) complex 2.71 showed the most potent activity (IC50 = 17.90 ± 3.75 μM), comparable to that of the standard drug cisplatin (IC50 = 15.00 ± 2.65 μM).132
Fig. 30. Cu(ii) complex 2.71 with anticancer activity comparable to cisplatin.
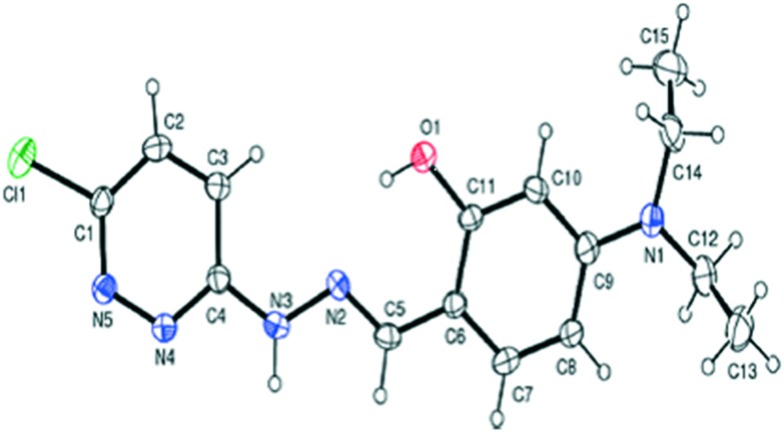
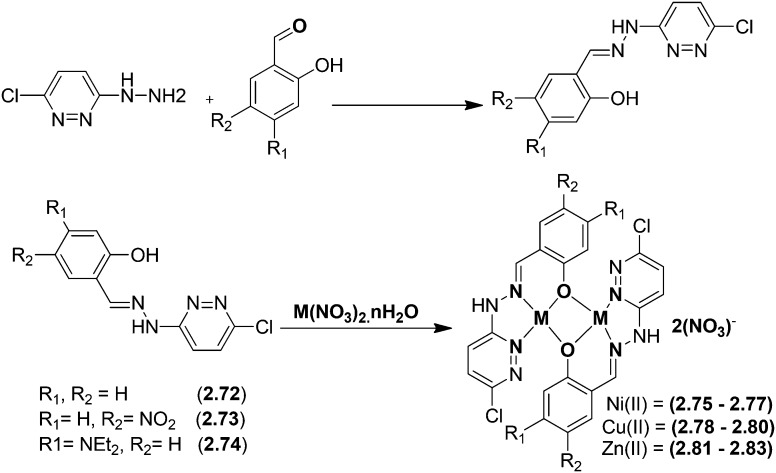
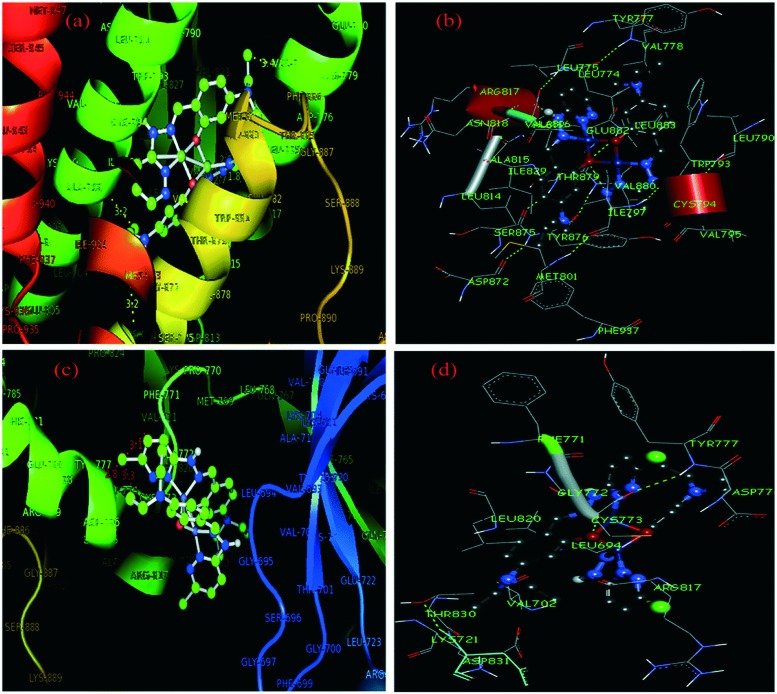
Pyridazine-based binuclear nickel(ii), copper(ii) and zinc(ii) complexes with tridentate Schiff base ligands have been synthesized as anticancer agents, targeting the protein EGFR kinase, which is over-expressed in a number of solid tumors. The crystal structure of the ligand 2.74 as observed by the single crystal X-ray diffraction method showed that it crystallizes in a monoclinic crystal system with the P21/n space group as shown in the ORTEP diagram in Fig. 31. Characterization of the ligands and their metal complexes through various spectroscopic studies revealed that the ligands behaved as tridentate ligands and coordinated to the metal atoms through the iminic nitrogen (–C N–), phenolic hydrogen and the pyridazine ring (Fig. 32). The emission studies proved that the metal complexes can be possibly used as fluorescent probes due to the enhancement of fluorescence intensity compared to the ligands, which could be due to the chelation-enhanced fluorescence effect (CHEF). On deciphering their anticancer potential, it was exposed that some of the synthesized metal complexes were competently more active against a breast cancer cell line (MDA-MB-231) compared to a normal cell line (L-6 myoblast), but complexes 2.75, 2.78 and 2.80 showed no difference in IC50 values between normal and cancer cells, which makes them unsuitable anticancer candidates, owing to their lack of selectivity. Complexes 2.76, 2.77 and 2.83 showed lower IC50 values against cancer cells and were relatively less toxic to normal cells, thereby exhibiting selectivity to these cancer cells as shown in Table 6. Further studies of the active metal complexes 2.77 and 2.83 on the MDA-MB-231 cancer cell line using clonogenic assay, apoptotic studies and cell cycle analysis ensured that these complexes inhibit the growth and proliferation of MDA-MB-231, causing cell cycle arrest in the S phase. The strong hydrophobic interactions, strong hydrogen bonding, and π–π interactions of these complexes with EGFR as evaluated by docking studies suggest that these complexes can be stored and released by the EGFR protein (Fig. 33), which is significant for further exploration of the protein interaction of these complexes (see Table 7). The relatively lower binding energy of complex 2.83 indicates its stronger binding affinity to EGFR than complex 2.77, because it contains a large hydrophobic cavity in subdomain IIA, which plays a key role in the metabolism and transportation of EGFR.133
Fig. 31. ORTEP diagram of the ligand 2.74 with the atomic numbering scheme. Copyright permission obtained from the Royal Society of Chemistry.133.
Fig. 32. Schematic representation of the preparation of pyrimidine core structure-based ligands and their complexes. The synthesized ligand acts as a tridentate ligand which upon complexation results in bimetallic complexes. For R1 and R2, groups are from the respective ligands and for metal complexes, see Table 6.
Table 6. IC50 values for complexes 2.75–2.83 and cisplatin against MDA-MB-231 and L-6 cell lines obtained using MTT assay.
| Ligand | Metal | Complexes | IC50 (μM) |
|
| MDA-MB-231 | L-6 | |||
| 2.72 | Ni(ii) | 2.75 | 0.36 | 0.40 |
| — | — | 2.76 | 59.29 | 67.48 |
| — | — | 2.77 | 74.65 | 94.63 |
| 2.73 | Cu(ii) | 2.78 | 0.34 | 0.36 |
| — | — | 2.79 | 306.2 | 386.1 |
| — | — | 2.80 | 12.0 | 14.17 |
| 2.74 | Zn(ii) | 2.81 | 512.5 | 741.2 |
| — | — | 2.82 | 462.1 | 470.0 |
| — | — | 2.83 | 36.48 | 47.27 |
| Cisplatin | 91.28 | — | ||
Fig. 33. Molecular docked model of complex 2.77 located within the hydrophobic pocket of EGFR (a) and the interaction mode between 2.77 and EGFR (b). Molecular docked model of complex 2.83 located within the hydrophobic pocket of EGFR (c) and the interaction mode between 2.83 and EGFR (d). Copyright permission obtained from the Royal Society of Chemistry.133.
Table 7. Protein EGFR docking results for complexes 2.77 and 2.83 (kcal mol–1).
| Complexes | vdW + H bond + desolv energy (ΔGvdW+hb+desolv) | Electrostatic energy (ΔGelec) | Total internal energy (ΔGtotal) | Torsional free energy (ΔGtor) | Unbound system's energy (ΔGunb) | Binding free energy (ΔGbinding) a |
| 2.77 | –7.86 | –0.18 | –0.82 | –1.34 | –0.42 | –7.10 |
| 2.83 | –9.02 | –0.20 | –0.83 | –1.65 | –0.55 | –7.86 |
aΔGbinding = ΔGvdW+hb+desolv + ΔGelec + ΔGtotal + ΔGtor + ΔGunb.
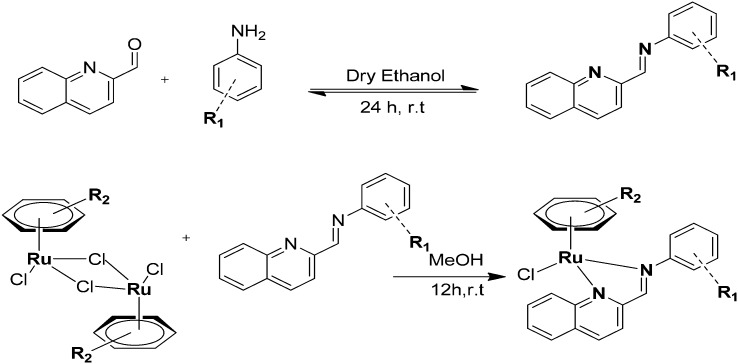
Most of the drugs developed to combat the alarming situation created by cancer are losing their importance due to the development of drug resistance and other associated multiple side-effects. An interesting methodology to deal with this resistance is to evaluate new anticancer medications exhibiting different modes of action. A distinctive series of ruthenium(ii) arene Schiff (RAS) base complexes (Fig. 34) were synthesized and assessed for their anticancer action via p53-independence. The synthesized RAS complexes exhibited significant cytotoxic effects on various cancer cell lines, depending on their structure and the presence of different hydrophobic moieties.134 The effect of the structure and hydrophobicity on the cytotoxicity profile of the complexes against colorectal cancer cell lines HCT116 and SW480 and gastric cancer cell lines AGS and KATOIII showed that the complexes bearing the more hydrophobic HMB and TPB arene ligands demonstrated the highest cytotoxicity in the cell lines, with IC50 values in the low μM range. Complex 2.88 displayed the greatest efficacies with IC50ca. 1.0 μM in HCT116, AGS, and KATOIII and 4.1 μM in SW480, which was 34- and 7-fold more cytotoxic than CDDP in AGS and KATOIII, respectively (see Table 8), suggesting a marked effect of hydrophobicity on cytotoxicity, which in turn was attributed to the higher cellular uptake of the more hydrophobic compounds via passive diffusion. These complexes also exerted their anticancer activities via p53-independent pathways, as confirmed by the lack of activation of p53 and downstream targets and their unchanged activity in the presence of small molecule p53 inhibitors.
Fig. 34. Synthesis of Ru(ii) arene SB complexes (RAS) as anticancer agents with p53-independent activity. For R1 and R2, see Table 8.
Table 8. Cytotoxicity data of RAS complexes.
| Complex |
|
|
IC50 (μM) |
|||
| HCT116 | SW480 | AGS | KATOIII | |||
| 2.84 | Benzene | 4-OMe | 288 ± 44 | 155 ± 4 | 300 ± 10 | 213 ± 24 |
| 2.85 | Toluene | — | 175 ± 22 | 62.1 ± 17.9 | 117 ± 10 | 103 ± 21 |
| 2.86 | Cymene | — | 25.5 ± 4.0 | 27.0 ± 12.0 | 12.4 ± 1.7 | 14.2 ± 2.5 |
| 2.87 | Hexamethylbenzene (HMB) | — | 5.76 ± 1.22 | 34.7 ± 19.3 | 3.04 ± 0.91 | 13.7 ± 5.0 |
| 2.88 | 1,3,5-Triisopropylbenzene (TPB) | — | 1.19 ± 0.12 | 4.07 ± 1.35 | 1.01 ± 0.07 | 1.22 ± 0.24 |
| 2.89 | 1,3,5-Triisopropylbenzene (TPB) |
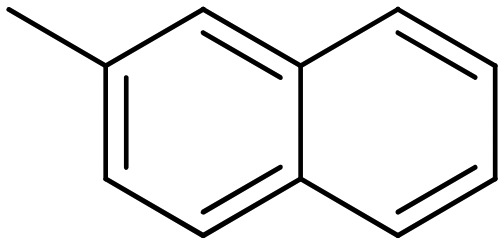
|
3.48 ± 0.36 | 5.32 ± 2.09 | 1.16 ± 0.28 | 1.86 ± 0.24 |
| 2.90 | 1,3,5-Triisopropylbenzene (TPB) |
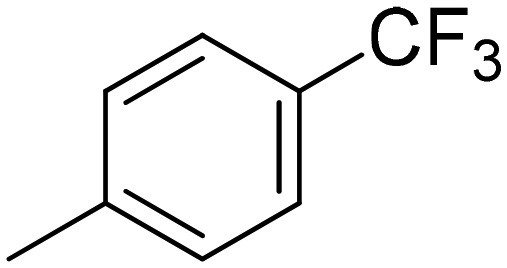
|
6.87 ± 1. 11 | 13.9 ± 5.5 | 3.07 ± 0.72 | 3.89 ± 0.15 |
| 2.91 | 1,3,5-Triisopropylbenzene (TPB) |
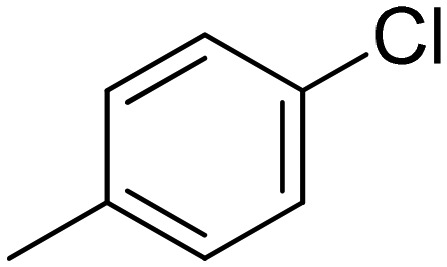
|
5.76 ± 0.13 | 11.0 ± 4.7 | 2.76 ± 0.82 | 3.28 ± 1.12 |
| 2.92 | Hexamethylbenzene (HMB) |
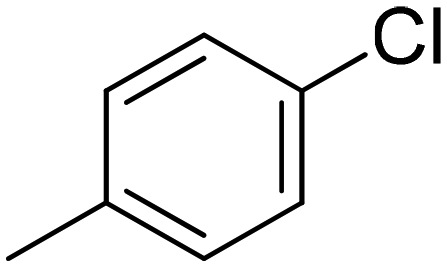
|
3.05 ± 0.26 | 16.0 ± 6.1 | 1.95 ± 0.40 | 2.22 ± 0.35 |
| Oxaliplatin | 1.22 ± 0.18 | 1.08 ± 0.36 | n.d. | n.d. | ||
| Cisplatin | n.d. | n.d. | 34.7 ± 0.8 | 8.64 ± 0.11 | ||
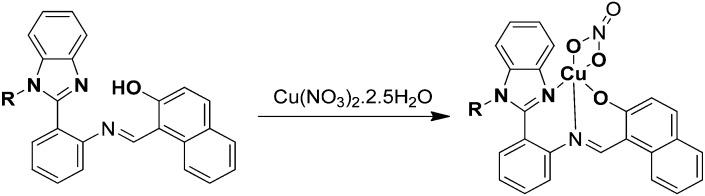
A series of Cu(ii) complexes 2.93–2.95 from an N-alkyl-substituted benzimidazole-based SB were synthesized and analyzed for their biological activities (Fig. 35). In vitro cytotoxicity results suggested that the complexes proved to be effective drug candidates against lung (A-549), breast (MDA-MB-231) and cervical (HeLa) cancer cell lines in contrast to the commonly used cisplatin (IC50 = 27.2 ± 1.71 μM) as can be evidenced from their IC50 values given in Table 9.135
Fig. 35. Synthesis of benzimidazole-based Cu(ii) SB complexes 2.93–2.95.
Table 9. IC50 values of metal complexes 2.93–2.95.
| Compound | R | IC50 (μM) |
||
| A-549 | MDA-MB-231 | HeLa | ||
| 2.93 | Methyl | 16.7 ± 0.29 | 29.58 ± 1.17 | 32.66 ± 0.58 |
| 2.94 | Ethyl | 24.0 ± 1.99 | 31.92 ± 0.73 | 33.79 ± 0.36 |
| 2.95 | Propyl | 29.1 ± 1.12 | 46.03 ± 1.12 | 45.27 ± 0.65 |
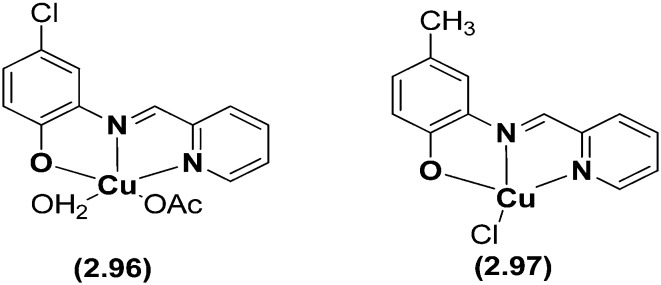
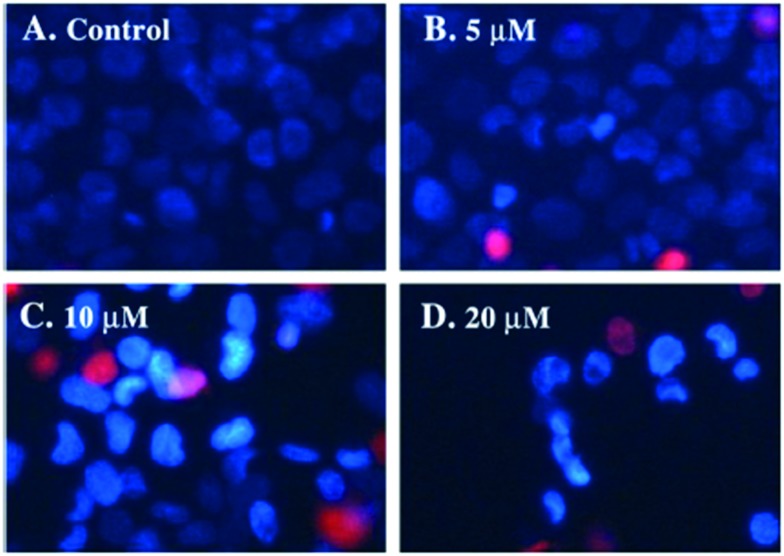
Copper(ii) complexes (Fig. 36) with the tridentate (NNO) SB N-2-pyridylmethylidene-2-hydroxy-5-chlorophenylamine have been synthesized and evaluated for their cytotoxic effects on human cancer cell lines.136 In these complexes, the copper atom showed a 4 + 1 pyramidal coordination with a water oxygen atom in the apical position, three of the basal positions occupied by the NNO tridentate ligand and the fourth by an acetate oxygen as shown in Fig. 37. Several techniques like fluorescence spectroscopy, UV-visible spectroscopy and agarose gel electrophoresis were employed to disclose their DNA interaction and binding energy constants. The binding constant (Kapp) value of 6.40 × 105 M–1 for 2.96 with DNA suggested a moderate intercalative binding mode and the efficient oxidative cleavage of supercoiled DNA at pH = 7.2 and 37 °C, involving hydroxyl radicals, singlet oxygen-like species, and hydrogen peroxide as reactive oxygen species, revealed that the complex could induce scission of pBR322 supercoiled DNA effectively without addition of external agents. In vitro cytotoxicity results established that 2.96 exhibited prominent cytotoxicity (IC50 = 16.123 ± 1.207 μM) and induced S-phase cell cycle arrest, cytopathologic effects and apoptosis of HeLa cells as can be seen in Fig. 38. The potential mechanism for apoptosis induction was attributed to the stimulation of an intrinsic mitochondrial apoptotic pathway owing to the activation of caspase-9 and caspase-3.136
Fig. 36. Structures of tridentate HSBs with Cu(OAc)2·H2O and Cu(Cl). Metal chelates of this kind exhibited potent anticancer activity on HeLa cells.
Fig. 37. OPTEP view of the molecular structure and atom labeling scheme of 2.96. Hydrogen atoms and solvent H2O molecules are omitted for clarity. Copyright permission obtained from Elsevier.136.
Fig. 38. Morphological changes of HeLa cells treated with various concentrations of 2.96 for 48 h which were double stained with Hoechst 33258/PI and detected using a fluorescence microscope.
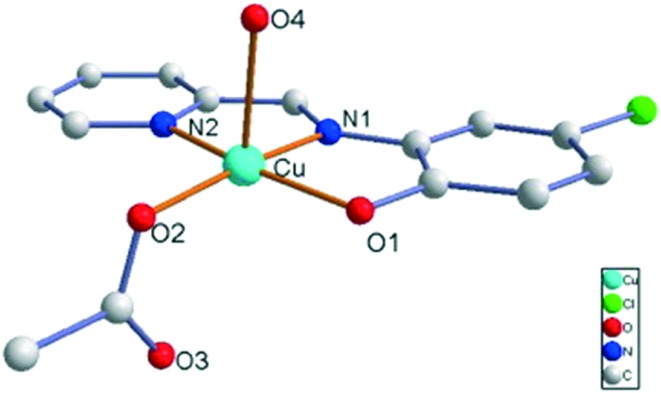
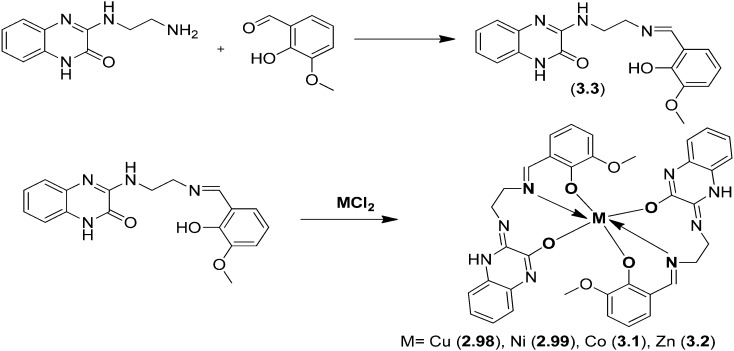
Cu(ii), Ni(ii), Co(ii) and Zn(ii) metal chelates 2.98–3.2 were prepared from a tridentate ONO donor ligand (3.3), which in turn was prepared by carrying out a simple condensation reaction between 3-(2-aminoethylamino)quinoxalin-2(1H)-one and o-vanillin (Fig. 39). The in vitro anticancer action of the blended ligands and complexes on a human cervical cancer cell line (HeLa) was explored and it was confirmed that the metal chelates act as potential anticancer agents compared to the ligand. The inhibitory effects obtained by MTT assay displayed IC50 values of 69.51 μM for the ligand 3.3 and 66.83, 33.23, 35.61 and 17.67 μM for complexes 2.98–3.2, respectively.137
Fig. 39. Scheme for the preparation of quinoxalinedione-tethered SB ligand 3.3 and its metal complexes 2.98–3.2.
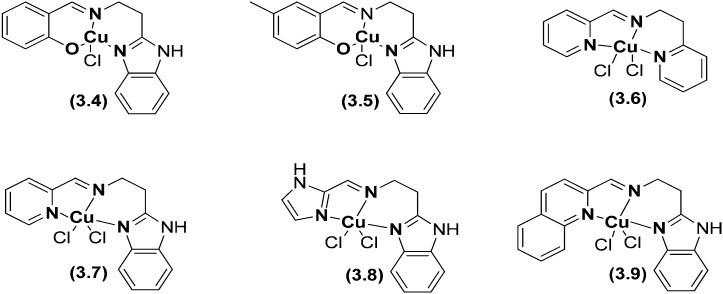
Phenolate ligand complexes 3.4 and 3.5 possessing a distorted square-based geometry and tridentate 3N ligand complexes 3.6–3.9 with pyridyl and quinolyl nitrogen donors possessing a distorted trigonal bipyramidal geometry showed interesting cell killing activities.138 All the complexes (Fig. 40) bind with calf thymus (CT) DNA through a covalent mode of DNA interaction involving the replacement of an easily removable chloride ion with DNA nucleobases and exhibit oxidative cleavage of supercoiled (SC) plasmid DNA in the presence of hydrogen peroxide as an activator. Complexes 3.8 and 3.9 completely degraded SC DNA into undetectable minor fragments and thus they act as efficient chemical nucleases. All the complexes displayed remarkable cytotoxicity against the HBL-100 human breast cancer cell line (IC50 = 8.6–21.4 μM) with a potency more than that of the widely used drug cisplatin (IC50 = 25 μM).138
Fig. 40. Structures of some imperative HSB metal complexes 3.4–3.9. The metal chelates demonstrated potential cytotoxic effects compared to the reference drug cisplatin.
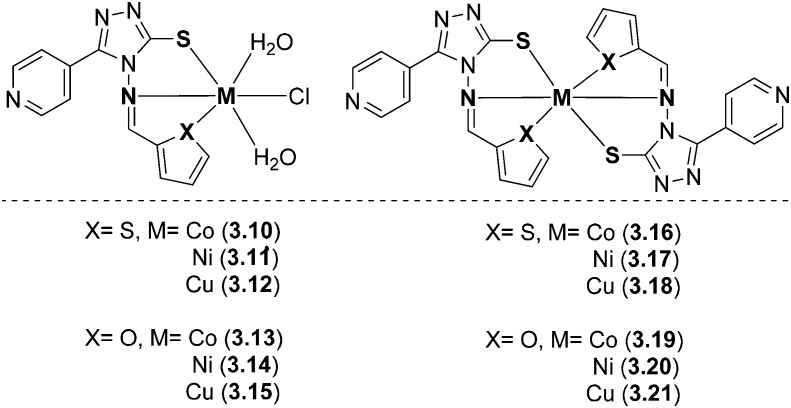
Metal complexes of bioactive SB ligands (E)-5-(pyridin-4-yl)-4-((thiophen-2-ylmethylene)amino)-4H-1,2,4-triazole-3-thiol and (E)-4-((furan-2-ylmethylene)amino)-5-(pyridin-4-yl)-4H-1,2,4-triazole-3-thiol were prepared and characterized (Fig. 41). An octahedral geometry was predicted for all the complexes 3.10–3.21. On analyzing them for their biological potential, it was confirmed that the complexes had a profound effect on cancer cell lines, viz., human breast cancer cell line (MCF-7) and human hepatocellular liver carcinoma cell line (Hep-G2), respectively. The results suggested that all the metal complexes showed moderate to significant % inhibition of cell proliferation. Complexes 3.15 and 3.18 showed 38% and 49% inhibition of cell proliferation against the HepG2 cell line, whereas complexes 3.18, 3.19 and 3.20 greatly reduced malignant MCF-7 cell growth by 34%, 38% and 33%, respectively.139
Fig. 41. Structures of biologically active triazole-based metal complexes with interesting anticancer activity.
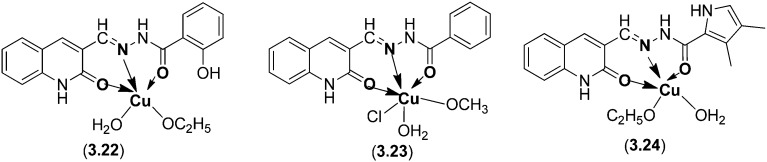
Novel 2-oxoquinoline-3-carbaldehyde SB ligands and their Cu(ii) complexes were prepared and analyzed by various spectroscopic techniques (Fig. 42). The prepared ligands and their Cu(ii) complexes were explored for their potential anticancer activity. MTT [3-(4,5-dimethyl-2-thiazoyl)-2,5-diphenyl-2H-tetrazolium bromide] and SRB (sulforhodamine B) assays indicated that the Cu(ii) complexes exhibited more effective cytotoxic activity against HL60 cells and HeLa cells and potential DNA binding efficiency compared to the corresponding ligands.140 Complex 3.24 showed higher cytotoxic activity (IC50 = 38 μM) than the metal complexes 3.22 (IC50 = 160 μM) and 3.23 (IC50 = 43 μM).
Fig. 42. SB ligand metal complexes 3.22–3.24 with versatile biological applications especially as anticancer agents.
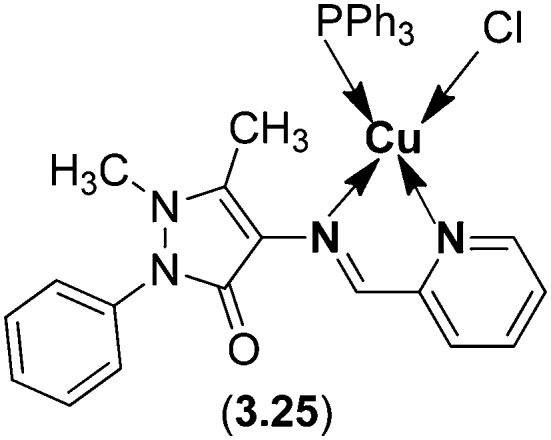
Copper(i) SB complex 3.25 with a distorted tetrahedral geometry has been found to show interaction through intercalation with CT-DNA using an absorption titration technique. The cytotoxic impact of the complex analyzed on carcinogenic cell lines (human cervical cancer cell line (HeLa), human laryngeal epithelial carcinoma cell line (Hep-2) and human breast cancer cell line (MCF-7)) demonstrated that the complex displayed considerable anticancer activity. The IC50 values of the Cu(i) complex for HeLa, Hep-2 and MCF-7 cancer cell lines were found to be 3.04, 19.25 and 4.34 μM, respectively. In addition, the Cu(i) complex (Fig. 43) displayed four and three times higher inhibitory activity against HeLa and MCF-7 than the widely used cisplatin.141
Fig. 43. Structure of Cu(i) complex 3.25 obtained from 4-aminoantipyrine and pyridinecarbaldehyde. This metal chelate has been proved to be a potential anticancer agent with lesser toxicity.
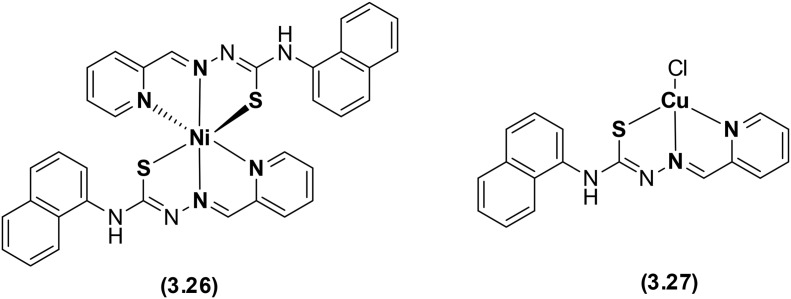
Pyridine-based heterocyclic thiosemicarbazone ligand-based Ni(ii) and Cu(ii) metal complexes have been prepared and thoroughly examined using several spectroscopic techniques (Fig. 44). The coordination of the mono-deprotonated anionic form of the ligand via NNS donor atoms yielded an octahedral Ni(ii) complex 3.26 and a distorted square planar Cu(ii) complex 3.27. Biological studies made it clear that the metal chelates exhibited significant interaction with DNA via intercalation but could not induce DNA cleavage, although complex 3.27 was able to strongly bind to DNA and interfere with its electrophoretic mobility and proteins. A comparative evaluation specified that complex 3.26 showed superior DNA binding, but complex 3.27 proved to be an effective anticancer chemotherapeutic agent against HeLa cells with an IC50 value of 31.27 μM.142
Fig. 44. Structures of the pyridine-based heterocyclic thiosemicarbazone ligand and its Ni(ii) and Cu(ii) complexes.
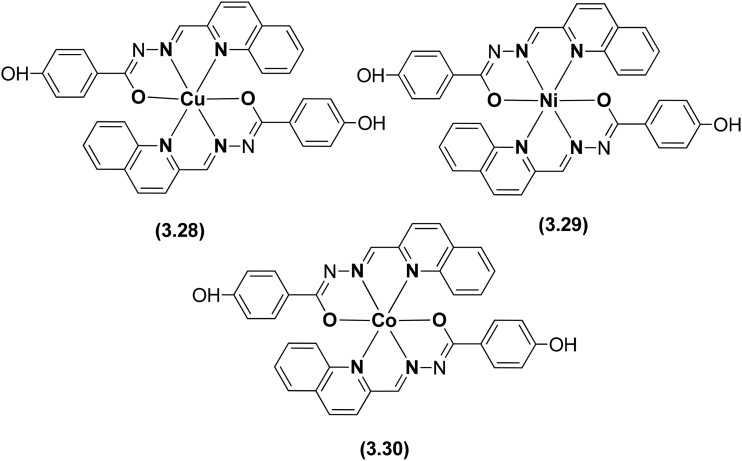
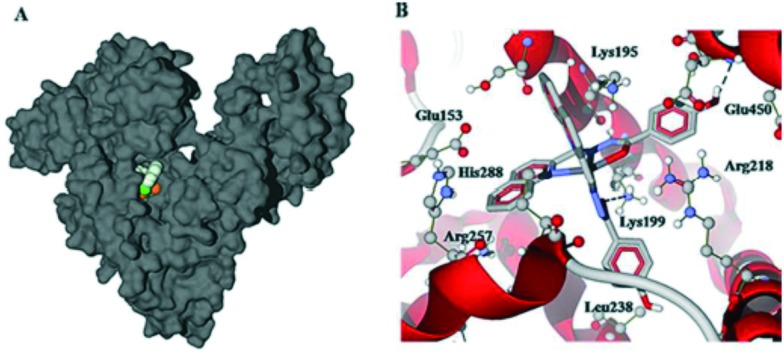
A tridentate heterocyclic hydrazone ligand was prepared and tethered with three transition metals to prepare the metal chelates 3.28–3.30 as anticancer agents (Fig. 45). The cytotoxicity profile revealed that among the three metal chelates, the Cu(ii) complex (3.28) displayed more efficient activity than Ni(ii) 3.29 and Co(ii) complex 3.30 due to significantly higher intracellular reactive oxygen species (ROS) generation.143 Complex 3.30 could induce caspase-mediated apoptosis in A549 cells through the activation of the mitochondrial signaling pathway and showed affinity towards human serum albumin (HSA) as evidenced by fluorescence spectroscopy and molecular docking. Complex 3.30 showed hydrophobic interaction with Leu-238, Leu-219, Ala-291, Phe-223 and Phe-157 residues and hydrogen bonds within the binding pocket of sub-domain IIA (the warfarin binding pocket). The phenolic hydroxyl group of 3.30 interacted with Glu-450 and the oxygen atom bound to Cu showed interaction with Lys-199. The Cu(ii) center of complex 3.30 located approximately 6.7 Å away from Trp-214 (see Fig. 46) allowed for quenching of fluorescence from the tryptophan residue of HSA, consistent with the fluorescence quenching studies.143
Fig. 45. Structures of Co(ii), Ni(ii) and Cu(ii) metal chelates 3.28–3.30 with the heterocyclic hydrazone ligand.
Fig. 46. Docking studies of 3.30 and HSA using Autodock Vina: (A) the Cu complex is buried beneath the HSA protein surface. (B) Cu complex interacting with a variety of amino acid residues. Copyright permission obtained from the Royal Society of Chemistry.143.
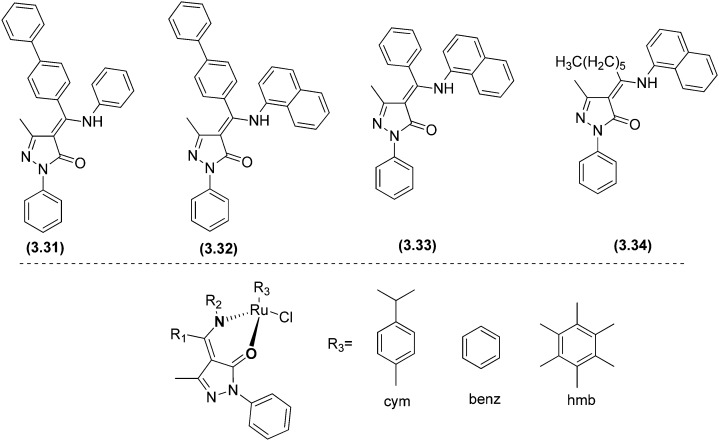
Interesting and efficient phenyl, naphthyl and biphenyl-substituted pyrazolone-based β-ketoamine proligands 3.31–3.34 and their ruthenium(ii) arene complexes (3.35–3.43) were synthesized and assessed for their in vitro anticancer activity toward the human ovarian cancer cell lines A2780 and A2780cisR.144 Biological studies showed that the ligands were not cytotoxic, indicating that DNA intercalation is unlikely. Nevertheless, some of the resulting compounds were rationally cytotoxic and, fascinatingly, the biphenyl-containing complex 3.35 showed the highest levels of cytotoxicity to the nonresistant A2780 cell line (IC50 = 7.6 ± 1.3 μM) and 3.36 also with a biphenyl ring was cytotoxic toward the resistant A2780cisR cell line (IC50 = 13 ± 1 μM) compared to the standard drug cisplatin (IC50 = 25 ± 1 μM). The difference in activity could be due to the different nature of the η6-arene ring, i.e., p-cymene in 3.35 and benzene in 3.36 (see Fig. 47 and Table 10). Complexes 3.39 and 3.40 were slightly more cytotoxic than cisplatin to this resistant cell line, although not as efficient as complex 3.36. Overall, the cytotoxicity of 3.35, 3.36, 3.39, and 3.40 was like that detected for many other series of organoruthenium compounds.
Fig. 47. Structures of ligands 3.31–3.34 and their Ru(ii) metal complexes 3.35–3.43. For complete structures, see Table 10.
Table 10. Cytotoxicity of the metal complexes 3.35–3.43 and cisplatin following exposure to the ovarian carcinoma cells A2780 and A2780cisR (cisplatin-resistant) for 72 h.
| Complex | R3 | Ligand | IC50 (μM) |
|
| A2780 | A2780cisR | |||
| 3.35 | cym | 3.31 | 7.6 ± 1.3 | 61 ± 5 |
| 3.36 | benz | 3.31 | 16.7 ± 1.2 | 13 ± 1 |
| 3.37 | hmb | 3.31 | 63.1 ± 1.2 | 442 ± 20 |
| 3.38 | cym | 3.32 | 96 ± 4 | 243 ± 19 |
| 3.39 | benz | 3.33 | 23 ± 2 | 20 ± 1 |
| 3.40 | hmb | 3.33 | 20 ± 1 | 21 ± 4 |
| 3.41 | cym | 3.33 | 20 ± 1 | 24 ± 3 |
| 3.42 | benz | 3.34 | 31 ± 2 | 30 ± 10 |
| 3.43 | cym | 3.34 | 126 ± 14 | 49 ± 1 |
| Cisplatin | 1.0 ± 0.2 | 25 ± 1 | ||
7. Conclusion and future prospects
There have been enormous advances in almost every sphere of science and technology during the last couple of decades. Despite these advances, the treatment of microbial infections and cancer is still far from over. The application of presently available antimicrobial and anticancer drugs is limited due to their side effects and drug resistance and there is no doubt that microbial infections and cancer are a big threat to human beings and a challenge to our society. The development of novel antimicrobial and anticancer agents is associated with many impediments and the main problem lies in the fact that no general guideline is available that could direct the synthesis of new active antimicrobial and anticancer agents with the least or no possibilities of the onset of resistance. To date, there have been significant advances in the understanding of the molecular aetiology of microbial infections and cancer, but ideal therapeutic modalities are still missing. In view of these facts, it is very urgent to speed up the development of new antimicrobial and anticancer agents. The therapeutic potential of the diversity of molecules (both ligands and metal complexes) can be fully harnessed for the design of novel and efficient antimicrobial and anticancer agents. Thus, it would be beneficial to explore other heterocyclic Schiff base metal complexes with diverse molecular features and topologies as antimicrobial and anticancer agents. Besides, targeting and activation strategies may be helpful in the development of future generations of antimicrobial and anticancer drugs with the ability to overcome the disadvantages of available agents. Modern theoretical methods such as density functional theory, molecular operating environments and techniques such as high-resolution electrospray mass spectrometry and multinuclear polarization transfer NMR (nuclear magnetic resonance) spectroscopy are greatly helpful and can surely improve our understanding of the chemical and biochemical reactivity of drugs, which might help in establishing some meaningful structure–activity relationships. Therefore, chemical studies of antimicrobial and anticancer agents under physiologically relevant conditions become very important in drug development rationales.
In the present review, we collected all the scattered information about transition metal complexes with bioactive HSB ligands. It is noteworthy to mention that transition metals containing heterocyclic scaffolds display promising pharmacological activities compared to conventional SBs, encouraging us to carry out further research in this field. As per the extent of this survey, we have attempted to give straightforward features accounting for the integrative uses of HSB–transition metal complexes. In view of these facts, the sensible design and synthesis of new HSB–transition metal complexes are exceptionally significant to design novel drug libraries with fewer side effects and expunge the dilemma of multiple drug resistance. Although plenty of Schiff-base metal complexes show excellent antimicrobial and anticancer profiles, none of these complexes have been put to advanced studies or have been brought to market, which could be due to a lack of attention from researchers or belief that Schiff-base complexes do not possess the potential to be used as antimicrobial or anticancer drugs. Through this review, we want to make it clear that Schiff-base transition metal complexes have all the inherent properties that are required to bring a drug to the market. The only thing required is more scientific attention and focus on this otherwise magnificent Schiff-base transition metal-based drug discovery field.
List of abbreviations
- SB
Schiff base
- HSB
Heterocyclic Schiff base
- HIV
Human immunodeficiency virus
- MIC
Minimum inhibitory concentration
- BHPP
1,4-Bis[(2-hydroxybenzaldehyde)propyl]piperazine
- IR
Infrared
- X
Halide
- IC50
Concentration at which 50% inhibition of cell growth occurs
- NMR
Nuclear magnetic resonance
- MS
Mass spectrometry
- DPPH
2,20-Diphenyl-1-picrylhydrazyl
- DNA
Deoxyribonucleic acid
- Salen
Salicylaldehyde ethylenediamine
- BHT
Butylated hydroxytoluene
- RAS
Ruthenium(ii) arene Schiff
- MTT
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- dppz
Dipyridophenazine
- PDT
Photodynamic therapy
- bpy
2,2′-Bipyridine
- SRB
Sulforhodamine B
Conflicts of interest
The authors declare no competing interests.
Acknowledgments
MAM and OAD are thankful to University Grants Commission (UGC), Government of India for financial support through a Central University doctoral fellowship.
References
- Li F., Collins J. G., Keene F. R. Chem. Soc. Rev. 2015;44:2529–2542. doi: 10.1039/c4cs00343h. [DOI] [PubMed] [Google Scholar]
- http://www.who.int/mediacentre/factsheets/fs297/en/ .
- Barot K. P., Manna K. S., Ghate M. D. J. Saudi Chem. Soc. 2013:S35–S43. [Google Scholar]
- Abd-El-Aziz A. S., Agatemor C., Etkin N. Biomaterials. 2017;118:27–50. doi: 10.1016/j.biomaterials.2016.12.002. [DOI] [PubMed] [Google Scholar]
- Dhanaraj C. J., Johnson J. Appl. Organomet. Chem. 2016;30:860–871. [Google Scholar]
- Krishnamoorthy P., Sathyadevi P., Cowley A. H., Butorac R. R., Dharmaraj N. Eur. J. Med. Chem. 2011;46:3376–3387. doi: 10.1016/j.ejmech.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Arjmand F., Jamsheera A., Mohapatra D. K. J. Photochem. Photobiol., B. 2013;121:75–85. doi: 10.1016/j.jphotobiol.2012.12.009. [DOI] [PubMed] [Google Scholar]
- Bharti S. K., Singh S. K. Pharm. Lett. 2009;1(2):39–51. [Google Scholar]
- Uivarosi V. Molecules. 2013:11153–11197. doi: 10.3390/molecules180911153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imran M., Iqbal J., Iqbal S., Ijaz N. Turk. J. Biol. 2007;31:67–72. [Google Scholar]
- Silva P. P., Guerra W., Silveira J. N., Maria A., Ferreira C., Bortolotto T., Fischer F. L., Neves A., Pereira-maia E. C., Prof A., Prestes L., Sp P. À., Federal U., Catarina D. S. Inorg. Chem. 2011;50:6414–6424. doi: 10.1021/ic101791r. [DOI] [PubMed] [Google Scholar]
- Shaikh A. R., Giridhar R., Yadav M. R. Int. J. Pharm. 2007;6:24–30. doi: 10.1016/j.ijpharm.2006.11.037. [DOI] [PubMed] [Google Scholar]
- Guerra W., de Andrade Azevedo E., de Souza Monteiro A. R., Bucciarelli-Rodriguez M., Chartone-Souza E., Nascimento A. M., Fontes A. P., Le Moyec L., Pereira-Maia E. C. J. Inorg. Biochem. 2005;99:2348–2354. doi: 10.1016/j.jinorgbio.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Ejidike I., Ajibade P. Int. J. Mol. Sci. 2016;17:60. doi: 10.3390/ijms17010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M. X., Chen C. L., Zhang D., Niu J. Y., Ji B. S. Eur. J. Med. Chem. 2010;45:3169–3177. doi: 10.1016/j.ejmech.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Manjunath M., Kulkarni A. D., Bagihalli G. B., Malladi S., Patil S. A. J. Mol. Struct. 2017;1127:314–321. [Google Scholar]
- Hranjec M., Starčević K., Pavelić S. K., Lučin P., Pavelić K., KarminskiZamola G. Eur. J. Med. Chem. 2011;46:2274–2279. doi: 10.1016/j.ejmech.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Huang H., Chen Q., Ku X., Meng L., Lin L., Wang X., Zhu C., Wang Y., Chen Z., Li M., Jiang H., Chen K., Ding J., Liu H. J. Med. Chem. 2010;53:3048–3064. doi: 10.1021/jm9014394. [DOI] [PubMed] [Google Scholar]
- Badea M., Calu L., Chifiriuc M. C., Bleotu C., Marin A., Ion S., IoniŢă G., Stanică N., MăruŢescu L., Lazăr V., Marinescu D., Olar R. J. Therm. Anal. Calorim. 2014;118:1145–1157. [Google Scholar]
- Sinha D., Tiwari A. K., Singh S., Shukla G., Mishra P., Chandra H., Mishra A. K. Eur. J. Med. Chem. 2008;43:160–165. doi: 10.1016/j.ejmech.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Sönmez M., Şekerci M., Chemistry A. Synth. React. Inorg. Met.-Org. Chem. 2010;5714:489–502. [Google Scholar]
- Tabassum S., Amir S., Arjmand F., Pettinari C., Marchetti F., Masciocchi N., Lupidi G., Pettinari R. Eur. J. Med. Chem. 2013;60:216–232. doi: 10.1016/j.ejmech.2012.08.019. [DOI] [PubMed] [Google Scholar]
- Grivani G., Bruno G., Rudbari H. A., Khalaji A. D., Pourteimouri P. Inorg. Chem. Commun. 2012;18:15–20. [Google Scholar]
- Cozzi P. G. Chem. Soc. Rev. 2004;33:410–421. doi: 10.1039/b307853c. [DOI] [PubMed] [Google Scholar]
- Gupta K. C., Sutar A. K. Coord. Chem. Rev. 2008;252:1420–1450. [Google Scholar]
- Zhang Z., Li X., Wang C., Zhang C., Liu P., Fang T., Xiong Y., Xu W. Dalton Trans. 2012;41:1252–1258. doi: 10.1039/c1dt11370d. [DOI] [PubMed] [Google Scholar]
- Abd El-halim H. F., Omar M. M., Mohamed G. G. Spectrochim. Acta, Part A. 2011;78:36–44. doi: 10.1016/j.saa.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Hambley T. W. Dalton Trans. 2007:4929–4937. doi: 10.1039/b706075k. [DOI] [PubMed] [Google Scholar]
- Arnesano F., Natile G. Coord. Chem. Rev. 2009;253:2070–2081. [Google Scholar]
- Jung Y. W., Lippard S. J. Chem. Rev. 2007;107:1387–1407. doi: 10.1021/cr068207j. [DOI] [PubMed] [Google Scholar]
- Boulikas T., Pantos A., Bellis E., Christofis P. Cancer Ther. 2007;5:537–583. [Google Scholar]
- van Zutphen S., Reedijk J. Coord. Chem. Rev. 2005;249:2845. [Google Scholar]
- Gust R., Beck W., Jaouen G., Schoenenberger H. Coord. Chem. Rev. 2009;253:2760–2779. [Google Scholar]
- Gust R., Beck W., Jaouen G., Schoenenberger H. Coord. Chem. Rev. 2009;253:2742–2759. [Google Scholar]
- Bruijnincx P. C. A., Sadler P. J. Curr. Opin. Chem. Biol. 2008;12:197–206. doi: 10.1016/j.cbpa.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-dief A. M., Mohamed I. M. A. Beni-Suef Univ. J. Basic Appl. Sci. 2015;4:119–133. doi: 10.1016/j.bjbas.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al W., Ali A., Al-hamdani S., Kaseem M. Appl. Organomet. Chem. 2016;30:810–817. [Google Scholar]
- Suvarapu L. N., Seo Y. K., Baek S. O., Ammireddy V. R. Eur. J. Chem. 2012;9:1288–1304. [Google Scholar]
- Sahu R., Thakur D. S., Kashyap P. Int. J. Pharm. Sci. Nanotechnol. 2012;5:IJPSN-4-27-12-SAHU. [Google Scholar]
- Kavitha H. Rasayan J. Chem. 2010;3:385–410. [Google Scholar]
- Prakash A., Adhikari D. Int. J. ChemTech Res. 2011;3:1891–1896. [Google Scholar]
- Jia Y., Li J. Chem. Rev. 2015;115:1597–1621. doi: 10.1021/cr400559g. [DOI] [PubMed] [Google Scholar]
- Rezaeivala M., Keypour H. Coord. Chem. Rev. 2014;280:203–253. [Google Scholar]
- Qin W., Long S., Panunzio M., Biondi S. Molecules. 2013;18:12264–12289. doi: 10.3390/molecules181012264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva C. M., da Silva D. L., Modolo L. V., Alves R. B., de Resende M. A., Martins C. V. B., de Fátima Â. J. Adv. Res. 2011;2:1–8. [Google Scholar]
- Abu-Dief A. M., Mohamed I. M. A. Beni-Suef Univ. J. Basic Appl. Sci. 2015;4:119–133. doi: 10.1016/j.bjbas.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodowska K., Łodyga-Chruścińska E. Chem. Commun. 2014;68:129–134. [Google Scholar]
- Belowich M. E., Stoddart J. F. Chem. Soc. Rev. 2012;41:2003. doi: 10.1039/c2cs15305j. [DOI] [PubMed] [Google Scholar]
- Arun T. R., Subramanian R., Packianathan S. J. Fluoresc. 2015:1127–1140. doi: 10.1007/s10895-015-1603-4. [DOI] [PubMed] [Google Scholar]
- Hameed A., Al-Rashida M., Uroos M., Abid Ali S., Khan K. M. Expert Opin. Ther. Pat. 2017;27:63–79. doi: 10.1080/13543776.2017.1252752. [DOI] [PubMed] [Google Scholar]
- Jana S., Dalapati S., Guchhait N. J. Phys. Chem. A. 2012;116:10948–10958. doi: 10.1021/jp3079698. [DOI] [PubMed] [Google Scholar]
- Biswas A., Das L. K., Drew M. G. B., Aromí G., Gamez P., Ghosh A. Inorg. Chem. 2012;51:7993–8001. doi: 10.1021/ic202748m. [DOI] [PubMed] [Google Scholar]
- Wilkinson S. M., Sheedy T. M., New E. J. J. Chem. Educ. 2016;93:351–354. [Google Scholar]
- Han F., Teng Q., Zhang Y., Wang Y., Shen Q. Inorg. Chem. 2011;50:2634–2643. doi: 10.1021/ic102529d. [DOI] [PubMed] [Google Scholar]
- Kajal A., Bala S., Kamboj S., Sharma N., Saini V. J. Catal. 2013;2013:1–14. [Google Scholar]
- Kushwah N., Pal M. K., Wadawale A., Sudarsan V., Manna D., Ghanty T. K., Jain V. K. Organometallics. 2012;31:3836–3843. [Google Scholar]
- Facchinetti V., da Reis R. R., Gomes C. R. B., Vasconcelos T. R. A. Mini-Rev. Org. Chem. 2012;9:44–53. doi: 10.2174/138955712800959099. [DOI] [PubMed] [Google Scholar]
- Kalanithi M., Rajarajan M., Tharmaraj P., Sheela C. D. Spectrochim. Acta, Part A. 2012;87:155–162. doi: 10.1016/j.saa.2011.11.031. [DOI] [PubMed] [Google Scholar]
- Chow M. J., Licona C., Yuan Qiang Wong D., Pastorin G., Gaiddon C., Ang W. H. J. Med. Chem. 2014;57:6043–6059. doi: 10.1021/jm500455p. [DOI] [PubMed] [Google Scholar]
- Lo K. K.-W., Li S. P.-Y. RSC Adv. 2014;4:10560–10585. [Google Scholar]
- Giannousi K., Avramidis I., Dendrinou-Samara C. RSC Adv. 2013;3:21743–21752. [Google Scholar]
- Jafarpour M., Rezaeifard A. Transition Met. Chem. 2011:685–690. [Google Scholar]
- Devi J., Batra N. Spectrochim. Acta, Part A. 2015;135:710–719. doi: 10.1016/j.saa.2014.07.041. [DOI] [PubMed] [Google Scholar]
- Saini A. K., Kumari P., Sharma V., Mathur P., Mobin S. M. Dalton Trans. 2016;45:19096–19108. doi: 10.1039/c6dt03573f. [DOI] [PubMed] [Google Scholar]
- Dhanaraj C. J., Johnson J. J. Photochem. Photobiol., B. 2016;161:108–121. doi: 10.1016/j.jphotobiol.2016.04.033. [DOI] [PubMed] [Google Scholar]
- Shekaari H., Kazempour A., Khoshalhan M. Phys. Chem. Chem. Phys. 2015;17:2179–2191. doi: 10.1039/c4cp04432k. [DOI] [PubMed] [Google Scholar]
- da Silva C. M., da Silva D. L., Modolo L. V., Alves R. B., de Resende M. A., Martins C. V. B., de Fátima Â. J. Adv. Res. 2011;2:1–8. [Google Scholar]
- Zhang S.-H., Feng C. J. Mol. Struct. 2010;977:62–66. [Google Scholar]
- Ali E., Naimi-Jamal M. R., Dekamin M. G. Sci. Iran. 2013;20:592–597. [Google Scholar]
- Liu M., Little M. A., Jelfs K. E., Jones J. T. A., Schmidtmann M., Chong S. Y., Hasell T., Cooper A. I. J. Am. Chem. Soc. 2014;136:7583–7586. doi: 10.1021/ja503223j. [DOI] [PubMed] [Google Scholar]
- Clarke R. M., Storr T. Dalton Trans. 2014;43:9380–9391. doi: 10.1039/c4dt00591k. [DOI] [PubMed] [Google Scholar]
- Rao V. K., Reddy S. S., Krishna B. S., Naidu K. R. M., Raju C. N., Ghosh S. K. Green Chem. Lett. Rev. 2010;3:217–223. [Google Scholar]
- Ren M., Xu Z.-L., Bao S.-S., Wang T.-T., Zheng Z.-H., Ferreira R. A. S., Zheng L.-M., Carlos L. D. Dalton Trans. 2016;45:2974–2982. doi: 10.1039/c5dt03897a. [DOI] [PubMed] [Google Scholar]
- Asatkar A. K., Senanayak S. P., Bedi A., Panda S., Narayan K. S., Zade S. S. Chem. Commun. 2014;50:7036–7039. doi: 10.1039/c4cc01360c. [DOI] [PubMed] [Google Scholar]
- Azam M., Hussain Z., Warad I., Al-Resayes S. I., Khan M. S., Shakir M., Trzesowska-Kruszynska A., Kruszynski R. Dalton Trans. 2012;41:10854–10864. doi: 10.1039/c2dt31143g. [DOI] [PubMed] [Google Scholar]
- Cheng J., Wei K., Ma X., Zhou X., Xiang H. J. Phys. Chem. C. 2013;117:16552–16563. [Google Scholar]
- Haikarainen A., Sipilä J., Pietikäinen P., Pajunen A., Mutikainen I. J. Chem. Soc., Dalton Trans. 2001:991–995. doi: 10.1016/s0968-0896(01)00053-0. [DOI] [PubMed] [Google Scholar]
- Whiteoak C. J., Salassa G., Kleij A. W. Chem. Soc. Rev. 2012;41:622–631. doi: 10.1039/c1cs15170c. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy P., Sathyadevi P., Butorac R. R., Cowley A. H., Bhuvanesh N. S. P., Dharmaraj N. Dalton Trans. 2012;41:4423–4436. doi: 10.1039/c2dt11938b. [DOI] [PubMed] [Google Scholar]
- Chew S. T., Lo K. M., Sinniah S. K., Sim K. S., Tan K. W. RSC Adv. 2014;4:61232–61247. [Google Scholar]
- Kitamura F., Sawaguchi K., Mori A., Takagi S., Suzuki T., Kobayashi A., Kato M., Nakajima K. Inorg. Chem. 2015;54:8436–8448. doi: 10.1021/acs.inorgchem.5b01128. [DOI] [PubMed] [Google Scholar]
- Bagherzadeh M., Zare M. J. Coord. Chem. 2012;65:4054–4066. [Google Scholar]
- Manikandan R., Viswanathamurthi P., Muthukumar M. Spectrochim. Acta, Part A. 2011;83:297–303. doi: 10.1016/j.saa.2011.08.033. [DOI] [PubMed] [Google Scholar]
- Dilworth J. R., Hueting R. Inorg. Chim. Acta. 2012;389:3–15. [Google Scholar]
- Chellan P., Land K. M., Shokar A., Au A., An S. H., Clavel C. M., Dyson P. J., de Kock C., Smith P. J., Chibale K., Smith G. S. Organometallics. 2012;31:5791–5799. [Google Scholar]
- Su W., Qian Q., Li P., Lei X., Xiao Q., Huang S., Huang C., Cui J. Inorg. Chem. 2013;52:12440–12449. doi: 10.1021/ic401362s. [DOI] [PubMed] [Google Scholar]
- Bal-Demirci T., Congur G., Erdem A., Erdem-Kuruca S., Özdemir N., Akgün-Dar K., Varol B., Ülküseven B. New J. Chem. 2015;39:5643–5653. [Google Scholar]
- Singh M., Singh S. K., Gangwar M., Nath G., Singh S. K. Med. Chem. Res. 2016;25:263–282. [Google Scholar]
- Prabhakaran R., Kalaivani P., Poornima P., Dallemer F., Paramaguru G., Vijaya Padma V., Renganathan R., Huang R., Natarajan K. Dalton Trans. 2012;41:9323–9336. doi: 10.1039/c2dt12231f. [DOI] [PubMed] [Google Scholar]
- Koley Seth B., Ray A., Saha A., Saha P., Basu S. J. Photochem. Photobiol., B. 2014;132:72–84. doi: 10.1016/j.jphotobiol.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Alaghaz A.-N. M. A., Bayoumi H. A., Ammar Y. A., Aldhlmani S. A. J. Mol. Struct. 2013;1035:383–399. [Google Scholar]
- Xu H., Chen R., Sun Q., Lai W., Su Q., Huang W., Liu X. Chem. Soc. Rev. 2014;43:3259. doi: 10.1039/c3cs60449g. [DOI] [PubMed] [Google Scholar]
- Song J.-F., Wang J., Li S.-Z., Li Y., Zhou R.-S. J. Mol. Struct. 2017;1129:1–7. [Google Scholar]
- Sathyadevi P., Krishnamoorthy P., Butorac R. R., Cowley A. H., Dharmaraj N. Metallomics. 2012;4:498–511. doi: 10.1039/c2mt00004k. [DOI] [PubMed] [Google Scholar]
- Nagesh G. Y., Mruthyunjayaswamy B. H. M. J. Mol. Struct. 2015;1085:198–206. [Google Scholar]
- Raman N., Pothiraj K., Baskaran T. J. Mol. Struct. 2011;1000:135–144. [Google Scholar]
- Wani M. Y., Ahmad A., Malik M. A., Sobral A. J. F. N. Med. Chem. Res. 2016;25:2557–2566. [Google Scholar]
- Tyagi P., Tyagi M., Agrawal S., Chandra S., Ojha H., Pathak M. Spectrochim. Acta, Part A. 2017;171:246–257. doi: 10.1016/j.saa.2016.08.008. [DOI] [PubMed] [Google Scholar]
- Singh B. K., Mishra P., Prakash A., Bhojak N. Arabian J. Chem. 2017;10:S472–S483. [Google Scholar]
- Bandyopadhyay D., Layek M., Fleck M., Saha R., Rizzoli C. Inorg. Chim. Acta. 2017;461:174–182. [Google Scholar]
- Zafar H., Ahmad A., Khan A. U., Khan T. A. J. Mol. Struct. 2015;1097:129–135. [Google Scholar]
- Chityala V. K., Sathish Kumar K., Subhashini N. J. P., Raghavaiah P. J. Coord. Chem. 2013;66:274–286. [Google Scholar]
- Ali A. A., Nimir H., Aktas C., Huch V., Rauch U., Schäfer K.-H., Veith M. Organometallics. 2012;31:2256–2262. [Google Scholar]
- Shaabani B., Khandar A. A., Mahmoudi F., Maestro M. A., Balula S. S., Cunha-Silva L. Polyhedron. 2013;57:118–126. [Google Scholar]
- Lukmantara A. Y., Kalinowski D. S., Kumar N., Richardson D. R. Org. Biomol. Chem. 2013;11:6414–6425. doi: 10.1039/c3ob41109e. [DOI] [PubMed] [Google Scholar]
- Shakir M., Azam M., Ullah M. F., Hadi S. M. J. Photochem. Photobiol., B. 2011;104:449–456. doi: 10.1016/j.jphotobiol.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Pettinari R., Pettinari C., Marchetti F., Clavel C. M., Scopelliti R., Dyson P. J. Organometallics. 2013:5–12. [Google Scholar]
- Mishra A. P., Mishra R., Jain R., Gupta S. Mycobiology. 2012;40:20–26. doi: 10.5941/MYCO.2012.40.1.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathiresan S., Anand T., Mugesh S., Annaraj J. J. Photochem. Photobiol., B. 2015;148:290–301. doi: 10.1016/j.jphotobiol.2015.04.016. [DOI] [PubMed] [Google Scholar]
- Karekal M. R., Biradar V., Bennikallu M., Mathada H. Bioinorg. Chem. Appl. 2013;2013:315972. doi: 10.1155/2013/315972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yernale N. G., Bennikallu M., Mathada H. Bioinorg. Chem. Appl. 2014;2014:314963. doi: 10.1155/2014/314963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azam M., Warad I., Al-resayes S. I., Alzaqri N., Rizwan M. J. Mol. Struct. 2013;1047:48–54. [Google Scholar]
- Raman N., Sakthivel A., Pravin N. Spectrochim. Acta, Part A. 2014;125:404–413. doi: 10.1016/j.saa.2014.01.108. [DOI] [PubMed] [Google Scholar]
- Leelavathy C., Antony S. A. Spectrochim. Acta, Part A. 2013;113:346–355. doi: 10.1016/j.saa.2013.04.055. [DOI] [PubMed] [Google Scholar]
- Raman N., Selvaganapathy M. Inorg. Chem. Commun. 2013;37:114–120. [Google Scholar]
- Manjula B., Antony S. A., Dhanaraj C. J. Spectrosc. Lett. 2014;47:518–526. [Google Scholar]
- Kumaran J. S., Priya S., Jayachandramani N., Mahalakshmi S. J. Chem. 2012;2013:1–10. [Google Scholar]
- Rosu T., Pahontu E., Reka-stefana M., Ilies D., Georgescu R., Shova S., Gulea A. Polyhedron. 2012;31:352–360. [Google Scholar]
- Joseph V. A., Vyas K. M., Pandya J. H., Gupta V. K., Jadeja R. N. J. Coord. Chem. 2013;8972:1094–1106. [Google Scholar]
- Surati K. R., Sathe P. A. Med. Chem. Res. 2016:0–1. [Google Scholar]
- Hunoor R. S., Patil B. R., Badiger D. S., Vadavi R. S., Gudasi K. B., Chandrashekhar V. M., Muchchandi I. S. Spectrochim. Acta, Part A. 2010;77:838–844. doi: 10.1016/j.saa.2010.08.015. [DOI] [PubMed] [Google Scholar]
- Shiva K., Shiva L., Chandan S., Kumar R. M. N., Revanasiddappa H. D. Spectrochim. Acta, Part A. 2013;107:108–116. doi: 10.1016/j.saa.2013.01.013. [DOI] [PubMed] [Google Scholar]
- Joseph J., Nagashri K., Rani G. A. B. J. Saudi Chem. Soc. 2013;17:285–294. [Google Scholar]
- Shedeed M., Abdou A., Sherif E., Hasan R., Taha A., Karim A. Eur. J. Chem. 2013;4:370–378. [Google Scholar]
- El-sherif A. A., Shehata M. R., Shoukry M. M., Barakat M. H. Spectrochim. Acta, Part A. 2012;96:889–897. doi: 10.1016/j.saa.2012.07.047. [DOI] [PubMed] [Google Scholar]
- Hanif M., Chohan Z. H. Spectrochim. Acta, Part A. 2013;104:468–476. doi: 10.1016/j.saa.2012.11.077. [DOI] [PubMed] [Google Scholar]
- Tyagi P., Chandra S., Saraswat B. S., Sharma D. Spectrochim. Acta, Part A. 2015;143:1–11. doi: 10.1016/j.saa.2015.02.027. [DOI] [PubMed] [Google Scholar]
- Arjmand F., Sayeed F., Muddassir M. J. Photochem. Photobiol., B. 2011;103:166–179. doi: 10.1016/j.jphotobiol.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Banerjee S., Dixit A., Maheswaramma K. S., Maity B., Mukherjee S., Kumar A., Karande A. A., Chakravarty A. R. J. Chem. Sci. 2016;128:165–175. [Google Scholar]
- Manikandan R., Viswanathamurthi P., Velmurugan K. J. Photochem. Photobiol., B. 2014;130:205–216. doi: 10.1016/j.jphotobiol.2013.11.008. [DOI] [PubMed] [Google Scholar]
- Zhang N., Fan Y., Zhang Z., Zuo J., Zhang P., Wang Q., Liu S., Bi C. Inorg. Chem. Commun. 2012;22:68–72. [Google Scholar]
- Creaven B. S., Duff B., Egan D. A., Kavanagh K., Rosair G., Reddy V., Walsh M. Inorg. Chim. Acta. 2010;363:4048–4058. [Google Scholar]
- Rafi U. M., Mahendiran D., Haleel A. K., Nankar R. P., Doble M., Rahiman A. K. New J. Chem. 2016;40:2451–2465. [Google Scholar]
- Chow M. J., Babak M. V., Wong D. Y. Q., Pastorin G., Gaiddon C., Ang W. H. Mol. Pharmaceutics. 2016;13:2543–2554. doi: 10.1021/acs.molpharmaceut.6b00348. [DOI] [PubMed] [Google Scholar]
- Paul A., Anbu S., Sharma G., Kuznetsov M. L., Koch B., Guedes da Silva M. F. C., Pombeiro A. J. L. Dalton Trans. 2015;44:19983–19996. doi: 10.1039/c5dt02880a. [DOI] [PubMed] [Google Scholar]
- Qiao X., Ma Z.-Y., Xie C.-Z., Xue F., Zhang Y.-W., Xu J.-Y., Qiang Z.-Y., Lou J.-S., Chen G.-J., Yan S.-P. J. Inorg. Biochem. 2011;105:728–737. doi: 10.1016/j.jinorgbio.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Chellaian J. D., Johnson J. Spectrochim. Acta, Part A. 2014;127:396–404. doi: 10.1016/j.saa.2014.02.075. [DOI] [PubMed] [Google Scholar]
- Rajarajeswari C., Loganathan R., Palaniandavar M., Suresh E., Riyasdeen A., Akbarsha M. A. Dalton Trans. 2013;42:8347. doi: 10.1039/c3dt32992e. [DOI] [PubMed] [Google Scholar]
- Tyagi P., Chandra S., Saraswat B. S., Yadav D. Spectrochim. Acta, Part A. 2015;145:155–164. doi: 10.1016/j.saa.2015.03.034. [DOI] [PubMed] [Google Scholar]
- Liu Z.-C., Wang B.-D., Li B., Wang Q., Yang Z.-Y., Li T.-R., Li Y. Eur. J. Med. Chem. 2010;45:5353–5361. doi: 10.1016/j.ejmech.2010.08.060. [DOI] [PubMed] [Google Scholar]
- Sathiyaraj S., Sampath K., Butcher R. J., Pallepogu R., Jayabalakrishnan C. Eur. J. Med. Chem. 2013;64:81–89. doi: 10.1016/j.ejmech.2013.03.047. [DOI] [PubMed] [Google Scholar]
- Basu A., Thiyagarajan D., Kar C., Ramesh A., Das G. RSC Adv. 2013;3:14088. [Google Scholar]
- Du Y., Chen W., Fu X., Deng H., Deng J. RSC Adv. 2016;6:109718–109725. [Google Scholar]
- Pettinari R., Marchetti F., Pettinari C., Petrini A., Scopelliti R., Clavel C. M., Dyson P. J. Inorg. Chem. 2014;53:13105–13111. doi: 10.1021/ic502274b. [DOI] [PubMed] [Google Scholar]