Abstract
Background:
Night shift work, exposure to light at night (ALAN) and circadian disruption may increase the risk of hormone-dependent cancers.
Objectives:
We evaluated the association of exposure to ALAN during sleeping time with breast and prostate cancer in a population based multicase–control study (MCC-Spain), among subjects who had never worked at night. We evaluated chronotype, a characteristic that may relate to adaptation to light at night.
Methods:
We enrolled 1,219 breast cancer cases, 1,385 female controls, 623 prostate cancer cases, and 879 male controls from 11 Spanish regions in 2008–2013. Indoor ALAN information was obtained through questionnaires. Outdoor ALAN was analyzed using images from the International Space Station (ISS) available for Barcelona and Madrid for 2012–2013, including data of remotely sensed upward light intensity and blue light spectrum information for each geocoded longest residence of each MCC-Spain subject.
Results:
Among Barcelona and Madrid participants with information on both indoor and outdoor ALAN, exposure to outdoor ALAN in the blue light spectrum was associated with breast cancer [adjusted odds ratio (OR) for highest vs. lowest tertile, ; 95% CI: 1.00, 2.17] and prostate cancer (; 95% CI: 1.38, 3.03). In contrast, those exposed to the highest versus lowest intensity of outdoor ALAN were more likely to be controls than cases, particularly for prostate cancer. Compared with those who reported sleeping in total darkness, men who slept in “quite illuminated” bedrooms had a higher risk of prostate cancer (; 95% CI: 1.55, 5.04), whereas women had a slightly lower risk of breast cancer (; 95% CI: 0.39, 1.51).
Conclusion:
Both prostate and breast cancer were associated with high estimated exposure to outdoor ALAN in the blue-enriched light spectrum. https://doi.org/10.1289/EHP1837
Introduction
The increase of artificial light at night (ALAN) in cities has altered the natural light levels in the nocturnal environment and extended human activities into the usually dark hours (Falchi et al. 2011). It has been estimated that more than 80% of the world population (99% of the population from the United States and Europe) and almost one-fifth of the world terrain is under light-polluted skies that suffer from an excessive, misdirected, or obtrusive artificial (usually outdoor) light (Cinzano et al. 2001; Falchi et al. 2011, 2016). Migration toward the light emitting diode (LED) technology in urban settings has resulted to an increase in ALAN and particularly an increase of the blue light spectrum due to the use of white LED as the new urban light standard (Kyba et al. 2017).
In 2007, the International Agency for Research on Cancer (IARC) concluded that shift work that involves circadian disruption is “probably carcinogenic to humans” (IARC 2007). The epidemiological evidence mostly focused on breast cancer, but since 2007 studies on night shift have examined other cancers and several have identified modest increased risks for prostate cancer (Behrens et al. 2017), particularly among advanced tumors (Papantoniou et al. 2016). Several mechanisms related to the circadian system and exposure to light at night were examined by IARC involving suppression of melatonin production, alterations of sleep–activity patterns, and deregulation of circadian genes. Depending on light intensity and wavelength, exposure to ALAN may affect human health by decreasing the production and secretion of pineal melatonin (N-acetyl-5-methoxytriptamine), which is a hormone normally produced in the dark phase of the 24-h cycle (Brainard et al. 2001; Chang et al. 2014; Escofet and Bará 2015; Thapan et al. 2013). Melatonin is related to cancer through several pathways (IARC 2010; Korkmaz and Reiter 2008), including effects on estrogen-receptor positive human breast cancer cells (Hill et al. 2015). Studies in day and night shift workers have shown a delay in peak time and lower melatonin levels in night workers with mean urinary 6-sulfatoxymelatonin (aMT6s) levels of creatinine per hour compared with 15.4 in day workers (Papantoniou et al. 2014). Data showing similar patterns in the general population in relation to ALAN are limited. For example, subjects reading light-emitting devices (e.g., eBook) before sleeping compared with a printed book, took longer to fall asleep, had reduced evening sleepiness, reduced melatonin secretion, later timing of their circadian clock, and reduced next-morning alertness (Chang et al. 2015).
Genetic background has been shown to be related to preferential day or night profile (chronotype) (Jones et al. 2016), adaptation to night work, and changes in sleep and wake schedules. For instance, Papantoniou et al. (2014) identified the lowest melatonin levels among night shift workers with the morning-preference chronotype, an individual characteristic that may relate to night shift work adaptation. Age, sex, and probably other factors such as living indoors and nighttime illumination (Roenneberg and Merrow 2016) or personality traits (Antypa et al. 2017) may also be related to chronotype.
The IARC evaluation examined occupational rather than environmental exposures and only a few studies, most of them based on ecological comparisons, have measured the direct impact of ALAN in cities on circadian rhythms and hormone-dependent cancers. Nighttime satellite photometry, collected in the framework of the U.S. Air Force Defense Meteorological Satellite Program—Operational Linescan System (DMSP-OLS), has been used for mapping sky brightness and built surfaces (Falchi 1999; Cinzano et al. 2000). Even though data obtained from satellite images are only able to detect the intensity of light but not to measure the spectrum of nighttime lighting emissions, different studies used this source of information to link the ALAN intensities captured by DMSP-OLS with incidence rates of breast and prostate cancer and found a significant positive association (Kloog et al. 2009, 2010; Keshet-Sitton et al. 2017). Rybnikova et al. (2015, 2016), reanalyzed Kloog et al.’s work, using GLOBOCAN (US-DMSP and World Bank’s 2002 and 2012 databases), controlling for several country-level predictors including birth rates, percent of urban population, and per capita GDP and electricity consumption. They found a significant positive breast and prostate cancer–ALAN association once the data were reorganized in geographic clusters of similarly developed countries. In addition, further studies (Bauer et al. 2013; Hurley et al. 2014; Keshet-Sitton et al. 2016; Kloog et al. 2011) combined indoor ALAN estimates, based on questionnaire data regarding sleep habits and use of nighttime lighting, with estimates of outdoor ALAN obtained from DMSP-OLS or also from questionnaires, to evaluate the association with breast cancer, concluding that decreasing nighttime light exposure diminished breast cancer risk. A recent analysis of the Nurses’ Health Study II (James et al. 2017) reported a small increased breast cancer risk among premenopausal women associated with exposure to residential outdoor light at night.
We have shown in a population based case–control study in Spain (estudio español multi-caso control; MCC-Spain) an overall higher risk of breast and prostate cancer among night shift workers (Papantoniou et al. 2015a, 2016). In the present analysis, we evaluated in the same study among non–night shift workers, the association of breast cancer and prostate cancer risk with the level of reported indoor ALAN during sleeping time and with remotely sensed levels of outdoor ALAN light intensity and color (spectral content), individually assigned to geocoded addresses of study participants. In this study we did not measure nighttime melatonin levels among subjects.
Materials and Methods
Study Population
The MCC-Spain is a population based multicase–control study (http://www.mccspain.org) on frequent tumors in Spain that includes cases and population controls from the catchment areas of 23 hospitals in 12 regions and assesses five types of cancer (breast, colorectal, prostate, and stomach cancers and chronic lymphocytic leukemia) using the same series of population controls for all cases (Castaño-Vinyals et al. 2015). In this analysis we include subjects from the 11 regions enrolling breast and prostate cancer cases and corresponding controls (Asturias, Barcelona, Cantabria, Girona, Granada, Guipúzcoa, Huelva, León, Madrid, Navarra, and Valencia). Images from the International Space Station (ISS) used to evaluate outdoor ALAN were only available for the two largest centers, Barcelona and Madrid, and tables in the main text present results only for these two centers.
Recruitment of incident cancer cases and population controls 20–85 y of age took place from 2008 to 2013. We recruited cases with an incident histologically confirmed diagnosis of cancer, living in the catchment area of each selected hospital for at least 6 months. Controls were randomly selected from the Primary Health Centers located in the same catchment area as cases with no history of cancer and were frequency matched to cases by sex, age in 5-y age groups and study area. They were contacted on behalf of their General Practitioner and invited to participate in the study. Excluded subjects included those incapable of participating in the interview due to communication difficulties (i.e., mental or speaking problems) and/or excess impairment of physical ability. Response rates varied by center with an average 72% response rate among cases and 52% among controls with valid telephone numbers in the primary health centers’ rosters. All models were adjusted for individual and area-based socioeconomic status to, in part, take into account a potential bias from differential participation among cases and controls.
To analyze the effect of ALAN during sleeping time, we excluded subjects who had ever worked in night-shift (i.e., working schedule that involved working partly or entirely between 0000 and 0600 hours, at least three times per month) so as to avoid potential exposure misclassification given that, at least some nights, those subjects would not be at their home. Due to this condition we excluded 224 breast cases, 208 female controls, 327 prostate cases, and 353 male controls.
The population having both indoor and outdoor ALAN exposure estimates from Barcelona and Madrid includes 380 breast cancer cases, 490 female controls, 359 prostate cancer cases and 544 male controls (Table 1). The study population (Barcelona, Madrid, and all other areas) for which only indoor ALAN estimates are presented includes 1,219 breast cancer cases, 1,385 female controls, 623 prostate cancer cases, and 879 male controls.
Table 1.
Distribution of potential breast and prostate cancer risk factors among non–shift workers from Barcelona and Madrid (MCC-Spain) included in the artificial light-at-night (ALAN) models.
| Characteristic | Breast cancer | Prostate cancer | ||
|---|---|---|---|---|
| Controls [n (%)] | Cases [n (%)] | Controls [n (%)] | Cases [n (%)] | |
| Age (y) [mean (SD)] | 60.4 (12.2) | 55.8 (11.9) | 66 (8.3) | 65.1 (6.8) |
| Educational level | ||||
| Less than primary school | 91 (18.6) | 47 (12.4) | 71 (13.1) | 58 (16.2) |
| Primary school | 151 (30.8) | 134 (35.3) | 164 (30.1) | 140 (39.0) |
| Secondary school | 152 (31.0) | 124 (32.6) | 159 (29.2) | 86 (24.0) |
| University | 96 (19.6) | 75 (19.7) | 150 (27.6) | 75 (20.9) |
| Socioeconomic scorea | ||||
| Low | 131 (29.2) | 120 (31.6) | 131 (26.8) | 145 (40.4) |
| Medium | 240 (53.6) | 208 (54.7) | 245 (50.2) | 167 (46.5) |
| High | 77 (17.2) | 52 (13.7) | 112 (23.0) | 47 (13.1) |
| Missing values (n) | 42 | 0 | 56 | 0 |
| Urban vulnerability [mean (SD)]b | 0.5 (0.2) | 0.5 (0.1) | 0.5 (0.2) | 0.5 (0.2) |
| BMI | ||||
| 238 (48.6) | 170 (44.7) | 143 (26.3) | 93 (25.9) | |
| 25–30 | 162 (33.1) | 148 (38.9) | 284 (52.2) | 192 (53.5) |
| 90 (18.4) | 62 (16.3) | 117 (21.5) | 74 (20.6) | |
| Smoking (ever)c | ||||
| No | 194 (39.7) | 177 (46.6) | 408 (75.0) | 264 (73.5) |
| Yes | 295 (60.3) | 203 (53.4) | 136 (25.0) | 95 (26.5) |
| Missing values (n) | 1 | 0 | 0 | 0 |
| Family history of breast/prostate cancer | ||||
| No | 439 (89.6) | 310 (81.6) | 506 (93) | 299 (83.3) |
| Yes | 51 (10.4) | 70 (18.4) | 38 (7.0) | 60 (16.7) |
| Alcohol consumption [mean (SD)] | 5 (8.2) | 6.2 (10.5) | 29.6 (33.3) | 30.8 (34.4) |
| Missing values (n) | 56 | 55 | 44 | 37 |
| Chronotype | ||||
| Morning type | 228 (47.5) | 164 (43.6) | 292 (55.7) | 196 (54.9) |
| Intermediate chronotypes | 186 (38.8) | 150 (39.9) | 171 (32.6) | 118 (33.1) |
| Evening type | 66 (13.8) | 62 (16.5) | 61 (11.6) | 43 (12.0) |
| Missing values (n) | 10 | 4 | 20 | 2 |
| Menopausal status | ||||
| Premenopausal | 112 (22.9) | 136 (35.8) | — | — |
| Postmenopausal | 378 (77.1) | 244 (64.2) | — | — |
| Participating centers | ||||
| Madrid | 254 (51.8) | 193 (50.8) | 239 (43.9) | 172 (47.9) |
| Barcelona | 236 (48.2) | 187 (49.2) | 305 (56.1) | 187 (52.1) |
Note: —, not applicable.
Socioeconomic score based on a combination of information on education of parents and on occupation and education of the subject.
Urban vulnerability index to measure socioeconomic status at area level was coded from 0 to 1 [Ministry of Public Works (Spain); http://atlasvulnerabilidadurbana.fomento.es/#v=map2;l=en].
Smoking habits (ever smoked at least 100 cigarettes or 360 g of tobacco vs. none).
Data collection.
Data was collected through face-to-face interviews performed by trained personnel and included lifetime residential and occupational history. Information on other risk factors for breast or prostate cancer was collected, including age, educational level, family socioeconomic level, race, body mass index (BMI), family history of cancer, smoking status, and in women reproductive history (see full questionnaire at http://www.mccspain.org/wp-content/uploads/2016/07/Quest_MCCSpain.pdf). Chronotype was assessed through a follow-up telephone interview and the use of the Munich Chronotype Questionnaire (MCTQ). Chronotype () was estimated as the mid-sleep time on free days [], corrected for oversleep on free days compared with working days []. Chronotype was assessed using categorical variables with three categories: morning type (corresponding to ); intermediate/neither type (); and evening type () (Papantoniou et al. 2015a, 2016).
The MCC-Spain study followed the national and international directives, namely the deontological code and declaration of Helsinki and the Spanish law on confidentiality of data (Ley Organica 15/1999 de 13 Diciembre de Proteccion de Datos de carácter personal; LOPD). All subjects who agreed to participate and fulfilled the eligibility criteria signed an informed consent form before participating in the study. The corresponding ethics committees of the participating centers and hospitals reviewed the protocol of the study.
Tumor subphenotypes.
Clinical information from medical records analyzed for 350 breast cancer cases, including tumor hormonal receptor status, and for 355 prostate cases with information on Gleason score. Breast cancer cases were subclassified into three subtypes based on local pathology reports: a) estrogen-receptor positive and progesterone-receptor positive () tumors with luminal human epidermal growth factor receptor 2 negative () and estrogen-receptor positive () or progesterone-receptor positive (); b) tumors with luminal human epidermal growth factor receptor 2 positive () irrespective of estrogen or progesterone receptor results; c) TN (triple-negative) tumors with , , and . Prostate cancer cases were evaluated by degree of differentiation/grade using the prostate biopsy Gleason score (: well or moderately differentiated; : poorly differentiated/more aggressive).
ALAN Exposures
We evaluated indoor ALAN through the MCC-Spain questionnaire where it was defined as the level of light in the bedroom during sleeping time when the participants were at 40 y of age. This was a subjective measure requested during the face-to-face interview using a four-digit Likert scale: a) total darkness, b) almost dark, c) dim light, and d) quite illuminated. No additional specification of the scale was provided. For subjects of age, we recorded their response at the age of diagnosis or interview. We analyzed responses for subjects who were 40 y of age to capture average exposures in adult life but had requested the same information for indoor light during the last year before diagnosis or interview. Responses were similar to those reported for subjects 40 y of age with Pearson correlation coefficients of 0.90 for breast cancer study subjects and 0.91 for prostate cancer study subjects.
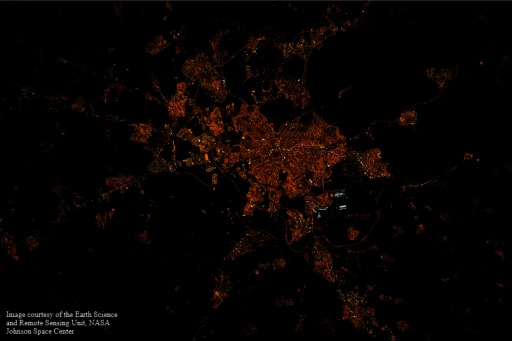
For the evaluation of outdoor ALAN, we used images of Madrid (Figure 1) and Barcelona (Figure 2) taken by astronauts aboard the ISS in 2012 (ISS030-E-82052) and 2013 (ISS035-E-23385), respectively. The images were downloaded from the Earth Science and Remote Sensing Unit, NASA Johnson Space Center (https://eol.jsc.nasa.gov). There were no major changes in the two cities on their street lighting from 2001 to 2014, according to local experts, ISS images, and statistics of the energy consumption of the town halls (Sánchez de Miguel et al. 2014). Because there are no ISS images for earlier years and for the period 2001–2014, we evaluated available images and selected those appearing most informative that corresponded to the years 2012–2013. Those images were taken with commercial Digital Single-Lens Reflex (DSLR) cameras providing image information in three spectral bands in the visual range [red (R), green (G), blue (B); i.e., RGB], and with the European Space Agency NightPod system (installed in 2012). These instruments may provide ground-level resolutions of (Kyba 2016), but in the images included in the present analysis, the spatial resolution was about . The images were calibrated applying the procedure described in Sánchez de Miguel (2015) by using existing databases of standard typical emission spectra of known types of outdoor lighting (e.g., white LED, low-pressure sodium, metal halide) and inferring the observed lighting type from the RGB signature (Sánchez de Miguel 2007; Sánchez de Miguel et al. 2014). More specifically, we used the G/R ratio to proceed to the classification of the ground-level spectral type of the lamps, and then we used a lamp spectral database to estimate the ground-based spectrum of the light emissions (see Figure S1). In the estimation we assume the atmospheric transfer function and the ground reflectance to not much affect the classification process.
Figure 1.
International Space Station night image of Madrid 12 February 2012, time: 02:22:46 GMT (local time 03:22:46) (ISS030-E-82052; NASA Johnson Space Center; https://eol.jsc.nasa.gov).
Figure 2.
International Space Station night image of Barcelona 18 April 2013, time: 22:10:46 GMT (local time 00:10:46) (ISS035-E-23385; NASA Johnson Space Center; https://eol.jsc.nasa.gov).
An estimate of the visual light was done using a relationship between the ratio of the photopic visual light over the green band fluxes detected from the ISS () to the ratio of the green to the red bands (G/R). This relationship has been determined for a variety of lighting technologies by Sánchez de Miguel (2015) (see Figure S2).
We also calculated an index of outdoor blue light spectrum using an approach described in Aubé et al. (2013) to calculate the melatonin suppression index (MSI) at each pixel of the image. The MSI is related to exposure to blue light and is a metric designed to scale the spectral interaction between a given light spectrum and the published measurements of the melatonin suppression action spectrum (MSAS) (Brainard et al. 2001; Thapan et al. 2001). The MSI has been designed to separate the effect of the shape of a spectrum from its averaged luminous intensity by making use of the MSAS. The MSI determinations were done for the house location of each participant involved in the study and derived as a number generally ranging from 0 to 1. The MSI represents to what extent the spectrum shape of different lights are efficient to suppress the melatonin production compared with the spectrum shape of the International Commission on Illumination (CIE) illuminant D65 that has been arbitrarily set to the highest value (one). The CIE Standard Illuminant D65 corresponds approximately to the average midday sunlight in Western and Northern Europe.
Therefore, two quantitative indices of outdoor ALAN were estimated from space based color imagery: a) outdoor visual-ALAN, as a proxy for luminance; and b) MSI, which is highly linked to blue light spectrum and melatonin suppression action spectrum.
A Geographic Information System (GIS), QGIS (QGIS Development Team 2015), was used to assign outdoor ALAN levels of visual light (outdoor visual-ALAN) and MSI to each individual cases and controls locations from the MCC-Spain study, selecting the geocoded residence with the longest duration for each participant. Residential mobility was low in this study population and the longest residence was, on average, and coincided with the last residence for 80.2% of the subjects.
Statistical Analysis
We applied unconditional logistic regression and calculated adjusted odds ratios (ORs) and 95% confidence intervals (CIs) in separate and combined models of indoor and/or outdoor ALAN exposures for each of the two cancers.
Models were adjusted a priori (basic adjustment) for age, center (participant cities), and educational level (less than primary school, primary school, secondary school and university); breast cancer models also included adjustment for menopausal status (pre- or postmenopausal based on self-report in combination with additional criteria, specifically number of menstruations during the last year, age of stopping menstruation, and cause of nonmenstruating, including surgeries). A further adjustment was also carried out including the previous variables in addition to the following: BMI, [treated as a categorical variable: normal weight (0 to ), overweight (25–30), obese ()]; socioeconomic status score [ranged from 0 to 7 and categorized in three levels: high (scores 6–7), medium (scores 3–5), low (scores 0–3); the score was based on a combination of information on education of parents (range 0–2), socioeconomic status derived from the occupation of the subject (range 0–2), and education of the subject (range 0–3)]; urban vulnerability to measure socioeconomic status at area level coded from 0 to 1 [Ministry of Public Works (Spain); http://atlasvulnerabilidadurbana.fomento.es/#v=map2;l=en]; family history of breast cancer in women and prostate cancer in men in first-degree relatives; smoking habits (ever smoked at least 100 cigarettes or 360 g of tobacco vs. none); chronotype information (morning, evening vs. intermediate), and mutual adjustment for each type of light exposure. Adjustment for alcohol made minimal difference (data not shown) and we present results without adjustment because 10.7% of the study population had missing values for alcohol. In all models, we completed case analyses such that observations with missing data for any covariate were excluded from models. Outdoor ALAN variables were analyzed as categorical variables using tertiles of exposure among controls. We used generalized additive models (GAMs) to examine the shape of the dose-response relationship between outdoor ALAN exposure and risk of cancer.
We explored effect modification by menopausal status using the likelihood ratio test, comparing the model including the interaction term to the model without the interaction term. ORs for light exposure variables by menopausal status and chronotype are reported from the stratified models.
We analyzed effects on subphenotypes of the diseases using multinomial logistic regression, applying the basic adjustment for breast and prostate cancer. Chronotype was also examined in a stratified analysis. In a sensitivity analysis, we provide results that include subjects who reported also having done night shift work.
All statistical analyses were performed using Stata S.E. (version 12.1; StataCorporation) and the R software environment (R Core Development Team).
Results
Study Population
The distribution of potential breast and prostate cancer risk factors among 1,773 participants for indoor and outdoor ALAN models (Barcelona and Madrid) are shown in Table 1. Around 17% of the female population and 21% of the male population were obese, with a BMI of . Female cases were slightly younger than controls (56 vs. 60 y of age), less often postmenopausal (64% vs. 77%), and more frequently reported family history of breast cancer (18% vs. 10%) compared with controls. Male cases also more frequently reported family history of prostate cancer than controls (17% vs. 7%). The chronotype questionnaire was completed by 99% of breast and prostate cancer cases, and by 98% and 96% of breast and prostate cancer controls, respectively.
The same information is provided in Table S1 for the total MCC-Spain population () for which only indoor ALAN information is available. For nearly all variables, distributions are very similar to those presented in Table 1 for the main study population (, Barcelona and Madrid).
Indoor and Outdoor ALAN Models
Visual light data (units proportional to the luminance, a quantity expressed in units of ) had an average of 0.064 [SD (minimum, maximum): 0.034 (0.010, 0.219)] for breast cancer cases and 0.065 [0.034 (0.009, 0.225)] for controls, and an average of 0.061 [0.032 (0.005, 0.175)] for prostate cancer cases and 0.066 [0.033 (0.002, 0.225)] for corresponding controls. Values of MSI had an average of 0.155 [0.051 (0.053, 0.407)] for breast cancer cases and 0.150 [0.045 (0.041, 0.361)] for controls, and an average of 0.153 [0.051 (0.025, 0.413)] for prostate cancer cases, and 0.148 [0.044 (0.017, 0.365)] for corresponding controls. No correlation was found between indoor ALAN (categorical variable) and outdoor ALAN (continuous variables) with coefficients of determination (R2) of 2.36% for visual and 1.62% for MSI for breast cancer, and corresponding R2 of 2.95% and 0.99% for prostate cancer. There was no correlation between outdoor ALAN-visual and MSI (blue light spectrum) with Spearman correlation coefficients of 0.1 for breast cancer and 0.06 for prostate cancer cases and controls.
ORs for indoor and outdoor ALAN and breast or prostate cancer in the Barcelona and Madrid study populations are shown for in Table 2. Patterns of ORs were similar for the basic and fully adjusted models; ORs for the fully adjusted models are reported in the text unless otherwise noted. Sleeping in more illuminated bedrooms (indoor ALAN) compared with sleeping in total darkness was positively associated with prostate cancer (; 95% CI: 1.55, 5.04) but was inversely associated breast cancer (; 95% CI: 0.39, 1.51). Exposure to the higher versus lowest tertile of blue light spectrum (outdoor ALAN-MSI) was positively associated with both breast (; 95% CI: 1.00, 2.17) and prostate cancer (; 95% CI: 1.38, 3.03) cancers. The positive association between the highest versus lowest tertile of blue light spectrum (MSI) and breast cancer was slightly higher in postmenopausal women (, 95% CI: 0.84, 2.03) compared with premenopausal women (; 95% CI: 0.57, 2.09) although this difference was not statistically significant () (see Table S2). Visual light (outdoor ALAN) was inversely associated with prostate cancer (; 95% CI: 0.38, 0.84 for the highest vs. lowest tertile), but the association was close to the null for breast cancer.
Table 2.
Associations of indoor and outdoor artificial light at night (ALAN) with breast and prostate cancer among non–shift workers from Barcelona and Madrid (MCC-Spain).
| Exposure | Breast cancer | Prostate cancer | ||||
|---|---|---|---|---|---|---|
| Controls [n (%)] | Cases [n (%)] | ORs (95% CI) | Controls [n (%)] | Cases [n (%)] | ORs (95% CI) | |
| Basic adjustment (n)a | 490 | 380 | — | 544 | 359 | — |
| Indoor ALAN | — | — | ||||
| 59 (12.0) | 50 (13.2) | 1.00 | 120 (22.1) | 73 (20.3) | 1.00 | |
| Almost dark | 186 (38.0) | 119 (31.3) | 0.88 (0.55, 1.41) | 210 (38.6) | 92 (25.6) | 0.66 (0.44, 1.00) |
| Dim light | 208 (42.4) | 180 (47.4) | 1.26 (0.78, 2.03) | 181 (33.3) | 140 (39.0) | 1.17 (0.78, 1.75) |
| Quite illuminated | 37 (7.6) | 31 (8.2) | 1.08 (0.57, 2.02) | 33 (6.1) | 54 (15.0) | 2.82 (1.65, 4.83) |
| Outdoor ALAN-Visual Lightc | ||||||
| (lowest) | 157 (32.0) | 133 (35.0) | 1.00 | 162 (29.8) | 139 (38.7) | 1.00 |
| 2nd tertile | 170 (34.7) | 121 (31.8) | 0.86 (0.60, 1.21) | 182 (33.5) | 119 (33.1) | 0.72 (0.52, 1.02) |
| 3rd tertile (highest) | 163 (33.3) | 126 (33.2) | 0.86 (0.59, 1.26) | 200 (36.8) | 101 (28.1) | 0.54 (0.37, 0.78) |
| Outdoor ALAN-MSI (blue light)d | ||||||
| (lowest) | 164 (33.5) | 126 (33.2) | 1.00 | 189 (34.7) | 114 (31.8) | 1.00 |
| 2nd tertile | 174 (35.5) | 116 (30.5) | 0.80 (0.56, 1.15) | 184 (33.8) | 116 (32.3) | 1.14 (0.80, 1.61) |
| 3rd tertile (highest) | 152 (31.0) | 138 (36.3) | 1.16 (0.81, 1.66) | 171 (31.4) | 129 (35.9) | 1.45 (1.02, 2.07) |
| Further adjustment (n)b | 444 | 376 | — | 472 | 357 | — |
| Indoor ALAN | ||||||
| 46 (10.4) | 49 (13.0) | 1.00 | 94 (19.9) | 72 (20.2) | 1.00 | |
| Almost dark | 173 (39.0) | 118 (31.4) | 0.73 (0.44, 1.21) | 185 (39.2) | 92 (25.8) | 0.68 (0.43, 1.06) |
| Dim light | 192 (43.2) | 178 (47.3) | 1.01 (0.60, 1.69) | 165 (35.0) | 139 (38.9) | 1.17 (0.75, 1.81) |
| Quite illuminated | 33 (7.4) | 31 (8.2) | 0.77 (0.39, 1.51) | 28 (5.9) | 54 (15.1) | 2.79 (1.55, 5.04) |
| Outdoor ALAN-Visual Lightc | ||||||
| (lowest) | 153 (34.5) | 132 (35.1) | 1.00 | 155 (32.8) | 138 (38.7) | 1.00 |
| 2nd tertile | 150 (33.8) | 121 (32.2) | 0.87 (0.60, 1.24) | 165 (35.0) | 119 (33.3) | 0.70 (0.49, 1.00) |
| 3rd tertile (highest) | 141 (31.8) | 123 (32.7) | 0.81 (0.54, 1.20) | 152 (32.2) | 100 (28.0) | 0.56 (0.38, 0.84) |
| Outdoor ALAN-MSI (blue light)d | ||||||
| (lowest) | 158 (35.6) | 124 (33.0) | 1.00 | 175 (37.1) | 113 (31.7) | 1.00 |
| 2nd tertile | 153 (34.5) | 114 (30.3) | 0.91 (0.62, 1.32) | 162 (34.3) | 115 (32.2) | 1.33 (0.92, 1.93) |
| 3rd tertile (highest) | 133 (30.0) | 138 (36.7) | 1.47 (1.00, 2.17) | 135 (28.6) | 129 (36.1) | 2.05 (1.38, 3.03) |
Note: BMI, body mass index; CI, confidence interval; MSI, melatonin suppression index; OR, odds ratio; tert, tertile; UVI, urban vulnerability index; —, not applicable.
Basic adjustment: age, center, educational level, and menopausal status (in breast cancer).
Further adjustment: age, center, educational level, socioeconomic status, UVI, BMI, tobacco, family history of breast/prostate cancer, chronotype, menopausal status (breast cancer), and mutual adjustment for other light exposures.
Tertiles of ALAN-visual for breast (no units): ; ; . Tertiles of visual for prostate (no units): ; ; .
Tertiles of ALAN-MSI for breast (no units): ; ; . Tertiles of MSI for prostate (no units): ; ; .
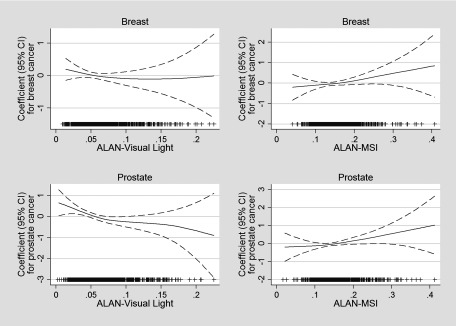
In GAM models evaluating outdoor ALAN (overall light visual and MSI-blue light) (Figure 3), all p-values for departure of linearity for were not statistically significant (). Similar to the analysis by exposure tertiles, estimated associations with outdoor ALAN-MSI (blue light) were positive and approximately linear for both prostate and breast cancer, whereas associations with outdoor ALAN-visual were approximately linear and inverse for prostate cancer, and essentially null for breast cancer.
Figure 3.
Generalized additive models (binomial distribution with a logit link function) for breast and prostate cancer and exposure to visual light and blue light (MSI). Models were adjusted by age, center, educational level, and menopausal status (breast cancer) and are mutually adjusted for visual light and blue light (MSI). Dashed lines represent 95% CI bands and the density of observations according to exposure level is indicated in the bottom part of each graph. All p-values for departure of linearity for were not statistically significant (). CI, confidence interval; MSI, melatonin suppression index.
Associations between indoor ALAN in the entire MCC-Spain study population (see Table S3) were similar to estimates based on participants from the Barcelona and Madrid centers only (Table 2). Prostate cancer was positively associated with the highest level of indoor illumination during bedtime compared with no indoor illumination (; 95% CI: 1.18, 2.76), but there was no evidence of an association with breast cancer (; 95% CI: 95%: 0.70, 1.27). Estimated associations for breast and prostate cancer in the Barcelona and Madrid study populations were consistent with the primary analyses when participants who reported ever working a night shift were included in models that were adjusted for night shift work as a covariate, as well as age, center, education, all light exposure variables, and (for breast cancer) menopausal status (see Table S4).
Chronotype and Tumor Subphenotypes
For stratified analyses by chronotype and tumor subphenotypes, we present results for the basic adjustment models so as to have a larger population sample size. Estimated associations for indoor ALAN and outdoor ALAN-MSI (blue light) with prostate cancer indicated higher ORs for evening types compared with intermediate or morning types, but these comparisons were based on small number of subjects and tests for interaction were not statistically significant (Table 3). There were no clear patterns by chronotype observed for breast cancer. Results on indoor ALAN for the total MCC-Spain population by chronotype are shown in Table S5 and are consistent with those observed in the population of Barcelona and Madrid.
Table 3.
Associations of indoor and outdoor-MSI artificial light at night (ALAN) with breast and prostate cancer among non–shift workers from Barcelona and Madrid (MCC-Spain) by chronotype.
| Characteristic | Morning chronotype | Neither Chronotype | Evening Chronotype | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Controlsa | Casesa | OR (95% CI)b | Controlsa | Casesa | OR (95% CI)b | Controlsa | Casesa | OR (95% CI)b | p-Valuec | |
| Breast cancer (n) | 228 | 164 | 186 | 150 | 66 | 62 | ||||
| Indoor ALAN | ||||||||||
| 27 (11.8) | 17 (10.4) | 1.00 | 23 (12.4) | 22 (14.7) | 1.00 | 7 (10.6) | 10 (16.1) | 1.00 | 0.974 | |
| Almost dark | 84 (36.8) | 51 (31.1) | 1.09 (0.52, 2.28) | 74 (39.8) | 49 (32.7) | 0.86 (0.41, 1.81) | 25 (37.9) | 18 (29.0) | 0.49 (0.13, 1.90) | |
| Dim light | 102 (44.7) | 85 (51.8) | 1.67 (0.80, 3.46) | 74 (39.8) | 66 (44.0) | 1.09 (0.52, 2.31) | 29 (43.9) | 27 (43.5) | 0.65 (0.17, 2.55) | |
| Quite illuminated | 15 (6.6) | 11 (6.7) | 1.29 (0.47, 3.53) | 15 (8.1) | 13 (8.7) | 0.80 (0.30, 2.16) | 5 (7.6) | 7 (11.3) | 1.20 (0.23, 6.28) | |
| Outdoor ALAN-MSI (blue light) | ||||||||||
| (lowest) | 81 (35.5) | 57 (34.8) | 1.00 | 58 (31.2) | 46 (30.7) | 1.00 | 22 (33.3) | 21 (33.9) | 1.00 | 0.870 |
| 2nd tertile | 83 (36.4) | 47 (28.7) | 0.78 (0.45, 1.33) | 61 (32.8) | 50 (33.3) | 0.97 (0.55, 1.74) | 25 (37.9) | 17 (27.4) | 0.65 (0.24, 1.76) | |
| 3rd tertile (highest) | 64 (28.1) | 60 (36.6) | 1.28 (0.75, 2.18) | 67 (36.0) | 54 (36.0) | 1.03 (0.58, 1.83) | 19 (28.8) | 24 (38.7) | 1.09 (0.36, 3.30) | |
| Prostate cancer | 292 | 196 | 171 | 118 | 61 | 43 | ||||
| Indoor ALAN | ||||||||||
| 61 (20.9) | 37 (18.9) | 1.00 | 29 (17.0) | 26 (22.0) | 1.00 | 23 (37.7) | 9 (20.9) | 1.00 | 0.310 | |
| Almost dark | 113 (38.7) | 53 (27.0) | 0.66 (0.38, 1.16) | 77 (45.0) | 29 (24.6) | 0.46 (0.22, 0.96) | 15 (24.6) | 10 (23.3) | 2.34 (0.66, 8.22) | |
| Dim light | 98 (33.6) | 81 (41.3) | 1.20 (0.69, 2.08) | 58 (33.9) | 46 (39.0) | 0.90 (0.42, 1.90) | 20 (32.8) | 12 (27.9) | 1.71 (0.51, 5.74) | |
| Quite illuminated | 20 (6.8) | 25 (12.8) | 2.16 (1.04, 4.46) | 7 (4.1) | 17 (14.4) | 3.13 (1.08, 9.03) | 3 (4.9) | 12 (27.9) | 12.83 (2.71, 60.82) | |
| ALAN-MSI | ||||||||||
| (lowest) | 109 (37.3) | 73 (37.2) | 1.00 | 57 (33.3) | 31 (26.3) | 1.00 | 17 (27.9) | 9 (20.9) | 1.00 | 0.864 |
| 2nd tertile | 90 (30.8) | 60 (30.6) | 1.08 (0.68, 1.71) | 62 (36.3) | 42 (35.6) | 1.29 (0.70, 2.38) | 24 (39.3) | 13 (30.2) | 1.12 (0.37, 3.37) | |
| 3rd tertile (highest) | 93 (31.8) | 63 (32.1) | 1.20 (0.75, 1.92) | 52 (30.4) | 45 (38.1) | 1.83 (0.98, 3.43) | 20 (32.8) | 21 (48.8) | 2.55 (0.86, 7.57) | |
Note: CI, confidence interval; LRT, ; MSI, melatonin suppression index; OR, odds ratio.
Basic adjustment: age, center, educational level, and menopausal status (in breast cancer).
Number of subjects and percentage in brackets.
p-Value for interaction (LRT).
Breast cancers that were estrogen- or progesterone-receptor positive but Her2 negative (267 cases total) were positively associated with the highest versus lowest tertile of outdoor ALAN-MSI (; 95% CI: 0.8, 1.88), but associations were close to the null for Her2 positive breast cancers (57 cases total) and inverse for triple negative tumors (26 cases total) (Table 4). For prostate cancer, associations with outdoor ALAN-MSI were similar for more aggressive (Gleason score ) and less aggressive (Gleason score ) tumors (Table 4).
Table 4.
Association of outdoor ALAN-MSI (blue light) with breast and prostate cancer subphenotypes. Non–shift workers from Barcelona and Madrid (MCC-Spain).
| Outdoor ALAN-MSI | 1st tertile | 2nd tertile | 3rd tertile | |||
|---|---|---|---|---|---|---|
| (%) | RRRa | (%) | RRR (95% CI) | (%) | RRR (95% CI) | |
| Breast cancer () | ||||||
| Controls | 164 (33.5) | — | 174 (35.5) | — | 152 (31.0) | — |
| or and | 84 (31.5) | 1.00 | 82 (30.7) | 0.86 (0.6, 1.28) | 101 (37.8) | 1.26 (0.8, 1.88) |
| 18 (31.6) | 1.00 | 19 (33.3) | 0.80 (0.4, 1.65) | 20 (35.1) | 0.99 (0.5, 2.07) | |
| Triple negativeb | 13 (50.0) | 1.00 | 7 (26.9) | 0.59 (0.2, 1.60) | 6 (23.1) | 0.64 (0.2, 1.85) |
| Prostate cancer () | ||||||
| Controls | 189 (34.7) | — | 184 (33.8) | — | 171 (31.4) | — |
| Gleason score | 56 (31.8) | 1.00 | 56 (31.8) | 1.16 (0.8, 1.80) | 64 (36.4) | 1.55 (1.0, 2.40) |
| Gleason score | 54 (30.2) | 1.00 | 60 (33.5) | 1.24 (0.8, 1.91) | 65 (36.3) | 1.54 (1.0, 2.39) |
Note: CI, confidence interval; ER, estrogen receptor; HER, human epidermal growth factor receptor; MSI, melatonin suppression index; RRR, Relative Risk Ratios; —, not applicable.
Polytomous regression models. Basic adjustment: age, center, educational level, and menopausal status (in breast cancer).
Triple negative: , , and .
Discussion
We evaluated the association between exposure to indoors and outdoors ALAN during sleep time and breast and prostate cancer risk, two cancers that have been associated with circadian disruption (IARC 2010; Behrens et al. 2017). We found that prostate cancer was positively associated with outdoor light at night in the blue light spectrum and with self-reported indoor light at night, but was inversely associated with outdoor light in the visible spectrum. Breast cancer also was associated with outdoor light in the blue spectrum, but was not associated with outdoor light in the visible spectrum or with self-reported indoor light at night. We did not find clear differences in associations with exposures to light at night according to chronotype. In this study, we applied more elaborate methods for the evaluation of light exposure compared with previous studies, but exposure assessment remains a key issue when examining the potential health effects of ALAN in human studies.
Exposure to ALAN is ubiquitous and whether the spread of exposure to ALAN may increase cancer risk and how this could be prevented are public health issues. Exposure to short wavelength light color during the hours before bedtime (Gringras et al. 2015) has been shown to suppress nocturnal melatonin production (Cajochen et al. 2005; Chang et al. 2014), which, in turn, could be associated with an increased risk of hormone-dependent type of cancers such as breast and prostate cancer (Stevens and Zhu 2015).
Estimates for outdoor light were based on ISS images in 2012–2013 that were selected as the most representative of the period examined. ISS light data were not available for early periods prior to the commencement of the study, although this mismatch in timing of exposure assessment and study inclusion may be less important than expected. There are no major changes in Barcelona and Madrid on their street lighting from 2003 to 2014, according to local experts and ISS images, and statistics of the energy consumption of the town halls (Sánchez de Miguel et al. 2014). Except for major street light replacements like those happening in Milan or Madrid in 2014, the street lights in many western European cities have been stable for decades, although many cities across Europe are recently experiencing marked increases in nighttime brightness (Bennie et al. 2014), especially with the massive arrival and exponential growth of LEDs in the way of replacing the incandescent and high-pressure sodium lamps (Sánchez de Miguel et al. 2017).
Existing studies examining ALAN exposures and cancer risk rates have relied almost exclusively on satellite data, primarily from the Defense Meteorological Satellite Program/Operational Linescan System (DMSP/OLS; e.g., Cinzano et al. 2001, Bennie et al. 2014) and more recently the Visible Infrared Imaging Radiometer Suite (VIIRS) with its Day-Night Band camera onboard the Suomi National Polar-Orbiting Partnership (Suomi NPP) satellite (e.g., Baugh et al. 2013). In particular, the satellite sensors from which the data have been obtained are effectively color blind; that is, they are able to detect the intensity of light integrated across a range of wavelengths but not to measure the spectrum of nighttime lighting emissions. Moreover both satellite platforms are insensitive to the blue content of the light. As a consequence, very little is known about the spatial and temporal dynamics of the spectrum of artificial nighttime lighting systems (Gaston et al. 2015). This is critical for at least two reasons. First, almost all known environmental impacts of artificial nighttime lighting are sensitive to the spectrum of that lighting, including melatonin production (Aubé et al. 2013). Second, these changes in physiological parameters may in turn influence circadian rhythms and, hence, the timing of sleep, blood pressure regulation, and seasonal reproduction and the role of melatonin as an antioxidant (Korkmaz and Reiter 2008), with consequences for the prevalence of some kinds of cancer (e.g., Cajochen et al. 2005).
We applied new methods now available that make it feasible to convert ISS images with simple three-band spectral information into ecological risk maps, using known spectral responses of key physiological and ecological processes with a higher spatial resolution (up to ), rather than those images obtained from the VIIRS/DNB platform () or DMSP/OLS () (Elvidge et al. 2013). In common with other remotely sensed data on ALAN, the maps we produced also represent light emitted or reflected upwards towards the sensor, assuming that this is a good proxy for the intensity and density of light sources at ground level. It would be interesting in further studies to include information about the aerosol content of the atmosphere in order to correct the ISS images by differential atmospheric absorption. In this research, we could not estimate the potential exposure reaching the retinae of each subject. Eyelids act as filters and, actually, as color filters transforming the incoming spectrum before reaching the retinae. Estimating individual exposures reaching the retinae would require an experimental design or much more extensive knowledge on shedding and absorption of blue light so as to develop quantitative exposure models.
We found evidence of positive associations for breast and prostate cancer with exposure to outdoor light in the blue spectrum (MSI), whereas exposure to outdoor light in the visible spectrum (i.e., luminance) was associated with lower odds of prostate cancer and was not clearly related to breast cancer. Visual light estimates are based on what the cameras detect from space, although there is a part of the light emitted that might never enter the houses. Moreover, the luminance at the window level is linked in a complex way to ground-based light emissions while taking into account atmospheric-induced optical distortion as well as spectral and geometrical transformations from the underlying ground surfaces and obstacles (Aubé 2015). In other words, the light output pattern of the light fixtures cannot be assessed from space, and it is possible that the upward light remains weakly correlated to the horizontal light that enters the houses. There is less of this problem with MSI. The only variation on the spectrum can come from different combinations of direct and reflected lights as a function of the angle; however, generally the most important contribution to the light entering a window is the direct light, and MSI does not depend on the angle for that component. Visual response that we and others have used to evaluate the outdoor visual-ALAN is not necessarily well correlated to blue light, which is the light spectrum most likely to be relevant when evaluating biological responses related to cancer. In this population there was no correlation between estimates of outdoor visual light with blue light exposure.
We did not find clear evidence that chronotype modifies associations between light at night and prostate or breast cancer, consistent with previous findings for associations with night shift work in the MCC-Spain study as a whole (Papantoniou et al. 2015b, 2016). Although chronotype is related to preference for morningness or eveningness, and has been hypothesized as a potential modifier of effects of ALAN exposure (Erren et al. 2017), the potential influence of chronotype on cancer risk is still unknown. We found a higher risk of prostate cancer among participants with a more illuminated bedroom at night (indoor ALAN). There was no association between outdoor visual-ALAN and indoor ALAN. This lack of correlation could be due to the use of shutters at night among subjects with high outdoor visual-ALAN, or perhaps a lack of relationship between the light reaching the ISS and the light reaching the house’s windows. Similar results were described in a previous study carried out by Rea et al. (2011) concluding that satellite-measured sky brightness (visual light) was unrelated to personal light exposures. In addition, sources other than street light might be contributing to indoor ALAN exposures, such as light coming from neighbors or the use of portable electronic devices with self-luminous displays and energy-efficient lighting (LEDs). The use of such devices is increasing and has a significant effect on decreasing melatonin production if they are used before bedtime (Bonmati-Carrion et al. 2014; Chang et al. 2014).
In further studies it will be interesting to measure indoor light levels rather than using only questionnaire-based methodology, which is more subjective although it may capture a longer time span of exposure. Improvements in modelling exposure such as the inclusion of the height of the residence buildings and of different obstacles in the street (e.g., trees or other buildings that could protect subjects from the received outdoor light) would have been advantageous but also should be validated with light measurements. Such approaches could help explain our observations where outdoor visual-ALAN (i.e., luminance) was associated with no or a negative effect that is opposite to that observed for blue light (MSI index), which might still penetrate the curtains or shutters (Aubé et al. 2013).
Summarizing, in this study we used modelled images provided by the ISS to map the spatial variation of artificial nighttime lighting exposure, including blue light spectrum, combined with data from questionnaires on exposure to indoor light at night, and related this information with the risk of developing the two most common hormone dependent cancers (breast and prostate). The main strengths of this study are the use of individual information rather than relying on ecological comparisons as most other studies have and the possibility, therefore, of developing personal estimates of exposure and adjusting for potential confounding factors. In addition, we used new methods for the evaluation of the blue light spectrum. The main limitation of the study is exposure misclassification because we used proxy estimates for the evaluation of both indoor ALAN and for outdoor visual-ALAN exposure (although not for MSI). We adjusted for socioeconomic status both at the individual and area level to take into account, at least in part, a potential bias due to an association of socioeconomic factors with urban structure and light, although it is unlikely that this would result in differential misclassification between cases and controls.
Conclusions
Findings from this large case–control study of two cancers that have been associated with circadian disruption and light at night during shift work provide some support for the influence of ALAN for the development of cancer in the general population. Men who reported the highest level of exposure to indoor ALAN were at greater risk of prostate cancer than men who reported no indoor illumination at night. Although both cancers were less likely among those in the highest versus lowest tertile of exposure to outdoor ALAN in the visible spectrum, outdoor ALAN in the blue-light spectrum, which is believed to be the most biologically relevant exposure, was positively associated with prostate cancer and, to a lesser extent, with breast cancer.
Supplemental Material
Acknowledgments
The study was partially funded by the Accion Transversal del Cancer, approved by the Spanish Ministry Council on 11 October 2007, by the Instituto de Salud Carlos III-FEDER (PI08/1770, PI08/0533, PI08/1359, PS09/00773-Cantabria, PS09/01286-León, PS09/01903-Valencia, PS09/02078-Huelva, PS09/01662-Granada, PI11/01889-FEDER, PI11/02213, PI12/00488, PI12/00265, PI12/01270, PI12/00715, PI14/0613), by the Fundación Marqués de Valdecilla (API 10/09), by the ICGC International Cancer Genome Consortium CLL [the ICGC CLL-Genome Project is funded by Spanish Ministerio de Economía y Competitividad (MINECO) through the Instituto de Salud Carlos III (ISCIII) and Red Temática de Investigación del Cáncer (RTICC) del ISCIII (RD12/0036/0036)], by the Junta de Castilla y León (LE22A10-2), by the Consejería de Salud of the Junta de Andalucía (PI-0571-2009, PI-0306-2011, salud201200057018tra), by the Conselleria de Sanitat of the Generalitat Valenciana (AP_061/10), by the Regional Government of the Basque Country, by the Consejería de Sanidad de la Región de Murcia, by the European Commission grants FOOD-CT-2006-036224-HIWATE, by the Spanish Association Against Cancer (AECC) Scientific Foundation, by the Catalan Government–Agency for Management of University and Research Grants (AGAUR) grants 2014SGR647 and 2014SGR850, by the Fundación Caja de Ahorros de Asturias, and by the University of Oviedo. ISGlobal is a member of the Centres de Recerca de Catalunya (CERCA) Programme, Generalitat de Catalunya.
This research was also supported in part by the STARS4ALL project funded by the H2020-ICT-2015-688135 program of the European Union, the ORISON project funded by the H2020-INFRASUPP-2015-2 program of the European Union, and through the resources of researchers and collaborators of the Cities at Night project.
J.G.-P. was funded by the Scientific Foundation of the Spanish Association against Cancer (Fundación Científica de la Asociación Española Contra el Cáncer (AECC), EVP-1178/14).
We acknowledge the participants in the study, the data manager, and all the interviewers and technicians involved in the data collection.
References
- Antypa N, Verkuil B, Molendijk M, Schoevers R, Penninx BWJH, Van Der Does W. 2017. Associations between chronotypes and psychological vulnerability factors of depression. Chronobiol Int 34(8):1125–1135, PMID: 28759270, 10.1080/07420528.2017.1345932. [DOI] [PubMed] [Google Scholar]
- Aubé M. 2015. Physical behaviour of anthropogenic light propagation into the nocturnal environment. Phil Trans R Soc Lond B Biol Sci 370(1667):20140117, PMID: 25780231, 10.1098/rstb.2014.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubé M, Roby J, Kocifaj M. 2013. Evaluating potential spectral impacts of various artificial lights on melatonin suppression, photosynthesis, and star visibility. PLoS One 8(7):e67798, PMID: 23861808, 10.1371/journal.pone.0067798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer SE, Wagner SE, Burch J, Bayakly R, Vena JE. 2013. A case-referent study: light at night and breast cancer risk in Georgia. Int J Health Geogr 12:23, PMID: 23594790, 10.1186/1476-072X-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baugh K, Hsu FC, Elvidge CD, Zhizhin M. 2013. Nighttime lights compositing using the VIIRS day-night band: preliminary results. Proc Asia-Pac Adv Network 35:70–86, 10.7125/APAN.35.8. [DOI] [Google Scholar]
- Behrens T, Rabstein S, Wichert K, Erbel R, Eisele L, Arendt M, et al. 2017. Shift work and the incidence of prostate cancer: a 10-year follow-up of a German population-based cohort study. Scand J Work Environ Health 43(6):560–568, PMID: 28879368, 10.5271/sjweh.3666. [DOI] [PubMed] [Google Scholar]
- Bennie J, Davies TW, Duffy JP, Inger R, Gaston KJ. 2014. Contrasting trends in light pollution across Europe based on satellite observed night time lights. Sci Rep 4:3789, PMID: 24445659, 10.1038/srep03789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonmati-Carrion MA, Arguelles-Prieto R, Martinez-Madrid MJ, Reiter R, Hardeland R, Rol MA, et al. 2014. Protecting the melatonin rhythm through circadian healthy light exposure. Int J Mol Sci 15(12):23448–23500, PMID: 25526564, 10.3390/ijms151223448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, et al. 2001. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci 21(16):6405–6412, PMID: 11487664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajochen C, Münch M, Kobialka S, Kräuchi K, Steiner R, Oelhafen P, et al. 2005. High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J Clin Endocrinol Metab 90(3):1311–1316, PMID: 15585546, 10.1210/jc.2004-0957. [DOI] [PubMed] [Google Scholar]
- Castaño-Vinyals G, Aragonés N, Pérez-Gómez B, Martín V, Llorca J, Moreno V, et al. 2015. Population-based multicase-control study in common tumors in Spain (MCC-Spain): rationale and study design. Gac Sanit 29(4):308–315, PMID: 25613680, 10.1016/j.gaceta.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Chang AM, Aeschbach D, Duffy JF, Czeisler CA. 2015. Evening use of light-emitting eReaders negatively affects sleep, circadian timing, and next-morning alertness. Proc Natl Acad Sci USA 112(4):1232–1237, PMID: 25535358, 10.1073/pnas.1418490112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinzano P, Falchi F, Elvidge CD. 2001. The first World Atlas of the artificial night sky brightness. Mon Not R Astron Soc 328(3):689–707, 10.1046/j.1365-8711.2001.04882.x. [DOI] [Google Scholar]
- Cinzano P, Falchi F, Elvidge CD, Baugh KE. 2000. The artificial night sky brightness mapped from DMSP satellite Operational Linescan System measurements. Mon Not R Astron Soc 318(3):641–657, 10.1046/j.1365-8711.2000.03562.x. [DOI] [Google Scholar]
- Elvidge CD, Baugh KE, Zhizhin M, Hsu FC. 2013. Why VIIRS data are superior to DMSP for mapping nighttime lights. Proc Asia-Pac Adv Network 35:62–69, 10.7125/APAN.35.7. [DOI] [Google Scholar]
- Erren TC, Groß JV, Fritschi L. 2017. Focusing on the biological night: towards an epidemiological measure of circadian disruption. Occup Environ Med 74(3):159–160, PMID: 27852644, 10.1136/oemed-2016-104056. [DOI] [PubMed] [Google Scholar]
- Escofet J, Bará S. 2015. Reducing the circadian input from self-luminous devices using hardware filters and software applications. Lighting Res Technol 49(4):481–496, 10.1177/1477153515621946. [DOI] [Google Scholar]
- Falchi F. 1999. Artificial_Luminance_of_the_Night_Sky_in_Italy. https://www.researchgate.net/publication/266557297_LUMINANZA_ARTIFICIALE_DEL_CIELO_NOTTURNO_IN_ITALIA_Artificial_luminance_of_the_night_sky_in_Italy (accessed 4 October 2017).
- Falchi F, Cinzano P, Duriscoe D, Kyba CCM, Elvidge CD, Baugh K, et al. 2016. The new World Atlas of artificial night sky brightness. Sci Adv 2(6):e1600377, PMID: 27386582, 10.1126/sciadv.1600377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falchi F, Cinzano P, Elvidge CD, Keith DM, Haim A. 2011. Limiting the impact of light pollution on human health, environment and stellar visibility. J Environ Manage 92(10):2714–2722, PMID: 21745709, 10.1016/j.jenvman.2011.06.029. [DOI] [PubMed] [Google Scholar]
- Gaston KJ, Visser ME, Hölker F. 2015. The biological impacts of artificial light at night: the research challenge. Phil Trans R Soc Lond B Biol Sci 370(1667):20140133, PMID: 25780244, 10.1098/rstb.2014.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gringras P, Middleton B, Skene DJ, Revell VL. 2015. Bigger, brighter, bluer-better? Current light-emitting devices – adverse sleep properties and preventative strategies. Front Public Health 3:233, PMID: 26528465, 10.3389/fpubh.2015.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SM, Belancio VP, Dauchy RT, Xiang S, Brimer S, Mao L, et al. 2015. Melatonin: an inhibitor of breast cancer. Endocr Relat Cancer 22(3):R183–R204, PMID: 25876649, 10.1530/ERC-15-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley S, Goldberg D, Nelson D, Hertz A, Horn-Ross PL, Bernstein L, et al. 2014. Light at night and breast cancer risk among California teachers. Epidemiology 25(5):697–706, PMID: 25061924, 10.1097/EDE.0000000000000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC (International Agency for Research on Cancer). 2010. “Painting, Firefighting, and Shiftwork.” http://monographs.iarc.fr/ENG/Monographs/vol98/mono98.pdf [accessed 26 March 2018].
- James P, Bertrand KA, Hart JE, Schernhammer ES, Tamimi RM, Laden F. 2017. Outdoor Light at Night and Breast Cancer Incidence in the Nurses’ Health Study II. Environ Health Perspect 125(8):087010, PMID: 28886600, 10.1289/EHP935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SE, Tyrrell J, Wood AR, Beaumont RN, Ruth KS, Tuke MA, et al. 2016. Genome–Wide Association Analyses in 128,266 Individuals Identifies New Morningness and Sleep Duration Loci. PLoS Genet 12(8):e1006125, PMID: 27494321, 10.1371/journal.pgen.1006125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshet-Sitton A, Or-Chen K, Huber E, Haim A. 2017. Illuminating a risk for breast cancer: a preliminary ecological study on the association between streetlight and breast cancer. Integr Cancer Ther 16(4):451–463, PMID: 27899698, 10.1177/1534735416678983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshet-Sitton A, Or-Chen K, Yitzhak S, Tzabary I, Haim A. 2016. Can avoiding light at night reduce the risk of breast cancer? Integr Cancer Ther 15(2):145–152, PMID: 26631258, 10.1177/1534735415618787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloog I, Haim A, Stevens RG, Portnov BA. 2009. Global co-distribution of light at night (LAN) and cancers of prostate, colon, and lung in men. Chronobiol Int 26(1):108–125, PMID: 19142761, 10.1080/07420520802694020. [DOI] [PubMed] [Google Scholar]
- Kloog I, Portnov BA, Rennert HS, Haim A. 2011. Does the modern urbanized sleeping habitat pose a breast cancer risk? Chronobiol Int 28(1):76–80, PMID: 21182407, 10.3109/07420528.2010.531490. [DOI] [PubMed] [Google Scholar]
- Kloog I, Stevens RG, Haim A, Portnov BA. 2010. Nighttime light level co-distributes with breast cancer incidence worldwide. Cancer Causes Control 21(12):2059–2068, PMID: 20680434, 10.1007/s10552-010-9624-4. [DOI] [PubMed] [Google Scholar]
- Korkmaz A, Reiter RJ. 2008. Epigenetic regulation: a new research area for melatonin? J Pineal Res 44(1):41–44, PMID: 18078446, 10.1111/j.1600-079X.2007.00509.x. [DOI] [PubMed] [Google Scholar]
- Kyba CC. 2016. Defense Meteorological Satellite Program data should no longer be used for epidemiological studies. Chronobiol Int 33(8):943–945, PMID: 27254140, 10.1080/07420528.2016.1189432. [DOI] [PubMed] [Google Scholar]
- Kyba CCM, Kuester T, Sánchez de Miguel A, Baugh K, Jechow A, Hölker F, et al. 2017. Artificially lit surface of Earth at night increasing in radiance and extent. Sci Adv 3(11):e1701528, PMID: 29181445, 10.1126/sciadv.1701528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papantoniou K, Castaño-Vinyals G, Espinosa A, Aragonés N, Pérez-Gómez B, Ardanaz E, et al. 2016. Breast cancer risk and night shift work in a case–control study in a Spanish population. Eur J Epidemiol 31(9):867–878, PMID: 26205167, 10.1007/s10654-015-0073-y. [DOI] [PubMed] [Google Scholar]
- Papantoniou K, Castaño-Vinyals G, Espinosa A, Aragonés N, Pérez-Gómez B, Burgos J, et al. 2015a. Night shift work, chronotype and prostate cancer risk in the MCC-Spain case-control study. Int J Cancer 137:(5):1147–1157, PMID: 25530021, 10.1002/ijc.29400. [DOI] [PubMed] [Google Scholar]
- Papantoniou K, Pozo OJ, Espinosa A, Marcos J, Castaño-Vinyals G, Basagaña X, et al. 2014. Circadian variation of melatonin, light exposure, and diurnal preference in day and night shift workers of both sexes. Cancer Epidemiol Biomarkers Prev 23(7):1176–1186, PMID: 24812038, 10.1158/1055-9965.EPI-13-1271. [DOI] [PubMed] [Google Scholar]
- Papantoniou K, Pozo OJ, Espinosa A, Marcos J, Castaño-Vinyals G, Basagaña X, et al. 2015b. Increased and mistimed sex hormone production in night shift workers. Cancer Epidemiol Biomarkers Prev 24(5):854–863, PMID: 25737330, 10.1158/1055-9965.EPI-14-1271. [DOI] [PubMed] [Google Scholar]
- QGIS Development Team. 2015. QGIS Geographic Information System. http://www.qgis.org/ [accessed 26 March 2018].
- Rea MS, Brons JA, Figueiro MG. 2011. Measurements of light at night (LAN) for a sample of female school teachers. Chronobiol Int 28(8):673–680, PMID: 21867367, 10.3109/07420528.2011.602198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roenneberg T, Merrow M. 2016. The circadian clock and human health. Curr Biol 26(10):R432–R443, PMID: 27218855, 10.1016/j.cub.2016.04.011. [DOI] [PubMed] [Google Scholar]
- Rybnikova NA, Haim A, Portnov BA. 2015. Artificial light at night (ALAN) and breast cancer incidence worldwide: a revisit of earlier findings with analysis of current trends. Chronobiol Int 32(6):757–773, PMID: 26102518, 10.3109/07420528.2015.1043369. [DOI] [PubMed] [Google Scholar]
- Rybnikova NA, Haim A, Portnov BA. 2016. Does artificial light-at-night exposure contribute to the worldwide obesity pandemic? Int J Obes (Lond) 40(5):815–823, PMID: 26795746, 10.1038/ijo.2015.255. [DOI] [PubMed] [Google Scholar]
- Sánchez de Miguel A. 2007. Differential Photometry Study of the European Light Emission to the Space. In: World Conference in Defence of the Night Sky and the Right to Observe the Stars, 20–23 April 2007, La Palma, Canary Islands. [Google Scholar]
- Sánchez de Miguel A. 2015. Variación espacial, temporal y espectral de la contaminación lumínica y sus fuentes: metodología y resultados [in Spanish], 10.13140/RG.2.1.2233.7127, http://www.researchgate.net/publication/280077947 [accessed 26 March 2018]. [DOI]
- Sánchez de Miguel A, Aubé M, Zamorano J, Kocifaj M, Roby J, Tapia C. 2017. Sky Quality Meter measurements in a colour-changing world. Mon Not R Astron Soc 467(3):2966–2979. [Google Scholar]
- Sánchez de Miguel A, Zamorano J, Castaño JG, Pascual S. 2014. Evolution of the energy consumed by street lighting in Spain estimated with DMSP-OLS data. J Quant Spectrosc Radiat Transf 139:109–117, 10.1016/j.jqsrt.2013.11.017. [DOI] [Google Scholar]
- Stevens RG, Zhu Y. 2015. Electric light, particularly at night, disrupts human circadian rhythmicity: is that a problem? Phil Trans R Soc Lond B Biol Sci 370(1667):20140120, PMID: 25780233, 10.1098/rstb.2014.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapan K, Arendt J, Skene DJ. 2001. An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol 535(pt 1):261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.