 A new series of 2-arylaminobenzothiazole-arylpropenone conjugates were designed based on 5F-203, synthesized and evaluated for their cytotoxic potency as well as inhibition of tubulin polymerization.
A new series of 2-arylaminobenzothiazole-arylpropenone conjugates were designed based on 5F-203, synthesized and evaluated for their cytotoxic potency as well as inhibition of tubulin polymerization.
Abstract
A new series of 2-arylaminobenzothiazole-arylpropenone conjugates 5–6(a–r) was designed, synthesized and investigated for their cytotoxic potency against the various human cancer cell lines. Most of these conjugates exhibited cytotoxic activity and inhibited in vitro tubulin polymerization effectively. Conjugates 5d and 6d cause cell cycle blocks in the G2/M phase in HeLa cells and treatments with 5d and 6d manifested increased mRNA and protein levels of the G2/M marker, cyclin B1. Immunocytochemistry revealed loss of intact microtubule structure in cells treated with 5d and 6d. Western blot analysis revealed that these conjugates accumulate more tubulin in the soluble fraction. Moreover, the triggering of apoptotic cell death after mitotic arrest was investigated by studying their effect on Hoechst staining, mitochondrial membrane potential, ROS generation.
1. Introduction
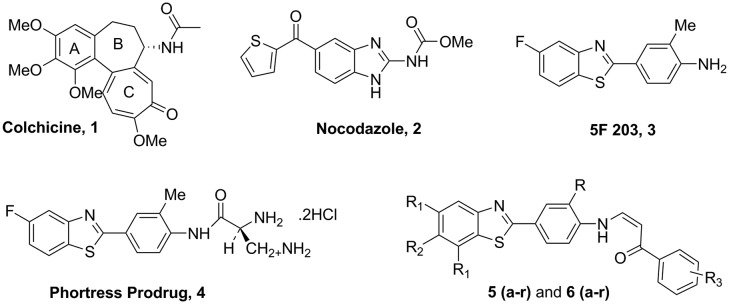
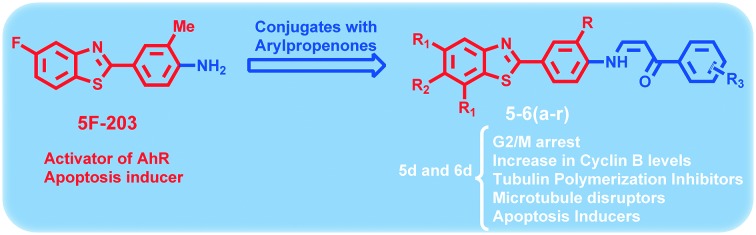
Despite much progress in the development of antiproliferative drugs that inhibit the function of various target proteins, we are still faced with some significant deficits in finding effective drugs. Thus the search for new anti-tumor active agents is of great importance and challenging of medicinal chemistry research. Microtubules are the key motif in the process of cell division in eukaryotic cells makes them an important target for anticancer drugs.1 The microtubules that make up the mitotic spindle are in a particularly delicate state of balance between assembly and disassembling into their constituent subunits of αβ-tubulin heterodimers. These are essential for many cellular processes, such as maintenance of cellular shape, intracellular transport and mitotic spindle assembly during cell division.2 Generally, drugs that target microtubules bind to one of three main sites of tubulin3 which includes the taxane-site for the microtubule stabilizing agents,4,5 the vinca domain6 maytansine binding site7 and the colchicine domain8 for the destabilizing agents. Interfering with the dynamic stability of microtubules, these agents act as spindle poisons arresting the dividing cells in G2/M phase of the cell cycle, causing mitotic catastrophy and finally leading to apoptotic cell death.9 Some of the well-known naturally occuring tubulin binding ligand that affect the microtubule dynamics by binding to distinct colchicine domain of tubulin are colchicine (1).10 Nocodazole (2) is well-known inhibitor of tubulin polymerization which inhibits cell proliferation and is largely used as a pharmacological tool and positive control.11 Benzoheterocycles such as benzothiazoles, benzimidazoles and benzoxazoles can serve as unique and versatile scaffolds for experimental drug design. Among the all benzoheterocycles, benzothiazole has considerable place in research area especially in synthetic as well as in pharmaceutical chemistry because of its potent and significant pharmacological activities. Benzothiazole is a privileged bicyclic ring system and its derivatives have long been therapeutically used for the treatment of various diseases.12–18 However, in recent years, 2-(4 aminophenyl)benzothiazoles have emerged as an important pharmacophore in the development of antitumor agents.19 Among them (5F-203) (3)20 and its prodrug, phortress (4)21 which is water soluble and chemically stable, is found to rapidly and quantitatively revert to its parent amine in mice, rats and dogs (in vivo). Thus, clinical evaluation of phortress has demonstrated potent and selective antitumor activity via a different mechanism of action which provides substantial scope for the development of benzothiazole-based derivatives as anticancer agents.22 Considering the biological importance of these moieties, an attempt has been made in the present study to synthesize new benzothiazole derivatives. Our recent research studies have been mainly focused on the synthesis, evaluation and mechanistic aspects of newer molecules based on different heterocyclic scaffolds as potential anticancer agents particularly, the targeting of tubulin by using new diversified ligands.23–25 In continuation to our efforts for identifying new potent and selective anticancer agents, in view of considering the biologic importance of 2-(4-aminophenyl)benzothiazole derivatives, we design and synthesis of 2-(4-aminophenyl)benzothiazole-arylpropionone conjugates (5a–r and 6a–r) (Fig. 1). These were evaluated for cytotoxic potential followed by structure–activity relationship (SAR) and mode of action of mechanism is elucidated.
Fig. 1. Tubulin polymerization inhibitors (1, 2, 5 and 6a–r) and antitumor agents (3 and 4).
2. Results and discussions
2.1. Chemistry
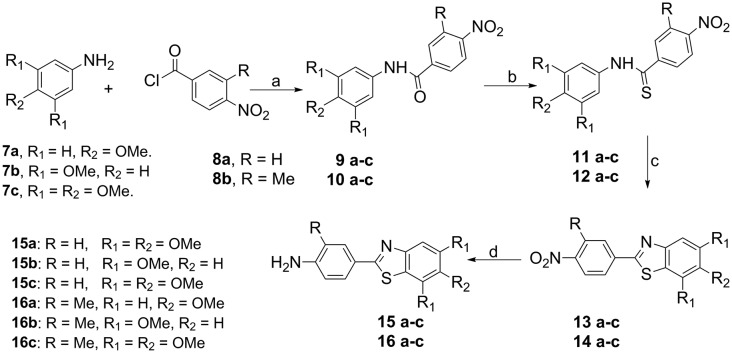
2.1.1. Synthesis of substituted 2-(4-amino-phenyl)benzothiazoles 15–16(a–c)
The preparation of various substituted 2-(4-aminophenyl)benzothiazole precursors (15a–c) and (16a–c) was achieved by the Jacobson thioanilide radical cyclization. Thus, reaction of substituted anilines (7a–c) with substituted p-nitrobenzoylchlorides (8a–b) in pyridine gave the benzanilides (9a–c) and (10a–c) which were further converted to their corresponding thiobenzanilides (11a–c) and (12a–c) using Lawesson's reagent. These were cyclized by using Jacobson method to afford nitrobenzothiazole derivatives (13a–c) and (14a–c) using potassium ferricyanide and aqueous sodium hydroxide which upon reduction of these nitro compounds with stannous chloride yielded the corresponding substituted 2-(4-amino-phenyl)benzothiazoles (15a–c) and (16a–c) as illustrated in Scheme 1.
Scheme 1. Reagents and conditions: (a) pyridine, reflux, 3 h, 90%; (b) Lawesson's reagent, toluene, reflux, 8 h, 80%; (c) K3Fe(CN)6, aq. NaOH, EtOH, 90 °C, 2–3 h, 60–70%; (d) SnCl2.2H2O, EtOH, 3 h, 75–80%.
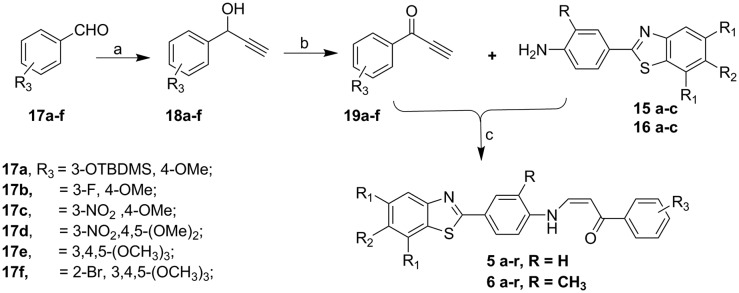
2.1.2. Synthesis of (Z)-3-((4-(benzo[d]thiazol-2-yl)aryl)amino)-1-arylprop-2-en-1-ones 5 and 6(a–r)
The synthesis of target compounds (Z)-3-((4-(benzo[d]thiazol-2-yl)aryl)amino)-1-arylprop-2-en-1-ones 5 and 6(a–r) were illustrated in Scheme 2. Arylaldehydes (17a–f) were treated with ethynylmagnesium bromide in dry tetrahydrofuran (THF) at 0 °C to room temperature, producing 1-aryl-2-propyn-1-ol (18a–f). Oxidation of (18a–f) with 2-iodoxybenzoic acid (IBX) in the presence of dimethyl sulfoxide (DMSO) gave 1-aryl-2-propyn-1-one (19a–f). Condensation of (19a–f) with aryl amines (15a–c and 16a–c) in ethanol at room temperature resulted in the formation of (Z)-3-((4-(benzo[d]thiazol-2-yl)aryl)amino)-1-arylprop-2-en-1-ones 5 and 6(a–r) in good yields and their structures have shown in Table 1 were confirmed by 1H, 13C NMR and HRMS spectral analysis.
Scheme 2. Reagents and conditions: (a) ethynylmagnesium bromide, THF, 0 °C rt, 8–9 h, 72–78%; (b) 2-iodoxybenzoic acid, DMSO, rt, 5 h, 70–76%; (c) ethanol, rt, 4 h, 75–82%.
Table 1. Structures of 2-arylaminobenzothiazole-arylpropenone conjugates 5–6(a–r).
| Compound | R | R1 | R2 | R3 |
| 5a | H | H | OMe | 3-OH,4-OMe |
| 5b | H | OMe | H | 3-OH,4-OMe |
| 5c | H | OMe | OMe | 3-OH,4-OMe |
| 5d | H | H | OMe | 3-F,4-OMe |
| 5e | H | OMe | H | 3-F,4-OMe |
| 5f | H | OMe | OMe | 3-F,4-OMe |
| 5g | H | H | OMe | 3-NO2,4-OMe |
| 5h | H | OMe | H | 3-NO2,4-OMe |
| 5i | H | OMe | OMe | 3-NO2,4-OMe |
| 5j | H | H | OMe | 3-NO2,4,5-diOMe |
| 5k | H | OMe | H | 3-NO2,4,5-diOMe |
| 5l | H | OMe | OMe | 3-NO2,4,5-diOMe |
| 5m | H | H | OMe | 3,4,5-triOMe |
| 5n | H | OMe | H | 3,4,5-triOMe |
| 5o | H | OMe | OMe | 3,4,5-triOMe |
| 5p | H | H | OMe | 2-Br,3,4,5-triOMe |
| 5q | H | OMe | H | 2-Br,3,4,5-triOMe |
| 5r | H | OMe | OMe | 2-Br,3,4,5-triOMe |
| 6a | CH3 | H | OMe | 3-OH,4-OMe |
| 6b | CH3 | OMe | H | 3-OH,4-OMe |
| 6c | CH3 | OMe | OMe | 3-OH,4-OMe |
| 6d | CH3 | H | OMe | 3-F,4-OMe |
| 6e | CH3 | OMe | H | 3-F,4-OMe |
| 6f | CH3 | OMe | OMe | 3-F,4-OMe |
| 6g | CH3 | H | OMe | 3-NO2,4-OMe |
| 6h | CH3 | OMe | H | 3-NO2,4-OMe |
| 6i | CH3 | OMe | OMe | 3-NO2,4-OMe |
| 6j | CH3 | H | OMe | 3-NO2,4,5-diOMe |
| 6k | CH3 | OMe | H | 3-NO2,4,5-diOMe |
| 6l | CH3 | OMe | OMe | 3-NO2,4,5-diOMe |
| 6m | CH3 | H | OMe | 3,4,5-triOMe |
| 6n | CH3 | OMe | H | 3,4,5-triOMe |
| 6o | CH3 | OMe | OMe | 3,4,5-triOMe |
| 6p | CH3 | H | OMe | 2-Br,3,4,5-triOMe |
| 6q | CH3 | OMe | H | 2-Br,3,4,5-triOMe |
| 6r | CH3 | OMe | OMe | 2-Br,3,4,5-triOMe |
2.2. Biology
2.2.1. Antiproliferative activity
All newly synthesized conjugates of arylaminobenzothiazole-arylpropenone conjugates 5–6(a–r) were evaluated for their antiproliferative activity in a concentration-dependent manner against a panel of four different human cancer cell lines A549, HeLa, MDAMB-231 and MIA PaCa-2 using the SRB assay. The results of growth inhibitory activities (IC50 values) are presented in Table 2 in micromolar concentrations, nocodazole used as the reference compound.
Table 2. Cytotoxic effects on arylaminobenzothiazole-arylpropenone conjugates 5–6(a–r).
| IC50
a
| ||||
| Compound | A549 b | HeLa c | MDAMB-231 d | MIA PaCa-2 e |
| 5a | 12.8 ± 0.47 | 6.9 ± 0.04 | 18.9 ± 0.17 | 21.9 ± 0.29 |
| 5b | 10.61 ± 01 | 12.1 ± 0.03 | 7.61 ± 25 | 11.6 ± 0.39 |
| 5c | 23.0 ± 0.79 | 14.1 ± 0.05 | 18.1 ± 0.23 | 32.77 ± 0.02 |
| 5d | 0.9 ± 0.42 | 0.5 ± 0.02 | 1.1 ± 0.33 | 1.3 ± 0.12 |
| 5e | 5.3 ± 0.4 | 7.3 ± 0.1 | 6.7 ± 0.4 | 4.3 ± 0.36 |
| 5f | 15.5 ± 58 | 7.3 ± 0.06 | 16.5 ± 0.33 | 19.4 ± 0.16 |
| 5g | 13.6 ± 0.06 | 17.6 ± 0.03 | 17.6 ± 0.09 | 23.6 ± 0.01 |
| 5h | 5.8 ± 0.06 | 12.9 ± 0.04 | 12.9 ± 0.07 | 2.9 ± 0.09 |
| 5i | 21.2 ± 0.2 | 11.4 ± 0.7 | 17.1 ± 0.7 | 15.9 ± 0.5 |
| 5j | 21.61 ± 01 | 16.1 ± 0.03 | 7.61 ± 05 | 25.6 ± 0.09 |
| 5k | 13.0 ± 0.09 | 19.01 ± 0.5 | 3.06 ± 0.03 | 12.7 ± 0.09 |
| 5l | 11.8 ± 0.02 | 7.8 ± 0.02 | 9.8 ± 0.03 | 15.8 ± 0.05 |
| 5m | 12.1 ± 0.11 | 14.1 ± 0.13 | 19.61 ± 0.25 | 31.1 ± 0.73 |
| 5n | 21.8 ± 0.92 | 17.8 ± 0.14 | 15.8 ± 0.11 | 26.8 ± 0.87 |
| 5o | 15.5 ± 0.88 | 5.3 ± 0.26 | 16.5 ± 0.53 | 29.05 ± 0.06 |
| 5p | 6.0 ± 0.89 | 8.02 ± 0.15 | 11.06 ± 0.23 | 14.7 ± 0.49 |
| 5q | 12.03 ± 0.24 | 10.3 ± 0.41 | 16.09 ± 0.03 | 19.03 ± 0.96 |
| 5r | 13.6 ± 0.46 | 6.6 ± 0.23 | 15.6 ± 0.39 | 23.2 ± 0.51 |
| 6a | 18.02 ± 66 | 12.01 ± 71 | 14.02 ± 06 | 17.01 ± 0.89 |
| 6b | 14.3 ± 0.11 | 16.1 ± 0.62 | 3.1 ± 0.49 | 22.1 ± 0.27 |
| 6c | 15.4 ± 0.48 | 11.4 ± 0.13 | 12.4 ± 0.57 | 15.4 ± 0.06 |
| 6d | 1.4 ± 0.46 | 0.6 ± 0.29 | 2.0 ± 0.09 | 2.3 ± 0.02 |
| 6e | 15.3 ± 0.3 | 14.7 ± 0.5 | 13.3 ± 0.7 | 16.3 ± 0.17 |
| 6f | 1.69 ± 0.05 | 1.2 ± 0.02 | 2.9 ± 0.01 | 1.8 ± 0.07 |
| 6g | 13.6 ± 0.06 | 17.6 ± 0.03 | 17.6 ± 0.09 | 23.6 ± 0.01 |
| 6h | 12.1 ± 0.5 | 9.3.9 ± 0.3 | 10.8 ± 0.6 | 13.9 ± 0.8 |
| 6i | 9.1 ± 0.7 | 13.4 ± 0.6 | 12.4 ± 0.7 | 23. 4 ± 0.3 |
| 6j | 22.06 ± 0.01 | 12.1 ± 0.05 | 5.05 ± 0.09 | 12.0 ± 0.07 |
| 6k | 15.4 ± 0.08 | 11.4 ± 0.03 | 7.4 ± 0.03 | 10.4 ± 0.01 |
| 6l | 17.4 ± 0.06 | 13.1 ± 0.09 | 12.8 ± 0.09 | 22.03 ± 0.09 |
| 6m | 11.06 ± 0.35 | 16.1 ± 0.43 | 17.01 ± 0.62 | 10.03 ± 0.77 |
| 6n | 14.4 ± 0.07 | 16.4 ± 0.69 | 13.8 ± 0.43 | 17.03 ± 0.24 |
| 6o | 23.29 ± 0.37 | 11.09 ± 0.72 | 12.9 ± 0.21 | 22.8 ± 0.27 |
| 6p | 6.4 ± 0.04 | 13.4 ± 0.13 | 9.4 ± 0.73 | 23.4 ± 0.51 |
| 6q | 14.03 ± 0.43 | 14.7 ± 0.55 | 17.3 ± 0.37 | 16.03 ± 0.27 |
| 6r | 10.3 ± 0.24 | 14.3 ± 0.41 | 7.3 ± 0.18 | 24.3 ± 0.53 |
| Nocodazole | 0.82 ± 06 | 0.84 ± 0.05 | 0.71 ± 0.05 | 0.91 ± 0.02 |
aIC50 = compound concentration required to inhibit tumor cell proliferation by 50% after 48 h of drug treatment.
bLung cancer.
cCervical cancer.
dBreast cancer and.
ePancreatic cancer.
2.2.2. Structure–activity relationships (SAR)
These studies reveal that the cytotoxicity of the arylaminobenzothiazole-arylpropenone conjugates 5–6(a–r) was totally dependent on the nature and position of the substituents present on the two scaffold rings containing A, B and C-rings. The cytotoxicity data (Table 2) clearly showed that the conjugates (5d, 6d and 6f) exhibited promising cytotoxicity in HeLa cells (IC50 range 0.5–1.2 μM) and the results were comparable with positive control, nocodazole. These conjugates are more active when benzothiazole ring (A-ring) possessing methoxy group at 6th position with unsubstitution or monosubstituted (methyl) group 3rd position of phenyl ring (B-ring) and with a combination of a methoxy and a halogen atom (F) on C-ring (5d and 6d). On the basis of this observation, additional methoxy groups were introduced on the benzothiazole ring, while keeping a methoxy groups on the C-ring to evaluate the influence of these groups on the cytotoxicity of the molecules (5e–f and 6e–f). From the cytotoxicity data (Table 2), it was clearly indicate that 5,7-dimethoxy substitution (5e and 6e) moderately decreased the potency of the molecules while 5,6,7-trimethoxy substitution (5f) resulted in the loss of the cytotoxicity to several fold than 5d and wherein case of 6f enhanced the cytotoxicity with comparable to 6d. In addition we replaced the fluoro atom (F) with hydroxyl (OH) group in C-ring and the resulted molecules are 5a–c and 6a–c lost the activity, indicating that electronegative group at that position is required for the activity of the molecule.
When electron withdrawing nitro group introduced in place of fluoro atom on the C-ring resulted molecules were (5g–i and 6g–i) lost their cytotoxic potential on cancer cells, showed that the fluoro (F) atom at those position is critical for the activity of the molecules with exception of 5h with 2.9 μM. Additional nitro group was introduced on the C-ring of the molecules i.e.5g–i and 6g–i resulted molecules were 5j–l and 6j–l completely loss of cytotoxicity with few exceptions on certain cell lines. Also to evaluate the importance of the of 3,4,5-trimethoxy groups on C-ring, we switched these methoxy groups on to the benzothiazole rings (A and B) and tested the resulted molecules (5m–o and 6m–o) for the cytotoxicity. The loss of cytotoxicity activity observed when compare to 5d and 6d indicating that hydrophilic group at that positions are critical for activity. Among them, 5o showed significant activity with 5.3 μM on HeLa cell line. Surprisingly, in addition to these molecules we introduced bromo (Br) atom on C-ring of the 5m–o and 6m–o conjugates, the resulted conjugates (5p–r and 6p–r) are enhanced the cytotoxicity than counterparts.
The best activity in these conjugate series could be obtained only when a molecule bears 6-methoxy on the A ring and methyl group on the B-ring with combination methoxy and fluoro atom on C-ring. After identifying the most potent conjugates (5d and 6d) in the series towards HeLa cell lines, these conjugates taken for further detailed studies with a view to understand the cell growth inhibition mechanisms.
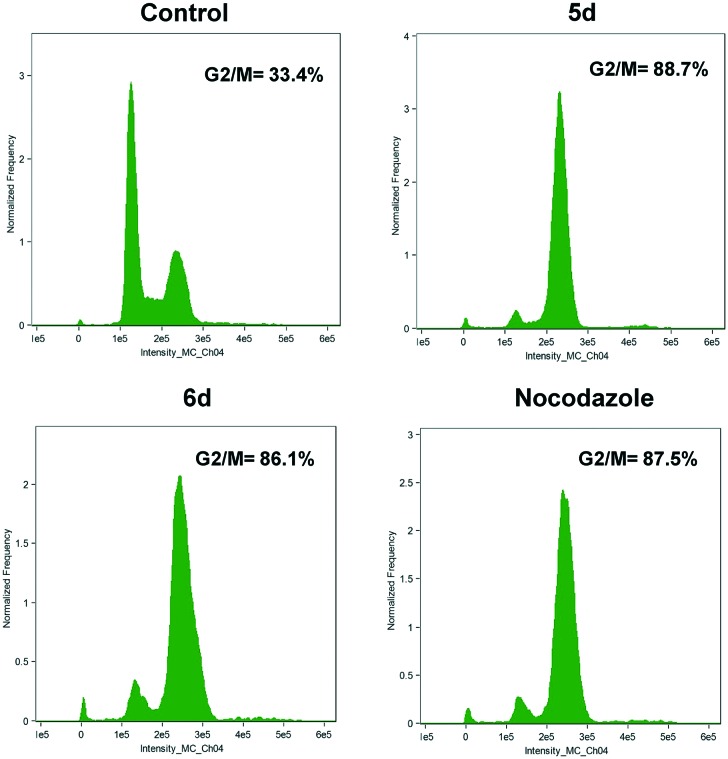
2.2.3. Effect on cell cycle
Many anticancer compounds exert their growth inhibitory effect either by arresting the cell cycle at a particular checkpoint of cell cycle or by induction of apoptosis or a combined effect of both cycle block and apoptosis.26,27 Furthermore regulation of the cell cycle and apoptosis are considered to be effective cancer therapeutic methods.28 To evaluate this possibility, HeLa cells were treated with 5d, 6d and nocodazole used as positive control at 3 μM for 48 h. The data obtained from the study as presented in Fig. 2 show that treatment with 5d, 6d and nocodazole resulted in the accumulation of cells in the G2/M phase of the cell cycle with 88.7%, 86.1% and 87.5% respectively. Whereas, DMSO treated cells showed predominant G1 phase, with 33.4% of cells in G2/M. Overall, these results suggest that these conjugates efficiently stall cells at G2/M phase.
Fig. 2. FACS analysis of cell cycle distribution of HeLa cells after treatment with 5d and 6d at 3 μM concentrations and nocodazole for 24 h. Cell cycle analysis was performed employing propidium iodide as indicated under Materials and methods.
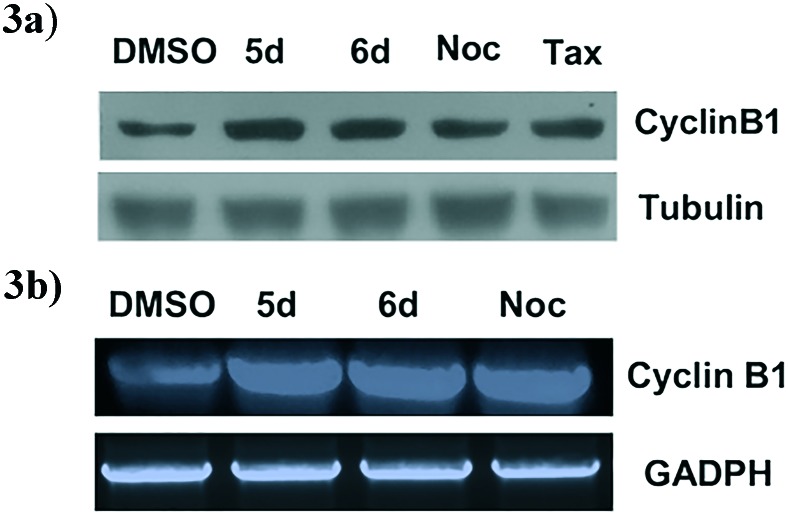
2.2.4. Effect on cellular cyclin-B1 levels by immunoblot analysis
Cyclin-B1 is induced at the G2/M boundary to promote cell division. This protein is one of the important regulatory proteins of mitosis and accumulation of cyclin-B1 is an indication for G2/M arrest.29 Since these compounds arrest the cells at G2/M phase, we investigated their effect on cyclin B1 protein levels. Therefore, HeLa cells were treated with 3 μM concentration of these compounds for 24 h and performed immunoblot analysis for cyclin-B1. For comparison, nocodazole and taxol were included as positive controls and tubulin as loading control. Immunoblot analysis revealed that these compounds 5d and 6d strongly induce cyclin B1 levels, a well-recognized G2/M marker (Fig. 3a). In addition, to further validate that the increase in protein levels of cyclin B1 was not due to increased stability of cyclin B1 protein in the presence of compounds 5d and 6d by any cross-reactivity of the compounds with cyclin B1 protein. Thus we performed semi-quantitative RT-PCR analysis for mRNA levels of cyclin B1 in control and compound treated cells. GAPDH was used as an internal control. Notably, treatments with the conjugates 5d and 6d showed robustly activated cyclin B1 mRNA levels compared to control (Fig. 3b). Thus our results support the suggestion that conjugates cause an accumulation of cells at the G2/M phase of the cell cycle.
Fig. 3. Western blot and RT-PCR analysis of cyclin B1 in 5d and 6d treated HeLa cells. 3a). The cells were treated with 3 μM of 5d, 6d, nocodazole and taxol for 24 h. Tubulin was used as loading for equal loading of protein samples and DMSO as vehicle control. 3b). Semi-quantitative RT-PCR analysis of cyclin B1 gene expression in conjugates 5d, 6d and Nocodazole treated HeLa cells. GAPDH was used as an internal control and DMSO as vehicle control.
2.2.5. Effect of 5d and 6d on in vitro and in vivo tubulin polymerization
Since 5d and 6d robustly activate G2/M arrest in cells and the compounds possess moieties that interact with tubulin, we assessed their ability to inhibit in vitro tubulin assembly. Nocodazole was used as a positive control. Whereas, DMSO served as a vehicle control. The compounds at five different doses were pre-incubated with tubulin protein. Later the tubulin polymerization was initialed by the addition of 4 mM GTP. After 30 min, the assays revealed that DMSO control did not inhibit tubulin assembly. In comparison, nocodazole, 5d and 6d acted as tubulin inhibitors. 5d and 6d showed an IC50 of 2.2 μM and 2.9 μM respectively (Table 3). Next, microtubule depolymerizing drugs cause improper chromosome separation by inhibiting the organization of the mitotic spindle, and predominantly arrest chromosomes in metaphase of mitosis.30 Occurrence of irregular spindle fibers due to disrupted microtubule network is a hallmark of cells treated with antitubulin agents.
Table 3. Tubulin polymerization inhibitory effect of compounds 5d and 6d.
| Compound | Anti-tubulin activity (IC50) |
| 5d | 2.2 ± 0.23 |
| 6d | 2.9 ± 0.26 |
| Nocodazole | 1.8 ± 0.04 |
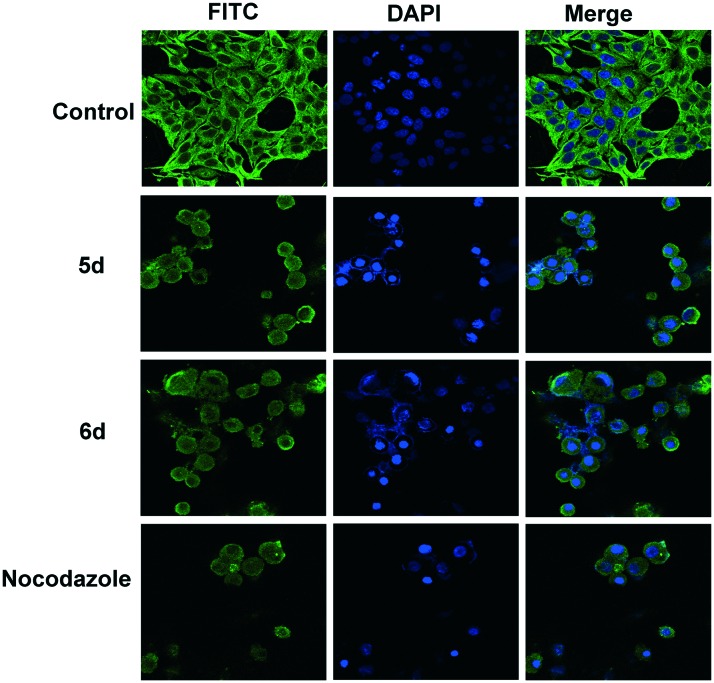
The inhibitors of tubulin assembly cause severe perturbation in the microtubule dynamics leading to irregular morphology. Since these benzothiazole derivatives exhibit profound inhibition of tubulin assembly and arrest cells at G2/M phase of cell cycle, we investigated their ability to alter the microtubule network. Therefore, it was of interest to examine the intracellular effect of 5d and 6d by monitoring cellular microtubules, as well as nuclear condensation. HeLa cells were treated with 3 μM of these compounds for 24 h cells upon staining and showed severe disruption in microtubule organization. However, vehicle-control/DMSO treated cells manifested normal microtubule network as shown in Fig. 4.
Fig. 4. Effect of 5d and 6d on microtubule network: immunofluorescence images of HeLa cells stained with anti-b-tubulin antibody FITC-conjugated and then observed by confocal microscopy, magnification at 60×. Cells were exposed to 3 μM of representative compounds for 24 h and then fixed and analyzed by fluorescence microscopy. The potent inhibitors (5d and 6d) of tubulin assembly show an irregular or rounded morphology. Cells were also counterstained with DAPI to visualize the nuclei.
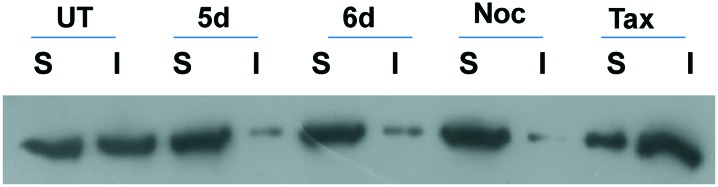
2.2.6. Distribution of soluble versus polymerized tubulin in cells
Since inhibition of tubulin polymerization disturbs the microtubule dynamics, we evaluated the levels of soluble (S) versus polymerized (I) forms of tubulin in HeLa cells following treatment with 3 μM of 5d and 6d for 24 h. In addition, cells were treated with nocodazole, as positive and taxol as negative controls in parallel experiments. Western blot analysis reveals that the amount of tubulin protein in both soluble and polymerized fractions was approximately the same in DMSO treated cells. Nocodazole treated cells exhibited a shift of tubulin from the polymerized fraction into the soluble fraction.
In comparison, paclitaxel a microtubule polymerization agent showed more amount of tubulin in the polymerized fraction. As expected the cells treated with 5d and 6d significantly increased the tubulin content in the soluble fraction, with almost all tubulin present in the soluble fraction similar to that of the positive control. Therefore, increased tubulin in soluble fraction of cells treated by these hybrids corroborated with the inhibition of tubulin assembly and arrest of cells in G2/M phase as shown in Fig. 5.
Fig. 5. HeLa cells were treated with 3 μM of 5d and 6d for 24 h. Nocodazole and taxol were used as reference standards. Levels of tubulin was detected by Western blot analysis. S: soluble fraction. I: polymerized fraction.
2.2.7. Hoechst staining
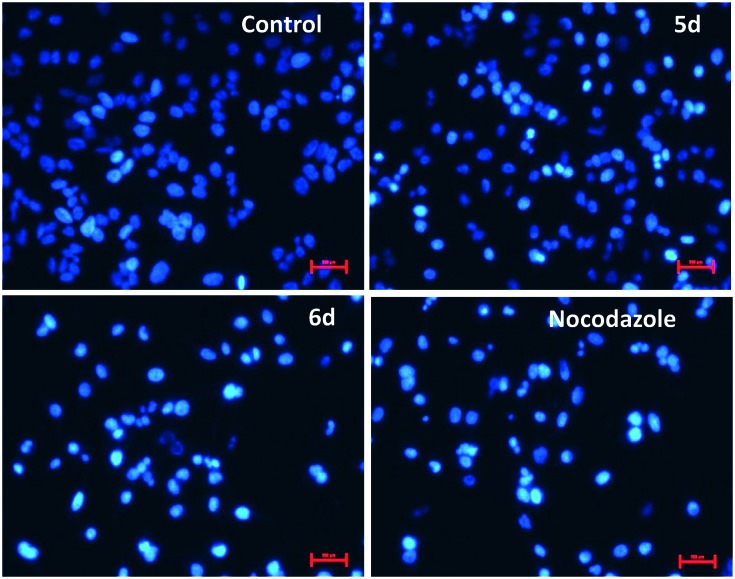
To investigate the apoptotic inducing effect of these conjugates (5d and 6d), Hoechst staining assay was carried out on HeLa cells. Hoechst 33242 is a cell membrane permeable nuclear staining dye, which emits blue fluorescence and stains the live cell nuclei as light blue, whereas the apoptotic cell nuclei appear as bright blue due to chromatin condensation. HeLa cells were treated with 5d and 6d at concentration of 1 μM for 24 h and stained with Hoechst and nocodazole was used as a reference compound.
The results from Fig. 6 indicated that in the control group, the untreated cells did not show obvious morphological changes (all the cells exhibited uniform rounded cell morphology), however in compounds (5d, 6d and nocodazole) treated group, the cells exhibited typical apoptotic morphology such as highly condensed nuclei (brightly stained). This observation demonstrates that these conjugates 5d and 6d are able to induce apoptosis in HeLa cells.
Fig. 6. Conjugates 5d and 6d induced nuclear morphological changes of HeLa cells.
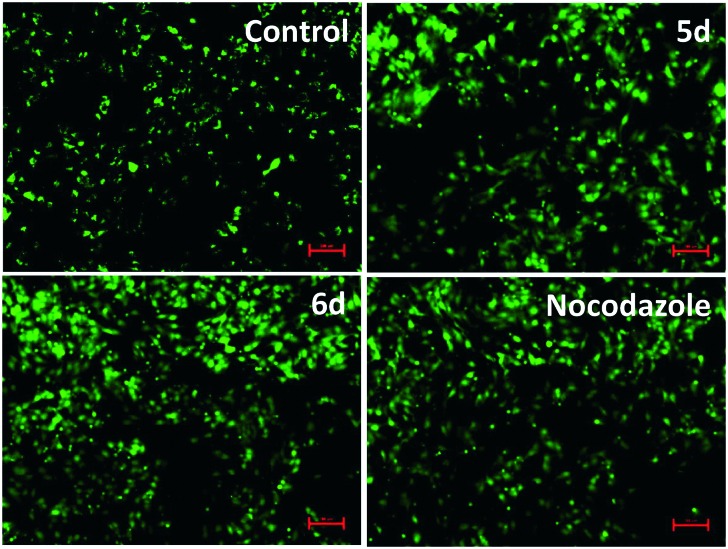
2.2.8. Mitochondrial membrane potential (ΔΨm)
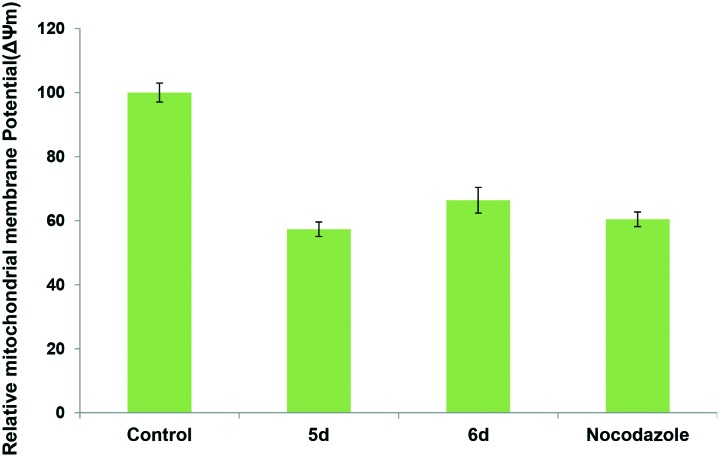
Previous reports have suggested that the loss of ΔΨm leads to apoptosis.31 In order to further investigate the apoptosis-inducing effect of target compounds, changes in ΔΨm was measured after staining with Rhodamine-123. Mitochondria that maintain normal ΔΨm can retain the Rhodamine 123, which give strong green fluorescence. Disruption of ΔΨm was associated with a decrease in green fluorescence due to a lack of Rh-123 retention.32 Thus the relationship between ΔΨm and Rh-123 fluorescence is considered as linear. HeLa cells were treated with these hybrids 5d and 6d at 1 μM concentration for 24 h and stained with Rh-123 and the intensity of Rh-123 fluorescence was measured by spectrofluorometer. The results from the Fig. 7 revealed that these conjugates induced 40–60% loss of ΔΨm in HeLa cells. Conjugate 5d is more potent in causing drop in ΔΨm, which showed 40% of collapse of ΔΨm followed by the conjugate 6d that induced 30% losses of ΔΨm, respectively. These results demonstrate that these compounds induce apoptosis in HeLa cells through changes in ΔΨm.
Fig. 7. Conjugates 5d and 6d induced loss of mitochondrial membrane potential (ΔΨm) in HeLa cells analyzed using Rhodamine 123 staining. The loss in intensity of fluorescence was measured by spetrofluorometer. Data are mean ± SD from three independent experiments.
2.2.9. Activation of reactive oxygen species
Loss of ΔΨm and elevation of intracellular ROS levels are two closely related events that occur during apoptosis.33 The levels of ROS were measured in HeLa cells by using a fluorescent probe, 2′,7′-dichlorofluorescein diacetate (H2DCFDA) to examine whether the drop in ΔΨm was on account of the production of ROS.34 The non-fluorescent DCFH-DA is oxidized to green fluorescent DCFH by intracellular esterases when it diffuses into cells. Thus, the intracellular ROS levels can be expressed as mean fluorescence intensity (MFI) of DCF. As shown in Fig. 8, HeLa cells treated with conjugates 5d, 6d and nocodazole at 1 μM concentration showed increase in green fluorescence compared to the control (untreated cells). Hence, it could be concluded that these conjugates significantly elevate the intracellular ROS levels.
Fig. 8. Effect of conjugates 5d and 6d on the intracellular levels of ROS. HeLa cells were treated with 5d and 6d for 24 h and stained with carboxy DCFH-DA. Images were captured by a fluorescence microscope (Nikon). The intensity of DCF-DA fluorescence was measured by spetrofluorometer using an excitation wavelength of 485 nm and an emission wavelength of 535 nm.
3. Conclusions
In conclusion, we designed and synthesized a series of 2-arylaminobenzothiazole-arylpropeonones conjugates based on the structure 3 and developed a potent conjugates 5d and 6d which displayed a strong cytotoxic activity with IC50 values of 0.5 and 0.6 μM against the human HeLa cancer cell lines. Flow cytometry analysis indicates that these compounds induce cell cycle arrest at G2/M phase in HeLa cells. Treatments with 5d and 6d manifested increased mRNA and protein levels of the G2/M marker, cyclin B1 which is corroborated cell cycle arrest at G2/M phase. Tubulin polymerization assay showed that they are potent inhibitors of tubulin polymerization. Increased levels of tubulin in the soluble fraction of cells remarkably corroborated with inhibition of tubulin polymerization by these conjugates 5d and 6d. In addition, they induce apoptosis in HeLa cells by studying their effect on Hoechst staining, collapse of ΔΨm, elevation of ROS production. Overall, these results demonstrate that the arylaminobenzothiazole-arylpropenone conjugates have the potential to be developed as leads and their further amenable structural modifications may produce promising anticancer agents for HeLa cancer cells.
4. Experimental section
4.1. Chemistry
4.1.1. Materials and methods
1H NMR spectra were recorded on Avance 300, Inova 400, Avance 500, and Bruker 600 MHz spectrometers using tetramethylsilane (TMS) as the internal standard. Chemical shifts are reported in parts per million (ppm) downfield from tetramethyl silane. Spin multiplicities are described as s (singlet), brs (broad singlet), d (doublet), dd (double doublet), t (triplet), q (quartet), and or m (multiplet). Coupling constants are reported in Hertz (Hz). Melting points were determined in a capillary tube using an electrothermal apparatus (Model IA9200) and are uncorrected. The IR spectra were recorded by employing a Nicolet FTIR model MX-1spectrophotometer. Analytical thin layer chromatography (TLC) was performed on MERCK precoated silica gel 60- F254 (0.5 mm) glass plates. Visualization of the spots on TLC plates was achieved either by exposure to iodine vapour or UV light or by dipping the plates into methanolic sulphuric acid-β-naphthol or to ethanolic anisaldehyde-sulphuric acid–acetic acid or to ethanolic ninhydrin solution and heating the plates to 120 °C. Column chromatography was performed using silica gel 60–120 and 100–200 mesh. Moisture sensitive reactions were carried out using standard syringe septum Techniques and under inert atmosphere of nitrogen. All solvents and reagents were purified by standard techniques. All evaporation of solvents was carried out under reduced pressure on Laborota-4000 rotary evaporator below 45 °C. The names of all the compounds given in the experimental section were taken from Chem Ultra, Version 11.0.
4.1.1.1. General method a for the synthesis of substituted benzanilides 9–10(a–c)
Substituted 4-nitrobenzoyl chlorides (8a–b) (0.029 mol) were added slowly to a solution of the appropriately substituted anilines (7a–c) (0.04 mol) in pyridine (110 mL). The resulting solution was stirred under reflux for 3 h and then poured into ice water (400 mL). The precipitate formed was collected and washed with 2 N HCl (100 mL), followed by water and methanol, to afford the respective benzanilides 9–10(a–c) as a yellow solids.
4.1.1.1.1. N-(4-Methoxyphenyl)-4-nitrobenzamide (9a)
Yellow solid, yield 85%; mp: 199–202 °C; 1H NMR (300 MHz, DMSO): δ 10.47 (s, 1H), 8.36 (d, J = 8.8 Hz, 2H), 8.17 (d, J = 8.8 Hz, 2H), 7.68 (d, J = 9.0 Hz, 2H), 6.95 (d, J = 9.0 Hz, 2H), 3.75 (s, 3H); MS (ESI): m/z 273 (M + H)+.
4.1.1.1.2. N-(4-Methoxyphenyl)-3-methyl-4-nitrobenzamide (10a)
Yellow solid, yield 71%; mp: 152–154 °C; 1H NMR (300 MHz, CDCl3): δ 8.00 (d, J = 8.4 Hz, 1H), 7.91 (s, 1H), 7.83 (s, 1H), 7.77 (d, J = 8.2 Hz, 1H), 7.52 (d, J = 8.8 Hz, 2H), 6.90 (d, J = 9.0 Hz, 2H), 3.82 (s, 3H), 2.64 (s, 3H); MS (ESI): m/z 287 (M + H)+.
4.1.1.1.3. N-(3,5-Dimethoxyphenyl)-4-nitrobenzamide (9b)
Yellow solid, yield 88%; mp: 211–213 °C; 1H NMR (300 MHz, CDCl3 + DMSO): δ 10.22 (s, 1H), 8.31 (d, J = 8.9 Hz, 2H), 8.20 (d, J = 8.9 Hz, 2H), 7.07 (d, J = 2.2 Hz, 2H), 6.25 (t, J = 2.2 Hz, 1H), 3.80 (s, 6H); MS (ESI): m/z 303 (M + H)+.
4.1.1.1.4. N-(3,5-Dimethoxyphenyl)-3-methyl-4-nitrobenzamide (10b)
Yellow solid, yield 72%; mp: 189–192 °C; 1H NMR (500 MHz, CDCl3): δ 8.03 (s, 1H), 7.98 (d, J = 8.4 Hz, 1H), 7.81 (s, 1H), 7.75 (dd, J = 8.4, 1.5 Hz, 1H), 6.87 (d, J = 2.0 Hz, 2H), 6.29 (t, J = 2.2 Hz, 1H), 3.79 (s, 6H), 2.62 (s, 3H); MS (ESI): m/z 317 (M + H)+.
4.1.1.1.5. 4-Nitro-N-(3,4,5-trimethoxyphenyl)benzamide (9c)
Yellow solid, yield 88%; mp: 220–224 °C; 1H NMR (300 MHz, CDCl3 + DMSO): δ 10.12 (s, 1H), 8.32 (d, J = 8.8 Hz, 2H), 8.20 (d, J = 8.8 Hz, 2H), 7.19 (s, 2H), 3.88 (s, 6H), 3.81 (s, 3H); MS (ESI): m/z 333 (M + H)+.
4.1.1.1.6. 3-Methyl-4-nitro-N-(3,4,5-trimethoxyphenyl)benzamide (10c)
Yellow solid, yield 76%; mp: 210–214 °C; 1H NMR (500 MHz, CDCl3): δ 8.23 (s, 1H), 8.03 (s, 1H), 8.02 (s, 1H), 7.51 (s, 1H), 6.85–6.58 (m, 2H), 3.84–3.82 (m, 6H), 3.81–3.80 (m, 3H), 2.44–2.42 (m, 3H); MS (ESI): m/z 347 (M + H)+.
4.1.1.2. General method B for the synthesis of substituted thiobenzanilides 11–12(a–c)
To a solution of substituted benzanilides 9–10(a–c) (6 g, 0.018 mol) in toluene (40 mL), Lawesson's reagent (0.9 eq.) was added and reflux it for 8 h. After completion of the reaction, cool to rt and solvent was removed in vacuo and work up with ethylacetate and water. Separation of the organic layer and evaporation followed by column chromatography gave yellow solids 11–12(a–c)
4.1.1.2.1. N-(4-Methoxyphenyl)-4-nitrobenzothioamide (11a)
Yellow solid, yield 84%; mp: 173–175 °C; MS (ESI): m/z 289 (M + H)+.
4.1.1.2.2. N-(4-Methoxyphenyl)-3-methyl-4-nitrobenzothioamide (12a)
Yellow solid, yield 72%; mp: 160–162 °C; MS (ESI): m/z 303 (M + H)+.
4.1.1.2.3. N-(3,5-Dimethoxyphenyl)-4-nitrobenzothioamide (11b)
Yellow solid, yield 90%; mp: 138–140 °C; MS (ESI): m/z 319 (M + H)+.
4.1.1.2.4. N-(3,5-Dimethoxyphenyl)-3-methyl-4-nitrobenzothioamide (12b)
Yellow solid, yield 74%; mp: 178–180 °C; MS (ESI): m/z 333 (M + H)+.
4.1.1.2.5. 4-Nitro-N-(3,4,5-trimethoxyphenyl)benzothioamide (11c)
Yellow solid, yield 84%; mp: 198–200 °C; MS (ESI): m/z 349 (M + H)+.
4.1.1.2.6. 3-Methyl-4-nitro-N-(3,4,5-trimethoxyphenyl)benzothioamide (12c)
Yellow solid, yield 72%; mp: 136–138 °C; MS (ESI): m/z 363 (M + H)+.
4.1.1.3. General method C for the Jacobson synthesis of substituted 2-(4-nitrophenyl)benzothiazoles 13–14(a–c)
A solution of the substituted thiobenzanilides 11–12(a–c) (0.017 mol) in aqueous sodium hydroxide (8 eq. in 50 mL of water) containing ethanol (3 mL) was added dropwise to a pre-heating solution of potassium ferricyanide (4 eq.) in water (30 mL) taken in a 250 mL RB flask at 90 °C over a period of 1 h. The resulting solution was stirred at 90 °C for a further 2 h and then cooled to room temperature. The precipitate formed was filtered and washed with water. Products were purified by column chromatography (ethylacetate/hexane) and to furnish the 4-nitrophenyl-benzothiazoles 13–14(a–c) as yellow solids.
4.1.1.3.1. 6-Methoxy-2-(4-nitrophenyl)benzo[d]thiazole (13a)
Yellow solid, yield 62%; mp: 216–217 °C; 1H NMR (300 MHz, CDCl3): δ 8.34 (d, J = 8.9 Hz, 2H), 8.21 (d, J = 8.9 Hz, 2H), 8.00 (d, J = 9.0 Hz, 1H), 7.39 (d, J = 2.5 Hz, 1H), 7.15 (dd, J = 9.0, 2.5 Hz, 1H), 3.92 (s, 3H); MS (ESI): m/z 287 (M + H)+.
4.1.1.3.2. 6-Methoxy-2-(3-methyl-4-nitrophenyl)benzo[d]thiazole (14a)
Yellow solid, yield 64%; mp: 195–197 °C; 1H NMR (300 MHz, CDCl3): δ 8.10 (d, J = 8.5 Hz, 1H), 8.04 (s, 1H), 7.99 (t, J = 8.4 Hz, 2H), 7.39 (d, J = 2.4 Hz, 1H), 7.15 (dd, J = 9.0, 2.5 Hz, 1H), 3.93 (s, 3H), 2.72 (s, 3H); MS (ESI): m/z 301 (M + H)+.
4.1.1.3.3. 5,7-Dimethoxy-2-(4-nitrophenyl)benzo[d]thiazole (13b)
Yellow solid, yield 60%; mp: 238–239 °C; 1H NMR (300 MHz, CDCl3): δ 8.37–8.32 (m, 2H), 8.24 (d, J = 8.9 Hz, 2H), 7.22 (d, J = 1.9 Hz, 1H), 6.56 (d, J = 1.9 Hz, 1H), 3.99 (s, 3H), 3.92 (s, 3H); MS (ESI): m/z 317 (M + H)+.
4.1.1.3.4. 5,7-Dimethoxy-2-(3-methyl-4-nitrophenyl)benzo[d]thiazole (14b)
Yellow solid, yield 65%; mp: 223–225 °C; 1H NMR (500 MHz, CDCl3): δ 8.00 (dd, J = 1.9, 1.2 Hz, 1H), 7.82 (d, J = 8.4 Hz, 1H), 7.06–7.02 (m, 2H), 6.83 (d, J = 2.4 Hz, 1H), 3.93 (s, 3H), 3.80 (s, 3H), 2.65 (s, 3H); MS (ESI): m/z 330 (M + H)+.
4.1.1.3.5. 5,6,7-Trimethoxy-2-(4-nitrophenyl)benzo[d]thiazole (13c)
Yellow solid, Yield 66%; mp 160–168 °C; 1H NMR (300 MHz, CDCl3): δ 8.36 (d, J = 8.8 Hz, 2H), 8.23 (d, J = 8.8 Hz, 2H), 7.40 (s, 1H), 4.14 (s, 3H), 3.99 (d, J = 6.4 Hz, 6H); MS (ESI): m/z 347 (M + H)+.
4.1.1.3.6. 5,6,7-Trimethoxy-2-(3-methyl-4-nitrophenyl)benzo[d]thiazole (14c)
Yellow solid, yield 69%; mp 176–179 °C; 1H NMR (300 MHz, CDCl3): δ 7.96 (d, J = 8.4 Hz, 1H), 7.86 (s, 1H), 7.61 (d, J = 8.3 Hz, 1H), 7.63–7.38 (m, 1H), 7.37 (s, 1H), 4.11 (d, J = 5.7 Hz, 6H), 3.97 (d, J = 5.6 Hz, 6H); MS (ESI): m/z 361 (M + H)+.
4.1.1.4. General method D for the reduction of substituted 2-(4-nitrophenyl)benzothiazoles 15–16(a–c)
To a solution of substituted 2-(4-nitrophenyl)benzothiazoles 13–14(a–c) (3 g, 8.66 mmol) in ethanol, tin(ii) chloride dihydrate (3 eq., 25.98 mmol) was added and refluxed it for 3 h. The solvent was removed under vacuum and the resulting oil taken up in chloroform (75 mL) was quenched with aq. NaHCO3 solution. The resulting organic layer was separated and evaporated to leave a residue of the amine which was purified by column chromatography (eluent: ethylacetate/hexane) to furnish the 4-aminophenyl-benzothiazoles 15–16(a–c) as yellow solids.
4.1.1.4.1. 4-(6-Methoxybenzo[d]thiazol-2-yl)aniline (15a)
Yellow solid, yield 92%; mp 191–193 °C; 1H NMR (300 MHz, CDCl3): δ 7.88 (t, J = 8.6 Hz, 3H), 7.34 (d, J = 2.4 Hz, 1H), 7.07 (dd, J 8.9, 2.5 Hz, 1H), 6.75 (d, J = 8.6 Hz, 2H), 3.99 (s, 1H), 3.90 (s, 3H); MS (ESI): m/z 257 (M + H)+.
4.1.1.4.2. 4-(6-Methoxybenzo[d]thiazol-2-yl)-2-methylaniline (16a)
Yellow solid, yield 95%; mp 151–153 °C; 1H NMR (300 MHz, CDCl3): δ 7.87 (d, J = 8.9 Hz, 1H), 7.78 (d, J = 1.2 Hz, 1H), 7.69 (dd, J = 8.2, 1.9 Hz, 1H), 7.31 (d, J = 2.5 Hz, 1H), 7.04 (dd, J = 8.9, 2.6 Hz, 1H), 6.71 (d, J = 8.3 Hz, 1H), 3.91 (s, 1H), 3.87 (s, 3H), 2.23 (s, 3H); MS (ESI): m/z 271 (M + H)+.
4.1.1.4.3. 4-(5,7-Dimethoxybenzo[d]thiazol-2-yl)aniline (15b)
Yellow solid, yield 90%; mp 150–152 °C; 1H NMR (500 MHz, CDCl3): δ 7.90–7.85 (m, 2H), 7.14 (d, J = 2.0 Hz, 1H), 6.75–6.69 (m, 2H), 6.45 (d, J = 2.0 Hz, 1H), 3.98 (s, 1H), 3.95 (s, 3H), 3.88 (s, 3H); MS (ESI): m/z 286 (M)+.
4.1.1.4.4. 4-(5,7-Dimethoxybenzo[d]thiazol-2-yl)-2-methylaniline (16b)
Yellow solid, yield 91%; mp: 142–145 °C; 1H NMR (300 MHz, CDCl3): δ 7.84 (s, 1H), 7.75 (dd, J = 8.3, 2.0 Hz, 1H), 7.16 (d, J = 2.0 Hz, 1H), 6.73 (d, J = 8.3 Hz, 1H), 6.47 (d, J = 2.0 Hz, 1H), 3.97 (s, 3H), 3.96–3.93 (m, 1H), 3.91 (s, 3H), 2.26 (s, 3H); MS (ESI): m/z 301 (M + H)+.
4.1.1.4.5. 4-(5,6,7-Trimethoxybenzo[d]thiazol-2-yl)aniline (15c)
Yellow solid, yield 89%; mp: 145–147 °C; 1H NMR (300 MHz, CDCl3): δ 7.85 (d, J = 8.6 Hz, 2H), 7.30 (s, 1H), 6.73 (d, J = 8.6 Hz, 2H), 4.09 (s, 3H), 3.99 (s, 2H), 3.94 (d, J = 4.4 Hz, 6H); MS (ESI): m/z 317 (M + H)+.
4.1.1.4.6. 2-Methyl-4-(5,6,7-trimethoxybenzo[d]thiazol-2-yl)aniline (16c)
Yellow solid, yield 90%; mp: 150–152 °C; 1H NMR (500 MHz, CDCl3): δ 7.79 (s, 1H), 7.71 (dd, J = 8.2, 2.0 Hz, 1H), 7.30 (s, 1H), 6.71 (d, J = 8.2 Hz, 1H), 4.09 (s, 3H), 3.94 (d, J = 7.0 Hz, 8H). MS (ESI): m/z 331 (M + H)+.
4.1.1.5. General procedure for the synthesis of 1-aryl-2-propyn-1-ol (18a–f)
A solution of aldehyde 17a–f (5 mmol) in dry tetrahydrofuran (THF) was added to a stirred solution of ethynylmagnesium bromide in THF (0.5 M solution, 7.5 mmol) at 0 °C. The solution was stirred at 0 °C for 2 h and then warmed to room temperature and stirred for another 6–7 h. Saturated aqueous ammonium chloride solution 5 mL was added, and the mixture was evaporated in vacuo and partitioned between ethyl acetate and saturated ammonium chloride solution. The organic layer was washed with brine, dried over anhydrous Na2SO4, and evaporated in vacuo to get pure compounds and were used for next step without further purification.
4.1.1.6. General procedure of 1-arylprop-2-yn-1-one (19a–f)
To the stirred solution of 1-arylprop-2-yn-1-ol (1 mmol) in dimethylsulfoxide (DMSO), a solution of 2-iodoxy-benzoic acid (IBX) (1.1 mmol) in dimethyl sulfoxide (DMSO) (10 mL) was added at 10–15 °C. Then, the reaction mixture was slowly rise the temperature to RT and allowed to stir for 3–4 h. the reaction was monitored by TLC using ethyl acetate/hexane (3 : 7) as a solvent system. Appropriate amount of ice water was added, and the reaction mixture was filtered through celite, and the aqueous layer was extracted with ethyl acetate. The organic layer was washed with water, brine, dried over anhydrous Na2SO4, and evaporated by using vacuum to get crude compounds. The compound was purified by column chromatography and the compound was eluted in ethyl acetate/hexane (3 : 7) as solvent system.
4.1.1.6.1. 1-(4-((tert-Butyldimethylsilyl)oxy)-3-methoxyphenyl)prop-2-yn-1-one (19a)
Compound 13a was prepared according to the method described, employing 1-(3-((tert-butyldimethylsilyl)oxy)-4-methoxyphenyl)prop-2-yn-1-ol (18a, 750 mg, 2.57 mmol) and IBX (790 mg, 2.82 mmol) to obtain the pure product 19a as a pale yellow solid. (620 mg, 83% yield) mp 125–126 °C; 1H NMR (CDCl3, 300 MHz) δ (ppm):7.67 (dd, J = 6.41 Hz, 0.92 Hz, 1H), 7.43 (dd, J = 0.92 Hz, 1.52 Hz), 6.73 (d, J = 8.54 Hz, 1H), 3.71 (s, 3H), 3.19 (s, 1H), 0.83 (s, 9H); MS (ESI) m/z 291 [M + H]+.
4.1.1.6.2. 1-(3-Fluoro-4-methoxyphenyl)prop-2-yn-1-one (19b)
Compound 19b was prepared according to the method described, employing 1-(3-fluoro-4-methoxyphenyl)prop-2-yn-1-ol (18b, 750 mg, 2.57 mmol) and IBX (790 mg, 2.82 mmol) to obtain the pure product 19b as a yellow colour solid. (620 mg, 83% yield) mp 125–126 °C; 1H NMR (300 MHz, CDCl3 + DMSO): δ 7.79 (d, J = 8.6 Hz, 1H), 7.63 (dd, J = 11.5, 2.1 Hz, 1H), 6.90 (dd, J = 11.3, 5.3 Hz, 1H), 3.80 (s, 3H), 3.54 (s, 1H).
4.1.1.6.3. 1-(3-Methoxy-4-nitrophenyl)prop-2-yn-1-one (19c)
Compound 19c was prepared according to the method described employing 1-(4-methoxy-3-nitrophenyl)prop-2-yn-1-ol (18c, 750 mg, 3.62 mmol) and IBX (1.12 g, 3.98 mmol) to obtain the pure product 19c as a pale yellow solid. (500 mg, 74% yield) mp 125–126 °C; 1H NMR (CDCl3, 500 MHz) δ (ppm): 8.61 (d, J = 2.0 Hz, 1H), 8.31 (dd, J = 2.0 Hz, 6.99 Hz, 1H), 7.19 (d, J = 7.99 Hz, 1H), 4.06 (s, 3H), 3.51 (s, 1H). MS (ESI) m/z 206 [M + H]+.
4.1.1.6.4. 1-(3,4-Dimethoxy-5-nitrophenyl)prop-2-yn-1-one (19d)
Compound 19d was prepared according to the method described by employing 1-(3,4-dimethoxy-5-nitrophenyl)prop-2-yn-1-ol (18d, 750 mg, 3.16 mmol) and IBX (973 mg, 3.48 mmol) to obtain the pure product 19d as a pale yellow solid. (617 mg, 83% yield); mp: 103–104 °C; 1H NMR (CDCl3, 300 MHz): δ 8.18 (s, 1H), 7.81 (s, 1H), 4.08 (s, 3H), 4.00 (s, 3H), 3.54 (s, 1H) ppm; MS (ESI) m/z 236 [M + H]+.
4.1.1.6.5. 1-(3,4,5-Trimethoxyphenyl)prop-2-yn-1-one (19e)
Compound 19e was prepared according to the method described by employing 1-(3,4,5-trimethoxyphenyl)prop-2-yn-1-ol (18e, 750 mg, 3.38 mmol) and IBX (1.04 g, 3.72 mmol) to obtain the pure product 19e as a light yellow colour solid. (654 mg, 88% yield) mp: 123–126 °C; 1H NMR (CDCl3, 300 MHz): δ 7.43 (s, 2H), 3.95 (s, 3H), 3.93 (s, 6H), 3.43 (s, 1H) ppm; MS (ESI) m/z 221 [M + H]+.
4.1.1.6.6. 1-(2-Bromo-3,4,5-trimethoxyphenyl)prop-2-yn-1-one (19f)
Compound 19f was prepared according to the method described by employing 1-(2-bromo-3,4,5-trimethoxyphenyl)prop-2-yn-1-ol (18f, 750 mg, 2.50 mmol) and IBX (770 mg, 2.75 mmol) to obtain the pure product 19f as a pale yellow colour solid. (638 mg, 86% yield); mp: 80–81 °C; 1H NMR (CDCl3, 300 MHz): δ 7.46 (s, 1H), 3.98 (s, 3H), 3.93 (s, 3H), 3.89 (s, 3H), 3.50 (s, 1H) ppm; MS (ESI) m/z 298 [M + H]+.
4.1.1.1.7. General procedure for the synthesis of substituted (Z)-3-((4-(benzo[d]thiazol-2-yl)phenyl)amino)-1-phenylprop-2-en-1-ones 5a–r and 6a–r
To the stirred solution of Aryl propynones 19(a–f) (5 mmol) in absolute ethanol, 4-aminophenyl-benzothiazoles 15–16(a–c) (5 mmol) was added. The reaction was stirred for 4 h at room temperature. After the completion of reaction (checked by TLC), the reaction mixture was diluted with water and the crude product was filtered. The crude product was recrystallized from methanol to get pure yellow coloured compounds 5a–r and 6a–r.
4.1.1.7.1. (Z)-1-(3-Hydroxy-4-methoxyphenyl)-3-((4-(6-methoxybenzo[d]thiazol-2-yl)phenyl)amino)prop-2-en-1-one (5a)
Compound 5a was prepared according to the method described by 4-(6-methoxybenzo[d]thiazol-2-yl)aniline (15a) (100 mg, 0.39 mmol) and 1-(3-((tert-butyldimethylsilyl)oxy)-4-methoxyphenyl)prop-2-yn-1-one (19a) (113 mg, 0.39 mmol) to obtain the pure product 5a1 as a yellow colour solid. 1 M TBAF in THF (0.87 mL, 3.3 mmol) was added to a stirred solution of 5a1 (160 mg, 0.29 mmol) in THF (15 mL) at 10–15 °C. Then, the temperature of the mixture was slowly increased to RT, and the mixture was stirred for 6 h. The progress of the reaction was monitored by TLC. Upon completion of the reaction, THF was evaporated, and the mixture was partitioned between water and EtOAc. The compound was purified by column chromatography to obtain pure product 5a as a yellow colour solid. 143 mg, 85% yield; mp: 180–182 °C; 1H NMR (300 MHz, CDCl3): δ 12.12 (d, J = 12.7 Hz, 1H), 7.54 (d, J = 5.8 Hz, 3H), 7.41 (dd, J = 12.4, 7.8 Hz, 1H), 7.06 (d, J = 8.9 Hz, 3H), 6.91 (dd, J = 8.9, 3.2 Hz, 4H), 5.93 (d, J = 7.8 Hz, 1H), 5.69 (s, 1H), 3.97 (s, 3H), 3.82 (s, 3H). 13C NMR (75 MHz, CDCl3 + TFA): δ 191.24, 168.89, 151.12, 145.98, 145.38, 142.62, 136.28, 131.68, 130.76, 128.62, 127.34, 122.87, 119.86, 118.67, 114.39, 110.65, 105.10, 104.86, 95.68, 56.47, 56.19. MS (ESI): m/z 433 [M + H]+; HRMS calcd for C24H21O4N2S[M + H]+ 433.12202, found 447.12175.
4.1.1.7.2. (Z)-1-(3-Hydroxy-4-methoxyphenyl)-3-((4-(6-methoxybenzo[d]thiazol-2-yl)-2-methylphenyl)amino)prop-2-en-1-one (6a)
Compound 6a was prepared according to the method described by 4-(6-methoxybenzo[d]thiazol-2-yl)-2-methylaniline (16a) (100 mg, 0.37 mmol) and 1-(3-((tert-butyldimethylsilyl)oxy)-4-methoxyphenyl)prop-2-yn-1-one (19a) (100 mg, 0.37 mmol) to obtain the pure product 6a1 as a yellow colour solid. 1 M TBAF in THF (0.88 mL, 3.3 mmol) was added to a stirred solution of 6a1 (165 mg, 0.247 mmol) in THF (15 mL) at 10–15 °C. Then, the temperature of the mixture was slowly increased to RT, and the mixture was stirred for 6 h. The progress of the reaction was monitored by TLC. Upon completion of the reaction, THF was evaporated, and the mixture was partitioned between water and EtOAc. The compound was purified by column chromatography to obtain pure product 6a as a yellow colour solid. 137 mg, 83% yield; mp: 230–232 °C; 1H NMR (500 MHz, DMSO): δ 12.37 (d, J = 11.7 Hz, 1H), 9.32 (s, 1H), 8.00 (dd, J = 11.6, 7.9 Hz, 1H), 7.92 (s, 1H), 7.88 (dd, J = 8.9, 3.4 Hz, 2H), 7.65 (d, J = 2.5 Hz, 1H), 7.59 (d, J = 8.7 Hz, 1H), 7.50 (dd, J = 8.5, 2.0 Hz, 1H), 7.44 (d, J = 2.0 Hz, 1H), 7.11 (dd, J = 8.9, 2.5 Hz, 1H), 7.00 (d, J = 8.5 Hz, 1H), 6.20 (d, J = 7.9 Hz, 1H), 3.86 (s, 6H), 2.45 (s, 3H). 13C NMR (75 MHz, CDCl3 + TFA): δ 193.01, 169.73, 151.12, 144.98, 144.47, 144.23, 134.88, 131.38, 130.52, 128.40, 127.60, 122.27, 119.86, 118.67, 114.39, 113.99, 110.65, 105.10, 104.86, 56.29, 56.24, 17.11. MS (ESI): m/z 447 [M + H]+; HRMS calcd for C25H23O4N2S[M + H]+ 447.13005, found 447.13737.
4.1.1.7.3. (Z)-3-((4-(5,7-Dimethoxybenzo[d]thiazol-2-yl)phenyl)amino)-1-(3-hydroxy-4-methoxyphenyl)prop-2-en-1-one (5b)
Compound 5b was prepared according to the method described by 4-(5,7-dimethoxybenzo[d]thiazol-2-yl)aniline (15b) (100 mg, 0.37 mmol) and 1-(3-((tert-butyldimethylsilyl)oxy)-4-methoxyphenyl)prop-2-yn-1-one (19a) (103 mg, 0.37 mmol) to obtain the pure product 5b1 as a yellow colour solid. 1 M TBAF in THF (0.85 mL, 3.2 mmol) was added to a stirred solution of 5b1 (163 mg, 0.282 mmol) in THF (15 mL) at 10–15 °C. Then, the temperature of the mixture was slowly increased to RT, and the mixture was stirred for 6 h. The progress of the reaction was monitored by TLC. Upon completion of the reaction, THF was evaporated, and the mixture was partitioned between water and EtOAc. The compound was purified by column chromatography to obtain pure product 5b as a yellow colour solid. Yield: 138 mg, 86%; m.p: 222–224 °C; 1H NMR (300 MHz, CDCl3 + DMSO): δ 10.17 (d, J = 12.7 Hz, 1H), 9.20 (d, J = 4.0 Hz, 1H), 7.98 (t, J = 12.7 Hz, 1H), 7.86 (dd, J = 8.5, 5.4 Hz, 2H), 7.75 (dd, J = 12.1, 8.2 Hz, 1H), 7.33 (t, J = 7.2 Hz, 1H), 7.25 (dd, J = 9.8, 5.7 Hz, 1H), 7.14 (d, J = 8.7 Hz, 1H), 7.06–7.02 (m, 1H), 6.86 (dd, J = 8.4, 3.9 Hz, 1H), 6.51 (t, J = 2.2 Hz, 1H), 6.36 (d, J = 12.6 Hz, 1H), 3.80 (s, 3H), 3.70 (d, J = 7.1 Hz, 6H). 13C NMR (75 MHz, CDCl3 + TFA): δ 192.05, 171.51, 163.94, 154.78, 151.48, 144.87, 142.60, 130.23, 124.84, 122.65, 120.39, 119.57, 117.71, 114.95, 113.98, 111.21, 110.80, 100.00, 91.45, 56.58, 56.39, 56.22. MS (ESI): m/z 463 [M + H]+; HRMS calcd for C25H23O5N2S[M + H]+ 463.13222, found 463.13149.
4.1.1.7.4. (Z)-3-((4-(5,7-Dimethoxybenzo[d]thiazol-2-yl)-2-methylphenyl)amino)-1-(3-hydroxy-4-methoxyphenyl)prop-2-en-1-one (6b)
Compound 6b was prepared according to the method described by 4-(5,7-dimethoxybenzo[d]thiazol-2-yl)-2-methylaniline (16b) (100 mg, 0.34 mmol) and 1-(3-((tert-butyldimethylsilyl)oxy)-4-methoxyphenyl)prop-2-yn-1-one (19a) (96 mg, 0.33 mmol) to obtain the pure product 6b1 as a yellow colour solid. 1 M TBAF in THF (0.79 mL, 3.0 mmol) was added to a stirred solution of 6b1 (156 mg, 0.264 mmol) in THF (15 mL) at 10–15 °C. Then, the temperature of the mixture was slowly increased to RT, and the mixture was stirred for 6 h. The progress of the reaction was monitored by TLC. Upon completion of the reaction, THF was evaporated, and the mixture was partitioned between water and EtOAc. The compound was purified by column chromatography to obtain pure product 6b as a yellow colour solid. (134 mg, 85% yield); mp: 242–244 °C; 1H NMR (300 MHz, CDCl3 + DMSO): δ 10.17 (d, J = 12.7 Hz, 1H), 9.20 (d, J = 4.0 Hz, 1H), 7.98 (t, J = 12.7 Hz, 1H), 7.86 (dd, J = 8.5, 5.4 Hz, 2H), 7.75 (dd, J = 12.1, 8.2 Hz, 1H), 7.33 (t, J = 7.2 Hz, 1H), 7.25 (dd, J = 9.8, 5.7 Hz, 1H), 7.14 (d, J = 8.7 Hz, 1H), 7.06–7.02 (m, 1H), 6.86 (dd, J = 8.4, 3.9 Hz, 1H), 6.51 (t, J = 2.2 Hz, 1H), 6.36 (d, J = 12.6 Hz, 1H), 3.80 (s, 3H), 3.70 (d, J = 7.1 Hz, 6H). 13C NMR (75 MHz, CDCl3 + TFA): δ 174.34, 149.49, 148.87, 139.22, 135.85, 135.12, 133.18, 132.03, 129.97, 128.59, 125.46, 124.57, 123.00, 120.27, 120.06, 119.17, 117.67, 109.66, 109.27, 60.92, 60.72, 60.24, 21.50. MS (ESI): m/z 477 [M + H]+; HRMS calcd for C26H25O5N2S[M + H]+ 477.14787, found 477.14906.
4.1.1.7.5. (Z)-1-(3-Hydroxy-4-methoxyphenyl)-3-((4-(5,6,7-trimethoxybenzo[d]thiazol-2-yl)phenyl)amino)prop-2-en-1-one (5c)
Compound 5c was prepared according to the method described by 4-(5,6,7-trimethoxybenzo[d]thiazol-2-yl)aniline (15c) (100 mg, 0.31 mmol) and 1-(3-((tert-butyldimethylsilyl)oxy)-4-methoxyphenyl)prop-2-yn-1-one (19a) (91 mg, 0.31 mmol) to obtain the pure product 5c1 as a yellow colour solid. 1 M TBAF in THF (0.79 mL, 2.9 mmol) was added to a stirred solution of 5c1 (158 mg, 0.260 mmol) in THF (15 mL) at 10–15 °C. Then, the temperature of the mixture was slowly increased to RT, and the mixture was stirred for 6 h. The progress of the reaction was monitored by TLC. Upon completion of the reaction, THF was evaporated, and the mixture was partitioned between water and EtOAc. The compound was purified by column chromatography to obtain pure product 5c as a yellow colour solid. (135 mg, 87% yield); mp: 216–218 °C; 1H NMR (300 MHz, CDCl3 + DMSO): δ 8.54 (s, 1H), 8.01 (d, J = 8.4 Hz, 2H), 7.63–7.44 (m, 5H), 7.21 (d, J = 8.5 Hz, 2H), 6.91 (d, J = 8.4 Hz, 1H), 6.08 (d, J = 8.0 Hz, 1H), 4.10 (s, 3H), 3.98–3.91 (m, 9H). 13C NMR (75 MHz, DMSO): δ 189.16, 166.34, 153.85, 151.23, 149.99, 146.39, 143.72, 143.55, 142.66, 142.09, 139.20, 139.13, 132.09, 131.44, 128.52, 128.38, 127.26, 126.26, 119.81, 119.68, 118.90, 118.76, 116.33, 115.52, 114.28, 114.06, 111.28, 100.88, 99.24, 94.53, 60.98, 60.31, 56.23, 55.61. MS (ESI): m/z 493 [M + H]+; HRMS calcd for C26H25O6N2S[M + H]+ 493.14278, found 493.14189.
4.1.1.7.6. (Z)-1-(3-Hydroxy-4-methoxyphenyl)-3-((2-methyl-4-(5,6,7-trimethoxybenzo[d]thiazol-2-yl)phenyl)amino)prop-2-en-1-one (6c)
Compound 6c was prepared according to the method described by 2-methyl-4-(5,6,7-trimethoxybenzo[d]thiazol-2-yl)aniline (16c) (100 mg, 0.3 mmol) and 1-(3-((tert-butyldimethylsilyl)oxy)-4-methoxyphenyl)prop-2-yn-1-one (19a) (87 mg, 0.3 mmol) to obtain the pure product 6c1 as a yellow colour solid. 1 M TBAF in THF (0.72 mL, 2.7 mmol) was added to a stirred solution of 6c1 (148 mg, 0.238 mmol) in THF (15 mL) at 10–15 °C. Then, the temperature of the mixture was slowly increased to RT, and the mixture was stirred for 6 h. The progress of the reaction was monitored by TLC. Upon completion of the reaction, THF was evaporated, and the mixture was partitioned between water and EtOAc. The compound was purified by column chromatography to obtain pure product 6c as a yellow colour solid. 127 mg, 83% yield; mp: 190–192 °C; 1H NMR (500 MHz, CDCl3): δ 12.34 (d, J = 11.8 Hz, 1H), 7.92 (d, J = 1.2 Hz, 1H), 7.89 (dd, J = 8.4, 2.0 Hz, 1H), 7.62–7.58 (m, 1H), 7.58–7.54 (m, 2H), 7.33 (s, 1H), 7.24 (d, J = 7.8 Hz, 1H), 6.92–6.90 (m, 1H), 6.09 (d, J = 7.9 Hz, 1H), 5.70 (s, 1H), 4.10 (s, 3H), 3.95 (d, J = 7.8 Hz, 10H), 3.94 (s, 4H), 2.52 (s, 3H). 13C NMR (75 MHz, TFA): δ 192.77, 171.22, 157.51, 150.94, 146.70, 145.23, 144.51, 143.54, 140.92, 137.48, 131.24, 131.07, 128.10, 127.61, 121.87, 119.86, 114.90, 114.11, 113.92, 110.48, 94.51, 61.84, 61.38, 56.72, 56.14, 17.20. MS (ESI): m/z 507 [M + H]+; HRMS calcd for C27H27O6N2S[M + H]+ 507.15843, found 507.15696.
4.1.1.7.7. (Z)-1-(3-Fluoro-4-methoxyphenyl)-3-((4-(6-methoxybenzo[d]thiazol-2-yl)phenyl)amino)prop-2-en-1-one (5d)
Compound 15a was prepared according to the method described by 4-(6-methoxybenzo[d]thiazol-2-yl)aniline (9a) (100 mg, 0.39 mmol) and 1-(3-fluoro-4-methoxyphenyl)prop-2-yn-1-one (13b) (70 mg, 0.39 mmol) to obtain the pure product 5d as a yellow colour solid (149 mg, 88% yield); mp: 189–191 °C; 1H NMR (400 MHz, CDCl3): δ 12.11 (d, J = 12.3 Hz, 1H), 8.40 (d, J = 1.5 Hz, 1H), 8.09–8.05 (m, 1H), 7.94 (d, J = 9.0 Hz, 1H), 7.73–7.68 (m, 2H), 7.41 (dd, J = 12.4, 7.7 Hz, 1H), 7.06–7.03 (m, 2H), 6.99 (t, J = 8.5 Hz, 1H), 6.92–6.87 (m, 2H), 5.89 (d, J = 7.8 Hz, 1H), 3.95 (s, 3H), 3.80 (s, 3H). 13C NMR (75 MHz, CDCl3 + TFA): δ 190.09, 169.87, 155.87, 151.12, 146.78, 146.47, 138.98, 134.88, 131.64, 130.52, 128.26, 127.61, 122.53, 119.86, 118.67, 114.54, 113.99, 105.10, 104.86, 94.93, 56.38, 56.29. MS (ESI): m/z 435 [M + H]+; HRMS calcd for C24H20O3N2SF[M + H]+ 435.1100, found 435.1172.
4.1.1.7.8. (Z)-1-(3-Fluoro-4-methoxyphenyl)-3-((4-(6-methoxybenzo[d]thiazol-2-yl)-2-methylphenyl)amino)prop-2-en-1-one (6d)
Compound 6d was prepared according to the method described by 4-(6-methoxybenzo[d]thiazol-2-yl)-2-methylaniline (10a) (100 mg, 0.37 mmol) and 1-(3-fluoro-4-methoxyphenyl)prop-2-yn-1-one (13b) (65 mg, 0.37 mmol) to obtain the pure product 6d as a yellow colour solid. (135 mg, 82% yield); mp: 132–135 °C; 1H NMR (500 MHz, DMSO): δ 12.31 (d, J = 12.27 Hz, 1H), 9.33 (s, 1H), 8.01 (dd, J = 12.31, 7.9 Hz, 1H), 7.93 (s, 1H), 7.89 (dd, J = 8.8, 3.4 Hz, 2H), 7.65 (d, J = 2.5 Hz, 1H), 7.59 (d, J = 8.7 Hz, 1H), 7.50 (dd, J = 8.5, 2.0 Hz, 1H), 7.44 (d, J = 2.0 Hz, 1H), 7.11 (dd, J = 8.8, 2.5 Hz, 1H), 7.00 (d, J = 8.5 Hz, 1H), 6.20 (d, J = 7.9 Hz, 1H), 3.86 (s, 6H), 2.45 (s, 3H). MS (ESI): m/z 449 [M + H]+; HRMS calcd for C25H22O3N2SF[M + H]+ 449.13320, found 449.13378.
4.1.1.7.9. (Z)-3-((4-(5,7-Dimethoxybenzo[d]thiazol-2-yl)phenyl)amino)-1-(3-fluoro-4-methoxyphenyl)prop-2-en-1-one (5e)
Compound 5e was prepared according to the method described by 4-(5,7-dimethoxybenzo[d]thiazol-2-yl)aniline (9b) (100 mg, 0.35 mmol) and 1-(3-fluoro-4-methoxyphenyl)prop-2-yn-1-one (13b) (62 mg, 0.35 mmol) to obtain the pure product 5e as a yellow colour solid. (134 mg, 83% yield); mp: 190–192 °C; 1H NMR (400 MHz, CDCl3): δ 12.17 (d, J = 12.1 Hz, 1H), 8.05 (d, J = 8.7 Hz, 1H), 7.75–7.68 (m, 1H), 7.53 (dd, J = 12.1, 8.0 Hz, 1H), 7.20–7.15 (m, 1H), 7.01 (dd, J = 10.8, 6.2 Hz, 1H), 6.49 (d, J = 2.0 Hz, 1H), 6.02 (d, J = 8.0 Hz, 1H), 3.96 (d, J = 2.5 Hz, 6H), 3.90 (s, 3H). 13C NMR (75 MHz, CDCl3 + TFA): δ 191.20, 185.30, 172.58, 171.80, 164.69, 155.36, 146.62, 145.87, 143.20, 130.44, 128.00, 125.97, 124.22, 120.22, 118.36, 112.84, 108.99, 100.75, 100.20, 91.46, 56.65, 56.28. MS (ESI): m/z 465 [M + H]+; HRMS calcd for C25H22O4N2FS[M + H]+ 465.12788, found 465.12727.
4.1.1.7.10. (Z)-3-((4-(5,7-Dimethoxybenzo[d]thiazol-2-yl)-2-methylphenyl)amino)-1-(3-fluoro-4-methoxyphenyl)prop-2-en-1-one (6e)
Compound 6e was prepared according to the method described by 4-(5,7-dimethoxybenzo[d]thiazol-2-yl)-2-methylaniline (10b) (100 mg, 0.33 mmol) and 1-(3-fluoro-4-methoxyphenyl)prop-2-yn-1-one (13b) (62 mg, 0.33 mmol) to obtain the pure product 6e as a yellow colour solid. (128 mg, 80% yield); mp: 229–231 °C; 1H NMR (500 MHz, CDCl3): δ 12.34 (d, J = 11.9 Hz, 1H), 7.93 (dd, J = 14.0, 5.6 Hz, 1H), 7.73 (dd, J = 16.1, 5.1 Hz, 2H), 7.62 (dd, J = 11.8, 8.0 Hz, 1H), 7.25 (d, J = 6.4 Hz, 2H), 7.17 (d, J = 2.0 Hz, 1H), 7.01 (t, J = 8.5 Hz, 1H), 6.49 (d, J = 2.0 Hz, 1H), 6.07 (d, J = 7.9 Hz, 1H), 3.96 (d, J = 2.8 Hz, 6H), 3.90 (s, 3H), 2.52 (s, 3H). 13C NMR (75 MHz, CDCl3 + TFA): δ 192.04, 172.29, 163.92, 154.79, 152.18, 152.04, 144.92, 144.45, 142.57, 131.33, 130.52, 128.61, 127.59, 125.50, 120.15, 115.80, 115.55, 114.60, 113.14, 110.84, 99.92, 91.45, 56.56, 56.42, 56.21, 17.04. MS (ESI): m/z 479 [M + H]+; HRMS calcd for C26H23O4N2FS[M + H]+ 479.1362, found 479.1432.
4.1.1.7.11. (Z)-1-(3-Fluoro-4-methoxyphenyl)-3-((4-(5,6,7-trimethoxybenzo[d]thiazol-2-yl)phenyl)amino)prop-2-en-1-one (5f)
Compound 5f was prepared according to the method described by 4-(5,6,7-trimethoxybenzo[d]thiazol-2-yl)aniline (9c) (100 mg, 0.31 mmol) and 1-(3-fluoro-4-methoxyphenyl)prop-2-yn-1-one (13b) (56 mg, 0.31 mmol) to obtain the pure product 5f as a yellow colour solid. (132 mg, 85% yield); mp: 143–145 °C; 1H NMR (400 MHz, CDCl3): δ 12.17 (d, J = 12.1 Hz, 1H), 8.02 (d, J = 8.6 Hz, 2H), 7.73 (d, J = 7.3 Hz, 1H), 7.69 (d, J = 1.9 Hz, 1H), 7.53 (dd, J = 12.1, 8.0 Hz, 1H), 7.33 (s, 1H), 7.17 (d, J = 8.7 Hz, 2H), 7.01 (t, J = 8.5 Hz, 1H), 6.01 (d, J = 8.0 Hz, 1H), 4.10 (s, 3H), 3.96 (d, J = 1.6 Hz, 6H), 3.94 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 189.04, 167.10, 154.03, 150.85, 150.56, 146.77, 143.47, 142.21, 139.69, 132.13, 132.08, 128.85, 124.25, 120.05, 116.19, 115.32, 115.13, 112.46, 100.59, 94.41, 61.51, 60.57, 56.29. MS (ESI): m/z 495 [M + H]+; HRMS calcd for C26H24O5N2FS[M + H]+ 495.13845, found 495.13760.
4.1.1.7.12. (Z)-1-(3-Fluoro-4-methoxyphenyl)-3-((2-methyl-4-(5,6,7-trimethoxybenzo[d]thiazol-2-yl)phenyl)amino)prop-2-en-1-one (6f)
Compound 6f was prepared according to the method described by 2-methyl-4-(5,6,7-trimethoxybenzo[d]thiazol-2-yl)aniline (10c) (100 mg, 0.30 mmol) and 1-(3-fluoro-4-methoxyphenyl)prop-2-yn-1-one (13b) (59 mg, 0.30 mmol) to obtain the pure product 6f as a yellow colour solid. (126 mg, 82% yield); mp: 190–191 °C; 1H NMR (400 MHz, CDCl3): δ 12.34 (d, J = 11.8 Hz, 1H), 7.94–7.88 (m, 2H), 7.77–7.70 (m, 2H), 7.61 (dd, J = 11.8, 8.0 Hz, 1H), 7.33 (s, 1H), 7.24 (s, 1H), 7.01 (t, J = 8.5 Hz, 1H), 6.07 (d, J = 7.9 Hz, 1H), 4.10 (s, 3H), 3.96 (s, 6H), 3.94 (s, 3H), 2.52 (s, 3H). 13C NMR (75 MHz, CDCl3 + DMSO) δ 188.20, 166.47, 153.23, 149.74, 145.93, 143.10, 140.11, 138.80, 131.36, 129.02, 127.48, 125.73, 125.56, 123.63, 119.07, 114.43, 114.18, 112.67, 111.81, 99.80, 94.04, 60.69, 59.76, 55.53, 16.94. MS (ESI): m/z 509 [M + H]+; HRMS calcd for C27H26O5N2FS[M + H]+ 509.15410, found 509.15216.
4.1.1.7.13. (Z)-1-(4-methoxy-3-nitrophenyl)-3-((4-(6-methoxybenzo[d]thiazol-2-yl)phenyl)amino)prop-2-en-1-one (5g)
Compound 5g was prepared according to the method described by 4-(6-methoxybenzo[d]thiazol-2-yl)aniline (9a) (100 mg, 0.39 mmol) and 1-(4-methoxy-3-nitrophenyl)prop-2-yn-1-one (13c) (80 mg, 0.39 mmol) to obtain the pure product 5g as a yellow colour solid. (142 mg, 79% yield); mp: 240–243 °C; 1H NMR (400 MHz, CDCl3): δ 12.11 (d, J = 12.1 Hz, 1H), 8.40 (d, J = 1.5 Hz, 1H), 8.08–8.04 (m, 1H), 7.96 (d, J = 9.0 Hz, 1H), 7.72–7.66 (m, 2H), 7.42 (dd, J = 12.1, 7.7 Hz, 1H), 7.03–7.01 (m, 2H), 6.97 (t, J = 8.5 Hz, 1H), 6.91–6.86 (m, 2H), 5.85 (d, J = 7.7 Hz, 1H), 3.94 (s, 3H), 3.81 (s, 3H). 13C NMR (75 MHz, TFA): δ 191.22, 170.05, 146.12, 138.90, 137.28, 134.76, 134.61, 130.96, 130.28, 126.27, 121.27, 120.65, 118.47, 118.21, 114.40, 105.34, 100.35, 57.04, 56.27. MS (ESI): m/z 462 [M + H]+; HRMS calcd for C24H20O5N3S[M + H]+ 462.11285, found 462.11243.
4.1.1.7.14. (Z)-1-(4-methoxy-3-nitrophenyl)-3-((4-(6-methoxybenzo[d]thiazol-2-yl)-2-methylphenyl)amino)prop-2-en-1-one (6g)
Compound 6g was prepared according to the method described by 4-(6-methoxybenzo[d]thiazol-2-yl)-2-methylaniline (10a) (100 mg, 0.37 mmol) and 1-(4-methoxy-3-nitrophenyl)prop-2-yn-1-one (13c) (76 mg, 0.37 mmol) to obtain the pure product 6g as a yellow colour solid. (143 mg, 82% yield); mp: 270–272 °C; 1H NMR (500 MHz, DMSO): δ 12.21 (d, J = 12.11 Hz, 1H), 9.29 (s, 1H), 8.00 (dd, J = 12.11, 7.9 Hz, 1H), 7.94 (s, 1H), 7.84 (dd, J = 8.8, 3.4 Hz, 2H), 7.64 (d, J = 2.5 Hz, 1H), 7.57 (d, J = 8.7 Hz, 1H), 7.49 (dd, J = 8.5, 2.0 Hz, 1H), 7.41 (d, J = 2.0 Hz, 1H), 7.10 (dd, J = 8.8, 2.5 Hz, 1H), 7.00 (d, J = 8.5 Hz, 1H), 6.20 (d, J = 7.9 Hz, 1H), 3.86 (s, 6H). 13C NMR (75 MHz, TFA): δ 190.97, 170.12, 146.07, 144.31, 138.86, 134.61, 131.32, 130.91, 130.27, 129.12, 127.71, 126.25, 120.48, 118.44, 115.07, 114.30, 105.28, 98.40, 57.01, 56.24, 16.96. MS (ESI): m/z 476 [M + H]+; HRMS calcd for C25H22O5N3S[M + H]+ 476.12834, found 476.12784.
4.1.1.7.15. (Z)-3-((4-(5,7-dimethoxybenzo[d]thiazol-2-yl)phenyl)amino)-1-(4-methoxy-3-nitrophenyl)prop-2-en-1-one (5h)
Compound 5h was prepared according to the method described by 4-(5,7-dimethoxybenzo[d]thiazol-2-yl)aniline (9b) (100 mg, 0.35 mmol) and 1-(4-methoxy-3-nitrophenyl)prop-2-yn-1-one (13c) (71 mg, 0.35 mmol) to obtain the pure product 5h as a yellow colour solid. (133 mg, 78% yield); mp: 229–232 °C; 1H NMR (400 MHz, CDCl3): δ 12.20 (d, J = 12.1 Hz, 1H), 8.44 (d, J = 2.2 Hz, 1H), 8.18 (dd, J = 8.8, 2.2 Hz, 1H), 8.07 (d, J = 8.7 Hz, 2H), 7.60 (dd, J = 12.3, 7.9 Hz, 1H), 7.20 (d, J = 8.7 Hz, 2H), 7.17 (dd, J = 5.4, 3.4 Hz, 2H), 6.50 (d, J = 2.0 Hz, 1H), 6.04 (d, J = 7.9 Hz, 1H), 4.04 (s, 3H), 3.97 (s, 3H), 3.91 (s, 3H). 13C NMR (75 MHz, CDCl3 + TFA): δ 172.41, 164.10, 155.00, 145.77, 142.56, 138.93, 134.37, 130.27, 126.08, 120.82, 118.08, 114.28, 111.11, 100.10, 91.49, 57.04, 56.63, 56.23. MS (ESI): m/z 514 [M + Na]+; HRMS calcd for C25H21O6N3SNa[M + H]+ 514.10433, found 514.10371.
4.1.1.7.16. (Z)-3-((4-(5,7-Dimethoxybenzo[d]thiazol-2-yl)-2-methylphenyl)amino)-1-(4-methoxy-3-nitrophenyl)prop-2-en-1-one (6h)
Compound 6h was prepared according to the method described by 4-(5,7-dimethoxybenzo[d]thiazol-2-yl)-2-methylaniline (10b) (100 mg, 0.33 mmol) and 1-(4-methoxy-3-nitrophenyl)prop-2-yn-1-one (13c) (68 mg, 0.33 mmol) to obtain the pure product 6h as a yellow colour solid. (131 mg, 78% yield); mp: 272–274 °C; 1H NMR (500 MHz, CDCl3): δ 12.24 (d, J = 11.9 Hz, 1H), 7.91 (dd, J = 14.0, 5.6 Hz, 1H), 7.77 (dd, J = 16.1, 5.1 Hz, 2H), 7.61 (dd, J = 11.9, 7.9 Hz, 1H), 7.23 (d, J = 6.4 Hz, 2H), 7.14 (d, J = 2.0 Hz, 1H), 7.00 (t, J = 8.5 Hz, 1H), 6.45 (d, J = 2.0 Hz, 1H), 6.05 (d, J = 7.9 Hz, 1H), 3.96 (d, J = 2.8 Hz, 6H), 3.90 (s, 3H), 2.52 (s, 3H). 13C NMR (75 MHz, CDCl3 + TFA): δ 190.58, 172.34, 163.91, 156.60, 154.79, 145.36, 144.32, 142.60, 138.99, 134.18, 131.34, 130.18, 128.70, 127.66, 125.97, 120.38, 114.77, 114.05, 110.88, 99.94, 91.53, 57.00, 56.59, 56.22, 17.27. MS (ESI): m/z 506 [M + H]+; HRMS calcd for C26H24O6N3S[M + H]+ 506.13832, found 506.13793.
4.1.1.7.17. (Z)-1-(4-Methoxy-3-nitrophenyl)-3-((4-(5,6,7-trimethoxybenzo[d]thiazol-2-yl)phenyl)amino)prop-2-en-1-one (5i)
Compound 5i was prepared according to the method described by 4-(5,6,7-trimethoxybenzo[d]thiazol-2-yl)aniline (9c) (100 mg, 0.31 mmol) and 1-(4-methoxy-3-nitrophenyl)prop-2-yn-1-one (13c) (64 mg, 0.31 mmol) to obtain the pure product 5i as a yellow colour solid. (136 mg, 83% yield); mp: 194–196 °C; 1H NMR (500 MHz, CDCl3): δ 12.20 (d, J = 12.2 Hz, 1H), 8.44 (d, J = 2.0 Hz, 1H), 8.16 (d, J = 8.8 Hz, 1H), 8.03 (d, J = 8.5 Hz, 2H), 7.58 (dd, J = 12.2, 7.9 Hz, 1H), 7.33 (s, 1H), 7.19 (d, J = 8.5 Hz, 2H), 7.16 (d, J = 8.8 Hz, 1H), 6.03 (d, J = 7.9 Hz, 1H), 4.10 (s, 3H), 4.03 (s, 3H), 3.96 (s, 3H), 3.94 (s, 3H). 13C NMR (101 MHz, CDCl3): δ 187.55, 166.95, 155.37, 154.07, 150.52, 146.75, 144.38, 141.87, 139.73, 139.30, 133.19, 131.29, 129.21, 128.87, 125.16, 120.07, 116.41, 113.26, 100.59, 93.91, 61.51, 60.58, 56.83, 56.31. MS (ESI): m/z 522 [M + H]+; HRMS calcd for C26H24O7N3S[M + H]+ 522.13295, found 522.13245.
4.1.1.7.18. (Z)-1-(4-Methoxy-3-nitrophenyl)-3-((2-methyl-4-(5,6,7-trimethoxybenzo[d]thiazol-2-yl)phenyl)amino)prop-2-en-1-one (6i)
Compound 6i was prepared according to the method described by 4-(5,6,7-trimethoxybenzo[d]thiazol-2-yl)aniline (10c) (100 mg, 0.30 mmol) and 1-(4-methoxy-3-nitrophenyl)prop-2-yn-1-one (13c) (62 mg, 0.3 mmol) to obtain the pure product 6i as a yellow colour solid. (128 mg, 79% yield); mp: 210–211 °C; 1H NMR (400 MHz, CDCl3): δ 12.38 (d, J = 12.0 Hz, 1H), 8.46 (d, J = 2.2 Hz, 1H), 8.18 (dd, J = 8.8, 2.2 Hz, 1H), 7.91 (dd, J = 12.5, 4.0 Hz, 1H), 7.66 (dd, J = 12.0, 7.9 Hz, 1H), 7.33 (s, 1H), 7.27 (d, J = 6.9 Hz, 1H), 7.16 (d, J = 8.9 Hz, 1H), 6.09 (d, J = 7.8 Hz, 1H), 4.10 (s, 3H), 4.03 (s, 3H), 3.96 (s, 3H), 3.94 (s, 3H), 2.52 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 187.60, 167.26, 155.33, 154.04, 150.54, 146.76, 144.53, 140.53, 139.68, 139.33, 133.17, 131.37, 129.95, 128.83, 126.75, 126.49, 125.16, 120.03, 113.59, 113.23, 100.56, 94.23, 61.51, 60.57, 56.82, 56.31, 17.70. MS (ESI): m/z 536 [M + H]+; HRMS calcd for C27H26O7N3S[M + H]+ 536.14860, found 536.14724.
4.1.1.7.19. (Z)-1-(3,4-Dimethoxy-5-nitrophenyl)-3-((4-(6-methoxybenzo[d]thiazol-2-yl)phenyl)amino)prop-2-en-1-one (5j)
Compound 5j was prepared according to the method described by 4-(6-methoxybenzo[d]thiazol-2-yl)aniline (9a) (100 mg, 0.39 mmol) and 1-(3,4-dimethoxy-5-nitrophenyl)prop-2-yn-1-one (13d) (91 mg, 0.39 mmol) to obtain the pure product 5j as a yellow colour solid. (157 mg, 82% yield); mp: 238–241 °C; 1H NMR (400 MHz, CDCl3): δ 12.21 (d, J = 12.3 Hz, 1H), 8.04 (d, J = 8.7 Hz, 2H), 7.92 (d, J = 8.9 Hz, 1H), 7.87 (d, J = 1.9 Hz, 1H), 7.76 (d, J = 1.9 Hz, 1H), 7.60 (dd, J = 12.3, 7.9 Hz, 1H), 7.35 (d, J = 2.5 Hz, 1H), 7.20 (d, J = 8.7 Hz, 2H), 7.09 (dd, J = 8.9, 2.5 Hz, 1H), 6.03 (d, J = 7.9 Hz, 1H), 4.05 (s, 3H), 4.01 (s, 3H), 3.89 (s, 3H). MS (ESI): m/z 492 [M + H]+; HRMS calcd for C25H22O6N3S[M + H]+ 492.1151, found 492.1229.
4.1.1.7.20. (Z)-1-(3,4-Dimethoxy-5-nitrophenyl)-3-((4-(6-methoxybenzo[d]thiazol-2-yl)-2-methylphenyl)amino)prop-2-en-1-one (6j)
Compound 6j was prepared according to the method described by 4-(6-methoxybenzo[d]thiazol-2-yl)-2-methylaniline (10a) (100 mg, 0.37 mmol) and 1-(3,4-dimethoxy-5-nitrophenyl)prop-2-yn-1-one (13d) (187 mg, 0.37 mmol) to obtain the pure product 6j as a yellow colour solid. (157 mg, 84% yield); mp: 220–223 °C; 1H NMR (400 MHz, CDCl3): δ 12.35 (d, J = 12.1 Hz, 1H), 7.94–7.91 (m, 2H), 7.89 (t, J = 2.4 Hz, 2H), 7.78 (d, J = 1.9 Hz, 1H), 7.70–7.64 (m, 1H), 7.35 (d, J = 2.5 Hz, 1H), 7.28 (d, J = 8.8 Hz, 1H), 7.09 (dd, J = 8.9, 2.6 Hz, 1H), 6.08 (d, J = 7.8 Hz, 1H), 4.05 (s, 3H), 4.02 (s, 3H), 3.89 (s, 3H), 2.53 (s, 3H). MS (ESI): m/z 506 [M + H]+; HRMS calcd for C26H24O6N3S[M + H]+ 506.13803, found 506.13987.
4.1.1.7.21. (Z)-1-(3,4-Dimethoxy-5-nitrophenyl)-3-((4-(5,7-dimethoxybenzo[d]thiazol-2-yl)phenyl)amino)prop-2-en-1-one (5k)
Compound 5k was prepared according to the method described by 4-(5,7-dimethoxybenzo[d]thiazol-2-yl)aniline (9b) (100 mg, 0.35 mmol) and 1-(3,4-dimethoxy-5-nitrophenyl)prop-2-yn-1-one (13d) (82 mg, 0.35 mmol) to obtain the pure product 5k as a yellow colour solid. (147 mg, 81% yield); mp: 174–175 °C; 1H NMR (500 MHz, CDCl3 + TFA): δ 12.18–12.12 (m, 1H), 8.13 (s, 2H), 7.90 (s, 1H), 7.77 (s, 1H), 7.65 (s, 1H), 7.49 (s, 2H), 7.08 (s, 1H), 6.72 (s, 1H), 6.22 (s, 1H), 4.10 (s, 3H), 4.06 (s, 3H), 4.02 (s, 3H), 3.93 (s, 3H). 13C NMR (75 MHz, CDCl3 + TFA): δ 190.34, 172.12, 163.91, 154.81, 154.46, 146.13, 145.44, 143.82, 142.37, 133.19, 130.13, 120.82, 117.93, 116.34, 114.80, 111.00, 99.99, 91.32, 62.40, 56.43, 56.06. MS (ESI): m/z 522 [M + H]+; HRMS calcd for C26H24O7N3S[M + H]+ 522.13295, found 522.13227.
4.1.1.7.22. (Z)-1-(3,4-Dimethoxy-5-nitrophenyl)-3-((4-(5,7-dimethoxybenzo[d]thiazol-2-yl)-2-methylphenyl)amino)prop-2-en-1-one (6k)
Compound 6k was prepared according to the method described by 4-(5,7-dimethoxybenzo[d]thiazol-2-yl)-2-methylaniline (10b) (100 mg, 0.33 mmol) and 1-(3,4-dimethoxy-5-nitrophenyl)prop-2-yn-1-one (13d) (78 mg, 2.75 mmol) to obtain the pure product 6k as a yellow colour solid. (142 mg, 80% yield); mp: 246–248 °C; 1H NMR (500 MHz, CDCl3): δ 12.37–12.29 (m, 1H), 8.05 (s, 1H), 7.92 (d, J = 1.6 Hz, 2H), 7.76 (d, J = 1.6 Hz, 2H), 7.49 (s, 1H), 7.31 (d, J = 11.0 Hz, 1H), 7.10 (s, 1H), 6.69 (s, 1H), 6.30–6.23 (m, 1H), 4.05 (d, J = 17.7 Hz, 9H), 3.93 (s, 3H), 2.55 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 189.22, 163.61, 154.56, 154.30, 146.07, 144.34, 144.26, 143.84, 133.42, 131.11, 127.97, 127.86, 123.07, 120.03, 115.71, 114.43, 114.00, 110.66, 99.51, 62.10, 56.47, 56.44, 56.11, 17.25. MS (ESI): m/z 536 [M + H]+; HRMS calcd for C27H26O7N3S[M + H]+ 536.14132, found 536.1498.
4.1.1.7.23. (Z)-1-(3,4-Dimethoxy-5-nitrophenyl)-3-((4-(5,6,7-trimethoxybenzo[d]thiazol-2-yl)phenyl)amino)prop-2-en-1-one (5l)
Compound 5l was prepared according to the method described by 4-(5,6,7-trimethoxybenzo[d]thiazol-2-yl)aniline (9c) (100 mg, 0.31 mmol) and 1-(3,4-dimethoxy-5-nitrophenyl)prop-2-yn-1-one (13d) (74 mg, 2.75 mmol) to obtain the pure product 5l as a yellow colour solid. (144 mg, 83% yield); mp: 175–178 °C; 1H NMR (400 MHz, CDCl3): δ 12.22 (d, J = 12.3 Hz, 1H), 8.05 (d, J = 8.5 Hz, 2H), 7.88 (d, J = 1.6 Hz, 1H), 7.77 (d, J = 1.6 Hz, 1H), 7.62 (dd, J = 12.3, 7.9 Hz, 1H), 7.34 (s, 1H), 7.21 (d, J = 8.6 Hz, 2H), 6.05 (d, J = 7.9 Hz, 1H), 4.11 (s, 3H), 4.05 (s, 3H), 4.02 (s, 3H), 3.96 (d, J = 7.9 Hz, 6H). 13C NMR (100 MHz, CDCl3): δ 187.65, 166.89, 154.22, 154.10, 150.52, 146.76, 145.67, 144.75, 144.36, 141.73, 139.76, 134.25, 129.42, 128.90, 120.10, 116.51, 115.43, 114.31, 100.60, 93.97, 62.17, 61.51, 60.60, 56.60, 56.31. MS (ESI): m/z 552 [M + H]+; HRMS calcd for C27H26O8N3S[M + H]+ 552.14351, found 552.14277.
4.1.1.7.24. (Z)-1-(3,4-Dimethoxy-5-nitrophenyl)-3-((2-methyl-4-(5,6,7-trimethoxybenzo[d]thiazol-2-yl)phenyl)amino)prop-2-en-1-one (6l)
Compound 6l was prepared according to the method described by 2-methyl-4-(5,6,7-trimethoxybenzo[d]thiazol-2-yl)aniline (10c) (100 mg, 0.3 mmol) and 1-(3,4-dimethoxy-5-nitrophenyl)prop-2-yn-1-one (13d) (71 mg, 0.3 mmol) to obtain the pure product 6l as a yellow colour solid. (143 mg, 84% yield); mp: 232–234 °C; 1H NMR (500 MHz, CDCl3): δ 12.36 (d, J = 12.0 Hz, 1H), 7.95 (s, 1H), 7.92 (d, J = 8.4 Hz, 1H), 7.90 (d, J = 1.8 Hz, 1H), 7.79 (d, J = 1.7 Hz, 1H), 7.69 (dd, J = 12.0, 7.8 Hz, 1H), 7.34 (s, 1H), 7.29 (d, J = 8.5 Hz, 1H), 6.10 (d, J = 7.8 Hz, 1H), 4.11 (s, 3H), 4.05 (s, 3H), 4.02 (s, 3H), 3.96 (d, J = 9.4 Hz, 6H), 2.53 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 187.72, 167.22, 154.24, 154.07, 146.77, 145.61, 144.87, 144.38, 140.43, 134.40, 129.96, 129.04, 126.83, 126.54, 123.47, 120.08, 115.62, 115.44, 114.37, 113.79, 104.25, 100.58, 94.33, 62.17, 61.52, 60.59, 56.65, 56.32, 17.71. MS (ESI): m/z 566 [M + H]+; HRMS calcd for C26H24O7N3S[M + H]+ 566.15968, found 566.15829.
4.1.1.7.25. (Z)-3-((4-(6-Methoxybenzo[d]thiazol-2-yl)phenyl)amino)-1-(3,4,5-trimethoxyphenyl)prop-2-en-1-one (5m)
Compound 5m was prepared according to the method described by 4-(6-methoxybenzo[d]thiazol-2-yl)aniline (9a) (100 mg, 0.39 mmol) and 1-(3,4,5-trimethoxyphenyl)prop-2-yn-1-one (13e) (85 mg, 0.85 mmol) to obtain the pure product 5m as a yellow colour solid. (159 mg, 86% yield); mp: 208–210 °C; 1H NMR (300 MHz, CDCl3): δ 12.23 (d, J = 12.1 Hz, 1H), 8.02 (d, J = 8.6 Hz, 2H), 7.92 (d, J = 8.9 Hz, 1H), 7.54 (dd, J = 12.1, 8.0 Hz, 1H), 7.34 (d, J = 2.4 Hz, 1H), 7.21 (s, 2H), 7.17 (d, J = 8.7 Hz, 2H), 7.08 (dd, J = 8.9, 2.5 Hz, 1H), 6.05 (d, J = 8.0 Hz, 1H), 3.94 (d, J = 6.7 Hz, 9H), 3.89 (s, 4H). 13C NMR (75 MHz, CDCl3): δ 190.20, 164.64, 157.64, 153.06, 148.67, 143.54, 141.91, 141.47, 136.21, 134.25, 128.96, 128.80, 123.43, 116.10, 115.59, 104.68, 104.13, 94.58, 60.97, 56.23, 55.77. MS (ESI): m/z 477 [M + H]+; HRMS calcd for C26H25O5N2S[M + H]+ 477.14787, found 477.14705.
4.1.1.7.26. (Z)-3-((4-(6-Methoxybenzo[d]thiazol-2-yl)-2-methylphenyl)amino)-1-(3,4,5-trimethoxyphenyl)prop-2-en-1-one (6m)
Compound 6m was prepared according to the method described by 4-(6-methoxybenzo[d]thiazol-2-yl)-2-methylaniline (10a) (100 mg, 0.37 mmol) and 1-(3,4,5-trimethoxyphenyl)prop-2-yn-1-one (13e) (81 mg, 0.37 mmol) to obtain the pure product 6m as a yellow colour solid. (159 mg, 88% yield); mp: 207–209 °C; 1H NMR (300 MHz, CDCl3): δ 12.33 (d, J = 11.9 Hz, 1H), 7.91 (t, J = 7.8 Hz, 3H), 7.62 (dd, J = 11.7, 8.0 Hz, 1H), 7.35 (d, J = 2.3 Hz, 1H), 7.29–7.21 (m, 3H), 7.08 (dd, J = 8.9, 2.4 Hz, 1H), 6.10 (d, J = 7.9 Hz, 1H), 3.96 (s, 6H), 3.93 (s, 3H), 3.89 (s, 3H), 2.53 (s, 3H). 13C NMR (75 MHz, CDCl3): δ 190.38, 164.99, 157.65, 153.10, 148.73, 143.66, 141.53, 140.67, 136.22, 134.47, 129.89, 128.61, 126.55, 126.44, 123.39, 115.53, 113.34, 104.85, 104.23, 95.04, 60.97, 56.30, 55.80, 17.75. MS (ESI): m/z 491 [M + H]+; HRMS calcd for C27H27O5N2S[M + H]+ 491.16352, found 491.16282.
4.1.1.7.27. (Z)-3-((4-(5,7-Dimethoxybenzo[d]thiazol-2-yl)phenyl)amino)-1-(3,4,5-trimethoxyphenyl)prop-2-en-1-one (5n)
Compound 5n was prepared according to the method described by 4-(5,7-dimethoxybenzo[d]thiazol-2-yl)aniline (9b) (100 mg, 0.35 mmol) and 1-(3,4,5-trimethoxyphenyl)prop-2-yn-1-one (13e) (76 mg, 0.35 mmol) to obtain the pure product 5n as a yellow colour solid. (153 mg, 87% yield); mp: 175–177 °C; 1H NMR (500 MHz, CDCl3): δ 12.23 (d, J = 12.0 Hz, 1H), 8.06 (d, J = 8.3 Hz, 2H), 7.55 (dd, J = 12.1, 8.0 Hz, 1H), 7.26 (s, 1H), 7.23–7.15 (m, 4H), 6.49 (s, 1H), 6.06 (d, J = 7.8 Hz, 1H), 3.96 (d, J = 6.8 Hz, 9H), 3.93 (s, 3H), 3.90 (s, 3H). 13C NMR (75 MHz, CDCl3): δ 190.24, 168.34, 160.41, 155.85, 154.30, 153.09, 143.48, 142.21, 141.58, 134.26, 128.95, 116.11, 104.79, 97.43, 96.77, 94.69, 60.97, 56.26, 55.93, 55.77. MS (ESI): m/z 507[M + H]+; HRMS calcd for C27H27O6N2S[M + H]+ 507.15843, found 507.15661.
4.1.1.7.28. (Z)-3-((4-(5,7-Dimethoxybenzo[d]thiazol-2-yl)-2-methylphenyl)amino)-1-(3,4,5-trimethoxyphenyl)prop-2-en-1-one (6n)
Compound 6n was prepared according to the method described by 4-(5,7-dimethoxybenzo[d]thiazol-2-yl)-2-methylaniline (10b) (100 mg, 0.33 mmol) and 1-(3,4,5-trimethoxyphenyl)prop-2-yn-1-one (13e) (73 mg, 0.35 mmol) to obtain the pure product 6n as a yellow colour solid. (152 mg, 88% yield); mp: 180–182 °C; 1H NMR (500 MHz, CDCl3): δ 12.33 (d, J = 11.8 Hz, 1H), 7.96 (s, 1H), 7.93 (d, J = 8.5 Hz, 1H), 7.63 (dd, J = 11.8, 8.0 Hz, 1H), 7.26 (t, J = 4.2 Hz, 1H), 7.23 (s, 2H), 7.17 (d, J = 1.9 Hz, 1H), 6.49 (d, J = 1.9 Hz, 1H), 6.11 (d, J = 7.9 Hz, 1H), 3.96 (d, J = 4.6 Hz, 9H), 3.93 (s, 3H), 3.90 (s, 3H), 2.53 (s, 3H). 13C NMR (75 MHz, CDCl3): δ 190.37, 168.66, 160.40, 155.87, 154.30, 153.10, 143.57, 143.25, 141.55, 140.90, 134.45, 129.98, 128.53, 126.56, 115.93, 113.28, 104.86, 97.43, 96.71, 95.10, 60.96, 56.30, 55.93, 55.77, 17.73. MS (ESI): m/z 521 [M + H]+; HRMS calcd for C28H29O6N2S[M + H]+ 521.17408, found 521.17337.
4.1.1.7.29. (Z)-3-((4-(5,6,7-Trimethoxybenzo[d]thiazol-2-yl)phenyl)amino)-1-(3,4,5-trimethoxyphenyl)prop-2-en-1-one (5o)
Compound 5o was prepared according to the method described by 4-(5,6,7-trimethoxybenzo[d]thiazol-2-yl)aniline (9c) (100 mg, 0.31 mmol) and 1-(3,4,5-trimethoxyphenyl)prop-2-yn-1-one (13e) (69 mg, 0.31 mmol) to obtain the pure product 5o as a yellow colour solid. (148 mg, 88% yield); mp: 142–144 °C; 1H NMR (500 MHz, CDCl3): δ 12.24 (d, J = 12.1 Hz, 1H), 8.04 (d, J = 8.6 Hz, 2H), 7.56 (dd, J = 12.1, 8.0 Hz, 1H), 7.33 (s, 1H), 7.22 (s, 2H), 7.19 (d, J = 8.7 Hz, 2H), 6.07 (d, J = 8.0 Hz, 1H), 4.11 (s, 3H), 3.96 (s, 3H), 3.95 (d, J = 4.1 Hz, 9H), 3.93 (s, 3H). 13C NMR (75 MHz, CDCl3): δ 190.31, 167.06, 154.03, 153.10, 150.54, 146.74, 143.51, 142.17, 141.62, 139.70, 134.27, 128.86, 120.05, 116.18, 104.83, 100.58, 94.73, 61.49, 60.96, 60.56, 56.28. MS (ESI): m/z 537 [M + H]+; HRMS calcd for C28H29O7N2S[M + H]+ 537.16900, found 537.16716.
4.1.1.7.30. (Z)-3-((2-Methyl-4-(5,6,7-trimethoxybenzo[d]thiazol-2-yl)phenyl)amino)-1-(3,4,5-trimethoxyphenyl)prop-2-en-1-one (6o)
Compound 6o was prepared according to the method described by 2-methyl-4-(5,6,7-trimethoxybenzo[d]thiazol-2-yl)aniline (10c) (100 mg, 0.3 mmol) and 1-(3,4,5-trimethoxyphenyl)prop-2-yn-1-one (13e) (66 mg, 0.3 mmol) to obtain the pure product 6o as a yellow colour solid. (139 mg, 84% yield); mp: 173–175 °C; 1H NMR (300 MHz, CDCl3): δ 12.33 (d, J = 11.9 Hz, 1H), 7.92 (d, J = 11.2 Hz, 2H), 7.63 (dd, J = 11.8, 8.0 Hz, 1H), 7.34 (s, 1H), 7.27 (t, J = 4.2 Hz, 1H), 7.23 (s, 2H), 6.11 (d, J = 7.9 Hz, 1H), 4.11 (s, 3H), 3.98–3.92 (m, 16H), 2.53 (s, 3H). 13C NMR (75 MHz, CDCl3): δ 190.44, 167.38, 154.01, 153.12, 150.57, 146.76, 143.62, 141.58, 140.87, 139.66, 134.46, 129.91, 128.48, 126.59, 126.47, 120.02, 113.37, 104.88, 100.56, 95.15, 61.49, 60.96, 60.55, 56.31, 17.74. MS (ESI): m/z 551 [M + H]+; HRMS calcd for C29H31O7N2S[M + H]+ 551.18465, found 551.18456.
4.1.1.7.31. (Z)-1-(2-Bromo-3,4,5-trimethoxyphenyl)-3-((4-(6-methoxybenzo[d]thiazol-2-yl)phenyl)amino)prop-2-en-1-one (5p)
Compound 5p was prepared according to the method described by 4-(6-methoxybenzo[d]thiazol-2-yl)aniline (9a) (100 mg, 0.38 mmol) and 1-(2-bromo-3,4,5-trimethoxyphenyl)prop-2-yn-1-one (13f) (116 mg, 0.38 mmol) to obtain the pure product 5p as a yellow colour solid. (194 mg, 90% yield); mp: 202–204 °C; 1H NMR (300 MHz, CDCl3): δ 11.97 (d, J = 12.4 Hz, 1H), 8.04 (d, J = 8.6 Hz, 2H), 7.93 (d, J = 8.9 Hz, 1H), 7.54 (dd, J = 12.3, 7.9 Hz, 1H), 7.36 (d, J = 2.4 Hz, 1H), 7.21 (d, J = 8.6 Hz, 2H), 7.09 (dd, J = 8.9, 2.5 Hz, 1H), 6.87 (s, 1H), 5.80 (d, J = 7.8 Hz, 1H), 3.93 (d, J = 2.4 Hz, 6H), 3.90 (d, J = 3.5 Hz, 6H). 13C NMR (125 MHz, CDCl3): δ 193.10, 164.60, 157.73, 152.83, 151.10, 148.72, 144.47, 143.57, 141.79, 137.89, 136.27, 129.35, 128.85, 123.52, 116.45, 115.66, 108.08, 106.48, 104.23, 99.08, 61.21, 61.11, 56.25, 55.84. MS (ESI): m/z 577 [M + Na]+; HRMS calcd for C26H23O5N2SBr[M + Na]+ 577.04033, found 577.03935.
4.1.1.7.32. (Z)-1-(2-Bromo-3,4,5-trimethoxyphenyl)-3-((4-(6-methoxybenzo[d]thiazol-2-yl)-2-methylphenyl)amino)prop-2-en-1-one (6p)
Compound 6p was prepared according to the method described by 4-(6-methoxybenzo[d]thiazol-2-yl)-2-methylaniline (10a) (100 mg, 0.37 mmol) and 1-(2-bromo-3,4,5-trimethoxyphenyl)prop-2-yn-1-one (13f) (110 mg, 0.37 mmol) to obtain the pure product 6p as a yellow colour solid. (180 mg, 86% yield); mp: 187–189 °C; 1H NMR (500 MHz, CDCl3): δ 12.15 (d, J = 12.1 Hz, 1H), 7.94–7.91 (m, 2H), 7.91–7.88 (m, 1H), 7.60 (dd, J = 12.1, 7.8 Hz, 1H), 7.35 (d, J = 2.5 Hz, 1H), 7.28–7.25 (m, 1H), 7.09 (dd, J = 8.9, 2.5 Hz, 1H), 6.90 (s, 1H), 5.86 (d, J = 7.6 Hz, 1H), 3.93 (d, J = 5.4 Hz, 6H), 3.89 (s, 6H), 2.52 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 193.06, 164.94, 157.68, 152.85, 151.05, 148.72, 144.44, 143.66, 140.46, 137.99, 136.24, 129.95, 128.93, 126.78, 126.48, 123.44, 115.60, 113.62, 108.16, 106.47, 104.24, 99.51, 61.22, 61.12, 56.26, 55.84, 17.70. MS (ESI): m/z 591 [M + Na]+; HRMS calcd for C27H25O5N2SBr[M + Na]+ 591.05598, found 591.05626.
4.1.1.7.33. (Z)-1-(2-Bromo-3,4,5-trimethoxyphenyl)-3-((4-(5,7-dimethoxybenzo[d]thiazol-2-yl)phenyl)amino)prop-2-en-1-one (5q)
Compound 5q was prepared according to the method described by 4-(5,7-dimethoxybenzo[d]thiazol-2-yl)aniline (9b) (100 mg, 0.35 mmol) and 1-(2-bromo-3,4,5-trimethoxyphenyl)prop-2-yn-1-one (13f) (104 mg, 0.35 mmol) to obtain the pure product 5q as a yellow colour solid. (179 mg, 88% yield); mp: 193–194 °C; 1H NMR (300 MHz, CDCl3): δ 11.98 (d, J = 12.5 Hz, 1H), 8.08 (d, J = 8.5 Hz, 2H), 7.55 (dd, J = 12.3, 7.9 Hz, 1H), 7.26–7.16 (m, 3H), 6.89 (s, 1H), 6.52 (s, 1H), 5.81 (d, J = 7.8 Hz, 1H), 3.98 (s, 3H), 3.94 (d, J = 2.3 Hz, 6H), 3.91 (d, J = 4.8 Hz, 6H). 13C NMR (125 MHz, DMSO): δ 188.37, 163.53, 155.69, 151.11, 149.58, 148.08, 146.35, 139.73, 138.75, 137.28, 133.13, 124.53, 124.22, 111.67, 111.26, 103.33, 101.72, 94.40, 92.70, 92.09, 56.46, 56.36, 51.50, 51.23, 51.06. MS (ESI): m/z 585 [M + H]+; HRMS calcd for C27H26O6N2SBr[M + H]+ 585.06895, found 585.06795.
4.1.1.7.34. (Z)-1-(2-Bromo-3,4,5-trimethoxyphenyl)-3-((4-(5,7-dimethoxybenzo[d]thiazol-2-yl)-2-methylphenyl)amino)prop-2-en-1-one (6q)
Compound 6q was prepared according to the method described by 4-(5,7-dimethoxybenzo[d]thiazol-2-yl)-2-methylaniline (10b) (100 mg, 0.33 mmol) and 1-(2-bromo-3,4,5-trimethoxyphenyl)prop-2-yn-1-one (13f) (99 mg, 0.33 mmol) to obtain the pure product 6q as a yellow colour solid. (165 mg, 83% yield); mp: 222–223 °C; 1H NMR (300 MHz, CDCl3): δ 12.16 (d, J = 12.0 Hz, 1H), 7.98 (s, 1H), 7.95 (d, J = 8.5 Hz, 1H), 7.62 (dd, J = 12.0, 7.8 Hz, 1H), 7.29 (d, J = 6.8 Hz, 1H), 7.19 (d, J = 1.7 Hz, 1H), 6.91 (s, 1H), 6.51 (d, J = 1.7 Hz, 1H), 5.88 (d, J = 7.7 Hz, 1H), 3.98 (s, 3H), 3.94 (d, J = 3.3 Hz, 6H), 3.92 (d, J = 1.5 Hz, 6H), 2.54 (s, 3H). 13C NMR (125 MHz, CDCl3): δ 193.10, 168.64, 160.43, 155.85, 154.33, 152.85, 151.06, 144.44, 143.61, 140.71, 137.99, 130.06, 128.86, 126.78, 126.61, 115.97, 113.59, 108.15, 106.46, 99.58, 97.41, 96.79, 61.22, 61.12, 56.26, 55.98, 55.82, 17.69. MS (ESI): m/z 601 [M + H]+; HRMS calcd for C28H28O6N2SBr[M + H]+ 601.0773, found 601.0832.
4.1.1.7.35. (Z)-1-(2-Bromo-3,4,5-trimethoxyphenyl)-3-((4-(5,6,7-trimethoxybenzo[d]thiazol-2-yl)phenyl)amino)prop-2-en-1-one (5r)
Compound 5r was prepared according to the method described by 4-(5,6,7-trimethoxybenzo[d]thiazol-2-yl)aniline (9c) (100 mg, 0.31 mmol) and 1-(2-bromo-3,4,5-trimethoxyphenyl)prop-2-yn-1-one (13f) (94 mg, 0.31 mmol) to obtain the pure product 5r as a yellow colour solid. (172 mg, 89% yield); mp: 230–233 °C; 1H NMR (500 MHz, CDCl3): δ 11.96 (d, J = 12.3 Hz, 1H), 8.05 (d, J = 8.6 Hz, 2H), 7.53 (dd, J = 12.4, 7.8 Hz, 1H), 7.34 (s, 1H), 7.21 (d, J = 8.7 Hz, 2H), 6.87 (s, 1H), 4.11 (s, 2H), 3.97 (s, 3H), 3.95 (s, 3H), 3.93 (d, J = 4.0 Hz, 6H), 3.89 (s, 3H). 13C NMR (75 MHz, CDCl3): δ 193.15, 167.01, 154.04, 152.82, 151.08, 150.53, 146.76, 144.45, 143.56, 143.43, 141.96, 139.70, 137.86, 129.18, 128.92, 128.79, 116.46, 108.10, 107.96, 106.45, 100.59, 100.52, 100.05, 99.23, 99.08, 98.25, 61.59, 61.45, 56.37, 56.32, 56.24, 56.17. MS (ESI): m/z 637 [M + Na]+; HRMS calcd for C28H27O7N2SBr[M + Na]+ 637.06144, found 637.06012.
4.1.1.7.36. (Z)-1-(2-Bromo-3,4,5-trimethoxyphenyl)-3-((2-methyl-4-(5,6,7-trimethoxybenzo[d]thiazol-2-yl)phenyl)amino)prop-2-en-1-one (6r)
Compound 6r was prepared according to the method described by 4-(5,6,7-trimethoxybenzo[d]thiazol-2-yl)aniline (10c) (100 mg, 0.30 mmol) and 1-(2-bromo-3,4,5-trimethoxyphenyl)prop-2-yn-1-one (13f) (90 mg, 0.30 mmol) to obtain the pure product 6r as a yellow colour solid. (171 mg, 90% yield); mp: 178–179 °C; 1H NMR (300 MHz, CDCl3): δ 12.14 (d, J = 11.8 Hz, 1H), 7.96–7.87 (m, 2H), 7.61 (dd, J = 11.9, 7.9 Hz, 1H), 7.35–7.24 (m, 2H), 6.89 (s, 1H), 5.87 (d, J = 7.6 Hz, 1H), 4.11 (s, 3H), 3.99–3.87 (m, 15H), 2.53 (s, 3H). 13C NMR (100 MHz, CDCl3): δ 193.07, 167.34, 154.01, 152.80, 151.01, 150.41, 146.71, 144.41, 143.53, 140.64, 139.65, 137.91, 129.93, 126.78, 126.46, 119.97, 113.60, 108.10, 106.41, 100.48, 99.56, 61.46, 61.15, 61.05, 60.54, 56.27, 17.63. MS (ESI): m/z 651 [M + Na]+; HRMS calcd for C29H29O7N2SBr[M + Na]+ 651.07711, found 651.07834.
4.2. Biology
4.2.1. Cell cultures, maintenance and anti-proliferative evaluation
The cell lines, A549, HeLa, MDAMB-231 and MIA PaCa-2 (lung, cervical, breast, and pancreatic) used in this study were procured from American Type Culture Collection (ATCC), USA. The synthesized test compounds were evaluated for their in vitro anti-proliferative activity in these four different human cancer cell lines. A protocol of 48 h continuous drug exposure was used, and an SRB cell proliferation assay was used to estimate cell viability or growth. All the cell lines were grown in Dulbecco's modified Eagle's medium (containing 10% FBS in a humidified atmosphere of 5% CO2 at 37 °C). Cells were trypsinized when sub-confluent from T25 flasks/60 mm dishes and seeded in 96-well plates in 100 μL aliquots at plating densities depending on the doubling time of individual cell lines. The microtitre plates were incubated at 37 °C, 5% CO2, 95% air, and 100% relative humidity for 24 h prior to the addition of experimental drugs and were incubated for 48 h with different doses (0.01, 0.1, 1, 10, 100 μM) of the prepared derivatives. After incubation at 37 °C for 48 h, the cell monolayers were fixed by the addition of 10% (wt/vol) cold trichloroacetic acid and incubated at 4 °C for 1 h and were then stained with 0.057% SRB dissolved in 1% acetic acid for 30 min at room temperature. Unbound SRB was washed with 1% acetic acid. The protein-bound dye was dissolved in 10 mM Tris base solution for OD determination at 510 nm using a microplate reader (Enspire, Perkin Elmer, USA). Using the seven absorbance measurements [time zero (Tz), control growth (C), and test growth in the presence of drug at the five concentration levels (Ti)], the percentage growth was calculated at each of the drug concentration levels. Percentage growth inhibition was calculated as follows: [(Ti – Tz)/Tz] × 100 for concentrations for which Ti < Tz. The dose-response parameter, growth inhibition of 50% (GI50), was calculated from [(Ti – Tz)/(IJC – Tz)] × 100 = 50, which is the drug concentration resulting in a 50% reduction in the net protein increase (as measured by SRB staining) in control cells during the drug incubation. Values were calculated for this parameter if the level of activity is reached; however, if the effect is not reached or is exceeded, the value for that parameter was expressed as greater or less than the maximum or minimum concentration tested.
4.2.2. Analysis of cell cycle
HeLa cells in 60 mm dishes were incubated for 24 h in the presence or absence of test compounds 5d, 6d and nocodazole (3 μM). Cells were harvested with trypsin–EDTA, fixed with ice-cold 70% ethanol at 4 °C for 30 min, ethanol was removed by centrifugation and cells were stained with 1 mL of DNA staining solution [0.2 mg of propidium iodide (PI), and 2 mg RNase A] for 30 min as described earlier. The DNA contents of 20 000 events were measured by flow cytometer (BD FACSC anto II). Histograms were analyzed using FCS express 4 plus.35
4.2.3. Dot-blot assay
Cells were trypsinized when sub-confluent from T25 flasks/60 mm dishes and seeded in 6-well plates. These conjugates (5d and 6d) were evaluated for their activity against cyclin B1. HeLa cells were treated with 3 μM concentrations of 5d, 6d and nocodazole for 24 h. Subsequently, cells were harvested and proteins were quantified using Amido Black followed by densitomerty analysis. Equal amount of protein were blotted on nitrocellulose membrane using Bio-Dot SF microfiltration apparatus (Bio-Rad). Briefly, nitrocellulose membrane and 3 filters papers (Whatmann 3) were soaked in IX TBS solution for 10 min. Later, the filter papers, membrane were arranged in the apparatus and connected to vacuum pump (Millipore). The membranes were rehydrated using 100 μl of 1× TBS by vacuum filtration. Subsequently, 50 μl volumes of equal protein samples were blotted on the membrane and washed with 200 μl of 1× TBS through application of vacuum . The blot was blocked with 5% blotto for 1 h at room temperature. Immunoblot analysis was performed using UVP, biospectrum 810 imaging system. Semi-quantitative reverse transcription PCR (RT-PCR)-Total RNA was extracted using RNase mini kit (Qiagen) and reverse transcribed into cDNA using superscript II reverse transcriptase (Invitrogen life technologies). The PCR primers for cyclin B1 were 5′-CTACCTTTGCACTTCCTTCGG-3′ as forward and 5′-CCTGCTGCAATTTGAGAAGG-3′ as reverse primers. For GAPDH, the forward primer was 5′-CAAGGTCATCCATGACAACTTTG-3′ and the reverse was 5′-CTTCACCACCTTCTTGATGTCATC-3′. The PCR conditions for cyclin B1 and GAPDH were, 95 °C 2 minutes, followed by 21 cycles of 95 °C-15 s, 60 °C-30 s, and 72 °C-30 s. GAPDH was used as an internal control. The products were electrophoresed on agarose gel (2.5%) followed by staining with ethidium bromide and visualized under U.V. light. The signal intensity of respective bands was measured by means of the quantity one version 4.1.1 software using Bio-Rad image analysis system.25
4.2.4. Tubulin polymerization assay
An in vitro assay for monitoring the time-dependent polymerization of tubulin to microtubules was performed employing a fluorescence-based tubulin polymerization assay kit (BK011, Cytoskeleton, Inc.) according to the manufacturer's protocol. The reaction mixture in a final volume of 10 μl in PEM buffer (80 mM PIPES, 0.5 mM EGTA, 2 mM MgCl2, pH 6.9) in 384 well plates contained 2 mg mL–1 bovine brain tubulin, 10 μM fluorescent reporter, 1 mM GTP in the presence or absence of test compounds at 37 °C. Tubulin polymerization was followed by monitoring the fluorescence enhancement due to the incorporation of a fluorescence reporter into microtubules as polymerization proceeds. Fluorescence emission at 420 nm (excitation wavelength is 360 nm) was measured for 1 h at 1 min intervals in a multimode plate reader (Tecan M200). Nocodazole was used as positive control under similar experimental conditions. To determine the IC50 values of the conjugates against tubulin polymerization, the conjugates were pre-incubated with tubulin at varying concentrations (1, 2, 3, 4 and 5 μM).
4.2.5. Immunohistochemistry of tubulin and analysis of nuclear morphology
HeLa cells were seeded on glass cover slip, incubated for 24 h in the presence or absence of test conjugates 5d and 6d (3 μM). Cells grown on cover slips were fixed in 3.5% formaldehyde in phosphate-buffered saline (PBS) pH 7.4 for 10 minutes at room temperature. Cells were permeabilized for 6 minutes in PBS containing 0.5% Triton X-100 (Sigma) and 0.05% Tween-20 (Sigma). The permeabilized cells were blocked with 2% BSA (Sigma) in PBS for 1 h. Later, the cells were incubated with primary antibody for tubulin from (sigma) at (1 : 200) diluted in blocking solution for 4 h at room temperature. Subsequently the antibodies were removed and the cells were washed thrice with PBS. Cells were then incubated with FITC labeled anti-mouse secondary antibody (1 : 500) for 1 h at room temperature. Cells were washed thrice with PBS and mounted in medium containing DAPI. Images were captured using the Olympus confocal microscope and analyzed with Provision software.
4.2.6. Western blot analysis of soluble versus polymerized tubulin
Cells were seeded in 12-well plates at 1 × 105 cells per well in complete growth medium. Following treatment of cells with respective compounds (5d, 6d, nocodazole and paclitaxel) for a duration of 24 h, cells were washed with PBS and subsequently soluble and insoluble tubulin fractions were collected. To collect the soluble tubulin fractions, cells were permeabilized with 200 μL of pre-warmed lysis buffer [80 mM Pipes-KOH (pH 6.8), 1 mM MgCl2, 1 mM EGTA, 0.2% Triton X-100, 10% glycerol, 0.1% protease inhibitor cocktail (Sigma-Aldrich)] and incubated for 3 min at 30 °C. Lysis buffer was gently removed, and mixed with 100 μL of 3 × Laemmli's sample buffer (180 mM Tris-Cl pH 6.8, 6% SDS, 15% glycerol, 7.5% β-mercaptoethanol and 0.01% bromophenol blue). Samples were immediately heated to 95 °C for 3 min. To collect the insoluble tubulin fraction, 300 μL of 1 × Laemmli's sample buffer was added to the remaining cells in each well, and the samples were heated to 95 °C for 3 min. Equal volumes of samples were run on an SDS-10% polyacrylamide gel and were transferred to a nitrocellulose membrane employing semidry transfer at 50 mA for 1 h. Blots were probed with mouse anti-human α-tubulin diluted 1 : 2000 ml (Sigma) and stained with rabbit anti-mouse secondary antibody coupled with horseradish peroxidase, diluted 1 : 5000 ml (Sigma). Bands were visualized using an enhanced chemiluminescence protocol (Pierce) and radiographic film (Kodak).
4.2.7. Hoechst staining
HeLa cells (5 × 104 cells per well) at their growth phase were seeded in 12 well plates and allowed to adhere for 24 h. The culture medium containing the test conjugates 5d and 6d at 1 μM concentration were added to the cells and incubated for 24 h at 37 °C. After 24 h, culture medium was removed; cells were washed with PBS and fixed with 4% para formaldehyde solution at 4 °C for 10 min. The cells were washed twice with PBS and stained with Hoechst 33 242 (5 μg mL–1) for 30 min at room temp. The excess dye was removed by washing twice with PBS and cells were examined for morphological changes under fluorescence microscope using 350 nm excitation and 460 nm emission (Nikon, magnification 10×).
4.2.8. Measurement of mitochondrial membrane potential (MMP)
HeLa cells were cultured in 6 well plates at a density of 5 × 105 cells per mL and allowed to grow over night. The cells were treated with the test conjugates 5d and 6d with 1 μM concentration for 24 h. After 24 h treatment, the adherent cells were collected by trypsinisation, washed with PBS and resuspended in solution of PBS containing Rhodamine-123 (10 μg mL–1). After incubation for 30 min at room temp, cells were washed twice with PBS and resuspended in PBS. The samples were analyzed for fluorescence using spectroflourimeter.
4.2.9. Measurement of reactive oxygen species (ROS) levels
Intracellular ROS levels were measured by using carboxy-2′,7′-dichlorofluorescin diacetate (carboxy-DCFH-DA). U87MG cells were plated in 6 well plates at a density of 5×105 cells per mL and allowed to grow overnight. The cells were incubated with the at 1 μM concentration of the conjugates 5d and 6d for 24 h. After treatment, the medium was replaced with the culture medium containing DCFH-DA (10 μM) and further incubated for 30 min at room temperature in dark. Cells were washed with PBS, collected from the plate and resuspended in PBS at 5 × 104 cells per mL. The fluorescence intensity of the 2′,7′-dichlorofluorscein formed by the hydrolysis of carboxy-DCFH-DA from each sample was analyzed by spectroflourimeter at an excitation and emission wavelength of 488 and 525 nm, respectively. Qualitative cellular fluorescence images were captured by using Nikon ECLIPSETE2000-S fluorescence microscope.
Acknowledgments
The authors A. V. S. R., S. P. S., B. S. would like to thank to CSIR, New Delhi, India for the award of research fellowships and S. S. acknowledge the CSIR-UGC, New Delhi (India), for the award of senior research fellowships. The authors are also thankful to CSIR for financial support under the 12th Five Year plan project “Affordable Cancer Therapeutics (ACT)” (CSC0301).
Footnotes
†The authors declare no competing interests.
References
- Jordan M. A., Wilson L. Nat. Rev. Cancer. 2004;4:253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- Jordan M. Curr. Med. Chem.: Anti-Cancer Agents. 2002;2:1–17. doi: 10.2174/1568011023354290. [DOI] [PubMed] [Google Scholar]
- Hsieh H. P., Liou J. P., Lin Y. T., Mahindroo N., Chang J. Y., Yang Y. N., Chern S. S., Tan U. K., Chang C. W., Chen T. W., Lin C. H., Chang Y. Y., Wang C. C. Bioorg. Med. Chem. Lett. 2003;13:101–105. doi: 10.1016/s0960-894x(02)00850-8. [DOI] [PubMed] [Google Scholar]
- Belotti D., Vergani V., Drudis T., Borsotti P., Pitelli M. R., Viale G., Giavazzi R., Taraboletti G. Clin. Cancer Res. 1996;2:1843–1849. [PubMed] [Google Scholar]
- Mekhail T. M., Markman M. Expert Opin. Pharmacother. 2002;3:755–766. doi: 10.1517/14656566.3.6.755. [DOI] [PubMed] [Google Scholar]
- Rowinsky E. K., Donehower R. C. Pharmacol. Ther. 1991;52:35–84. doi: 10.1016/0163-7258(91)90086-2. [DOI] [PubMed] [Google Scholar]
- Prota A. E., Bargsten K., Northcote P. T., Marsh M., Altmann K. H., Miller J. H., Diaz J. F., Steinmetz M. O. Angew. Chem., Int. Ed. 2014;53:1621–1625. doi: 10.1002/anie.201307749. [DOI] [PubMed] [Google Scholar]
- Nam N. H. Curr. Med. Chem. 2003;10:1697–1722. doi: 10.2174/0929867033457151. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya B., Panda D., Gupta S., Banerjee M. Med. Res. Rev. 2008;28:155–183. doi: 10.1002/med.20097. [DOI] [PubMed] [Google Scholar]
- Jordan A., Hadfield J. A., Lawrence N. J., McGown A. T. Med. Res. Rev. 1998;18:259–296. doi: 10.1002/(sici)1098-1128(199807)18:4<259::aid-med3>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- (a) Duanmu C., Shahrik L. K., Holly H. H., Hamel E. Cancer Res. 1989;49:1344–1348. [PubMed] [Google Scholar]; (b) Vasquez R. J., Howell B., Yvon A. M., Wadsworth P., Cassimeris L. Mol. Biol. Cell. 1997;8:973–985. doi: 10.1091/mbc.8.6.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban M., Taguchi H., Katsushima T., Takahashi M., Shinoda K., Watanabe A., Tominaga T. Bioorg. Med. Chem. 1998;6:1069–1076. doi: 10.1016/s0968-0896(98)00065-0. [DOI] [PubMed] [Google Scholar]
- Papadopoulou C., Geronikaki A., Hadjipavlou-Litina D. Farmaco. 2005;60:969–973. doi: 10.1016/j.farmac.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Chung Y., Shin Y. K., Zhan C. G., Lee S., Cho H. Arch. Pharmacal Res. 2004;27:893–900. doi: 10.1007/BF02975839. [DOI] [PubMed] [Google Scholar]
- McFadyen M. C. E., Melvin W. T., Murray G. I. Mol. Cancer Ther. 2004;3:363–371. [PubMed] [Google Scholar]
- Yoshida M., Hayakawa I., Hayashi N., Agatsuma T., Oda Y., Tanzawa F., Iwasaki S., Koyama K., Furukawa H., Kurakata S. Bioorg. Med. Chem. Lett. 2005;15:3328–3332. doi: 10.1016/j.bmcl.2005.05.077. [DOI] [PubMed] [Google Scholar]
- Starčević K., Ćaleta I., Cinčić D., Kaitner B., Kralj M., Ester K., Karminski-Zamola G. Heterocycles. 2006;68:2285–2299. [Google Scholar]
- Ćaleta I., Kralj M., Bertoša B., Tomić S., Pavlović G., Pavelić K., Karminski-Zamola G. J. Med. Chem. 2009;52:1744–1756. doi: 10.1021/jm801566q. [DOI] [PubMed] [Google Scholar]
- Shi D. F., Bradshaw T. D., Wrigley S., McCall C. J., Lelieveld P., Fichtner I., Stevens M. F. G. J. Med. Chem. 1996;37:3375–3384. doi: 10.1021/jm9600959. [DOI] [PubMed] [Google Scholar]
- Leong C. O., Suggitt M., Swaine D. J., Bibby M. C., Stevens M. F. G., Bradshaw T. D. Mol. Cancer Ther. 2004;3:1565–1575. [PubMed] [Google Scholar]
- Hutchinson I., Jennings S. A., Vishnuvajjala B. R., Westwell A. D., Stevens M. F. G. J. Med. Chem. 2002;45:744–747. doi: 10.1021/jm011025r. [DOI] [PubMed] [Google Scholar]
- Subba Rao A. V., Swapna K., Shaik S. P., Lakshma Nayak V., Srinivasa Reddy T., Sunkari S., Shaik T. B., Bagul C., Kamal A. Bioorg. Med. Chem. 2017;25:977–999. doi: 10.1016/j.bmc.2016.12.010. [DOI] [PubMed] [Google Scholar]
- Kamal A., Subba Rao A. V., Lakshma Nayak V., Subba Reddy N. V., Swapna K., Ramakrishna Mallika Alvala G. Org. Biomol. Chem. 2014;12:9864–9880. doi: 10.1039/c4ob01930j. [DOI] [PubMed] [Google Scholar]
- Kamal A., Subba Rao A. V., Vishnuvardhan M. V. P. S., Srinivas Reddy T., Swapna K., Subba Reddy N. V., Bagul C., Srinivasulu V. Org. Biomol. Chem. 2015;13:4879–4895. doi: 10.1039/c5ob00232j. [DOI] [PubMed] [Google Scholar]
- Kamal A., Subba Rao A. V., Srinivas Reddy T., Polepalli S., Pasha Shaik S., Bagul C., Vishnuvardhan M. V. P. S. MedChemComm. 2015;6:1842–1856. [Google Scholar]
- Chan K. T., Meng F. Y., Li Q., Ho C. Y., Lam T. S., To Y., Lee W. H., Li M., Chu K. H., Toh M. Cancer Lett. 2010;294:118–124. doi: 10.1016/j.canlet.2010.01.029. [DOI] [PubMed] [Google Scholar]
- Shen J. K., Du H. P., Yang M., Wang Y. G., Jin J. Ann. Hematol. 2009;88:743–752. doi: 10.1007/s00277-008-0677-3. [DOI] [PubMed] [Google Scholar]
- Blank M., Shiloh Y. Cell Cycle. 2007;6:686–695. doi: 10.4161/cc.6.6.3990. [DOI] [PubMed] [Google Scholar]
- Maity H. A., McKenna W. G., Muschel R. J. J. Biol. Chem. 1995;270:28419–28424. doi: 10.1074/jbc.270.47.28419. [DOI] [PubMed] [Google Scholar]
- Wolfe A. L., Duncan K. K., Parelkar N. K., Weir S. J., Vielhauer G. A., Boger D. L. J. Med. Chem. 2012;55:5878–5886. doi: 10.1021/jm300330b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. D. Genes Dev. 2001;15:2922–2933. [PubMed] [Google Scholar]
- Miao R. D., Han Y., An L. Z., Yang J. B., Wang Q. Cell Biol. Int. 2008;32:217–223. doi: 10.1016/j.cellbi.2007.08.034. [DOI] [PubMed] [Google Scholar]
- Simon H. U., Yehia A. H., Schaffer F. L. Apoptosis. 2000;5:415–418. doi: 10.1023/a:1009616228304. [DOI] [PubMed] [Google Scholar]
- LeBel C. P., Ischiropoulos H., Bondy S. C. Chem. Res. Toxicol. 1992;5:227–231. doi: 10.1021/tx00026a012. [DOI] [PubMed] [Google Scholar]
- Riccardi C., Nicoletti I. Nat. Protoc. 2006;13:1458–1461. doi: 10.1038/nprot.2006.238. [DOI] [PubMed] [Google Scholar]