Abstract
Background:
Organophosphorus (OP) compounds are the most widely used group of insecticides in the world. Risk assessments for these chemicals have focused primarily on 10% inhibition of acetylcholinesterase in the brain as the critical metric of effect. Aside from cholinergic effects resulting from acute exposure, many studies suggest a linkage between cognitive deficits and long-term OP exposure.
Objective:
In this proof-of-concept study, we focused on one of the most widely used OP insecticides in the world, chlorpyrifos (CPF), and utilized an existing physiologically based pharmacokinetic (PBPK) model and a novel pharmacodynamic (PD) dose–response model to develop a point of departure benchmark dose estimate for cognitive deficits following long-term, low-dose exposure to this chemical in rodents.
Methods:
Utilizing a validated PBPK/PD model for CPF, we generated a database of predicted biomarkers of exposure and internal dose metrics in both rat and human. Using simulated peak brain CPF concentrations, we developed a dose–response model to predict CPF-induced spatial memory deficits and correlated these changes to relevant biomarkers of exposure to derive a benchmark dose specific to neurobehavioral changes. We extended these cognitive deficit predictions to humans and simulated corresponding exposures using a model parameterized for humans.
Results:
Results from this study indicate that the human-equivalent benchmark dose (BMD) based on a 15% cognitive deficit as an end point is lower than that using the present threshold for 10% brain AChE inhibition. This predicted human-equivalent subchronic BMD threshold compares to occupational exposure levels determined from biomarkers of exposure and corresponds to similar exposure conditions where deficits in cognition are observed.
Conclusions:
Quantitative PD models based on neurobehavioral testing in animals offer an important addition to the methodologies used for establishing useful environmental public health indicators and BMDs, and predictions from such models could help inform the human health risk assessment for chlorpyrifos. https://doi.org/10.1289/EHP1743
Introduction
Organophosphorus (OP) insecticides are among the most widely used synthetic chemicals for the control of agricultural and domestic insect pests. Approximately 70% of the insecticides in current use in the United States are OP-based, which amounts to a total of approximately of these chemicals applied each year (U.S. EPA 2011). OP insecticides phosphorylate numerous enzymes including a large number of B-esterases whose primary function is to hydrolyze choline-based esters such as acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE) (Chambers and Levi 1992). These esterases are present throughout the body, with high abundances in the plasma, hematocrit, and brain. The primary mechanism of action of OP insecticide–induced toxicity is the inhibition of AChE by active oxon metabolites, resulting in the accumulation of acetylcholine neurotransmitter within the cholinergic synapses (Casida 2009), resulting in cholinergic toxicity due to continuous stimulation of cholinergic receptors throughout the central and peripheral nervous system. Symptoms of acute exposure to organophosphates or similar cholinesterase-inhibiting compounds may include headache, dizziness, numbness, tremor, nausea, sweating, blurred vision, respiratory depression, and slow heartbeat (Calvert et al. 2008; Eaton et al. 2008). Very high doses may result in unconsciousness, incontinence, and convulsions or fatality (Eaton et al. 2008).
Currently, the most sensitive biomarker of effect of exposure to a variety of OP insecticides, in both animals and humans, is the inhibition of cholinesterases (Clegg and van Gemert 1999; Nigg and Knaak 2000; Reiss et al. 2012). With cholinergic biochemical changes as the primary end point of toxicity, risk assessments have been conducted to determine benchmark doses corresponding to a given percentage of cholinesterase inhibition. In its most comprehensive OP insecticide cumulative risk assessment update, the U.S. Environmental Protection Agency (EPA) elected to use 10% AChE inhibition in the brain () as the point of departure (PoD) response level, stating that “The 10% response level is health protective in that no functional or behavioral effects have been noted below this level in adult or juvenile animals” (U.S. EPA 2006). In addition to the formal 10% brain AChE inhibition guideline set by the U.S. EPA for organophosphorus insecticide exposure, Reiss et al. considered a 20% AChE inhibition in red blood cells (RBC) as a reasonable guideline for protection against OP insecticide toxicity. Following consideration of a number of additional studies (U.S. EPA 2014, 2015, 2016b), the U.S. EPA’s 2016 revised human health risk assessment indicated that additional, more sensitive, end points may exist and should be considered in assessing risk associated with exposure to this chemical (U.S. EPA 2016c). In particular, this realization has led to increased efforts to understand the risk associated with CPF exposure during prenatal and postnatal windows of exposure and the potential neurodevelpomental outcomes associated with early exposure and to the consideration of appropriate biomarkers of effect linked to adverse neurodevelopmental outcomes (U.S. EPA 2016b, 2016c). In reviewing the available information and potential risks from CPF exposure, the U.S. EPA’s updated assessment stated, “The agency has endeavored to derive PoDs and uncertainty/safety factors for risk assessment that are protective of both AChE inhibition and any adverse effects that could occur at lower doses” (U.S. EPA 2016c).
Although the methodology for developing PoDs for neurodevelopmental end points is still under consideration (U.S. EPA 2016c), the method for determining the PoD benchmark doses in the context of biochemical changes commonly involves developing a cholinesterase inhibition dose–response curve following OP exposure in rats. Specifically, using an appropriate pharmacodynamic relationship between known external dose and resulting cholinesterase inhibition, a benchmark dose, usually defined for oral, dermal, or inhalation exposure in mg/kg/day, is estimated based on the designated biomarker of effect level: for example, 10% AChE inhibition in the brain. These dose–response results in rodents are then extrapolated to humans to derive acceptable exposure thresholds. Within the context of developing neurodevelopmental PoDs, the most recent Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) Science Advisory Panel (SAP) on chlorpyrifos aimed to expand the current understanding of neurodevelopmental effects following prenatal exposure to CPF and considered whether an internal dose metric might be used to determine a relevant PoD (U.S. EPA 2016b). Although the members of the FIFRA SAP did not reach a consensus on the appropriate measure for setting a neurodevelopmental PoD, multiple members embraced the effort to utilize physiologically based pharmacokinetic (PBPK) modeling to predict time-weighted averages of internal dosimetry from relevant exposure scenarios.
In addition to the studies associating neurodevelopmental effects and CPF exposure, two recent reviews concluded that the weight of evidence supports adverse neurobehavioral effects of chronic low-dose exposures to OP insecticides in occupational settings (Rohlman et al. 2011; Ross et al. 2013). For their analyses, these two reviews cited studies that utilized neuropsychological testing to uncover impairments to a variety of neurobehavioral functions, such as memory (visual, working, and auditory), perception, and information processing (Blanc-Lapierre et al. 2013; Farahat et al. 2003; Mackenzie Ross et al. 2010; Reidy et al. 1994; Roldán-Tapia et al. 2005). Furthermore, these performance deficits occurred at OP insecticide exposure levels that did not produce overt signs of cholinergic toxicity. Although the two systematic reviews suggest that the weight of evidence supports cognitive deficits associated with long-term exposure to OP insecticides, there is significant uncertainty in the dose, frequency, and duration of exposure that give rise to the observed deficits (Ross et al. 2013). This lack of exposure characterization has prohibited the creation of a source-to-outcome model that would link OP exposure to neurobehavioral outcomes, ultimately allowing the development of a BMD based on this potentially more sensitive end point.
Several studies have been conducted in the rat to investigate neurobehavioral dose–responses following a known dose administered over a sustained period to represent chronic exposure to OP insecticides. These studies have employed a variety of neurobehavioral assessments during the course of the OP exposure, including the Morris water maze, which utilizes the delay-to-platform measurement to assess spatial memory deficits (Ivens et al. 1998; Johnson et al. 2009; López-Granero et al. 2013; Terry et al. 2011, 2012, 2007; Yan et al. 2012); the 5-choice serial reaction time test (5C-SRTT), which determines sustained attention deficits (Middlemore-Risher et al. 2010; Samsam et al. 2005; Terry et al. 2012, 2007); and repeated acquisition tasks for assessing the ability to learn and maintain new information (Cohn and MacPhail 1997). Although some of these studies utilized CPF doses high enough to cause significant brain AChE inhibition, (Johnson et al. 2009; Middlemore-Risher et al. 2010; Samsam et al. 2005; Yan et al. 2012), most doses administered across these studies led to neurobehavioral effects below the PoD threshold of 10% brain AChE inhibition (López-Granero et al. 2013; Terry et al. 2012, 2007; Yan et al. 2012). In addition, all studies reported evidence of dose–response neurobehavioral deficits during or after subacute exposure to OPs (i.e., doses below the threshold required to elicit overt cholinergic toxicity). These findings suggest that cognitive outcomes related to memory, attention, and learning may be more sensitive markers of adverse health effects than AChE inhibition.
Finally, the mechanisms behind the observed OP-induced neurobehavioral deficits are currently unknown, but hypotheses resulting from recent studies include disruption of neurotrophin-mediated cognitive processes in the CA1 region of the hippocampus (Lee et al. 2016); impaired axon transport and growth (Eaton et al. 2008; Gearhart et al. 2007; Ruiz-Muñoz et al. 2011; Yang et al. 2008), including deficits to vesicle movement in rats at submicromolar concentrations (Gao et al. 2017); and dose-dependent changes in gene expression in the brain (Eaton et al. 2008; Lee et al. 2016; Ray et al. 2010). Furthermore, a consensus of the literature points to changes in cognitive pathways resulting from localized oxidative stress and inflammation in the brain (Casida and Quistad 2005; Eaton et al. 2008; Lee et al. 2016; Lukaszewicz-Hussain 2010; Pancetti et al. 2007; Rohlman et al. 2011; Soltaninejad and Abdollahi 2009).
To quantify the implications of changes in cognition following CPF exposure and to better understand how a health-based end point can be used to estimate a benchmark dose, we developed a methodology and computational framework that integrated a validated PBPK model, a new pharmacodynamic dose–response model, and both pharmacokinetic and pharmacodynamic data in rats and in humans. In addition to a benchmark dose estimate, we derived threshold environmental public health indicators and benchmark external exposure conditions specific to CPF. Although this proof-of-principle methodology concerns subacute exposure to CPF, this procedure could be applied to other scenarios where there is a need to extrapolate observed health effects in animals to potential effects in humans.
Materials and Methods
Model Overview
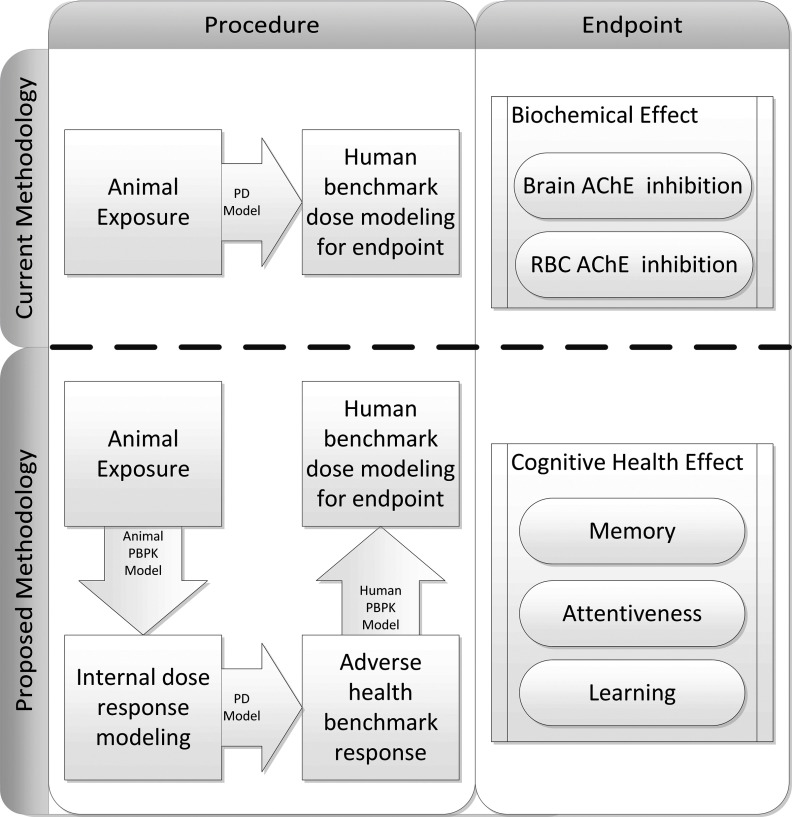
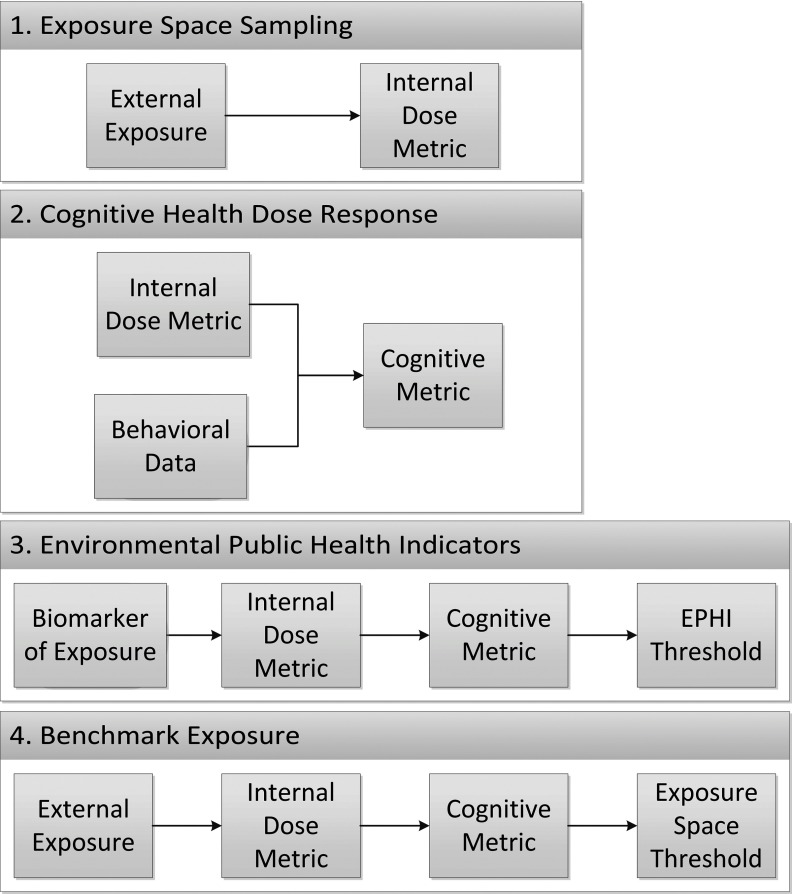
What follows is a brief overview of the proposed methodology. The following sections provide a more extensive description of each stage of the analysis. As illustrated in Figure 1, the workflow behind the proposed computational framework differs from the current PoD benchmark dose modeling procedure, which utilizes animal studies to make benchmark dose predictions based on a biochemical effect rather than on an observable health outcome. As depicted in Figure 2, our approach involved four fundamental steps. In step 1, we used dosing parameters corresponding to a large exposure space (i.e., a range of subacute chronic exposure scenarios reflecting differences in dose magnitude, duration, frequency, and route of exposure) as inputs to a well-validated PBPK model for CPF developed by Poet et al. (2014). This procedure produced an exposure space database (ESD) of simulated pharmacokinetic and pharmacodynamic results for each exposure condition that included brain and plasma CPF concentrations and percent inhibition of RBC AChE and plasma BuChE. In step 2, we used the PBPK model estimates corresponding to dosing conditions associated with neurobehavioral outcomes in a previous rat study (Yan et al. 2012) to develop a dose–response model relating peak CPF concentrations in the brain to cognitive deficits in rats. In step 3, we input the brain CPF concentrations from the ESD into the dose–response model to predict a cognitive deficit for each exposure scenario. Using the predicted cognitive deficit, we then developed a mathematical correlation between simulated biomarkers of exposure and the predicted cognitive effect. These biomarkers could serve as environmental public health indicators (Dreyling et al. 2007; Egorov et al. 2013; Furgal and Gosselin 2002) (EPHIs) to relate readily measurable chemical species to the health status of a population with respect to CPF exposure. Finally, in step 4, we divided the exposure space into risk categories based on the predicted resulting level of cognitive deficit.
Figure 1.
Overview of the procedure used to develop dose–response relationship for cognitive health effect end points. The top row shows a current methodology for determining point-of-departure estimates of acetylcholinesterase (AChE) inhibition using animal exposure models, and the bottom row illustrates the proposed methodology for using internal dose metrics to predict cognitive health end points. PD, pharmacodynamic; PBPK, physiologically based pharmacokinetic; RBC, red blood cells.
Figure 2.
Outline of the required inputs for each step of the proposed methodology for determining Environmental Public Health Indicators (EPHIs) and Exposure Space Thresholds. The cognitive metric represents the predicted neurobehavioral deficit using data from the animal study. The EPHI threshold represents the biomarker of exposure corresponding to a defined change in the cognitive metric, and the exposure space threshold represents the external exposure conditions giving rise to that same change in cognitive metric. The values for “Behavioral Data” and “Biomarker of Exposure” are measured values from published neurobehavioral and biomonitoring studies, respectively.
Step 1: Exposure Space Sampling
Four independent variables defined the exposure space: route of exposure, dose amount (D), exposure periodicity (), and duration of exposure (), with exposure periodicity and duration of exposure representing the time between exposure events and the amount of time over which the exposure event occurred, respectively. For example, a daily oral exposure to CPF through the ingestion of food would result in a of 24 h and a of approximately 0.083 h (). Comparatively, a daily inhalation exposure in an occupational setting would have a of 8 h representing a continuous 8-h exposure. The exposure space database was constructed by running the PBPK/PD model for a total of 10,000 Monte-Carlo (MC) sampling draws using uniform distributions for each of the quantitative parameters (Table 1). One month of exposure was simulated for each MC simulation, with the final simulated day representing the steady-state window over which PK/PD end points were calculated.
Table 1.
Sampled exposure space parameters.
| Route of exposure | D (units) | (h) | (h) |
|---|---|---|---|
| Oral | 4–24 | ||
| Dermal | 4–24 | ||
| Inhalation | 4–24 |
Note: Parameters comprising the exposure space database (ESD) were sampled from a uniform distribution where D is the administered dose, represents the time between dosing events, and represents the duration of exposure. Here, the periodicity () represents the amount of time (in hours) between the start of each dosing event, and the duration () represents how long the dosing event lasted.
Step 2: Cognitive Health Dose–response Development
Despite promising studies aimed at elucidating the mechanisms behind observed OP-induced neurobehavioral deficits (Casida and Quistad 2005; Eaton et al. 2008; Gao et al. 2017; Gearhart et al. 2007; Lee et al. 2016; Pancetti et al. 2007; Ray et al. 2010; Rohlman et al. 2011; Ruiz-Muñoz et al. 2011; Soltaninejad and Abdollahi 2009; Yang et al. 2008), there is currently no well-developed adverse outcome pathway (AOP) to quantify the weight of evidence surrounding each hypothesized mode of action. Lacking an AOP, we used peak CPF concentration in the brain as the internal-dose metric for neurobehavioral deficits based on biological plausibility and prior practices for internal dosimetry for PoD development (U.S. EPA 2016b).
Using the chosen metric (peak brain CPF), we utilized an model (Felmlee et al. 2013; Reisfeld et al. 2007) to describe the relationship between predicted peak CPF concentration, , and observed fractional cognitive deficit, CD:
| (1) |
Here, the maximum effect level represents a 100% deficit in cognitive ability, and and represent the Hill coefficient and peak CPF concentration to produce half of the effect, respectively.
In addition to the (Hill) equation form, we evaluated power-law and exponential models, which are commonly used to describe dose–response in such applications (U.S. EPA 2016a). We then quantified the quality of each model fit using the Akaike Information Criteria (AIC), which resulted in the following scores: power-law: , Hill: , and exponential: . We selected the (Hill) model because it represented the best balance of goodness-of-fit and relative model simplicity; moreover, as a direct-effect model, Equation 1 is consistent with a currently hypothesized biological mechanism that OP metabolism to reactive oxygen species is a significant contributor to insecticide-induced neurobehavioral deficits (Eaton et al. 2008; Lukaszewicz-Hussain 2010).
We estimated the parameters in Equation 1 using data obtained using the Morris water maze latency-to-platform tests (Yan et al. 2012), in which a delay in platform discovery relative to control indicates a cognitive deficit in spatial learning (Terry et al. 2003; Vorhees and Williams 2006). Values for the simulated peak CPF concentrations from Yan et al. (2012) are given in Table 2, and specific dosing conditions for this animal study are outlined in Methods, “Rodent data utilized for model evaluation.” Using the parameter distributions determined from the nonlinear least squares fit to Equation 1, we determined the peak CPF concentration in the brain giving rise to a 15% cognitive deficit in spatial memory performance as the PoD benchmark dose (). We chose the 15% cognitive deficit as the PoD because it represented the smallest statistically significant departure in cognitive deficit relative to control. Using the Equation 1 parameter distributions, we also computed the lower-limit benchmark dose () with the lower 95% confidence interval from the peak CPF brain concentration.
Table 2.
Predicted peak brain chlorpyrifos (CPF) concentrations for the rat using the three dosing conditions (once daily, oral gavage CPF) reported by Yan et al. (2012).
| Dose (mg/kg) | Peak brain CPF () |
|---|---|
| 0 | 0 |
| 1 | |
| 5 | |
| 10 |
Note: Peak brain CPF represents the simulated peak concentration of CPF in the brain for each exposure using the Poet et al. (2014) physiologically based pharmacokinetic (PBPK) model.
Step 3: Environmental Public Health Indicators
Using the set of biomarkers of exposure and the corresponding simulated peak CPF brain concentration from the ESD, we predicted spatial memory deficits using the dose–response relationship, Equation 1. This calculation led to a predicted cognitive deficit for each of the 10,000 simulated exposure conditions for each biomarker. We then related the cognitive deficit end point, CD, to a biomarker of exposure, BM, using the following equation:
| (2) |
where αi = parameters used for fitting; log = natural log.
We selected the specific form for Equation 2 because it was the simplest representation we found to accurately relate cognitive deficit as a function of all three biomarkers of exposure across a broad range of exposure conditions. Here, the variable BM is a normalized value that can represent both measurable concentrations and percent enzyme inhibitions. Specifically, if RBC AChE or plasma BuChE served as the biomarker of exposure, BM was calculated using , where is the simulated percent of enzyme available for reaction from the ESD for a given exposure; alternatively, if peak plasma CPF was chosen, BM was simply the measured CPF concentration. In the text and tables to follow, predicted biomarkers of exposure are denoted by for peak plasma CPF concentrations, for transformed RBC AChE inhibition, and for transformed plasma BuChE inhibition. Using a cognitive deficit threshold of 0.15, we computed a corresponding threshold biomarker of exposure at the 95th percentile curve predicted from Equation 2. We chose the upper 95th percentile because it provided a protective EPHI for a majority of the population within the large exposure space from the ESD. Finally, we tested Equation 2 using two studies (one rodent, one human) in which both biomarkers of exposure and neurobehavioral deficits were reported (Farahat et al. 2003; Terry et al. 2007). As noted earlier, such biomarkers could be used as EPHIs in tracking the health status of a target population through correlation of the measurable biomarker of exposure with the predicted cognitive health outcome.
Step 4: Benchmark Exposure
Exposure space mapping.
We mapped the external exposure to the CPF brain concentrations for rats and humans using the exposure space from the initial Monte Carlo draws of the PBPK/PD model. This mapping allowed a given external dosing scenario to be compared directly to the and derived earlier. The independent exposure parameters (duration, periodicity, and dose) were transformed to allow for a two-dimensional representation of total absorbed dose (TAD) versus fraction of day (FOD) exposed, with FOD defined as
| (3) |
where is the duration of exposure, and is the time between exposure events. Therefore, FOD ranged from 0 to 1 and represented the fraction of the day over which the total absorbed dose was administered. The TAD variable (mg/kg) is characteristic of the total amount of external exposure applied to the organism over the course of a single day.
Benchmark CPF exposure.
Using the exposure space of the two independent exposure parameters, FOD and TAD, we determined exposure cutoffs for the peak CPF concentrations using a second-order polynomial:
| (4) |
where is the route-specific exposure cutoff for reaching the benchmark internal dose, and are parameters used for fitting. We then classified exposures giving rise to a predicted peak brain concentration below the as “safe,” and exposures resulting in peak brain CPF concentrations above the as “hazardous.”
Rodent Data Utilized for Model Evaluation
When selecting rodent CPF study data to use for model evaluation, we focused on studies that used Morris water maze latency-to-platform tests as the outcome because of evidence suggesting that these tests are more sensitive to OP exposure than other neurobehavioral tests. Specifically, multiple rat behavioral studies (Cañadas et al. 2005; Ivens et al. 1998; Terry et al. 2011, 2012, 2007; Yan et al. 2012) consistently found deficits in latency to platform at daily CPF doses lower than those reported in repeated acquisition (Middlemore-Risher et al. 2010) and performance tests (Cohn and MacPhail 1997). Moreover, this outcome is considered to be analogous to tests of visuospatial working memory in humans (Netherton et al. 1989; Webster et al. 2014), and deficits in overall spatial reference memory are thought to reflect effects localized to the hippocampus (Lee et al. 2016; Vorhees and Williams 2006), suggesting the relevance of local concentrations of CPF in the brain. Of the previous rat studies of CPF that used Morris water maze latency-to-platform tests (Cañadas et al. 2005; Terry et al. 2012, 2007; Yan et al. 2012), we identified two that we felt were appropriate as sources of data for our analysis: Yan et al. (2012), which was used for model calibration, and Terry et al. (2007), which we used for verification and testing.
Yan et al. (2012) reported a 4-wk study in which rats were orally administered 0, 1, 5, and CPF. This specific study was chosen because it provided exposure conditions readily simulated in the PBPK model and corresponding neurobehavioral performance data. The authors reported that latency to platform was significantly reduced in the 5- and 10-mg/kg/day groups (relative to vehicle controls), but brain AChE inhibition (measured after 4 wk of exposure) was significantly affected only in the 10-mg/kg/day group. To calibrate our model, we determined peak CPF brain concentrations in rat using the exposure conditions in the experimental study and rat-specific parameters of the PBPK model (Poet et al. 2014). By normalizing the reported seconds-to-platform for each exposure group to the control, we determined the fractional cognitive deficit to be 0.26, 0.34, and 0.40 for 1, 5, and repeated dosing, respectively. We then used the CPF PBPK model (Poet et al. 2014) to simulate corresponding peak brain CPF concentrations (Table 2) to use as the internal-dose metric for Equation 1. Parameterization of Equation 1 produced a pharmacodynamic model to predict a fractional decrease in Morris water maze latency to platform as a function of peak brain CPF concentration.
Terry et al. (2007) administered CPF to rats by subcutaneous (SC) injection every other day over a 30-d period at doses of 0, 2.5, 10, and . We could not estimate internal doses because the SC route of exposure is not a validated component of the PBPK model. Therefore, we compared peak blood CPF values (measured in samples collected once per week during the 30-d dosing period and the following two weeks) with predicted Morris water maze latency-to-platform fractional deficit values based on our computational methodology.
Human Data Utilized for Model Evaluation
As described in the introduction, the two systematic reviews cited numerous studies indicating changes in neurobehavioral performance following subacute exposure to CPF. In addition, several biomonitoring studies have assessed CPF exposure for various cohorts of workers in different occupational settings (Alexander et al. 2006; Callahan et al. 2017; Farahat et al. 2011; Wang et al. 2016). One such study that both measured biomarkers of exposure and assessed changes in neurobehavioral performance for the same cohort was performed by Farahat el al. (2003). From this study, we utilized the data from the results of the Benton Visual Retention Test (BVRT), a human-equivalent test for assessing spatial working memory deficits analogous to the Morris water maze test in rats (Netherton et al. 1989). Here, 52 men with a history of working in the pesticide application department served as the exposed group, and 50 male clerks and administrators served as the control. Using the reported mean, standard error (SEM), and number of participants in each group, we generated normal distributions for the BVRT of the control and insecticide-exposed groups. To compute the fractional deficit, we took the ratio of the distributions, resulting in a Cauchy distribution with a mean fractional deficit of 0.13. In addition, this study presented results for serum AChE activity for groups of unexposed () and CPF-exposed workers (). For the unexposed group, the average AChE activity was , whereas the average AChE activity for workers exposed to CPF for was , indicating that the exposed group had approximately 80% activity compared with the control group. Although this study represents only a small cohort of exposed individuals, it does contain biomonitoring and corresponding cognitive assessment data useful in a coarse test of the proposed methodology.
To develop realistic external exposure conditions and to verify exposure thresholds, we used results from the Farm Family exposure study (Alexander et al. 2006). The Farm Family study investigated CPF exposures in 28 farmers from South Carolina and six from Minnesota. Applicators were required to live on a farm, have a spouse and at least one child between 4 and 17 y old, and plan to apply CPF on , some of which had to be within 1 mile (1.61 Km) of the farmer’s residence. Alexander et al. (2006) used daily 3,5,6-trichloro-2-pyridinol (TCP) urinary concentrations to characterize the exposure profiles of the applicators and their spouses and children. Using these biomarker data, they estimated the total absorbed CPF dose for the applicator cohort to be with an upper 90th percentile exposure of . Although this is not a definitive measure of all occupational exposure to CPF, it provides an estimate of the total absorbed dose of an occupational cohort.
Software and Computing Platform
We conducted CPF PBPK/PD model simulations and Monte Carlo sampling using MCSim (version 5.6; GNU) (Bois 2009). Parameter estimation of the model was performed using the Python package lsqfit (v.7.1; G.P. Lepage). Determination of candidate model structures for correlating biomarkers of exposure to peak CPF concentrations in the brain was carried out in Eureqa (version 1.24.0; Nutonian). Data from the literature published in graphical form (mean and reported error) were digitized using DigitizeIt (version 1.5.8; I. Bormann, bormisoft@digitizeit.de).
Results
Internal-Dose Prediction from Known Exposure
Using the methodology described in step 1 in “Methods,” we estimated peak CPF concentrations in the brain (Table 2) and subsequently used these results in the specification of the dose–response model with reported cognitive deficit as the end point.
Dose–response Modeling
Dose–response curve.
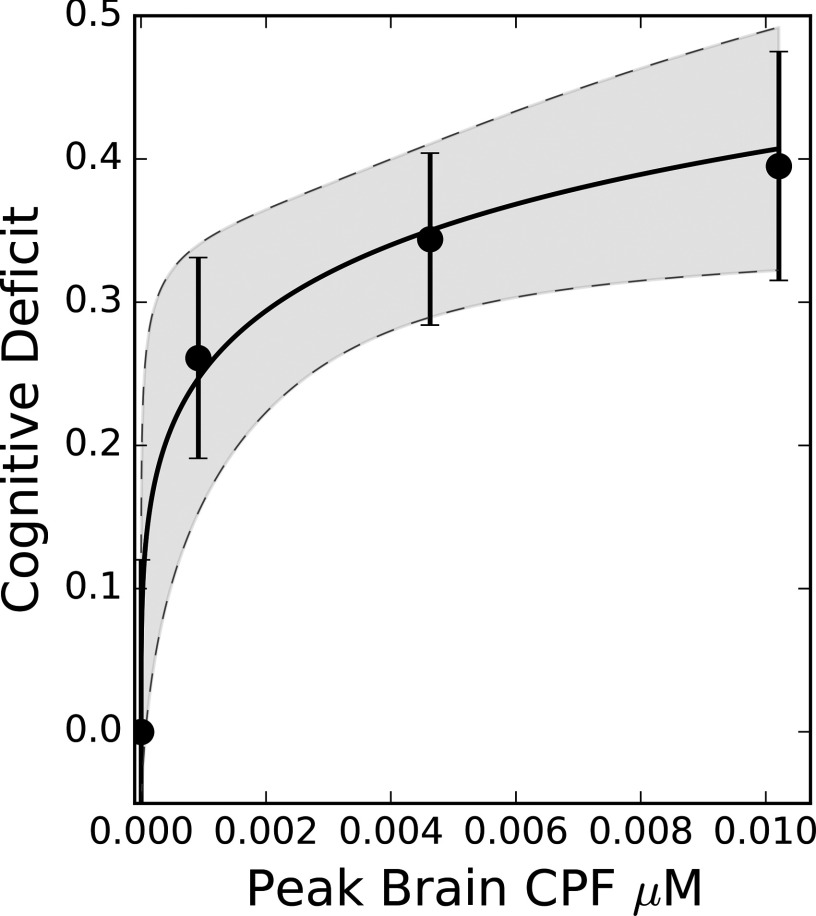
Using the results in Table 2 and the normalized latency-to-platform results from Yan et al. (2012) as the cognitive deficit training set, we employed a nonlinear least squares approach to determine the unknown parameters in Equation 1, resulting in mean [coefficient of variation (CV)] values of (0.47) and (0.18). With these parameters, the fit of data to the model equation and the resulting uncertainty envelope could be illustrated and examined (Figure 3).
Figure 3.
Dose–response curve for spatial memory fractional deficit corresponding to a chlorpyrifos (CPF)-induced change in Morris water maze latency to platform. Solid and dashed lines represent the mean and 95% prediction intervals, respectively. Circles represent reported fractional change in Morris water maze latency to platform for the exposed groups (Yan et al. 2012). Peak CPF concentrations for these data are the values predicted from the physiologically based pharmacokinetic (PBPK)/pharmacodynamic (PD) model based on the reported once daily oral gavage exposures of 1, 5, and .
Benchmark dose calculation.
Employing the calibrated model (Equation 1), we determined benchmark internal doses for a 15% deficit in spatial memory function using peak CPF concentrations in the brain as the internal dose metric (Table 3). Also in this table are the current BMD/BMDL for 10% brain AChE inhibition and 20% RBC AChE inhibition (Reiss et al. 2012).
Table 3.
Comparison of benchmark doses for various points of departure.
| End point | Peak brain CPF concentration | |
|---|---|---|
| BMD () | BMDL () | |
| 15% Cognitive deficit | ||
| 20% RBC AChE inhibition | ||
| 10% Brain AChE inhibition | ||
Note: Twenty percent RBC AChE inhibition and 10% brain AChE inhibition were determined by Reiss et al. (2012). Peak brain chlorpyrifos (CPF) concentrations were determined from the human exposure space database for all three end points. AChE, acetylcholinesterase; BMD, predicted benchmark dose corresponding to each end point; BMDL, lower limit benchmark dose corresponding to each end point; RBC, red blood cells.
Biomarkers of Exposure as EPHIs
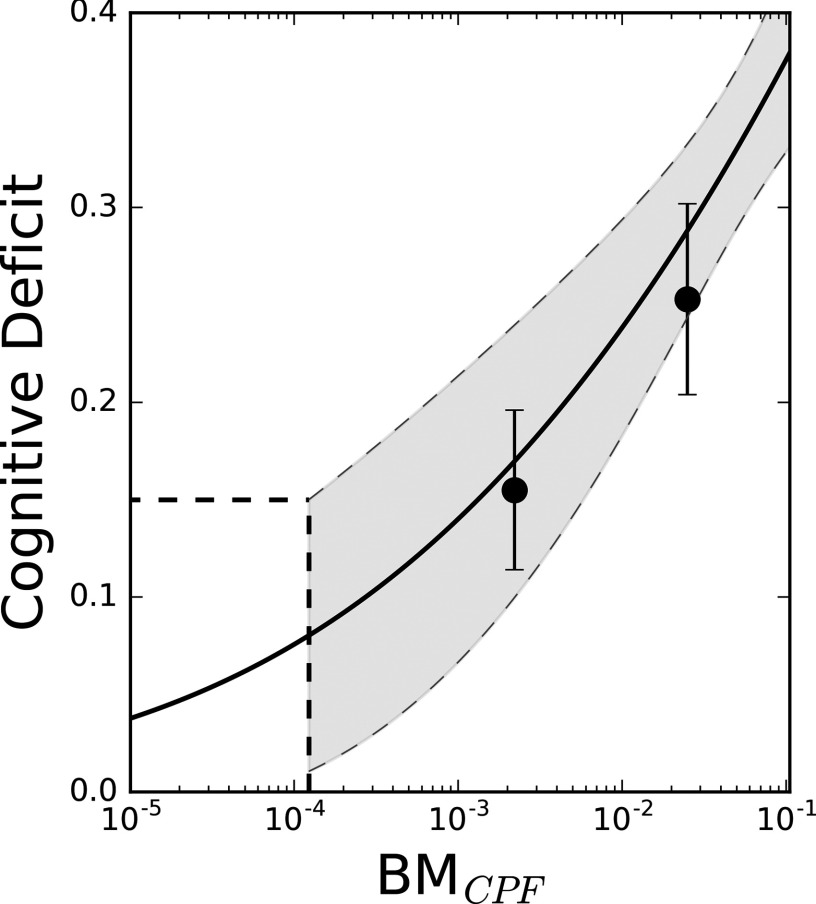
We correlated measurable biomarkers of exposure to predicted cognitive deficits to quantify the EPHI. In particular, we used Equation 2 to relate fractional cognitive deficit, CD, to peak CPF plasma concentrations in the rat. Using the upper 95th percentile predicted from Equation 2, we derived the threshold biomarker of exposure giving rise to a cognitive deficit of 0.15 of for peak CPF plasma concentrations. A comparison of predictions from this correlation and measured values from an in vivo experiment (Terry et al. 2007) is presented in Figure 4. The computed parameter values [mean (CV)] in this case were , , , and .
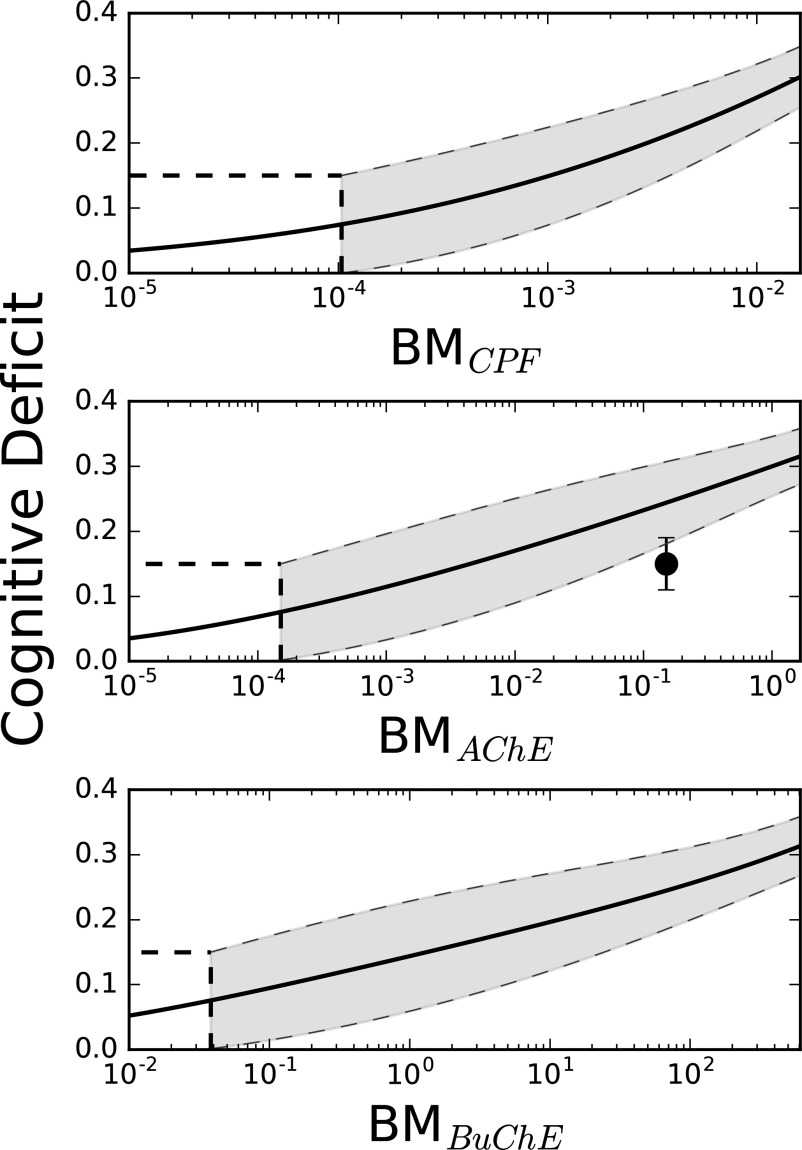
Figure 4.
Predictions of spatial memory cognitive deficit (Morris water maze latency to platform) for the rat. Dashed lines represent 95% prediction intervals, solid circles (•) represent reported plasma chlorpyrifos (CPF) concentrations from Terry et al. (2007) for the rats dosed subcutaneously every other day at 2.5 and . corresponds to peak plasma CPF biomarker of exposure used in Equation 2. Horizontal and vertical dashed lines demonstrate the threshold biomarker of exposure for a 15% cognitive deficit.
We then applied this same correlation method using three different biomarkers of exposure contained in the human ESD: CPF plasma concentration, RBC AChE inhibition, and plasma BuChE inhibition. Figure 5 shows a graphical representation of the resulting predictions, where the underlying parameter values [mean (CV)] are as follows: peak CPF concentrations in the plasma [, , , and ], minimum RBC AChE inhibition [, , , and ], and minimum plasma BuChE inhibition [, , , and ]. Using each BM curve developed in Figure 5, threshold biomarkers of exposure levels were determined using the upper 95th percentile confidence interval, ensuring that health indicators provided a level of protection for a given population. After transforming each and back to percent enzyme available, we estimated the following exposure thresholds: for peak CPF plasma concentration, 99% available RBC AChE, and 90% available plasma BuChE.
Figure 5.
Predictions of human-specific cognitive deficits from measurable biomarkers of exposure. , , and , are the independent biomarker of exposure variables for Equation 2 and correspond to peak plasma chlorpyrifos (CPF) concentration, minimum blood acetylcholinesterase (AChE) inhibition and minimum blood butyrylcholinesterase (BuChE) inhibition, respectively. Dashed lines represent 95% prediction intervals, and the solid circle (•) represents measured fractional cognitive deficit data from Farahat et al. (2003) using the differences in performance of the Benton Visual Retention Test between the exposed and unexposed groups and fractional plasma AChE activity from the reported plasma AChE activity.
We then utilized results from an occupational exposure study in humans (Farahat et al. 2003) (vide supra) to test the prediction of cognitive deficits from measurable biomarkers of exposure. In that study, the biomarker of exposure used to assess CPF exposure was RBC AChE inhibition, and investigators reported an approximate 13% deficit in the BVRT for exposed groups compared with control. Using the measured RBC AChE activity as the biomarker of exposure, we compared the reported cognitive deficits from this study to model predictions (Figure 5). Because Farahat et al. (2003) reported 80% activity of RBC AChE in exposed versus control groups, the resulting biomarker of exposure used for cognitive prediction within our computational framework (Equation 2) was .
Exposure Space
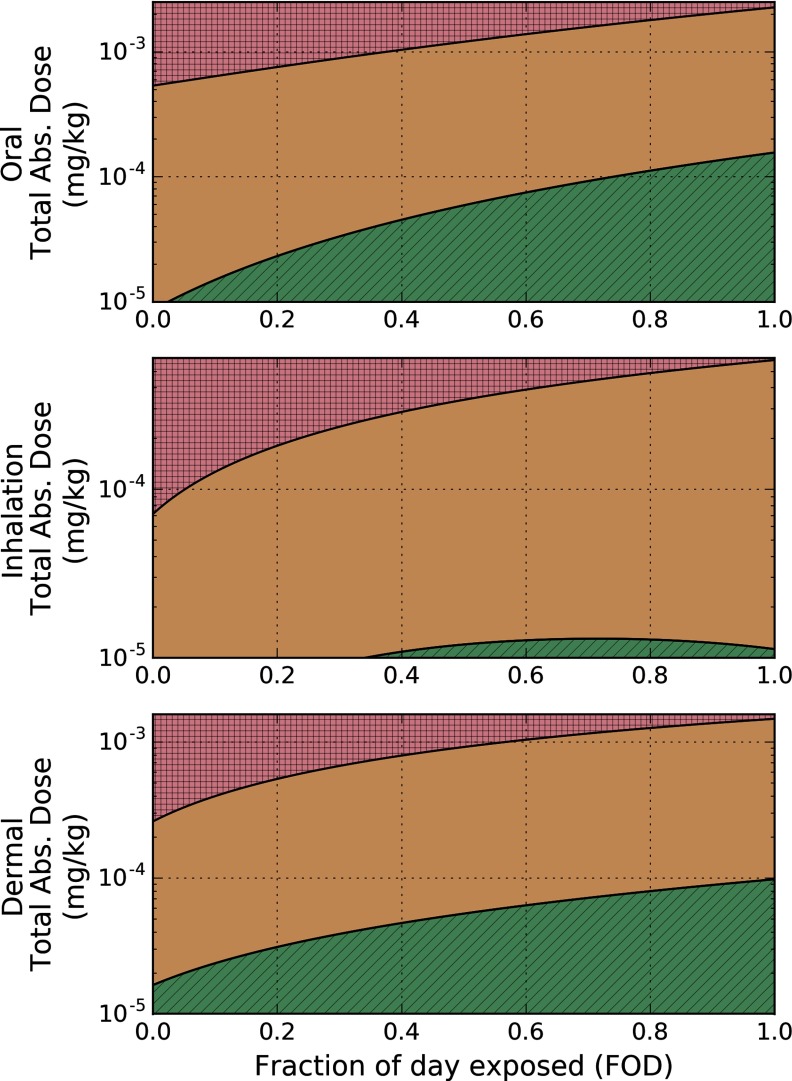
Using the effective internal dose metric calculated from the dose–response modeling in the rat, we computed the exposure space resulting in concentrations below the and the . Figure 6 depicts this space for oral, dermal, and inhalation routes of exposure for the rat, where the ordinate represents the total amount of CPF administered over the duration of exposure. From these results, we estimated the rat-equivalent oral exposure resulting in peak brain CPF concentrations equal to the and the and fit these results using Equation 4, where relevant model parameters for the rat are presented in Table 4.
Figure 6.
External exposure space using the preliminary benchmark dose giving rise to a 15% cognitive deficit () and lower limit benchmark dose giving rise to a 15% cognitive deficit () for rats. Boundaries for the and were derived from Equation 4 when the external exposure scenario gives rise to a CPF brain concentration at the proposed and in the rat. Fraction of day exposed (FOD) represents how much of the day a rat is exposed to the total applied dose (TAD). Red (square hatching), orange (no hatching), and green (diagonal hatching) shading indicate exposure scenarios that fall above the , between the and the , and below the , respectively.
Table 4.
External exposure space parameters for second-order polynomial fit to the simulated exposure space using a nonlinear least squares fit to Equation 4.
| Derived BMD | Route of exposure | Species | |||
|---|---|---|---|---|---|
| Oral | Rat | 0.148 | 0.253 | 0.227 | |
| Human | 0.00848 | 0.0106 | 0.00787 | ||
| Inhalation | Rat | 0.779 | 6.052 | ||
| Human | 1.334 | 3.386 | |||
| Dermal | Rat | 68.564 | 372.216 | ||
| Human | 0.288 | 1.619 | |||
| Oral | Rat | 0.00241 | 0.0148 | 0.0262 | |
| Human | |||||
| Inhalation | Rat | 0.022 | 0.334 | ||
| Human | 0.0694 | 0.133 | |||
| Dermal | Rat | 4.299 | 18.99 | 2.718 | |
| Human | 0.069 | 0.133 |
Note: Polynomial coefficients () are tabulated for each species, and route of exposure with corresponding dose–response curves are shown in Figure 6 (rat) and Figure 7 (human). Parameters for determine the total absorbed dose (TAD) versus fraction of day (FOD) exposed boundary resulting in a chlorpyrifos (CPF) brain concentration at the derived . Similarly, parameters for determine the boundary resulting in a brain CPF concentration at . Differences in physiologically based pharmacokinetic (PBPK) model parameters between rat and human simulations resulted in differences in the and cutoff for each species. BMD, benchmark dose; , benchmark dose at 15% cognitive deficit; , lower limit benchmark dose.
As an example of the application of this methodology, for a once-daily oral gavage exposure, (five-minute exposure) and (once-daily exposure). Therefore, the fraction of day exposed for this exposure scenario is , and the threshold repeated oral exposure based on the is with a of . An analogous analysis for humans resulted in the and displayed in Figure 7.
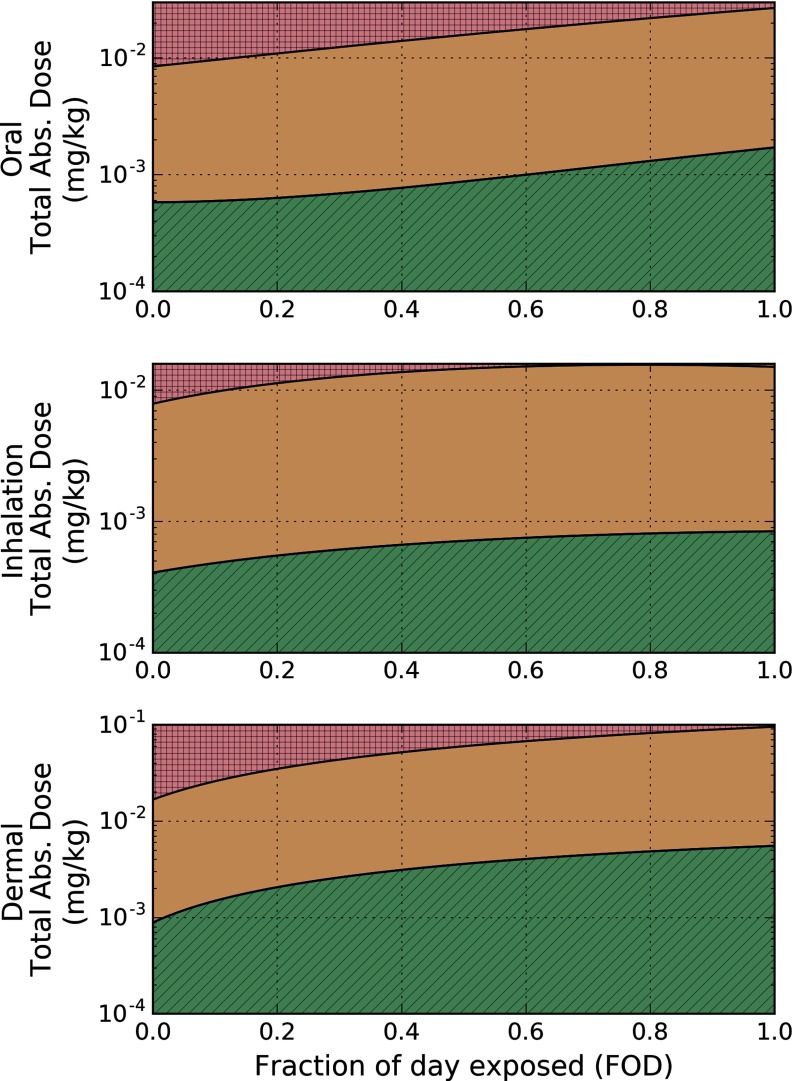
Figure 7.
External exposure space using the preliminary benchmark dose giving rise to a 15% cognitive deficit () and lower limit benchmark dose giving rise to a 15% cognitive deficit ()for humans. Boundaries for the and were derived from Equation 4 when the external exposure scenario gives rise to a chlorpyrifos (CPF) brain concentration at the proposed and in the human. Fraction of day exposed (FOD) represents how much of the day a human is exposed to the total applied dose (TAD). Red (square hatching), orange (no hatching), and green (diagonal hatching) shading indicate exposure scenarios that fall above the , between the and the , and below the , respectively.
As with the analysis for the rodent data, we determined the human equivalent oral exposure resulting in peak brain CPF concentrations equal to the and the and computed the fit using Equation 4 with respect to the fraction of day exposed. The resulting best-fit model parameters are presented in Table 4. Assuming a once-daily oral exposure, , and the resulting benchmark exposure was .
Discussion and Conclusions
Methodology
The present study provides a proof-of-principle methodology for not only determining a point-of-departure for cognitive deficits through benchmark dose modeling but also utilizing measurable biomarkers of exposure to develop public health indicators for monitoring this health outcome. The methodology presented in this study is agnostic to a specific chemical and requires a validated interspecies PBPK model for internal dose prediction, a critical dose metric to serve as the internal dose across species, and a quantifiable behavioral outcome observed in dose–response in animals. In the case of chlorpyrifos toxicity, the use of a neurobehavioral end point offers several advantages compared with an end point based on cholinesterase inhibition. First, the neurobehavioral end point represents an overt change in the health of the individual, whereas the inhibition of cholinesterase merely represents a biochemical change that may or may not represent an adverse health effect. Second, a variety of neurobehavioral end points can be used depending on the application and availability of data. For example, coordination and motor deficits could be the metric of interest instead of spatial memory as illustrated herein. Third, neurobehavioral studies conducted in animals can be utilized for prediction of a potentially more sensitive end point.
Employing the dose–response for a given cognitive deficit facilitated the identification and description of EPHIs from measurable biomarkers of exposure. In specifying a threshold for the given cognitive deficit, for example, 15% deficit in spatial memory, biomonitoring data from a population could be used to ascertain whether a given cohort is protected.
Although this study was intended as a proof of principle of the application of results from neurobehavioral studies in animals to predict health-based outcomes in humans, we believe that the cognitive deficit BMDs we have estimated are relevant to subacute CPF exposure and that these results suggest that selected end points based on neurobehavioral deficit may be more sensitive than the biochemical end points used to derive the current BMDs for CPF. With additional data from animal and human studies that collect both biomonitoring and neurobehavioral test results, we expect that approaches like the one presented here will provide more definitive quantitative measures that can be used to inform the risk-assessment process for a variety of chemicals.
Novel Features and Advantages of the Present Methodology
By utilizing a well-validated PBPK/PD model coupled with appropriate pharmacodynamic data, we developed a computational methodology for predicting neurobehavioral changes based on measurable biomarkers of exposure and corresponding internal concentrations at the proposed site of action, rather than using external exposure metrics. This difference in approach is meaningful because the amount of organophosphorus insecticide that reaches the brain will differ based on the route, frequency, and duration of exposure. In addition, the approach we developed for determining EPHIs is not specific to the end point illustrated here; it can be applied to a variety of studies where a dose–response neurobehavioral change is observed. As an example, in the present study, we utilized peak CPF concentrations in the rat following subcutaneous administration of CPF to predict spatial reference memory deficits following a route of exposure not characterized in the current PBPK model. Although not described here, we similarly applied this approach (Zurlinden 2016) to a separate set of CPF data related to repeated acquisition (short-term memory) tests in rats exposed to CPF through oral gavage (Cohn and MacPhail 1997). Although the experimental study showed a dose–response effect on repeated acquisition following chronic exposure to CPF, the present methodology predicted a BMD for CPF above the based on brain AChE inhibition in the rat presented by Reiss et al. (2012). By comparing these cases, it seems clear that examining BMDs across neurobehavioral outcomes may lead to information useful in determining the most sensitive cognitive end points for further study.
Another useful, and perhaps broadly applicable, feature of this study was the generation of what we believe is a meaningful exposure space to quantify how exposure to CPF compares to the target end point. Many of the current point-of-departure benchmark doses are determined for a single route of exposure and assume the same dosing frequencies: for example, an oral dose administered repeatedly once per day. Figures 6 and 7 illustrate how exposure parameters are collapsed into a two-dimensional space for each route of exposure. Therefore, exposure thresholds for oral, dermal, and inhalation routes of exposure can be determined based on the fraction of day exposed.
Limitations, Deficiencies, and Uncertainties of the Present EPHI Framework
The suggested method for developing EPHIs and for determining a safe exposure space suffers from several limitations. First, behavioral end points in the rats, such as spatial memory deficits from the Morris water maze and repeated acquisition results, are not adequately correlated to cognitive deficits in humans. Results from the Morris water maze and the corresponding changes due to exposure are indicative of a hippocampal synaptic plasticity and receptor function (Vorhees and Williams 2006). Although the BVRT in humans is also correlated strongly with changes in the dentate gyrus hippocampal subregion (Brickman et al. 2011), results from the Morris water maze in the rat have not been related to those from this test. Second, the mechanism of action of CPF on cognitive deficits is unknown. Hypotheses from studies reviewed in the introduction suggest a noncholinergic affinity for the OP insecticide in various parts of the brain, specifically the hippocampus. To elucidate a potential mechanism of action to describe deficits in cognitive function, a recent study (Lee et al. 2016) focused on determining gene transcription levels in the hippocampus following subchronic exposures to CPF in rats. In that study, investigators determined significant differential expression of several genes coding for neuropeptides specific to cognitive function in this brain region. Based on those results, a proposed mechanism of toxicity was presented by the study authors in which CPF induces transcriptomic changes in the brain resulting in an increased secretion of neuropeptides associated with neurocognitive disorders. Although differences in OP-induced effects between cognitive tests demonstrate the OP’s affinity to different regions of the brain, it is expected that further in vivo and in vitro experimentation will clarify this mechanism of action and its relevance to humans and lead to improved dose–response models. Third, utilizing the method described in this study relies on a measurable biomarker of exposure to be used as an EPHI. A problem communicated in the 2016 FIFRA SAP (U.S. EPA 2016b) pertained to the limitation of current analytical techniques to quantify very low concentrations of CPF in cord blood under realistic exposure conditions. Although this is also a concern for the present study, the methodology presented herein considers additional, or supplementary, biomarkers of exposure, such as RBC AChE and plasma BuChE inhibition, to be used as EPHIs for cognitive deficits. Other measures, such as CPF concentrations in a different compartment, metabolite concentrations (e.g., 3,5,6-trichloro-2-pyridinol in the urine), or additional enzyme activity assays, could readily be employed within the framework if they prove to be more appropriate biomarkers of exposure. Finally, the present methodology utilized peak brain CPF concentrations as the critical dose metric for linking CPF exposure to neurobehavioral deficits. Using this dose metric assumes that peak CPF concentrations in the brain will yield similar neurobehavioral outcomes between rats and humans. Although extrapolation of the neurobehavioral changes across species through peak CPF brain concentrations is addressed using the available cross-species data, differences in cognitive effects at the equivalent critical dose metric will lead to more uncertainty in the predicted benchmark effect. However, as described earlier, the EPHI framework developed in this study is sufficiently flexible to accommodate other types of internal dose metrics and exposure/neurobehavioral data, depending on the application.
Future Directions
Using the method detailed herein as a foundation, evaluation and quantitation of the effect of additional sensitive neurobehavioral end points can be undertaken. Because CPF concentrations in the brain will have localized effects in different regions, the degree of damage to one cognitive pathway may be different from that to a different pathway. Therefore, establishing EPHI thresholds for different types of learning and memory may allow for better protection of certain populations.
To characterize CPF’s effects on additional cognitive pathways, animal studies should be undertaken using explicit exposure conditions with doses much lower than those currently used to elicit cholinergic responses. For maximum benefit, the route of exposure chosen for these studies must be incorporated into the PBPK/PD model for the determination of internal dose metrics. Finally, it is critical that relevant biomarkers of exposure and corresponding neuropsychological end points be measured in various exposed cohorts representing different exposure scenarios.
Acknowledgments
The authors thank S. Hays for counsel and valuable input regarding various aspects of this work. The project from which these results were obtained was funded in part by a U.S. Environmental Protection Agency Science to Achieve Results fellowship for T. J. Zurlinden (U.S. EPA STAR: FP – 91730301).
References
- Alexander BH, Burns CJ, Bartels MJ, Acquavella JF, Mandel JS, Gustin C, et al. 2006. Chlorpyrifos exposure in farm families: results from the farm family exposure study. J Expo Sci Environ Epidemiol 16(5):447–456, PMID: 16570094, 10.1038/sj.jes.7500475. [DOI] [PubMed] [Google Scholar]
- Blanc-Lapierre A, Bouvier G, Gruber A, Leffondré K, Lebailly P, Fabrigoule C, et al. 2013. Cognitive disorders and occupational exposure to organophosphates: results from the PHYTONER study. Am J Epidemiol 177(10):1086–1096, PMID: 23535900, 10.1093/aje/kws346. [DOI] [PubMed] [Google Scholar]
- Bois FY. 2009. GNU MCSim: Bayesian statistical inference for SBML-coded systems biology models. Bioinformatics 25(11):1453–1454, PMID: 19304877, 10.1093/bioinformatics/btp162. [DOI] [PubMed] [Google Scholar]
- Brickman AM, Stern Y, Small SA. 2011. Hippocampal subregions differentially associate with standardized memory tests. Hippocampus 21(9):923–928, PMID: 20824727, 10.1002/hipo.20840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan CL, Hamad LA, Olson JR, Ismail AA, Abdel-Rasoul G, Hendy O, et al. 2017. Longitudinal assessment of occupational determinants of chlorpyrifos exposure in adolescent pesticide workers in Egypt. Int J Hyg Environ Health 220(8):1356–1362, PMID: 28939184, 10.1016/j.ijheh.2017.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert GM, Karnik J, Mehler L, Beckman J, Morrissey B, Sievert J, et al. 2008. Acute Pesticide Poisoning Among Agricultural Workers in the United States, 1998–2005. Am J Ind Med 51(12):883–898, PMID: 18666136, 10.1002/ajim.20623. [DOI] [PubMed] [Google Scholar]
- Cañadas F, Cardona D, Dávila E, Sánchez-Santed F. 2005. Long-term neurotoxicity of chlorpyrifos: spatial learning impairment on repeated acquisition in a water maze. Toxicol Sci 85(2):944–951, PMID: 15772369, 10.1093/toxsci/kfi143. [DOI] [PubMed] [Google Scholar]
- Casida JE, Quistad GB. 2005. Serine hydrolase targets of organophosphorus toxicants. Chem Biol Interact 157–158:277–283, PMID: 16243304, 10.1016/j.cbi.2005.10.036. [DOI] [PubMed] [Google Scholar]
- Casida JE. 2009. Pest toxicology: the primary mechanisms of pesticide action. Chem Res Toxicol 22(4):609–619, PMID: 19284791, 10.1021/tx8004949. [DOI] [PubMed] [Google Scholar]
- Chambers J, Levi P. 1992. Organophosphates: Chemistry, Fate and Effects. Academic Press:San Diego, CA. [Google Scholar]
- Clegg DJ, van Gemert M. 1999. Determination of the reference dose for chlorpyrifos: proceedings of an expert panel. J Toxicol Environ Health B Crit Rev 2(3):211–255, PMID: 10429680, 10.1080/109374099281179. [DOI] [PubMed] [Google Scholar]
- Cohn J, MacPhail RC. 1997. Chlorpyrifos produces selective learning deficits in rats working under a schedule of repeated acquisition and performance. J Pharmacol Exp Ther 283(1):312–320, PMID: 9336338. [PubMed] [Google Scholar]
- Dreyling E, Dederick EJ, Chari R, Resnick B, Malecki KC, Burke T, et al. 2007. Tracking health and the environment: a pilot test of environmental public health indicators. J Environ Health 70(5):9–16, PMID: 18189034. [PubMed] [Google Scholar]
- Eaton DL, Daroff RB, Autrup H, Bridges J, Buffler P, Costa LG, et al. 2008. Review of the toxicology of chlorpyrifos with an emphasis on human exposure and neurodevelopment. Crit Rev Toxicol 38(suppl 2):1–125, PMID: 18726789, 10.1080/10408440802272158. [DOI] [PubMed] [Google Scholar]
- Egorov AI, Dalbokova D, Krzyzanowski M. 2013. Biomonitoring-based environmental public health indicators. Methods Mol Biol 930:275–293, PMID: 23086846, 10.1007/978-1-62703-059-5_12. [DOI] [PubMed] [Google Scholar]
- Farahat F, Ellison CA, Bonner MR, McGarrigle B, Crane A, Fenske RA, et al. 2011. Biomarkers of chlorpyrifos exposure and effect in Egyptian cotton field workers. Environ Health Perspect 119(6):801–806, PMID: 21224175, 10.1289/ehp.1002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farahat TM, Abdelrasoul GM, Amr MM, Shebl MM, Farahat FM, Anger WK. 2003. Neurobehavioural effects among workers occupationally exposed to organophosphorous pesticides. Occup Environ Med 60(4):279–286, PMID: 12660376, 10.1136/oem.60.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmlee MA, Morris ME, Mager DE. 2013. Mechanism-based pharmacodynamic modeling. In: Computational Toxicology of Methods in Molecular Biology, Vol. 930 Reisfeld B, Mayeno, AN, eds. Totowa, NJ:Humana Press, 583–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furgal C, Gosselin P. 2002. Challenges and directions for environmental public health indicators and surveillance. Can J Public Health 93(suppl 1):S5–S8, PMID: 12425168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Naughton SX, Beck WD, Hernandez CM, Wu G, Wei Z, et al. 2017. Chlorpyrifos and chlorpyrifos oxon impair the transport of membrane bound organelles in rat cortical axons. Neurotoxicology 62:111–123, PMID: 28600141, 10.1016/j.neuro.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearhart DA, Sickles DW, Buccafusco JJ, Prendergast MA, Terry AV. 2007. Chlorpyrifos, chlorpyrifos-oxon, and diisopropylfluorophosphate inhibit kinesin-dependent microtubule motility. Toxicol Appl Pharmacol 218(1):20–29, PMID: 17123561, 10.1016/j.taap.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Ivens IA, Schmuck G, Machemer L. 1998. Learning and memory of rats after long-term administration of low doses of parathion. Toxicol Sci 46(1):101–111, PMID: 9928673, 10.1006/toxs.1998.2508. [DOI] [PubMed] [Google Scholar]
- Johnson FO, Chambers JE, Nail CA, Givaruangsawat S, Carr RL. 2009. Developmental chlorpyrifos and methyl parathion exposure alters radial-arm maze performance in juvenile and adult rats. Toxicol Sci 109(1):132–142, PMID: 19293373, 10.1093/toxsci/kfp053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Lewis JA, Ippolito DL, Hussainzada N, Lein PJ, Jackson DA, et al. 2016. Repeated exposure to neurotoxic levels of chlorpyrifos alters hippocampal expression of neurotrophins and neuropeptides. Toxicology 340:53–62, PMID: 26775027, 10.1016/j.tox.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Granero C, Cardona D, Giménez E, Lozano R, Barril J, Sánchez-Santed F, et al. 2013. Chronic dietary exposure to chlorpyrifos causes behavioral impairments, low activity of brain membrane-bound acetylcholinesterase, and increased brain acetylcholinesterase-R mRNA. Toxicology 308:41–49. [DOI] [PubMed] [Google Scholar]
- Lukaszewicz-Hussain A. 2010. Role of oxidative stress in organophosphate insecticide toxicity - Short review. Pestic Biochem Physiol 98(2):145–150, 10.1016/j.pestbp.2010.07.006. [DOI] [Google Scholar]
- Mackenzie Ross SJ, Brewin CR, Curran HV, Furlong CE, Abraham-Smith KM, Harrison V. 2010. Neuropsychological and psychiatric functioning in sheep farmers exposed to low levels of organophosphate pesticides. Neurotoxicol Teratol 32(4):452–459, PMID: 20227490, 10.1016/j.ntt.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlemore-Risher ML, Buccafusco JJ, Terry AV. 2010. Repeated exposures to low-level chlorpyrifos results in impairments in sustained attention and increased impulsivity in rats. Neurotoxicol Teratol 32(4):415–424, PMID: 20350597, 10.1016/j.ntt.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netherton SD, Elias JW, Albrecht NN, Acosta C, Hutton JT, Albrecht JW. 1989. Changes in the performance of parkinsonian patients and normal aged on the Benton Visual Retention Test. Exp Aging Res 15(1–2):13–18, PMID: 2583210, 10.1080/03610738908259753. [DOI] [PubMed] [Google Scholar]
- Ruiz-Muñoz AM, Nieto-Escamez FA, Aznar S, Colomina MT, Sanchez-Santed F. 2011. Cognitive and histological disturbances after chlorpyrifos exposure and chronic Aβ(1-42) infusions in Wistar rats. Neurotoxicology 32(6):836–844, PMID: 21669222, 10.1016/j.neuro.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Nigg HN, Knaak JB. 2000. Blood cholinesterases as human biomarkers of organophosphorus pesticide exposure. Rev Environ Contam Toxicol 163:29–111, PMID: 10771584, 10.1007/978-1-4757-6429-1_2. [DOI] [PubMed] [Google Scholar]
- Pancetti F, Olmos C, Dagnino-Subiabre A, Rozas C, Morales B. 2007. Noncholinesterase effects induced by organophosphate pesticides and their relationship to cognitive processes: implication for the action of acylpeptide hydrolase. J Toxicol Environ Health B Crit Rev 10(8):623–630, PMID: 18049927, 10.1080/10937400701436445. [DOI] [PubMed] [Google Scholar]
- Poet TS, Timchalk C, Hotchkiss JA, Bartels MJ. 2014. Chlorpyrifos PBPK/PD model for multiple routes of exposure. Xenobiotica 44(10):868–881, PMID: 24839995, 10.3109/00498254.2014.918295. [DOI] [PubMed] [Google Scholar]
- Ray A, Liu J, Ayoubi P, Pope C. 2010. Dose-related gene expression changes in forebrain following acute, low-level chlorpyrifos exposure in neonatal rats. Toxicol Appl Pharmacol 248(2):144–155, PMID: 20691718, 10.1016/j.taap.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidy TJ, Bolter JF, Cone JE. 1994. Neuropsychological sequelae of methyl bromide: a case study. Brain Inj 8(1):83–93, PMID: 8124320, 10.3109/02699059409150961. [DOI] [PubMed] [Google Scholar]
- Reisfeld B, Mayeno AN, Lyons MA, Yang, RSH. 2007. Physiologically based pharmacokinetic and pharmacodynamic modeling. In: Computational Toxicology: Risk Assessment for Pharmaceutical and Environmental Chemicals. Ekins S, ed Hoboken, NJ:John Wiley & Sons, Inc, 33–69. [Google Scholar]
- Reiss R, Neal B, Lamb JC 4th, Juberg DR. 2012. Acetylcholinesterase inhibition dose-response modeling for chlorpyrifos and chlorpyrifos-oxon. Regul Toxicol Pharmacol 63(1):124–131, PMID: 22446730, 10.1016/j.yrtph.2012.03.008. [DOI] [PubMed] [Google Scholar]
- Rohlman DS, Anger WK, Lein PJ. 2011. Correlating neurobehavioral performance with biomarkers of organophosphorous pesticide exposure. Neurotoxicology 32(2):268–276, PMID: 21182866, 10.1016/j.neuro.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roldán-Tapia L, Parrón T, Sánchez-Santed F. 2005. Neuropsychological effects of long-term exposure to organophosphate pesticides. Neurotoxicol Teratol 27(2):259–266, PMID: 15734277, 10.1016/j.ntt.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Ross SM, McManus IC, Harrison V, Mason O. 2013. Neurobehavioral problems following low-level exposure to organophosphate pesticides: a systematic and meta-analytic review. Crit Rev Toxicol 43(1):21–44, PMID: 23163581, 10.3109/10408444.2012.738645. [DOI] [PubMed] [Google Scholar]
- Samsam TE, Hunter DL, Bushnell PJ. 2005. Effects of chronic dietary and repeated acute exposure to chlorpyrifos on learning and sustained attention in rats. Toxicol Sci 87(2):460–468, PMID: 16033991, 10.1093/toxsci/kfi264. [DOI] [PubMed] [Google Scholar]
- Soltaninejad K, Abdollahi M. 2009. Current opinion on the science of organophosphate pesticides and toxic stress: a systematic review. Med Sci Monit 15(3):RA75–RA90, PMID: 19247260. [PubMed] [Google Scholar]
- Terry AV Jr, Beck WD, Warner S, Vandenhuerk L, Callahan PM. 2012. Chronic impairments in spatial learning and memory in rats previously exposed to chlorpyrifos or diisopropylfluorophosphate. Neurotoxicol Teratol 34(1):1–8, PMID: 22024239, 10.1016/j.ntt.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry AV Jr, Buccafusco JJ, Gearhart DA, Beck WD, Middlemore-Risher M-L, Truan JN, et al. 2011. Repeated, intermittent exposures to diisopropylfluorophosphate in rats: protracted effects on cholinergic markers, nerve growth factor-related proteins, and cognitive function. Neuroscience 176:237–253, PMID: 21185910, 10.1016/j.neuroscience.2010.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry AV Jr, Gearhart DA, Beck WD Jr, Truan JN, Middlemore M-L, Williamson LN, et al. 2007. Chronic, intermittent exposure to chlorpyrifos in rats: protracted effects on axonal transport, neurotrophin receptors, cholinergic markers, and information processing. J Pharmacol Exp Ther 322(3):1117–1128, PMID: 17548533, 10.1124/jpet.107.125625. [DOI] [PubMed] [Google Scholar]
- Terry AV Jr, Stone JD, Buccafusco JJ, Sickles DW, Sood A, Prendergast MA. 2003. Repeated exposures to subthreshold doses of chlorpyrifos in rats: hippocampal damage, impaired axonal transport, and deficits in spatial learning. J Pharmacol Exp Ther 305(1):375–384, PMID: 12649392, 10.1124/jpet.102.041897. [DOI] [PubMed] [Google Scholar]
- U.S. EPA (U.S. Environmental Protection Agency). 2006. “Organophosphate Pesticides (OP) Cumulative Assessment. 2006 Update.” Washington, DC:U.S. EPA. http://citeseerx.ist.psu.edu/viewdoc/download;jsessionid=56FFA70B754032348E3E2DCA9BE40B91?doi=10.1.1.645.5687&rep=rep1&type=pdf [accessed 23 March 2016].
- U.S. EPA. 2011. “Pesticides Industry Sales and Usage 2006 and 2007 Market Estimates.” Washington, DC. https://www.epa.gov/sites/production/files/2015-10/documents/market_estimates2007.pdf [accessed 10 June 2016].
- U.S. EPA. 2014. “Chlorpyrifos: revized Human Health Risk Assessment for Registration Review.” Washington DC. D424485. https://www.regulations.gov/document?D=EPA-HQ-OPP-2008-0850-0195 [accessed 10 May 2017].
- U.S. EPA. 2015. “Literature Review on Neurodevelopmental Effects & FQPA Safety Factor Determination for the Organophosphate Pesticides.” D331251. https://www.regulations.gov/document?D=EPA-HQ-OPP-2008-0883-0012 [accessed 9 May 2017]
- U.S. EPA. 2016a. Benchmark Dose (BMD) Methods. https://www.epa.gov/bmds/benchmark-dose-bmd-methods [accessed 23 March 2016].
- U.S. EPA. 2016b. U.S. EPA Federal Insecticide, Fungicide, and Rodenticide Act Scientific Advisory Panel (FIFRA SAP) April 19-21, 2016 meeting on Chlorpyrifos: Analysis of Biomonitoring Data. https://www.regulations.gov/document?D=EPA-HQ-OPP-2016-0062-0140 [accessed 8 May 2017].
- U.S. EPA. 2016c. “Chlorpyrifos: Revised Human Risk Assessment for Registration Review.” https://www.regulations.gov/document?D=EPA-HQ-OPP-2015-0653-0454 [accessed 11 May 2017].
- Vorhees CV, Williams MT. 2006. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 1(2):848–858, PMID: 17406317, 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Liu Z, Zhang J, Wu Y, Sun H. 2016. Chlorpyrifos exposure in farmers and urban adults: Metabolic characteristic, exposure estimation, and potential effect of oxidative damage. Environ Res 149:164–170, PMID: 27208467, 10.1016/j.envres.2016.05.011. [DOI] [PubMed] [Google Scholar]
- Webster SJ, Bachstetter AD, Nelson PT, Schmitt FA, Van Eldik LJ. 2014. Using mice to model Alzheimer’s dementia: an overview of the clinical disease and the preclinical behavioral changes in 10 mouse models. Front Genet 5:88, PMID: 24795750, 10.3389/fgene.2014.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C, Jiao L, Zhao J, Yang H, Peng S. 2012. Repeated exposures to chlorpyrifos lead to spatial memory retrieval impairment and motor activity alteration. Neurotoxicol Teratol 34(4):442–449, PMID: 22640976, 10.1016/j.ntt.2012.05.053. [DOI] [PubMed] [Google Scholar]
- Yang D, Howard A, Bruun D, Ajua-alemanj M, Pickart C, Lein PJ. 2008. Chlorpyrifos and chlorpyrifos-oxon inhibit axonal growth by interfering with the morphogenic activity of acetylcholinesterase. Toxicol Appl Pharmacol 228(1):32–41, PMID: 18076960, 10.1016/j.taap.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurlinden TJ. 2016. Computational Modeling of the Pharmacokinetics and Pharmacodynamics of Selected Xenobiotics [Dissertation]. Fort Collins, CO:Colorado State University. [Google Scholar]