Abstract
GM3, a precursor for synthesis of a- and b-series gangliosides, is elevated in adipocytes of obese model animals and in sera of obese human patients with type 2 diabetes and/or dyslipidemia. GM3 synthase (GM3S)-KO C57BL/6 mice display enhanced insulin sensitivity and reduced development of high-fat diet-induced insulin resistance. However, the pathophysiological roles of GM3 and related gangliosides in the central control of feeding and metabolism remain unclear. We found that a mouse model (KKAy GM3S KO) generated by KO of the GM3S gene in the yellow obese strain, KKAy, displayed significant amelioration of obese phenotype. Whereas KKAy mice were hyperphagic and developed severe obesity, KKAy GM3S KO mice had significantly lower body weight and food intake, and greater glucose and insulin tolerance. The hypothalamic response to intraperitoneal administration of leptin was greatly reduced in KKAy mice, but was retained in KKAy GM3S KO mice. In studies of a cultured mouse hypothalamic neuronal cell line, enhanced leptin-dependent phosphorylation of ERK was observed in GM3S-deficient cells. Furthermore, KKAy GM3S KO mice did show altered coat color, suggesting that GM3S is also involved in melanocortin signaling. Our findings, taken together, indicate that GM3-related gangliosides play key roles in leptin and melanocortin signaling.
Keywords: animal models, diabetes, brain, receptors/hormone, GM3, hypothalamus, leptin resistance, GM3 synthase knockout
Gangliosides [glycosphingolipids (GSLs) that contain sialic acid] are key components of membrane microdomains, where they participate in a variety of biological processes, including cell growth, adhesion, and signal transduction (1). GM3, a precursor molecule in synthesis of a- and b-series gangliosides, shows increased levels in association with obesity, inflammation, and certain metabolic diseases (2–4). GM3 synthase (GM3S)-null mice display enhanced insulin sensitivity and reduced development of high-fat diet (HFD)-induced insulin resistance and chronic low-grade inflammatory states (4, 5). Our group observed associations between numerous metabolic disease risk factors and GM3 molecular species in human serum (6).
The mediobasal hypothalamus, which includes the arcuate nucleus (ARC) and paraventricular nucleus (PVH), plays essential roles in control of feeding, body weight, and energy expenditure. ARC contains two interconnected groups of neurons; one is orexigenic (appetite-stimulating) and releases agouti-related peptide (AgRP) and neuropeptide Y (NPY), while the other is anorexigenic (appetite-suppressing) and releases α-melanocyte-stimulating hormone (α-MSH) produced from proopiomelanocortin (POMC) precursors. α-MSH binds to melanocortin receptor 4 (MC4R) in PVH and other hypothalamic areas, and exerts anorectic effects. NPY binds to its cognate receptor, stimulates feeding, and reduces basal energy expenditure. AgRP acts on MC4R as an inverse agonist and inhibits α-MSH (7). AgRP/NPY neurons and POMC neurons thus regulate melanocortin signaling in a coordinated manner to maintain energy balance.
Leptin, an adipocyte-derived circulating hormone whose expression is correlated with adipose mass, is essential for maintenance of energy homeostasis and body weight. Increased levels of leptin lead to reduction of food intake and body weight through the long form of its receptor, LepRb (8), which is highly expressed in ARC, ventromedial hypothalamic nucleus, and other hypothalamic nuclei. LepRb is expressed in both AgRP/NPY neurons and POMC neurons. Leptin inhibits AgRP neuronal activity and NPY and AgRP expression in AgRP/NPY neurons, and stimulates POMC synthesis in POMC neurons (9).
Diet-induced obesity leads to a state of low-grade inflammation in peripheral insulin target tissues that is associated with development of insulin resistance and type 2 diabetes (10). Diet-induced activation of inflammatory pathways in the mediobasal hypothalamus has been reported (11). There is increasing evidence for an association between hypothalamic inflammation and development of leptin resistance and obesity (12), but the detailed molecular basis for such an association is unclear.
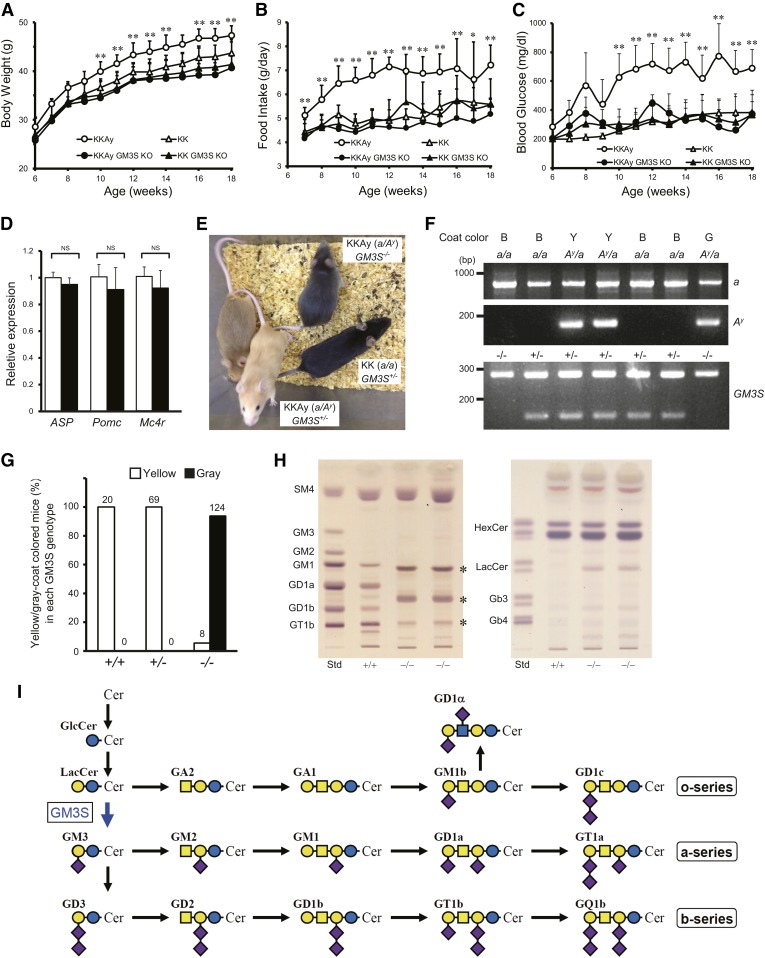
An obese diabetic mouse model, KKAy, was established by introducing Ay mutation into strain KK, resulting in ectopic expression of agouti signaling protein (ASP), which is homologous to AgRP. ASP acts as an antagonist at the melanocortin 1 receptor (MC1R) on hair follicular melanocytes to block α-MSH-induced eumelanin production, resulting in the subterminal band of pheomelanin (13). In the KKAy mouse, it is believed that the ectopic expression of ASP causes obesity due to disruption of melanocortin signaling outside the hair follicle. Onset of obesity and diabetic pathology appear earlier and are more severe in KKAy mice than in KK mice (14, 15). We and Proia’s group observed previously that body weight following a HFD feeding regime did not differ significantly between GM3S-null C57BL/6 mice and WT mice (4, 5). In the present study, we generated GM3S-null mice in a KKAy genetic background (KKAy GM3S KO) to evaluate the pathophysiological roles of GM3 and related gangliosides in obesity. KKAy mice are hyperphagic and typically develop leptin resistance and morbid obesity. In contrast, KKAy GM3S KO mice were less likely to develop leptin resistance and had significantly lower body weight and food intake, comparable to values in control KK mice. The coat color was grayish in homozygous KKAy GM3S KO mice, but yellow in heterozygotes (similar to KKAy mice), suggesting that GM3 is involved in melanin synthesis, possibly through MC1R signaling. Our findings indicate that GM3 and/or related ganglioside species play an essential role in leptin and melanocortin signaling.
MATERIALS AND METHODS
Reagents
Phospho-STAT3 (Tyr705, D3A7), STAT3 (79D7), ERK1/2, and c-fos antibodies were from Cell Signaling Technology (Beverly, MA). Phospho-ERK (E-4) antibody was from Santa Cruz Biotechnology (Santa Cruz, CA). Recombinant mouse leptin was from R&D Systems (Minneapolis, MN).
Generation of GM3S-deficient mice in KK/KKAy background
KK (a/a) and KKAy (Ay/a) mice were from CLEA Japan. GM3S (St3gal5)-deficient mice were generated from C57BL/6 mice as described previously (16). These mice were backcrossed over eight times into the KK background, and KK/GM3S+/− or KK/GM3S−/− females were crossed with KKAy males to obtain KKAy/GM3S+/− mice. Genotyping for the a/Ay alleles was performed by PCR of ear DNA using primers 5′-GGCCACACTTAGGGAGTTCA-3′ (forward primer for a ‘nonagouti’ and Ay ‘lethal yellow’ alleles), 5′-TGCCTTATTCTGTTTTTGTTCC-3′ (reverse primer for a allele), and 5′-GGTTGGCCACCATGTCTAGT-3′ (reverse primer for Ay allele), according to the Jackson Laboratory website (KK.Cg-Ay/J 002468). Genotyping PCR for the GM3S gene was performed using the following primers: 5′-GGAATCCATCCCTTTTCTCACAGAG-3′ and 5′-TGAACTCACTTGGCATTGCTGG-3′ for WT allele, and 5′-ACTGGGCACAACAGACAATCGG-3′ and 5′-TGGATACTTTCTCGGCAGGAGC-3′ for the KO allele (17). KK and KKAy mice with WT GM3S alleles were used as control for each experiment. Body weight and food consumption were measured once per week.
GSL analysis
Lyophilized brain tissue was extracted with chloroform/methanol 2:1, 1:1, 1:2 (v/v), in succession. Total lipids were purified, separated into acidic and neutral fractions, deesterified by methanolysis, and analyzed by HPTLC as described previously (18).
RNA isolation, reverse transcription, and quantitative PCR
Total RNA was extracted using the RNeasy Lipid Tissue Mini kit (Qiagen). cDNA was generated from 500 ng of RNA, and quantitative PCR was performed on a StepOnePlus real-time PCR system (Applied Biosystems) using SYBR Premix Ex Taq II (TaKaRa Shuzo, Kyoto, Japan). Relative mRNA levels were calculated either by a standard curve and normalized to peptidylprolyl isomerase B (Ppib) expression or as 2–ΔΔ±CT with glyceraldehyde-3-phosphate dehydrogenase.
Glucose and insulin tolerance tests
Mice were either fasted for 16 h and administered glucose solution (1.5 g/kg body weight) orally or fasted for 6 h and injected intraperitoneally with insulin solution (0.75 U/kg body weight). Glucose levels in blood (from tail vein) at various time points before and after these treatments were measured with an Accu-Chek Aviva glucometer (Roche Applied Science).
Plasma parameters
Plasma levels of triglycerides (TGs), NEFAs, total cholesterol, and HDL cholesterol were determined using (respectively) the Triglyceride E-test kit, the NEFA C-test kit, the Cholesterol E-test kit, and the HDL-Cholesterol E-test kit (Wako Pure Chemical, Japan). Serum insulin and leptin levels were determined using ELISA kits (Morinaga Institute of Biological Science, Yokohama, Japan).
Generation of GM3S-deficient N41 cells
Exon 5 of the mouse St3gal5 (GM3S) gene, which contains the coding sequence for sialyl motif L, was chosen to design targeting guide RNAs using the online CRISPR Design Tool (19). A primer pair (5′-CACCGCAGGTACGCGAAGACGGCTA-3′ and 5′-AAACTAGCCGTCTTCGCGTACCTGC-3′) was cloned into plasmid pSpCas9(BB)-2A-GFP (Addgene; #48138). The plasmid was introduced into embryonic mouse hypothalamic N41 cells (CELLutions Biosystems, Ontario, Canada) using a Nucleofector device with Cell Line Nucleofector Kit V (Amaxa), as per the manufacturer’s protocol. The above plasmid without targeting sequence served as control. GFP+ cells were isolated using a FACSAria II flow cytometer (BD Biosciences); clonal cell lines were obtained by limiting dilution; and expression levels of gangliosides were evaluated by TLC.
Leptin administration and immunohistochemistry
Intraperitoneal injection of leptin and immunohistochemical detection of c-fos protein were performed as described previously (20, 21). In brief, mice were fasted for 24 h, intraperitoneally injected with either leptin (4 mg/kg body weight) or saline, anesthetized 2 h later with pentobarbital, and perfused transcardially with saline followed by 4% paraformaldehyde in PBS. Brains were removed, postfixed in the same fixative overnight, embedded in OCT compound (Sakura Finetek, Tokyo, Japan), frozen, and sectioned coronally (thickness 40 μm). Floating sections were pretreated for 10 min with 3% hydrogen peroxide, blocked with 3% BSA for 1 h, incubated in c-fos antibody (dilution 1:2,000) for 72 h at 4°C, washed, incubated with Simple Stain MAX-PO (Nichirei Biosciences, Tokyo, Japan) for 1 h, washed, incubated in 0.05% DAB solution for 10 min (for visualization of c-fos protein), stained with hematoxylin, dehydrated, and mounted on coverslips. The c-fos-positive cells in ARC were counted at regular intervals in three sections for each animal.
Lentiviral construct and establishment of stably LepRb-expressing cells
To generate a lentiviral construct for stable expression of full-length mouse leptin receptor (mLepRb), a 3.5 kb mLepRb cDNA fragment was subcloned into CSII-EF-RfA vector (developed by Dr. H. Miyoshi, Keio University, Tokyo, Japan and provided by RIKEN BioResource Research Center, Tsukuba, Japan). HEK293T cells cultured in DMEM supplemented with 10% FBS were transfected with mLepRb/CSII-EF-RfA, pCAG-HIVgp, and pCMV-VSV-G-RSV-Rev plasmids using Lipofectamine 2000 (Invitrogen), and incubated for 24 h at 37°C. Medium was replaced by fresh medium containing 10 μM forskolin, and cells were cultured for another 24 h at 32°C. Lentivirus-containing supernatant was harvested by centrifugation at 200 g for 3 min. For lentiviral transduction of cultured N41 cells, the supernatant was added; the cells were incubated for 24 h at 32°C; the temperature was increased to 37°C; the incubation was continued for 48 h; the medium was replaced; and the culture was continued for 1 week prior to experiments. Cells established by this procedure were designated as N41-LepRb cells.
Leptin treatment of N41 cells and immunoblotting
Phosphorylation assays of ERK and STAT3 were performed using N41 and N41-LepRb cells, respectively. Near-confluent cells were washed twice with DMEM, incubated for 2 h with DMEM containing 0.5% FBS, stimulated for various durations with 0.5 μg/ml leptin, washed twice with ice-cold PBS, lysed, and analyzed by Western blotting with appropriate antibodies, as described previously (2).
Statistical analysis
Data were analyzed by Student’s t-test and ANOVA followed by Tukey’s post hoc test, using the GraphPad Prism software program.
RESULTS
Mouse strain KKAy was established in 1969 by Ay mutation of strain KK, resulting in ectopic expression of ASP and associated hyperphagia, obesity, and hyperglycemia (22). To investigate the roles of GM3 and/or related gangliosides in the pathogenesis of obesity, we knocked out the GM3S gene in KKAy to generate strain KKAy GM3S KO. These mice showed notable amelioration of obese phenotype in terms of reduced body weight, daily food intake, and blood glucose level (Fig. 1A–C). mRNA expression levels of ASP, Pomc, and Mc4r did not differ significantly in the hypothalami of KKAy versus KKAy GM3S KO mice (Fig. 1D). The coat color was grayish for KKAy GM3S KO homozygotes, but yellow (similar to KKAy) for heterozygotes (Fig. 1E). The a/Ay genotype was confirmed by genotyping PCR (Fig. 1F) and it was found that whereas all the KKAy GM3S+/+ or GM3S+/− mice had yellow coat color, only 6.1% of KKAy GM3S−/− mice had yellow and all of the rest had gray coat color. Brain ganglioside expression patterns for KKAy GM3S KO were similar to those reported previously for GM3S-deficient mice in a C57BL/6 background (5, 17). Brains of KKAy GM3S KO animals lacked a- and b-series gangliosides (GM1, GD1a, GD1b, and GT1b), the major ganglioside species in WT brain; rather, they expressed predominantly o-series gangliosides, such as GM1b and GD1α (Fig. 1H, I).
Fig. 1.
GM3S KO prevented development of hyperphagia and obesity in KKAy mice. A–C: Body weight (A), food intake (B), and nonfasting blood glucose levels (C) of KKAy and KK mice with or without deletion of the GM3S gene (GM3S KO) (n ≥ 5 for each group). *P < 0.05, **P < 0.01 versus KKAy GM3S KO. D: Quantitative RT-PCR analysis of mRNA for ASP, Pomc, and Mc4r in KKAy and KKAy GM3S KO hypothalami. White bar, KKAy; black bar, KKAy GM3S KO (n = 3 for each group). *P < 0.05, **P < 0.01 versus KKAy GM3S KO. E: Coat color phenotype of GM3S KO (GM3S−/−) observed in the KKAy (a/Ay) background. F: Representative genotyping PCR results for KKAy GM3S KO mice. Top panel: Genotyping results for the a/Ay allele. The a and Ay alleles generate PCR products of 839 bp and 163 bp, respectively. Coat color: B, black; Y, yellow; G, gray. Bottom panel: Genotyping results for GM3S KO. The WT and mutant alleles generate PCR products of 276 bp and 120 bp, respectively. G: The percentage of yellow- or gray-coat-colored offspring compared with the total number of Ay/a offspring born with each GM3S genotype. The number of each group is indicated on the top of each bar. Because of the infertility of KKAy females, KKAy males (Ay/a, GM3S+/− or −/−) and KK females (a/a, GM3S+/− or −/−) were used for breeding. Data of the a/a offspring (black coat color) were excluded. H: TLC of acidic (left) and neutral (right) GSLs from KKAy (+/+) and KKAy GM3S KO (−/−) brains. GSLs were detected using orcinol-sulfuric acid reagent. The asterisks indicate o-series gangliosides whose biosynthesis is independent of GM3S [see text and references (5) and (17) for details]. (I): Biosynthetic pathway of ganglio-series gangliosides. GM3S is a sialyltransferase required for initiation of synthesis of a- and b-series gangliosides.
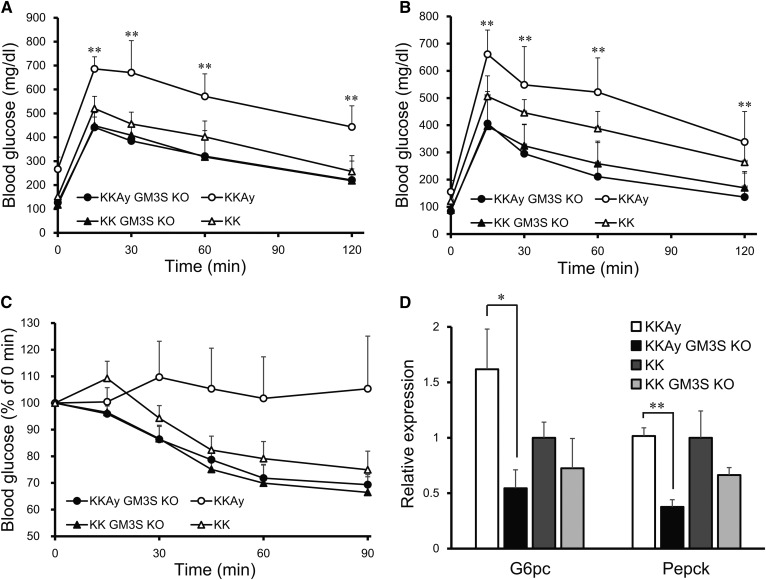
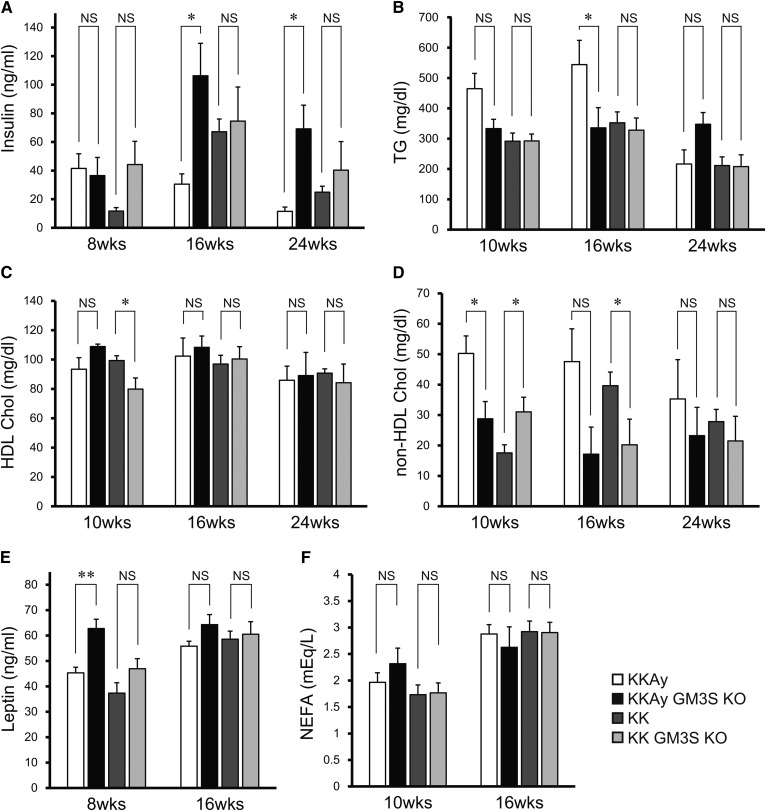
At age 14 weeks, the glucose tolerance of KKAy GM3S KO mice was significantly greater than that of KKAy mice (Fig. 2A). Even in the KK background, GM3S KO mice showed a slight improvement of glucose tolerance at age 24 weeks (Fig. 2B), similarly to our previous findings for HFD-fed C57BL/6 GM3S KO mice (4). KKAy GM3S KO mice also showed greatly increased insulin tolerance at age 24 weeks (Fig. 2C), and reduced expression levels of the gluconeogenesis genes, G6pc and Pepck, in liver (Fig. 2D). Plasma insulin levels at ages 16 and 24 weeks were low in KKAy mice, but significantly higher in KKAy GM3S KO mice, probably due to preserved insulin secretion (Fig. 3A). Plasma TG and non-HDL cholesterol levels at ages 10 and 16 weeks in KKAy mice were higher than in KK mice, while these levels in KKAy GM3S KO mice were lower than in KKAy mice (similar to those in KK) (Fig. 3B, D). Plasma leptin levels at age 8 weeks in KKAy GM3S KO mice were significantly higher than in KKAy mice (Fig. 3E). HDL cholesterol and NEFA levels did not differ notably between KKAy and KKAy GM3S KO mice (Fig. 3C, F).
Fig. 2.
GM3S KO-enhanced glucose and insulin tolerance in KKAy mice. A, B: Glucose tolerance tests at age 14 weeks (A) and 24 weeks (B). C: Insulin tolerance tests at age 24 weeks. D: Quantitative analysis of mRNA for G6pc and Pepck in liver at age 24 week (n ≥ 4 for each group). *P < 0.05, **P < 0.01 vs. KKAy GM3S KO.
Fig. 3.
Blood parameters in KKAy GM3S KO and control mice. Blood plasma or serum samples were collected from mice fed ad libitum at the indicated times.Insulin (A), TG (B), HDL cholesterol (HDL Chol) (C), non-HDL Chol (D), leptin (E), and NEFAs (F) (n ≥ 4 for each group). *P < 0.05, **P < 0.01.
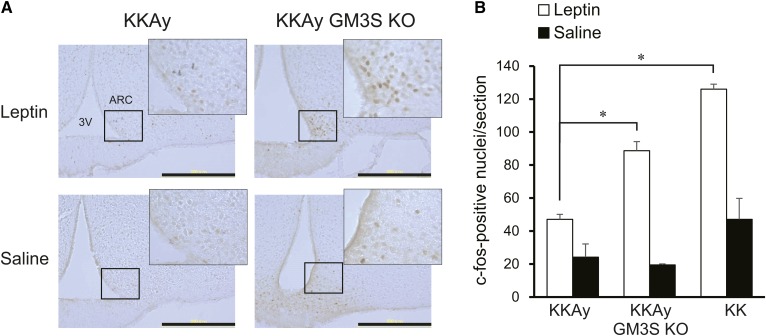
KKAy mice develop obesity (associated with hyperglycemia and hyperinsulinemia), hyperleptinemia, and leptin resistance (23). We examined possible changes in central responsiveness to leptin in KKAy GM3S KO mice. In KKAy mice, intraperitoneal injection of leptin had only a slight effect on neuron activation, as assayed by c-fos expression in ARC (Fig. 4A), supporting the concept that development of obesity in these mice induces increased leptin resistance. In contrast, injection of leptin in KKAy GM3S KO mice caused a notable increase of c-fos expression (Fig. 4A, B), indicating that responsiveness to leptin is maintained in these mice.
Fig. 4.
KKAy GM3S KO mice retained leptin responsiveness in ARC. A: Representative images of basal (saline) and leptin-induced c-fos immunoreactivity in ARC of KKAy and KKAy GM3S KO mice (male, age 10 weeks). Fasted mice were injected intraperitoneally with either saline or leptin. ARC sections were stained with c-fos antibody. Scale bars: 300 μm. B: The c-fos positive cells in ARC were counted (n = 3 for each group). *P < 0.05.
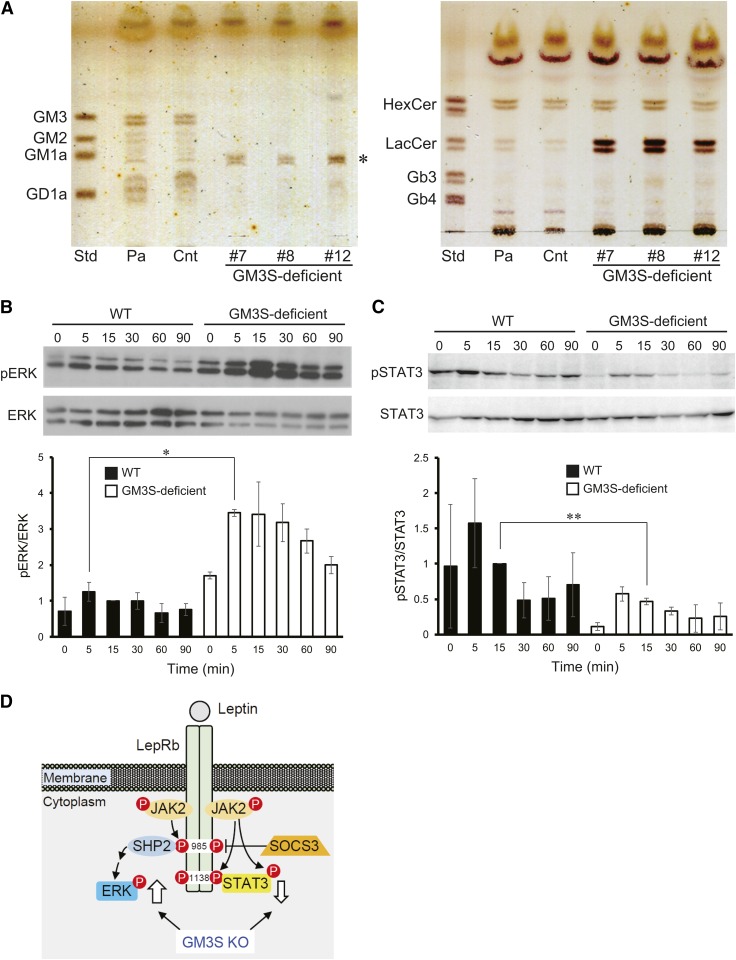
To examine the possible effects of loss of GM3S activity on leptin receptor signaling, we established a GM3S-deficient mutant of mouse hypothalamic neuronal N41 cells. We used the CRISPR/Cas9 system to target exon 5, which contains sialyl motif L, a conserved sequence motif essential for catalytic activity of mammalian sialyltransferases (24). GSL analysis showed that several obtained clones lacked GM3 and related gangliosides (Fig. 5A, left), but contained LacCer (Fig. 5A, right). A major GM1-like band (Fig. 5A, left) was found to be GM1 by LC/MS/MS multiple reaction monitoring (supplemental data), and most likely GM1b, an o-series ganglioside also observed in KKAy GM3S KO brain (Fig. 1D). LepRb is highly expressed in the mediobasal hypothalamus and mediates anti-obesity signaling by leptin via the Janus-activated kinase 2/signal transducers and activators of transcription 3 (JAK2-STAT3) pathway. The hypothalamic ERK pathway plays a key role in mediating control of food intake and thermogenesis by leptin (25). Leptin, at a concentration 0.5 μg/ml, greatly enhanced ERK phosphorylation in GM3S-deficient N41 cells (Fig. 5B). In contrast, we could not detect STAT3 phosphorylation by leptin, even at 1 μg/ml, in either parental or GM3S-deficient N41 cells (data not shown). To evaluate leptin-induced phosphorylation of STAT3, we established stable expression of LepRb by lentiviral transfer of the mouse LepRb gene. LepRb-expressing cells responded to leptin at 0.5 μg/ml, and STAT3 phosphorylation was slightly reduced in GM3S-deficient cells (Fig. 5C). These findings indicate that the leptin-dependent ERK pathway was enhanced by deficient ganglioside synthesis in KKAy GM3S KO.
Fig. 5.
Leptin-induced ERK phosphorylation was greatly enhanced in GM3S-deficient N41 cells. A: TLC of acidic (left) and neutral (right) GSLs from control and GM3S-deficient cell clones generated from N41 cells. Std, standard; Pa, parental cells; Cnt, control cells transfected with vector without targeting sequence. An asterisk indicates a GM1-like band, which is most likely GM1b, an o-series ganglioside. B, C: WT and GM3S-deficient cells were treated with leptin and then lysed at the indicated times. For pSTAT3 assay (C), the LepRb gene was introduced for stable expression (see text). The blots are representative of three independent experiments. Bottom: quantification of immunoblots (n = 3). *P < 0.05, **P < 0.01. D: Leptin receptor signaling pathway. Thick arrows: increased and decreased phosphorylation of ERK and STAT3 (respectively) observed in GM3S-deficient cells.
The observed coat color change and reduction of food intake and body weight in KKAy GM3S KO mice suggested that GM3 and related gangliosides play an important role in melanocortin receptor signaling. We examined possible effects of GM3 deficiency on MC4R signaling in HEK293T cells. Parental and GM3-deficient cells were transfected with the mouse Mc4r gene; intracellular cAMP production was evaluated; and G protein-coupled receptor activation was assayed by TGF-α shedding assay, as described previously (26), using α-MSH with or without synthetic mouse AgRP or ASP. No clear difference was observed between parental and GM3-deficient cells (data not shown; see Discussion).
DISCUSSION
Previous reports by our group and others have demonstrated the increased insulin sensitivity of GM3S KO mice in a C57BL/6 background relative to WT mice. KO mice developed HFD-induced obesity (as did WT mice), but exhibited reduced development of insulin resistance and chronic low-grade inflammatory states (4, 5). In the present study, we used a GM3S KO strain of obese mouse model KKAy to evaluate the pathophysiological effects of GM3 or related gangliosides in hyperphagic and obese phenotypes. The coat color was grayish in KKAy GM3S KO homozygotes, but yellow (similar to KKAy) in heterozygotes. The yellow Ay mutation (in the agouti gene) causes ectopic expression of the gene product, ASP, resulting in a switch of follicular melanocytes from synthesis of eumelanin (black) to phaeomelanin (yellow) through inhibition of MC1R signaling as an endogenous antagonist, by blocking α-MSH (13). The observed coat color change of KKAy GM3S KO mice may suggest involvement of GM3/related gangliosides in MC1R signaling in melanocytes, possibly through direct effect on MC1R function (e.g., cell surface expression or α-MSH binding) or direct or indirect modulation of ASP binding to MC1R, although functional analysis will be required to establish this point. Mutations of the GM3S (ST3GAL5) gene were identified in patients with salt-and-pepper syndrome, an autosomal recessive neurocutaneous disorder characterized by altered skin pigmentation (27).
In contrast to the previous results from GM3S KO C57BL/6 mice, the obese model KKAy mice that have perturbed melanocortin signaling that thereby cause defects in the central control of feeding behavior, showed remarkable reductions of body weight and food intake by GM3S KO, suggesting that the anti-obese effects observed in the present study are mediated at least in part by the central defects in the synthesis of GM3/ related gangliosides. Given that the reduced body weight and food intake in the KKAy GM3S KO mice were observed as early as 6 weeks of age, we believe that the food intake effects are central in origin. The observed food intake effects suggest that hypothalamic MC4R function may also be modulated by GM3/related gangliosides (Fig. 6, a schematic illustration of our current model). We used GM3-deficient HEK293T cells to examine α-MSH-dependent MC4R activation in the presence/absence of ASP and AgRP. For this assay, we used commercially available synthetic mouse ASP(93-132)-NH2 and AgRP(82-131)-NH2, which are conserved Cys-rich C-terminal domains sufficient for melanocortin receptor antagonism (28). The synthetic ASP and AgRP both strongly inhibited MC4R activation, but the inhibitory effect did not differ notably in parental versus GM3S-deficient cells. (data not shown). However, we cannot exclude a possibility that the cell system may not reflect the conditions in neurons (e.g., intrinsic properties and ganglioside expression pattern) and thus, further investigation will be needed to conclude whether gangliosides are involved in the MC4R function.
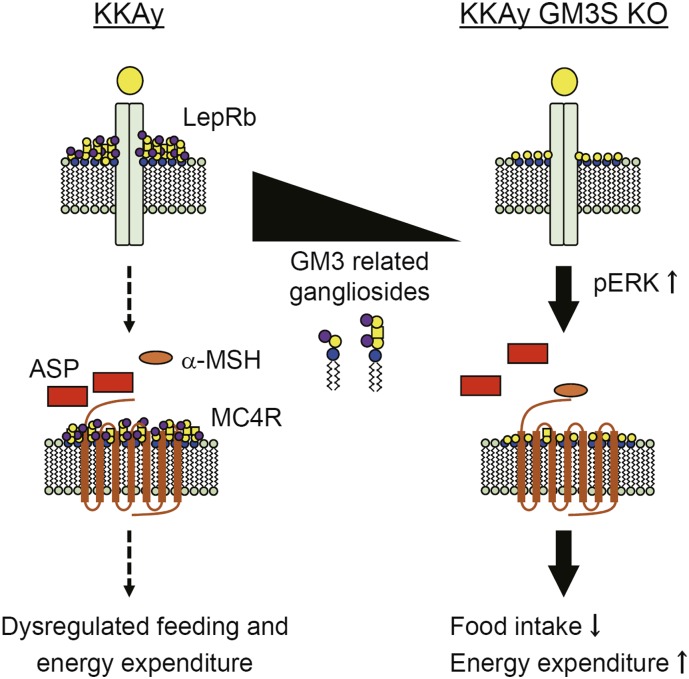
Fig. 6.
A working hypothesis of the involvement of GM3/ related gangliosides in leptin and melanocortin receptor signaling. Elimination of GM3/related gangliosides ameliorated the obese phenotype of KKAy mice. Ectopic expression of ASP caused alteration of MC4R signaling and leptin resistance in hypothalamic neurons, resulting in dysregulated feeding and energy expenditure. Elimination of GM3/related gangliosides restored leptin sensitivity. These findings and the observed coat color change suggest that GM3/related gangliosides are involved in MC1R/MC4R signaling (see Discussion).
Body weight and food intake were notably lower in KKAy GM3S KO mice than in KKAy mice, but no striking difference was observed between KK GM3S KO and KK mice (Fig. 1A, B). These findings suggest a function of GM3/related gangliosides in hyperphagia and obesity development, perhaps through altered ASP expression or function in KKAy mice. No significant changes were observed in Asp, Pomc, or Mc4r gene expression in KKAy GM3S KO hypothalamus at age 6 weeks (Fig. 1D).
In the leptin administration experiments, KKAy GM3S KO mice were less susceptible to central leptin resistance than were KKAy mice. In GM3S-deficient hypothalamic neuronal cells, leptin-dependent STAT3 phosphorylation was slightly reduced, whereas leptin-dependent ERK phosphorylation was greatly enhanced, suggesting differential roles of specific ganglioside(s) in LepRb signaling. Numerous studies have demonstrated an essential role of the JAK2-STAT3 pathway in the anorectic and metabolic effects of leptin. Leptin binding to LepRb activates JAK2, and JAK2 subsequently phosphorylates other Tyr residues within LepRb and JAK2 itself (29, 30). Phospho-Tyr1138 serves as a binding site for STAT3, and STAT3 is subsequently phosphorylated by JAK2 and translocated to the nucleus to modulate expression of its target genes, which include POMC and suppressor of cytokine signaling 3 (SOCS3) (31). Protein tyrosine phosphatase 2 (SHP2) is recruited to phospho-Tyr985 in response to leptin, and phosphorylated by JAK2. Phosphorylated SHP2 can bind the adaptor protein, growth factor receptor-bound protein 2 (Grb2), which facilitates ERK activation. The SHP2/ERK pathway is involved in the anti-obesity effect of leptin (25, 32).
Nordstrom et al. (33) reported that loss of GlcCer synthase in hypothalamic neurons inhibited LepRb signaling, using mice with deletion of inducible neuron-specific Ugcg, the gene encoding GlcCer synthase. These mice developed progressive obesity, and the obese phenotype was significantly ameliorated by adeno-associated virus-mediated Ugcg delivery to ARC. GM1 and GD1a in N41 cells interacted with LepRb, suggesting that these a-series gangliosides enhance leptin signaling in hypothalamic neurons. In a study of GD3S-null mice, which lack all b-series gangliosides, Ji et al. (34) observed enhanced LepRb signaling in the hypothalamus, as indicated by phosphorylated STAT3 levels. Overexpression of GD3S in N41 cells reduced responsiveness to leptin, supporting the concept that certain a-series gangliosides positively regulate LepRb signaling. The GM3S KO mice used in the present study lacked all a-series gangliosides (Fig. 1F, G), but still retained hypothalamic responsiveness to leptin. Our findings in GM3S-deficient N41 cells (Fig. 5B, D) suggest that GM3S KO mice may be protected from central leptin resistance by enhancement of leptin-dependent ERK phosphorylation. We speculate that there are at least two possibilities; that i) loss of GM3, GM1a, or GD1a or ii) increased GM1b, enhanced the ERK signaling. Future studies will address the detailed mechanism of this effect.
Increasing evidence indicates that hypothalamic inflammation contributes to the development of leptin resistance and obesity (11, 12). A HFD may result in activation of inflammatory pathways in the hypothalamus even in the absence of notable weight gain. In animal obesity models, GM3 and GM3S gene expression is upregulated in adipose tissues (2, 4). In obese human patients, serum GM3 levels are elevated and associated with metabolic risk factors (3, 6). It is conceivable that ganglioside expression in the hypothalamus is altered by HFD- and/or obesity-induced inflammation. Such alteration may play a pathophysiological role in the regulation of energy homeostasis through effects on receptor signaling involved in feeding behavior and energy expenditure.
Supplementary Material
Acknowledgments
The authors thank all members of the Inokuchi laboratory for fruitful discussions and technical assistance, Dr. S. Anderson for helpful comments and English editing of the manuscript, and Tohoku Medical and Pharmaceutical University Center for Laboratory Animal Science for their services.
Footnotes
Abbreviations:
- AgRP
- agouti-related peptide
- ARC
- arcuate nucleus
- ASP
- agouti signaling protein
- α-MSH
- α-melanocyte-stimulating hormone
- GM3S
- GM3 synthase
- GSL
- glycosphingolipid
- HFD
- high-fat diet
- JAK2-STAT3
- Janus-activated kinase 2/signal transducers and activators of transcription 3
- LepRb
- leptin receptor long form
- MC1R
- melanocortin receptor 1
- MC4R
- melanocortin receptor 4
- NPY
- neuropeptide Y
- POMC
- proopiomelanocortin
- PVH
- paraventricular nucleus
- TG
- triglyceride
This study was supported by Ministry of Education, Culture, Sports, Science and Technology Grants-in-Aid for Scientific Research 26440061 (K-i.I.); and 16H04767 (J-i.I.), the Uehara Memorial Foundation (K-i.I.), the Kato Memorial Bioscience Foundation (K-i.I.), the Novartis Foundation (Japan) for the Promotion of Science (K-i.I.), and the Suzuken Memorial Foundation (K-i.I.).
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Hakomori S. 2002. The glycosynapse. Proc. Natl. Acad. Sci. USA. 99: 225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tagami S., Inokuchi J., Kabayama K., Yoshimura H., Kitamura F., Uemura S., Ogawa C., Ishii A., Saito M., Ohtsuka Y., et al. . 2002. Ganglioside GM3 participates in the pathological conditions of insulin resistance. J. Biol. Chem. 277: 3085–3092. [DOI] [PubMed] [Google Scholar]
- 3.Sato T., Nihei Y., Nagafuku M., Tagami S., Chin R., Kawamura M., Miyazaki S., Suzuki M., Sugahara S., Takahashi Y, et al. . 2008. Circulating levels of ganglioside GM3 in metabolic syndrome: a pilot study. Obes. Res. Clin. Pract. 2: I–II. [DOI] [PubMed] [Google Scholar]
- 4.Nagafuku M., Sato T., Sato S., Shimizu K., Taira T., and Inokuchi J.. 2015. Control of homeostatic and pathogenic balance in adipose tissue by ganglioside GM3. Glycobiology. 25: 303–318. [DOI] [PubMed] [Google Scholar]
- 5.Yamashita T., Hashiramoto A., Haluzik M., Mizukami H., Beck S., Norton A., Kono M., Tsuji S., Daniotti J. L., Werth N., et al. . 2003. Enhanced insulin sensitivity in mice lacking ganglioside GM3. Proc. Natl. Acad. Sci. USA. 100: 3445–3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Veillon L., Go S., Matsuyama W., Suzuki A., Nagasaki M., Yatomi Y., and J. Inokuchi. 2015. Identification of ganglioside GM3 molecular species in human serum associated with risk factors of metabolic syndrome. PLoS One. 10: e0129645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ollmann M. M., Wilson B. D., Yang Y. K., Kerns J. A., Chen Y., Gantz I., and G. S. Barsh. 1997. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 278: 135–138. [DOI] [PubMed] [Google Scholar]
- 8.Tartaglia L. A., Dembski M., Weng X., Deng N., Culpepper J., Devos R., Richards G. J., Campfield L. A., Clark F. T., Deeds J., et al. . 1995. Identification and expression cloning of a leptin receptor, OB-R. Cell. 83: 1263–1271. [DOI] [PubMed] [Google Scholar]
- 9.Myers M. G. Jr., Munzberg H., Leinninger G. M., and Leshan R. L.. 2009. The geometry of leptin action in the brain: more complicated than a simple ARC. Cell Metab. 9: 117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wellen K. E., and Hotamisligil G. S.. 2005. Inflammation, stress, and diabetes. J. Clin. Invest. 115: 1111–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valdearcos M., Xu A. W., and Koliwad S. K.. 2015. Hypothalamic inflammation in the control of metabolic function. Annu. Rev. Physiol. 77: 131–160. [DOI] [PubMed] [Google Scholar]
- 12.Jais A., and Bruning J. C.. 2017. Hypothalamic inflammation in obesity and metabolic disease. J. Clin. Invest. 127: 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu D., Willard D., Patel I. R., Kadwell S., Overton L., Kost T., Luther M., Chen W., Woychik R. P., Wilkison W. O., et al. . 1994. Agouti protein is an antagonist of the melanocyte-stimulating-hormone receptor. Nature. 371: 799–802. [DOI] [PubMed] [Google Scholar]
- 14.Iwatsuka H., Shino A., and Suzuoki Z.. 1970. General survey of diabetic features of yellow KK mice. Endocrinol. Jpn. 17: 23–35. [DOI] [PubMed] [Google Scholar]
- 15.Fan W., Boston B. A., Kesterson R. A., Hruby V. J., and Cone R. D.. 1997. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 385: 165–168. [DOI] [PubMed] [Google Scholar]
- 16.Tsukamoto K., Kohda T., Mukamoto M., Takeuchi K., Ihara H., Saito M., and S. Kozaki. 2005. Binding of Clostridium botulinum type C and D neurotoxins to ganglioside and phospholipid. Novel insights into the receptor for clostridial neurotoxins. J. Biol. Chem. 280: 35164–35171. [DOI] [PubMed] [Google Scholar]
- 17.Yoshikawa M., Go S., Takasaki K., Kakazu Y., Ohashi M., Nagafuku M., Kabayama K., Sekimoto J., Suzuki S., Takaiwa K., et al. . 2009. Mice lacking ganglioside GM3 synthase exhibit complete hearing loss due to selective degeneration of the organ of Corti. Proc. Natl. Acad. Sci. USA. 106: 9483–9488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inokuchi J., Momosaki K., Shimeno H., Nagamatsu A., and Radin N. S.. 1989. Effects of D-threo-PDMP, an inhibitor of glucosylceramide synthetase, on expression of cell surface glycolipid antigen and binding to adhesive proteins by B16 melanoma cells. J. Cell. Physiol. 141: 573–583. [DOI] [PubMed] [Google Scholar]
- 19.Ran F. A., Hsu P. D., Wright J., Agarwala V., Scott D. A., and Zhang F.. 2013. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 8: 2281–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsukita S., Yamada T., Uno K., Takahashi K., Kaneko K., Ishigaki Y., Imai J., Hasegawa Y., Sawada S., Ishihara H., et al. . 2012. Hepatic glucokinase modulates obesity predisposition by regulating BAT thermogenesis via neural signals. Cell Metab. 16: 825–832. [DOI] [PubMed] [Google Scholar]
- 21.Yamada T., Katagiri H., Ishigaki Y., Ogihara T., Imai J., Uno K., Y. Hasegawa, J. Gao, H. Ishihara, A. Niijima, et al. . 2006. Signals from intra-abdominal fat modulate insulin and leptin sensitivity through different mechanisms: neuronal involvement in food-intake regulation. Cell Metab. 3: 223–229. [DOI] [PubMed] [Google Scholar]
- 22.Nishimura M. 1969. Breeding of mice strains for diabetes mellitus. Exp. Anim. 18: 147–157. [Google Scholar]
- 23.Masuzaki H., Ogawa Y., Aizawa-Abe M., Hosoda K., Suga J., Ebihara K., N. Satoh, H. Iwai, G. Inoue, H. Nishimura, et al. . 1999. Glucose metabolism and insulin sensitivity in transgenic mice overexpressing leptin with lethal yellow agouti mutation: usefulness of leptin for the treatment of obesity-associated diabetes. Diabetes. 48: 1615–1622. [DOI] [PubMed] [Google Scholar]
- 24.Datta A. K., Chammas R., and Paulson J. C.. 2001. Conserved cysteines in the sialyltransferase sialylmotifs form an essential disulfide bond. J. Biol. Chem. 276: 15200–15207. [DOI] [PubMed] [Google Scholar]
- 25.Rahmouni K., Sigmund C. D., Haynes W. G., and Mark A. L.. 2009. Hypothalamic ERK mediates the anorectic and thermogenic sympathetic effects of leptin. Diabetes. 58: 536–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inoue A., Ishiguro J., Kitamura H., Arima N., Okutani M., Shuto A., S. Higashiyama, T. Ohwada, H. Arai, K. Makide, et al. . 2012. TGFα shedding assay: an accurate and versatile method for detecting GPCR activation. Nat. Methods. 9: 1021–1029. [DOI] [PubMed] [Google Scholar]
- 27.Boccuto L., Aoki K., Flanagan-Steet H., Chen C. F., Fan X., Bartel F., M. Petukh, A. Pittman, R. Saul, A. Chaubey, et al. . 2014. A mutation in a ganglioside biosynthetic enzyme, ST3GAL5, results in salt & pepper syndrome, a neurocutaneous disorder with altered glycolipid and glycoprotein glycosylation. Hum. Mol. Genet. 23: 418–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dinulescu D. M., and Cone R. D.. 2000. Agouti and agouti-related protein: analogies and contrasts. J. Biol. Chem. 275: 6695–6698. [DOI] [PubMed] [Google Scholar]
- 29.Couturier C., and Jockers R.. 2003. Activation of the leptin receptor by a ligand-induced conformational change of constitutive receptor dimers. J. Biol. Chem. 278: 26604–26611. [DOI] [PubMed] [Google Scholar]
- 30.Taniguchi T. 1995. Cytokine signaling through nonreceptor protein tyrosine kinases. Science. 268: 251–255. [DOI] [PubMed] [Google Scholar]
- 31.Banks A. S., Davis S. M., Bates S. H., and Myers M. G. Jr.. 2000. Activation of downstream signals by the long form of the leptin receptor. J. Biol. Chem. 275: 14563–14572. [DOI] [PubMed] [Google Scholar]
- 32.You J., Yu Y., Jiang L., Li W., Yu X., Gonzalez L., G. Yang, Z. Ke, W. Li, C. Li, et al. . 2010. Signaling through Tyr985 of leptin receptor as an age/diet-dependent switch in the regulation of energy balance. Mol. Cell. Biol. 30: 1650–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nordström V., Willershauser M., Herzer S., Rozman J., von Bohlen Und Halbach O., Meldner S., U. Rothermel, S. Kaden, F. C. Roth, C. Waldeck, et al. . 2013. Neuronal expression of glucosylceramide synthase in central nervous system regulates body weight and energy homeostasis. PLoS Biol. 11: e1001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ji S., Tokizane K., Ohkawa Y., Ohmi Y., Banno R., Okajima T., H. Kiyama, K. Furukawa, and K. Furukawa. 2016. Increased a-series gangliosides positively regulate leptin/Ob receptor-mediated signals in hypothalamus of GD3 synthase-deficient mice. Biochem. Biophys. Res. Commun. 479: 453–460. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.