Abstract
Intercellular communication has been known for decades to involve either direct contact between cells or to operate via circulating molecules, such as cytokines, growth factors, or lipid mediators. During the last decade, we have begun to appreciate the increasing importance of intercellular communication mediated by extracellular vesicles released by viable cells either from plasma membrane shedding (microvesicles, also named microparticles) or from an intracellular compartment (exosomes). Exosomes and microvesicles circulate in all biological fluids and can trigger biological responses at a distance. Their effects include a large variety of biological processes, such as immune surveillance, modification of tumor microenvironment, or regulation of inflammation. Extracellular vesicles can carry a large array of active molecules, including lipid mediators, such as eicosanoids, proteins, and nucleic acids, able to modify the phenotype of receiving cells. This review will highlight the role of the various lipidic pathways involved in the biogenesis and functions of microvesicles and exosomes.
Keywords: lipolytic enzymes, lipid kinases, lipid transporters, membrane asymmetry, membrane fusion
EXOSOME AND MICROVESICLE BIOGENESIS: IS IT AN INTERCONNECTED PROCESS?
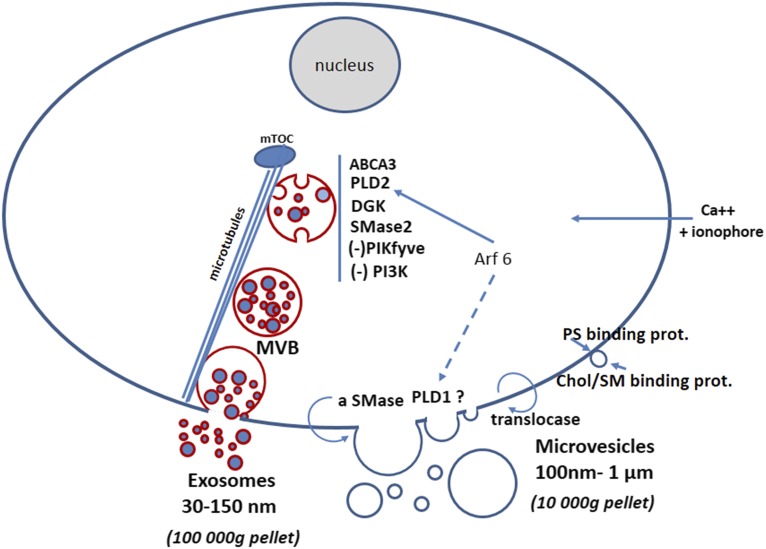
Exosome biogenesis begins by a process of microautophagy (1) occurring at the level of late endosomes (Fig. 1). This process ends by an inward budding of the endosome membrane generating the accumulation of nano-vesicles (30–150 nm diameter) inside the late endosome subset of multivesicular bodies (MVBs). Moving along the microtubules by a dynamic process regulated by cholesterol (2), MVBs reach the plasma membrane, fuse with it, and release their content of intraluminal vesicles (ILVs), which become named exosomes (3). A comprehensive review updating the cell biology of extracellular vesicles was recently issued (4). However, the difference between ILVs and exosomes might not be just a matter of name because ILVs are generated in an acidic pH environment (pH 5.5) inside the MVB, and they are released into a neutral pH environment to become “exosomes”. Such a pH variation might affect their membrane organization because it has been shown that the membrane rigidity of exosomes increases between pH 5 and 7 (5).
Fig. 1.
Lipid-related partners of exosome and microvesicle biogenesis. Exosomes and microvesicles (also called ectosomes or microparticles) are recovered by ultracentrifugation from viable cells. Microvesicles sediment in the 10,000 g pellet and their size ranges between 100 and 1,000 nm. The 10,000 g supernatant is then used to recover exosomes by a 100,000 g centrifugation (82); exosome size ranges from 30 to 150 nm, with an average around 100 nm. Some markers to discriminate between these two types of extracellular vesicles have been reported (21, 40, 83). MVB, multivesicular body; mTOC, microtubule organizing center.
Enhanced exosome production involves lipid transporters, such as ABCA3 (84), and requires the activities of PLD2 (11), diglyceride kinase (DGK) (85), and neutral sphingomyelinase (46), but the inhibition of phosphoinositide kinases, such as the PI3 kinase (25, 42) and PIKfyve (86). Translocation on the outer leaflet of the plasma membrane of the acid sphingomyelinase (aSMase) promotes the budding of microvesicles (10). This budding process also involves the small G proteins, such as Arf6 and RhoA (87), which are activators of PLD1 and PLD2, supporting the proposition that, in some situations, the production of both exosomes and microvesicles could be coordinated via PLD activity. Microvesicles can also be produced by modification of plasma membrane asymmetry by the aminophospholipid translocases (12), or by modification of the lateral pressure of phospholipids via PS binding protein on the inner leaflet (17) or sphingomyelin/cholesterol binding protein (16) on the outer leaflet. Calcium loading into cells by means of a calcium ionophore can trigger the production of microvesicles (18) or exosomes (20). MVB and exosomes are circled in red in the figure to represent the BMP content of their membrane, which definitely discriminates between exosomes and microvesicles because BMP intracellular localization is strictly restricted to late endosomes and lysosomes (22, 26).
ILV biogenesis involves interactions between the protein sorting machinery of the MVB membrane, i.e., the endosomal sorting complex required for transport (ESCRT), and various lipidic molecules. Associated with the ESCRT complexes I–III are vacuolar protein sorting (Vps)4 and Alg2-interacting protein X (Alix). In yeast, Vps4 has been shown to interact with the oxysterol binding proteins, Osh6 and Osh7 (6). Oxysterols have specific pharmacological and physiological properties (7). Alix is recruited on the endosome membrane via a specific domain binding the endosomal lipid, bis(monoacylglycero)phosphate (BMP) (8), also inappropriately named LBPA for lyso(bis)phosphatidic acid. An ESCRT-independent pathway involving ceramides and the neutral sphingomyelinase 2 has also been characterized (9). Remarkably another sphingomyelinase, the acid sphingomyelinase, is involved in microvesicle formation following translocation of the enzyme to the plasma membrane outer leaflet where it generates ceramides triggering microvesicle budding (10).
More recently another pathway for exosome biogenesis involving the syndecan/syntenin complex has been characterized. This pathway requires the activity of phospholipase D (PLD)2 (11). Indeed the inactivation of PLD2 prevents the formation of ILVs inside the MVBs (11). PLD activities could be a coordinating process between exosomes and microvesicle formation. PLD2 is activated by the small G protein, Arf6, and interestingly, Arf6 is also involved in microvesicle formation from plasma membrane shedding (12). Arf6 activity leads to the localization of the myosin-light chain kinase at the neck of the newly forming vesicles, promoting their release by fission from the plasma membrane. The plasma membrane contains mainly the PLD1 isoform. Although Arf1 is the most common PLD1 activator, stimulation by Arf6 has also been reported (13). Thus, a coordinated secretion of exosomes and microvesicles involving the Arf6/Arf1/PLD2/PLD1 pathways deserves further study. These pathways could occur in tumor cells, which constitutively release exosomes and microvesicles. It is known that PLDs are overexpressed in cancer cells (14). However, the pioneering study showing that the same cell can produce both exosomes and microvesicles was performed using activated platelets (15).
Disruption of the plasma membrane lipid organization appears to be critical to allow microvesicle (microparticle) formation. Indeed modification of the outer membrane leaflet by a cholesterol/sphingomyelin binding protein promotes microvesicle formation (16), which can occur following outer membrane sphingomyelin hydrolysis (10). On the inner leaflet, proteinase 3, an autoantigen that binds to phosphatidylserine (PS), promotes the formation of microvesicles (17). Translocation of PS to the outer leaflet upon cellular activation is a prerequisite for microvesicle biogenesis (18, 19). This occurs during platelet activation by natural agonists or calcium ionophore (19); notably, a calcium ionophore also promotes exosome production (20).
In summary, although the biogenesis of microvesicles and exosomes may be coordinated, the molecular pathways involved in their respective formation appear different. Indeed, discrimination between microvesicles and exosomes can be demonstrated through use of a lipid marker (21), the BMP that is present on the peripheral membrane of the MVBs (22) and on the ILVs (23). BMP has been shown to trigger the inward budding of a reconstituted MVB membrane in vitro, leading to the formation of intra-liposomal vesicles similar to the ILVs observed in cells (24). In this respect, we previously reported the presence of BMP in exosomes with a similar amount as in the parental cells, i.e., about 1% of total phospholipids (5). However, a recent report indicated an enrichment of BMP on exosomes released upon inactivation of the class III PI3 kinase Vps34 in parental cells (25). On the other hand, no BMP labeling has been observed in the cell plasma membrane by immunoelectron microscopy (22) or by immunostaining using the highly specific anti-BMP antibody, 6C4 (26–28), thus ruling out the possibility that this lipid is present on microvesicles. Moreover, the negatively charged BMP could account for the increase in exosome electronegativity, as compared with the plasma membrane. Indeed exosomes (77–93 nm diameter) (20) derived from rat mast cells exhibited a zeta-potential between −12.9 and −15.7 mV, whereas that of parent cells was of −8.5 mV (20). In summary, both the BMP content and electronegativity may allow discrimination between vesicles having endosomal versus plasma membrane origins.
EXOSOMES AND MICROVESICLES CONCENTRATE SIGNALING MOLECULES IN DISTINCT POPULATIONS FROM MULTIPLE BIOGENESIS PATHWAYS
Exosomes were originally described about 30 years ago as a mechanism for clearing unneeded molecules from reticulocytes during their maturation into erythrocytes (29). This led to the concept that the function of exosomes was to eliminate biological material. The idea expanded when exosomes were shown to participate in immune surveillance (30) and to transport molecules, such as miRNA and mRNA (31), able to initiate functional responses in target cells. In the meantime, it was shown that lipid molecules and related enzymes can participate in the signaling function of exosomes because they carry eicosanoids together with functional phospholipases and enzymes of the prostaglandin (3, 20) and leukotriene pathways (32). The same conceptual evolution occurred for microvesicles, moving from the idea of “artefact no more” (33) to the demonstration of their involvement in various biological events (12).
Comparative proteomic and lipidomic analysis of exosomes and microvesicles has identified about 3,500 proteins and 2,000 lipid species (34). A recent review of lipids in exosomes has been released (35). Also, it was reported that exosomes contained 1,300 mRNAs (i.e., about 10% of mRNAs from the parent cells) and 120 miRNAs (31), together with various other types of RNAs and of double-stranded DNA fragments (36). The presence of so many molecules in a single type of vesicle seemed unlikely. Instead, it has been shown that the bulk of material isolated as an “exosome pellet” or a “microvesicle pellet” is representative of several populations of microvesicles or exosomes (37–40).
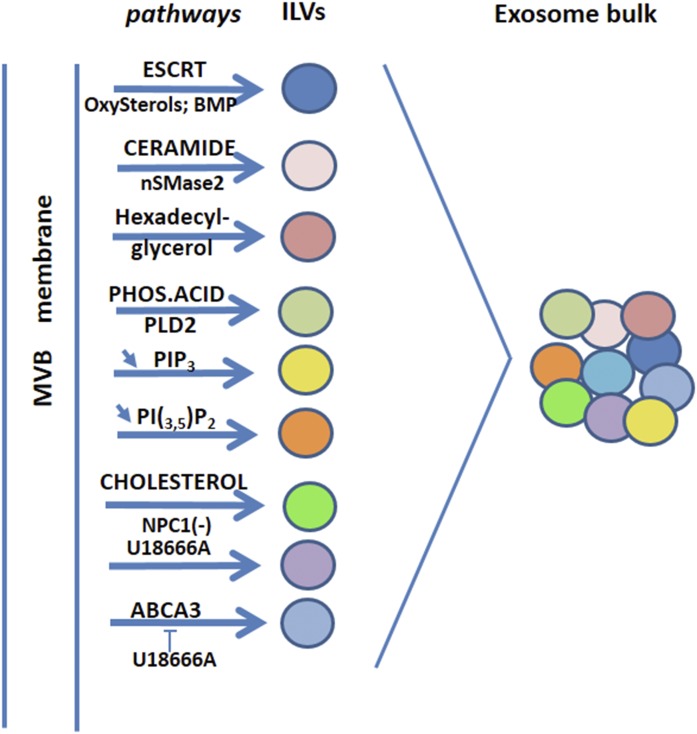
The existence of these various exosome populations suggests that there are distinct biogenesis pathways for different populations (Fig. 2). It has been shown that exosomes containing miRNA were preferentially produced via the SMase2/ceramide pathway, independently of the canonical ESCRT pathway (41). Mobilization of the various pathways reported in the Fig. 2 might operate differently according to the cell type and cell activation conditions, making a combination of pathways leading to a combination of exosome populations constituting the bulk of exosomes. In that respect, the ESCRT-associated member, Alix, not only binds the endosomal lipid BMP (8) but also binds syntenin, which interacts directly with phosphatidic acid produced by the PLD2 (11). Whereas the syndecan/syntenin pathway enhanced angiopoietin-containing exosome secretion from endothelial cells, the PI3K/Akt/eNOS pathway had an opposite effect in the same cells (42), reducing angiopoietin secretion. Thus, the two opposite pathways promote the release of distinct exosome populations with different contents, including angiopoietin, which plays multiple roles in blood and lymphatic vessel growth. Differential labeling of exosome lipids and proteins had already indicated the existence of various exosome populations (37). This observation was confirmed by inactivation of different elements of the ESCRT machinery (38, 39) and by means of fine-tuned gradient fractionations to accurately characterize exosome and microvesicle populations (40). With respect to microvesicles, it is also conceivable that distinct parts of the plasma membrane generate several different microvesicle populations.
Fig. 2.
Lipid pathways involved in exosome biogenesis processes. Connections with lipid pathways are present within the ESCRT, the first pathway described for ILV biogenesis (38). Indeed, Vps4, a member of the AAA family of proteins (ATPases associated with a variety of activities), interacts with oxysterol binding proteins (Osh6 and Osh7) (6), and Alix interacts with BMP via a specific sequence domain (8). Independent of the ESCRT machinery are lipid pathways involving ceramides (nSMase2) (46) and, to some extent, phosphatidic acid (PHOS.ACID/PLD2) (11). Another neutral lipid, precursor of ether-linked phospholipids, hexadecyl-glycerol, stimulates exosome production (88). The phosphoinositides, PIP3 and PI(3,5)P2, act in a negative way because inhibition of PI3K/Akt (25, 42) and PIKfyve (86) favor exosome production. Because PI3K is involved in macroautophagy (89), this observation indicates that there is a requirement to block macroautophagy to promote the microautophay process involved in exosome biogenesis (90). Indeed, rapamycin, the inhibitor of mammalian target of rapamycin (mTOR)C1 induces macroautophagy (90, 91) and decreases exosome production (84). However an alternative pathway for exosome production via a secretory autophagy process, distinct from the degradative autophagy, has been reported following PIKfyve inhibition (86). Because PIKfyve activity promotes mTORC1 translocation to the plasma membrane (92), the reverse PIKfyve inhibition might retain mTORC1 on the late endosome membrane. mTORC1 is located on the endosome membrane (93) and could have a direct function at this location to generate ILVs. mTORC1 is activated by the endosomal cholesterol (93) and enhanced exosome production was reported when cholesterol was supplied to glial cells either directly or by means of U18666A (94). mTORC1 activation by cholesterol is reversed by NPC1, which effluxes cholesterol from endosomes (93). Consistently, NPC1 inactivation is required to enhance exosome production (94). U18666A displays an opposite effect on lymphoblastoma cells where it inhibits exosome production, which is mediated in these cells by the lipid transporter, ABCA3 (84). It is worth noting that NPC1 and ABCA3-containing endosomes are distinct populations (95). In addition, the effect of U18666A cannot be restricted to cholesterol because U18666A also promotes the accumulation of oxysterols in cells (cholesterol 5,6 epoxides) and cholesterol precursors (zymostenol, desmosterol) (96). In the figure, each pathway leading to a specific vesicle content is a graphical simplification. Combination of the various pathways reported could depend upon cell type and activation conditions or mobilization of distinct late endosome populations, and several pathways can probably be triggered at the same time.
Microvesicles have lost the plasma membrane phospholipid asymmetry of the parent cell (18). Consequently microvesicles expose their PS on the outer membrane leaflet. Similarly, exosomes lack transmembrane phospholipid asymmetry, as demonstrated by the presence of 50–70% of aminophospholipids (PE and PS) on the outer leaflet (5). This is consistent with the presence of a phospholipid scramblase in the exosome membrane (20, 43) and with the rapid flip-flop reported between the two membrane leaflets (5). Consistently, exosomes were shown to be stained by annexin V (44). Only some transmembrane asymmetry of aminophospholipids was reported in exosomes derived from guinea pig reticulocytes (45), although how this asymmetry was maintained was not determined. Indeed, exosomes lack an organized cytoskeleton that participates in maintaining the phospholipid asymmetry of the cellular plasma membrane.
Exosomes are enriched in disaturated molecular species of phospholipids (5, 46, 47). This accounts, in part, for their increased membrane rigidity relative to parent cell membranes. The high ratio of protein/lipid in exosomes (48, 49) also enhances their membrane rigidity. The increased membrane rigidity of exosomes could be important in ensuring that exosomes are not easily degraded and are readily able to circulate in biological fluids (50). Consistently, exosomes have been found to be more resistant than microvesicles toward detergent treatments, representative of a higher membrane lipid order (49). The only elimination process reported so far for circulating exosomes occurs via their surface lipid lysophosphatidylcholines, which bind IgM-type immunoglobulins. This facilitates interaction with apoptotic circulating cells whose elimination can lead to exosome clearance (51). Pharmacokinetics of injected exosomes in mice revealed an average half-life of 4 min with 10% of the initial amount remaining at 4 h (52). In contrast, direct degradation of microvesicles can occur in the circulation because their membrane lipids can be hydrolyzed by secreted phospholipases (18, 53), suggesting a shorter half-life than exosomes in biological fluids.
In summary, many lipid-related pathways appear to be involved in the biogenesis of exosomes (Fig. 2). Mobilization of these pathways may depend upon the type of parent cell, the nature of the initial stimulus, and the micro-environment, making possible combinations of biogenesis pathways leading to vesicles with diverse contents. Material packaging into exosomes and microvesicles appears to be highly dynamic process.
EXOSOMES AND MICROVESICLES ARE INTERCELLULAR TRANSPORTERS OF LIPID MOLECULES AND RELATED ENZYMES INVOLVED IN VARIOUS PATHOPHYSIOLOGIES
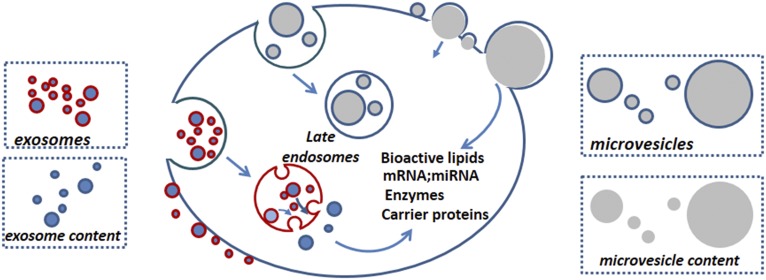
The interaction of exosomes and microvesicles with surrounding cells is a stochastic process (i.e., it is both random and organized). The vesicles randomly move in the intercellular space or in biological fluids until they find a way to interact with cells. If they fail to interact, they are eliminated by degradation or clearance. Interaction with cells is the “organized process” that can involve either receptor-mediated endocytosis, macropinocytosis, or membrane fusion (Fig. 3).
Fig. 3.
Exosome and microvesicle-mediated transfer of biologically active material into recipient cells. Importantly, both exosomes (31) and microvesicles (12) carry functional molecules able to modify the phenotype of recipient cells. Exosomes are preferentially endocytosed and may release their contents by fusion with the recipient endosomal membrane by a process called back-fusion (8). This may be mediated by the BMP (highlighted in red) present both on the exosome and endosome membrane, because BMP is fusogenic in an acidic pH environment (23). Even though some microvesicles are internalized by receptor-mediated endocytosis (61), the microvesicle membrane is devoid of BMP, and how microvesicles transfer their contents inside target cells is unclear. Instead, the fusion of large microvesicles with the peripheral cell membrane has been observed (97) and fusion between microvesicles and the cellular plasma membrane might represent the preferential transfer mechanism of material from microvesicles to recipient cells.
Because exosomes and microvesicles expose peripheral PS, they can interact with cells exposing the PS receptors, Tim1 or Tim4, on their surfaces (54). Exosomes released from activated T cells and internalized via the PS receptor on monocytes have been shown to induce cholesterol accumulation, suggesting that exosomes could be an atherogenic factor (55). Other endocytic receptors are involved in exosome internalization by recipient cells, such as the scavenger receptor, CD36 (56), or the LDL receptor-related protein 1 (LRP1) (CD91/LRP1) (57), which binds the heat-shock proteins, Hsp90 or Hsp70, that are enriched in exosome membranes (3). Interestingly LRP1 regulates phosphorylation of cPLA2 as well as cholesterol efflux (58).
Microvesicles recovered in the 10,000 g pellet from ATP-activated microglia cells have been shown to stimulate synaptic activity in neurons by activation of sphingolipid metabolism (59). Microglia cell activation provokes the acid sphingomyelinase to move to the plasma membrane outer leaflet, where it generates ceramides that trigger microvesicle budding (10). Functional effects of microglia-derived microvesicles involve lipid components of their membrane (59) because liposomes prepared from the microvesicle lipid extract reproduce the effects. Microvesicle treatment with annexin V prevented their biological effect, suggesting that the PS receptor is required for their interaction with and activity on the target cells (59). Remarkably, microvesicles released from ATP-stimulated microglia cells carry the endocannabinoid molecule, N-arachidonylethanolamine (anandamide), and such microvesicles are able to activate the type 1 cannabinoid receptor, CB1 (60). Microglia cells released both microvesicles and exosomes, but the anandamide was three times more enriched in microvesicles than in exosomes (60).
In platelet-derived microvesicles (microparticles) the lipid component triggering the functional effect was identified as being 12-HETE (61). BLT2 is the HETE receptor that mediates the internalization of microparticles into neutrophils (61). In this work, microparticles are designated as microvesicles with an average size of 350 nm. HETE biosynthesis occurs in platelet microparticles themselves. The successive steps of this process involve the action of a phospholipase A2 [secreted (s)PLA2-IIA] present in the extracellular milieu that acts on phospholipid microparticles to release free arachidonic acid that is then converted to HETE by the 12-lipoxygenase present in microparticles (61). Interestingly, this study suggests a possible cooperation between exosomes and microparticles. Activated platelets release both exosomes and microvesicles (15), and it has been shown that exosomes carry the sPLA2-IIA (20). Exosomal sPLA2-IIA could act on microparticle phospholipids to release arachidonic acid required for HETE formation, leading to amplification of inflammation in arthritis (62). Platelet microparticles are a critical component of inflammation and immunity-induced thrombotic disorders (63). In addition to its importance in arachidonate mobilization, PLA2 activity generates lysophosphatidylcholine that acts as a substrate for autotaxin activity. Autotaxin generates lysophosphatidic acid that acts as an agonist for cell surface G-protein-coupled receptors. Autotaxin has been demonstrated to be bound to a population of exosomes (64). Thus, the PLA2 associated with microparticles and/or exosomes promotes activation of two (or more) signaling cascades.
Exosomes carry functional enzymes related to eicosanoid biosynthesis as well as their products. Antigen-presenting cell-derived exosomes from human macrophages and dendritic cells (DCs) incubated with the leukotriene precursor, LTA4, synthesized the end-products, LTB4 and LTC4, in amounts exceeding those generated by the parent cells (32). Exosomes contain enzymes of the leukotriene pathway [i.e., 5-lipoxygenase (weakly active), LTA4 hydrolase, and LTC4 synthase]. Activated antigen-presenting cell-derived exosomes promoted granulocyte migration (32). Exosomes recovered from fluids of patients with allergic asthma contributed to leukotriene production (65).
Exosomes also transport prostaglandins. Exosomes produced from rat basophilic leukemia cells contain three types of PLA2s (cPLA2, iPLA2, and sPLA2) and cyclooxygenases-1 and -2 (COX-1 and COX-2). These exosomes are also enriched in free fatty acids (including arachidonic acid) and in the immunosuppressive prostaglandin, PGE2 (20). Tumor-derived exosomes enriched in PGE2 participate largely to turn the tumor micro-environment into one that permits tumor growth. Indeed PGE2 inhibits immune responses toward the tumor at different steps of its development (66). For instance PGE2-enriched exosomes released from the intestinal mucosa can be transferred to the liver where they induce anergy of natural killer T cells (67).
Other enzymes related to fatty acid metabolism play a role in exosome function. Exosomes derived from adipocytes supply two enzymes of the β-oxidation pathway to melanoma cells (68), namely enoyl-CoA hydratase (ECHA) and hydroxyacyl-CoA dehydrogenase (HCDH). Adipocyte exosome internalization into melanoma cells increased fatty acid oxidation and promoted melanoma cell migration. Cell migration was suppressed upon inhibition of fatty acid oxidation (68). Thus exosomes may be central in linking obesity and cancer (69). Besides enzymes related to fatty acid metabolism, exosomes also carry lipid kinases, such as diacylglycerol-kinase (70) or sphingosine kinase, that promote hepatocyte proliferation and liver regeneration (71). Exosomes also contain phospholipid hydrolases, such as phospholipases (20, 51), and the phosphoinositide (PIP3)-phosphatase (PTEN) (72). Secretion of PTEN by exosomes requires its ubiquitination, and PTEN retains its PIP3-phosphatase activity when exosomes are transferred into recipient cells (72). Consequently exosomes can participate in reducing tumor growth through their delivered PTEN content. Whereas some exosomes contain PTEN, others derived from astrocytes in the central nervous system carry miRNAs triggering PTEN degradation in brain-infiltrating tumor cells, allowing tumors to develop in the brain (73). Thus, the delivery of different exosome contents to recipient cells can produce opposing physiological outcomes.
CONCLUSIONS
In summary, both exosomes and microvesicles contribute their lipid molecules or their lipid-related enzymes to several pathophysiologies, including inflammation, tumor development, and atherogenesis. However, extracellular vesicles also act positively within the immune system in lymph nodes (74) or in the physiology of the brain through neuron activation (10) and endocannabinoid transfer (60). Whereas research on exosomes and microvesicles has previously been conducted somewhat independently by distinct scientific communities, more recent publications deal with parallel studies on both types of vesicles and have demonstrated that both are produced by the same cells, suggesting a possible coordinated biogenesis. Exosome and microvesicle functions appear to be either complementary or in opposition. Complementary, for instance, in the case of transfer of oncogenic material to recipient cells (75), but in opposition in the immune system. Indeed exosomes act positively in the immune response by extending the duration of antigen presentation in lymph nodes (74) and appear as a tool for immunotherapy against cancer (76), whereas microvesicles (ectosomes) turn off the immune response by inducing differentiation of CD4 T cells into T regulatory lymphocytes (77). DC-derived exosomes are more efficient than DC-derived microvesicles in eliciting an antigen-specific immune response in vivo (78). Similar to agonist/antagonist molecules interacting with receptors, further studies on extracellular vesicles may reveal the presence of “ago-vesicles” and “antago-vesicles” in the regulation of several physiological processes.
Another aspect of the growing interest in extracellular vesicles is the detection of biomarkers borne by circulating exosomes or microvesicles in the blood. Indeed, isolating circulating exosomes might allow enrichment of a specific marker by 105 times (M. Record, unpublished observation). In this respect an exosomal marker of pancreatic cancer has recently been identified (79). One can conceive that isolating PGE2-carrying exosomes or 12-HETE-carrying microvesicles will permit monitoring of the efficiency of a related therapy. Thus, “nano-devices” able to monitor extracellular vesicles have begun to appear (80), and a continuous-flow chip with multiplexed exosomal marker detection for blood-based ovarian cancer detection has recently been devised (81). In conclusion, intercellular communication by exosomes and microvesicles has opened a new era in studying pathophysiological processes.
Footnotes
Abbreviations:
- Alix
- Alg2-interacting protein X
- BMP
- bis(monoacylglycero)phosphate
- DC
- dendritic cell
- ESCRT
- endosomal sorting complex required for transport
- ILV
- intraluminal vesicle
- LRP1
- LDL receptor-related protein 1
- MVB
- multivesicular body
- PLA
- phospholipase A
- PLD
- phospholipase D
- PS
- phosphatidylserine
- sPLA2
- secreted phospholipase A2
- Vps
- vacuolar protein sorting
REFERENCES
- 1.Sahu R., Kaushik S., Clement C. C., Cannizzo E. S., Scharf B., Follenzi A., Potolicchio I., Nieves E., Cuervo A. M., and Santambrogio L.. 2011. Microautophagy of cytosolic proteins by late endosomes. Dev. Cell. 20: 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huotari J., and Helenius A.. 2011. Endosome maturation. EMBO J. 30: 3481–3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Record M., Subra C., Silvente-Poirot S., and Poirot M.. 2011. Exosomes as intercellular signalosomes and pharmacological effectors. Biochem. Pharmacol. 81: 1171–1182. [DOI] [PubMed] [Google Scholar]
- 4.van Niel G., D’Angelo G., and Raposo G.. 2018. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 19: 213–228. [DOI] [PubMed] [Google Scholar]
- 5.Laulagnier K., Motta C., Hamdi S., Roy S., Fauvelle F., Pageaux J. F., Kobayashi T., Salles J. P., Perret B., Bonnerot C., et al. 2004. Mast cell- and dendritic cell-derived exosomes display a specific lipid composition and an unusual membrane organization. Biochem. J. 380: 161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang P., Zhang Y., Li H., Chieu H. K., Munn A. L., and Yang H.. 2005. AAA ATPases regulate membrane association of yeast oxysterol binding proteins and sterol metabolism. EMBO J. 24: 2989–2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poirot M., and Silvente-Poirot S.. 2013. Oxysterols and related strerols:implications in pharmacology and physiopathology. Biochem. Pharmacol. 86: 1–2. [DOI] [PubMed] [Google Scholar]
- 8.Bissig C., Lenoir M., Velluz M. C., Kufareva I., Abagyan R., Overduin M., and Gruenberg J.. 2013. Viral infection controlled by a calcium-dependent lipid-binding module in ALIX. Dev. Cell. 25: 364–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trajkovic K., Hsu C., Chiantia S., Rajendran L., Wenzel D., Wieland F., Schwille P., Brugger B., and Simons M.. 2008. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319: 1244–1247. [DOI] [PubMed] [Google Scholar]
- 10.Bianco F., Perrotta C., Novellino L., Francolini M., Riganti L., Menna E., Saglietti L., Schuchman E. H., Furlan R., Clementi E., et al. 2009. Acid sphingomyelinase activity triggers microparticle release from glial cells. EMBO J. 28: 1043–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghossoub R., Lembo F., Rubio A., Gaillard C. B., Bouchet J., Vitale N., Slavik J., Machala M., and Zimmermann P.. 2014. Syntenin-ALIX exosome biogenesis and budding into multivesicular bodies are controlled by ARF6 and PLD2. Nat. Commun. 5: 3477. [DOI] [PubMed] [Google Scholar]
- 12.Tricarico C., Clancy J., and D’Souza-Schorey C.. 2017. Biology and biogenesis of shed microvesicles. Small GTPases. 8: 220–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vitale N., Mawet J., Camonis J., Regazzi R., Bader M. F., and Chasserot-Golaz S.. 2005. The small GTPase RalA controls exocytosis of large dense core secretory granules by interacting with ARF6-dependent phospholipase D1. J. Biol. Chem. 280: 29921–29928. [DOI] [PubMed] [Google Scholar]
- 14.Brown H. A., Thomas P. G., and Lindsley C. W.. 2017. Targeting phospholipase D in cancer, infection and neurodegenerative disorders. Nat. Rev. Drug Discov. 16: 351–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heijnen H. F., Schiel A. E., Fijnheer R., Geuze H. J., and Sixma J. J.. 1999. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 94: 3791–3799. [PubMed] [Google Scholar]
- 16.Skočaj M., Yu Y., Grundner M., Resnik N., Bedina Zavec A., Leonardi A., Krizaj I., Guella G., Macek P., Kreft M. E., et al. 2016. Characterisation of plasmalemmal shedding of vesicles induced by the cholesterol/sphingomyelin binding protein, ostreolysin A-mCherry. Biochim. Biophys. Acta. 1858: 2882–2893. [DOI] [PubMed] [Google Scholar]
- 17.Martin K. R., Kantari-Mimoun C., Yin M., Pederzoli-Ribeil M., Angelot-Delettre F., Ceroi A., Grauffel C., Benhamou M., Reuter N., Saas P., et al. 2016. Proteinase 3 is a phosphatidylserine-binding protein that affects the production and function of microvesicles. J. Biol. Chem. 291: 10476–10489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lhermusier T., Chap H., and Payrastre B.. 2011. Platelet membrane phospholipid asymmetry: from the characterization of a scramblase activity to the identification of an essential protein mutated in Scott syndrome. J. Thromb. Haemost. 9: 1883–1891. [DOI] [PubMed] [Google Scholar]
- 19.Morel O., Jesel L., Freyssinet J. M., and Toti F.. 2011. Cellular mechanisms underlying the formation of circulating microparticles. Arterioscler. Thromb. Vasc. Biol. 31: 15–26. [DOI] [PubMed] [Google Scholar]
- 20.Subra C., Grand D., Laulagnier K., Stella A., Lambeau G., Paillasse M., De Medina P., Monsarrat B., Perret B., Silvente-Poirot S., et al. 2010. Exosomes account for vesicle-mediated transcellular transport of activatable phospholipases and prostaglandins. J. Lipid Res. 51: 2105–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Record M., Carayon K., Poirot M., and Silvente-Poirot S.. 2014. Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochim. Biophys. Acta. 1841: 108–120. [DOI] [PubMed] [Google Scholar]
- 22.Möbius W., van Donselaar E., Ohno-Iwashita Y., Shimada Y., Heijnen H. F., Slot J. W., and Geuze H. J.. 2003. Recycling compartments and the internal vesicles of multivesicular bodies harbor most of the cholesterol found in the endocytic pathway. Traffic. 4: 222–231. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi T., Beuchat M. H., Chevallier J., Makino A., Mayran N., Escola J. M., Lebrand C., Cosson P., and Gruenberg J.. 2002. Separation and characterization of late endosomal membrane domains. J. Biol. Chem. 277: 32157–32164. [DOI] [PubMed] [Google Scholar]
- 24.Matsuo H., Chevallier J., Mayran N., Le Blanc I., Ferguson C., Faure J., Blanc N. S., Matile S., Dubochet J., Sadoul R., et al. 2004. Role of LBPA and Alix in multivesicular liposome formation and endosome organization. Science. 303: 531–534. [DOI] [PubMed] [Google Scholar]
- 25.Miranda A. M., Lasiecka Z. M., Xu Y., Neufeld J., Shahriar S., Simoes S., Chan R. B., Oliveira T. G., Small S. A., and Di Paolo G.. 2018. Neuronal lysosomal dysfunction releases exosomes harboring APP C-terminal fragments and unique lipid signatures. Nat. Commun. 9: 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobayashi T., Beuchat M. H., Lindsay M., Frias S., Palmiter R. D., Sakuraba H., Parton R. G., and Gruenberg J.. 1999. Late endosomal membranes rich in lysobisphosphatidic acid regulate cholesterol transport. Nat. Cell Biol. 1: 113–118. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi T., Startchev K., Whitney A. J., and Gruenber J.. 2001. Localization of lysobisphosphatidic acid-rich membrane domains in late endosomes. Biol. Chem. 382: 483–485. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi T., Stang E., Fang K. S., de Moerloose P., Parton R. G., and Gruenberg J.. 1998. A lipid associated with the antiphospholipid syndrome regulates endosome structure and function. Nature. 392: 193–197. [DOI] [PubMed] [Google Scholar]
- 29.Johnstone R. M., Adam M., Hammond J. R., Orr L., and Turbide C.. 1987. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 262: 9412–9420. [PubMed] [Google Scholar]
- 30.Zitvogel L., Regnault A., Lozier A., Wolfers J., Flament C., Tenza D., Ricciardi-Castagnoli P., Raposo G., and Amigorena S.. 1998. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat. Med. 4: 594–600. [DOI] [PubMed] [Google Scholar]
- 31.Valadi H., Ekstrom K., Bossios A., Sjostrand M., Lee J. J., and Lotvall J. O.. 2007. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9: 654–659. [DOI] [PubMed] [Google Scholar]
- 32.Esser J., Gehrmann U., D’Alexandri F. L., Hidalgo-Estevez A. M., Wheelock C. E., Scheynius A., Gabrielsson S., and Radmark O.. 2010. Exosomes from human macrophages and dendritic cells contain enzymes for leukotriene biosynthesis and promote granulocyte migration. J. Allergy Clin. Immunol. 126: 1032–1040. [DOI] [PubMed] [Google Scholar]
- 33.Cocucci E., Racchetti G., and Meldolesi J.. 2009. Shedding microvesicles: artefacts no more. Trends Cell Biol. 19: 43–51. [DOI] [PubMed] [Google Scholar]
- 34.Haraszti R. A., Didiot M. C., Sapp E., Leszyk J., Shaffer S. A., Rockwell H. E., Gao F., Narain N. R., DiFiglia M., Kiebish M. A., et al. 2016. High-resolution proteomic and lipidomic analysis of exosomes and microvesicles from different cell sources. J. Extracell. Vesicles. 5: 32570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skotland T., Sandvig K., and Llorente A.. 2017. Lipids in exosomes: current knowledge and the way forward. Prog. Lipid Res. 66: 30–41. [DOI] [PubMed] [Google Scholar]
- 36.Kahlert C., Melo S. A., Protopopov A., Tang J., Seth S., Koch M., Zhang J., Weitz J., Chin L., Futreal A., et al. 2014. Identification of double-stranded genomic DNA spanning all chromosomes with mutated KRAS and p53 DNA in the serum exosomes of patients with pancreatic cancer. J. Biol. Chem. 289: 3869–3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laulagnier K., Vincent-Schneider H., Hamdi S., Subra C., Lankar D., and Record M.. 2005. Characterization of exosome subpopulations from RBL-2H3 cells using fluorescent lipids. Blood Cells Mol. Dis. 35: 116–121. [DOI] [PubMed] [Google Scholar]
- 38.Colombo M., Moita C., van Niel G., Kowal J., Vigneron J., Benaroch P., Manel N., Moita L. F., Thery C., and Raposo G.. 2013. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J. Cell Sci. 126: 5553–5565. [DOI] [PubMed] [Google Scholar]
- 39.Bobrie A., Colombo M., Krumeich S., Raposo G., and Thery C.. 2012. Diverse subpopulations of vesicles secreted by different intracellular mechanisms are present in exosome preparations obtained by differential ultracentrifugation. J. Extracell. Vesicles. 1: 18397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kowal J., Arras G., Colombo M., Jouve M., Morath J. P., Primdal-Bengtson B., Dingli F., Loew D., Tkach M., and Thery C.. 2016. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA. 113: E968–E977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kosaka N., Iguchi H., Yoshioka Y., Takeshita F., Matsuki Y., and Ochiya T.. 2010. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J. Biol. Chem. 285: 17442–17452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ju R., Zhuang Z. W., Zhang J., Lanahan A. A., Kyriakides T., Sessa W. C., and Simons M.. 2014. Angiopoietin-2 secretion by endothelial cell exosomes: regulation by the phosphatidylinositol 3-kinase (PI3K)/Akt/endothelial nitric oxide synthase (eNOS) and syndecan-4/syntenin pathways. J. Biol. Chem. 289: 510–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mathivanan S., Lim J. W., Tauro B. J., Ji H., Moritz R. L., and Simpson R. J.. 2010. Proteomics analysis of A33 immunoaffinity-purified exosomes released from the human colon tumor cell line LIM1215 reveals a tissue-specific protein signature. Mol. Cell. Proteomics. 9: 197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fitzner D., Schnaars M., van Rossum D., Krishnamoorthy G., Dibaj P., Bakhti M., Regen T., Hanisch U. K., and Simons M.. 2011. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J. Cell Sci. 124: 447–458. [DOI] [PubMed] [Google Scholar]
- 45.Vidal M., Sainte-Marie J., Philippot J. R., and Bienvenue A.. 1989. Asymmetric distribution of phospholipids in the membrane of vesicles released during in vitro maturation of guinea pig reticulocytes: evidence precluding a role for “aminophospholipid translocase”. J. Cell. Physiol. 140: 455–462. [DOI] [PubMed] [Google Scholar]
- 46.Trajkovic K., Hsu C., Chiantia S., Rajendran L., Wenzel D., Wieland F., Schwille P., Brugger B., and Simons M.. 2008. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 319: 1244–1247. [DOI] [PubMed] [Google Scholar]
- 47.Llorente A., Skotland T., Sylvanne T., Kauhanen D., Rog T., Orlowski A., Vattulainen I., Ekroos K., and Sandvig K.. 2013. Molecular lipidomics of exosomes released by PC-3 prostate cancer cells. Biochim. Biophys. Acta. 1831: 1302–1309. [DOI] [PubMed] [Google Scholar]
- 48.Subra C., Laulagnier K., Perret B., and Record M.. 2007. Exosome lipidomics unravels lipid sorting at the level of multivesicular bodies. Biochimie. 89: 205–212. [DOI] [PubMed] [Google Scholar]
- 49.Osteikoetxea X., Balogh A., Szabo-Taylor K., Nemeth A., Szabo T. G., Paloczi K., Sodar B., Kittel A., Gyorgy B., Pallinger E., et al. 2015. Improved characterization of EV preparations based on protein to lipid ratio and lipid properties. PLoS One. 10: e0121184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alvarez-Erviti L., Seow Y., Yin H., Betts C., Lakhal S., and Wood M. J.. 2011. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 29: 341–345. [DOI] [PubMed] [Google Scholar]
- 51.Blanc L., Barres C., Bette-Bobillo P., and Vidal M.. 2007. Reticulocyte-secreted exosomes bind natural IgM antibodies: involvement of a ROS-activatable endosomal phospholipase iPLA2. Blood. 110: 3407–3416. [DOI] [PubMed] [Google Scholar]
- 52.Charoenviriyakul C., Takahashi Y., Morishita M., Matsumoto A., Nishikawa M., and Takakura Y.. 2017. Cell type-specific and common characteristics of exosomes derived from mouse cell lines: yield, physicochemical properties, and pharmacokinetics. Eur. J. Pharm. Sci. 96: 316–322. [DOI] [PubMed] [Google Scholar]
- 53.Fourcade O., Simon M. F., Viode C., Rugani N., Leballe F., Ragab A., Fournie B., Sarda L., and Chap H.. 1995. Secretory phospholipase A2 generates the novel lipid mediator lysophosphatidic acid in membrane microvesicles shed from activated cells. Cell. 80: 919–927. [DOI] [PubMed] [Google Scholar]
- 54.Freeman G. J., Casasnovas J. M., Umetsu D. T., and DeKruyff R. H.. 2010. TIM genes: a family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunol. Rev. 235: 172–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zakharova L., Svetlova M., and Fomina A. F.. 2007. T cell exosomes induce cholesterol accumulation in human monocytes via phosphatidylserine receptor. J. Cell. Physiol. 212: 174–181. [DOI] [PubMed] [Google Scholar]
- 56.Record M., Poirot M., and Silvente-Poirot S.. 2014. Emerging concepts on the role of exosomes in lipid metabolic diseases. Biochimie. 96: 67–74. [DOI] [PubMed] [Google Scholar]
- 57.Skokos D., Botros H. G., Demeure C., Morin J., Peronet R., Birkenmeier G., Boudaly S., and Mecheri S.. 2003. Mast cell-derived exosomes induce phenotypic and functional maturation of dendritic cells and elicit specific immune responses in vivo. J. Immunol. 170: 3037–3045. [DOI] [PubMed] [Google Scholar]
- 58.Zhou L., Choi H. Y., Li W. P., Xu F., and Herz J.. 2009. LRP1 controls cPLA2 phosphorylation, ABCA1 expression and cellular cholesterol export. PLoS One. 4: e6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Antonucci F., Turola E., Riganti L., Caleo M., Gabrielli M., Perrotta C., Novellino L., Clementi E., Giussani P., Viani P., et al. 2012. Microvesicles released from microglia stimulate synaptic activity via enhanced sphingolipid metabolism. EMBO J. 31: 1231–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gabrielli M., Battista N., Riganti L., Prada I., Antonucci F., Cantone L., Matteoli M., Maccarrone M., and Verderio C.. 2015. Active endocannabinoids are secreted on extracellular membrane vesicles. EMBO Rep. 16: 213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Duchez A. C., Boudreau L. H., Naika G. S., Bollinger J., Belleannee C., Cloutier N., Laffont B., Mendoza-Villarroel R. E., Levesque T., Rollet-Labelle E., et al. 2015. Platelet microparticles are internalized in neutrophils via the concerted activity of 12-lipoxygenase and secreted phospholipase A2-IIA. Proc. Natl. Acad. Sci. USA. 112: E3564–E3573. [Erratum. 2015. Proc. Natl. Acad. Sci. USA. 112: E6825.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boilard E., Nigrovic P. A., Larabee K., Watts G. F., Coblyn J. S., Weinblatt M. E., Massarotti E. M., Remold-O’Donnell E., Farndale R. W., Ware J., et al. 2010. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science. 327: 580–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morel O., Morel N., Jesel L., Freyssinet J. M., and Toti F.. 2011. Microparticles: a critical component in the nexus between inflammation, immunity, and thrombosis. Semin. Immunopathol. 33: 469–486. [DOI] [PubMed] [Google Scholar]
- 64.Jethwa S. A., Leah E. J., Zhang Q., Bright N. A., Oxley D., Bootman M. D., Rudge S. A., and Wakelam M. J.. 2016. Exosomes bind to autotaxin and act as a physiological delivery mechanism to stimulate LPA receptor signalling in cells. J. Cell Sci. 129: 3948–3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Torregrosa Paredes P., Gutzeit C., Johansson S., Admyre C., Stenius F., Alm J., Scheynius A., and Gabrielsson S.. 2014. Differences in exosome populations in human breast milk in relation to allergic sensitization and lifestyle. Allergy. 69: 463–471. [DOI] [PubMed] [Google Scholar]
- 66.Srivastava M. K., Zhu L., Harris-White M., Huang M., St John M., Lee J. M., Salgia R., Cameron R. B., Strieter R., Dubinett S., et al. 2012. Targeting myeloid-derived suppressor cells augments antitumor activity against lung cancer. ImmunoTargets Ther. 2012: 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Deng Z. B., Zhuang X., Ju S., Xiang X., Mu J., Liu Y., Jiang H., Zhang L., Mobley J., McClain C., et al. 2013. Exosome-like nanoparticles from intestinal mucosal cells carry prostaglandin E2 and suppress activation of liver NKT cells. J. Immunol. 190: 3579–3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lazar I., Clement E., Dauvillier S., Milhas D., Ducoux-Petit M., LeGonidec S., Moro C., Soldan V., Dalle S., Balor S., et al. 2016. Adipocyte exosomes promote melanoma aggressiveness through fatty acid oxidation: a novel mechanism linking obesity and cancer. Cancer Res. 76: 4051–4057. [DOI] [PubMed] [Google Scholar]
- 69.Clement E., Lazar I., Muller C., and Nieto L.. 2017. Obesity and melanoma: could fat be fueling malignancy? Pigment Cell Melanoma Res. 30: 294–306. [DOI] [PubMed] [Google Scholar]
- 70.Alonso R., Mazzeo C., Merida I., and Izquierdo M.. 2007. A new role of diacylglycerol kinase alpha on the secretion of lethal exosomes bearing Fas ligand during activation-induced cell death of T lymphocytes. Biochimie. 89: 213–221. [DOI] [PubMed] [Google Scholar]
- 71.Nojima H., Freeman C. M., Schuster R. M., Japtok L., Kleuser B., Edwards M. J., Gulbins E., and Lentsch A. B.. 2016. Hepatocyte exosomes mediate liver repair and regeneration via sphingosine-1-phosphate. J. Hepatol. 64: 60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Putz U., Howitt J., Doan A., Goh C. P., Low L. H., Silke J., and Tan S. S.. 2012. The tumor suppressor PTEN is exported in exosomes and has phosphatase activity in recipient cells. Sci. Signal. 5: ra70. [DOI] [PubMed] [Google Scholar]
- 73.Zhang L., Zhang S., Yao J., Lowery F. J., Zhang Q., Huang W. C., Li P., Li M., Wang X., Zhang C., et al. 2015. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature. 527: 100–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Luketic L., Delanghe J., Sobol P. T., Yang P., Frotten E., Mossman K. L., Gauldie J., Bramson J., and Wan Y.. 2007. Antigen presentation by exosomes released from peptide-pulsed dendritic cells is not suppressed by the presence of active CTL. J. Immunol. 179: 5024–5032. [DOI] [PubMed] [Google Scholar]
- 75.Milani G., Lana T., Bresolin S., Aveic S., Pasto A., Frasson C., and Te Kronnie G.. 2017. Expression profiling of circulating microvesicles reveals intercellular transmission of oncogenic pathways. Mol. Cancer Res. 15: 683–695. [DOI] [PubMed] [Google Scholar]
- 76.Besse B., Charrier M., Lapierre V., Dansin E., Lantz O., Planchard D., Le Chevalier T., Livartoski A., Barlesi F., Laplanche A., et al. 2016. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology. 5: e1071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sadallah S., Amicarella F., Eken C., Iezzi G., and Schifferli J. A.. 2014. Ectosomes released by platelets induce differentiation of CD4+T cells into T regulatory cells. Thromb. Haemost. 112: 1219–1229. [DOI] [PubMed] [Google Scholar]
- 78.Wahlund C. J. E., Gucluler G., Hiltbrunner S., Veerman R. E., Naslund T. I., and Gabrielsson S.. 2017. Exosomes from antigen-pulsed dendritic cells induce stronger antigen-specific immune responses than microvesicles in vivo. Sci. Rep. 7: 17095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Melo S. A., Luecke L. B., Kahlert C., Fernandez A. F., Gammon S. T., Kaye J., LeBleu V. S., Mittendorf E. A., Weitz J., Rahbari N., et al. 2015. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 523: 177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fouet M., Mader M-A., Iraïn S., Yanha Z., Naillon A., Cargou S., Gué A-M., and Joseph P.. 2016. Filter-less submicron hydrodynamic size sorting. Lab Chip. 16: 720–733. [DOI] [PubMed] [Google Scholar]
- 81.Zhao Z., Yang Y., Zeng Y., and He M.. 2016. A microfluidic ExoSearch chip for exosome detection towards blood-based ovarian cancer diagnosis. Lab Chip. 16: 489–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Théry C., Amigorena S., Raposo G., and Clayton A.. 2006. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell. Biol. Chapter 3: Unit 3.22. [DOI] [PubMed] [Google Scholar]
- 83.Théry C., Ostrowski M., and Segura E.. 2009. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 9: 581–593. [DOI] [PubMed] [Google Scholar]
- 84.Aung T., Chapuy B., Vogel D., Wenzel D., Oppermann M., Lahmann M., Weinhage T., Menck K., Hupfeld T., Koch R., et al. 2011. Exosomal evasion of humoral immunotherapy in aggressive B-cell lymphoma modulated by ATP-binding cassette transporter A3. Proc. Natl. Acad. Sci. USA. 108: 15336–15341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Alonso R., Mazzeo C., Rodriguez M. C., Marsh M., Fraile-Ramos A., Calvo V., Avila-Flores A., Merida I., and Izquierdo M.. 2011. Diacylglycerol kinase alpha regulates the formation and polarisation of mature multivesicular bodies involved in the secretion of Fas ligand-containing exosomes in T lymphocytes. Cell Death Differ. 18: 1161–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hessvik N. P., Overbye A., Brech A., Torgersen M. L., Jakobsen I. S., Sandvig K., and Llorente A.. 2016. PIKfyve inhibition increases exosome release and induces secretory autophagy. Cell. Mol. Life Sci. 73: 4717–4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Muralidharan-Chari V., Clancy J., Plou C., Romao M., Chavrier P., Raposo G., and D’Souza-Schorey C.. 2009. ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr. Biol. 19: 1875–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Phuyal S., Skotland T., Hessvik N. P., Simolin H., Overbye A., Brech A., Parton R. G., Ekroos K., Sandvig K., and Llorente A.. 2015. The ether lipid precursor hexadecylglycerol stimulates the release and changes the composition of exosomes derived from PC-3 cells. J. Biol. Chem. 290: 4225–4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Young L. N., Cho K., Lawrence R., Zoncu R., and Hurley J. H.. 2016. Dynamics and architecture of the NRBF2-containing phosphatidylinositol 3-kinase complex I of autophagy. Proc. Natl. Acad. Sci. USA. 113: 8224–8229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Baixauli F., Lopez-Otin C., and Mittelbrunn M.. 2014. Exosomes and autophagy: coordinated mechanisms for the maintenance of cellular fitness. Front. Immunol. 5: 403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fader C. M., Sanchez D., Furlan M., and Colombo M. I.. 2008. Induction of autophagy promotes fusion of multivesicular bodies with autophagic vacuoles in k562 cells. Traffic. 9: 230–250. [DOI] [PubMed] [Google Scholar]
- 92.Bridges D., Ma J. T., Park S., Inoki K., Weisman L. S., and Saltiel A. R.. 2012. Phosphatidylinositol 3,5-bisphosphate plays a role in the activation and subcellular localization of mechanistic target of rapamycin 1. Mol. Biol. Cell. 23: 2955–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Castellano B. M., Thelen A. M., Moldavski O., Feltes M., van der Welle R. E., Mydock-McGrane L., Jiang X., van Eijkeren R. J., Davis O. B., Louie S. M., et al. 2017. Lysosomal cholesterol activates mTORC1 via an SLC38A9-Niemann-Pick C1 signaling complex. Science. 355: 1306–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Strauss K., Goebel C., Runz H., Mobius W., Weiss S., Feussner I., Simons M., and Schneider A.. 2010. Exosome secretion ameliorates lysosomal storage of cholesterol in Niemann-Pick type C disease. J. Biol. Chem. 285: 26279–26288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.van der Kant R., Zondervan I., Janssen L., and Neefjes J.. 2013. Cholesterol-binding molecules MLN64 and ORP1L mark distinct late endosomes with transporters ABCA3 and NPC1. J. Lipid Res. 54: 2153–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chapuy-Regaud S., Subra C., Requena M., de Medina P., Amara S., Delton-Vandenbroucke I., Payre B., Cazabat M., Carriere F., Izopet J., et al. 2013. Progesterone and a phospholipase inhibitor increase the endosomal bis(monoacylglycero)phosphate content and block HIV viral particle intercellular transmission. Biochimie. 95: 1677–1688. [DOI] [PubMed] [Google Scholar]
- 97.Howcroft T. K., Zhang H. G., Dhodapkar M., and Mohla S.. 2011. Vesicle transfer and cell fusion: Emerging concepts of cell-cell communication in the tumor microenvironment. Cancer Biol. Ther. 12: 159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]