An additional mechanism of intercellular communication has been unveiled by the characterization of extracellular vesicles, namely exosomes and microvesicles, released from viable cells and concentrating selected molecules from the parental cells and able to function far from the emitting cells. Exosomes are naturally engineered nanovesicles, while microvesicles scale up to micron size. Among the numerous and diverse molecules they carry, these extracellular vesicles also contain a variety of lipid mediators. The purpose of this Thematic Review Series is to give an overview of the role of lipid molecules on the biogenesis and functions of exosomes and microvesicles.
Extracellular vesicles are formed in the parental cell by a budding process occurring either at the membrane of a late endosome or at the plasma membrane. Membrane biogenesis and budding is a coordinated process between the bilayer organization of lipids and the insertion of proteins. Both proteins and lipids define the specificity of each type of membrane within the cell. The distinct lipid composition of the various cellular membranes has subsequent effects on their biophysical properties and their functional roles in the cell. The same observations apply to the extracellular vesicles considered in this Thematic Review Series; i.e., vesicles released from intact viable cells, namely microvesicles (microparticles) shed from the plasma membrane, and exosomes released from multivesicular bodies (MVBs), a subset of the late endosome compartment. An overview of the cell biology of extracellular vesicles was recently presented (1). The lipid content of extracellular vesicles plays a key role in their involvement in various pathophysiological processes. This Thematic Review Series will emphasize the lipid sorting machinery involved in extracellular vesicle biogenesis, with their subsequent lipid composition, and their functionality as conveyers of bioactive lipids in intercellular communication.
For decades, particulate material released from cells had been considered as unneeded material, often representative of dying cells. For instance, microvesicles released from platelets were qualified as “platelet dust” (2), and the first observations related to exosomes reported between 1983 and 1987 by Philip D. Stahl’s laboratory (Washington University, Saint Louis, MO) (3) and Rose Johnstone’s laboratory at McGill University in Montreal (4) were made in the context of reticulocyte differentiation, leading to the idea that exosomes were conveyers of unneeded material for erythrocyte function.
Science moves in a stepwise manner. The first new step in this story was the observation by Graça Raposo in Willem Stoorvogel’s laboratory at Utrecht University, more than 20 years ago, that a B cell line was releasing nanovesicles enriched in antigen-presenting molecules (5), and that this was occurring from intact viable cells. Another step, a few years later, was the observation that these vesicles were functional. Indeed Laurence Zitvogel and Graça Raposo, at the Curie Institute, reported that exosomes derived from dendritic cells pulsed with tumor peptides stimulated the immune system with subsequent efficient eradication of tumors in mice (6). Since then, the characterization and function of exosomes has been developed largely by Clotilde Thery’s team at the Curie Institute (7–9). The first workshop on exosomes, organized by Rose Johnstone in Montreal in 2005 gathered only about 20 scientists, but discussions pointed out that these vesicles would become important in the future (10). A new step indeed confirmed that intuition, as a couple of years later, Hari Valadi and Jan Lötval at Göteborg University found that exosomes were carrying genetic material, which was exchanged between cells, and was functional in target cells (11). Thus the role of exosomes has evolved toward a function of intercellular “signalosomes” and pharmacological effectors (12).
Remarkably, exosomes concentrate a mixture of proteins, lipids, and nucleic acids from the parental cells, subsequent to a sorting process occurring at the level of the MVBs. Exosome constituents are functional, whatever they are. In addition, exosomes can travel long distances, being potentially able to circulate in the whole body (13). For scientists working in cell signaling, exosomes have opened a new era.
In the meantime, another community of scientists was investigating other types of vesicles derived from the plasma membrane. Interestingly the observation that shed plasma membrane vesicles could have a functional effect, in that case as a mechanism for tumor development, started at the same period of time as the first characterizations of exosomes in Douglas D. Taylor’s laboratory at Boston University (14, 15). Microvesicles derived from platelets have received ongoing interest because of their phospholipid-dependent pro-coagulant activity (16). In recent years, the exponentially growing interest in exosomes has moved forward that for microvesicles in various cell systems, in as much as both types of vesicles appear released from the same cell in some cell models. In this context, the creation of the International Society for Extracellular Vesicles has federated the two research communities (www.isev.org) and promoted research on both types of vesicles.
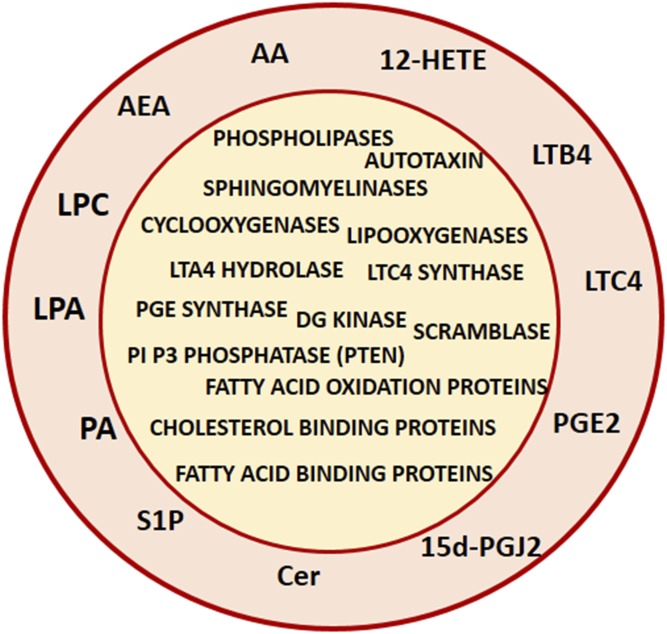
Generating a vesicle requires the parental membrane to bend and this is one function of lipids, whose structural modifications by specific enzymes will modify the molecular area of some lipids in one leaflet of the membrane, generating subsequent changes in membrane curvature. This applies both to exosomes and microvesicles whose biogenesis might be coordinated (reviewed in this series by M. Record et al.). Within this Thematic Review Series, the importance of sphingomyelinases (reviewed by C. Verderio et al.) and phospholipases D (reviewed by A. L. Egea-Jimenez et al.) in this process is considered. Exosomes can trigger changes in the phospholipid metabolism of receiving cells following their uptake, and can also modify the fatty acid oxidation status of target cells with a subsequent increase in the migration of cancer cells (reviewed by I. Lazar et al.). Internalization of platelet-derived microvesicles via their eicosanoid content into neutrophils promotes inflammation (reviewed by E. Boilard). In addition, modification of the cellular content of some lipids, such as cholesterol (reviewed by F.W. Pfrieger et al.), ether-linked phospholipids, or phosphoinositides PI(3,5)P2 (reviewed by T. Skotland et al.) enhances exosome production. Modification of the membrane distribution of phospholipids between the two leaflets, termed phospholipid asymmetry, is a key process of microvesicle biogenesis (reviewed by H. Chap et al.). Exosome biogenesis occurs in the MVB compartment, which partly originates from the plasma membrane, and consistently, peripheral lipid rafts participate in exosomes biogenesis (reviewed by R. C. Lai). As mentioned above, in this regard, it is worth noting that both tumor-derived exosomes and microvesicles display immunosuppressive activity (see the review from E. Boilard); the presence of immunosuppressive eicosanoids account in part for this observation. Additionally, the exosome-mediated internalization into target cells of a prostaglandin such as 15d-PGJ2 allows the bypass of a putative specific receptor or transporter for this prostaglandin, neither of which have been characterized so far (17). Further, considering lipidic mediators, extracellular vesicles are also carriers of endocannabinoids (see the review from C. Verderio). The panel of bioactive lipids and their related enzymes or transporters that have been reported so far in extracellular vesicles are summarized in Fig. 1.
Fig. 1.
Lipid mediators and lipid-related proteins carried by extracellular vesicles. AA, arachidonic acid; 12-HETE, 12-hydroxyeicosatetraenoic acid; LTB4, leukotriene B4; LTC4, leukotriene C4; PGE2 and 15d-PGJ2, prostaglandins; Cer, ceramide; S1P, sphingosine 1 phosphate; PA, phosphatidic acid; LPA, lyso-phosphatidic acid; LPC, lyso phosphatidylcholine; AEA, N-arachidonoylethanolamine (endocannabinoid). For graphical simplification, lipid-related proteins are plotted inside the vesicle; localization for some of them in the membrane of a native extracellular vesicle cannot be excluded.
In summary, extracellular vesicles participate in intercellular communications via exchange of bioactive lipids, and the biogenesis of these vesicles is in part dependent upon different lipid pathways, which will be developed in this series of reviews. Although the articles are related to mammalian cells, it should be borne in mind that vesicle-mediated communication is an evolutionarily conserved process (18). In that respect, a striking report is the characterization of nanovesicles isolated from edible plants, very rich in lipids and particularly in phosphatidic acids, which might display some therapeutic efficacy (19, 20). Whether extracellular vesicles might become a therapeutic tool is an open question. Indeed, because of their stability ex vivo, exosomes can be manipulated by inserting nonpolar molecules such as curcumin into their lipid phase with subsequent potential therapeutic effects (21). Therefore, encapsulating lipidic molecules into exosomes might lead to a more efficient drug delivery tool than the various liposome formulations elaborated so far, as exosomes are protected from macrophage phagocytosis by the presence of an integrin-associated protein (IAP, CD47) on their surface (22).
References
- 1.van Niel G., D’Angelo G., and Raposo G.. 2018. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 19: 213–228. [DOI] [PubMed] [Google Scholar]
- 2.van der Pol E., and Harrison P.. 2017. From platelet dust to gold dust: physiological importance and detection of platelet microvesicles. Platelets. 28: 211–213. [DOI] [PubMed] [Google Scholar]
- 3.Harding C., Heuser J., and Stahl P.. 1983. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J. Cell Biol. 97: 329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnstone R. M., Adam M., Hammond J. R., Orr L., and Turbide C.. 1987. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 262: 9412–9420. [PubMed] [Google Scholar]
- 5.Raposo G., Nijman H. W., Stoorvogel W., Liejendekker R., Harding C. V., Melief C. J., and Geuze H. J.. 1996. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 183: 1161–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zitvogel L., Regnault A., Lozier A., Wolfers J., Flament C., Tenza D., Ricciardi-Castagnoli P., Raposo G., and Amigorena S.. 1998. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat. Med. 4: 594–600. [DOI] [PubMed] [Google Scholar]
- 7.Théry C., Zitvogel L., and Amigorena S.. 2002. Exosomes: composition, biogenesis and function. Nat. Rev. Immunol. 2: 569–579. [DOI] [PubMed] [Google Scholar]
- 8.Théry C., Ostrowski M., and Segura E.. 2009. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 9: 581–593. [DOI] [PubMed] [Google Scholar]
- 9.Tkach M., and Thery C.. 2016. Communication by extracellular vesicles: where we are and where we need to go. Cell. 164: 1226–1232. [DOI] [PubMed] [Google Scholar]
- 10.Johnstone R. M. 2005. Revisiting the road to the discovery of exosomes. Blood Cells Mol. Dis. 34: 214–219. [DOI] [PubMed] [Google Scholar]
- 11.Valadi H., Ekstrom K., Bossios A., Sjostrand M., Lee J. J., and Lotvall J. O.. 2007. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9: 654–659. [DOI] [PubMed] [Google Scholar]
- 12.Record M., Subra C., Silvente-Poirot S., and Poirot M.. 2011. Exosomes as intercellular signalosomes and pharmacological effectors. Biochem. Pharmacol. 81: 1171–1182. [DOI] [PubMed] [Google Scholar]
- 13.Alvarez-Erviti L., Seow Y., Yin H., Betts C., Lakhal S., and Wood M. J.. 2011. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 29: 341–345. [DOI] [PubMed] [Google Scholar]
- 14.Taylor D. D., and Black P. H.. 1985. Inhibition of macrophage Ia antigen expression by shed plasma membrane vesicles from metastatic murine melanoma lines. J. Natl. Cancer Inst. 74: 859–867. [PubMed] [Google Scholar]
- 15.Taylor D. D., and Black P. H.. 1986. Shedding of plasma membrane fragments. Neoplastic and developmental importance. In Developmental Biology. Vol 3. Springer, New York: 33–57. [DOI] [PubMed] [Google Scholar]
- 16.Zwaal R. F., Comfurius P., and Bevers E. M.. 1992. Platelet procoagulant activity and microvesicle formation. Its putative role in hemostasis and thrombosis. Biochim. Biophys. Acta. 1180: 1–8. [DOI] [PubMed] [Google Scholar]
- 17.Subra C., Grand D., Laulagnier K., Stella A., Lambeau G., Paillasse M., De Medina P., Monsarrat B., Perret B., Silvente-Poirot S., et al. 2010. Exosomes account for vesicle-mediated transcellular transport of activatable phospholipases and prostaglandins. J. Lipid Res. 51: 2105–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schatz D., and Vardi A.. 2018. Extracellular vesicles - new players in cell-cell communication in aquatic environments. Curr. Opin. Microbiol. 43: 148–154. [DOI] [PubMed] [Google Scholar]
- 19.Ju S., Mu J., Dokland T., Zhuang X., Wang Q., Jiang H., Xiang X., Deng Z. B., Wang B., Zhang L., et al. 2013. Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mol. Ther. 21: 1345–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Record M. 2013. Exosome-like nanoparticles from food: protective nanoshuttles for bioactive cargo. Mol. Ther. 21: 1294–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun D., Zhuang X., Xiang X., Liu Y., Zhang S., Liu C., Barnes S., Grizzle W., Miller D., and Zhang H. G.. 2010. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol. Ther. 18: 1606–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamerkar S., LeBleu V. S., Sugimoto H., Yang S., Ruivo C. F., Melo S. A., Lee J. J., and Kalluri R.. 2017. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 546: 498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]