Abstract
Neutrophils form neutrophil extracellular traps (NETs), which have been implicated in microcirculatory plugging. NET formation (NETosis) involves the fusion of granule and nuclear contents, which are then released in the extracellular space. Myeloperoxidase (MPO) plays a major role in NETosis leading to the dissociation of DNA from histones. During neutrophil activation, MPO is released and activated to convert hydrogen peroxide and chloride to hypochlorous acid (HOCl). HOCl targets plasmalogens leading to the production of the chlorinated lipids, 2-chlorofatty aldehyde and 2-chlorofatty acid (2-ClFA). Here, we tested the hypothesis that 2-ClFAs are important lipid mediators of NETosis. Human neutrophils treated with physiological levels of 2-ClFAs formed NETs, characterized by MPO association with DNA and neutrophil elastase (NE) redistribution to the perinuclear area. 2-ClFA-induced NETs reduced Escerichia coli colony forming units. 2-ClFA-induced NETosis is calcium- and protein arginine deiminase 4-dependent. Interestingly, unlike PMA, 2-ClFA initiates the NETosis process without neutrophil activation and degranulation. Furthermore, 2-ClFA elicits NETosis in bone-marrow derived neutrophils from MPO-deficient mice. Taken together, these findings suggest 2-ClFA as an MPO product that triggers the NETosis pathway following neutrophil activation.
Keywords: neutrophils, fatty acids, cell signaling, myeloperoxidase, oxidized lipids, plasmalogens
During neutrophil activation the respiratory burst is responsible for the production of hydrogen peroxide. At the same time, neutrophils release some of their cytosolic granules and vesicles by fusing them with the plasma membrane. This results in the release of the granule content in the extracellular space, but it also leads to a substantial rearrangement in the expression profile of the molecules present on the neutrophil membrane surface. An increased surface expression of CD11b facilitates the rolling and adhesion of the neutrophils to the endothelial cells, facilitating the transmigration to the site of inflammation (1–3). At the same time, CD62L surface expression is known to decrease during neutrophil activation (4). Additionally, neutrophils may also release extracellular traps (NETs) during activation (5, 6). NET formation (NETosis) involves the dissolution of both storage granule membrane and nuclear membrane, leading to physical interactions between released granular and nuclear content (7). Subsequent histone modification and protease-mediated degradation of chromatin leads to DNA unwinding (8). Granule proteins adhere to the DNA that is released in the extracellular environment to form a trap decorated with histones, proteases, and bactericidal proteins (9). Once released, NETs are capable of localized bacterial trapping.
During NETosis, the granule content is released inside the cell, with subsequent neutrophil elastase (NE) degradation of actin in the cytosol (10) followed by its translocation to the nucleus where it targets histone H4, which is an important step facilitating the decondensation of the chromatin and the release of the DNA (the trap) (11). Myeloperoxidase (MPO), the main component of neutrophil granules, is also a crucial player in NETosis. While adhering to DNA NETs, MPO converts hydrogen peroxide to hypochlorous acid (HOCl), which potentially mediates bacterial killing (12, 13). One mechanism for MPO-mediated NETosis may be through the cationic properties of MPO leading to competition with histones for binding to negatively charged DNA, which is believed to promote the dissociation of the DNA NET from the chromatin (11, 14). However, a role for HOCl produced by MPO is suggested by the ability of HOCl to elicit NETosis in MPO-deficient neutrophils (15). Another crucial step in NETosis is the citrullination of histone H3, a modification mediated by protein arginine deiminase 4 (PAD4) that facilitates the dissociation of the DNA from the chromatin (8, 16–18). Calcium also has a role in NETosis being necessary for PAD4 activity and for the activation of NADPH oxidase (19). NADPH oxidase is one of the main mediators in the NETosis pathway, as it is responsible for starting the respiratory burst during neutrophil activation, which is considered essential for NETosis (7, 20, 21).
NETs are potentially harmful to the host (19, 22–24). NETosis is involved in numerous pathological conditions involving neutrophil activation. Autoimmune disease, renal disease, thrombotic events, pulmonary disease, sepsis, and metastasis of cancer cells are examples of conditions in which NETosis has a pathological role (6, 16, 25–34). Therefore, a better understanding of the mechanisms mediating NETosis may lead to new pharmacological targets to reduce deleterious roles of NETs. Interestingly, the trigger that initiates the dissolution of the granule and nuclear membranes, starting the process of NETosis, remains to be determined. Because MPO has a critical role in the canonical pathway for NETosis, the role of the MPO-dependent lipid, 2-chlorofatty acid (2-ClFA), in NETosis was investigated. MPO-derived HOCl can target the vinyl ether bond found at the sn-1 position of plasmalogen phospholipids. These are found abundant in the plasma membrane of neutrophils as well as endothelial cells. 2-Chlorofatty aldehyde (2-ClFALD) represents the first member of the chlorinated lipidome to be released from plasmalogens after HOCl oxidation of the plasmalogen vinyl ether bond. 2-ClFA is then produced following 2-ClFALD oxidation (35–38). In this study, we demonstrate that 2-ClFAs function as lipid mediators of NETosis. Overall, 2-ClFAs may have an important role in determining neutrophil fate following activation, mediating the production of NETs and providing a new pharmacological target to alter NETosis during inflammation.
METHODS
Materials
2-ClFAs were synthesized and prepared as described previously using palmitic or stearic acid as precursor (38). PMA was purchased from EMD Millipore.
Neutrophil isolation
Neutrophils were prepared from whole blood of healthy volunteers as previously described (37), and as authorized by Saint Louis University Institutional Review Board Protocol 9952. Briefly, whole blood was carefully layered on the density gradient in a scale 1:1. After centrifugation (500 gmax for 30 min), the band containing the polymorphonuclear cells (PMNs) was isolated and washed with HBSS. Following a lysis step to remove the contaminating red blood cells from the isolated PMNs, neutrophils were washed twice and suspended in HBSS at a final concentration of 0.5×106 cells/ml.
Cell treatment
Isolated human neutrophils were suspended in HBSS (0.5×106 cells/ml) to a final volume of 2 ml. The indicated conditions were delivered in 0.1% ethanol (EtOH). Neutrophils were then incubated at 37°C for the indicated amount of time. For lipid analyses, methanol was added to cells at the end of incubation periods prior to lipid extraction.
Extracellular DNA detection
Neutrophils were suspended in HBSS (106 cells/ml). The indicated conditions were delivered in 0.1% EtOH in the presence of 10 μM Sytox Green. Cells were then incubated at 37°C in a 96-well black plate with clear bottom. At the described time points, extracellular DNA was detected by measuring fluorescence at 523 nm. Because neutrophils display the tendency to aggregate and distribute unevenly throughout the well during NETosis, in every well the fluorescent signal was detected in 21 different spots using SpectraMax i3 Multi-Mode Detection PlatForm molecular devices. The average of those 21 readings was used as a single value for n = 1. The experiment was carried out in triplicate. A total of 20 mM saponin was used to lyse the cells and obtain the value considered the total extracellular DNA (which represents 100% extracellular DNA release). The ratio of that value was used to calculate the percentage of extracellular DNA in response to the treatments.
Immunofluorescence staining
Isolated human neutrophils were suspended in Hank’s Buffer (2×106 cells/ml) to a final volume of 2 ml and seated on coverslips in the presence of the described conditions for the indicated time. After incubation, cells were fixed in 10% formalin for 20 min. Fixed cells were washed with PBS, permeabilized with 0.3% Triton-X, washed and blocked in 2% BSA. The fixed cells were then incubated with the primary antibody in 2% BSA for 1 h at room temperature in a humidified chamber (anti-MPO 1:1000 or anti-NE 1:50). Cells were then washed and incubated with the secondary antibody in the dark with the same conditions (anti-rabbit 1:1000). After washing the cells again, the coverslips were mounted on the slides using VECTASHIELD oil containing DAPI. Images for the figures were captured with an Olympus FV 1000 laser scanning confocal microscope. Images in the figures represent the z-stack maximum projection. Background for MPO was adjusted so that the field without cells does not show signal. For neutrophil elastase analyses and image quantification, images were captured with a Leica DM5000 B microscope using 100× oil objective lenses with a Leica DFC350FX digital camera.
NE activity assay
After isolation, neutrophils were suspended in Hank’s Buffer (106 cells/ml) and incubated with either vehicle (0.1% EtOH), PMA, palmitic acid (PA), or the 2-ClFA, 2-chloropalmitic acid (2-ClPA) in the presence of 200 μM of NE chromogenic substrate (MeOSuc-AAPV-pNA, Cayman chemical, catalog #: 70967-90-7). Cells in the indicated conditions were incubated at 37°C for 30 min. NE activity was quantified by measuring absorbance at 405 nm using ThermoMax microplate reader molecular devices.
Surface marker expression
Human neutrophils were isolated, suspended in HBSS (106 cells/ml) with 1% BSA, and incubated in the described conditions at 37°C for 15 min. After the incubation, cells were centrifuged at 1,000 gmax for 5 min. The cell pellets were suspended in 1%BSA-PBS containing 0.1% sodium azide. Next, cells were incubated for 30 min with FITC-conjugated CD62L or CD11b antibody. Again, cells were washed and suspended in PBS-BSA. Finally, cells were fixed by adding paraformaldehyde to a final concentration of 4% while vortexing. Levels of CD62L and CD11b surface expression were measure via flow cytometry (excitation, 488 nm and emission, 525 nm).
Lysozyme ELISA assay
Following isolation, human peripheral blood neutrophils were suspended in HBSS (106 cells/ml) and incubated with the described conditions at 37°C for 10 min. After the treatment, cells were centrifuged for 10 min at 3,000 gmax to collect the supernatant, which was diluted 1:100 with the buffer from the ELISA kit (Abcam, ab108880). Instructions from the kit manufacturer were followed for lysozyme detection. Results are presented in absorbance units at 450 nm reflecting the amount of colorimetric substrate generated with the kit, which is directly proportional to the amount of lysozyme captured in the plate.
Inhibitor treatment
Several inhibitors were used to dissect the 2-ClFA-induced NETosis pathway. To this end, neutrophils were preincubated with indicated inhibitors for 20 min at 37°C. Diphenyleneiodonium chloride (10 μM, Sigma, D2926), was used to inhibit NADPH oxidase activity. Cl-Amidine (Cl-Am, 50 μM, Cayman Chemical, 10599) was used to inhibit PAD4 activity. 2-Aminoethoxydiphenylborane (100 μM, TOCRIS, 1224) was used to inhibit calcium release.
Neutrophil isolation from mouse bone marrow
Bone marrow neutrophils were isolated from 40-week-old male and female C57BL/6J and 32-week-old male and female MPO−/− mice fed chow. All animal experiments were conducted with the approval of the Institutional Animal Care and Use Committee at Saint Louis University. Following euthanasia, femur and tibia were extracted and cleaned with 70% EtOH in HBSS. After removing the epiphyses from the clean bones, HBSS, supplemented with 10% fetal bovine serum and 2 mM EDTA, was used to flush the bone marrow out of the bone and into a tube through a 100 μm cell strainer. Bone marrow cells were centrifuged at 368 gmax for 7 min. To lyse red blood cells contaminating the preparation, 20 ml of 0.2% NaCl was added to the cells, followed by 20 ml ice-cold 1.6% NaCl. Cells were centrifuged again (500 gmax for 7 min) and resuspended in 1 ml of buffer. The solution was then layered on a density gradient comprised of 3 ml of histopaque (1.119 gm/ml) and 3 ml of histopaque (1.077 gm/ml). PMNs were sequentially collected following centrifugation (30 min at 738 gmax), washed with HBSS, and suspended in HBSS to a final concentration of 106 cells/ml.
2-ClFA analysis
Mouse bone marrow neutrophils were isolated as described above and suspended in 0.5x106/ml. Human peripheral blood neutrophils were isolated as described above. Neutrophils were then incubated in either 0.1% EtOH or 100 nM PMA at 37°C for 1 h, shaking. After incubation, lipids were first extracted using a Bligh and Dyer extraction with 20 pmol of 2-chloro-[d4]hexadecanoic acid added as internal standard. Ten percent of the lipid extract was transferred to another tube and dried under nitrogen. Lipids were then suspended in 85/15 methanol/water with 0.1% formic acid. 2-ClFA levels, including both 2-ClPA and 2-chlorostearic acid (2-ClSA), were analyzed via ESI-MS as previously described (39, 40).
Trapping assay
Human neutrophils (2×106 cells/ml HBSS) were plated in 12-well plates in the presence of the indicated treatments and centrifuged at 300 gmax for 5 min. Cells were then incubated at 37°C for 3 h to induce the formation of NETs. After incubation, samples were treated with or without 100 units/ml of DNase for 20 min to dissolve the DNA backbone of NETs, making the structure no longer functional. 2×106 JM109 E. coli (1:1 ratio) cells were then added to the neutrophil NETs, followed by centrifugation at 700 gmax. The mixture of NETs and bacteria was incubated at 37°C for another hour. Samples were collected after scraping the wells and were subsequently suspended in Luria Broth at 1:10, 1:100, and 1:1000 dilutions. The diluted samples were plated on Luria Broth agar plates and incubated overnight at 37°C. Colony forming units (CFUs) were counted the next day and the percentage of NET trapping resulting in lower CFUs was calculated for each experiment using the formula
where df stands for “dilution factor”. That value was then used to obtain the average for an n = 4.
Statistics
Multiple comparisons were performed using ANOVA with Dunnetts posthoc test.
RESULTS
2-ClFAs induce extracellular DNA release in a time and concentration dependent manner
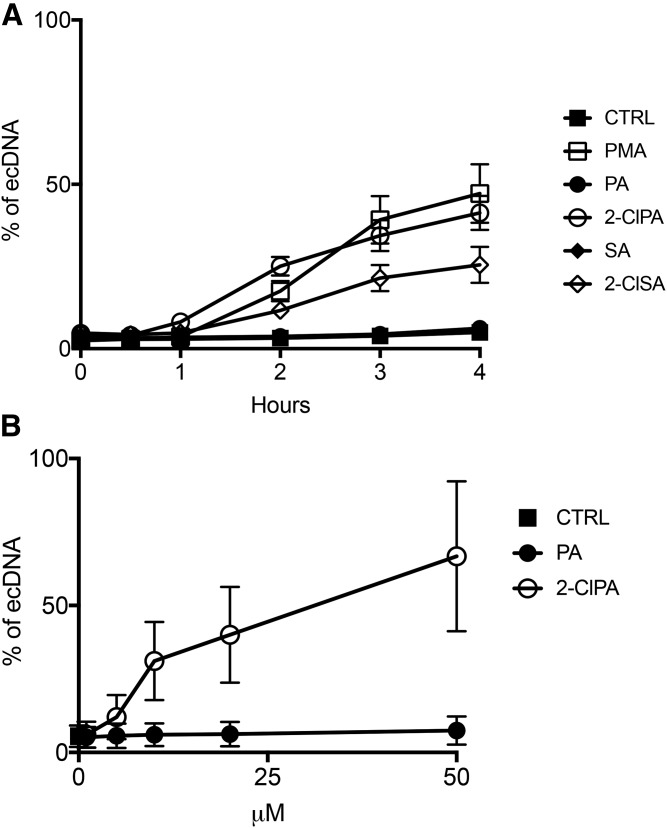
To investigate the role of 2-ClFAs on neutrophils, we tested the hypothesis that 2-ClFAs function as lipid mediators of NETosis by first assessing 2-ClFA-elicited extracellular DNA release. Data shown in Fig. 1A demonstrate the two common 2-ClFA molecular species produced as a result of HOCl targeting plasmalogens, 2-ClPA and 2-ClSA, elicit an increase in the release of extracellular DNA (ecDNA) detected using the cell membrane-impermeable fluorescent DNA stain, Sytox Green. Release of ecDNA in response to these 2-ClFAs was similar in magnitude and temporal course to that of the known activator of NETosis, PMA. In contrast, the nonchlorinated fatty acids, PA, or stearic acid did not elicit ecDNA release. Data in Fig. 1B show that the release of ecDNA in response to 2-ClPA was also concentration-dependent within physiological ranges of 2-ClFA found in activated neutrophils (36).
Fig. 1.
ecDNA quantification in response to 2-ClFA treatment. Human neutrophils were isolated from peripheral blood and treated at 37°C with either vehicle (0.1% EtOH, CTRL), 100 nM phorbol myristate acetate (PMA), 10 μM palmitic acid (PA), 10 μM 2-chloropalmitic acid (2-ClPA), 10 μM stearic acid (SA), or 10 μM 2-chlorostearic acid (2-ClSA) as indicated with extracellular DNA (ecDNA) detection with Sytox Green as described in the Materials and Methods. ecDNA accumulation was monitored by measuring fluorescence for up to 4 h of treatment as indicated in A, and after 3 h, in B. Fluorescence values represent mean ± SEM, n = 3.
2-ClFAs induce neutrophil extracellular trap formation in human neutrophils
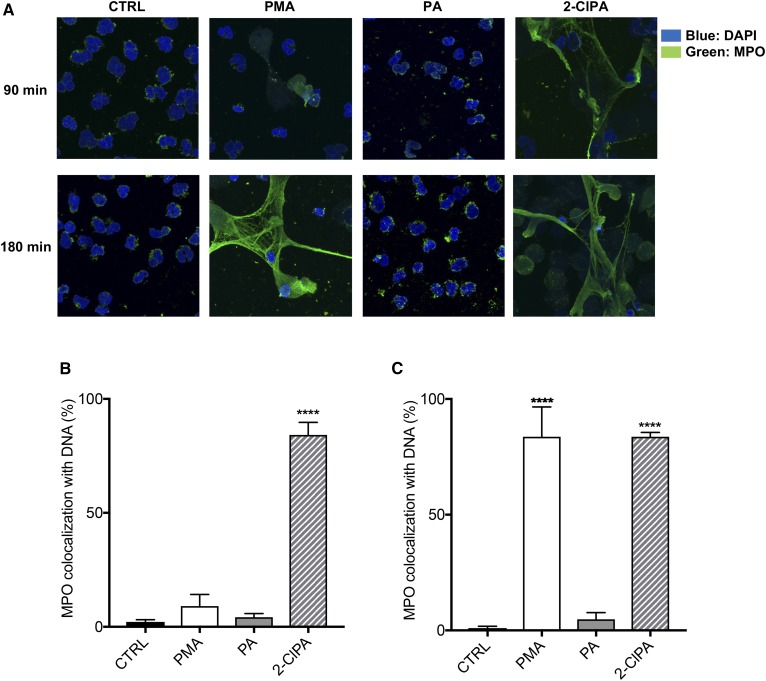
To confirm that the ecDNA release observed in human neutrophils in response to 2-ClFAs was the result of NET production, extracellular DNA strand formation, as well as the colocalization of MPO with DNA, were examined. Neutrophils treated with vehicle or PA for both 1.5 and 3 h displayed common characteristics of neutrophils: multi-lobular nuclei (stained with DAPI, in blue) and MPO stored in the primary granules in a punctate distribution throughout the cytoplasm (41) (Fig. 2A). In contrast, NETosis was apparent in neutrophils treated with 2-ClPA-treatment for 1.5 h, as indicated by the loss of nuclear structure and significant colocalization of MPO with the DNA associated with extracellular DNA strands (Fig. 2A). NETosis in PMA-treated neutrophils at 1.5 h post treatment was not as robust as that observed with 2-ClPA treatment. After 3 h, neutrophils treated with PMA and 2-ClFA displayed significant characteristics of NETosis including MPO colocalization with DNA on extracellular DNA strands (Figs. 2A–C). Data shown in Fig. 2A are also displayed in gray scale in supplemental Fig. S1, which further illustrate the colocalization of DNA staining with MPO staining under conditions of NETosis. Additional confocal analyses are shown in supplemental Fig. S2 with neutrophils stimulated with either 2-ClPA or PMA with an increased microscopic field to show multiple neutrophils forming NETs under these conditions. Other analyses demonstrated NE distribution in 2-ClPA treated neutrophils was perinuclear, which is characteristic of NETosis (supplemental Fig. S3). After 3 h of treatment, neutrophils treated with vehicle or PA maintained the same characteristics of nonactivated neutrophils, while PMA- and 2-ClFA-treated neutrophils both displayed perinuclear NE distribution. Together, these fluorescence microscopy studies indicate 2-ClFA, a product of neutrophil activation, functions as a strong stimulus for NETosis.
Fig. 2.
MPO colocalization with DNA in 2-ClFA-treated neutrophils. Human neutrophils were isolated from peripheral blood, suspended in HBSS, and seeded on coverslips in 6-well plates. Cells were then treated with either vehicle (0.1% EtOH, CTRL), 100 nM PMA, 10 μM PA, or 10 μM 2-ClPA for 90 or 180 min at 37°C. After incubation, cells were fixed, permeabilized, and analyzed via immunofluorescence for MPO (green) and DAPI (nuclei, blue). A shows images acquired with confocal microscope, and B and C show the quantification of samples at 90 and 180 min, respectively (mean ± SEM). ****P < 0.0001 for comparisons with CTRL treatment.
Interestingly, as shown in Figs. 1 and 2, 2-ClFA-induced NETosis is faster than PMA-induced NETosis. These data suggest that the MPO-derived product of neutrophil activation, 2-ClFA, functions as a lipid mediator of NETosis. With this paradigm, 2-ClFA-elicited NETosis would occur more rapidly than that elicited by PMA because PMA-elicited NETosis requires sequential neutrophil activation, HOCl production, HOCl oxidation of plasmalogens, and subsequent 2-ClFA production.
2-ClFAs-induced NETs are capable of trapping bacteria
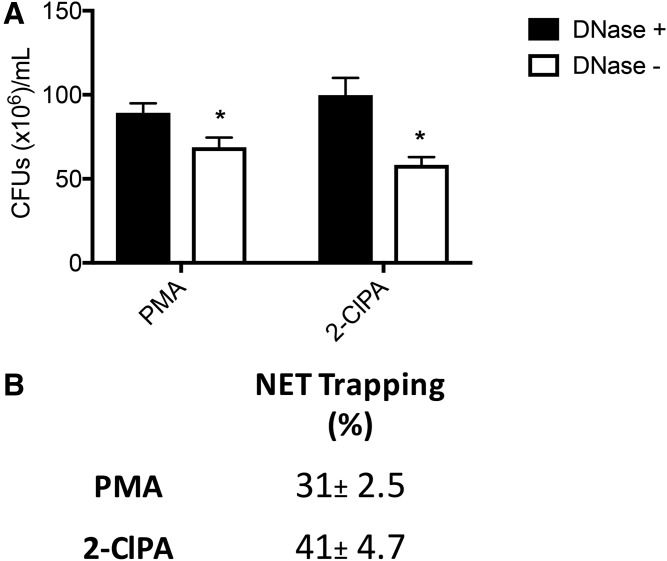
NETosis is a final mechanism for neutrophils to capture bacteria to prevent microbes from invading surrounding tissue and entering the systemic circulation. Accordingly, a functional assay was employed to analyze the ability of 2-ClFA-induced NETs to trap bacteria and compare 2-ClFA-induced NETs to PMA-induced NETs. For these studies, human neutrophils were treated with 2-ClFA or PMA for 3 h to induce NET formation. Bacteria were then added to the treated neutrophils for 1 h, and bacterial proliferation was tested by counting CFUs. As a negative control, DNase was added to degrade the DNA to make NETs ineffective. As shown in Fig. 3A and B, the efficacy of bacteria trapped by 2-ClFA-induced NETs in 1 h was at least as powerful as that of PMA-induced NETs. Taken together, the data suggest that 2-ClFAs induce the formation of extracellular traps that not only display the markers of NETs, but are also capable of carrying out their physiological function of trapping bacteria.
Fig. 3.
2-ClFA induces functional NETs. Human neutrophils were stimulated with either PMA or 2-ClFA for 3 h and then treated with or without DNase. The stimulated neutrophils were then incubated with an equal number of E. coli for 1 h. A shows colony forming unit (CFU) count, n = 4. B represents the average of four different experiments (mean ± SEM). Bacterial trapping was measured as described in the Materials and Methods. *P < 0.05 for t-test comparisons between conditions with and without DNase treatment.
2-ClFA-induced NETosis is Ca2+- and PAD4-dependent
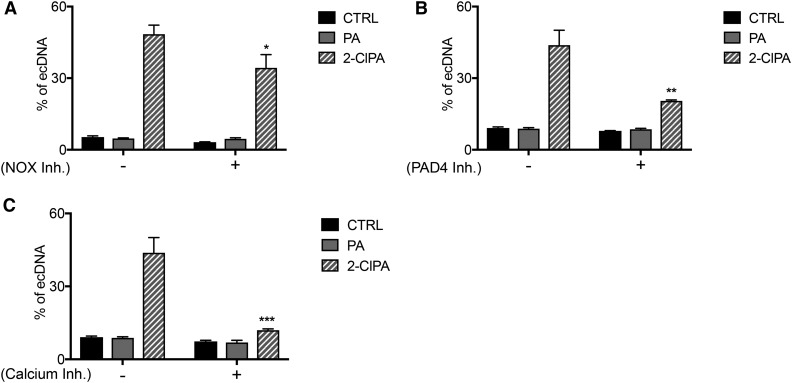
To further characterize the NETosis pathway induced by 2-ClFA treatment, inhibitors of Ca2+ release, NADPH oxidase and PAD4, were used. For these studies, we used ecDNA as a surrogate indicator of NETosis based on our findings shown in Figs. 1 and 2. 2-ClFA-stimulated ecDNA release was reduced in the presence of either PAD4 or Ca2+ release inhibitors (Fig. 4B and C). The main role of PAD4 and Ca2+ in NET formation was not surprising, as calcium is involved in NADPH oxidase activation and functions as cofactor for PAD4 activity (42). PAD4 was also demonstrated to be an essential mediator in NETosis due to its role in histone citrullination, a chromatin modification that removes positive charges from histones facilitating chromatin decondensation and NET formation (8, 43). Interestingly, NADPH oxidase inhibitor caused a less significant decrease in ecDNA release (Fig. 4A), suggesting that NADPH oxidase plays a minor role in 2-ClFA-induced NETosis. A possible interpretation is that NADPH oxidase plays a crucial role in neutrophil activation, which precedes 2-ClFA accumulation and NET formation. In these experiments, 2-ClPA, which is endogenously produced as a result of NADPH oxidase and MPO activity during neutrophil activation, is delivered exogenously, and because it is downstream from these enzymes, it is capable of eliciting ecDNA release in the presence of NADPH oxidase inhibitor. Together, these data helped further characterize this phenomenon, leading to the conclusion that 2-ClFA-induced NETosis is Ca2+- and PAD4-dependent.
Fig. 4.
Dissection of the canonical NETosis pathway in the presence of 2-ClFA. Human neutrophils were isolated from peripheral blood and treated with either vehicle (0.1% EtOH, CTRL), 100 nM PMA, 10 μM PA, or 10 μM 2-ClPA in the presence or absence of 10 μM diphenyleneiodonium chloride [DPI, NADPH oxidase inhibitor (NOX inhibitor)], 50 μM chloro-amidine (Cl-Am, PAD4 inhibitor), or 100 μM of 2- aminoethyldiphenyl borate (2-APB, calcium release inhibitor). Neutrophils were incubated at 37°C in the presence of Sytox Green. Extracellular DNA (ecDNA) accumulation was measured by fluorescence analysis after 3 h of treatment. Fluorescence values represent mean ± SEM, n = 3, as described in the Materials and Methods. *P < 0.05, **P < 0.005, ***P < 0.001 for t-test comparisons between conditions with and without inhibitor treatment.
2-ClFAs do not activate neutrophils
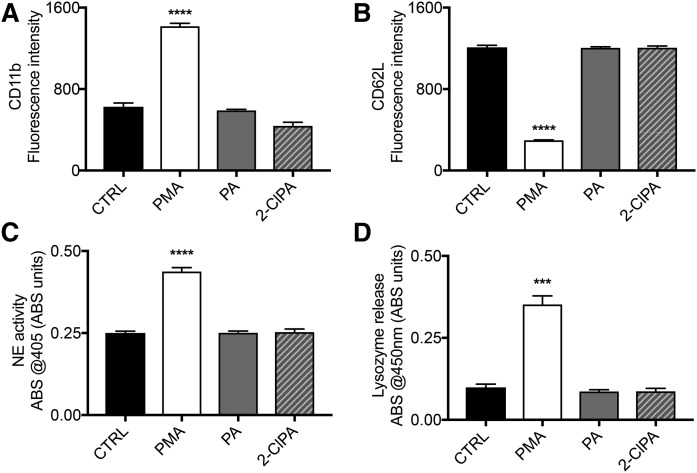
To further investigate the role of 2-ClFA on neutrophils, we tested the hypothesis that 2-ClFA elicits neutrophil activation. Data in Fig. 5A and B show PMA-treated, but not 2-ClPA-treated, neutrophils display a typical expression profile of activated neutrophils with a significant increase in the CD11b plasma membrane surface expression and a significant decrease in the CD62L plasma membrane surface expression level compared with control treatments. Extracellular degranulation is another step in neutrophil activation, which can be monitored through the quantification of the cytosolic granule content released in the extracellular environment. To this end, we quantitatively investigated the amount of lysozyme released by neutrophils in response to the different stimuli. As shown in Fig. 5D, PMA-treated, but not 2-ClPA-treated, neutrophils have significant increases in extracellular lysozyme release compared with the control conditions. Once neutrophils are activated, the enzymes in the granules are released and activated. Here, we used an activity assay to detect NE proteolytic activity in the extracellular space of neutrophils. Again, PMA, but not 2-ClPA, induced a significant increase in NE activity compared with control conditions (Fig. 5C). To summarize, unlike PMA, 2-ClFAs do not induce a significant change in the surface marker expression profile typical of activated neutrophils and they do not result in increased extracellular indicators of degranulation. Together, these observations lead to the conclusion that 2-ClFAs are a product of neutrophil activation, but they do not elicit further neutrophil activation.
Fig. 5.
Neutrophil activation marker analysis in response to PMA and 2-ClFA treatment. Human neutrophils were isolated from peripheral blood and treated with either vehicle (0.1% EtOH, CTRL), 100 nM phorbol myristate acetate (PMA), 10 μM palmitic acid (PA) or 10 μM chloropalmitic acid (2-ClPA) and incubated at 37°C for 15 min. Neutrophils were then fixed and stained with antibodies against CD11b and CD62L as described in the Materials and Methods and the expression level of the markers was analyzed by flow cytometry (A, B). In C, after the incubation with the same conditions, neutrophils were pelleted, and the supernatant was tested via ELISA for lysozyme release. In D, after neutrophils were incubated in the same conditions as above, neutrophil elastase (NE) activity was measured as indicated in the Materials and Methods. Values are expressed in arbitrary units and represent mean ± SEM, n = 3 (A–C) or n = 5 (D). ***P < 0.001, ****P < 0.0001 for comparisons with CTRL treatment.
2-ClFAs rescue NETosis in MPO−/− mice
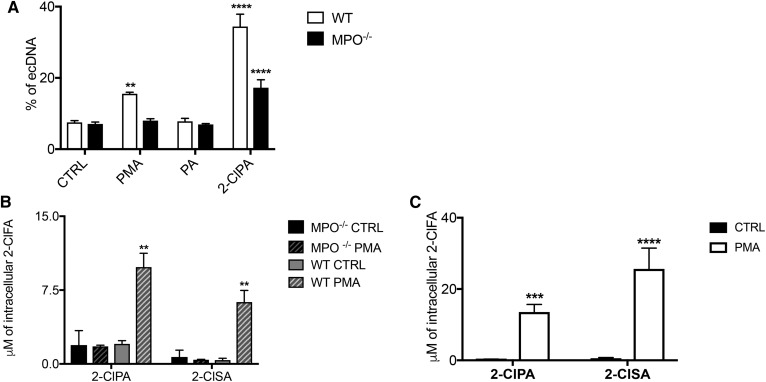
MPO activity is essential during NETosis (10, 44). The role of MPO in NETosis is particularly interesting in this study as its activity constitutes a sine qua non for 2-ClFA production. Therefore, to investigate the role of MPO in 2-ClFA-mediated NETosis, we isolated neutrophils from WT mice and MPO−/− mice to observe the effects of the different stimuli in either the presence or absence of neutrophil MPO. As shown in Fig. 6A, PMA induced significant ecDNA release in WT- but not in MPO−/−-neutrophils compared with control. However, bypassing the physiological formation of 2-ClFA by treating murine neutrophils with 2-ClFA led to significant ecDNA release in both WT- and MPO−/−-neutrophils. Interestingly, the percentage of ecDNA released in WT neutrophils was higher than that elicited in MPO−/− neutrophils. This difference may be due to the following two reasons. First, the absence of MPO in the MPO−/− neutrophils compromised chromatin decondensation, the other important role of MPO in NETosis. Second, the difference may be due to MPO producing additional endogenous 2-ClFA in WT neutrophils, which may enhance the effect of the 2-ClFA delivered exogenously. This latter possibility is supported by data shown in Fig. 6B demonstrating increased levels of 2-ClFA in PMA-treated WT neutrophils compared with control conditions. No detectable levels of 2-ClFA were observed under these same conditions in MPO−/− neutrophils. Another interesting observation is that the effect elicited by PMA in murine neutrophils is not as robust as that observed in human neutrophils, and this could be explained by the fact that murine neutrophil MPO content is less than that of human neutrophils (45, 46). This can lead to less chlorinated lipid formation, which, according to our findings, would explain a weaker response of murine NETosis compared with human NETosis. Indeed, comparisons of 2-ClFA accumulation in peripheral blood isolated human neutrophils in response to PMA stimulation (Fig. 6C) is greater than that observed in murine WT neutrophils (Fig. 6B). Overall, these data show MPO is essential for PMA elicited NETosis, but this can be bypassed by stimulation with exogenously provided 2-ClFA.
Fig. 6.
Murine WT- and MPO−/−-neutrophil NETosis. Murine neutrophils were isolated from bone marrow from either WT or MPO−/− mice as described in the Materials and Methods. Neutrophils were suspended in HBSS and treated with either vehicle (0.1% EtOH, CTRL), 100 nM PMA, 10 μM PA, or 10 μM 2-ClPA at 37°C. A: ecDNA accumulation using Sytox Green was measured by fluorescence analysis after 3 h of treatment. Fluorescence values represent mean ± SEM, n = 3, as described in the Materials and Methods. B: after an incubation interval of 1 h, lipids were extracted in the presence of internal standard, and 2-ClFA levels were quantified as described in the Materials and Methods. In C, human neutrophils were isolated from peripheral blood. After 1 h incubation, lipids were extracted in the presence of internal standard, and 2-ClFA levels were quantified as described in the Materials and Methods. Values represent the mean ± SEM (n = 3). **P < 0.005, ****P < 0.0001 for comparisons with CTRL treatment.
DISCUSSION
In 2004, Brinkmann et al. (5) demonstrated that human neutrophils are capable of releasing granule proteins and chromatin that together form traps capable of binding Gram-positive and Gram-negative bacteria. NETs were classified as a form of innate response that binds microorganisms, preventing them from spreading. However, as efficient as NETs are considered to be in trapping bacteria, they are also detrimental in several pathological conditions in which the formation of NETs mediate, in part, inflammatory sequelae leading to tissue damage and potentially contribute to the formation of thrombi with subsequent tissue ischemia. NETs have been reported to be involved in thrombosis, autoimmune diseases, metabolic disorders, lung diseases, fibrosis, diabetes, rheumatoid arthritis, sepsis, and cancer (16, 25, 47). Accordingly, characterizing the mechanisms underlying the formation of NETs is crucial as it may provide new therapeutic targets for these disease states.
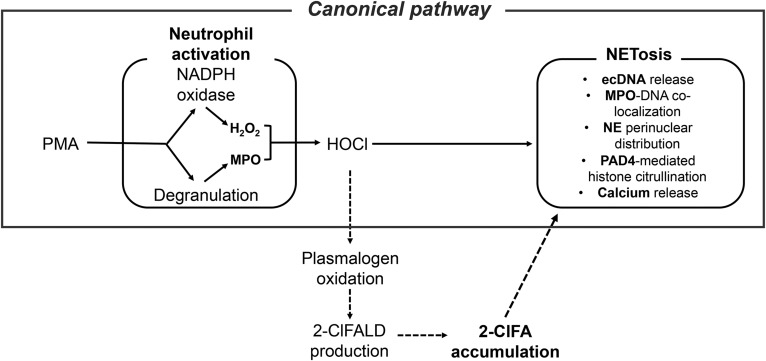
Because MPO has an important role in NETosis and 2-ClFA levels increase in activated neutrophils, these novel lipids were investigated as potential triggers of NETosis. 2-ClFAs are produced during inflammation as a consequence of MPO-derived HOCl targeting plasmalogen phospholipids. Neutrophil 2-ClFA levels peak 1 h after activation (36, 38), and in activated neutrophils and monocytes, 2-ClFA levels reach 10–30 μM levels (36, 48). This study shows that exposing neutrophils to 2-ClFAs results in the formation of functional NETs. These NETs were shown to be calcium- and PAD4-dependent. The inhibition of NADPH oxidase induced a less significant decrease in the ecDNA release, which is due to the fact that NADPH oxidase is upstream from the production of 2-ClFA. In the case of in vitro 2-ClFA-induced NETs, the role of NADPH oxidase is bypassed as the NADPH oxidase/MPO product is being given exogenously to the neutrophils. Furthermore, the role of MPO in NETosis was analyzed. Neutrophils lacking MPO (bone marrow-derived neutrophils from MPO−/− mice) did not release extracellular DNA in response to PMA treatment. In contrast, bone marrow-derived neutrophils from MPO−/− mice formed NETs in response to 2-ClFA treatment. In addition to the known role of MPO in chromatin decondensation (10, 11), the data in this study support an additional role for MPO in NETosis through the MPO oxidation product, 2-ClFA. These studies using the MPO−/− mouse neutrophils are also consistent with the findings of Akong-Moore and colleagues (15) that HOCl treatment of MPO−/− mouse neutrophils rescues NETosis capacity. HOCl would lead to 2-ClFA production in these cells (35, 36, 38). Based on these findings, a model is proposed with an additional mediator to the canonical pathway for NETosis (Fig. 7). Thus, while NETosis elicited by PMA is dependent on NADPH oxidase, MPO, calcium mobilization, and PAD4, NETosis elicited by exogenous 2-ClFA is only dependent on calcium mobilization and PAD4. Because 2-ClFA is produced as a result of NADPH oxidase and MPO activity, the addition of exogenous 2-ClFA to neutrophils bypasses the NADPH oxidase and MPO requirements for NETosis. Furthermore, these findings suggest that 2-ClFA may be an essential mediator of the canonical pathway for NETosis.
Fig. 7.
Canonical pathway for NETosis. Known mediators and mechanisms of NETosis are shown by solid arrows. Proposed mechanistic refinement of the model is shown in the broken arrows.
Previous studies have shown the essential roles of neutrophil elastase and PAD4 in NETosis, and have targeted these mechanisms to inhibit and attenuate the toxic effects of NETs (8, 10, 11). Additionally, DNase and antithrombotic drugs have been used in combination to attack DNA and degrade NETs. These approaches help in minimizing but not preventing NETs from forming. Many of the mechanisms involved in NETosis, including MPO, involve critical mediators that may also mediate microbe killing and infection resolution, which make their pharmacological manipulation difficult due to potential unwanted side effects. The identification of 2-ClFA as an MPO product that mediates NET formation might represent a step forward in the development of a more targeted therapeutic strategy to help prevent significant damage caused by NETs formed during inflammation. Future studies directed at identifying the specific targets of 2-ClFA that elicit NETosis may provide a new rationale for drug design to modulate NETosis.
Supplementary Material
Footnotes
Abbreviations:
- Cl-Am
- chloro-amidine
- 2-ClFA
- 2-chlorofatty acid
- 2-ClFALD
- 2-chlorofatty aldehyde
- 2-ClPA
- 2-chloropalmitic acid
- 2-ClSA
- 2-chlorostearic acid
- CFU
- colony forming unit
- EtOH
- ethanol
- ecDNA
- extracellular DNA
- HOCl
- hypochlorous acid
- MPO
- myeloperoxidase
- NET
- neutrophil extracellular trap
- NETosis
- NET formation
- NE
- neutrophil elastase
- PA
- palmitic acid
- PMN
- polymorphonuclear cell
- PAD4
- protein arginine deiminase 4
This study was supported by research funding from the National Institutes of Health Grant R01 GM-115553 to D.A.F. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Gao J. X., and Issekutz A. C.. 1996. Mac-1 (cd11b/cd18) is the predominant beta 2 (cd18) integrin mediating human neutrophil migration through synovial and dermal fibroblast barriers. Immunology. 88: 463–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao J. X., and Issekutz A. C.. 1995. Polymorphonuclear leucocyte migration through human dermal fibroblast monolayers is dependent on both beta 2-integrin (cd11/cd18) and beta 1-integrin (cd29) mechanisms. Immunology. 85: 485–494. [PMC free article] [PubMed] [Google Scholar]
- 3.Gao J. X., Wilkins J., and Issekutz A. C.. 1995. Migration of human polymorphonuclear leukocytes through a synovial fibroblast barrier is mediated by both beta 2 (cd11/cd18) integrins and the beta 1 (cd29) integrins vla-5 and vla-6. Cell. Immunol. 163: 178–186. [DOI] [PubMed] [Google Scholar]
- 4.Mastej K., and Adamiec R.. 2008. Neutrophil surface expression of cd11b and cd62l in diabetic microangiopathy. Acta Diabetol. 45: 183–190. [DOI] [PubMed] [Google Scholar]
- 5.Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D. S., Weinrauch Y., and Zychlinsky A.. 2004. Neutrophil extracellular traps kill bacteria. Science. 303: 1532–1535. [DOI] [PubMed] [Google Scholar]
- 6.Kolaczkowska E., and Kubes P.. 2013. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 13: 159–175. [DOI] [PubMed] [Google Scholar]
- 7.Fuchs T. A., Abed U., Goosmann C., Hurwitz R., Schulze I., Wahn V., Weinrauch Y., Brinkmann V., and Zychlinsky A.. 2007. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 176: 231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li P., Li M., Lindberg M. R., Kennett M. J., Xiong N., and Wang Y.. 2010. Pad4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J. Exp. Med. 207: 1853–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brinkmann V., and Zychlinsky A.. 2012. Neutrophil extracellular traps: Is immunity the second function of chromatin? J. Cell Biol. 198: 773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Metzler K. D., Goosmann C., Lubojemska A., Zychlinsky A., and Papayannopoulos V.. 2014. A myeloperoxidase-containing complex regulates neutrophil elastase release and actin dynamics during netosis. Cell Reports. 8: 883–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Papayannopoulos V., Metzler K. D., Hakkim A., and Zychlinsky A.. 2010. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 191: 677–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parker H., Albrett A. M., Kettle A. J., and Winterbourn C. C.. 2013. Myeloperoxidase associated with neutrophil extracellular traps is active and mediates bacterial killing in the presence of hydrogen peroxide. J. Leukoc. Biol. 91: 369–376. [DOI] [PubMed] [Google Scholar]
- 13.Parker H., and Winterbourn C. C.. 2012. Reactive oxidants and myeloperoxidase and their involvement in neutrophil extracellular traps. Front. Immunol. 3: 424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klinke A., Nussbaum C., Kubala L., Friedrichs K., Rudolph T. K., Rudolph V., Paust H. J., Schroder C., Benten D., Lau D., et al. . 2011. Myeloperoxidase attracts neutrophils by physical forces. Blood. 117: 1350–1358. [DOI] [PubMed] [Google Scholar]
- 15.Akong-Moore K., Chow O. A., von Kockritz-Blickwede M., and Nizet V.. 2012. Influences of chloride and hypochlorite on neutrophil extracellular trap formation. PLoS One. 7: e42984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sørensen O. E., and Borregaard N.. 2016. Neutrophil extracellular traps - the dark side of neutrophils. J. Clin. Invest. 126: 1612–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leshner M., Wang S., Lewis C., Zheng H., Chen X. A., Santy L., and Wang Y.. 2012. Pad4 mediated histone hypercitrullination induces heterochromatin decondensation and chromatin unfolding to form neutrophil extracellular trap-like structures. Front. Immunol. 3: 307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y., Li M., Stadler S., Correll S., Li P., Wang D., Hayama R., Leonelli L., Han H., Grigoryev S. A., et al. . 2009. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J. Cell Biol. 184: 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaplan M. J., and Radic M.. 2012. Neutrophil extracellular traps: double-edged swords of innate immunity. J. Immunol. 189: 2689–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bianchi M., Hakkim A., Brinkmann V., Siler U., Seger R. A., Zychlinsky A., and Reichenbach J.. 2009. Restoration of net formation by gene therapy in cgd controls aspergillosis. Blood. 114: 2619–2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steinberg B. E., and Grinstein S.. 2007. Unconventional roles of the nadph oxidase: signaling, ion homeostasis, and cell death. Sci. STKE. 2007: pe11. [DOI] [PubMed] [Google Scholar]
- 22.Cooper P. R., Palmer L. J., and Chapple I. L.. 2013. Neutrophil extracellular traps as a new paradigm in innate immunity: friend or foe? Periodontol. 2000. 63: 165–197. [DOI] [PubMed] [Google Scholar]
- 23.Saffarzadeh M., Juenemann C., Queisser M. A., Lochnit G., Barreto G., Galuska S. P., Lohmeyer J., and Preissner K. T.. 2012. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One. 7: e32366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Medina E. 2009. Neutrophil extracellular traps: a strategic tactic to defeat pathogens with potential consequences for the host. J. Innate Immun. 1: 176–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitsios A., Arampatzioglou A., Arelaki S., Mitroulis I., and Ritis K.. 2017. Netopathies? Unraveling the dark side of old diseases through neutrophils. Front. Immunol. 7: 678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Porto B. N., and Stein R. T.. 2016. Neutrophil extracellular traps in pulmonary diseases: too much of a good thing? Front. Immunol. 7: 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Döring Y., Soehnlein O., and Weber C.. 2017. Neutrophil extracellular traps in atherosclerosis and atherothrombosis. Circ. Res. 120: 736–743. [DOI] [PubMed] [Google Scholar]
- 28.Shen X. F., Cao K., Jiang J. P., Guan W. X., and Du J. F.. 2017. Neutrophil dysregulation during sepsis: an overview and update. J. Cell. Mol. Med. 21: 1687–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Camicia G., Pozner R., and de Larranaga G.. 2014. Neutrophil extracellular traps in sepsis. Shock. 42: 286–294. [DOI] [PubMed] [Google Scholar]
- 30.Barnado A., Crofford L. J., and Oates J. C.. 2016. At the bedside: neutrophil extracellular traps (nets) as targets for biomarkers and therapies in autoimmune diseases. J. Leukoc. Biol. 99: 265–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pfeiler S., Stark K., Massberg S., and Engelmann B.. 2017. Propagation of thrombosis by neutrophils and extracellular nucleosome networks. Haematologica. 102: 206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Delabranche X., Stiel L., Severac F., Galoisy A. C., Mauvieux L., Zobairi F., Lavigne T., Toti F., Angles-Cano E., Meziani F., et al. . 2017. Evidence of netosis in septic shock-induced disseminated intravascular coagulation. Shock. 47: 313–317. [DOI] [PubMed] [Google Scholar]
- 33.Cools-Lartigue J., Spicer J., McDonald B., Gowing S., Chow S., Giannias B., Bourdeau F., Kubes P., and Ferri L.. 2013. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J. Clin. Invest. 123: 3446–3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma A. C., and Kubes P.. 2008. Platelets, neutrophils, and neutrophil extracellular traps (nets) in sepsis. J. Thromb. Haemost. 6: 415–420. [DOI] [PubMed] [Google Scholar]
- 35.Albert C. J., Crowley J. R., Hsu F. F., Thukkani A. K., and Ford D. A.. 2001. Reactive chlorinating species produced by myeloperoxidase target the vinyl ether bond of plasmalogens: Identification of 2-chlorohexadecanal. J. Biol. Chem. 276: 23733–23741. [DOI] [PubMed] [Google Scholar]
- 36.Anbukumar D. S., Shornick L. P., Albert C. J., Steward M. M., Zoeller R. A., Neumann W. L., and Ford D. A.. 2010. Chlorinated lipid species in activated human neutrophils: Lipid metabolites of 2-chlorohexadecanal. J. Lipid Res. 51: 1085–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thukkani A. K., Hsu F. F., Crowley J. R., Wysolmerski R. B., Albert C. J., and Ford D. A.. 2002. Reactive chlorinating species produced during neutrophil activation target tissue plasmalogens: production of the chemoattractant, 2-chlorohexadecanal. J. Biol. Chem. 277: 3842–3849. [DOI] [PubMed] [Google Scholar]
- 38.Wildsmith K. R., Albert C. J., Anbukumar D. S., and Ford D. A.. 2006. Metabolism of myeloperoxidase-derived 2-chlorohexadecanal. J. Biol. Chem. 281: 16849–16860. [DOI] [PubMed] [Google Scholar]
- 39.Wang W. Y., Albert C. J., and Ford D. A.. 2013. Approaches for the analysis of chlorinated lipids. Anal. Biochem. 443: 148–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wacker B. K., Albert C. J., Ford B. A., and Ford D. A.. 2013. Strategies for the analysis of chlorinated lipids in biological systems. Free Radic. Biol. Med. 59: 92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Borregaard N. 1997. Development of neutrophil granule diversity. Ann. N. Y. Acad. Sci. 832: 62–68. [DOI] [PubMed] [Google Scholar]
- 42.Wang S., and Wang Y.. 2013. Peptidylarginine deiminases in citrullination, gene regulation, health and pathogenesis. Biochim. Biophys. Acta. 1829: 1126–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kolaczkowska E., Jenne C. N., Surewaard B. G., Thanabalasuriar A., Lee W. Y., Sanz M. J., Mowen K., Opdenakker G., and Kubes P.. 2015. Molecular mechanisms of net formation and degradation revealed by intravital imaging in the liver vasculature. Nat. Commun. 6: 6673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Metzler K. D., Fuchs T. A., Nauseef W. M., Reumaux D., Roesler J., Schulze I., Wahn V., Papayannopoulos V., and Zychlinsky A.. 2011. Myeloperoxidase is required for neutrophil extracellular trap formation: Implications for innate immunity. Blood. 117: 953–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rausch P. G., and Moore T. G.. 1975. Granule enzymes of polymorphonuclear neutrophils: a phylogenetic comparison. Blood. 46: 913–919. [PubMed] [Google Scholar]
- 46.Zschaler J., Schlorke D., and Arnhold J.. 2014. Differences in innate immune response between man and mouse. Crit. Rev. Immunol. 34: 433–454. [PubMed] [Google Scholar]
- 47.Yang H., Biermann M. H., Brauner J. M., Liu Y., Zhao Y., and Herrmann M.. 2016. New insights into neutrophil extracellular traps: Mechanisms of formation and role in inflammation. Front. Immunol. 7: 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang W. Y., Albert C. J., and Ford D. A.. 2014. Alpha-chlorofatty acid accumulates in activated monocytes and causes apoptosis through reactive oxygen species production and endoplasmic reticulum stress. Arterioscler. Thromb. Vasc. Biol. 34: 526–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.