Abstract
Background:
Despite the importance of understanding the connection between air pollution exposure and diabetes, studies investigating links between air pollution and glucose metabolism in nondiabetic adults are limited.
Objective:
We aimed to estimate the association of medium-term air pollution exposures with blood glucose and glycated hemoglobin A1c (HbA1c) among nondiabetics.
Methods:
This study included observations from nondiabetic participants () of the population-based Heinz Nixdorf Recall study at baseline (2000–2003) and follow-up examination (2006–2008). Daily fine particulate matter (; ), accumulation mode particle number (), and nitrogen dioxide () exposures were estimated at participants’ residences using the spatiotemporal European Air Pollution Dispersion (EURAD) chemistry transport model. We evaluated the associations between medium-term air pollution exposures (28- and 91-d means) and glucose metabolism measures using mixed linear regression and adjusting for season, meteorology, and personal characteristics. A range of other exposure windows (1-, 2-, 3-, 7-, 14-, 45-, 60-, 75-, 105-, 120-, and 182-d means) were also evaluated to identify potentially relevant biological windows.
Results:
We observed positive associations between and exposures and blood glucose levels [e.g., 28-d : (95% CI: 0.38, 1.44) per ]. , , and exposures were positively associated with HbA1c [e.g., 91-d : (95% CI: 0.04, 0.10) per ]. Mean exposures during longer exposure windows (75- to 105-d) were most strongly associated with HbA1c, whereas 7- to 45-d exposures were most strongly associated with blood glucose. exposure was not associated with blood glucose or with HbA1c.
Conclusions:
Medium-term PM and exposures were positively associated with glucose measures in nondiabetic adults. These findings indicate that reducing ambient air pollution levels may decrease the risk of diabetes. https://doi.org/10.1289/EHP2561
Introduction
Exposure to particulate matter (PM) in ambient air is a major environmental health risk, accounting for an estimated 3.1 million deaths and 3.1% of global disability-adjusted life years lost worldwide in 2010 (Lim et al. 2010). Short- and long-term exposure to these inhalable particles can aggravate respiratory and cardiovascular conditions, increase hospital admissions, and result in mortality from cardiovascular and respiratory diseases as well as from lung cancer (WHO 2006). Emerging evidence suggests that exposure to PM may also increase the risk of type 2 diabetes mellitus (T2DM) in the general population (Balti et al. 2014). Although specific pathophysiological mechanisms that might contribute to associations between PM and T2DM are unclear at present, one plausible hypothesis is that inhalation of particulate matter causes oxidative stress in the lungs that can lead to systemic inflammation, inflammation of adipose tissue, and insulin resistance. Together, these intermediate outcomes may contribute to a diabetogenic metabolism and eventually lead to the onset of T2DM (Franklin et al. 2015; Rajagopalan and Brook 2012).
In an effort to clarify these pathways, several epidemiological studies have explored whether higher air pollution exposure is associated with elevated blood glucose levels, a potential sign of increased insulin resistance. Short-term (days to weeks; Peng et al. 2016), medium-term (weeks to months; Peng et al. 2016; Sade et al. 2015, 2016), and long-term (; Cai et al. 2017; Chuang et al. 2011; Liu et al. 2016; Ward-Caviness et al. 2015; Wolf et al. 2016) exposure studies have shown positive associations between a variety of air pollution measures and blood glucose levels. Nevertheless, study findings have been inconsistent for specific pollutants [e.g., particulate matter (PM), nitrogen dioxide (), sulfur dioxide ()], and the latency period required for a cause–effect relationship has received little attention.
Because blood glucose measures are subject to high intrapersonal variability, glycated hemoglobin A1c (HbA1c), a biomarker that reflects average blood glucose levels over the previous 6–8 wk, is a useful instrument for assessing glucose levels and potential insulin resistance. At present, the epidemiological studies that have evaluated associations between long-term outdoor air pollution exposures (PM, , ) and HbA1c level show mixed results (Chuang et al. 2011; Honda et al. 2017; Liu et al. 2016; Tamayo et al. 2014, 2016; Wolf et al. 2016). The few studies investigating medium-term exposure periods, which may be the more relevant period for HbA1c levels, have also yielded mixed results (Sade et al. 2015, 2016).
Additionally, few studies have examined these associations in persons without diabetes (Brook et al. 2013; Chen et al. 2016; Honda et al. 2017; Kim and Hong 2012; Peng et al. 2016; Wolf et al. 2016), an important study group for bettering our understanding of how air pollution exposure may play a role in the early development of diabetes. We examined whether exposure to medium-term (28-, 91-d mean) air pollution [particulate matter with aerodynamic diameter (), particulate matter with aerodynamic diameter (), , and accumulation mode particle number ()] is associated with blood glucose and HbA1c levels in nondiabetic participants of the German population-based prospective Heinz Nixdorf Recall (HNR) study using data from two examination times. Because blood glucose levels vary more with daily changes than HbA1c levels do, we hypothesized that 28-d mean exposure windows would be more strongly associated with blood glucose measures, whereas 91-d mean exposure windows would be more strongly associated with HbA1c levels.
Methods
Study Design
This study was conducted using data from the baseline (2000–2003) and first follow-up (2006–2008) examinations of the HNR study, an ongoing prospective population-based cohort study located in three adjacent cities (Bochum, Essen, and Mülheim) within the highly urbanized German Ruhr area. The study design has been described in detail elsewhere (Schmermund et al. 2002; Stang et al. 2005). Briefly, potential participants between the ages of 45 and 75 were identified through random sampling of local residency lists and were recruited via letter or telephone. Subjects were not eligible for study inclusion if they were institutionalized, had died or moved away at time of recruitment, had an incorrect or nonexistent address, did not speak a sufficient level of German, could not be interviewed because of severe illness, or were pregnant. In all, 4,814 participants were enrolled into the HNR study between December 2000 and August 2003 (recruitment efficacy proportion: 55.8%; Stang et al. 2005), and 4,157 participants returned for a follow-up examination between 2006 and 2008. Assessment at both examinations included a self-administered questionnaire, face-to-face interviews, clinical examinations, and comprehensive laboratory tests following standard protocols. The study was approved by the institutional ethics committees of the University of Duisburg–Essen and the University Hospital of Essen and adhered to strict internal and external quality assurance protocols. All participants gave informed consent.
Environmental Exposures
Air pollution.
In this study, we used the validated, time-dependent, three-dimensional European Air Pollution Dispersion (EURAD) chemistry transport model (Büns et al. 2012; Hass et al. 1993; Memmesheimer et al. 2004) to estimate daily mass concentrations of , , , and (particle number with aerodynamic diameter between ; Nonnemacher et al. 2014). The EURAD model is a multilayer, multigrid model system that simulates the transport, chemical transformation, and deposition of tropospheric constituents (Büns et al. 2012). It employs four sequential nesting grid sizes (, , , ) for assigning exposure to Europe, central Europe, North Rhine–Westphalia in Germany, and the Ruhr area (Duisburg–Mülheim–Essen–Bochum), respectively (Büns et al. 2012; Memmesheimer et al. 2004). Specific information concerning the input data for the EURAD model have been published previously (Memmesheimer et al. 2004). The EURAD model produces hourly estimates for each grid square for a set of chemical compounds and a set of volatile organic compounds. Additionally, an assimilation process for and concentrations integrating measurement information from routine state-operated monitoring was conducted (measurement data on and for this study region and period were only available from individual measurement campaigns and therefore were not used for the assimilation process). estimates from the EURAD model have been validated against measurements obtained between January 2011 and December 2014 by the Institute of Energy and Environmental Technology (IUTA) at its measuring station in Mülheim–Styrum using a TSI 3926 scanning mobility particle sizer spectrometer (size range: ; TSI Inc.; for more details, see Birmili et al. 2016). For the accumulation mode (), Pearson correlation coefficients between the model estimates and measured averages were calculated by season (spring: 1 March–31 May; summer: 1 June–31 August; fall: 1 September–30 November; winter: 1 December–29 February) and ranged between 0.51 and 0.61, with the highest correlation occurring during winter and fall seasons (H. Jakobs, unpublished data, 2016).
The HNR study area covers approximately in the Ruhr area of Germany. Each participant in the HNR was assigned daily mean , , , and concentrations from the grid cell corresponding to his/her given residential address (ArcView, version 9.2, ESRI; Hennig et al. 2016; Nonnemacher et al. 2014). Residential mean exposure values were calculated for each participant for the 28- and 91-d before each examination. Additional means for 1-, 2-, 3-, 7-, 14-, 45-, 60-, 75-, 105-, 120-, and 182-d exposure windows were calculated for use in exploring the temporal shape of the association of interest. Interquartile range (IQR) values were calculated for each exposure using all observations across both examinations.
Meteorological variables.
Meteorological data for humidity and temperature were calculated as averages for the same 28- and 91-d time periods before examination using the Mesoscale Meteorological Model component of the EURAD model (Memmesheimer et al. 2004). In the modeling process, meteorological exposures were estimated for each grid using centralized data from the German weather service (Deutscher Wetterdienst) center in Essen, Germany. Means for 1-, 2-, 3-, 7-, 14-, 45-, 60-, 75-, 105-, 120-, and 182-d meteorological windows were also calculated.
Noise measures.
Long-term road noise was modeled for the year 2007 at façade points according to European Union Directive 2002/49/EC (European Commission 2002) and was calculated as the weighted 24-h mean (). Residential exposures were then assigned to participants using the maximum noise value at the most exposed façade point for the building of residence or, if building-specific information was lacking, the maximum value in a 10-m buffer around each participant’s address. Noise was included as a categorical variable with the following groups: , 45–50, 50–55, 55–60, 60–65, 65–70, 70–75, and .
Traffic indicators.
Proximity (in meters) of a participant’s residence to a major road, as defined by a traffic count of , was calculated using official digitized maps with a precision of at least . The reference line was the median strip between oncoming traffic lanes.
Blood Glucose and HbA1c Measures
Blood glucose levels (mg/dL) were measured at each examination with participants being advised to fast before the visit. Information on time since last meal (hours) was also collected to facilitate classification of each test as random or fasting. Serum samples were centrifuged immediately after being obtained and were analyzed enzymatically by the hexokinase method (Burrin and Price, 1985). HbA1c levels (percentage points, p.p.) were also measured at each examination using an automated nephelometer (BN-II; Dade Behring, Inc.). All analyses were performed in the central laboratory of the University Hospital of Essen following a standard procedure.
Definition of Covariates
Individual socioeconomic status (SES) was defined as years of education in four categories (, 11–13, 14–17, and ) according to the International Standard Classification of Education (UNESCO 1997). Neighborhood unemployment rates (as percentages) were obtained from local census authorities for each administrative neighborhood (median size: 11,263 inhabitants) for the years of the baseline examination (2000–2003; Dragano et al. 2009). Smoking status was defined as current, former ( since quitting), or never smoker. Cumulative smoking exposure was assessed for former and current smokers using pack-years and accounting for periods of nonsmoking. Exposure to environmental tobacco smoke (ETS; yes/no) was defined as regular passive exposure to smoke at home, work, or other location. Physical activity (yes/no) was assessed as participation in regular sporting activities at least once a week for a minimum of 30 min. Alcohol consumption was obtained through a dietary questionnaire (0, 1–3, 4–6, ). Anthropometric measurements (height, weight) were measured at examinations according to standard protocols, and body mass index (BMI; ) was calculated. Nutrition status was assessed using a dietary pattern index created by incorporating consumption frequency of 13 food items and diet quality classifications used in previous studies (Winkler and Döring 1995, 1998). Possible scores ranged from 0 to 26, with 26 representing an ideal diet, and were categorized into quartiles (, 11–12, 13–15, ). Diabetes mellitus status (yes/no) was classified as positive if the participant reported a physician diagnosis or was taking an antihyperglycemic drug. No differentiation was made by diabetes type (1 or 2). For medication use, participants were asked to bring all medication taken in the last seven days to each examination, where confirmed use was assigned using the WHO Anatomical Therapeutic Chemical classification system (ATC; Collaborating Centre for Drug Statistics Methology, 2014). Statin use (yes/no) was thus assigned. Seasonality at the time of blood draw was modeled using Fourier series terms cos() and sin(), where doy represents day of year (Peng et al. 2016). Updated information on all baseline characteristics was obtained at the follow-up study visit, with the exception of neighborhood unemployment rate and cumulative smoking exposure.
Study Population
A total of 8,971 observations were collected over the two examinations of the HNR study. Observations were excluded from the analysis if the participant had a diagnosis of diabetes or was taking antihyperglycemic medication at the time of the examination (). Participant observations from the baseline examination were not excluded if participants first reported a diabetes diagnosis or antihyperglycemic medication at follow-up. Additionally, 890 observations were excluded owing to missing variable information at baseline () or at the follow-up examination (). Of these, air pollution exposure (), HbA1c measure (), information on statin use (), alcohol consumption (), and nutrition index () were most commonly missing. After exclusions, the main analyses for blood glucose and HbA1c included 7,108 total observations from 4,176 participants.
Statistical Analyses
We evaluated the association between air pollution exposures (, , , ) and blood sugar measures (blood glucose, HbA1c) using a linear mixed-effects regression model with a random participant intercept to account for the correlation between individuals at baseline and at the follow-up examination. Separate 28- and 91-d mean exposure models were run for each air pollutant. Model estimates were calculated per IQR increase of exposure and are presented as mean exposure point estimates with 95% confidence intervals (CIs). Because significant nonlinearity was present for age, temperature, and humidity, these variables were modeled using restricted cubic splines with four knots. The normality of the model residuals was checked in a pre-analysis.
Three models (Models 1–3) of increasing covariate adjustment were conducted for each air pollutant exposure. Updated covariate information was used for all follow-up observations, with the exception of neighborhood unemployment and cumulative smoking (information only available at baseline). In Model 1, we adjusted for temperature, humidity, and examination. In Model 2, we additionally adjusted for age, season, nutrition index, smoking status, and BMI. For blood glucose analyses, time since last meal was also included. Covariates included for adjustment in Model 2 were identified using a directed acyclic graph (DAG; see Figure S1; Textor et al. 2011). To assess whether residual confounding was present, we further adjusted in Model 3 (the main model) for sex, education level, and additional lifestyle factors, including physical activity, alcohol consumption, statin use, ETS exposure, cumulative smoking exposure, and neighborhood unemployment. Covariates included in Model 3 were selected based on inclusion in prior studies (Peng et al. 2016; Wolf et al. 2016).
To explore whether associations between air pollution exposure and glucose metabolism varied according to exposure time window, we ran the main model analysis using a range of exposure windows before examination (1-, 2-, 3-, 7-, 14-, 28-, 45-, 60-, 75-, 91-, 105-, 120-, and 182-d means) for each pollutant. We chose the 28- and 91-d exposures a priori for the main analyses and treated the additional time points as an exploratory analysis.
Sensitivity Analyses
For , we repeated analyses using multipollutant models adjusted for , , or (as continuous variables) to determine whether associations with this exposure metric were independent of associations with more traditional air pollution markers. Spearman correlation coefficients were calculated between all air pollutant exposures (28- and 91-d means).
Analyses were also conducted to investigate whether different associations were apparent in observations designated as prediabetic () based on a fasting blood glucose level between or an HbA1c level between 5.7% and 6.5% (American Diabetes Association 2016) compared with observations having no indication of prediabetes. We also looked at whether associations differed by working status ( vs. ), because persons who worked less had a greater likelihood of spending their time at home and thus theoretically more accurate exposure assignments.
To evaluate the robustness of our results to the inclusion of traffic noise exposure, we added noise () and proximity to major roads to the main model individually as well as jointly. We also repeated analyses after excluding participants with a high blood glucose test (fasting blood glucose or nonfasting blood glucose ) as well as participants who had a diagnosis of diabetes or who were using antihyperglycemic medication at the time of examination. The blood glucose analyses were also run after excluding participants who were not fasting at the time of blood draw (; time since last meal ) to limit any effects due to incomplete fasting or to differences in fasting between examinations of the same individual.
Effect Modification
We evaluated potential effect modification by sex (male, female), smoking status (current/former/never), examination (baseline/follow-up), age (), season (spring/summer/fall/winter), and regular physical activity (yes/no) through addition of a multiplicative interaction term between air pollution exposure (28- and 91-d means; continuous) and the covariate of interest into the main analysis model. Interactions were considered significant if the corresponding likelihood ratio test yielded a .
All statistical analyses were conducted in R version 3.3.0 (R Core Team).
Results
Demographic Characteristics
A total of 7,108 blood glucose and HbA1c measurements were collected from 4,176 participants in the HNR study without diabetes and with complete covariate data (Table 1). Among fasting participants, mean blood glucose levels [] were and at baseline and at the follow-up examination, respectively. Glucose levels were similar for observations from nonfasting participants ( at baseline and at follow-up), possibly explained by the fact that although these participants did not fast the complete 8 h, the mean fasting time was still approximately 4.5 h. Mean HbA1c levels () at baseline and at follow-up were and , respectively. Compared with information reported by participants at the baseline examination (), participants at the follow-up examination () reported higher consumption of alcoholic drinks per week and lower exposure to ETS and were more likely to be former smokers and to report statin use. For all air pollutants, mean exposure levels decreased between visits (e.g., from 17.4 to for 28-d ; Table 2). exposure levels were moderately correlated with other air pollutants (, 0.51, and 0.33 for 28-d , , and , respectively; see Table S1).
Table 1.
Demographic characteristics of the Heinz Nixdorf Recall study participant observations included in this study, stratified by examination time (baseline: 2000–2003; follow-up: 2006–2008).
| Variable | Baseline () or (%) | Follow-up () or (%) |
|---|---|---|
| Age (y) | ||
| HbA1c (percentage points) | ||
| Blood glucose (mg/dL) | ||
| Time since last meal (h) | ||
| Fasting Status (yes) | 2,442 (63.3) | 2,527 (77.8) |
| BMI () | ||
| Neighborhood unemployment (%) | – | |
| Cumulative smoking (pack-years)a | – | |
| Sex (male) | 1,879 (48.7) | 1,551 (47.7) |
| Regular physical activity (yes) | 2,149 (55.7) | 1,911 (58.8) |
| Statin use (yes) | 378 (9.8) | 551 (16.9) |
| ETS exposure (yes) | 1,378 (35.7) | 807 (24.8) |
| Season at blood draw | ||
| Spring | 1,032 (26.8) | 897 (27.6) |
| Summer | 1,098 (28.5) | 731 (22.5) |
| Fall | 917 (23.8) | 797 (24.5) |
| Winter | 810 (21.0) | 826 (25.4) |
| Smoking status | ||
| Never smoker | 1,634 (42.4) | 1,409 (43.3) |
| Former smoker | 1,321 (34.2) | 1,266 (38.9) |
| Current smoker | 902 (23.4) | 576 (17.7) |
| Education level | ||
| 397 (10.3) | 304 (9.4) | |
| 11–13 years | 2,154 (55.8) | 1,830 (56.3) |
| 14–17 years | 883 (22.9) | 730 (22.5) |
| 423 (11.0) | 387 (11.9) | |
| Nutrition index | ||
| 650 (16.9) | 462 (14.2) | |
| 11–12 | 1,281 (33.2) | 1,073 (33.0) |
| 13–15 | 880 (22.8) | 737 (22.7) |
| 1,046 (27.1) | 979 (30.9) | |
| Alcoholic drinks per week | ||
| 0 | 1,877 (48.7) | 1,170 (36.0) |
| 1–3 | 693 (18.0) | 670 (20.6) |
| 4–6 | 378 (9.8) | 248 (7.6) |
| 909 (23.6) | 1,163 (35.8) | |
| Noise density (dB) | ||
| 633 (16.5) | 547 (17.0) | |
| 45–50 | 873 (22.8) | 767 (23.8) |
| 50–55 | 783 (20.4) | 641 (19.9) |
| 55–60 | 490 (12.8) | 419 (13.0) |
| 60–65 | 431 (11.3) | 345 (10.7) |
| 65–70 | 424 (11.1) | 349 (10.8) |
| 70–75 | 164 (4.3) | 133 (4.1) |
| 31 (0.8) | 18 (0.6) | |
| Missing | 28 (0.7) | 32 (1.0) |
Note: –, no information was collected at that particular examination point; BMI, body mass index; ETS, environmental tobacco smoke; HbA1c, glycated hemoglobin A1c; SD, standard deviation.
Among current or former smokers only.
Table 2.
Summary statistics for residential 28- and 91-day mean exposure levels at the baseline (2000–2003) and follow-up (2006–2008) examinations from the EURAD model.
| Exposure | Baseline () | Follow-up () | IQR | ||
|---|---|---|---|---|---|
| Range | Range | ||||
| 28-Day Exposures | |||||
| () | 10.3–31.2 | 7.5–33.7 | 5.7 | ||
| () | 11.1–44.3 | 8.7–40.6 | 7.4 | ||
| () | 18.9–104.2 | 15.6–80.5 | 15.7 | ||
| (n/mL) | 888.4–15,540.0 | 691.5–9,961.0 | 2,142.3 | ||
| Temperature () | 0.8–24.7 | 11.5 | |||
| Humidity (%) | 2.9–11.1 | 3.6–10.9 | 3.8 | ||
| 91-Day Exposures | |||||
| () | 12.3–27.3 | 9.6–25.2 | 4.0 | ||
| () | 13.2–36.3 | 10.6–34.9 | 5.5 | ||
| () | 20.7–76.7 | 18.1–65.9 | 15.0 | ||
| (n/mL) | 1,662.0–9,775.0 | 1,457.0–6,781.0 | 1,352.7 | ||
| Temperature () | 0.1–20.1 | 3.5–20.5 | 10.6 | ||
| Humidity (%) | 3.7–10.0 | 4.3–10.0 | 3.5 | ||
Note: EURAD, European Air Pollution Dispersion; IQR, interquartile range; , nitrogen dioxide; , particulate matter with aerodynamic diameter ; , particulate matter with aerodynamic diameter ; , accumulation mode particle number; SD, standard deviation.
Participants excluded because of missing data (covariate, exposure, and/or outcome) at the baseline examination were more likely to have attended their examination in the winter, to report exposure to ETS, to have of education, and to report being physically inactive than participants with complete data from the baseline examination (see Table S2). Participants excluded because of missing data at the follow-up examination were more likely to have attended their examination in the spring and to be former smokers than participants with complete data at follow-up.
Main Analysis: Air Pollution and Glucose Metabolism
In Model 1, (28- and 91-d), (28-d), and (28- and 91-d) exposures were positively associated with blood glucose (Table 3). Similarly, , , and exposures (28 d and 91 d) were positively associated with HbA1c. Further adjustment for additional covariates identified using a DAG (Model 2) slightly reduced the point estimates for blood glucose but yielded similar estimates for HbA1c.
Table 3.
Estimated association (95% confidence interval) between interquartile range increase in 28- and 91-day mean air pollution and HbA1c and blood glucose ().
| Exposure | Blood glucose (mg/dL)a | HbA1c (percentage points) | ||
|---|---|---|---|---|
| 28-Day estimate (95% CI) | 91-Day estimate (95% CI) | 28-Day estimate (95% CI) | 91-Day estimate (95% CI) | |
| Model 1b | 1.04 (0.50, 1.58) | 0.84 (0.15, 1.52) | 0.03 (0.02, 0.05) | 0.04 (0.01, 0.06) |
| Model 2c | 0.97 (0.44, 1.50) | 0.78 (0.01, 1.55) | 0.03 (0.01, 0.05) | 0.07 (0.04, 0.10) |
| Model 3 (Main)d | 0.91 (0.38, 1.44) | 0.81 (0.05, 1.58) | 0.03 (0.01, 0.05) | 0.07 (0.04, 0.10) |
| Model 1b | 0.77 (0.21, 1.34) | 0.37 (, 0.98) | 0.04 (0.02, 0.06) | 0.03 (0.00, 0.05) |
| Model 2c | 0.70 (0.15, 1.26) | 0.21 (, 0.86) | 0.04 (0.02, 0.06) | 0.04 (0.02, 0.06) |
| Model 3 (Main)d | 0.59 (0.04, 1.14) | 0.10 (, 0.75) | 0.04 (0.02, 0.06) | 0.04 (0.02, 0.06) |
| Model 1b | 0.45 (, 1.18) | 0.19 (, 1.17) | 0.02 (, 0.04) | (, 0.02) |
| Model 2c | 0.48 (, 1.13) | (, 0.83) | 0.01 (, 0.04) | 0.00 (, 0.04) |
| Model 3 (Main)d | 0.27 (, 0.99) | (, 0.65) | 0.01 (, 0.04) | 0.00 (, 0.04) |
| Model 1b | 0.74 (0.17, 1.31) | 0.86 (0.29, 1.44) | 0.04 (0.02, 0.06) | 0.09 (0.07, 0.11) |
| Model 2c | 0.75 (0.17, 1.32) | 0.75 (0.19, 1.32) | 0.03 (0.01, 0.06) | 0.09 (0.07, 0.11) |
| Model 3 (Main)d | 0.64 (0.07, 1.21) | 0.67 (0.10, 1.24) | 0.03 (0.01, 0.05) | 0.09 (0.07, 0.11) |
Note: All models were linear mixed-effects regression models with random participant intercepts. Interquartile range values are provided in Table 2. CI, confidence interval; HbA1c, glycated hemoglobin A1c; , nitrogen dioxide; , particulate matter with aerodynamic diameter ; , particulate matter with aerodynamic diameter ; , accumulation mode particle number concentration.
All blood glucose models were additionally adjusted for time since last meal (hours).
Adjusted for humidity, temperature, and examination.
, smoking status, nutrition index, season, and BMI.
, alcohol consumption, statin use, and exposure to environmental tobacco smoke at each visit; and pack-years of smoking and neighborhood unemployment at baseline.
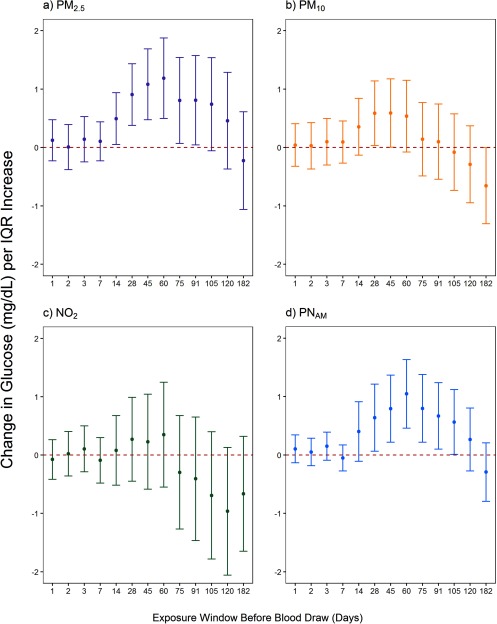
In Model 3, IQR increases in 28-d mean exposure to , , and were positively associated with blood glucose levels (Table 3). Significant positive associations were also seen for 91-d mean exposure to . For , neither 28- nor 91-d exposure levels were clearly associated with blood glucose levels. After evaluation of the association between a range of short- and medium-term exposures and blood glucose, we observed the strongest positive associations with 28-, 45-, and 60-d exposure windows for PM and (Figure 1). Associations between and blood glucose levels were close to the null for most exposure windows, although nonsignificant negative associations were estimated for the longest time windows (75- to 182-d).
Figure 1.
Associations between an interquartile range (IQR) increase in mean air pollution exposure and blood glucose level (mg/dL) using a range of short- and medium-term exposure windows before blood draw. Linear mixed-effects regression models with random participant intercepts were run using the main model (covariates provided in Table 3) and are shown stratified by air pollutant (, nitrogen dioxide; , particulate matter with aerodynamic diameter ; , particulate matter with aerodynamic diameter ; , accumulation mode particle number concentration). Error bars represent the 95% confidence interval for each point estimate. IQR values are provided in Table 2.
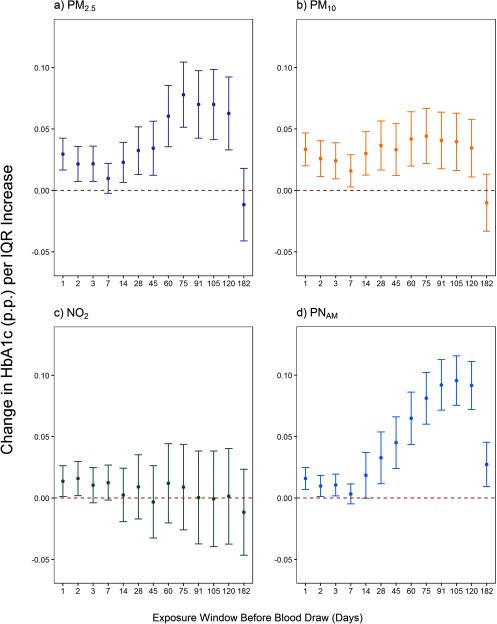
For HbA1c, significant positive associations were observed for 28-d and 91-d mean exposure to , , and using Model 3 (Table 3), with stronger point estimates being observed for 91-d than 28-d mean exposures. For , neither 28- nor 91-d exposure levels were associated with HbA1c. Using the range of exposure windows, we observed the strongest positive associations with slightly longer exposure windows for , , and (Figure 2) than for glucose. Associations between and HbA1c levels were null for all exposure windows.
Figure 2.
Associations between an interquartile range (IQR) increase in mean air pollution exposure and glycated hemoglobin A1c (HbA1c; percentage points) using a range of short- and medium-term exposure windows before blood draw. Linear mixed-effects models with random participant intercepts were run using the main model (covariates provided in Table 3) and are shown stratified by air pollutant (, nitrogen dioxide; , particulate matter with aerodynamic diameter ; , particulate matter with aerodynamic diameter ; , accumulation mode particle number concentration). Error bars represent the 95% confidence interval for each point estimate. IQR values are provided in Table 2.
Sensitivity Analyses
In the multipollutant models, the association between and blood glucose was attenuated when was included in the model but remained after adjustment for and (see Figure S2). All associations between and HbA1c were robust to adjustment for , , and exposure (see Figure S2).
When considering nondiabetic and prediabetic participants, associations for both blood glucose and HbA1c were attenuated among prediabetic participants (), but they remained in the same direction as those in the main analysis (see Figures S3 and S4). Estimates did not differ greatly by working status ( working part-time or less), when additional observations were excluded based on a broader definition of diabetes ( additionally excluded), or when analyses were limited to only fasting participants (). Model estimates were also very robust to single and simultaneous addition of proximity of the participant’s residence to a major road and chronic noise exposure ().
Effect Modification
For blood glucose, we observed evidence of effect modification by examination for (28-, 91-d) with associations that were positive for the baseline examination but close to the null at the follow-up (; see Figures S5 and S6). We also observed significant effect modification by season for (91-d; ), (91-d; ), and (28-d; ), with no clear seasonal pattern across pollutants. In analyses of HbA1c, we observed evidence of effect modification by season for all air pollutants, with stronger associations between air pollutants and HbA1c during the spring season (see Figures S7 and S8). Effect modification by examination was also present for 91-d , , and , with stronger associations apparent at the baseline examination [e.g., 0.09 (95% CI: 0.06, 0.13) vs. 0.06 (95% CI: 0.03, 0.09)] for ). Additionally, stronger associations were seen among former smokers than among current smokers and nonsmokers for (28- and 91-d p-values: 0.04 and 0.02, respectively) and (91-d; ). Although not statistically significant, the same pattern was also observed for and .
Discussion
In the present study, we showed that higher levels of residential medium-term exposure to , , and were positively associated with blood glucose levels and HbA1c among nondiabetic adults. These associations were robust to extensive adjustment for lifestyle factors, personal characteristics, traffic noise, and meteorological covariates. Importantly, we were able to observe a consistent temporal pattern of associations between air pollution exposure and glucose metabolism measures across a range of short- and medium-term exposure windows. The slightly different patterns observed for blood glucose and HbA1c are biologically plausible because more recent exposures were most strongly associated with blood glucose, whereas longer exposure windows were most strongly associated with HbA1c levels. To our knowledge, this is the first study to also examine the effects of particle number concentration (accumulation mode), a submicron particle metric of growing interest in the health community, on blood glucose levels and HbA1c using a spatiotemporal exposure model.
Biological Mechanisms
As described in previous reviews (Liu et al. 2013; Rajagopalan and Brook 2012; Rao et al. 2015), several pathophysiologic pathways may explain the associations between air pollution exposures and glucose metabolism. Inhalation of air pollution has been shown to induce low-grade oxidative stress and inflammation in the lungs (Rajagopalan and Brook 2012), and these effects do not remain localized in the lung but can lead to systemic oxidative stress and inflammation, including in the adipose tissue (Rao et al. 2015; Sun et al. 2005, 2009). Systemic and adipose tissue inflammation have been observed to lead to impaired insulin signaling in the pathways that moderate glucose metabolism (Haberzettl et al. 2016; Rao et al. 2015).
Evidence from several epidemiologic studies supports this connection between air pollution and glucose metabolism given that researchers have observed associations between short- and medium-term air pollution exposure and increased inflammation (Rajagopalan and Brook 2012) as well as worsening insulin resistance (Brook et al. 2013; Chen et al. 2016; Kelishadi et al. 2009; Kim and Hong 2012). Similarly, ongoing research suggests that medium-term exposures to air pollution during pregnancy may be related to abnormal glucose regulation (Fleisch et al. 2014; Lu et al. 2017; Robledo et al. 2015) and incidence of gestational diabetes mellitus among pregnant women (Hu et al. 2015). The results from the present study provide additional evidence that short- and medium-term air pollution exposures may represent biologically relevant exposure windows for influencing glucose metabolism measures. These associations with glucose metabolism may help explain emerging epidemiologic data that there is an association between long-term air pollution exposure and incidence of diabetes mellitus (Balti et al. 2014).
Comparison with Prior Studies
The observed association between medium-term exposure to PM and and blood glucose concentration has been previously shown for PM measures (Kim and Hong 2012; Peng et al. 2016). In a recent study among nondiabetic participants in the Normative Aging Study using a hybrid spatiotemporal prediction model, Peng et al. (2016) observed a significant positive association between 28-d exposure and fasting blood glucose ( per increase). Chen et al. (2016) also observed associations between centrally measured short-term exposures and fasting blood glucose among a cohort of Mexican Americans with high risk of diabetes (e.g., per 7-d mean ). In contrast to several previous studies (Chen et al. 2016; Chuang et al. 2011; Honda et al. 2017; Kim and Hong 2012; Sade et al. 2015; Wolf et al. 2016), we did not observe an association between exposure and blood glucose level, for which we have no clear explanation.
We specifically investigated nondiabetic participants, an important group for evaluating how air pollution exposure may influence early disease mechanisms that lead to a diabetogenic metabolism and eventually to T2DM in the general population. Three other studies including persons of mixed diabetes status have reported positive associations between air pollution exposures and blood glucose levels (Sade et al. 2015, 2016; Ward-Caviness et al. 2015). However, effects of diet, physical activity, and medication on blood glucose levels in participants with diabetes may overshadow relatively small effects of air pollution, suggesting that these effects might be easier to detect among metabolically healthy persons with intact glucose metabolism.
An important and novel finding of our study is that we found a positive association between medium-term PM and exposure and HbA1c levels in persons with no known metabolic disorder. This finding is of pathophysiological importance because it suggests that air pollution exposure may lead to a diabetogenic metabolism, manifesting itself as increased glucose levels over periods of several weeks to months. Of the two prior studies among nondiabetic participants, one reported no associations with , , or a variety of other exposure metrics (Wolf et al. 2016), whereas the second reported an association with but not with (Honda et al. 2017). Although all three studies were conducted in Western populations, the divergence in the results may be due to the difference in exposure windows used. With our spatiotemporal exposure model, we were able to choose biologically motivated medium-term (28- and 91-d mean) exposures before the blood draw for our analysis, whereas Wolf et al. (2016) utilized a temporally static long-term mean, and Honda et al. (2017) used 1- to 5-y moving average exposure windows. From a biological standpoint, medium-term exposures (averaged over weeks to months) would be more likely to be associated with HbA1c than exposures averaged over longer periods of time given that HbA1c levels in individual red blood cells increase with age, and the lifespan of a typical red blood cell is approximately 115 d (Franco 2012).
In recent years, experimental and epidemiologic evidence has emerged suggesting that submicron and ultrafine (UFP; aerodynamic diameter ) particles may have significant adverse health effects owing to their large surface area–to–mass ratio, their ability to generate reactive oxygen species, and their small size, which enables them to penetrate deeply into the respiratory system (Li et al. 2016). Nevertheless, epidemiologic studies linking exposure to submicron particles or UFPs with health effects are limited because they are not routinely measured by monitoring networks, and their heterogeneous distribution in the atmosphere makes them challenging to model and measure. To our knowledge, only three studies have investigated whether UFP exposure is associated with diabetes-related outcomes. One small study, conducted among 58 Danish households of unknown diabetes status, observed that 48-h exposure to indoor UFPs was associated with HbA1c levels, but it did not observe any connection between 48-h outdoor UFP exposure (assigned from a central monitoring station) and HbA1c (Karottki et al. 2014). In a study of near-highway households, no association was observed between annual UFP exposure and prevalent diabetes (Li et al. 2017). In contrast, a recent Canadian cohort study by Bai et al. (2018) observed a positive association between annual ambient UFP exposure and risk of diabetes. In the present study, we considered the accumulation mode rather than ultrafine particles specifically and found positive associations between both short-term (28-d) and medium-term (91-d) outdoor exposure and two measures of glucose metabolism that merit further investigation.
Clinical Importance
Although the increases in blood glucose and HbA1c levels observed in the present study (e.g., increase in HbA1c of 0.15 p.p. per ) are small, and the exposure range in the Ruhr area of Germany (e.g., 91-d mean IQR: ) is low, air pollution exposure is ubiquitous and unavoidable for all members of the population. Even small increases in blood glucose and HbA1c levels may increase the risk of cardiometabolic disease (Zhang et al. 2010) as well as of cardiovascular events and mortality (Cohen et al. 2009; Schöttker et al. 2016). Thus, even these small increases per unit of air pollution exposure may have relevant and wide-reaching impacts on health worldwide.
Strengths and Limitations
This study has several strengths. The HNR study is a well-characterized, population-based cohort with extensive covariate data, which allowed us to adjust for potential confounders as well as for the co-occurring exposure of long-term traffic noise. Additionally, the use of a spatiotemporal model allowed us to estimate a range of short- and medium-term exposures. The consistency and the temporal pattern across short- to medium-term exposure windows also support the hypothesis that a true association exists between air pollution exposure and glucose metabolism measures among nondiabetic adults.
Nevertheless, there are several limitations of this study. Because the EURAD model estimates urban background exposures on a grid and not directly at participants’ residences, some exposure misclassification, particularly in traffic-related air pollution, likely exists. We expect this misclassification to be nondifferential and thus, in principle, to bias our results towards the null. Additionally, it is possible that a background air pollution estimate for the around a residence may not represent a good estimate of overall exposure because people typically do not spend the entirety of their time at home. Assimilated estimates of and are unavailable in this study area owing to a small number of measurements; thus, error in these exposure estimates is likely greater than for and .
Conclusion
Medium-term exposure to PM and was positively associated with blood glucose and HbA1c levels, consistent with an adverse effect on glucose regulation in nondiabetic adults. Further studies investigating PM components and source-specific particulate matter as well as possible mediation pathways are needed to better understand the mechanisms by which air pollution exposure influences blood glucose and HbA1c levels in metabolically healthy persons.
Supplemental Material
Acknowledgments
We are indebted to the investigative group and the study personnel of the Heinz Nixdorf Recall study. We also thank the North Rhine–Westphalia State Agency for Nature, Environment and Consumer Protection for providing emission and land use data for North Rhine–Westphalia. We thank A. Buschka for her data management work and U. Quass from the Institute of Energy and Environmental Technology for his help in describing the validation study. The Heinz Nixdorf Recall study was funded by the Heinz Nixdorf Foundation, Germany [Chairman: M. Nixdorf; Past Chairman: Dr. jur. G. Schmidt (deceased)]. The HNR was also supported by grants from the German Research Council [Deutsche Forschungsgemeinschaft (DFG); ER 155/6-1, ER 155/6-2, SI 236/8-1, and SI 236/9-1] and the Kulturstiftung Essen, Germany. The AirFlamm III study was supported by the German Research Council (DFG; HO 3314/4-3). This work was completed in partial fulfillment of the requirements of the Ph.D. thesis for S.L.
References
- American Diabetes Association. 2016. Erratum. Classification and diagnosis of diabetes. Sec. 2. In Standards of Medical Care in Diabetes-2016. Diabetes Care 2016;39(Suppl. 1):S13-S22. Diabetes Care 39(9):1653 S13–S22, PMID: 26696675, 10.2337/dc16-S005. [DOI] [PubMed] [Google Scholar]
- Bai L, Chen H, Hatzopoulou M, Jerrett M, Kwong JC, Burnett RT, et al. 2018. Exposure to Ambient Ultrafine Particles and Nitrogen Dioxide and Incident Hypertension and Diabetes. Epidemiology [Epub ahead of print], PMID: 29319630, 10.1097/EDE.0000000000000798. [DOI] [PubMed] [Google Scholar]
- Balti EV, Echouffo-Tcheugui JB, Yako YY, Kengne AP. 2014. Air pollution and risk of type 2 diabetes mellitus: A systematic review and meta-analysis. Diabetes Res Clin Pract 106(2):161–172, PMID: 25262110, 10.1016/j.diabres.2014.08.010. [DOI] [PubMed] [Google Scholar]
- Birmili W, Weinhold K, Rasch F, Sonntag A, Sun J, Merkel M, et al. 2016. Long-term observations of tropospheric particle number size distributions and equivalent black carbon mass concentrations in the German Ultrafine Aerosol Network (GUAN). Earth Syst Sci Data 8(2):355–382, 10.5194/essd-8-355-2016. [DOI] [Google Scholar]
- Brook RD, Xu X, Bard RL, Dvonch JT, Morishita M, Kaciroti N, et al. 2013. Reduced metabolic insulin sensitivity following sub-acute exposures to low levels of ambient fine particulate matter air pollution. Sci Total Environ 448:66–71, PMID: 22901427, 10.1016/j.scitotenv.2012.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büns C, Klemm O, Wurzler S, Hebbinghaus H, Steckelbach I, Friesel J, et al. 2012. Comparison of four years of air pollution data with a mesoscale model. Atmos. Res 118:404–417, 10.1016/j.atmosres.2012.07.009. [DOI] [Google Scholar]
- Burrin JM, Price CP. 1985. Measurement of blood glucose. Ann Clin Biochem 22(Part 4):327–342, PMID: 3898972, 10.1177/000456328502200401. [DOI] [PubMed] [Google Scholar]
- Cai Y, Hansell AL, Blangiardo M, Burton PR, de Hoogh K, Doiron D, et al. 2017. Long-term exposure to road traffic noise, ambient air pollution, and cardiovascular risk factors in the HUNT and Lifelines cohorts. Eur. Heart J 44:1–8, PMID: 28575405, 10.1093/eurheartj/ehx263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Salam MT, Toledo-Corral C, Watanabe RM, Xiang AH, Buchanan TA, et al. 2016. Ambient air pollutants have adverse effects on insulin and glucose homeostasis in Mexican Americans. Diabetes Care 39(4):547–554, PMID: 26868440, 10.2337/dc15-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang KJ, Yan YH, Chiu SY, Cheng TJ. 2011. Long-term air pollution exposure and risk factors for cardiovascular diseases among the elderly in Taiwan. Occup Environ Med 68(1):64–68, PMID: 20833756, 10.1136/oem.2009.052704. [DOI] [PubMed] [Google Scholar]
- Cohen BE, Barrett-Connor E, Wassel CL, Kanaya AM. 2009. Association of glucose measures with total and coronary heart disease mortality: does the effect change with time? The Rancho Bernardo Study. Diabetes Res Clin Pract 86(1):67–73, PMID: 19671481, 10.1016/j.diabres.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragano N, Hoffmann B, Stang A, Moebus S, Verde PE, Weyers S, et al. 2009. Subclinical coronary atherosclerosis and neighbourhood deprivation in an urban region. Eur J Epidemiol 24(1):25–35, PMID: 18931923, 10.1007/s10654-008-9292-9. [DOI] [PubMed] [Google Scholar]
- European Commission. 2002. Directive 2002/49/EC of the European Parliament and of the Council of 25 June 2002 relating to the assessment and management of environmental noise. Off J Eur Commun L189 18.7 2002:12–25. http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32002L0049 [accessed 22 May 2017]. [Google Scholar]
- Fleisch AF, Gold DR, Rifas-Shiman SL, Koutrakis P, Schwartz JD, Kloog I, et al. 2014. Air pollution exposure and abnormal glucose tolerance during pregnancy: the Project Viva cohort. Environ Health Perspect 122(4):378–383, PMID: 24508979, 10.1289/ehp.1307065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco RS. 2012. Measurement of red cell lifespan and aging. Transfus Med Hemother 39(5):302–307, PMID: 23801920, 10.1159/000342232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin BA, Brook R, Arden Pope C. 2015. Air pollution and cardiovascular disease. Curr Probl Cardiol 40(5):207–238, PMID: 25882781, 10.1016/j.cpcardiol.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Haberzettl P, O’Toole TE, Bhatnagar A, Conklin DJ. 2016. Exposure to fine particulate air pollution causes vascular insulin resistance by inducing pulmonary oxidative stress. Environ Health Perspect 124(12):1830–1839, PMID: 27128347, 10.1289/EHP212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hass H, Ebel A, Feldmann H, Jakobs HJ, Memmesheimer M. 1993. Evaluation studies with a regional chemical transport model (EURAD) using air quality data from the EMEP monitoring network. Atmos Environ A Gen Top 27(6):867–887, 10.1016/0960-1686(93)90007-L. [DOI] [Google Scholar]
- Hennig F, Sugiri D, Tzivian L, Fuks K, Moebus S, Jöckel KH, et al. 2016. Comparison of land-use regression modeling with dispersion and chemistry transport modeling to assign air pollution concentrations within the Ruhr area. Atmosphere (Basel) 7(3):48, 10.3390/atmos7030048. [DOI] [Google Scholar]
- Honda T, Pun VC, Manjourides J, Suh H. 2017. Associations between long-term exposure to air pollution, glycosylated hemoglobin and diabetes. Int J Hyg Environ Health 220(7):1124–1132. PMID: 28712959, 10.1016/j.ijheh.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Ha S, Henderson BH, Warner TD, Roth J, Kan H, et al. 2015. Association of atmospheric particulate matter and ozone with gestational diabetes mellitus. Environ Health Perspect 123(9):853–859, PMID: 25794412, 10.1289/ehp.1408456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karottki DG, Bekö G, Clausen G, Madsen AM, Andersen ZJ, Massling A, et al. 2014. Cardiovascular and lung function in relation to outdoor and indoor exposure to fine and ultrafine particulate matter in middle-aged subjects. Environ Int 73:372–381, PMID: 25233101, 10.1016/j.envint.2014.08.019. [DOI] [PubMed] [Google Scholar]
- Kelishadi R, Mirghaffari N, Poursafa P, Gidding SS. 2009. Lifestyle and environmental factors associated with inflammation, oxidative stress and insulin resistance in children. Atherosclerosis 203(1):311–319, PMID: 18692848, 10.1016/j.atherosclerosis.2008.06.022. [DOI] [PubMed] [Google Scholar]
- Kim JH, Hong YC. 2012. GSTM1, GSTT1, and GSTP1 polymorphisms and associations between air pollutants and markers of insulin resistance in elderly Koreans. Environ Health Perspect 120(10):1378–1384, PMID: 22732554, 10.1289/ehp.1104406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Georas S, Alexis N, Fritz P, Xia T, Williams MA, et al. 2016. A work group report on ultrafine particles (American Academy of Allergy, Asthma & Immunology): Why ambient ultrafine and engineered nanoparticles should receive special attention for possible adverse health outcomes in human subjects. J Allergy Clin Immunol 138(2):386–396, PMID: 27130856, 10.1016/j.jaci.2016.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Lane KJ, Corlin L, Patton AP, Durant JL, Thanikachalam M, et al. 2017. Association of long-term near-highway exposure to ultrafine particles with cardiovascular diseases, diabetes and hypertension. Int J Environ Res Public Health 14(5):E461–16, PMID: 28445425, 10.3390/ijerph14050461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. 2012. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380(9859):2224–2260, PMID: 23245609, 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Yang C, Zhao Y, Ma Z, Bi J, Liu Y, et al. 2016. Associations between long-term exposure to ambient particulate air pollution and type 2 diabetes prevalence, blood glucose and glycosylated hemoglobin levels in China. Environ Int 92–93:416–421, PMID: 27148900, 10.1016/j.envint.2016.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Ying Z, Harkema J, Sun Q, Rajagopalan S. 2013. Epidemiological and experimental links between air pollution and type 2 diabetes. Toxicol Pathol 41(2):361–373, PMID: 23104765, 10.1177/0192623312464531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu MC, Wang P, Cheng TJ, Yang CP, Yan YH. 2017. Association of temporal distribution of fine particulate matter with glucose homeostasis during pregnancy in women of Chiayi City, Taiwan. Environ Res 152:81–87, PMID: 27743970, 10.1016/j.envres.2016.09.023. [DOI] [PubMed] [Google Scholar]
- Memmesheimer M, Friese E, Ebel A, Jakobs HJ, Feldmann H, Kessler C, et al. 2004. Long-term simulations of particulate matter in Europe on different scales using sequential nesting of a regional model. IJEP 22(1/2):108–132, 10.1504/IJEP.2004.005530. [DOI] [Google Scholar]
- Nonnemacher M, Jakobs H, Viehmann A, Vanberg I, Kessler C, Moebus S, et al. 2014. Spatio-temporal modelling of residential exposure to particulate matter and gaseous pollutants for the Heinz Nixdorf Recall Cohort. Atmos Environ 91:15–23, 10.1016/j.atmosenv.2014.03.052. [DOI] [Google Scholar]
- Peng C, Bind M-AC, Colicino E, Kloog I, Byun H-M, Cantone L, et al. 2016. Particulate air pollution and fasting blood glucose in non-diabetic individuals: associations and epigenetic mediation in the Normative Aging Study, 2000–2011. Environ. Health Perspect 124(11):1715–1721, PMID: 27219535, 10.1289/EHP183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan S, Brook RD. 2012. Air pollution and type 2 diabetes: Mechanistic insights. Diabetes 61(12):3037–3045, PMID: 23172950, 10.2337/db12-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao X, Patel P, Puett R, Rajagopalan S. 2015. Air pollution as a risk factor for type 2 diabetes. Toxicol Sci 143(2):231–241, PMID: 25628401, 10.1093/toxsci/kfu250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robledo CA, Mendola P, Yeung E, Männistö T, Sundaram R, Liu D, et al. 2015. Preconception and early pregnancy air pollution exposures and risk of gestational diabetes mellitus. Environ Res 137:316–322, PMID: 25601734, 10.1016/j.envres.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sade MY, Kloog I, Liberty IF, Katra I, Novack L, Novack V. 2015. Air pollution and serum glucose levels: a population-based study. Medicine (Baltimore) 94(27):e1093, PMID: 26166095, 10.1097/MD.0000000000001093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sade MY, Kloog I, Liberty IF, Schwartz J, Novack V. 2016. The association between air pollution exposure and glucose and lipids levels. J Clin Endocrinol Metab 101(6):2460–2467, PMID: 27218271, 10.1210/jc.2016-1378. [DOI] [PubMed] [Google Scholar]
- Schmermund A, Möhlenkamp S, Stang A, Grönemeyer D, Seibel R, Hirche H, et al. 2002. Assessment of clinically silent atherosclerotic disease and established and novel risk factors for predicting myocardial infarction and cardiac death in healthy middle-aged subjects: Rationale and design of the Heinz Nixdorf RECALL Study. Am Heart J 144(2):212–218, PMID: 12177636, 10.1067/mhj.2002.123579. [DOI] [PubMed] [Google Scholar]
- Schöttker B, Rathmann W, Herder C, Thorand B, Wilsgaard T, Njølstad I, et al. 2016. HbA1c levels in non-diabetic older adults - No J-shaped associations with primary cardiovascular events, cardiovascular and all-cause mortality after adjustment for confounders in a meta-analysis of individual participant data from six cohort studies. BMC Med 14:26, PMID: 26867584, 10.1186/s12916-016-0570-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stang A, Moebus S, Dragano N, Beck EM, Möhlenkamp S, Schmermund A, et al. 2005. Baseline recruitment and analyses of nonresponse of the Heinz Nixdorf Recall study: identifiability of phone numbers as the major determinant of response. Eur J Epidemiol 20(6):489–496, PMID: 16121757, 10.1007/s10654-005-5529-z. [DOI] [PubMed] [Google Scholar]
- Sun Q, Wang A, Jin X, Natanzon A, Duquaine D, Brook RD, et al. 2005. Acceleration of atherosclerosis and vascular inflammation in an animal model. JAMA 294(23):3003–3010, PMID: 16414948, 10.1001/jama.294.23.3003. [DOI] [PubMed] [Google Scholar]
- Sun Q, Yue P, Deiuliis JA, Lumeng CN, Kampfrath T, Mikolaj MB, et al. 2009. Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation 119(4):538–546, PMID: 19153269, 10.1161/CIRCULATIONAHA.108.799015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamayo T, Rathmann W, Krämer U, Sugiri D, Grabert M, Holl RW. 2014. Is particle pollution in outdoor air associated with metabolic control in type 2 diabetes? PLoS One 9(3):e91639, PMID: 24619127, 10.1371/journal.pone.0091639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamayo T, Rathmann W, Stahl-Pehe A, Landwehr S, Sugiri D, Krämer U, et al. 2016. No adverse effect of outdoor air pollution on HbA1c in children and young adults with type 1 diabetes. Int J Hyg Environ Health 219(4–5):349–355, PMID: 26935923, 10.1016/j.ijheh.2016.02.002. [DOI] [PubMed] [Google Scholar]
- Textor J, Hardt J, Knüppel S. 2011. DAGitty: A graphical tool for analyzing causal diagrams. Epidemiology 22(5):745, PMID: 21811114, 10.1097/EDE.0b013e318225c2be. [DOI] [PubMed] [Google Scholar]
- UNESCO. 1997. International standard classification of education ISCED 1997. http://www.unesco.org/education/information/nfsunesco/doc/isced_1997.htm [accessed 30 March 2017].
- Ward-Caviness CK, Kraus WE, Blach C, Haynes CS, Dowdy E, Miranda ML, et al. 2015. Association of roadway proximity with fasting plasma glucose and metabolic risk factors for cardiovascular disease in a cross-sectional study of cardiac catheterization patients. Environ Health Perspect 123(10):1007–1014, PMID: 25807578, 10.1289/ehp.1306980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (World Health Organization). 2006. WHO Air quality guidelines for particulate matter, ozone, nitrogen dioxide and sulfur dioxide-Global update 2005. http://www.who.int/phe/health_topics/outdoorair/outdoorair_aqg/en/index.html [accessed 29 September 2016].
- WHO Collaborating Centre for Drug Statistics Methodology. 2014. ATC/DDD Index 2014. http://www.whocc.no/atc_ddd_index/ [accessed 16 March 2017].
- Winkler G, Döring A. 1995. Kurzmethoden zur Charakterisierung des Ernährungsmusters: Einsatz und Auswertung eines Food-Frequency-Fragebogens. Ernährungs-Umschau 42(8):289–291. [Google Scholar]
- Winkler G, Döring A. 1998. Validation of a short qualitative food frequency list used in several German large scale surveys. Z Ernahrungswiss 37(3):234–241, PMID: 9800314, 10.1007/PL00007377. [DOI] [PubMed] [Google Scholar]
- Wolf K, Popp A, Schneider A, Breitner S, Hampel R, Rathmann W, et al. 2016. Association between long-term exposure to air pollution and biomarkers related to insulin resistance, subclinical inflammation, and adipokines. Diabetes 65(11):3314–3326, PMID: 27605624, 10.2337/db15-1567. [DOI] [PubMed] [Google Scholar]
- Zhang X, Gregg EW, Williamson DF, Barker LE, Thomas W, Bullard KM, et al. 2010. A1C level and future risk of diabetes: A systematic review. Diabetes Care 33(7):1665–1673, PMID: 20587727, 10.2337/dc09-1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.