Abstract
Background:
Aromatase (CYP19) is a key enzyme in estrogens biosynthesis. In the mammary gland, CYP19 gene is expressed at low levels under the regulation of its I.4 promoter. In hormone-dependent breast cancer, fibroblast cells surrounding the tumor express increased levels of CYP19 mRNA due to a decrease of I.4 promoter activity and an increase of PII, I.3, and I.7 promoter activity. Little is known about the effects of environmental chemicals on the promoter-specific CYP19 expression.
Objective:
We aimed to determine the effects of two neonicotinoids (thiacloprid and imidacloprid) on promoter-specific CYP19 expression in Hs578t breast cancer cells and understand the signaling pathways involved.
Methods:
Hs578t cells were exposed to various signaling pathway stimulants or neonicotinoids for 24 h. Promoter-specific expression of CYP19 was determined by real-time quantitative polymerase chain reaction and catalytic activity of aromatase by tritiated water release assay.
Results:
To our knowledge, we are the first to demonstrate that the normal I.4 promoter and the breast cancer-relevant PII, I.3, and I.7 promoters of CYP19 are active in these cells. We found that the expression of CYP19 via promoters PII, I.3, and I.7 in Hs578t cells was, in part, dependent on the activation of two VEGF signaling pathways: mitogen-activated protein kinase (MAPK) 1/3 and phospholipase C (PLC). Exposure of Hs578t cells to environmental concentrations of imidacloprid and thiacloprid resulted in a switch in CYP19 promoter usage, involving inhibition of I.4 promoter activity and an increase of PII, I.3, and I.7 promoter-mediated CYP19 expression and aromatase catalytic activity. Greater effects were seen at lower concentrations. Our results suggest that thiacloprid and imidacloprid exert their effects at least partially by inducing the MAPK 1/3 and/or PLC pathways.
Conclusions:
We demonstrated in vitro that neonicotinoids may stimulate a change in CYP19 promoter usage similar to that observed in patients with hormone-dependent breast cancer. https://doi.org/10.1289/EHP2698
Introduction
Background
In 2017, 26,300 women were diagnosed with breast cancer in Canada (Canadian Cancer Society’s Advisory Committee on Cancer Statistics 2017). In the United States, it was expected that 252,710 new cases of breast cancer would be diagnosed in 2017 (American Cancer Society 2017). Of these cases, 83% were estrogen-receptor and/or progesterone-receptor positive (American Cancer Society 2017). In this type of cancer, increased local estrogen is produced, resulting in greater concentrations in the tumor microenvironment, which stimulates the proliferation of breast cancer epithelial cells (Ghosh et al. 2009; Yamaguchi and Hayashi 2009). Aromatase (CYP19) is a key enzyme in the biosynthesis of estrogens, as it is responsible of the final conversion of androstenedione to estrone, and testosterone to estradiol (Bulun et al. 2003). The CYP19 gene is expressed in a tissue-specific manner by the activation of various promoters located in the noncoding region of the gene. In the normal breast, CYP19 is expressed at low levels in fibroblast cells (stromal preadipocytes) and driven by the I.4 promoter (Simpson and Davis 2001).
In breast cancer, a series of events leads to the inhibition of I.4 promoter activity (Agarwal et al. 1996; Harada et al. 1993) and the activation of several promoters that are normally inactive in the stromal cells of the mammary gland, namely PII, I.3, and I.7 (Irahara et al. 2006; Subbaramaiah et al. 2012; Zhou et al. 1997). This unique switch in promoter usage results in an increase of overall CYP19 gene expression, aromatase catalytic activity, and subsequent estrogen biosynthesis. Moreover, malignant epithelial cells synthesize prostaglandin (), which binds to its G-protein-coupled receptor to stimulate the production of cyclic AMP (cAMP), which results in increased CYP19 expression through activation of promoters PII and I.3 (Chen et al. 2007; Subbaramaiah et al. 2012). can also activate the orphan nuclear receptor homologue-1 (LRH-1), known to induce CYP19 expression in breast tissue (Zhou et al. 2005).
Increased levels of , and other inflammatory factors such as and IL-11 in the tumor microenvironment only partially explain the promoter-switch in regulation of CYP19 expression that occurs in hormone-dependent breast cancer patients. Another potential contributor to the promoter-switch in CYP19 expression is the vascular endothelial growth factor (VEGF) receptor signaling pathway. The VEGF receptor (VEGFR) signaling pathway plays a central role in angiogenesis. More precisely, secretion of VEGF is associated with proliferation of vascular endothelial cells (Schneider and Sledge 2007). It has been demonstrated that VEGF and its receptors are overexpressed in breast cancer (Adams et al. 2000; Konecny et al. 2004). Furthermore, we know that VEGF promotes angiogenesis and endothelial cell permeability by activating ERK 1/2 (MEK/MAPK1/3) (Breslin et al. 2003; Pai et al. 2001; Xu et al. 2008) and PLC/PKC (Cross and Claesson-Welsh 2001; Jiang et al. 2016).
Given the importance of aromatase in hormone-dependent breast cancer, understanding the regulation of the promoter-specific expression of CYP19 is paramount to assessing potential impacts of environmental contaminants on the development of this disease. Indeed, there is growing evidence that exposure to contaminants, such as pesticides, is a risk factor for hormone-dependent breast cancer (Cohn et al. 2007; Ibarluzea et al. 2004; Mathur et al. 2002; Xu et al. 2010). A lot of research has focused on effects of endocrine disruptors on the estrogen receptor (Bouskine et al. 2009; Roy et al. 2009; Rubin et al. 2001). The enzyme aromatase has been identified as a target for endocrine disrupting chemicals, including environmental pesticides (Sanderson 2006). However, we have little information on the roles that environmentally relevant levels of chemicals may play in the disruption of aromatase expression or activity. It has been demonstrated that the widely used herbicide atrazine induces estradiol synthesis in human cell lines by the activation of PII/I.3-mediated CYP19 expression (Caron-Beaudoin et al. 2016; Sanderson et al. 2002). Furthermore, our laboratory recently demonstrated that the neonicotinoids thiacloprid and thiamethoxam induced PII/I.3-mediated CYP19 expression as well as aromatase catalytic activity in a nonmonotonic manner in H295R adrenocortical carcinoma cells, at relatively low concentrations (Caron-Beaudoin et al. 2016). We also demonstrated that three neonicotinoids (thiacloprid, thiamethoxam, and imidacloprid) increased the production of estrone and estradiol, yet strongly inhibited the production of estriol in a fetoplacental coculture model of steroidogenesis during pregnancy (Caron-Beaudoin et al. 2017). To the best of our knowledge, the impacts of neonicotinoid insecticides on human health have not been studied in any detail, but an increasing body of evidence suggests they have the potential to disrupt endocrine functions (Bal et al. 2012; Hoshi et al. 2014; Kapoor et al. 2011; Şekeroğlu et al. 2014). For example, female rats exposed to imidacloprid through diet () showed decreased ovarian weights and alterations in progesterone and follicle-stimulating hormone levels (Kapoor et al. 2011).
Neonicotinoids are widely used pesticides that have been linked to Honey Bee Colony Collapse Disorder (Goulson 2013; Henry et al. 2012). In 2012, of active neonicotinoids were applied on 11 million hectares of land in Canada (Main et al. 2014). These insecticides exert their effects by binding to nicotinic acetylcholine receptors (Matsuda et al. 2001), and they are used as a seed coating in a variety of crops, fruits, and vegetables (Elbert et al. 2008). Neonicotinoid half-lives can reach 1,250 days for imidacloprid (Main et al. 2014), and these insecticides are detected in surface water and soil (Schaafsma et al. 2015; Starner and Goh 2012; Stokstad 2013). Due to their relative persistence in the environment, and because neonicotinoids are used as seed treatments and repeatedly applied, concerns regarding human exposure have been raised. Imidacloprid has been detected in 89% of water samples in California, and concentrations exceeded the U.S. Environmental Protection Agency’s aquatic life benchmark dose in 19% of samples (Starner and Goh 2012). In wetlands in Saskatchewan, Canada, concentrations of clothianidin and thiamethoxam were found to be as high as (Main et al. 2014). Furthermore, it was recently demonstrated that residues of at least one neonicotinoid were detected in vegetables and fruits purchased from grocery stores in Boston, Massachusetts, with concentrations reaching . In this study, at least two different neonicotinoids were detected in 72% of fruits and 45% of vegetables (Chen et al. 2014). Finally, a study conducted in Japan analyzed neonicotinoid metabolites in urine samples of farmers. 3-Furoic acid, the major metabolite of the neonicotinoid dinotefuran, was detected in all urine samples, with concentrations reaching (Nomura et al. 2013). Urinary neonicotinoid levels were also measured in females from the general Japanese population, and thiacloprid and imidacloprid were detected at concentrations up to (Ueyama et al. (2015). The human exposure to neonicotinoid insecticides highlights the need to investigate their potential endocrine disrupting effects, especially at environmentally relevant concentrations.
Objectives
Using Hs578t cells as a breast cancer-relevant in vitro model, we aimed to understand the signaling pathways implicated in the expression of CYP19 via the activity of promoters I.4, I.7, I.3, and PII and whether neonicotinoids can induce a promoter-switch in CYP19 expression, as has been described in breast cancer patients (Irahara et al. 2006).
Methods
Reagents
Thiacloprid (Pestanal®; cat. no. 37905, ) and imidacloprid (Pestanal®; cat. no. 37894, ) were obtained from Sigma-Aldrich (Saint-Louis, MO) and dissolved in sterile-filtered dimethylsulfoxide (DMSO; cat. no. 67-68-5, Sigma-Aldrich) as stock solutions. The MAPK 1/3 pathway inhibitor PD98059 was purchased from Fisher Scientific and dissolved in DMSO as a stock solution. The phospholipase C (PLC) inhibitor U73122 (Calbiochem) was dissolved in DMSO as a stock solution. Forskolin and dexamethasone were obtained from Sigma-Aldrich and dissolved in DMSO as and stock solutions, respectively. VEGF was purchased from the American Type Culture Collection (ATCC) at a concentration of .
Cell Culture and Experimental Design
Hs578t cells (ATCC, cat. no. HTB-126) are triple-negative breast cancer epithelial cells derived from a 74-y-old patient with mammary carcinoma. Cells from low passages (below 9) were cultured in Dulbecco’s modified Eagle medium (DMEM, cat. no. 30-2002, Sigma-Aldrich) containing L-glutamine, glucose, sodium pyruvate, and sodium bicarbonate. Medium was completed with 10% fetal bovine serum (FBS) and of bovine insulin (Sigma-Aldrich). Hs578t cells were exposed to various concentrations of each compound in culture medium at a final DMSO concentration of 0.1%.
Real-Time Quantitative Polymerase Chain Reaction
For the Real-Time Quantitative Polymerase Chain Reaction experiments, Hs578t cells were cultured for 24 h in 6-well plates (CellBind, Corning Incorporated) ( cells/well) containing medium/well. For subsequent exposures, medium was removed, Hs578t cells were washed with PBS (1X) and of treated medium was added. To determine which CYP19 promoters are active, Hs578t cells were exposed for 24 h to forskolin, dexamethasone, or VEGF. Dexamethasone () was used as a known inducer of I.4-mediated CYP19 expression, whereas forskolin () was used to induce PII/I.3-mediated CYP19 expression. VEGF () was used as a potential inducer of I.7-mediated CYP19 expression (Kalluri and Zeisberg 2006). Control cells were exposed to 0.1% DMSO. To determine which VEGF signaling pathways are implicated in the expression of CYP19 via promoters PII, I.3 or I.7, Hs578t cells were pretreated with a PLC inhibitor ( U73122) or a MAPK 1/3 inhibitor ( PD88059) 4 h prior to the addition of forskolin or VEGF for 24 h. Forskolin is known to increase calcium release (Schmidt et al. 2001), which can activate the PLC pathway. Furthermore, it is known that the MAPK 1/3 signaling pathway is activated by VEGF (Breslin et al. 2003; Cross and Claesson-Welsh 2001; Lee et al. 1998). Therefore, we tested the potential involvement of the PLC pathway in PII and I.3 promoter-specific CYP19 expression by pretreating Hs578t cells with U73122 4 h prior to the addition of forskolin, and the potential involvement of the MAPK 1/3 pathway in I.7 promoter-specific CYP19 expression by pretreating with PD98059 4 h prior to addition of VEGF.
Conditions for Neonicotinoid Exposures for Real-Time Quantitative PCR Experiments
Hs578t cells were exposed to thiacloprid (0.03, 0.1, 0.3, 3, and ) or imidacloprid (0.03, 0.1, 0.3, and ) for 24 h. These concentrations are similar to those found in urine samples of farmers and women from the general population in Japan (Nomura et al. 2013; Ueyama et al. 2015). Finally, to determine if neonicotinoids exert their effects on CYP19 expression via the PLC and/or MAPK1/3 pathways, Hs578t cells were pretreated with the selective inhibitors ( U73122 or PD88059) 4 h prior to a 24-h exposure to thiacloprid or imidacloprid. After treatment, medium was removed and Hs578t cells were washed twice with PBS (1X) prior to RNA isolation (see the section “RNA isolation and amplification by quantitative RT-PCR” below).
Exposure Conditions for the Aromatase Catalytic Activity Assay
Hs578t cells were cultured in 24-well plates (400,000 cells/well) containing of culture medium. After 24 h, medium was removed and Hs578t cells were washed with PBS (1X) before of treated medium was added. To determine the impact of changes in promoter-specific CYP19 expression on aromatase activity, Hs578t cells were exposed to forskolin, dexamethasone, or VEGF for 24 h. To determine the effects of neonicotinoids on aromatase activity, Hs578t cells were exposed to thiacloprid (0.03, 0.1, 0.3, 3, and ) or imidacloprid (0.03, 0.1, 0.3, and ) for 24 h. Control cells were exposed to 0.1% DMSO. Formestane (), a selective and irreversible aromatase inhibitor, was used to verify the specificity of the assay for the aromatization reaction. Prior to the aromatase assay, the treated medium was removed and the cells were washed twice with PBS (1X).
Cell Viability
The cytotoxicity of thiacloprid and imidacloprid was determined using a WST-1 kit (Roche), which measures mitochondrial reductase activity in viable cells. Hs578t cells were incubated for 24 h in 96-well plates ( cells/well) in culture medium. After this period, cells were exposed for 24 h to fresh medium containing various concentrations of thiacloprid (0.03, 0.1, 0.3, 3, and ) or imidacloprid (0.03, 0.1, 0.3, and ). Cells were then incubated with WST-1 substrate for 1.5 h, after which the formation of formezan was measured at an absorbance wavelength of using a SpectraMax M5 spectrophotometer (Molecular Devices).
RNA Isolation, Reverse Transcription, and Amplification by Quantitative PCR
Real-time quantitative PCR was designed and performed following recommendations from Taylor et al. (2010). RNA was isolated using an RNeasy mini-kit (Qiagen) according to the manufacturer’s instructions, and stored at . The absorbance ratio at was used to determine purity of the RNA samples. Reverse transcription was performed with an iScript cDNA Synthesis kit (BioRad) and T3000 Thermocycler (Biometra) using of RNA. Resultant cDNA was preamplified using SsoAdvanced PreAmp SuperMix (BioRad) and T3000 Thermocycler following the manufacturer’s instructions. cDNA and preamplified cDNA were stored at . Primer pairs were designed to selectively amplify mRNA species containing an untranslated 5′ region uniquely derived from the promoters I.4, PII, I.3, or I.7 of CYP19; a primer pair designed to recognize only the coding region (exons II–X) was used to amplify overall (promoter nondistinct) CYP19 transcript (Table 1). All the primer pairs were analyzed with Blast and Primer-Blast (National Center for Biotechnology Information) to ensure their selectivity. Real-time quantitative PCR was performed with EvaGreen MasterMix (BioRad) using a CFX96 Real-Time PCR Detection System (BioRad) (95°C for 5 min; 40 cycles of 95°C for 5 s, and 60°C for 15 s) (Table 1). Suitable reference genes were selected using the geNorm algorithm method (version 1.5; Biogazelle qbase Plus software). For treatments with thiacloprid, UBC (forward primer 5′–3′: ATTTGGGTCGCGGTTCTTG; reverse primer 5′–3′: TGCCTTGACATTCTCGATGGT) and RPLP0 (forward primer 5′–3′: GGCGACCTGGAAGTCCAACT; reverse primer 5′–3′: CCATCAGCACCACAGCCTTC) reference genes were used; for imidacloprid, we used UBC and PBGD (forward primer 5′–3′: GGCAATGCGGCTGCAA; reverse primer 5′–3′: GGGTACCCACGCGAATCAC). For forskolin, dexamethasone and VEGF treatments alone, UBC and RPLP0 were used as reference genes.
Table 1.
Primer pair sequences and amplification characteristics for the amplification of promoter-specific CYP19 expression in Hs578t cells.
| CYP19 promoter | Primer pairs (5′–3′) | Amplification characteristics in Hs578t | Tissue-specific expression | Reference and NCBI accession number |
|---|---|---|---|---|
| CYP19-coding region | Fw: TGTCTCTTTGTTCTTCATGCTATTTCTC | Standard curve: | Detects all aromatase transcripts regardless of promoter utilized | Sanderson et al. 2000) |
| Rv: TCACCAATAACAGTCTGGATTTCC | Efficiency: 92.8% | M22246 | ||
| CYP19-I.4 | Fw: GGCTCCAAGTAGAACGTGACCAACTG | Standard curve: | Expressed in fibroblasts in the normal mammary gland | Heneweer et al. 2004) |
| Rv: CAGCCCAAGTTTGCTGCCGAA | Efficiency: 101.9% | S52794 | ||
| CYP19-PII | Fw: TCTGTCCCTTTGATTTCCACAG | Standard curve: | Expressed in ovaries, testes and stroma of breast cancer patients | Heneweer et al. 2004) |
| Rv: GCACGATGCTGGTGATGTTATA | Efficiency: 108.9% | S52794 | ||
| CYP19-I.3 | Fw: GGGCTTCCTTGTTTTGACTTGTAA | Standard curve: | Expressed in ovaries, testes and stroma of breast cancer patients | Wang et al. 2008) |
| Rv: AGAGGGGGCAATTTAGAGTCTGTT | Efficiency: 95.7% | D30796 | ||
| CYP19-I.7 | Fw: ACACTCAGCTTTTTCCCAACA | Standard curve: | Expressed in endothelial cells and stroma of breast cancer patients | NM_001347251 |
| Rv: TTTCACCCCTTTCTCCGGTC | Efficiency: 90.7% |
Note: Fw, forward; NCBI, National Center Biotechnology Information; Rv, reverse.
Aromatase Catalytic Activity
Aromatase activity was measured using the tritiated water-release assay as described previously (Caron-Beaudoin et al. 2016; Sanderson et al. 2000). A volume of of culture medium (without phenol red) containing was added to each well, and cells were incubated for 150 min at 37°C (5% ). As described previously (Lephart and Simpson 1991; Sanderson et al. 2000), of culture medium underwent chloroform extraction to separate the substrate from tritiated water (), a product of the aromatization reaction. To remove any remaining substrate, the aqueous fraction treated with dextran-coated charcoal. The amount of tritiated water released was counted in 24-well plates containing liquid scintillation cocktail using a Microbeta Trilux (PerkinElmer, Waltham, MA). Counts per minute emitted from each sample were corrected for quenching to determine disintegrations per minute, which were then converted into aromatase activity (fg/h per 100,000 cells) and ultimately expressed as a percent of control activity (cells treated with 0.1% DMSO).
Statistical Analysis
Results are presented as means with standard errors of three independent experiments using different cell passages; per experiment, each treatment was tested in triplicate. The normal distribution of the residuals and the homoscedasticity of the variance were verified for each analysis using JMP Pro 13 Software (SAS Institute Inc.). Statistically significant differences (* = ; ** = ; *** = ) from control were determined by Student t-test or one-way analysis of variance (ANOVA) followed by a Dunnett post hoc test to correct for multiple comparisons to control using GraphPad Prism (version 5.04; GraphPad Software).
Results
Promoter-Specific Expression of CYP19 in Hs578t Cells
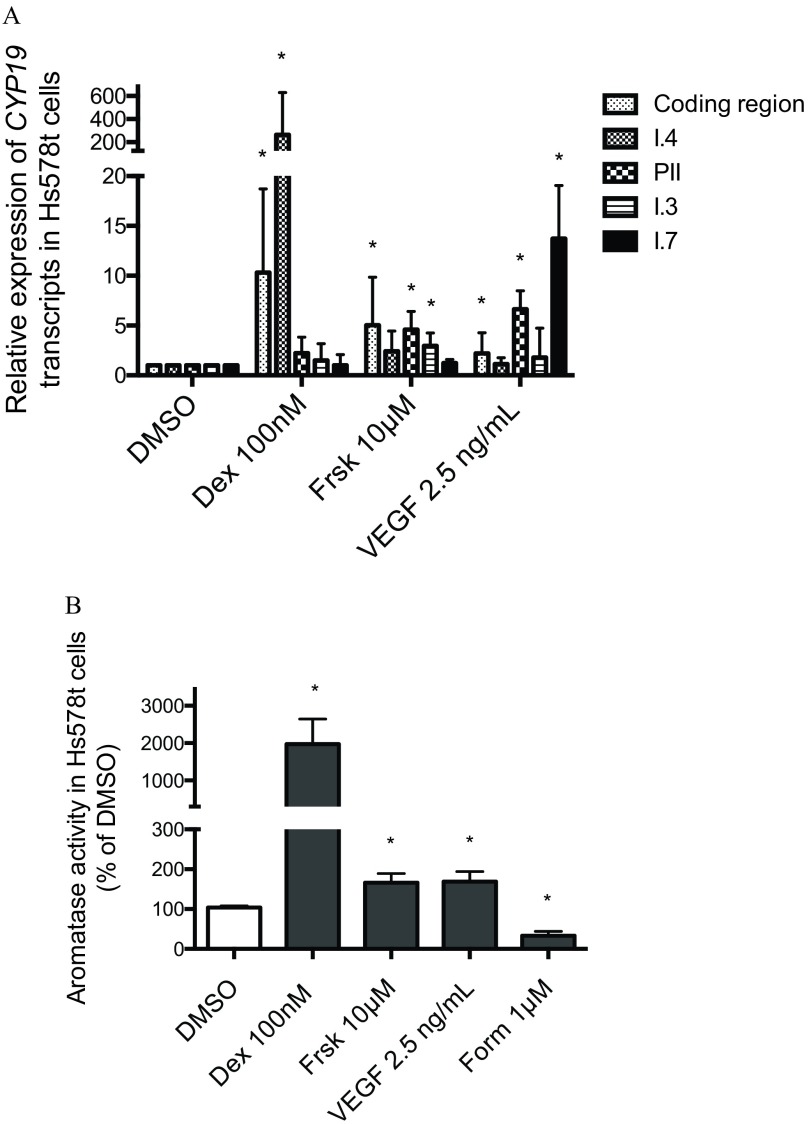
None of the tested neonicotinoid insecticides was cytotoxic at the tested concentrations of 0.03, 0.1, 0.3, 3, and (see Figure S1). We determined the effects of a 24-h exposure to various pharmacological compounds on the promoter-specific induction of CYP19 gene expression in Hs578t cells (Figure 1A). In Hs578t cells exposed to vehicle control (0.1% DMSO), basal CYP19 expression was driven by the I.4 promoter (; quantification cycle; the amplification cycle at which accurate quantification of expression levels can be made), the PII (), I.7 (), and I.3 () promoters of aromatase. In Hs578t cells exposed to dexamethasone, I.4 promoter-derived CYP19 mRNA levels were induced fold compared with DMSO control, whereas no significant differences were observed in PII, I.3, and I.7 promoter-derived CYP19 mRNA levels. Cells treated with forskolin () exhibited and fold higher PII and I.3 promoter-mediated CYP19 expression, respectively, than did DMSO controls. By contrast, no significant effects were observed on transcripts derived from the I.7 promoter (Figure 1A). Finally, Hs578t cells treated with VEGF exhibited significantly higher I.7 and PII-mediated CYP19 expression ( and fold, respectively) compared with DMSO control (Figure 1A).
Figure 1.
(A) Relative expression of CYP19 coding region (nonpromoter-specific or total), and I.4, PII, I.3, and I.7 promoter-derived CYP19 transcripts in Hs578t cells (fold DMSO control). (B) Aromatase catalytic activity in Hs578t cells exposed to dexamethasone (DEX) , forskolin (Frsk) , vascular endothelial growth factor (VEGF) , or formestane (Form) . Experiments were performed in triplicate with three different cell passages; per experiment, each treatment was tested in triplicate. Cells were exposed to treatments for 24 h. *, . Statistically significant difference between treatments compared with DMSO (Student t-test).
Aromatase Catalytic Activity in Hs578t Cells
Formestane inhibited aromatase catalytic activity in Hs578t cells. Dexamethasone (), forskolin (), and VEGF () induced aromatase catalytic activity in Hs578t cells by , , and fold, respectively, compared with DMSO controls (Figure 1B).
Effects of Inhibition of the PLC and MAPK 1/3 Pathways on Promoter-Specific Expression of CYP19 in Hs578t Cells
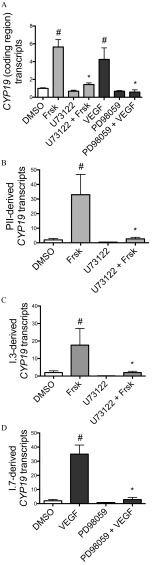
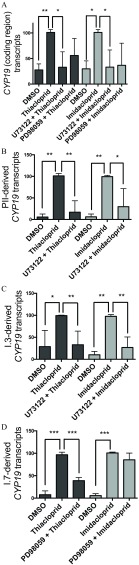
To assess the involvement of two VEGF signaling pathways (PLC and MAPK 1/3) in the promoter-specific expression of CYP19, Hs578t cells were pretreated with pathway-selective inhibitors 4 h prior to addition of VEGF or forskolin. Pretreatment of Hs578t cells with the PLC inhibitor U73122 () prior to forskolin () treatment resulted in significantly lower relative (to DMSO control) expression of CYP19 coding region than treatment with forskolin alone ( vs. fold; Figure 2A) this was also true for PII-mediated CYP19 expression ( vs. fold; Figure 2B), and I.3-mediated CYP19 expression ( vs. fold; Figure 2C). Furthermore, pretreatment of Hs578t cells with the MEK/MAPK 1/3 inhibitor PD98059 () prior to VEGF () treatment also resulted in a significantly lower relative expression of CYP19 coding region than treatment with VEGF alone ( vs. fold; Figure 2A); this was also the case for I.7-mediated CYP19 expression ( vs. fold; Figure 2D).
Figure 2.
Relative expression of (A) CYP19 coding region (nonpromoter-specific or total), and CYP19 transcripts derived from promoters (B) PII, (C) I.3, and (D) I.7 in Hs578t cells (fold DMSO control). Cells were exposed for 24 h to forskolin (Frsk) or VEGF, inducers of PII/I.3 or I.7 promoter-mediated CYP19 expression, in the presence or absence of selective inhibitors of the PLC (U73122; ) or MEK/MAPK 1/3 (PD98059; ) signaling pathways. Experiments were performed in triplicate with three different cell passages; per experiment, each treatment was tested in triplicate. *, . Statistically significant difference between Hs578t cells pretreated with U73122 compared with those treated with Frsk alone, or between Hs578t cells pretreated with PD98059 compared with those treated with VEGF alone (Student t-test). #, . Significantly different from DMSO control (Student t-test).
Effects of Neonicotinoids on Promoter-Specific Expression of CYP19 in Hs578t Cells
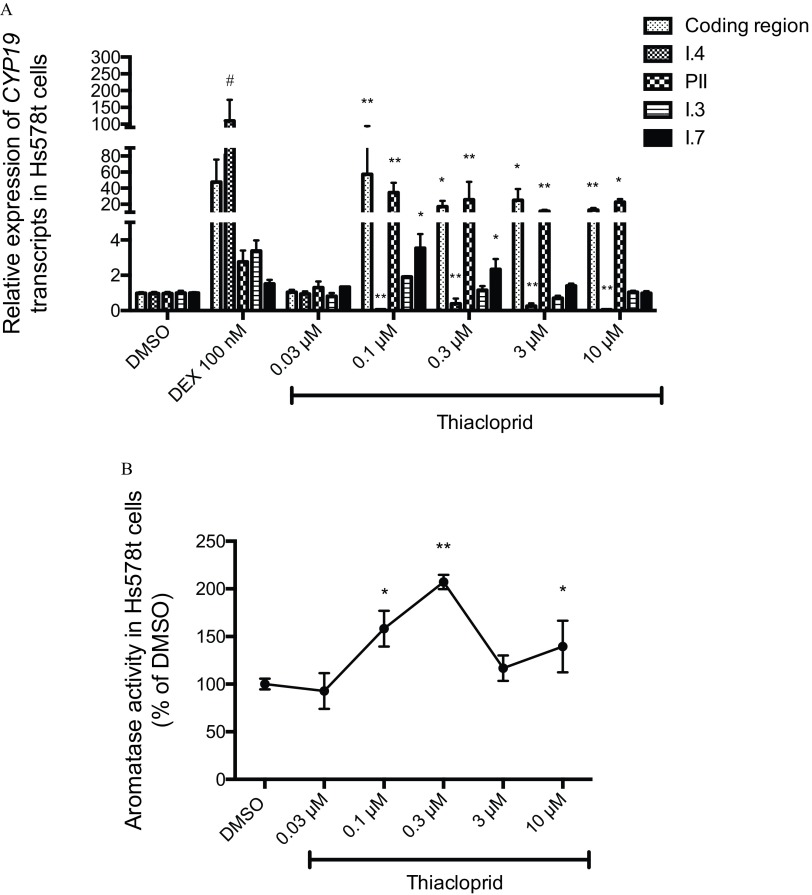
Generally, in Hs578t cells treated for 24 h with thiacloprid, at all concentrations above (), I.4 promoter-mediated CYP19 expression was lower than in control cells, whereas levels of PII, I.3, and I.7 promoter-derived CYP19 transcripts as well as overall (promoter-nonspecific coding region) CYP19 transcript were increased (Figure 3A). Above , relatively lower concentrations appeared to have greater effects. Hs578t cells exposed to thiacloprid had lower CYP19 expression via the I.4 promoter ( fold of DMSO controls), whereas PII ( fold), I.7-mediated CYP19 () and overall coding region expression ( fold) were significantly higher than DMSO controls (Figure 3A). I.3-mediated CYP19 expression was higher ( fold) than DMSO controls, although this was not statistically significant. We observed an increase of the catalytic activity of aromatase at 0.1, 0.3, and thiacloprid, with greater increases at relatively lower concentrations (Figure 3B).
Figure 3.
(A) Relative expression of CYP19 coding region (nonpromoter-specific or total), and I.4, PII, I.3, and I.7 promoter-derived CYP19 transcripts in Hs578t cells (fold DMSO control). (B) Aromatase catalytic activity in Hs578t cells exposed to thiacloprid (0.03, 0.1, 0.3, 3, and ). DEX () was used as a positive control for I.4 promoter-mediated CYP19 expression. Experiments were performed in triplicate with three different cell passages; per experiment, each treatment was tested in triplicate. DEX, dexamethasone. *, ; **, ). Statistically significant difference between thiacloprid and DMSO control (one-way ANOVA and Dunnett post hoc test). #, . Statistically significant difference between DEX treatment and DMSO control (Student t-test).
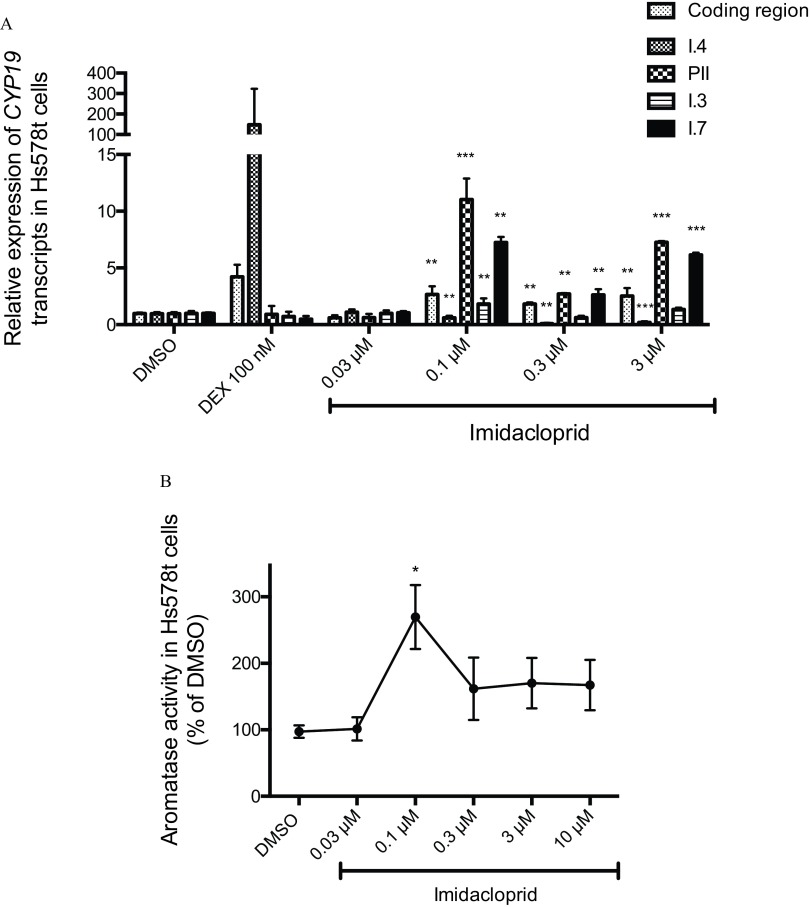
In Hs578t cells treated for 24 h with the neonicotinoid imidacloprid, differences in promoter-specific CYP19 expression were observed in concentrations of 0.1– (Figure 4A), compared with DMSO controls. Following a 24-h exposure to imidacloprid, the relative levels of I.4 promoter-derived CYP19 transcripts were significantly lower ( fold) whereas PII, I.3, and I.7 promoters-derived CYP19 transcripts was higher (, , and fold) than the DMSO control, with an overall higher coding region expression ( fold) relative to DMSO controls (Figure 4A). Compared with DMSO controls, the catalytic activity of aromatase was increased significantly after treatment with imidacloprid (Figure 4B).
Figure 4.
(A) Relative expression of CYP19 coding region (nonpromoter-specific or total), and I.4, PII, I.3, and I.7 promoter-derived CYP19 transcripts in Hs578t cells (fold DMSO control). (B) Aromatase catalytic activity in Hs578t cells exposed to imidacloprid (0.03, 0.1, 0.3, and ). DEX () was used as a positive control for I.4 promoter-mediated CYP19 expression. DEX, dexamethasone. Experiments were performed in triplicate with three different cell passages; per experiment, each treatment was tested in triplicate. *, ; **, ; ***, . Statistically significant difference between imidacloprid compared with DMSO control (one-way ANOVA and Dunnett post hoc test). #, . Statistically significant difference between DEX treatment and DMSO control (Student t-test).
Effects of Inhibition of the PLC and MAPK 1/3 Pathways on Neonicotinoid-Mediated Changes in CYP19 Expression in Hs578t Cells
To investigate whether the effects of neonicotinoids on promoter-specific CYP19 expression were due to an action on the PLC and/or MEK/MAPK 1/3 pathways, we determined the promoter-specific expression of CYP19 in Hs578t cells treated with either thiacloprid or imidacloprid () in the presence of a selective inhibitor of either the PLC (U73122, ) or MEK/MAPK 1/3 (PD98059, ) pathway. Hs578t cells pretreated with U73122 prior to thiacloprid had significantly lower expression of promoter-nonspecific CYP19 (coding region; ) than did Hs578t cells exposed to thiacloprid alone. When Hs478t cells were pretreated with PD98059, expression of promoter-nonspecific CYP19 transcripts were lower () than expression in cells exposed to thiacloprid alone, although this inhibition was not statistically significant (Figure 5A). Pretreatment of Hs578t cells with U73122 prior to thiacloprid resulted in significantly lower PII and I.3 promoter-mediated CYP19 expression ( and , respectively) than that measured in Hs578t cells exposed to thiacloprid alone (Figure 5B,C). Furthermore, pretreatment of Hs578t cells with PD98059 prior to thiacloprid resulted in lower I.7 promoter-mediated CYP19 expression () than that in Hs578t cells exposed to thiacloprid alone (Figure 5D).
Figure 5.
Relative expression of (A) CYP19 coding region (nonpromoter-specific or total), and CYP19 transcripts derived from promoters (B) PII, (C) I.3, and (D) I.7 in Hs578t cells exposed to thiacloprid () or imidacloprid () in the presence or absence of selective inhibitors of the PLC (U73122, ) or MEK/MAPK 1/3 (PD98059, ) signaling pathways. Relative transcript levels are expressed as a percentage (%) of the response of Hs578t cells exposed to thiacloprid or imidacloprid (100%). Experiments were performed in triplicate with three different cell passages; per experiment, each treatment was tested in triplicate. *, ; **, ; ***, . Statistically significant difference between inhibitor pretreatment and neonicotinoid treatment alone (Student t-test).
We observed a similar trend for imidacloprid. In Hs578t cells pretreated with U73122 prior to imidacloprid, expression of promoter-nonspecific CYP19 (coding region) transcripts was lower () than expression in Hs578t cells exposed to imidacloprid alone (Figure 5A); PII and I.3-mediated CYP19 expression was lower ( and , respectively) than expression in cells exposed to imidacloprid alone (Figure 5B,C). In Hs578t cells pretreated with PD98059 prior to imidacloprid, we observed a nonsignificant lower expression of CYP19 coding region () than expression when treated with imidacloprid alone (Figure 5A). However, the same pretreatment did not result in lower I.7 promoter-mediated CYP19 expression (Figure 5D).
Discussion
Hs578t Cells as a Suitable Model to Study the Promoter-Specific Expression of CYP19 in Hormone-Dependent Breast Cancer
In this study, we successfully developed robust and sensitive real-time quantitative PCR methods to evaluate the expression of CYP19 via four specific promoters, namely the normally active I.4 promoter and the breast cancer-associated promoters PII, I.3, and I.7, using Hs578t cells as a representative model of the aromatase-expressing and estrogen-producing cells typically found in the hormone-dependent breast tumor environment. Triple-negative cells do not express estrogen or progesterone receptors and do not display amplification of the human epidermal growth factor receptor 2 (HER2) (Chavez et al. 2010). Epithelial cells normally do not express aromatase, but it has been previously demonstrated that triple-negative breast cancer cells (MDA-MB-231) express CYP19 by the activation of the adipose I.4 promoter and breast cancer-associated proximal PII/I.3 promoters, and that aromatase is catalytically active in this cell line (Knower et al. 2010; Su et al. 2008).
In breast cancer, increased CYP19 expression and estrogen synthesis is driven by a promoter-switch involving the activation of the PII, I.3, and I.7 promoters, and inhibition of normal I.4 promoter activity (Irahara et al. 2006; Sebastian and Bulun 2001). To our knowledge, we are the first to demonstrate that Hs578t cells express CYP19 through these four breast cancer-relevant promoters, which is key to the relevance of this cell line as an in vitro model of the estrogen-producing cells present in the hormone-dependent breast tumor environment. The mechanisms leading to this switch in CYP19 promoter usage are not fully understood. Breast cancer epithelial cells synthesize prostaglandin (), a G-protein–coupled receptor that stimulates the production of cAMP. Cyclic AMP then activates the protein kinase A (PKA) pathway, leading to the phosphorylation of cAMP responsive element binding protein 1 (CREB1). CREB1 then translocates to the nucleus and binds to CRE-like sequences in the PII/I.3 promoter region to stimulate promoter activity, which leads to increased expression of CYP19 (Sofi et al. 2003; To et al. 2015; Zhao et al. 1996). PKA can also phosphorylate the transcription factor GATA-4, which recruits coactivators such as the CREB-binding protein (CBP). The resulting complex then binds to the PII promoter region of CYP19 (Tremblay and Viger 2003).
We know less about the endothelial I.7 promoter of CYP19. This promoter, originally characterized by Sebastian et al. (2002), may have a role in regulating the effects of estrogens on blood vessels through its main regulator, the transcription factor GATA-2. However, it has also been demonstrated that the I.7 promoter is overactive in breast cancer (Sebastian et al. 2002). As VEGF is involved in angiogenesis in breast cancer and has a role in increasing endothelial permeability (Breslin et al. 2003), we hypothesized that I.7 promoter activation is regulated by the VEGF/MEK/MAPK 1/3 signaling pathway in Hs578t cells. It is known that MEK/MAPK 1/3 is activated by the binding of VEGF to its receptors (VEGFR-1 and VEGFR-2) (Breslin et al. 2003; Cross and Claesson-Welsh 2001; Lee et al. 1998). We also know that the activation of VEGFR-2 in endothelial cells stimulates the PLC/PKC pathway (Cario et al. 2004; Cross and Claesson-Welsh 2001; Jiang et al. 2016), thus explaining the overexpression of PII-derived CYP19 in Hs578t cells exposed to VEGF (Figure 1A).
Angiogenesis is associated with tumor growth and metastasis in breast cancer (Adams et al. 2000) and increased expression of VEGF and its receptors has been denoted in invasive breast carcinomas (Yoshiji et al. 1996). In our study, VEGF stimulated I.7- and PII-mediated CYP19 expression, resulting in an increase in overall (nonpromoter-specific) expression of CYP19 (Figure 1A) and aromatase catalytic activity (Figure 1B). Using an inhibitor of the MEK/MAPK 1/3 pathway, we also demonstrated that the VEGF-mediated overexpression of I.7 promoter-derived CYP19 was at least in part mediated through the MEK/MAPK 1/3 pathway (Figure 2A,D). This finding supports our hypothesis and is consistent with the literature. Indeed, it has been demonstrated that VEGF increases human endothelial permeability via the MEK pathway (Breslin et al. 2003), that incubation of endothelial cells with PD98059 reduces MAPK 1 activity (Pai et al. 2001), and that VEGF induces the phosphorylation of MAPK 1/2 (Xu et al. 2008). We have also demonstrated that treatment with forskolin stimulates PII/I.3-mediated CYP19 expression and induces aromatase activity (Figure 1A,B), and that this effect is mediated at least in part through the PLC pathway (Figure 2A–C). This result is also supported by a study showing that in HEK-293 cells, forskolin induces calcium release (Schmidt et al. 2001), an important component of the PLC pathway. These results suggest that VEGF signaling pathways, and more specifically the PLC and MEK/MAPK 1/3 pathways, are involved in PII/I.3 and I.7-mediated CYP19 expression in Hs578t breast cancer cells.
Effects of Neonicotinoids on the Promoter-Specific Expression of CYP19
Certain contaminants such as atrazine exert estrogenic activity by increasing CYP19 expression and aromatase activity (Fan et al. 2007; Sanderson et al. 2002), which would result in increased biosynthesis of estrogens (Caron-Beaudoin et al. 2017; Eldridge et al. 1994). Moreover, we have previously shown that atrazine, and recently, that several neonicotinoid insecticides induce the promoter-specific expression of CYP19 and/or its catalytic activity in various in vitro cell systems (Caron-Beaudoin et al. 2017; Caron-Beaudoin et al. 2016).
In the present study, we have found that treatment of Hs578t breast cancer cells with the neonicotinoids thiacloprid and imidacloprid results in an overall increase in CYP19 expression and catalytic activity of aromatase compared with control (Figures 3 and 4), an observation consistent with a neonicotinoid-induced switch in CYP19 promoter usage. Results from pretreatment of cells with VEGF pathway inhibitors suggest that thiacloprid increases PII/I.3 and I.7 promoter-mediated CYP19 expression through activation of the PLC and MEK/MAPK 1/3 pathways (Figures 5 and 6). We observed a similar promoter-specific response in Hs578t cells exposed to imidacloprid, although inhibition of the MEK/MAPK 1/3 pathway did not statistically significantly alter the response of the cells to this neonicotinoid (Figures 5 and 6). Exposure of Hs578t cells to thiacloprid and imidacloprid resulted in an increase of predominantly PII promoter-derived CYP19 transcripts and a more modest increase in I.3 promoter-derived transcripts compared with control. This differential effect on the two promoters is not unusual because similar expression patterns have been observed in primary adipose stromal cells exposed to phorbol 12-myristate 13-acetate (PMA), , or forskolin (Heneweer et al. 2004; Zhao et al. 1996). We also previously observed an increase in PII/I.3 promoter-mediated CYP19 expression in H295R cells exposed to the neonicotinoids thiacloprid, imidacloprid, and thiamethoxam (Caron-Beaudoin et al. 2016).
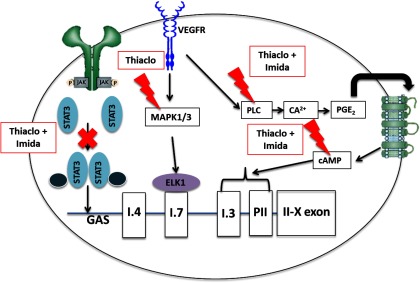
Figure 6.
Proposed signaling pathways implicated in the promoter-specific expression of CYP19 in Hs578t cells. It is proposed that thiacloprid and imidacloprid block the JAK/STAT3 pathway, which regulates I.4 promoter activity via a yet unknown mechanism. In addition, based on the effects of pathway inhibitors, the neonicotinoids are proposed to increase PII/I.3-mediated CYP19 expression in Hs578t cells via stimulation of the PLC pathway. Thiacloprid may also induce I.7-mediated CYP19 expression by stimulation of the MEK/MAPK 1/3 pathway. , calcium ion; cAMP, cyclic adenosine monophosphate; X, inhibition; ELK, electron transport system transcription factor; GAS, gamma interferon activation site; Imida, imidacloprid; JAK, Janus kinase; lightning bolt, activation/stimulation; MAPK, mitogen-activated protein kinases; , prostaglandin ; PLC, phospholipase C; STAT, signal transducer and activator of transcription protein; Thiaclo, thiacloprid; VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor.
Limitations and Perspectives
Cell–cell communication during hormone-dependent breast cancer progression has been widely studied. For instance, communication between epithelial cancer cells and fibroblastic cells surrounding the tumor leads to a desmoplastic reaction associated with the accumulation of fibroblasts (preadipocytes) due to inhibition of their differentiation into stromal adipocytes. Preadipocytes have greater CYP19 expression than differentiated stromal cells and are key actors in the overproduction of estrogens in the tumor microenvironment, leading to proliferation of cancer cells (Kalluri and Zeisberg 2006; Meng et al. 2001; Zhao et al. 1996). Therefore, using a single-cell bioassay has its limitations, given that we are not able to adequately mimic the cellular interactions during breast cancer progression. However, the present study in Hs578t cells provides crucial information for a better understanding of the mechanisms underlying the expression of CYP19 by breast cancer-relevant promoters, as well as the impacts of neonicotinoids on these processes. We are currently developing a cellular coculture model in which Hs578t cells together with estrogen-responsive epithelial breast cancer cells will produce a more representative model of the tumor microenvironment. This coculture model will provide a more physiologically and toxicologically relevant study tool to better understand impacts of environmental contaminants on hormone-dependent breast cancer.
Conclusions
To the best of our knowledge, the present study is the first to describe the promoter-specific expression of CYP19 via the normal mammary promoter I.4 and the breast cancer-relevant promoters PII, I.3, and I.7 in Hs578t cells. We have further shown that exposure of these cells to concentrations of the neonicotinoid insecticides thiacloprid and imidacloprid similar to what is found in urine of farmers and women from the general population in Japan increase CYP19 expression, associated with a decrease in I.4 promoter activity and an increase in the activities of promoters PII, I.3, and I.7. The observed promoter-switch appears to involve the VEGF-mediated PLC and MAPK 1/3 signaling pathways (Figure 6). This unique switch in promoter usage induced by thiacloprid and imidacloprid is a process usually observed in patients with progressive hormone-dependent breast cancer. However, the molecular targets of thiacloprid and imidacloprid involved in this promoter-switch remain unknown. Future work should also focus on investigating the signaling pathways implicated in the decrease of I.4-mediated CYP19 expression in Hs578t cells in response to the promoter-switch induced by exposed to neonicotinoids. Our findings highlight the need for further research to assess the potential impacts of low-dose and chronic exposure to neonicotinoids on endocrine processes affecting women’s health.
Supplemental Material
Acknowledgments
The authors thank J. St-Pierre for his expertise in preamplified quantitative polymerase chain reaction. This research was funded by a Natural Sciences and Engineering Research Council of Canada Discovery (NSERC; grant no. 313313-2012), a California Breast Cancer Research Program (CBCRP; grant no. 17UB-8703), and an Alternatives Research and Development Foundation (ARDF) grant to J.T.S. and doctoral studentships from the Fonds de recherche du Québec – Nature et technologies (FRQNT) and Fondation universitaire Armand-Frappier INRS to E.C.-B. This research was conducted in fulfillment of E.C-B.’s PhD requirements at the INRS Institut Armand-Frappier. This study was part of R.V.’s internship that was funded by the Fondation universitaire Armand-Frappier INRS.
References
- Adams J, Carder PJ, Downey S, Forbes MA, MacLennan K, Allgar V, et al. 2000. Vascular endothelial growth factor (VEGF) in breast cancer: comparison of plasma, serum, and tissue VEGF and microvessel density and effects of tamoxifen. Cancer Res 60(11):2898–2905, PMID: 10850435. [PubMed] [Google Scholar]
- Agarwal VR, Bulun SE, Leitch M, Rohrich R, Simpson ER. 1996. Use of alternative promoters to express the aromatase cytochrome P450 (CYP19) gene in breast adipose tissues of cancer-free and breast cancer patients. J Clin Endocrinol Metab 81(11):3843–3849, PMID: 8923826, 10.1210/jcem.81.11.8923826. [DOI] [PubMed] [Google Scholar]
- American Cancer Society. 2017. Breast Cancer Facts & Figures 2017–2018. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts-and-figures-2017-2018.pdf [accessed 5 April 2018].
- Bal R, Naziroğlu M, Türk G, Yilmaz Ö, Kuloğlu T, Etem E, et al. 2012. Insecticide imidacloprid induces morphological and DNA damage through oxidative toxicity on the reproductive organs of developing male rats. Cell Biochem Funct 30(6):492–499, PMID: 22522919, 10.1002/cbf.2826. [DOI] [PubMed] [Google Scholar]
- Bouskine A, Nebout M, Brücker-Davis F, Benahmed M, Fenichel P. 2009. Low doses of bisphenol A promote human seminoma cell proliferation by activating PKA and PKG via a membrane G-protein-coupled estrogen receptor. Environ Health Perspect 117(7):1053–1058, PMID: 19654912, 10.1289/ehp.0800367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslin JW, Pappas PJ, Cerveira JJ, Hobson RW 2nd, Durán WN. 2003. VEGF increases endothelial permeability by separate signaling pathways involving ERK-1/2 and nitric oxide. Am J Physiol Heart Circ Physiol 284(1):H92–H100, PMID: 12388327, 10.1152/ajpheart.00330.2002. [DOI] [PubMed] [Google Scholar]
- Bulun SE, Sebastian S, Takayama K, Suzuki T, Sasano H, Shozu M. 2003. The human CYP19 (aromatase P450) gene: Update on physiologic roles and genomic organization of promoters. J Steroid Biochem Mol Biol 86(3-5):219–224, PMID: 14623514. [DOI] [PubMed] [Google Scholar]
- Canadian Cancer Society’s Advisory Committee on Cancer Statistics. 2017. Canadian Cancer Statistics 2017. Toronto, ON, Canada:Canadian Cancer Society. [Google Scholar]
- Cario E, Gerken G, Podolsky DK. 2004. Toll-like receptor 2 enhances ZO-1-associated intestinal epithelial barrier integrity via protein kinase C. Gastroenterology 127(1):224–238, PMID: 15236188. [DOI] [PubMed] [Google Scholar]
- Caron-Beaudoin É, Denison MS, Sanderson JT. 2016. Effects of neonicotinoids on promoter-specific expression and activity of aromatase (CYP19) in human adrenocortical carcinoma (H295R) and primary umbilical vein endothelial (HUVEC) cells. Toxicol Sci 149(1):134–144, PMID: 26464060, 10.1093/toxsci/kfv220. [DOI] [PubMed] [Google Scholar]
- Caron-Beaudoin E, Viau R, Hudon Thibeault A-A, Vaillancourt C, Sanderson JT. 2017. The use of a unique fetoplacental steroidogenesis co-culture model as a screening tool for endocrine disruptors: the effects of neonicotinoids on aromatase activity and hormone production. Toxicol Appl Pharmacol 332:15–24, PMID: 28750898, 10.1016/j.taap.2017.07.018. [DOI] [PubMed] [Google Scholar]
- Chavez KJ, Garimella SV, Lipkowitz S. 2010. Triple negative breast cancer cell lines: one tool in the search for better treatment of triple negative breast cancer. Breast Dis 32(1–2):35–48, PMID: 21778573, 10.3233/BD-2010-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Reierstad S, Lin Z, Lu M, Brooks C, Li N, et al. 2007. Prostaglandin E2 induces breast cancer–related aromatase promoters via activation of p38 and c-Jun NH2-terminal kinase in adipose fibroblasts. Cancer Res 67(18):8914–8922, PMID: 17875734, 10.1158/0008-5472.CAN-06-4751. [DOI] [PubMed] [Google Scholar]
- Chen M, Tao L, McLean J, Lu C. 2014. Quantitative analysis of neonicotinoid insecticide residues in foods: implication for dietary exposures. J Agric Food Chem 62(26):6082–6090, PMID: 24933495, 10.1021/jf501397m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn BA, Wolff MS, Cirillo PM, Sholtz RI. 2007. DDT and breast cancer in young women: new data on the significance of age at exposure. Environ Health Perspect 115(10):1406, PMID: 17938728, 10.1289/ehp.10260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross MJ, Claesson-Welsh L. 2001. FGF and VEGF function in angiogenesis: signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol Sci 22(4):201–207, PMID: 11282421. [DOI] [PubMed] [Google Scholar]
- Elbert A, Haas M, Springer B, Thielert W, Nauen R. 2008. Applied aspects of neonicotinoid uses in crop protection. Pest Manag Sci 64(11):1099–1105, PMID: 18561166, 10.1002/ps.1616. [DOI] [PubMed] [Google Scholar]
- Eldridge JC, Tennant MK, Wetzel LT, Breckenridge CB, Stevens JT. 1994. Factors affecting mammary tumor incidence in chlorotriazine-treated female rats: hormonal properties, dosage, and animal strain. Environ Health Perspect 102(suppl 11):29–36, PMID: 7737039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W, Yanase T, Morinaga H, Gondo S, Okabe T, Nomura M, et al. 2007. Atrazine-induced aromatase expression is SF-1 dependent: implications for endocrine disruption in wildlife and reproductive cancers in humans. Environ Health Perspect 115(5):720–727, PMID: 17520059, 10.1289/ehp.9758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh D, Griswold J, Erman M, Pangborn W. 2009. Structural basis for androgen specificity and oestrogen synthesis in human aromatase. Nature 457(7226):219–223, PMID: 19129847, 10.1038/nature07614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulson D. 2013. Review: an overview of the environmental risks posed by neonicotinoid insecticides. J Appl Ecol 50(4):977–987, 10.1111/1365-2664.12111. [DOI] [Google Scholar]
- Harada N, Utsumi T, Takagi Y. 1993. Tissue-specific expression of the human aromatase cytochrome P-450 gene by alternative use of multiple exons 1 and promoters, and switching of tissue-specific exons 1 in carcinogenesis. Proc Natl Acad Sci USA 90(23):11312–11316, PMID: 8248245, 10.1073/pnas.90.23.11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneweer M, van den Berg M, Sanderson J. 2004. A comparison of human H295R and rat R2C cell lines as in vitro screening tools for effects on aromatase. Toxicol Lett 146(2):183–194, PMID: 14643970. [DOI] [PubMed] [Google Scholar]
- Henry M, Béguin M, Requier F, Rollin O, Odoux J-F, Aupinel P, et al. 2012. A common pesticide decreases foraging success and survival in honey bees. Science 336(6079):348–350, PMID: 22461498, 10.1126/science.1215039. [DOI] [PubMed] [Google Scholar]
- Hoshi N, Hirano T, Omotehara T, Tokumoto J, Umemura Y, Mantani Y, et al. 2014. Insight into the mechanism of reproductive dysfunction caused by neonicotinoid pesticides. Biol Pharm Bull 37(9):1439–1443, PMID: 25177026. [DOI] [PubMed] [Google Scholar]
- Ibarluzea Jm Jm, Fernández MF, Santa-Marina L, Olea-Serrano MF, Rivas AM, Aurrekoetxea JJ, et al. 2004. Breast cancer risk and the combined effect of environmental estrogens. Cancer Causes Control 15(6):591–600, PMID: 15280638, 10.1023/B:CACO.0000036167.51236.86. [DOI] [PubMed] [Google Scholar]
- Irahara N, Miyoshi Y, Taguchi T, Tamaki Y, Noguchi S. 2006. Quantitative analysis of aromatase mRNA expression derived from various promoters (I.4, I.3, PII and I.7) and its association with expression of TNF-α, IL-6 and COX-2 mRNAs in human breast cancer. Int J Cancer 118(8):1915–1921, PMID: 16287071, 10.1002/ijc.21562. [DOI] [PubMed] [Google Scholar]
- Jiang M, Qin C, Han M. 2016. Primary breast cancer induces pulmonary vascular hyperpermeability and promotes metastasis via the VEGF–PKC pathway. Mol Carcinog 55(6):1087–1095, PMID: 26152457, 10.1002/mc.22352. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Zeisberg M. 2006. Fibroblasts in cancer. Nat Rev Cancer 6(5):392–401, PMID: 16572188, 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- Kapoor U, Srivastava MK, Srivastava LP. 2011. Toxicological impact of technical imidacloprid on ovarian morphology, hormones and antioxidant enzymes in female rats. Food Chem Toxicol 49(12):3086–3089, PMID: 21946071, 10.1016/j.fct.2011.09.009. [DOI] [PubMed] [Google Scholar]
- Knower KC, To SQ, Simpson ER, Clyne CD. 2010. Epigenetic mechanisms regulating CYP19 transcription in human breast adipose fibroblasts. Mol Cell Endocrinol 321(2):123–130, PMID: 20211687, 10.1016/j.mce.2010.02.035. [DOI] [PubMed] [Google Scholar]
- Konecny GE, Meng YG, Untch M, Wang H-J, Bauerfeind I, Epstein M, et al. 2004. Association between HER-2/neu and vascular endothelial growth factor expression predicts clinical outcome in primary breast cancer patients. Clin Cancer Res 10(5):1706–1716, PMID: 15014023. [DOI] [PubMed] [Google Scholar]
- Lee AH, Dublin EA, Bobrow LG, Poulsom R. 1998. Invasive lobular and invasive ductal carcinoma of the breast show distinct patterns of vascular endothelial growth factor expression and angiogenesis. J Pathol 185(4):394–401, PMID: 9828838, . [DOI] [PubMed] [Google Scholar]
- Lephart ED, Simpson ER. 1991. [45] Assay of aromatase activity. In: Methods in Enzymology, Vol. 206, (Waterman MR, Johnson EF, ed) San Diego, CA:Academic Press, 477–483. [DOI] [PubMed] [Google Scholar]
- Main AR, Headley JV, Peru KM, Michel NL, Cessna AJ, Morrissey CA. 2014. Widespread use and frequent detection of neonicotinoid insecticides in wetlands of Canada’s Prairie Pothole Region. PLoS One 9(3):e92821, PMID: 24671127, 10.1371/journal.pone.0092821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur V, Bhatnagar P, Sharma RG, Acharya V, Sexana R. 2002. Breast cancer incidence and exposure to pesticides among women originating from Jaipur. Environ Int 28(5):331–336, PMID: 12437282. [DOI] [PubMed] [Google Scholar]
- Matsuda K, Buckingham SD, Kleier D, Rauh JJ, Grauso M, Sattelle DB. 2001. Neonicotinoids: insecticides acting on insect nicotinic acetylcholine receptors. Trends Pharmacol Sci 22(11):573–580, PMID: 11698101. [DOI] [PubMed] [Google Scholar]
- Meng L, Zhou J, Sasano H, Suzuki T, Zeitoun KM, Bulun SE. 2001. Tumor necrosis factor α and interleukin 11 secreted by malignant breast epithelial cells inhibit adipocyte differentiation by selectively down-regulating CCAAT/enhancer binding protein α and peroxisome proliferator-activated receptor γ: mechanism of desmoplastic reaction. Cancer Res 61(5):2250–2255, PMID: 11280794. [PubMed] [Google Scholar]
- Nomura H, Ueyama J, Kondo T, Saito I, Murata K, Iwata T, et al. 2013. Quantitation of neonicotinoid metabolites in human urine using GC-MS. J Chromatogr B Analyt Technol Biomed Life Sci 941:109–115, PMID: 24189204, 10.1016/j.jchromb.2013.10.012. [DOI] [PubMed] [Google Scholar]
- Pai R, Szabo IL, Soreghan BA, Atay S, Kawanaka H, Tarnawski AS. 2001. PGE2 stimulates VEGF expression in endothelial cells via ERK2/JNK1 signaling pathways. Biochem Biophys Res Commun 286(5):923–928, PMID: 11527387, 10.1006/bbrc.2001.5494. [DOI] [PubMed] [Google Scholar]
- Roy JR, Chakraborty S, Chakraborty TR. 2009. Estrogen-like endocrine disrupting chemicals affecting puberty in humans—a review. Med Sci Monit 15(6):RA137–RA145, PMID: 19478717. [PubMed] [Google Scholar]
- Rubin BS, Murray MK, Damassa DA, King JC, Soto AM. 2001. Perinatal exposure to low doses of bisphenol A affects body weight, patterns of estrous cyclicity, and plasma LH levels. Environ Health Perspect 109(7):675–680, PMID: 11485865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson JT. 2006. The steroid hormone biosynthesis pathway as a target for endocrine-disrupting chemicals. Toxicol Sci 94(1):3–21, PMID: 16807284, 10.1093/toxsci/kfl051. [DOI] [PubMed] [Google Scholar]
- Sanderson J, Boerma J, Lansbergen GWA, van den Berg M. 2002. Induction and inhibition of aromatase (CYP19) activity by various classes of pesticides in H295R human adrenocortical carcinoma cells. Toxicol Appl Pharmacol 182(1):44–54, PMID: 12127262, 10.1006/taap.2002.9420. [DOI] [PubMed] [Google Scholar]
- Sanderson J, Seinen W, Giesy JP, van den Berg M. 2000. 2-chloro-s-triazine herbicides induce aromatase (CYP19) activity in H295R human adrenocortical carcinoma cells: a novel mechanism for estrogenicity? Toxicol Sci 54(1):121–127, PMID: 10746939. [DOI] [PubMed] [Google Scholar]
- Schaafsma A, Limay-Rios V, Baute T, Smith J, Xue Y. 2015. Neonicotinoid insecticide residues in surface water and soil associated with commercial maize (corn) fields in southwestern Ontario. PLOS One 10(2):e0118139, PMID: 25710560, 10.1371/journal.pone.0118139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M, Evellin S, Weernink PA, von Dorp F, Rehmann H, Lomasney JW, et al. 2001. A new phospholipase-C-calcium signalling pathway mediated by cyclic AMP and a Rap GTPase. Nat Cell Biol 3(11):1020–1024, PMID: 11715024, 10.1038/ncb1101-1020. [DOI] [PubMed] [Google Scholar]
- Schneider BP, Sledge GW Jr.. 2007. Drug insight: VEGF as a therapeutic target for breast cancer. Nat Clin Pract Oncol 4(3):181–189, PMID: 17327858, 10.1038/ncponc0740. [DOI] [PubMed] [Google Scholar]
- Sebastian S, Bulun SE. 2001. A highly complex organization of the regulatory region of the human CYP19 (aromatase) gene revealed by the Human Genome Project. J Clin Endocrinol Metab 86(10):4600–4602, PMID: 11600509, 10.1210/jcem.86.10.7947. [DOI] [PubMed] [Google Scholar]
- Sebastian S, Takayama K, Shozu M, Bulun SE. 2002. Cloning and characterization of a novel endothelial promoter of the human CYP19 (aromatase P450) gene that is up-regulated in breast cancer tissue. Mol Endocrinol 16(10):2243–2254, PMID: 12351690, 10.1210/me.2002-0123. [DOI] [PubMed] [Google Scholar]
- Şekeroğlu V, Şekeroğlu ZA, Demirhan E. 2014. Effects of commercial formulations of deltamethrin and/or thiacloprid on thyroid hormone levels in rat serum. Toxicol Ind Health 30(1):40–46, PMID: 22677783, 10.1177/0748233712448114. [DOI] [PubMed] [Google Scholar]
- Simpson ER, Davis SR. 2001. Minireview: aromatase and the regulation of estrogen biosynthesis—some new perspectives. Endocrinology 142(11):4589–4594, PMID: 11606422, 10.1210/endo.142.11.8547. [DOI] [PubMed] [Google Scholar]
- Sofi M, Young MJ, Papamakarios T, Simpson ER, Clyne CD. 2003. Role of CRE-binding protein (CREB) in aromatase expression in breast adipose. Breast Cancer Res Treat 79(3):399–407, PMID: 12846424. [DOI] [PubMed] [Google Scholar]
- Starner K, Goh KS. 2012. Detections of the neonicotinoid insecticide imidacloprid in surface waters of three agricultural regions of California, USA, 2010–2011. Bull Environ Contam Toxicol 88(3):316–321, PMID: 22228315, 10.1007/s00128-011-0515-5. [DOI] [PubMed] [Google Scholar]
- Stokstad E. 2013. Pesticides under fire for risks to pollinators. Science 340(6133):674–676, PMID: 23661734, 10.1126/science.340.6133.674. [DOI] [PubMed] [Google Scholar]
- Su B, Díaz-Cruz ES, Landini S, Brueggemeier RW. 2008. Suppression of aromatase in human breast cells by a cyclooxygenase-2 inhibitor and its analog involves multiple mechanisms independent of cyclooxygenase-2 inhibition. Steroids 73(1):104–111, PMID: 18045633, 10.1016/j.steroids.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbaramaiah K, Morris PG, Zhou XK, Morrow M, Du B, Giri D, et al. 2012. Increased levels of COX-2 and prostaglandin E2 contribute to elevated aromatase expression in inflamed breast tissue of obese women. Cancer Discov 2(4):356–365, PMID: 22576212, 10.1158/2159-8290.CD-11-0241. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Taylor S, Wakem M, Dijkman G, Alsarraj M, Nguyen M. 2010. A practical approach to RT-qPCR—publishing data that conform to the MIQE guidelines. Methods 50(4):S1–S5, PMID: 20215014, 10.1016/j.ymeth.2010.01.005. [DOI] [PubMed] [Google Scholar]
- To SQ, Knower KC, Cheung V, Simpson ER, Clyne CD. 2015. Transcriptional control of local estrogen formation by aromatase in the breast. J Steroid Biochem Mol Biol 145:179–186, PMID: 24846828, 10.1016/j.jsbmb.2014.05.004. [DOI] [PubMed] [Google Scholar]
- Tremblay JJ, Viger RS. 2003. Transcription factor GATA-4 is activated by phosphorylation of serine 261 via the cAMP/protein kinase a signaling pathway in gonadal cells. J Biol Chem 278(24):22128–22135, PMID: 12670947, 10.1074/jbc.M213149200. [DOI] [PubMed] [Google Scholar]
- Ueyama J, Harada KH, Koizumi A, Sugiura Y, Kondo T, Saito I, et al. 2015. Temporal levels of urinary neonicotinoid and dialkylphosphate concentrations in Japanese women between 1994 and 2011. Environ Sci Technol 49(24):14522–14528, PMID: 26556224, 10.1021/acs.est.5b03062. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ye L, Leung LK. 2008. A positive feedback pathway of estrogen biosynthesis in breast cancer cells is contained by resveratrol. Toxicology 248(2–3):130–135, PMID: 18462857, 10.1016/j.tox.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Xu J, Liu X, Jiang Y, Chu L, Hao H, Liua Z, et al. 2008. MAPK/ERK signalling mediates VEGF‐induced bone marrow stem cell differentiation into endothelial cell. J Cell Mol Med 12(6A):2395–2406, PMID: 18266967, 10.1111/j.1582-4934.2008.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Dailey AB, Talbott EO, Ilacqua VA, Kearney G, Asal NR. 2010. Associations of serum concentrations of organochlorine pesticides with breast cancer and prostate cancer in U.S. adults. Environ Health Perspect 118(1):60–66, PMID: 20056587, 10.1289/ehp.0900919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Hayashi S. 2009. Estrogen-related cancer microenvironment of breast carcinoma. Endocr J 56(1):1–7, PMID: 18497452. [DOI] [PubMed] [Google Scholar]
- Yoshiji H, Gomez DE, Shibuya M, Thorgeirsson UP. 1996. Expression of vascular endothelial growth factor, its receptor, and other angiogenic factors in human breast cancer. Cancer Res 56(9):2013–2016, PMID: 8616842. [PubMed] [Google Scholar]
- Zhao Y, Agarwal VR, Mendelson CR, Simpson ER. 1996. Estrogen biosynthesis proximal to a breast tumor is stimulated by PGE2 via cyclic AMP, leading to activation of promoter II of the CYP19 (aromatase) gene. Endocrinology 137(12):5739–5742, PMID: 8940410, 10.1210/endo.137.12.8940410. [DOI] [PubMed] [Google Scholar]
- Zhou D, Zhou C, Chen S. 1997. Gene regulation studies of aromatase expression in breast cancer and adipose stromal cells. J Steroid Biochem Mol Biol 61(3–6):273–280, PMID: 9365201. [PubMed] [Google Scholar]
- Zhou J, Suzuki T, Kovacic A, Saito R, Miki Y, Ishida T, et al. 2005. Interactions between prostaglandin E2, liver receptor homologue-1, and aromatase in breast cancer. Cancer Res 65(2):657–663, PMID: 15695411. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.