The importance of amino acids and peptides in countering glycation and the formation of advanced glycation end-products (AGEs) is reviewed.
The importance of amino acids and peptides in countering glycation and the formation of advanced glycation end-products (AGEs) is reviewed.
Abstract
The importance of controlling or preventing protein glycation cannot be overstated and is of prime importance in the treatment of diabetes and associated complications including Alzheimer's disease, cataracts, atherosclerosis, kidney aliments among others. In this respect, simple molecules such as amino acids and peptides hold much promise both in terms of ease and scale-up of synthesis as well as in relation to negligible/low associated toxicity. In view of this, a comprehensive account of literature reports is presented, that documents the anti-glycation activity of natural and non-natural amino acids and peptides. This review also discusses the chemical reactions involved in glycation and the formation of advanced glycation end-products (AGEs) and possible/probable intervention sites and mechanism of action of the reported amino acids/peptides. This aspect of amino acids/peptides adds to their growing importance in medicinal and therapeutic applications.
Introduction
Glycation of proteins refers to the reversible reaction of amino groups in proteins, peptides, and lipids with reducing sugars1 and occurs by a complex series of sequential reactions that involve formation of a Schiff base, Amadori products, and eventually, advanced glycation end-products (AGEs). In conditions of low glucose levels, early glycation products are found to be in equilibrium with plasma glucose, and are capable of dissociating to the native proteins. On the other hand, in conditions of prevalently high glucose levels, molecular rearrangements take place, leading to the irreversible formation of AGEs2 which contribute to the development of various complications associated with diabetes mellitus (DM), atherosclerosis and neurodegenerative diseases.3 AGEs have primarily been detected in long-lived proteins, such as collagen and lens crystallins, with post-translational AGE-modifications predominantly occurring at the side-chain amino groups of lysine and arginine residues.4 This leads to structural alterations of the native protein, thereby impeding both protein function as well as increasing resistance to proteolytic removal.5 For example, protein glycation has been found to affect the function of cytoskeletal proteins and antioxidant enzymes, as well as result in protease-resistant and detergent-insoluble aggregates such as β-amyloid peptide deposits.4 AGEs, largely via the receptor for AGEs (RAGE), activate several signalling mechanisms that cause cell stress, contribute to cellular dysfunction, and damage target organs, leading to complications.6 RAGE is a transmembrane receptor of the immunoglobulin superfamily, and is prevalent at low concentrations in a variety of healthy human tissues, including the lungs, kidneys, liver, cardiovascular and nervous systems.7 It is a central signalling molecule in the innate immune system and is involved in the onset and sustainment of the inflammatory response.8
Chemistry of glycation
Glycation can be divided into three main stages (Fig. 1). In the initial stage, glucose reacts with an amine from the protein to form a metastable Schiff base which rearranges to the Amadori product. In this phase, glucose shows the slowest glycation rate when compared to other reducing sugars9 because of the maximal shift of the equilibrium towards its cyclic rather than open-chain aldehyde isoforms, unlike other natural monosaccharides such as ribose. In the intermediate stage, the Amadori product is fragmented to various reactive dicarbonyl compounds such as glyoxal, methylglyoxal and dexoyglucosones.10 Dicarbonyl compounds react more strongly than their parent sugars with amino groups of proteins to form inter- and intra-molecular cross-links,11 arginine and lysine being the main amino acid residues involved in cross-linking.12 The last stage of the reaction involves irreversible AGEs formation through a series of oxidation, dehydration and cyclization reactions. However, not all of the AGEs are derived from protein cross-links. The widely studied Nε-carboxymethyl-lysine (CML), for example, is derived from the modification of a single lysine residue.13 The heterogeneity of the AGEs formed depends wholly on the glycating agent. For example, glyoxal-derived AGEs include carboxymethyl-lysine (CML) and glyoxal lysine dimer (GOLD), while methylglyoxal-derived AGEs are carboxyethyl-lysine (CEL), argpyrimidine, and methylglyoxal lysine dimers (MOLD); 3-deoxyglucosone-derived AGEs are pyrraline and deoxyglucosone-derived lysine dimers (DOLD).10 Accumulation of AGEs is largely seen in long-lived proteins such as lens crystallins and tissue collagens owing to the slow process of their formation.13
Fig. 1. Pictorial representation of glycation.
Inhibitors of AGE formation
The molecular strategies to combat the formation and accumulation of AGEs necessitates consideration of different approaches in order to reduce the AGEs level in the body such as (a) early glycation inhibition (b) advanced glycation inhibition (c) carbonyl quenching (d) masking of lysine residues and (e) inhibition of glycation-induced aggregation,14 among others. This review is focused on amino acids and peptides against glycation. Besides amino acids and peptides, there exist several small molecules and drugs that possess anti-glycation properties; however, these are beyond the scope of this article.
Amino acids have received considerable attention as therapeutic agents in conditions like respiratory physiology, cardiology, renal failure, neurological disorders, congenital defects, etc.15 Likewise, many peptides have been reported to have properties such as anti-oxidant, anti-hypertensive, anti-atherosclerotic, immunomodulatory16 and anti-glycation activities17 in addition to nutrient roles. The role of amino acids and peptides in countering glycation has, however, not been accorded due importance. This review is therefore, aimed towards highlighting the utility benefits and application potential of amino acids and peptides as anti-glycation agents, and hopefully draw the attention of research towards this largely underexploited area.
Amino acid inhibitors of glycation
Some of the regulatory roles of amino acids include gene expression, synthesis and secretion of hormones, nutrient metabolism and oxidative defence, intracellular protein turnover, immune function, reproduction and obesity to name a few.18 In connection with glycation, free amino acids have been found to mitigate the glycation of lens protein, delay progression of cataract and also bring down blood sugar levels in diabetic rats. Some amino acids inhibit or reduce glycation by hampering the binding of glucose to proteins by competitive inhibition, thereby offering protection, while some amino acids influence pathological pathways resulting in increased tissue sensitivity towards insulin.19
Positively charged (cationic) amino acids
Cataract formation, a well-recognized consequence of protein glycation resulting from prolonged hyperglycaemia, was found to be delayed by the anti-glycation effect of free amino acid lysine (l-20,21 and d-lysine22). The reduction in the extent of glycation could be attributed to the competitive reaction between free lysine's amino groups and lens protein functionalities with the aldehyde group of glucose forming a Schiff's base, thus scavenging glucose from inside the cells, particularly in hyperglycaemic states.20 Arginine is known to impede the progression of cataract by acting as an AGE inhibitor and a protein stabilizing agent. The ability of arginine to suppress AGE formation was attributed to its ability to scavenge oxoaldehydes such as glyoxal and methylglyoxal, as well as dehydroascorbic acid (DHA) and its degradation products xylosone, erythrulose, and deoxythreosone.23 In another study, l-arginine and spermine were found to potently inhibit pyrraline formation in comparison to other polyamines such as putrescine, cadaverine and spermidine in vitro.24 This effect was probably a consequence of the guanidino group and four amino groups in arginine and spermine respectively, suggesting that the anti-glycation effect depended on the number of amino groups present, whereby l-arginine and polyamines are capable of forming a Schiff-base with the carbonyls of open-chain sugars. Glycation inhibition studies under oxidative and non-oxidative conditions led to the conclusion that under oxidative conditions, arginine's anti-glycation property was a result of its guanidinium group, whereas in non-oxidative conditions, it was the α-NH2 group that exerted this effect.25
Negatively charged (anionic) amino acids
The anti-glycation property and efficacy of aspartic acid (Asp) to inhibit AGEs formation was established by its protective effect in shielding the proteins like hemoglobin and albumin from exposure to reducing sugars like glucose, fructose and ribose.26 Molecular docking studies of BSA–Asp complex revealed hydrophobic – as well as hydrogen bonding interactions between Asp and BSA residues such as Trp, Leu, Val and Arg respectively, to be responsible for the reduced formation of AGEs, which presumably occurs through steric blocking of the reactive Arg guanidine functions from the sugars.
Glycine and hypoglycin A
Glycine exerts anti-diabetic effects in addition to being an insulin secretagogue.19 It has been reported to counter cataractogenesis by preventing glycation of lens proteins.20 Alvarado-Vásquez et al.27 reported that glycine and taurine diminished the concentration of glucose, triglycerides, total cholesterol, as well as the non-enzymatic glycation of hemoglobin in streptozotocin-treated rats. Although the specific mechanisms by which glycine or taurine induced diminution of glucose levels remained to be identified, this effect was attributed to the capability of these amino acids of interacting with the insulin receptor. The formation of Schiff base through the reaction of glycine with sugars such as glucose has also been observed,28 causing a decrease of the free glucose concentration in the medium. Glycine possibly reacts with glucose or dicarbonyl compounds via its amino group, thereby resulting in lower production of AGEs.29
The unusual amino acid, hypoglycin A (Fig. 2(a)), a plant toxin extracted from unripe fruits and seeds of ackee, Blighia sapida, has been reported to cause hypoglycaemia and depletion of glycogen in experimental animals, as a consequence of a decrease in gluconeogenesis resulting from impairment of long chain fatty acid metabolism.30
Fig. 2. Hypoglycin A (a) and Taurine (b).

Sulfur-containing amino acids
The use of sulfur-containing compounds in the treatment and prevention of diabetes and associated complications has been demonstrated.31 Taurine (2-aminoethanesulphonic acid, Fig. 2(b)) an intracellular amino acid, is one such compound.32 The anti-hyperglycaemic effect of taurine was reported to be a consequence of its reversible binding to a subunit of the insulin receptor, acting as an insulin agonist, and also causing many physiological actions of insulin. Additionally, the sulfur-containing acid group is also thought to be responsible, at least in part, to bring about the effect.33 Taurine was also shown to prevent glycation of lens protein by inhibiting the Schiff base formation between the protein and the reactive sugar,34 possibly a result of competitive reaction via its amino group, leading to a decrease in glucose content. The anti-oxidant activity of taurine and its high reactivity towards carbonyl compounds, both contribute to inhibition of protein glycation.32
The amino acid cysteine has also been reported to have hypoglycaemic activity and can be thought of as a multifunctional compound with hypoglycaemic, anti-oxidant and anti-inflammatory activities, that is capable of inhibiting protein glycation and trapping dicarbonyl compounds, besides inducing the glyoxalase system activity.35
Other amino acids
(4S)-4-Hydroxyisoleucine (Fig. 3(a)), first reported as a peculiar insulinotropic amino acid36 extracted from fenugreek seeds, is not found in mammalian tissues. Exerting a direct effect on pancreatic β-cells to stimulate insulin secretion, thereby causing hypoglycaemia, 4-hydroxyisoleucine was found to cause no change in pancreatic glucagon or somatostatin secretions. Importantly, the hypoglycaemic action of 4-hydroxyisoleucine was observed only in a state of high glucose concentration.36,37
Fig. 3. (4S)-4-Hydroxyisoleucine (a) and l-carnitine (b).

l-Carnitine (β-hydroxy-γ-trimethylamino butyrate, Fig. 3(b)) is widespread in mammalian plasma and tissues. l-Carnitine exhibits a higher anti-glycation effect than aminoguanidine (AG), acting by multiple modes including exerting anti-oxidant activity, enhancing glucose utilization, limiting methyl glyoxal formation and accumulation through activation of the glyoxalase I and II system in the cytosol and chelation of metal ions.38
Non-natural amino acids
From a series of urea/thiourea derivatives containing glycine- or proline-conjugated 6-fluoro-3-(4-piperidinyl)benzisoxazole (Fig. 4(a)), the Gly-containing compounds exhibited higher anti-glycation activity than the Pro-containing analogues, possibly due to enhanced interaction owing to their smaller size and simpler nature. The thiourea derivatives were found to be more active than the ureas. The greater nucleophilicity of sulfur (when compared to oxygen) could be responsible for this effect. Compounds containing –OCH3 and –Br substitutions were active inhibitors of glycation. Trapping of dicarbonyl species and preventing oxidation was the plausible mechanism of action suggested.39 Further, four series of amino acid (Glu, Tyr, Lys and Phe) dichlorophenylpiperazine-conjugated urea/thiourea derivatives (Fig. 4(b)) were reported.40 Compounds containing Glu (acidic) and Tyr (phenolic) showed higher anti-glycation activity when compared to Lys (basic) and Phe (phenyl)-containing analogues. In this latter study, unlike the previous report, there was no difference in the activity exhibited by urea or thiourea derivatives. However, electronegative substituents like halogens on the aromatic ring played a role in enhancing the anti-glycation activity of the resulting compounds.
Fig. 4. Urea/thiourea derivatives of amino acid-conjugated 6-fluoro-3-(4-piperidinyl)benzisoxazole (a) and 1-(2,3-dichlorophenyl)piperazine (b) heterocycles.
The synthesis, anti-glycation and anti-oxidant properties of a series of N-(3-aminoalkyl)proline derivatives was recently reported.41 These non-natural amino acid derivatives were shown to be capable of exerting good anti-oxidant properties in addition to anti-glycation. The compounds also displayed low cytotoxicity, enhancing their value as potential anti-glycation agents. The observed anti-glycation effect was due to the probable formation of a Schiff's base with glucose (Fig. 5).
Fig. 5. Probable mechanism for anti-glycation effect of a representative N-(3-aminoalkyl)proline derivative.
Peptides as anti-glycation agents
Proteins provide a rich source of biologically active peptides. Some peptides, that are largely inactive within the parent protein, exert their activity upon release by the action of proteolytic enzymes.42 The activity largely depends on the inherent amino acid composition and sequence, the length of active sequence varying from two to twenty amino acid residues.43 Thus peptides may act as alternatives to small molecule drugs offering many advantages such as high bioactivity and biospecificity to targets, wide spectrum of therapeutic action, low levels of toxicity, structural diversity and absence or low levels of accumulation in body tissues.44
Dipeptides
The naturally-occurring dipeptide, carnosine (β-alanyl-l-histidine), present in many organisms in muscle and nervous tissues, has been demonstrated to possess anti-glycation activity. Though multiple mechanisms are probably involved, carnosine was found to inhibit AGE formation by quenching reactive carbonyl species.45 Modifications at the carboxyl terminus or the β-alanine carbon skeleton were found to improve the pharmacokinetic profile without drastically affecting the quenching activity. A C-terminus capping strategy resulted in improved plasma stability as recognition by enzymes was hampered. Conversely, the primary amino group and the imidazole ring were found to play important roles in the observed activity and modifying them greatly reduced the efficiency and/or selectivity towards reactive carbonyl species.46 Carnosine is also capable of effecting transglycation.47 Carnosine may play a role in assisting the unfolding of damaged proteins and in solubilizing precipitated protein aggregates, possibly through disruption of complementary binding between AGE-related hydrophobic patches.48
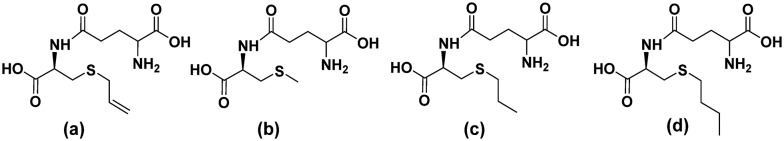
γ-Glutamyl-S-allyl-cysteine (GSAC, Fig. 6(a)) is a peptide isolated from fresh garlic scales. GSAC attenuated the Maillard reaction during the initial and late stages of glucose-induced protein damage. It significantly reduced structural and functional impairments in proteins caused due to glycation and blocked the exposed hydrophobic surfaces of proteins. Additional iron ion-chelating and radical-scavenging capacities of the peptide also contribute to its anti-glycation mechanism.49
Fig. 6. Organosulfur-containing anti-glycation dipeptides – (a) γ-glutamyl-S-allyl-cysteine (GSAC), (b) γ-glutamyl-methyl-cysteine (γ-GMC) and (c) γ-glutamyl-propyl-cysteine (γ-GPC) from fresh garlic and synthetically analogous (d) γ-glutamyl-butyl-cysteine (γ-GBC).
The anti-glycation behavior of γ-glutamylmethylcysteine (γ-GMC) and γ-glutamylpropylcysteine (γ-GPC), extracted from fresh garlic and synthetically prepared γ-glutamylbutylcysteine (γ-GBC) (Fig. 6(b)–(d) respectively) was attributed to their radical scavenging property.50 This scavenging activity was found to decrease in the order γ-GPC > γ-GMC > γ-GBC.
A computer-aided simulation study of yam dioscorin pepsin hydrolysis resulted in various peptides, of which, the tryptophan-containing dipeptide, Asn-Trp (NW) and its analogue, Gln-Trp (QW), were synthesized and found to possess anti-glycation properties, with NW showing higher activity than QW. Additionally, studies showed that both these dipeptides reduced in vitro AGE formation in BSA, although the mechanism is unclear, and could also reduce the production of ROS.51
Longer peptides
Animal-derived natural peptides
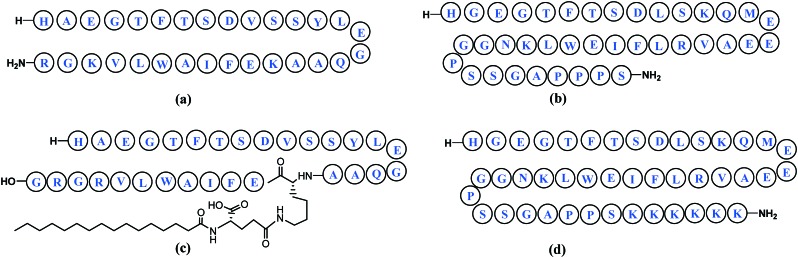
Insulin52 (Fig. 7) is highly effective in lowering extremely high glucose levels.53 Insulin binding to its receptors on insulin-sensitive cells results in activation of a tyrosine kinase which is present intrinsic to the receptor, triggering a signaling cascade and leading to the translocation of glucose transporters from an intracellular location to the cell membrane, which results in internalization and utilization of glucose.54 While managing hyperglycaemia has greatly improved in recent years, newer strategies focusing on aggressive glucose control have emerged since traditional insulin products do not provide optimal therapy.55 In spite of their clinical superiority, fundamental safety issues pertaining to these newer analogues are of concern.56
Fig. 7. Human insulin depicted using one-letter amino acid codes. Disulfide linkages are shown in blue.
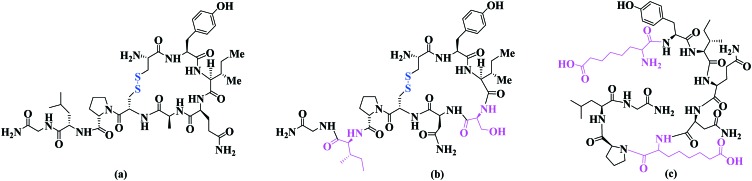
Safavi-Hemami and co-workers were the first group to report the presence of insulin (Fig. 8) in the venom of cone snails of the genus Conus.57 The study revealed a class of insulin that acted rapidly and potently to cause severe hypoglycaemia.
Fig. 8. Amino acid sequence of Conus insulin with inter- and intra-chain disulfide bridges shown in blue.
Oxytocin (Fig. 9(a)), a neuropeptide that plays an important role in labour and lactation, is also known to promote glucose uptake and stimulate insulin secretion.58–60 Oxytocin analogue [Ser4, Ile8]-oxytocin (Fig. 9(b)) was demonstrated to be superior to native oxytocin in combating glucose intolerance, while another analogue, [Asu1,6]-oxytocin, where Asu is aminosuberic acid (Fig. 9(c)), was found to have similar therapeutic efficacy as oxytocin.58 The improved activity of [Ser4, Ile8]-oxytocin was suggested to be a consequence of stronger α-helical structure, and consequently, a more stable peptide conformation as a result of Gln → Ser substitution at amino acid position 4.
Fig. 9. Oxytocin (a), [Ser4, Ile8]-oxytocin (b) and [Asu1,6]-oxytocin (c).
Plant-derived natural peptides
The purified leaf extract of Costus igneus was shown to contain an insulin-like peptide.61 Interestingly, oral administration of this peptide resulted in better hypoglycaemic activity than intraperitoneal. The hypoglycaemic action was found to occur via the insulin signaling pathway.62
A novel 68-residue insulin receptor (IR) binding protein was identified from the extract of Momordica charantia seeds. The protein was found to bind to the insulin receptor at a site distinct from the insulin-binding site, and could trigger the insulin signal transduction pathway and subsequently lead to reduced hyperglycaemia by enhancing the glucose uptake.63,64 Residues 50–68 were found to constitute the IR-binding-motif, RVRVWVTERGIVARPPTIG.65
Three short peptides from oat hydrolysates-FLQPNLDEH, DLELQNNVFPH and TPNAGVSGAAAGAGAGGKH were reported to reduce hyperglycaemia.66 These peptides exerted their action by stimulating insulin secretion and insulin sensitivity, and elevating glycogenesis in streptozotocin-induced diabetic mice.
Non-natural peptides
In 2015, a series of analogues of the N-terminal decameric peptide GHPYYSIKKS from Momordica charantia L. Var. abbreviata Ser. (MCV) were designed, synthesized and evaluated for anti-hyperglycaemic activity, as observed for the parent peptide.67 This peptide is distinct from the anti-glycating IR-binding peptide from Momordica charantia described above, and its mechanism of action is not fully understood. Alanine (Ala) scanning of the parent peptide revealed that the amino acids Pro, Ser, Ile and Ser at positions 3, 6, 7 and 10 respectively, were not necessary for activity. Conformationally constrained analogues of this peptide with a lactam bridge through a Glu-Xaa-Lys scaffold at positions 3, 6 or 10 were found to have increased activity, while introduction of the scaffold at position 7 led to a contrasting effect.67 Di-proline (-Pro-Pro-) segments, known to be templates for nucleation of folded structures in designed peptides,68 were further introduced into the peptide at the positions 3, 6, 7 or 10. All the di-proline-containing peptides were found to lower the blood glucose level compared to untreated control mice, with the peptide containing the di-proline segment at position 7 exhibiting significantly higher activity than the parent peptide.67
A synthetic selenocysteine analogue of Conus insulin, containing a diselenide bond as replacement of the intra-chain disulfide bond (Fig. 10(a)), was found to exhibit activity similar to human insulin.57 Two analogues of insulin glargine (Fig. 10(b)) containing a 1, 4-disubstituted 1,2,3-triazole group were synthesized to replace the disulfide bridge at positions 7 of the A- and B-chain (Fig. 10(c)) using Cu-assisted azide-alkyne ‘Click’ (CuAAC) chemistry to efficiently join the peptide chains.69 However CD studies showed that this modification altered the folding pattern of the native form, resulting in a loss in activity.
Fig. 10. (a) Seleno-cysteine analogue of Conus insulin, (b) insulin and insulin glargine, (c) Triazole analogue of insulin glargine.
Incretins, a group of hormones, stimulate a decrease in blood glucose levels and comprise mainly glucagon-like peptide-170 (GLP-1, Fig. 11(a)) and gastric inhibitory peptide or glucose-dependent insulinotropic polypeptide (GIP). The incretin effect may account for 50–70% of total insulin secretion after food intake, playing a crucial role in the maintenance of glycemic control. Incretin mimetics have been intensively developed71 in anti-diabetic therapies for providing effective glucose control, improved beta-cell function, body weight loss, and lowering of systolic blood pressure.72 Clinically, GLP-1 has been reported to be very effective in lowering blood glucose levels with very little risk of hypoglycaemia.73 However, due to rapid inactivation of GLP-1 by a few dominant enzymes like dipeptidyl peptidase IV (DPP IV)74 and a short lifespan in vivo (t1/2 ≈ 2 min), its effectiveness75 is severely restricted, creating a necessity for the development of longer-acting derivatives.76
Fig. 11. (a) Glucagon-like peptide-1 (GLP-1)70 and mimetics (b) exenatide,77 (c) liraglutide72 and (d) lixisenatide.81.
Exenatide77 a synthetic analogue of exendin-4,78 a 39-amino acid peptide produced by the salivary glands of the Gila monster (Heloderma suspectrum)79 (Fig. 11(b)), liraglutide72 (Fig. 11(c)), lixisenatide80,81 (Fig. 11(d)), albiglutide, semaglutide, dulaglutide, langlenatide, VRS-859 and CJC-1134-PC are some known GLP-1 agonists.70 These mimics differ from GLP-1 through the addition of amino acids or other functions, in attempts to increase their stability. Currently GLP-1 receptor agonists available in the market are derived from either the GLP-1 or Exendin-4 backbone.82 Other exendin-4 analogues reported in literature to possess glucose-lowering potency are conjugates with 2-sulfo-9-fluorenylmethoxycarbonyl group,83 biotin,84 hyaluronate (HA),85 carbohydrate moieties (glycosylated analogues),86 lithocholic acid,87 fatty acids (lauric acid or palmitic acid),88 truncated Evans Blue dye,89etc.
Further to the discovery of Xenopus GLP-1 peptides with potent GLP-1 receptor activation and insulinotropic activities,90 new Xenopus GLP-1 analogues have been reported in literature, such as site-specific mycophenolic acid-modified analogues with differing length of fatty acids,91 and PEGylated analogues,92 to name a few.
Thus, it is clear that stable GLP-1 peptide analogues with a long half-life would serve as an efficient therapy option for diabetes.73 Some noteworthy examples are described in Table 1.
Table 1. Some stable GLP-1 peptide analogues/GLP-1 receptor (GLP-1R) agonists.
| Sr. no | Study by | Study details | Ref. |
| 1 | Deacon et al. | N-terminal substitutions with amino acid residues such as threonine, glycine, serine, α-amino isobutyric acid | 93 |
| 2. | Knudsen et al. | Derivatisation with linear fatty acids up to the length of 16 carbon atoms or longer, almost anywhere in the C-terminal region | 94 |
| 3. | Kaiser et al. | Both the N- and C-termini engineered; l-Ala 8 replaced by d-alanine and Arg 36 deleted | 95 |
| 4. | Knudsen et al. | Fatty acid derivatisation and study of effect of polarity and bulkiness | 96 |
| 5. | Youn et al. | Site-specific PEGylation | 74 |
| 6. | Miranda et al. | Evaluation of the effect of helix-favouring amino acid residue substitutions (α-amino-isobutyric acid) and incorporation of a lactam bridge | 97 |
| 7. | Ueda et al. | Glycosylated analogues | 98 |
| 8. | Mapelli et al. | Optimisation of 9-mer peptide closely related to the N-terminal; substituted C-terminal biphenyl dipeptide | 99 |
| 9. | Haque et al. | Study involving 11-amino acid-containing non-natural peptides, including analogues with homohomophenylalanine at the C-terminal | 100 |
| 10. | Murage et al. | Multiple lactam bridges that stabilized both the N- and C-terminal α-helices simultaneously | 101 |
| 11. | Han et al. | Dicoumarol conjugates | 75 |
| 12. | Johnson et al. | Strategic replacement of native α-residues with conformationally constrained β-amino acid residues | 102 |
| 13. | Yang et al. | Structural modification at C-terminal with poly lysine, poly serine and poly valine | 103 |
| 14. | Hoang et al. | Cyclic constraints containing lactam bridges in short hydrophobic peptides | 104 |
Amino acids/peptides in clinical trials for anti-glycation activity
Since the discovery of insulin, its introduction in clinical practice has revolutionized the management of blood glucose levels.77 Newer insulin analogues like long-acting analogues (glargine, detemir) and rapid-acting analogues (lispro, aspart, glulisine), with subtle changes in their structure, have successfully made it beyond clinical trials and are commercially available in the market.56 To date, a few stable GLP-1 agonists have received FDA approval as anti-diabetic drugs while some are in clinical trials.69 Exenatide (approved by the US FDA as the first in the new class of incretin mimic drugs),78 liraglutide,72 albiglutide,70 semaglutide,105 dulaglutide,106etc., that act through the incretin effect, having overcome the clinical limitations of GLP-1, have received FDA approval and are currently available in the market. GLP-1 receptor agonists in clinical trials include, langlenatide, VRS-859, CJC-1134-PC, among others.70 As for amino acids, a double blind pilot clinical trial exhibited that oral supplementation with free amino acids for patients with type 2 diabetes mellitus appeared to decrease post-prandial plasma glucose without any change in plasma insulin levels.21 However, no further studies were reported, and to the best of our knowledge, no amino acid or derivative has been approved by the FDA as yet anti glycation.
Discussion
It is evident that the emergence of AGEs and associated complications is a consequence of persistent hyperglycaemia. This may be countered by multiple approaches – [1] increasing the glucose utilization/uptake, which in turn, could be achieved by increasing insulin sensitivity or stimulating its secretion (insulin secretagogues) or increasing its activity and production (insulinotropic agents). This may be achieved by insulin and analogues, which includes those that bind allosterically to the insulin receptor. [2] Decreasing gluconeogenesis. [3] Suppressing glucagon secretion. [4] Decreasing the extent of AGE formation in proteins, through competitive binding to glucose or reactive carbonyls. [5] Reduction in AGE formation through transglycation or reversal of glycation. [6] Shielding the exposed regions of proteins from exposure to sugars. [7] Preventing protein aggregation by increasing solubilization. [8] Ion-chelating. [9] Through the associated anti-oxidant properties of the agents. The heterogeneity of the AGE products, ranging from well-defined structures such as protein-linked – or free pentosidine and CML to ill-characterized AGE peptides, makes the study of their fate in vivo a difficult task.107 As a consequence, little attention has been paid to AGE disposal from the body.
The amino acids and derivatives prevent the formation of AGEs predominantly through competitive Schiff base formation by their amino groups, rather than the amino groups of proteins, with the carbonyls of sugars, trapping of reactive carbonyls, radical scavenging and through their anti-oxidant properties. These effects are more of a general nature. More specific mechanisms by which amino acids and derivatives act to prevent glycation are by binding to the insulin receptor (taurine, glycine) and acting as insulin secretagogues (glycine, cysteine). 4-Hydroxy-isoleucine is reported to be insulinotropic, while hypoglycin A indirectly effects anti-glycation through causing lower glucose levels by decreasing gluconeogenesis as a result of impaired long chain fatty acid metabolism. Some amino acids such as arginine also help to counter the effects of glycation through prevention of protein aggregation and increasing solubilization. Aspartic acid could shield the exposed hydrophobic regions of proteins from exposure to sugars and thus, from potential aggregation. Many of the amino acids and derivatives also possess considerable anti-oxidant activity, contributing to their potential utility value against glycation. The dipeptide carnosine is known to act by multiple mechanisms including an ion-chelating effect. Chelation of transition metal ions such as copper and iron by synthetic or natural compounds is one of the promising mechanisms for the inhibition of AGEs formation because transition metals, in presence of oxygen, catalyze autoxidation of glucose or lipid peroxidation and also assist the formation of oxygen free radicals such as hydroxyl radicals during Fenton reaction in states of hyperglycaemia.108 Carnosine is also involved in neutralization of reactive carbonyls, preventing protein aggregation and in the reversal of glycation through transglycation. The dipeptide GSAC was found to effectively shield exposed hydrophobic regions of proteins, preventing their aggregation, in addition to inhibiting early and late-stage Maillard reactions between protein and sugars, and having a radical-scavenging ability, this last property being shared with other dipeptides from garlic – γ-GMC and γ-GPC.
The anti-glycation effect of peptides described herein is more specific in nature, and is mostly insulin-related. Thus peptides and synthetic analogues could act as insulin secretagogues (oxytocin and oat hydrolysate peptides), bind to insulin receptor (insulin and analogues and insulin receptor-binding protein from Momordica charantia) and/or cause increased glucose uptake and utilization. Alternatively, peptides could act through the incretin effect, as incretin mimetics (e.g., GLP-1 mimetics), to bring about a decrease in the levels of blood glucose.
Scope for further development
The present review summarizes findings on the efficacy of some natural and non-natural amino acids and peptides to reduce, delay or even prevent complications that arise due to hyperglycaemia. They can be used to reduce glycaemia directly by inhibiting various stages of the Maillard reaction, while some of them act as insulin secretagogues or incretin mimetics, thereby reducing glucose levels. Considering the varied modes of action of the various amino acids and peptides described herein, it appears that application of a combination of mechanistic approaches would be desirable. Thus, the potency of amino acids in swiftly reducing the reactive sugar concentrations by Schiff base formation, for example, would benefit from being coupled to more specific receptor-based approaches, such as those involved in glucose uptake and utilization. If these can also be expanded to include anti-oxidant properties and prevention of protein aggregation, one can expect significantly improved anti-glycation, both in terms of potency as well as specificity. We believe that these would constitute directions for future research in this area, thus contributing to the already growing therapeutic appeal of peptides.
Conflicts of interest
The authors have no conflicts of interest to declare.
Acknowledgments
Research funding from CSIR project CSC0111 is gratefully acknowledged. CH acknowledges the Department of Science and Technology, Government of India for Women Scientist Scheme (SR/WOS-A/CS-44/2016).
Biographies

Harsha Chilukuri
Harsha Chilukuri obtained her Master's degree in Pharmaceutical Chemistry and is currently pursuing her doctoral studies from CSIR-National Chemical Laboratory (CSIR-NCL), Pune, India. She is a recipient of DST-Women Scientists Scheme-A (WOS-A). Her work comprises the design and synthesis of small molecules and peptides with therapeutic potential.

Mahesh J. Kulkarni
Mahesh J. Kulkarni is a Senior Scientist at CSIR-NCL, Pune, India. He obtained his Ph.D. degree from the University of Agricultural Sciences, Bengaluru, India. For the last 10 years, he has been working in the area of mass spectrometry and proteomics. The major focus of his research is to understand the role of advanced glycation end products (AGEs) in the development of diabetic complications. The long-term goal is to identify a diagnostic marker for diabetic complications, identify drug targets and develop intervention strategies.

Moneesha Fernandes
Moneesha Fernandes is a Senior Scientist at CSIR-NCL, Pune, India. Her research interests are in the biomolecular chemistry of peptides, nucleic acids and analogues. Work in her lab focuses on bio-organic chemistry approaches aimed towards the therapeutic and/or diagnostic application of peptides and nucleic acids.
References
- Frolov A., Schmidt R., Spiller S., Greifenhagen U., Hoffmann R., Agric J. Food Chem. 2014;62:3626–3635. doi: 10.1021/jf4050183. [DOI] [PubMed] [Google Scholar]
- Schmidt A. M., Hori O., Brett J., Yan S. D., Wautier J. L., Stern D. Arterioscler., Thromb., Vasc. Biol. 1994;14:1521–1528. doi: 10.1161/01.atv.14.10.1521. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Ames J. M., Smith R. D., Baynes J. W., Metz T. O. J. Proteome Res. 2009;8:754–769. doi: 10.1021/pr800858h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur H., Kamalov M., Brimble M. A. Acc. Chem. Res. 2016;49:2199–2208. doi: 10.1021/acs.accounts.6b00366. [DOI] [PubMed] [Google Scholar]
- Singh R., Barden A., Mori T., Beilin L. Diabetologia. 2001;44:129–146. doi: 10.1007/s001250051591. [DOI] [PubMed] [Google Scholar]
- Ramasamy R., Yan S. F., Schmidt A. M. Ann. N. Y. Acad. Sci. 2011;1243:88–102. doi: 10.1111/j.1749-6632.2011.06320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongarzone S., Savickas V., Luzi F., Gee A. D. J. Med. Chem. 2017;60:7213–7232. doi: 10.1021/acs.jmedchem.7b00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz G. Trends Biochem. Sci. 2011;36:625–632. doi: 10.1016/j.tibs.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Peng X., Ma J., Chen F., Wang M. Food Funct. 2011;2:289–301. doi: 10.1039/c1fo10034c. [DOI] [PubMed] [Google Scholar]
- Chinchansure A. A., Korwar A. M., Kulkarni M. J., Joshi S. P. RSC Adv. 2015;5:31113–31138. [Google Scholar]
- Alam S., Ahsan A., Alam S. J. Biochem. Technol. 2013;5:666–672. [Google Scholar]
- Seo S., Karboune S. J. Agric. Food Chem. 2014;62:12235–12243. doi: 10.1021/jf502497r. [DOI] [PubMed] [Google Scholar]
- Reddy V. P., Beyaz A. Drug Discovery Today. 2006;11:646–654. doi: 10.1016/j.drudis.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Awasthi S., Saraswathi N. T. RSC Adv. 2016;6:24557–24564. [Google Scholar]
- Shantharam C. S., Vardhan D. M. S., Suhas R., Sridhara M. B., Gowda D. C. Eur. J. Med. Chem. 2013;60:325–332. doi: 10.1016/j.ejmech.2012.12.029. [DOI] [PubMed] [Google Scholar]
- Han C. H., Lin Y. S., Lin S. Y., Hou W. C. Food Chem. 2014;147:195–202. doi: 10.1016/j.foodchem.2013.09.109. [DOI] [PubMed] [Google Scholar]
- Shi F., Bai B., Ma S., Ji S., Liu L. Food Chem. 2016;194:538–544. doi: 10.1016/j.foodchem.2015.07.140. [DOI] [PubMed] [Google Scholar]
- Wu G. Amino Acids. 2009;37:1–17. doi: 10.1007/s00726-009-0269-0. [DOI] [PubMed] [Google Scholar]
- Anuradha C. V. Curr. Protein Pept. Sci. 2009;10:8–17. doi: 10.2174/138920309787315194. [DOI] [PubMed] [Google Scholar]
- Ramakrishna S., Sulochana K. N. Exp. Eye Res. 1993;57:623–628. doi: 10.1006/exer.1993.1167. [DOI] [PubMed] [Google Scholar]
- Sulochana K. N., Lakshmi S., Punitham R., Arokiasamy T., Sukumar B., Ramakrishnan S. Med. Sci. Monit. 2002;8:CR131–CR137. [PubMed] [Google Scholar]
- Sensi M., Pricci F., De Rossi M. G., Morano S., Di Mario U. Clin. Chem. 1987;35:384–387. [PubMed] [Google Scholar]
- Fan X., Xiaoqin L., Potts B., Strauch C. M., Nemet I., Monnier V. M. Mol. Vision. 2011;17:2221–2227. [PMC free article] [PubMed] [Google Scholar]
- Méndez J. D., Leal L. I. Biomed. Pharmacother. 2004;58:598–604. doi: 10.1016/j.biopha.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Servetnick D. A., Bryant D., Wells-Knecht K. J., Wiesenfeld P. L. Amino Acids. 1996;11:69–81. doi: 10.1007/BF00805722. [DOI] [PubMed] [Google Scholar]
- Prasanna G., Saraswathi N. T. J. Biomol. Struct. Dyn. 2016;34:943–951. doi: 10.1080/07391102.2015.1060160. [DOI] [PubMed] [Google Scholar]
- Alvarado-Vásquez N., Zamudio P., Ceron E., Vanda B., Zenteno E., Sandoval G. C. Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol. 2003;134:521–527. doi: 10.1016/s1532-0456(03)00046-2. [DOI] [PubMed] [Google Scholar]
- Bahmani F., Bathaie S. Z., Aldavood S. J., Ghahghaei A. Mol. Vision. 2012;18:439–448. [PMC free article] [PubMed] [Google Scholar]
- Noe S. A., Mario G. L., Reyes G. D., Iván V. J. E., Javier A. A. F., Luis G. O. J. J. Exp. Clin. Med. 2013;5:109–114. [Google Scholar]
- Tanaka K. J. Biol. Chem. 1972;247:7465–7478. [PubMed] [Google Scholar]
- Manna P., Das J., Sil P. C. Curr. Diabetes Rev. 2013;9:237–248. doi: 10.2174/1573399811309030005. [DOI] [PubMed] [Google Scholar]
- Nandhini A. T. A., Thirunavukkarasu V., Anuradha C. V. Indian J. Med. Res. 2005;122:171–177. [PubMed] [Google Scholar]
- Maturo J., Kulakowski E. C. Biochem. Pharmacol. 1988;37:3755–3760. doi: 10.1016/0006-2952(88)90411-x. [DOI] [PubMed] [Google Scholar]
- Devamanoharan P. S., Ali A. H., Varma S. D. Mol. Cell. Biochem. 1997;177:245–250. doi: 10.1023/a:1006863322454. [DOI] [PubMed] [Google Scholar]
- Mahdavifard S., Bathaie S. Z., Nakhjavani M., Heidarzadeh H. Food Res. Int. 2014;62:909–916. [Google Scholar]
- Sauvaire Y., Petit P., Broca C., Manteghetti M., Baissac Y., Alvarez J. F., Gross R., Roye M., Leconte A., Gomis R., Ribes G. Diabetes. 1998;47:206–210. doi: 10.2337/diab.47.2.206. [DOI] [PubMed] [Google Scholar]
- Broca C., Manteghetti M., Gross R., Baissac Y., Jacob M., Petit P., Sauvaire Y., Ribes G. Eur. J. Pharmacol. 2000;390:339–345. doi: 10.1016/s0014-2999(00)00030-3. [DOI] [PubMed] [Google Scholar]
- Rajasekar P., Anuradha C. V. Acta Diabetol. 2007;44:83–90. doi: 10.1007/s00592-007-0247-5. [DOI] [PubMed] [Google Scholar]
- Shantharam C. S., Vardhan D. M. S., Suhas R., Sridhara M. B., Gowda D. C. Eur. J. Med. Chem. 2013;60:325–332. doi: 10.1016/j.ejmech.2012.12.029. [DOI] [PubMed] [Google Scholar]
- Vardhan D. M. S., Shantharam C. S., Suhas R., Gowda D. C. J. Saudi Chem. Soc. 2017;21:S248–S257. [Google Scholar]
- Chilukuri H., Kolekar Y. M., Bhosle G. S., Godbole R. K., Kazi R. S., Kulkarni M. J., Fernandes M. RSC Adv. 2015;5:77332–77340. [Google Scholar]
- Korhonen H., Pihlanto A. Int. Dairy J. 2006;16:945–960. doi: 10.1016/j.idairyj.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisel H., Fitz Gerald R. J. Curr. Pharm. Des. 2003;9:1289–1295. doi: 10.2174/1381612033454847. [DOI] [PubMed] [Google Scholar]
- Agyei D., Danquah M. K. Biotechnol. Adv. 2011;29:272–277. doi: 10.1016/j.biotechadv.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Pepper E. D., Farrell M. J., Nord G., Finkel S. E. Appl. Environ. Microbiol. 2010;76:7925–7930. doi: 10.1128/AEM.01369-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vistoli G., De Maddis D., Straniero V., Pedretti A., Pallavicini M., Valoti E., Carini M., Testa B., Aldini G. Eur. J. Med. Chem. 2013;66:153–160. doi: 10.1016/j.ejmech.2013.05.009. [DOI] [PubMed] [Google Scholar]
- Szwergold B. S. Biochem. Biophys. Res. Commun. 2005;336:36–41. doi: 10.1016/j.bbrc.2005.08.033. [DOI] [PubMed] [Google Scholar]
- Seidler N. W., Yeargans G. S., Morgan T. G. Arch. Biochem. Biophys. 2004;427:110–115. doi: 10.1016/j.abb.2004.04.024. [DOI] [PubMed] [Google Scholar]
- Tan D., Zhang Y., Chen L., Liu L., Zhang X., Wu Z., Bai B., Ji S. Nat. Prod. Res. 2015;29:2219–2222. doi: 10.1080/14786419.2014.1003065. [DOI] [PubMed] [Google Scholar]
- Shi F., Bai B., Ma S., Ji S., Liu L. Food Chem. 2016;194:538–544. doi: 10.1016/j.foodchem.2015.07.140. [DOI] [PubMed] [Google Scholar]
- Han C. H., Lin Y. S., Lin S. Y., Hou W. C. Food Chem. 2014;147:195–202. doi: 10.1016/j.foodchem.2013.09.109. [DOI] [PubMed] [Google Scholar]
- Banting F. G., Best C. H., Collip J. B., Campbell W. R., Fletcher A. A. Can. Med. Assoc. J. 1991;145:1281–1286. [PMC free article] [PubMed] [Google Scholar]
- Hirsch I. B., Bergenstal R. M., Parkin C. G., Wright Jr. E., Buse J. B. Clin. Diabetes. 2005;23:78–86. [Google Scholar]
- Kahn C. R. Annu. Rev. Med. 1985;36:429–451. doi: 10.1146/annurev.me.36.020185.002241. [DOI] [PubMed] [Google Scholar]
- Hartman I. Clin. Med. Res. 2008;6:54–67. doi: 10.3121/cmr.2008.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valla V., Exp. Diabetes Res., 2010, 2010 , 178372 10.1155/2010/178372 , , 14 pages . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safavi-Hemami H., Gajewiak J., Karanth S., Robinson S. D., Ueberheide B., Douglass A. D., Schlegel A., Imperial J. S., Watkins M., Bandyopadhyay P. K., Yandell M., Li Q., Purcell A. W., Norton R. S., Ellgaard L., Olivera B. M. Proc. Natl. Acad. Sci. U. S. A. 2015;112:1743–1748. doi: 10.1073/pnas.1423857112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Wu C., Chen Q., Chen X., Xu Z., Wu J., Cai D. PLoS One. 2013;8:e61477. doi: 10.1371/journal.pone.0061477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E. S., Uhm K.-O., Lee Y. M., Kwon J., Park S.-H., Soo K. H. Regul. Pept. 2008;151:71–74. doi: 10.1016/j.regpep.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Elabd S., Sabry I. Front. Endocrinol. 2015;6:121. doi: 10.3389/fendo.2015.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi B. N., Munot H., Hardikar M., Kulkarni A. A. Biochem. Biophys. Res. Commun. 2013;436:278–282. doi: 10.1016/j.bbrc.2013.05.093. [DOI] [PubMed] [Google Scholar]
- Hardikar M. R., Varma M. E., Kulkarni A. A., Kulkarni P. P., Joshi B. N. Phytochemistry. 2016;124:99–107. doi: 10.1016/j.phytochem.2016.02.001. [DOI] [PubMed] [Google Scholar]
- Lo H.-Y., Ho T.-Y., Lin C., Li C.-C., Hsiang C.-Y. J. Agric. Food Chem. 2013;61:2461–2468. doi: 10.1021/jf3042402. [DOI] [PubMed] [Google Scholar]
- Lo H.-Y., Ho T.-Y., Li C.-C., Chen J.-C., Liu J.-J., Hsiang C.-Y. J. Agric. Food Chem. 2014;62:8952–8961. doi: 10.1021/jf5002099. [DOI] [PubMed] [Google Scholar]
- Lo H.-Y., Li C.-C., Ho T.-Y., Hsiang C.-Y. Food Chem. 2016;204:298–305. doi: 10.1016/j.foodchem.2016.02.135. [DOI] [PubMed] [Google Scholar]
- Zhang H., Wang J., Liu Y., Sun B. J. Biomed. Sci. 2015;4:1–7. [Google Scholar]
- Yang B., Li X., Zhang C., Yan S., Wei W., Wang X., Deng X., Qian H., Lin H., Huang W. Org. Biomol. Chem. 2015;13:4551–4561. doi: 10.1039/c5ob00333d. [DOI] [PubMed] [Google Scholar]
- Chatterjee B., Saha I., Raghotama S., Aravinda S., Rai R., Shamala N., Balaram P. Chem. – Eur. J. 2008;14:6192–6204. doi: 10.1002/chem.200702029. [DOI] [PubMed] [Google Scholar]
- Williams G. M., Lee K., Li X., Cooper G. J. S., Brimble M. A. Org. Biomol. Chem. 2015;13:4059–4063. doi: 10.1039/c5ob00160a. [DOI] [PubMed] [Google Scholar]
- Manandhar B., Ahn J.-M. J. Med. Chem. 2015;58:1020–1037. doi: 10.1021/jm500810s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son S., Chae S. Y., Kim C. W., Choi Y. G., Jung S. Y., Lee S., Lee K. C. J. Med. Chem. 2009;52:6889–6896. doi: 10.1021/jm901153x. [DOI] [PubMed] [Google Scholar]
- Lau J., Bloch P., Schaffer L., Pettersson I., Spetzler J., Kofoed J., Madsen K., Knudsen L. B., McGuire J., Steensgaard D. B., Strauss H. M., Gram D. X., Knudsen S. M., Nielsen F. S., Thygesen P., Reedtz-Runge S., Kruse T. J. Med. Chem. 2015;58:7370–7380. doi: 10.1021/acs.jmedchem.5b00726. [DOI] [PubMed] [Google Scholar]
- Knudsen L. B. J. Med. Chem. 2004;47:4128–4134. doi: 10.1021/jm030630m. [DOI] [PubMed] [Google Scholar]
- Youn Y. S., Chae S. Y., Lee S., Jeon J. E., Shin H. G., Lee K. C. Biochem. Pharmacol. 2007;73:84–93. doi: 10.1016/j.bcp.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Han J., Sun L., Chu Y., Li Z., Huang D., Zhu X., Qian H., Huang W. J. Med. Chem. 2013;56:9955–9968. doi: 10.1021/jm4017448. [DOI] [PubMed] [Google Scholar]
- Garber A. J. Diabetes Care. 2011;34:S279–S284. doi: 10.2337/dc11-s231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechenov S., Bhattacharjee H., Yin D., Mittal S., Subramony J. A. Adv. Drug Delivery Rev. 2017;112:106–122. doi: 10.1016/j.addr.2017.01.006. [DOI] [PubMed] [Google Scholar]
- Davidson M. B., Bate G., Kirkpatrick P. Nat. Rev. Drug Discovery. 2005;4:713–714. doi: 10.1038/nrd1828. [DOI] [PubMed] [Google Scholar]
- Evers A., Haack T., Lorenz M., Bossart M., Elvert R., Henkel B., Stengelin S., Kurz M., Glien M., Dudda A., Lorenz K., Kadereit D., Wagner M. J. Med. Chem. 2017;60:4293–4303. doi: 10.1021/acs.jmedchem.7b00174. [DOI] [PubMed] [Google Scholar]
- Christensen M., Knop F. K., Holst J. J., Vilsboll T. IDrugs. 2009;12:503–513. [PubMed] [Google Scholar]
- Han J., Fei Y., Zhou F., Chen X., Zheng W., Fu J. Mol. Pharmaceutics. 2017;14:3954–3967. doi: 10.1021/acs.molpharmaceut.7b00632. [DOI] [PubMed] [Google Scholar]
- Park E. J., Lim S. M., Lee K. C., Na D. H. Expert Opin. Ther. Pat. 2016;26:833–842. doi: 10.1080/13543776.2016.1192130. [DOI] [PubMed] [Google Scholar]
- Shechter Y., Tsubery H., Fridkin M. Biochem. Biophys. Res. Commun. 2003;305:386–391. doi: 10.1016/s0006-291x(03)00715-0. [DOI] [PubMed] [Google Scholar]
- Jin C.-H., Chae S. Y., Son S., Kim T. H., Um K. A., Youn Y. S., Lee S., Lee K. C. J. Controlled Release. 2009;133:172–177. doi: 10.1016/j.jconrel.2008.09.091. [DOI] [PubMed] [Google Scholar]
- Kong J.-H., Oh E. J., Chae S. Y., Lee K. C., Hahn S. K. Biomaterials. 2010;31:4121–4128. doi: 10.1016/j.biomaterials.2010.01.091. [DOI] [PubMed] [Google Scholar]
- Ueda T., Ito T., Tomita K., Togame H., Fumoto M., Asakura K., Oshima T., Nishimura S.-I., Hanasaki K. Bioorg. Med. Chem. Lett. 2010;20:4631–4634. doi: 10.1016/j.bmcl.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Chae S. Y., Jin C.-H., Shin J. H., Son S., Kim T. H., Lee S., Youn Y. S., Byun Y., Lee M.-S., Lee K. C. J. Controlled Release. 2010;142:206–213. doi: 10.1016/j.jconrel.2009.10.025. [DOI] [PubMed] [Google Scholar]
- Chae S. Y., Choi Y. G., Son S., Jung S. Y., Lee D. S., Lee K. C. J. Controlled Release. 2010;144:10–16. doi: 10.1016/j.jconrel.2010.01.024. [DOI] [PubMed] [Google Scholar]
- Liu Y., Wang G., Zhang H., Ma Y., Lang L., Jacobson O., Kiesewetter D. O., Zhu L., Gao S., Ma Q., Chen X. Bioconjugate Chem. 2016;27:54–58. doi: 10.1021/acs.bioconjchem.5b00625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin D. M., Satkunarajah M., Wen Y., Brubaker P. L., Pederson R. A., Wheeler M. B. Proc. Natl. Acad. Sci. U. S. A. 1997;94:7915–7920. doi: 10.1073/pnas.94.15.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J., Fu J., Sun L., Han Y., Mao Q., Liao F., Zhenga X., Zhu K. Med. Chem. Commun. 2018;9:67–80. doi: 10.1039/c7md00471k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J., Wang Y., Meng Q., Li G., Huang F., Wu S., Fei Y., Zhou F., Fu J. Eur. J. Med. Chem. 2017;132:81–89. doi: 10.1016/j.ejmech.2017.03.032. [DOI] [PubMed] [Google Scholar]
- Deacon C. F., Knudsen L. B., Madsen K., Wiberg F. C., Jacobsen O., Holst J. J. Diabetologia. 1998;41:271–278. doi: 10.1007/s001250050903. [DOI] [PubMed] [Google Scholar]
- Knudsen L. B., Nielsen P. F., Huusfeldt P. O., Johansen N. L., Madsen K., Pedersen F. Z., Thogersen H., Wilken M., Agerso H. J. Med. Chem. 2000;43:1664–1669. doi: 10.1021/jm9909645. [DOI] [PubMed] [Google Scholar]
- Uckaya G., Delagrange P., Chavanieu A., Grassy G., Berthault M.-F., Ktorza A., Cerasi E., Leibowitz G., Kaiser N. J. Endocrinol. 2005;184:505–513. doi: 10.1677/joe.1.05818. [DOI] [PubMed] [Google Scholar]
- Madsen K., Knudsen L. B., Agersoe H., Nielsen P. F., Thogersen H., Wilken M., Johansen N. L. J. Med. Chem. 2007;50:6126–6132. doi: 10.1021/jm070861j. [DOI] [PubMed] [Google Scholar]
- Miranda L. P., Winters K. A., Gegg C. V., Patel A., Aral J., Long J., Zhang J., Diamond S., Guido M., Stanislaus S., Ma M., Li H., Rose M. J., Poppe L., Veniant M. M. J. Med. Chem. 2008;51:2758–2765. doi: 10.1021/jm701522b. [DOI] [PubMed] [Google Scholar]
- Ueda T., Tomita K., Notsu Y., Ito T., Fumoto M., Takakura T., Nagatome H., Takimoto A., Mihara S.-I., Togame H., Kawamoto K., Iwasaki T., Asakura K., Oshima T., Hanasaki K., Nishimura S.-I., Kondo H. J. Am. Chem. Soc. 2009;131:6237–6245. doi: 10.1021/ja900261g. [DOI] [PubMed] [Google Scholar]
- Mapelli C., Natarajan S. I., Meyer J.-P., Bastos M. M., Bernatowicz M. S., Lee V. G., Pluscec J., Riexinger D. J., Sieber-McMaster E. S., Constantine K. L., Smith-Monroy C. A., Golla R., Ma Z., Longhi D. A., Shi D., Xin L., Taylor J. R., Koplowitz B., Chi C. L., Khanna A., Robinson G. W., Seethala R., Antal-Zimanyi I. A., Stoffel R. H., Han S., Whaley J. M., Huang C. S., Krupinski J., Ewing W. R. J. Med. Chem. 2009;52:7788–7799. doi: 10.1021/jm900752a. [DOI] [PubMed] [Google Scholar]
- Haque T. S., Lee V. G., Riexinger D., Lei M., Malmstrom S., Xin L., Han S., Mapelli C., Coope C. B., Zhang G., Ewing W. R., Krupinski J. Peptides. 2010;31:950–955. doi: 10.1016/j.peptides.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Murage E. N., Gao G., Bisello A., Ahn J.-M. J. Med. Chem. 2010;53:6412–6420. doi: 10.1021/jm100602m. [DOI] [PubMed] [Google Scholar]
- Johnson L. M., Barrick S., Hager M. V., McFedries A., Homan E. A., Rabaglia M. E., Keller M. P., Attie A. D., Saghatelian A., Bisello A., Gellman S. H. J. Am. Chem. Soc. 2014;136:12848–12851. doi: 10.1021/ja507168t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Li Y., Wang Y., Zheng X., Kong W., Meng F., Zhou Z., Liu C., Li Y., Gong M. Mol. Pharmaceutics. 2014;11:4092–4099. doi: 10.1021/mp5002685. [DOI] [PubMed] [Google Scholar]
- Hoang H. N., Song K., Hill T. A., Derksen D. R., Edmonds D. J., Kok W. M., Limberakis C., Liras S., Loria P. M., Mascitti V., Mathiowetz A. M., Mitchell J. M., Piotrowski D. W., Price D. A., Stanton R. V., Suen J. Y., Withka J. M., Griffith D. A., Fairlie D. P. J. Med. Chem. 2015;58:4080–4085. doi: 10.1021/acs.jmedchem.5b00166. [DOI] [PubMed] [Google Scholar]
- Novo Nordisk: Company Announcement of the FDA approval of Semaglutide (Ozempic®) in the USA, Dec 2017, https://www.novonordisk.com/bin/getPDF.2154210.pdf.
- Sanford M. Drugs. 2014;74:2097–2103. doi: 10.1007/s40265-014-0320-7. [DOI] [PubMed] [Google Scholar]
- Miyata T., Ueda Y., Horie K., Nangaku M., Tanaka S., De Strihou C. V. Y., Kurokawa K. Kidney Int. 1998;53:416–422. doi: 10.1046/j.1523-1755.1998.00756.x. [DOI] [PubMed] [Google Scholar]
- Khangholi S., Majid F. A. A., Berwary N. J. A., Ahmad F., Aziz R. B. A. Planta Med. 2016;82:32–45. doi: 10.1055/s-0035-1558086. [DOI] [PubMed] [Google Scholar]