Abstract
Two new genera (Streptosarcina and Streptofilum) and three new species (Streptosarcina arenaria, S. costaricana and Streptofilum capillatum) of streptophyte algae were detected in cultures isolated from terrestrial habitats of Europe and Central America and described using an integrative approach. Additionally, a strain isolated from soil in North America was identified as Hormidiella parvula and proposed as an epitype of this species. The molecular phylogeny based on 18S rRNA and rbcL genes, secondary structure of ITS-2, as well as the morphology of vegetative and reproductive stages, cell ultrastructure, ecology and distribution of the investigated strains were assessed. The new genus Streptosarcina forms a sister lineage to the genus Hormidiella (Klebsormidiophyceae). Streptosarcina is characterized by packet-like (sarcinoid) and filamentous thalli with true branching and a cell organization typical for Klebsormidiophyceae. Streptofilum forms a separate lineage within Streptophyta. This genus represents an easily disintegrating filamentous alga which exhibits a cell coverage of unique structure: layers of submicroscopic scales of piliform shape covering the plasmalemma and exfoliate inside the mucilage envelope surrounding cells. The implications of the discovery of the new taxa for understanding evolutionary tendencies in the Streptophyta, a group of great evolutionary interest, are discussed.
Keywords: Streptosarcina, Streptofilum, Hormidiella, Streptophyta, integrative approach, ultrastructure
Introduction
Streptophycean algae are an extremely interesting and important group in plant evolution as they are related to the ancestry of embryophytes (Graham et al. 1991; Kranz et al. 1995; Marin and Melkonian 1999; Lewis and McCourt 2004; Wodniok et al. 2011). Investigation of these algae will contribute to a deeper understanding about the evolution of basal lineages of the Streptophyta and the transition to land plants.
At present lineages of Streptophyta include several algal classes: Mesostigmatophyceae, Chlorokybophyceae, Charophyceae, Coleochaetophyceae, Zygnematophyceae and Klebsormidiophyceae (Becker and Marin 2009; Friedl and Rybalka 2012; Lemieux et al. 2016; Lewis and McCourt 2004; Marin and Melkonian 1999; Van den Hoek et al. 1995). The three last mentioned classes exhibit considerable species biodiversity, with many taxa widely distributed and inhabiting aquatic and terrestrial environments (Delwiche et al. 2002; Gontcharov et al. 2003; Rindi et al. 2011). In contrast, the other mentioned lineages contain very low number of species which might be considered as rather rare or relict representatives (Leliaert et al. 2012; Lewis and McCourt 2004).
Streptophycean algae are a morphologically diverse group ranging from unicellular flagellates (Mesostigma Lauterborn) to coccoid (some Zygnematophyceae), filamentous (Klebsormidium Silva, Mattox et Blackwell, Hormidiella Iyengar et Kanthamma and others), sarcinoid taxa (Chlorokybus Geitler, some Interfilum Chodat) as well composite branched or parenchymatous thalli (Coleochaete Brébisson, Charophyceae) (Friedl and Rybalka 2012; Leliaert et al. 2012; Lewis and McCourt 2004). Also in terms of reproduction streptophycean algae exhibit numerous strategies: from simple binary fission to unique conjugation and highly developed oogamy in multicellular reproductive organs. Streptophycean algae are characterized by some ultrastructural characters typical for the majority of representatives: An asymmetrical cytoskeleton in flagellate cells with basal bodies connected with multilayered structures (MLS), presence of submicroscopic organic scales on plasmalemma and flagella (Lokhorst et al. 2000; Marin and Melkonian 1999; Mattox and Stewart 1984; Van den Hoek et al. 1995), open mitosis, persistent telophase spindle, cell division via a cleavage furrow or with formation of a phragmoplast in later diverging taxa (Lokhorst and Star 1985; Mattox and Stewart 1984; Pickett-Heaps 1972; Sluiman 1985; Van den Hoek et al. 1995) and presence of large peroxisomes located between chloroplast and nucleus (Lokhorst and Star 1985; Lokhorst et al. 2000; Melkonian 1989; Mikhailyuk et al. 2014; Stewart et al. 1972).
The phylogeny of streptophycean algae is still controversially discussed over the past decades. The discovery of a unique phylogenetic position of the scaly flagellate Mesostigma in the Streptophyta and description of its ultrastructure was a remarkable event in phycology, because it justified the concept of structure, development and evolution of the streptophycean lineage (Marin and Melkonian 1999). Later investigations specified the taxonomic position of some other genera: Chaetosphaeridium Klebahn inside Coleochaetophyceae (Delwiche et al. 2002), Spirotaenia Brébisson in close relationship with Chlorokybus and distant from Zygnematophyceae (Gontcharov and Melkonian 2004), and some other genera (e.g. Hormidiella, Entransia Hughes, Interfilum) were added to Klebsormidiophyceae (Cook 2004; Lokhorst et al. 2000; Mikhailyuk et al. 2008). Discussions concerning the possible sister group to the embryophytes also continue. Several lineages were proposed as putative ancestors of the embryophytes based on morphology, ultrastructure and molecular phylogeny (Bhattacharya and Medlin 1998; Graham 1984; Kranz et al. 1995; McCourt et al. 2004). More advanced analyses using multigene phylogenies showed a progress from early to the most recent studies, the latter included the largest data sets with better resolution than the previous ones (Cocquyt et al. 2010; Finet et al. 2010; Karol et al. 2001; Turmel et al. 2007; Wickett et al. 2014). The question of the closest relative of the embryophytes has been addressed by recent multigene-phylogenies, and it is currently thought that the Zygnematophyceae are the sister branch to embryophytes (Lemieux et al. 2007; Turmel et al. 2006; Wodniok et al. 2011). Phylotranscriptomic analysis using up to 852 nuclear genes clearly demonstrated, that the Zygnematophyceae is the sister lineage to embryophytes with maximal support (Wickett et al. 2014). However, the topology of the Streptophyta phylogeny is changing considerably depending on markers and datasets used (Lewis and McCourt 2004). The number of species known from this group is relatively small (except for the Zygnematophyceae) which represents a serious problem for resolving basal lineages of the Streptophyta in molecular phylogenies. The reason of the instability of this phylogeny is simply a lack of data, and hence it is reasonable to assume that not all streptophycean taxa existing in nature were discovered and investigated so far.
Studies on terrestrial streptophycean algae are especially important in the evolutionary context of invasion of the land by plants (‘terrestrialization’). Algae with a terrestrial life style were found mostly among Chlorokybophyceae and Klebsormidiophyceae, as well as some taxa of Zygnematophyceae, which all thrive in terrestrial and amphibian habitats (Ettl and Gärtner 2014). Over the years of research on terrestrial green algae from different biogeographic regions numerous new isolates were established as clonal cultures. The aim of the present study was to characterize undescribed isolates of the Streptophyta from terrestrial habitats in Europe, Central and North America. The selection of the strains derived from uncommon morphological traits, already observed in the light microscope. The description of new taxa included a polyphasic approach using a combination of molecular, morphological and ultrastructural markers.
Results
Molecular Phylogeny Based on 18S rRNA and rbcL Genes
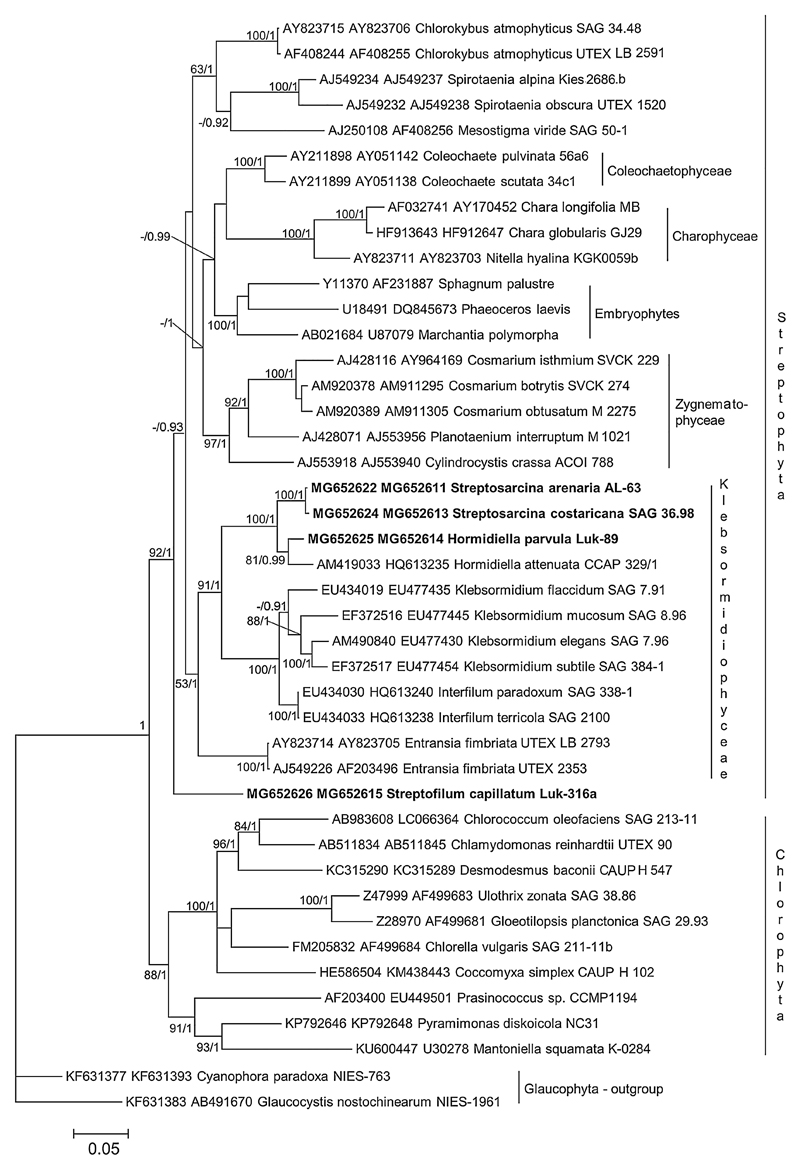
For the present study five unialgal terrestrial strains from different biogeographic regions were used. The phylogenetic analyses of a concatenated 18S rRNA – rbcL sequence dataset revealed that the newly sequenced isolates belonged to three different lineages in the Streptophyta (Fig. 1). Two of these lineages were assigned to the clade that corresponded to the Klebsormidiophyceae with the genera Klebsormidium, Interfilum, Hormidiella and Entransia. Strain Luk-89 fell into the clade designated as Hormidiella and therefore represented a species of this genus. Strains AL-63 and SAG 36.98 formed a separate clade with a high level of support, sister to Hormidiella. This new lineage corresponded to a separate genus, which was named Streptosarcina Mikhailyuk et Lukešová gen. nov. (see below). Strain Luk-316a formed a separate lineage in the monophyletic Streptophyta but its precise position was unresolved in our phylogeny. The isolated position of this strain in the 18S rRNA – rbcL phylogeny suggested that it represents a separate genus, which was named Streptofilum Mikhailyuk et Lukešová gen. nov. (see below).
Fig. 1.
Molecular phylogeny of Streptophyta based on concatenated dataset 18S rRNA and rbcL sequences. Phylogenetic tree was inferred by Bayesian method with Bayesian Posterior Probabilities (PP) and maximum likelihood (ML) bootstrap support (BP) indicated at nodes. From left to right, support values correspond to ML BP and Bayesian PP; BP values lower than 50% and PP lower than 0.8 not shown. Strains marked with bold are newly sequenced isolates. Clade designations follow Leliaert et al. (2012).
Comparison of ITS-2 Secondary Structures
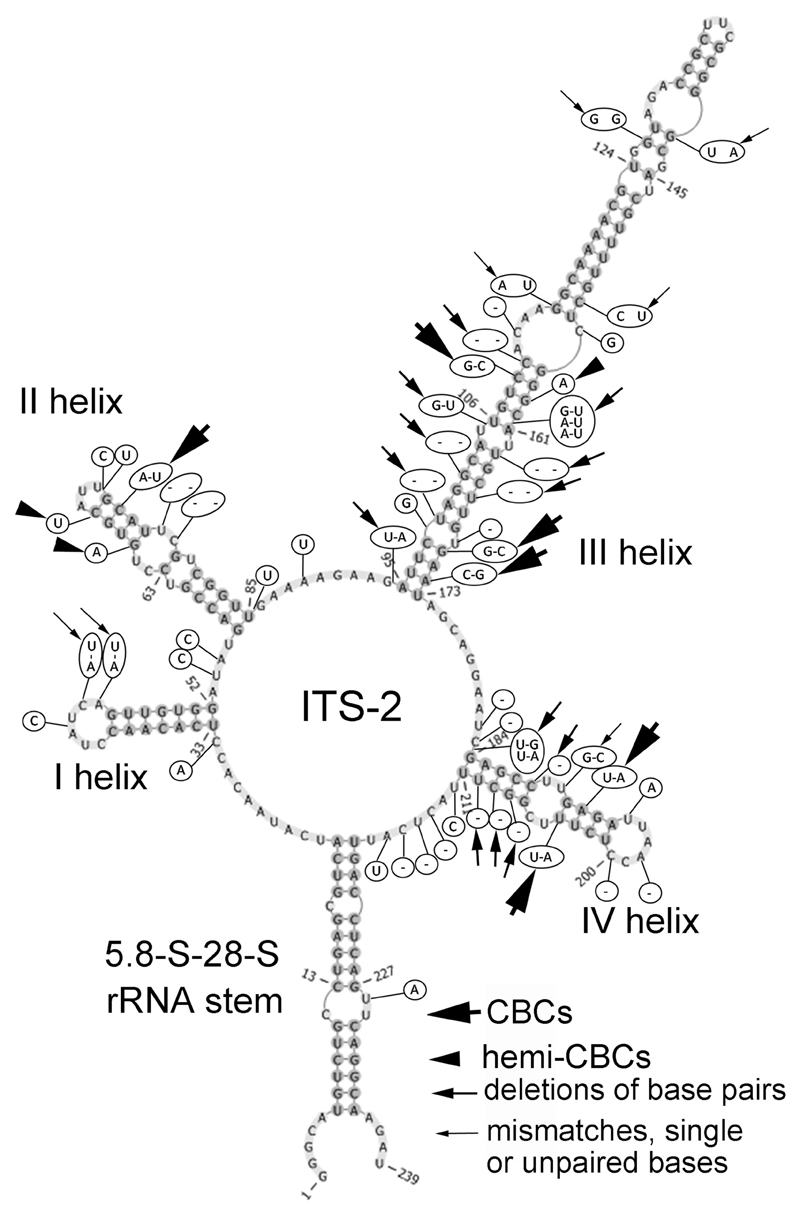
To obtain better resolution within clades formed by strains of Hormidiella and Streptosarcina, the secondary structure of ITS-2 was evaluated together with 5.8S–28S rRNA stem region in all new isolates and the authentic strain of Hormidiella attenuata Lokhorst (CCAP 329/1). Secondary structures of ITS-2 of both Hormidiella strains (Luk-89 and CCAP 329/1) were similar in the 5.8S–28S rRNA stem and in helices I and II, but essentially different in helices III and IV (Fig. 2). In general, 6 CBCs (in helices II–IV), 3 hemi-CBCs (in helices II and III), 16 deletions of base pairs (in helices III and IV) and 7 mismatches, unpaired or single bases (in helices I, III and IV) were determined. Most of these differences were localized in the conservative part of ITS-2 (4 CBCs, 3 hemi-CBCs, 10 deletions of base pairs and 4 mismatches, unpaired or single bases) (Fig. 2).
Fig. 2.
Comparison of ITS-2 secondary structure of Hormidiella strains. The structure of H. parvula (Luk-89) is presented with the differences to H. attenuata (CCAP 329/1) highlighted. Variable bases or base pairs of H. attenuata are shown with circles.
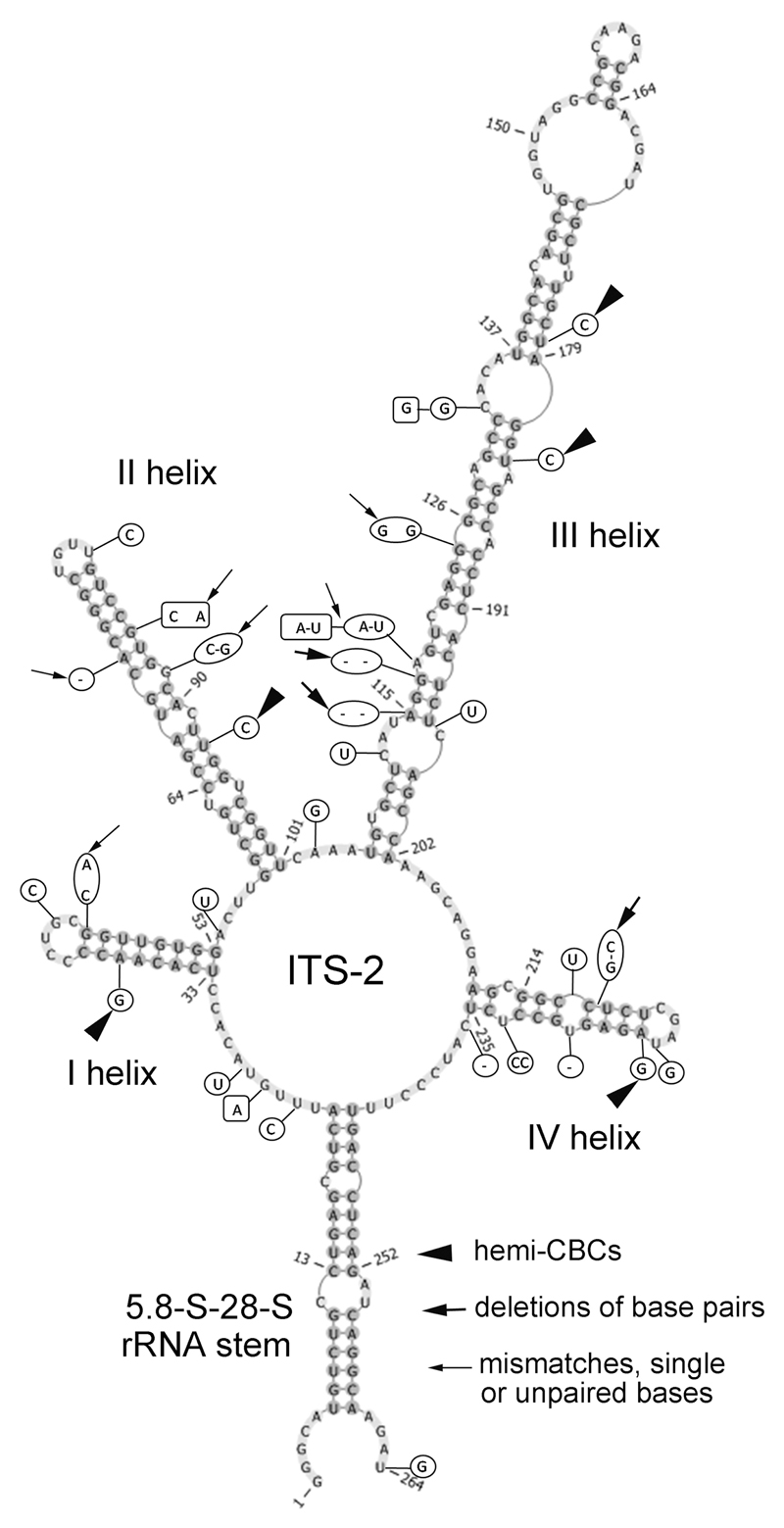
The ITS-2 secondary structure of the investigated Streptosarcina strains (Fig. 3) differed in some essential details. Most differences were found between strain SAG 36.98 and the new strains AL-63 and Prim 3-3. Five hemi-CBCs (in helices I–IV), 3 deletions of base pairs (in helices III and IV) and 4 mismatches, unpaired or single bases (in helices I–III) were determined. Most of these differences were localized in the conservative part of ITS-2 (3 hemi-CBCs, 2 deletions of base pairs and 1 mismatch). Strains AL-63 and Prim 3-3 exhibited similar ITS-2 sequences (differed by 4 nucleotides). The secondary structure of ITS-2 of these strains showed differences in 2 mismatches or unpaired bases (in helices II and III) and 2 different nucleotides in loops (Fig. 3).
Fig. 3.
Comparison of ITS-2 secondary structure of Streptosarcina strains. The structure of S. arenaria (AL-63) is presented with the differences to another strain of S. arenaria (Prim 3-3) and to S. costaricana (SAG 36.98). Variable bases or base pairs of S. arenaria (Prim 3-3) are shown with boxes and S. costaricana (SAG 36.98) with circles.
Morphology and Reproduction
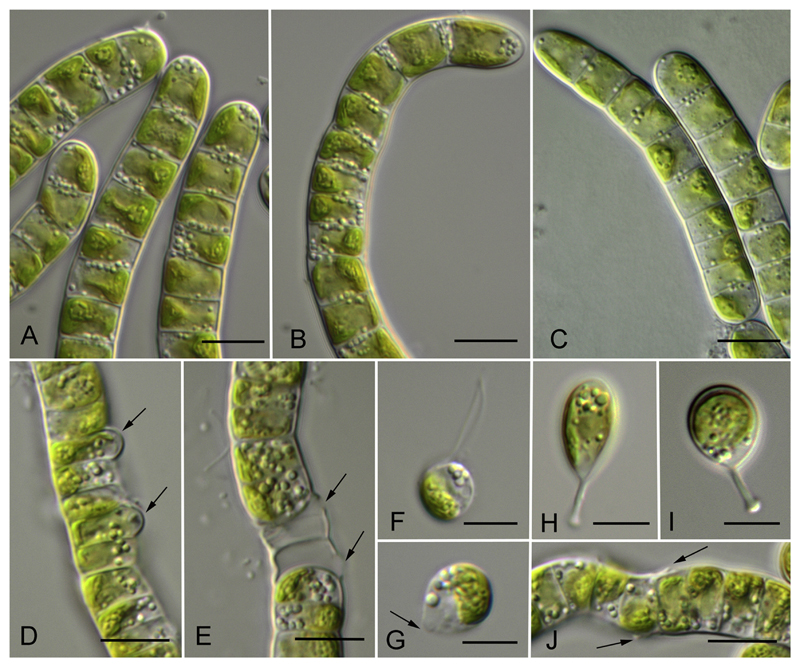
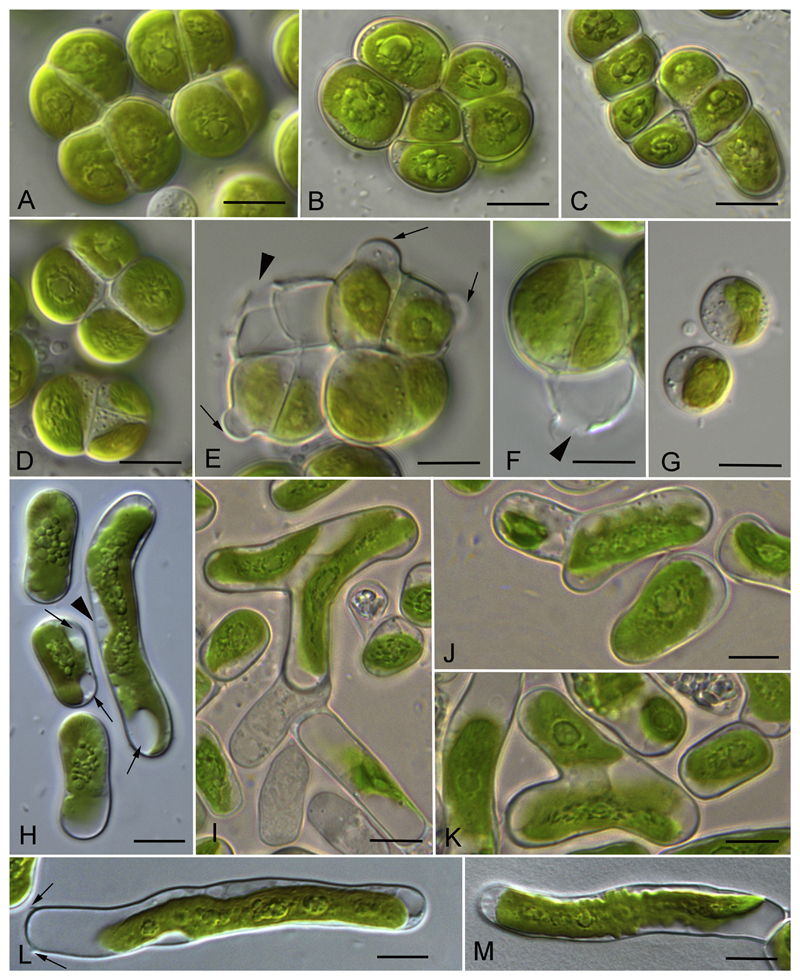
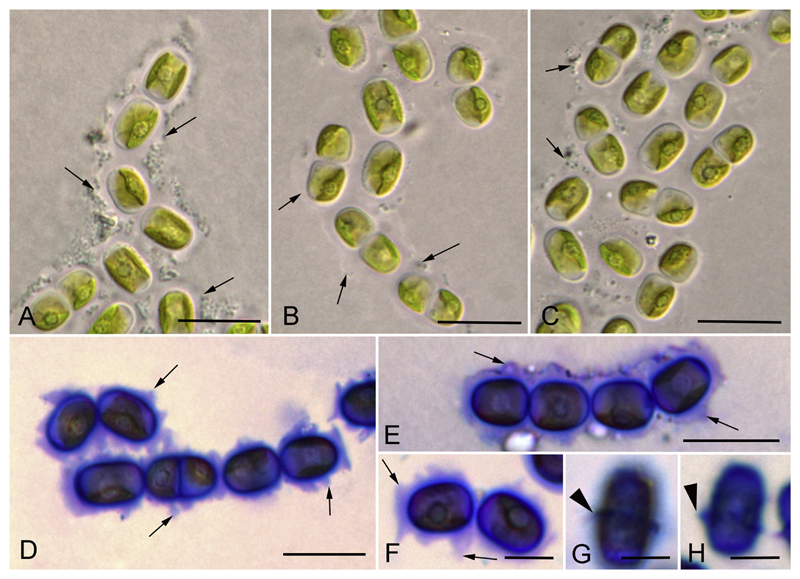
The newly investigated strains represented either a filamentous morphotype (Luk-89, Hormidiella) or filaments that easily disintegrated to diads and unicells (Luk-316a, Streptofilum, and SAG 36.98, Streptosarcina), or a sarcinoid packet-like morphotype (AL-63 and Prim 3-3, Streptosarcina), see Figs 4A–C, 5A–D, H–M, 6A–C, 7A, F, I–L. The Hormidiella strain (Luk-89) was characterized by strong or disintegrating filaments with short-cylindrical cells, partly slightly constricted at cross walls and with some attributes of heteropolarity (Figs 4A–C, 7A). H-like fragments sometimes formed (Fig. 4J). Two strains of Streptosarcina (AL-63 and Prim 3-3, S. arenaria Mikhailyuk et Lukešová gen. et sp. nov.) had spherical, hemispherical or widely ellipsoid cells grouped in three-four-celled packets or formed sometimes short filaments (Figs 5A–D, 7F). Another Streptosarcina strain (SAG 36.98, S. costaricana Mikhailyuk et Lukešová gen. et sp. nov.) exhibited elongated cylindrical cells which were separated or grouped in short easily disintegrated filaments or diads (Figs 5H, 7I). Also attributes of branching or budding (lateral bulging of the cell wall close to the cell’s apex) could be observed in this strain (Figs 5I–K, 7K). H-like fragments sometimes formed at the end of filaments (Figs 5L, 7J). Strain Luk-316a (Streptofilum) was characterized by ellipsoid cells, separated or grouped in diads or short filaments by a mucilaginous sheath that surrounded the cells (Figs 6A–C, 7L). All investigated strains showed a plate-shaped chloroplast that occupied 50–70% of inner cell circumference with smooth (Hormidiella and Streptofilum, Figs 4A–C, 6A–C, 7A, L) or waved and lobed edges (Streptosarcina strains, Figs 5A–D, H–M, 7F, I). All algal strains exhibited single pyrenoids surrounded by several or many starch grains, and in Streptosarcina strains numerous starch grains oriented in parallel rows (Figs 4A–C, 5A–D, H–M, 6A–C, 7A, F, I, L). The pyrenoid of Streptosarcina strain SAG 36.98 elongated in long cells and disintegrated into several small pyrenoids forming a line (Figs 5L, M, 7J). Cells were uninucleate with nuclei located opposite the pyrenoids (Figs 4A, C, 5H, I, K, 6B, 7A, F, I, L). The nucleus was located in a cytoplasmic bridge between two terminal vacuoles in the long cylindrical cells of Streptosarcina strain SAG 36.98 (Figs 5H, 7I). A mucilage sheath was observed only in the Streptofilum strain, while all other isolates lacked this feature. Mucilage showed a homogenous structure and a specific irregular to lobed surface (Figs 6D–F, 7L), as well as formation of a collar in the middle of the cell or cap-like structures during staining with methylene blue (Fig. 6G, H).
Fig. 4.
Morphology and reproduction of Hormidiella parvula (Luk-89). A–C) Vegetative filaments in adult (A, B) and young state (C). D) Formation of sporangia (arrows). E) Empty sporangia with openings in cell wall (arrows). F) Zoospore. G) Stopped zoospore, formation of papilla (arrow). H, I) Germination of young filament with the stalk. J) H-like fragments of cell wall (arrows). Scale bars: A–E, J are 10 μm, F–I are 5 μm.
Fig. 5.
Morphology and reproduction of Streptosarcina gen. nov. A–G) S. arenaria sp. nov. (AL-63 (B, C) and Prim-3-3 (A, D-G). A, B, D). Packet-like vegetative thallus. C) Filaments. E, F) Formation of sporangia (arrows) and empty sporangia with openings in cell wall (arrowheads). G) Stopped zoospores. H–M) S. costaricana sp. nov. (SAG 36.98). H) Unicellular stage, nucleus (arrowhead) and terminal vacuoles (arrows) are visible. I-K) Branching of filaments. L, M) Elongated cells with multiple pyrenoids from old culture, H-like fragments of cell wall are visible (arrows). Scale bars are 10 μm.
Fig. 6.
Morphology of Streptofilum capillatum gen. et sp. nov. (Luk-316a). A–C) Filaments and cell dyads surrounded by mucilage envelope (arrows). D–H) Mucilage staining by methylene blue. Homogenous mucilage envelope with lobbed edge (arrows) and collar structures (arrowheads) are visible. Scale bars: A–E are 10 μm, F–H are 5 μm.
Fig. 7.
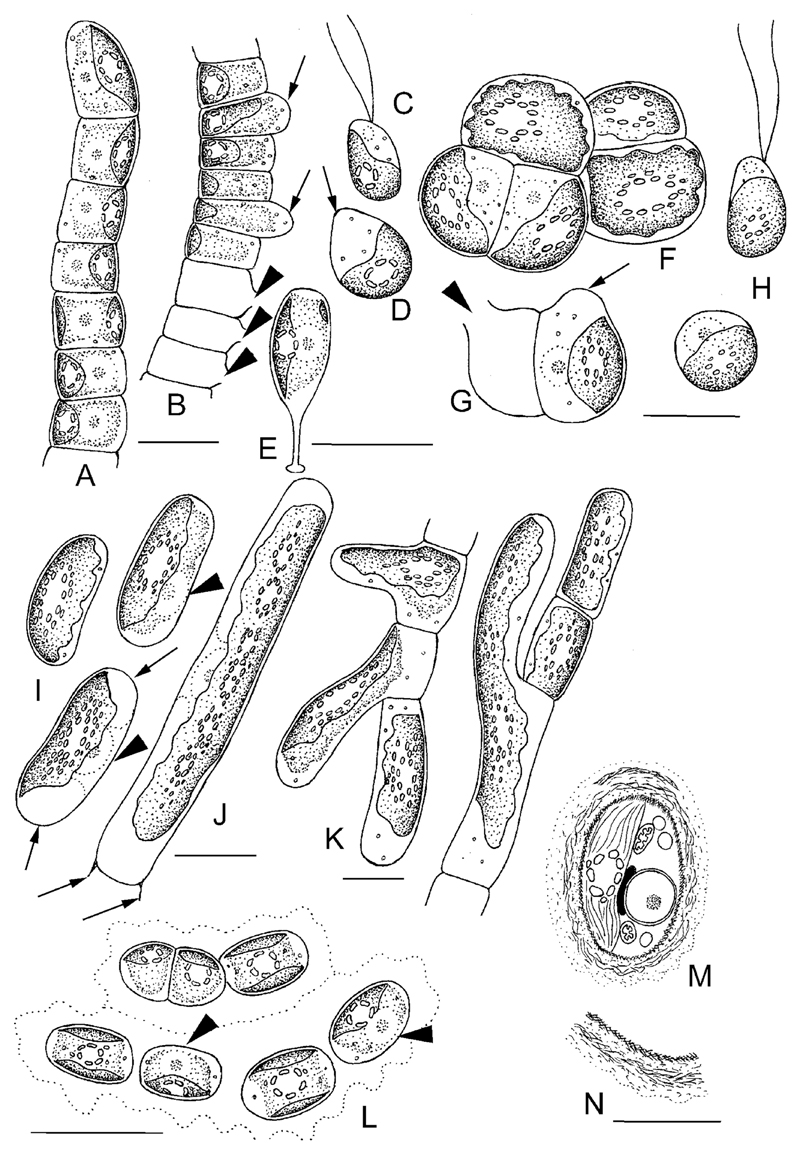
Drawings of Hormidiella, Streptosarcina and Streptofilum species. A–E) Morphology and reproduction of Hormidiella parvula A) Vegetative filament. B) Sporangia (arrows) and empty sporangia with openings in cell wall (arrowheads). C) Zoospore. D) Stopped zoospore, formation of papilla (arrow). E) Germination of young filament with the stalk. F–H) Streptosarcina arenaria sp. nov. F) Packet-like vegetative thallus. G). Sporangium (arrow) and empty sporangium with opening in cell wall (arrowhead). H) Zoospore and stopped zoospore. I–K) S. costaricana sp. nov. I) Unicellular stage, nuclei (arrowheads) and terminal vacuoles (arrows) are visible. J) Elongated cell with multiple pyrenoids from old culture, H-like fragments of cell wall are visible (arrows). K) Branching of filaments. L–N) Morphology and ultrastructure of Streptofilum capillatum gen. et sp. nov. L) Filament and cell dyad surrounded by mucilage envelope, nuclei (arrowheads) are visible. M, N) Reconstruction of TEM micrographs with details of cell ultrastructure and coverage. Scale bars: A–L are 10 μm, M, N is 5 μm.
Cells of Hormidiella and Streptofilum divided in one plane with the formation of uniseriate filaments or cell diads (Figs 4A–C, 6A–C, 7A, L). Cells of Streptosarcina divided in several planes with the formation of packets (AL-63 and Prim 3-3, Fig. 5A–D), and branch-like structures (SAG 36.98, Figs 5I–K, 7K). Reproduction by zoospores was induced in the new Hormidiella strain and in two strains of Streptosarcina (AL-63 and Prim 3-3). One zoospore per cell was formed. Zoosporangia formed a rounded protrusion which later ruptured by a small opening releasing zoospores (Figs 4D, E, 5E, F, 7B, G). Zoospores were ellipsoid, lacking cell wall and stigma, with two equal subapical flagella (Figs 4F, 7C, H). Vegetative cells originating from zoospores of Streptosarcina were spherical (Figs 5G, 7H). Zoospores of Hormidiella formed a papilla at the area where the flagella were inserted (Figs 4G, 7D). The papilla formed later a conspicuous stalk with a small non-ramified holdfast (foot) and young cells developed a Characium-like morphology (Figs 4H, I, 7E).
Ultrastructure
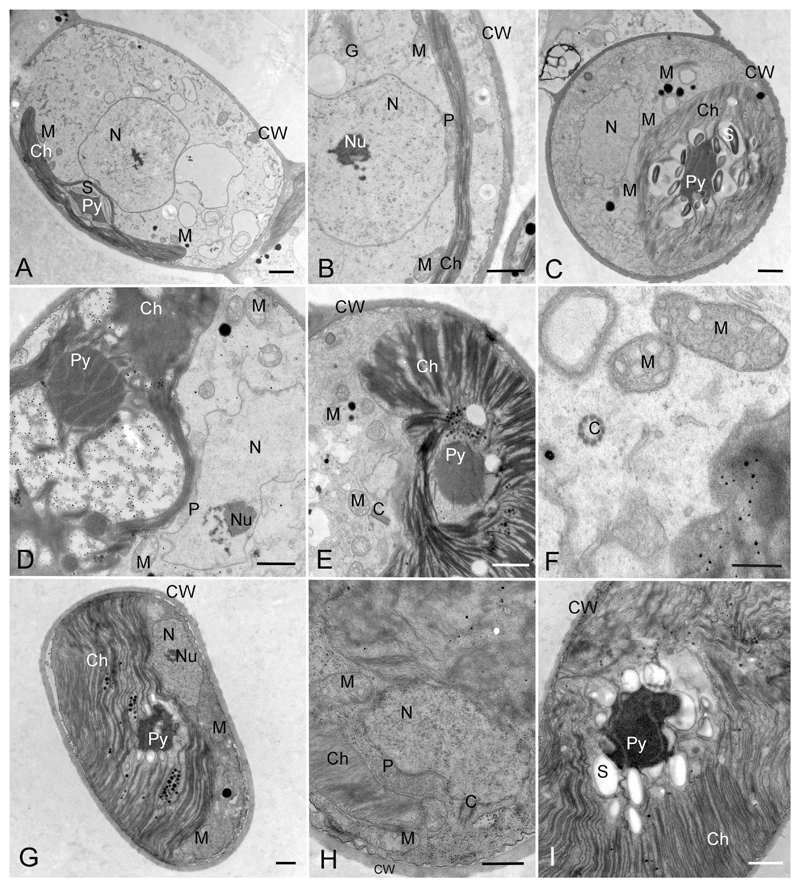
The ultrastructure of the cytoplasmic part of vegetative cells of Streptosarcina, Hormidiella and Streptofilum were similar. The chloroplast was always located at one side of the cell, covering half of the cell’s inner surface (Figs 7M, 8A, C, G, 9A, B), and it was richly lobed in Streptosarcina or had a smooth edge in Hormidiella and Streptofilum. The pyrenoid matrix was traversed by several parallel arranged thylakoid membranes in Streptosarcina, and often surrounded by several layers of small starch grains (Fig. 8C, D, G, I). In contrast, pyrenoids of Hormidiella and Streptofilum were surrounded by a layer of large starch grains (Figs 7M, 8A, 9A). The pyrenoid matrixes of these representatives were homogenous with thylakoid membranes invading the surrounding starch and continuing between starch grains and pyrenoid matrix (Figs 7M, 8A, 9A). Between the nucleus and chloroplast, one single peroxisome was located in all investigated strains, that showed a slightly curved bacilliform shape in cross-sections, occasionally several profiles of the peroxisome were observed (Figs 7M, 8B, D, H, 9B). Numerous mitochondrial profiles were observed in the cells of all strains and were mostly located close to the chloroplast (Figs 7M, 8A–H, 9A). The vegetative cells of Streptosarcina included a pair of centrioles (Fig. 8E, F, H).
Fig. 8.
Transmission electron micrographs of Hormidiella and Streptosarcina species. A, B) H. parvula (Luk-89). A) Cell in overview. B) Portion of cell showing closely arranged chloroplast, peroxisome and nucleus. C-F) S. arenaria gen. et sp. nov. (Prim-3-3). C) Cell in overview. D–F) Portions of cells showing closely arranged chloroplast, peroxisome and nucleus, pyrenoid traversable by parallel thylakoids, centrioles and chloroplast structure. G–I) S. costaricana gen. et sp. nov. (SAG 36.98). G) Cell in overview. H, I) Portions of cells showing closely arranged chloroplast, peroxisome and nucleus, pyrenoid traversed by parallel arranged thylakoids and chloroplast structure. Abbreviations: Ch, chloroplast; Py, pyrenoid; S, starch; N, nucleus, Nu, nucleolus; P, peroxisome; M, mitochondrium, G, Golgi body; C, centrioles; CW, cell wall. Scale bars: A–E, G–I are 1 μm, F is 0.5 μm.
Fig. 9.
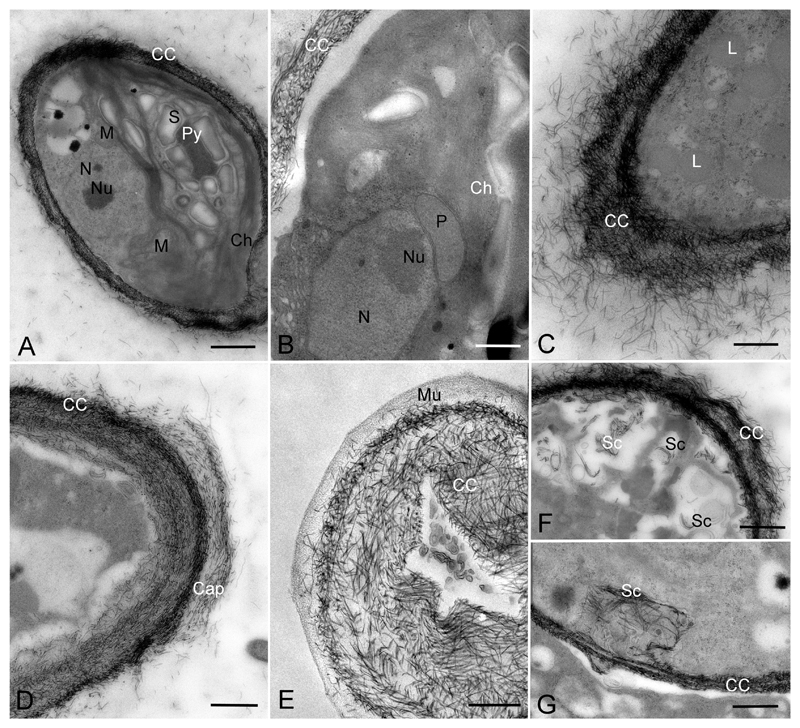
Transmission electron micrographs of Streptofilum capillatum gen. et sp. nov. (Luk-316a). A) Cell in overview showing pyrenoid, chloroplast and cell coverage by scales. B) Portion of cell showing closely arranged chloroplast, peroxisome and nucleus. C-E) Cell coverage forming by scales in section (C, D) and surface section (E). F, G) Origination of scales inside cells (likely transported to cell surface). Abbreviations: Ch, chloroplast; Py, pyrenoid; S, starch; N, nucleus; Nu, nucleolus; P, peroxisome; M, mitochondria; L, lipid globules; CC, cell coverage; Mu, mucilage, Sc, scales in the inner parts of the cell, likely prior to deposition to the cell surface. Scale bars: A, E are 1 μm, B-D, F, G are 0.5 μm.
Vegetative cells of all investigated strains of Streptosarcina and Hormidiella were surrounded by a cell wall of typical structure for Klebsormidiophyceae (Fig. 8A–C, E, G–I). Although cells of Streptofilum were lacking a classical cell wall, they were surrounded by a plasmalemma with many layers of specific scales submerged in the mucilage surrounding the cells (Figs 7M, N, 9A–G). These scales exhibited a piliform shape, oriented in different directions and formed two distinct layers. The scales of the inner layer were more densely arranged, while the outer layer was friable with waved edges and exfoliated scales (Figs 7N, 9C, D, F). Sometimes exfoliated layers of scales formed cap-like structures (Figs 7M, 9D). Some clusters of scales enclosed in the cytoplasm were observed, perhaps acting as transport mechanism to the cell surface (Fig. 9F, G).
Discussion
Phylogeny of Hormidiella, Streptosarcina and Streptofilum
Phylogenetic analysis based on a concatenated set of 18S rRNA and rbcL sequences mostly corresponded with the phylogeny of streptophycean algae provided in studies of Bhattacharya and Medlin (1998), Marin and Melkonian (1999), Delwiche et al. (2002), Mikhailyuk et al. (2008), Sluiman et al. (2008) and was also mostly in agreement with investigations using multigene phylogenies (Finet et al. 2010; Lemieux et al. 2007, 2016; Wodniok et al. 2011). Some differences between the mentioned and the phylogenetic trees of the present study are typical for the general topology of the Streptophyta tree which might strongly vary depending on the selection of data sets in terms of taxa or characters used (Friedl and Rybalka 2012; Leliaert et al. 2012; Lewis and McCourt 2004).
Strain Luk-89 showed clearly a very close position to Hormidiella with high support. Morphological characters of the vegetative thallus and reproductive stages of this strain confirmed the position of Luk-89 inside Hormidiella as well (see below). The resolution between the investigated strain Luk-89 and the authentic strain H. attenuata CCAP 329/1 was quite prominent. Comparison of the ITS-2 secondary structures of both strains showed some differences too: 6 CBCs, 3 hemi-CBCs, 23 deletions of base pairs, mismatches, unpaired or single bases, the most part of which are localized in conservative region of ITS-2. These conspicuous base pair differences are the basis for assigning both strains to two separate species (see Coleman 2009; Darienko et al. 2010; Demchenko et al. 2012). Sequence comparison of Luk-89 and CCAP 329/1 with other known species of Hormidiella (H. parvula Iyengar et Kanthamma and H. bharatiensis Subrahmanyan) is not possible because of lack of molecular data. However, comparison of morphological characters, ecology and distribution of the new isolate with other known species of Hormidiella showed that strain Luk-89 can be identified as H. parvula (type species of the genus). Therefore, we emended the diagnosis of this species and proposed strain Luk-89 as the epitype of H. parvula (see below).
The isolates SAG 36.98 and AL-63 formed a well-supported clade corresponding to the newly erected genus Streptosarcina. Resolution between the investigated strains of Streptosarcina in our 18S rRNA – rbcL phylogeny is not clear, although comparison of ITS-2 secondary structures of these strains showed some differences among two groups: European strains AL-63 and Prim 3-3 were different from the Costa-Rica isolate SAG 36.98. The observed differences (in general 5 hemi-CBCs, 3 deletions of base pairs and 4 mismatches, unpaired or single bases) are mainly localized in the conservative region and might be sufficient to justify two separate species. Therefore, two new species of Streptosarcina, namely Streptosarcina arenaria (AL-63 and Prim 3-3) and S. costaricana (SAG 36.98) were erected, based on ITS-2 secondary structure as well as on geographical distribution and morphology (see below).
Isolate Luk-316a was positioned in the phylum Streptophyta as a separate lineage. This new lineage is very distant from all known genera of Streptophyta. Therefore Luk-316a was erected as a new genus (Streptofilum gen. nov.). Unique ultrastructural characters, particularly the absence of a classical cell wall in this strain, confirmed its separate position in the Streptophyta (see below).
Definition and Phylogeny of the Genus Hormidiella
Isolate Luk-89 was assigned as member of Hormidiella. Morphology, ultrastructure and reproduction of Luk-89 completely corresponded with characters described for other members of Hormidiella: filaments with a tendency for heteropolarity, formed by narrow short-cylindrical cells, plate-shaped chloroplast with pyrenoid surrounded by several starch grains, reproduction by naked stigma-less zoospores with two subapical flagella, germination of zoospores with a stalk, large peroxisome between chloroplast and nucleus, homogenous pyrenoid matrix, etc. (Iyengar and Kanthamma 1940; Lokhorst et al. 2000). Further analysis of the ITS-2 secondary structure showed that Luk-89 and H. attenuata CCAP 329/1 differed considerably and hence represent two separate species.
Three species of Hormidiella are known: H. parvula (type of the genus), H. bharatiensis and H. attenuata which differ by width of filaments, number of pyrenoids per cell, shape of terminal cell, zoospores structure, absence/presence of sexual reproduction etc. (Iyengar and Kanthamma 1940; Subrahmanyan 1976; Lokhorst et al. 2000). All these species inhabit soils or amphibian habitats (rain puddle), and they were described from tropical regions (India and Brazil). But only H. attenuata was so far comprehensively investigated due to the availability of the authentic strain CCAP 329/1. Moreover, H. bharatiensis seems to be the most unique species (characterized by sexual reproduction via heterogamy and the presence of zoospores and gametes with uneven flagella) and probably represents another separate lineage outside of the Klebsormidiophyceae (Lokhorst et al. 2000).
Strain Luk-89 is morphologically similar to H. parvula especially with respect to the width of filaments, but differs by the absence of a stalk in adult filaments (perhaps due to culture conditions, although zoospores germinate with the formation of stalks with a non-ramified holdfast, the same as illustrated for H. parvula and H. attenuata (Iyengar and Kanthamma 1940; Lokhorst et al. 2000)). The place of origin of Luk-89 (isolated from soils of North America) is distant from the type locality of H. parvula (soils of India). Members of Hormidiella are rather rare algae, but some findings of H. parvula are also known for Europe, North and South America (Freitas and Loverde-Oliveira 2013; Paczuska and Paczuski 2015; Patrick 1996). Therefore, we identified Luk-89 as H. parvula, and propose Luk-89 as epitype of this species together with an emendation of the species diagnosis based on new data.
Hormidiella parvula Iyengar et Kanthamma (1940) J. Indian Bot. Soc. 19: 165, pl. V; text-figs 1–35 emend. Mikhailyuk et Lukešová (Figs 4A–J, 7A–E, 8A, B)
Emended description: Filaments long, unbranched, sometimes disintegrated to short filaments in old state, (6.0)7.8–8.8(10.2) μm width. Vegetative cells cylindrical to short-cylindrical and doliiform by slight constrictions, (4.4)5.3–8.5(14.6) μm length, to 2 times as long as wide in young state and (0.3)0.5–1(1.4) times as long as wide in mature filaments. Apical cell slightly narrowed and attenuated, especially in young filaments. Cell wall smooth, H-like fragments rarely observed. Chloroplast parietal, plate-shaped, with smooth margin and single pyrenoid surrounded by a layer of several starch grains. Vegetative reproduction by cell division in one plane (perhaps sporulation-like type) and fragmentation of thalli. Asexual reproduction by ellipsoid, naked, stigma-less zoospores with two equal subapical flagella. Zoosporangia originated from vegetative cells by formation of small rounded protrusion which later ruptured by small opening releasing zoospores. Zoospores formed one per sporangium and germinate with formation of a stalk with non-ramified holdfast. Germ has Characium-like morphology. Sexual reproduction not observed.
Type locality: India, Madras, soil.
Epitype (designated here): The authentic strain Luk-89 (BCCO 30_2136, SAG 2558) is permanently cryopreserved in metabolically inactive state (cryopreserved in liquid nitrogen) in the Culture collection of soil algae and cyanobacteria of the Institute of Soil Biology, České Budějovice, Czech Republic. Additionally culture material of strain Luk-89 (BCCO 30_2136, SAG 2558) preserved in 4% formaldehyde, Algotheca of M.G. Kholodny Institute of Botany of NASU of Ukraine (AKW-32371).
Epitype strain: Luk-89 was deposited in SAG, University of Göttingen, Germany under number SAG 2558 and BCCO, Institute of Soil Biology, České Budějovice, Czech Republic under number BCCO 30_2136.
Comments: The proposed epitype strain Luk-89 is morphologically similar to the original description, but differs by the absence of a stalk in adult filaments perhaps because of culture conditions, although zoospores germinate with the formation of stalks.
Definition and Phylogeny of the Genus Streptosarcina
Three isolates (AL-63, Prim 3-3 and SAG 36.98) were assigned to the clade of Klebsormidiophyceae, but formed a separate lineage which refers to a new genus (Streptosarcina gen. nov.). All investigated strains exhibited similar morphological and ultrastructural characters which are typical for Klebsormidiophyceae: plate-shaped chloroplast with several layers of small starch grains, pyrenoid matrix traversable by several single thylakoids, reproduction by naked stigma-less zoospores with two equal subapical flagella, large peroxisome between chloroplast and nucleus etc. (Cook 2004; Lokhorst 1996; Lokhorst et al. 2000; Mikhailyuk et al. 2008, 2014; Rindi et al. 2011). One particularly interesting ultrastructural feature is the occurrence of centrioles in strain Prim-3-3 (S. arenaria). This underpins also the phylogenetic position close to Hormidiella and Entransia. Centrioles have been observed in both genera (Cook 2004; Herburger et al. 2016; Lokhorst et al. 2000), and this was regarded as a clear difference from Klebsormidium and Interfilum, both lacking these structures.
The general morphology of the three isolates is quite different: packet-like thallus (AL-63 and Prim 3-3, S. arenaria) and filamentous thallus with tendency to branching (SAG 36.98, S. costaricana). Strain SAG 36.98 was formerly identified as Pseudopleurococcus speciosus nom. inval. (http://www.uni-goettingen.de/en/45175.html), its sequence was placed in NCBI under the species name Pseudopleurococcus sp. The genus Pseudopleurococcus Snow unites algae with branched, sarcinoid or pleurococcoid thalli (Ettl and Gärtner 2014). Some species of Pseudopleurococcus were transferred to the genus Dilabifilum Tschermak-Woess (Ulvophyceae), while others remained representatives of the mentioned genus (Trebouxiophyceae) (Ettl and Gärtner 2014). Darienko and Pröschold (2017) recently revised some members of the Ulvophyceae and transferred different Pseudopleurococcus species to the genera Pseudendoclonium Wille and Ctenocladus Borzi. It might be possible that initially SAG 36.98 represented a richly branched alga with pleurococcoid thallus, but growing in culture for decades influenced its morphology. This strain still forms branched filaments which appear extremely fragile, and therefore this alga in the light microscope appears as unicells or short filaments with a tendency to branching.
AL-63 and Prim 3-3 have identical morphology and represent strong sarcinoid aggregates composed mostly of 2-3-4 and more vegetative cells (up to 8–16), but sometimes form unicells and short non-branched filaments. Both strains of S. arenaria (AL-63 and Prim 3-3) were isolated from sandy soils, but from different regions (Slovakia and Ukraine). Both Streptosarcina species exhibit a similar type of cell division (sporulation-like type) that is typical for Klebsormidium and Interfilum (Mikhailyuk et al. 2014) and probably for some other streptophycean taxa (e.g. Chlorokybus). Differences in thallus morphology of Streptosarcina are caused by different shape of cells of both species: almost spherical in S. arenaria and elongated in S. costaricana. But in both species cells divide in several planes with the formation of packet-like aggregation of cells, non-branched or branched filaments. Branches in S. costaricana are formed by budding: branching starts by lateral bulging of the cell wall close to the cell’s apex, a process typical for many branched algae from different lineages (Starmach 1972; Moshkova 1979; Van den Hoek et al. 1995).
Streptosarcina Mikhailyuk et Lukešová gen. nov.
Diagnosis. Thallus with sarcinoid, packet-like or branched morphology, sometimes disintegrating to short filaments, cell diads and unicells. Vegetative cells wide-ellipsoid, hemispherical to ellipsoid and cylindrical. Cell wall smooth. Chloroplast parietal, plate-shaped, with waved or dissected margin and one or several pyrenoid surrounded by layers of small starch grains. Vegetative reproduction by cell division in several planes (sporulation-like type) and fragmentation of thalli. Formation of branches by budding. Asexual reproduction by naked, stigma-less zoospores with two equal subapical flagella. Zoospores formed one per sporangium and released through small opening in the cell wall. Sexual reproduction not observed.
Morphologically similar with other sarcinoid and filamentous algae from Streptophyta (Interfilum, Chlorokybus, Klebsormidium), but differs by true branching and absence of mucilage. Generally morphologically similar to some genera of Chlorophyta (Apatococcus Brand, Desmococcus Brand, Pseudendoclonium etc.), but differs by organization of vegetative and reproductive cells typical for Streptophyta, as well as by 18S rRNA and rbcL sequences.
Type species: Streptosarcina arenaria Mikhailyuk et Lukešová sp. nov.
Etymology: from the name of division – Streptophyta and Latin word sarcina – packet, cluster (referring to packet-like, sarcinoid morphology).
Streptosarcina arenaria Mikhailyuk et Lukešová sp. nov. (Figs. 5A–G, 7F–H, 8C–F)
Diagnosis. Thallus with sarcinoid, packet-like morphology, sometimes disintegrating to short filaments, cell diads and unicells. Vegetative cells wide-ellipsoid or hemispherical, 8.7–10.3(11.9) μm width, 14.2–15.5 μm length. Cell wall smooth. Chloroplast parietal, plate-shaped, with waved or dissected margin and single pyrenoid surrounded by layers of small starch grains. Vegetative reproduction by cell division in several planes (sporulation-like type) and fragmentation of thalli. Asexual reproduction by naked, stigma-less zoospores with two equal subapical flagella. Zoosporangia originated from vegetative cells by formation of small rounded protrusion which later ruptured by small opening releasing zoospores. Zoospores formed one per sporangium. Sexual reproduction not observed.
Differs from S. costaricana by shape of cells and general sarcinoid morphology. Morphologically similar with other sarcinoid algae from Streptophyta (Interfilum, Chlorokybus), but differs by absence of mucilage. Generally similar to some genera of Chlorophyta (Apatococcus, Desmococcus), but differs by organization of vegetative and reproductive cells typical for Streptophyta, as well as by 18S rRNA and rbcL sequences.
Habitat: sand or sandy soil.
Type locality: Slovakia, near town Malacky, Lozorno, completely burned out pine forest (pine plantation) in drift sand, sandy soil with thin layer of ash, altitude: 48.373890864, longitude: 17.029250428, 202 m a.s.l.
Holotype (designated here): The authentic strain AL-63 (BCCO 30_2608, SAG 2560) is permanently cryopreserved in metabolically inactive state (cryopreserved in liquid nitrogen) in the Culture collection of soil algae and cyanobacteria of the Institute of Soil Biology, České Budějovice, Czech Republic. Additionally culture material of authentic strain AL-63 (BCCO 30_2608, SAG 2560) preserved in 4% formaldehyde, Algotheca of M.G. Kholodny Institute of Botany of NASU of Ukraine (AKW-32372).
Iconotype (designated here in support of the holotype): Figure 7F–H.
Authentic strain: AL-63 was deposited in SAG, University of Göttingen, Germany under number SAG 2560 and BCCO, Institute of Soil Biology, České Budějovice, Czech Republic under number BCCO 30_2608.
Other strain: Prim-3-3 (Ukraine, Kiliya District, Odessa Region, vicinity of the Danube Delta Biosphere Reserve, the Black Sea coast, sand dunes, greenish crust, altitude: 45.544505, longitude: 29.66442, 4 m a.s.l.) was deposited in SAG, University of Göttingen, Germany under number SAG 2562 and BCCO, Institute of Soil Biology, České Budějovice, Czech Republic under number BCCO 30_2609.
Etymology: arenaria = from Latin word arena – sand (referring to type habitat).
Streptosarcina costaricana Mikhailyuk et Lukešová sp. nov. (Figs. 5H–M, 7I–K, 8G–I)
Diagnosis. Thallus disintegrating in culture to short filaments, cell diads and unicells, but with tendency to true branching. Vegetative cells ellipsoid to cylindrical and elongated cylindrical, (7.0)7.7–8.6 μm width, 13.2–30.0(41.9) μm length. H-like fragments of cell wall observed. Chloroplast parietal, plate-shaped, with waved or dissected margin. Pyrenoid single in young cells or several formed a line in old elongated cells. Pyrenoid surrounded by layers of small starch grains. Vegetative reproduction by cell division in several planes (sporulation-like type) and fragmentation of thalli. Formation of branches by budding. Asexual and sexual reproduction not observed.
Differs from S. arenaria by shape of cells and general filamentous branched morphology. Morphologically similar with other filamentous algae from Streptophyta (Interfilum, Klebsormidium), but differs by absence of mucilage and true branching. Generally similar with some genera of branching algae from Chlorophyta (Pseudendoclonium), but differs by organization of vegetative cells typical for Streptophyta, as well as by 18S rRNA and rbcL sequences.
Habitat: soil.
Type locality: Costa Rica, CICAFE, Suelo cafetal, sin sombra, soil, altitude: 10.033693, longitude: −84.137314, 50 m a.s.l.
Holotype (designated here): The authentic strain SAG 36.98 (BCCO 30_2610) is permanently cryopreserved in metabolically inactive state (cryopreserved in liquid nitrogen) in the Culture collection of soil algae and cyanobacteria of the Institute of Soil Biology, České Budějovice, Czech Republic. Additionally culture material of authentic strain SAG 36.98 (BCCO 30_2610) preserved in 4% formaldehyde, Algotheca of M.G. Kholodny Institute of Botany of NASU of Ukraine (AKW-32373).
Iconotype (designated here in support of the holotype): Figure 7I–K.
Authentic strain: SAG 36.98 was deposited in SAG, University of Göttingen, Germany in 1998, by U. Wydrzycka, original strain number GCh-05-1.
Etymology: costaricana = from Costa Rica (referring to type locality).
Definition and Phylogeny of the Genus Streptofilum
Isolate Luk-316a forms a separate lineage in the Streptophyta based on combined 18S rRNA and rbcL phylogeny. Therefore, it is possible to refer this to a new genus (Streptofilum gen. nov.). The investigated strain exhibited some morphological and ultrastructural characters typical for other streptophycean algae: general filamentous morphology, plate-shaped chloroplast, pyrenoid with a layer of starch grains, large peroxisome between the chloroplast and the nucleus (Cook 2004; Lokhorst et al. 1988, 2000; Lokhorst 1996; Marin and Melkonian 1999; Mikhailyuk et al. 2008, 2014; Rindi et al. 2011; Sluiman 1985). Morphologically Streptofilum looks similar to a species of Interfilum because of the cell shape, organization as disintegrating short filament surrounded by a mucilage envelope and formation of mucilaginous cap-like structures (Mikhailyuk et al. 2008). On the other hand, cells of Streptofilum are generally smaller, and mucilage has a different structure (striated with smooth margin in Interfilum, and morphologically homogenous with waved margin in Streptofilum).
TEM investigation of vegetative cells of Streptofilum showed cellular coverage by an unusual and unique structure: plasmalemma covered by a mucilage envelope with submerged layers of specific piliform scales. Such type of cell coverage can be compared with submicroscopic organic scales of some prasinophyte flagellate algae (Domozych and Korbusieski 1985; Manton 1966; McFadden et al. 1986; Melkonian 1982; Melkonian et al. 1991; Moestrup 1990, etc.). Organic scales are also typical for the cell and flagella surface of other representatives of the Streptophyta: Mesostigma, zoospores and gametes of Chaetosphaeridium, Coleochaete, Chlorokybus, genera of Charophyceae as well as some embryophytes (mosses and liverworts, Lycopodium L., Psilotum Sw. etc.) (Domozych et al. 1991, 1992; Duncan et al. 1997; Maden et al. 1996; Marin and Melkonian 1999; Moestrup 1970; Renzaglia et al. 2001; Van den Hoek et al. 1995). Scales of Streptofilum probably originated by the same mechanism as described for the other mentioned taxa, i.e. presumable originating from the Golgi bodies followed by a transport to the cell surface. Despite some similarity, scales of Streptofilum are completely different compared to the other taxa, because they are oriented irregularly on the cell surface and have a much more simple appearance. Scales of prasinophytes and other Streptophyta are morphologically very diverse, having a complicated structures and shapes. For example, Mesostigma has three different types of scales on the cell surface (Manton and Ettl 1965). Scales of variable structure and refined filigree shape are typical for the flagellar surface of zoospores and spermatozoids of Chara L., Chaetosphaeridium, Coleochaete (Duncan et al. 1997; Marin and Melkonian 1999). Usually these scales cover the cell surface overlying each other, similar to tile or fish scales. Structure and organization of Streptofilum scales appear rather unique. However, the chemical composition and structure of the cell coverage of Streptofilum remain unknown and will be subject for further investigation. This will allow better understanding of its origin and development, and could serve as an ultrastructural proxy for evolutionary differentiation among streptophycean algae.
Streptofilum Mikhailyuk et Lukešová gen. nov.
Description. Thallus filamentous, short, unbranched, often disintegrated to diads and unicells. Cells naked, surrounded by dense layers of piliform scales possibly organic nature (visible with TEM) and layer of homogenous mucilage. Vegetative cells ellipsoid to ovoid and hemispherical. Chloroplast parietal, plate-shaped, with single pyrenoid surrounded by a layer of starch grains. Vegetative reproduction by cell division in one plane (sporulation-like type) and fragmentation of thalli. Asexual and sexual reproduction not observed.
Morphologically similar to filamentous species of Interfilum (Streptophyta), but differs by homogenous mucilage and unique cell coverage formed by specific piliform scales as well as by 18S rRNA and rbcL sequences.
Type species: Streptofilum capillatum Mikhailyuk et Lukešová sp. nov.
Etymology: from the name of division – Streptophyta and Latin word filum – filament, thread (referring to filamentous morphology).
Streptofilum capillatum Mikhailyuk et Lukešová sp. nov. (Figs. 6, 7L–N, 9)
Description. Tallus filamentous, short, unbranched, often disintegrated to diads and unicells. Cells naked, surrounded by dense layers of piliform scales possibly of organic nature (visible with TEM) and layer of homogenous mucilage with waved lobbed edge. Sometimes mucilaginous caps on cells or specific collar in the middle of cells are formed. Vegetative cells ellipsoid to ovoid and hemispherical, (4.0)5.7–6.7(8.0) μm length, 4.6–5.0 μm width. Chloroplast parietal, plate-shaped, with smooth margin and single pyrenoid surrounded by a layer of starch grains. Vegetative reproduction by cell division in one plane (sporulation-like type) and fragmentation of thalli. Asexual and sexual reproduction not observed.
Habitat: sandy soil.
Type locality: Czech Republic, near Kamenice nad Lipou, Benešov, sandy soils, arable field, pH 6.4, altitude: 49.326089, longitude: 15.033993105, 602 m a.s.l.
Holotype (designated here): The authentic strain Luk-316a (BCCO 30 0050, SAG 2559) is permanently cryopreserved in metabolically inactive state (cryopreserved in liquid nitrogen) in the Culture collection of soil algae and cyanobacteria of the Institute of Soil Biology, České Budějovice, Czech Republic. Additionally culture material of authentic strain Luk-316a (BCCO 30 0050, SAG 2559) preserved in 4% formaldehyde, Algotheca of M.G. Kholodny Institute of Botany of NASU of Ukraine (AKW-32374).
Iconotype (designated here in support of the holotype): Figure 7L–N.
Authentic strain: Luk-316a was deposited in SAG, University of Göttingen, Germany under number SAG 2559 and BCCO, Institute of Soil Biology, České Budějovice, Czech Republic under number BCCO 30_0050.
Etymology: capillatum = from Latin words capillus – hair and capillatus – hairy (referring to unique cell coverage by piliform scales).
Relevance of the Newly Described Taxa in Evolution of Streptophycean Lineages
Investigation of algal taxa from early diverging lineages of Streptophyta is very important for understanding the evolutionary traits of this group, as well as questions on the origin of embryophytes and the process of terrestrialization. Some classes of streptophycean algae are represented only by one or few genera (Mesostigmatophyceae and Chlorokybophyceae) (Leliaert et al. 2012; Lewis and McCourt 2004; Marin and Melkonian 1999). Genetic distances between these taxa are large, as many representatives might be extinct or were not discovered in nature until now.
The newly described and investigated taxa supplement current knowledge about diversity of early diverging lineages of Streptophyta. These taxa eventually will shorten genetic distance between known genera and classes. Streptofilum represents a conspicuously separate lineage in the Streptophyta and due its unique phylogenetic position might be erected even to class level in the future. For this, a more detailed investigation of genetic, ultrastructural, biochemical and physiological traits is required. Such results might contribute to an improved phylogeny of the Streptophyta.
Finding a new alga with true thallus branching inside Klebsormidiophyceae is also a new observation. This class unites taxa of filamentous algae with uniseriate unbranched thalli (Ettl and Gärtner 2014; Lokhorst 1996; Lokhorst et al. 2000; Rindi et al. 2011; Van den Hoek et al. 1995). Some reports on branching in Klebsormidium, Interfilum or Entransia are related to cell division in several plains due to cell deformation or cellular organelles displacement (Mikhailyuk et al. 2014), as well as because of germination of aplanospores inside filaments (Cook 2004; Lokhorst 1996). These cases are considered as characters of so called “false branching”. Streptosarcina costaricana, however, showed features corresponding with the formation of true branched filaments. This type of branching is typical for algae with a filamentous branched (heterotrichal) thallus, such as Cladophora Kützing, Trentepohlia Martius, the recently described Ekerewekia Kastovský, Fučíková, Stenclová et Brewer-Carías etc. (Kaštovský et al. 2016; Moshkova 1979; Starmach 1972; Van den Hoek et al. 1995). Consequently, it is possible to state the occurrence of true branching in the Streptophyta inside the class Klebsormidiophyceae.
The most unique morphological observation of the present paper is the unusual cell coverage in Streptofilum capillatum. The authors are not aware on any similar cell coverage among algae. Although it is possible to superficially compare piliform coverage in S. capillatum with submicroscopic organic scales of early diverging representatives of the green algae: Different lineages of prasinophytes (Chlorophyta) and Mesostigmatophyceae (Streptophyta), as well as with flagellate cells of some streptophycean algae and early-diverging embryophytes (Domozych et al. 1991; Duncan et al. 1997; Maden et al. 1996; Moestrup 1970; Renzaglia et al. 2001; etc.). But the cell coverage of Streptofilum is highly specific. Therefore, Streptofilum represents a separate lineage of Streptophyta based on molecular and ultrastructural traits despite its simple morphology. Undoubtedly further investigation of the cell coverage of Streptofilum will give new material for understanding the structure and evolution of streptophycean algae.
It is amazing that Streptofilum originates from an usual terrestrial habitat in Central Europe (soil of Czech Republic), a region which is considered as one of the best investigated with respect to terrestrial algae in the world (Büdel et al. 2016; Rindi et al. 2009). Therefore, using an integrative approach (combination of molecular-phylogenetic, morphological and ultrastructural markers) is essential for exploring novel biodiversity of microalgae.
Morphological parallelism or similarity of representatives of different phylogenetic lineages is a typical phenomenon for various algal groups as a result of adaptation of algae to similar environmental conditions. The newly described taxa are difficult to identify at the morphological level. However, these algae show quite prominent morphological and ultrastructural characters typical for Streptophyta. On the other hand they are similar to other genera of streptophycean algae. Therefore, it is difficult to distinguish Hormidiella and Klebsormidium, Streptosarcina and Interfilum or Chlorokybus, Streptofilum and Interfilum (see Table 1). The useful morphological characters in these cases are not the general morphology, but small details of the structure of vegetative and reproductive stages described above.
Table 1.
Comparison of morphological and some ultarstructural characters of new and known genera of Streptophyta which characterized by similar morphology.1
| Character | Klebsormidium | Interfilum | Hormidiella | Entransia | Streptosarcina | Chlorokybus | Streptofilum |
|---|---|---|---|---|---|---|---|
| Thallus morphology | Filaments or short filaments disintegrating to unicells | Filaments surrounding by mucilage, easily disintegrating to unicells, or packets | Filaments with a stalk (or without it in culture conditions) | Filaments | Packets or branched filaments | Packets surrounding by strong mucilage envelope | Filaments surrounding by mucilage, easily disintegrating to unicells |
| Chloroplast morphology | Plate shaped, with smooth margin or with marginal dissections | Plate shaped, waved or dissected on several lobes | Plate shaped, with smooth margin | Plate shaped, deeply dissected on several long finger-like lobes | Plate shaped, waved or dissected on several lobes | Plate shaped with smooth margin | Plate shaped, with smooth margin |
| Pyrenoid morphology | Single, surrounded by several/many small starch grains | Single, surrounded by several starch grains | Single, surrounded by several starch grains | Several, surrounded by many small starch grains | Single or several in old stage, surrounded by many small starch grains | Single, surrounded by many small starch grains, presence of pseudopyrenoid (discrepancy of chloroplast lamellae) | Single, surrounded by several starch grains |
| Pyrenoid ultrastructure | Pyrenoid matrix traversed by several/many parallel thylakoids | Pyrenoid matrix traversed by several parallel thylakoids | Pyrenoid matrix homogenous | Pyrenoid matrix traversed by many parallel thylakoids | Pyrenoid matrix traversed by several parallel thylakoids | Pyrenoid matrix traversed by many parallel thylakoids | Pyrenoid matrix homogenous |
| Cell coverage | Cell wall, thin mucilage envelope in some species | Cell wall with mucilage envelope striated structure | Cell wall | Cell wall | Cell wall | Cell wall, strong mucilage envelope | Layers of piliform scales submerged in the mucilage with homogenous structure and waved margin |
| Cell wall/coverage remnants | H-like and cap-like (rarely) fragments | Cap-like fragments | H-like fragments, rarely | H-like fragments | H-like fragments | Cap-like fragments | Cap-like fragments |
| Cell division | Mostly in one plane (sporulation-like type) | In one/several planes (sporulation-like type) | In one plane (sporulation-like type) | In one plane (perhaps sporulation-like type) | In several planes (sporulation-like type) | In several planes (sporulation-like type) | In one plane (sporulation-like type) |
| Asexual reproduction | Zoospores, aplanospores | – | Zoospores, aplanospores | Zoospores, aplanospores | Zoospores, aplanospores | Zoospores, aplanospores | – |
| Sporangium morphology | One zoospore per sporangium, realized through opening in cell wall | – | One zoospore per sporangium, realized through opening in cell wall, sporangium with rounded protrusion | Zoospores realized through opening in cell wall | One zoospore per sporangium, realized through opening in cell wall, sporangium with rounded protrusion | One zoospore per sporangium, realized through opening in cell wall | – |
| Zoospores morphology | Wall- and stigma-less, with two equal subapical flagella | – | Wall- and stigma-less, with two equal or unequal (doubtful) subapical flagella | – | Wall- and stigma-less, with two equal subapical flagella | Wall-less (covered by submicroscopic scales) and stigma-less, with two equal subapical flagella | – |
| Zoospores germination | Ellipsoid cell with mucilage disc | – | Ellipsoid cell with stalk and holdfast | Ellipsoid cell with apical spine and mucilage adhesive | Spherical cell | Spherical cell | – |
| Young thallus originated from zoospore | Heteropolar filament with mucilage disc | – | Heteropolar filament with stalk | Heteropolar filament with mucilage adhesive and apical spine | Homopolar, sarcinoid packet | Homopolar, sarcinoid packet | – |
| Sexual reproduction | Isogamy, doubtful | – | Heterogamy, doubtful | – | – | – | – |
| Big single peroxisome | Present | Present | Present | Perhaps absent | Present | Present | Present |
| Ecology | Terrestrial and aquatic | Terrestrial | Terrestrial or amphibian | Aquatic (typical for peat bogs) | Terrestrial | Terrestrial | Terrestrial |
| Number of described species | About 20 | 3 | 3 | 2 | 2 | 1 | 1 |
Characters of known genera were provided on the base of following works: Lokhorst 1996; Mikhailyuk et al. 2008, 2014; Lokhorst et al. 1988, 2000; Cook 2004; Ettl and Gärtner 2014.
Conclusions
Two new genera and three new species were erected using an integrative approach in the Streptophyta, a group of great evolutionary and genomic interest related to the ancestry of embryophytes. Additionally, emendation of the diagnosis and epitypification of Hormidiella parvula (type species of the genus) were proposed. The phylogenetic position of the new genera affects the current classification of early-diverging lineages of Streptophyta and the new data presented contribute to a deeper understanding of biodiversity and evolutionary tendencies inside this group.
Methods
Strains, culture conditions, light microscopy, ultrastructure
As material for the present study five unialgal strains were used. They were isolated from terrestrial habitats (soil, sandy soil or biological soil crusts) of Europe (Ukraine, Czech Republic and Slovakia) and America (USA and Costa-Rica). A short information about sample areas and sampling sites is provided in Table 2. Four strains were isolated and preliminary investigated morphologically by Alena Lukešová and Tatiana Mikhailyuk during several projects devoted to studies on algal biodiversity in soils of different regions. One strain was isolated by Ursula Wydrzycka (Universidad de Costa-Rica) and deposited in the SAG collection in 1998, and was later morphologically investigated by us. All preliminary data already indicated that the 5 taxa have uncommon morphological traits, and comprise mainly unstudied isolates of Streptophyta. Later they were investigated in more detail using molecular phylogenetic methods in the frame of research projects devoted to Streptophyta (Mikhailyuk et al. 2008, 2014, 2015; Rindi et al. 2011).
Table 2.
Details of strains of new streptophycean algae examined in the present study.
| Species | Strain label | Collection information | Culture numbers | Gen Bank accession number |
||
|---|---|---|---|---|---|---|
| 18S rRNA | ITS-1, 5.8S rRNA, ITS-2 | rbcL | ||||
| Hormidiella parvula | Luk-89 | USA, Wyoming, Belle Ayr Mine near Gillette, natural prairie, soil, A. Lukešová, May 2008, altitude: 44.2910915, longitude: −105.50222050, 1395 m a.s.l. |
BCCO 30_2136 SAG 2558 |
MG652625 | MG652614 | |
| Streptosarcina arenaria gen. and sp. nov. | AL-63 | Slovakia, near town Malacky, Lozorno, completely burned out pine forest (pine plantation) in drift sand, sandy soil with thin layer of ash, A. Lukešová, October 1993, altitude: 48.373890864, longitude: 17.029250428, 202 m a.s.l. |
BCCO 30_2608 SAG 2560 |
MG652622 | MG652611 | |
| Prim-3-3 | Ukraine, Odessa oblast, Kiliya district, near the Danube Delta Biosphere Reserve, Black Sea coast, sand dunes, soil crust, T. Mikhailyuk, September 2013, altitude: 45.544505, longitude: 29.66442, 4 m a.s.l. |
BCCO 30_2609 SAG 2562 |
MG652623 | MG652612 | ||
| Streptosarcina costaricana gen. and sp. nov. | GCh-05-1 | Costa Rica, Cicafe, Suelocafetal, sin sombra, soil, U. Wydrzycka, 1991, altitude: 10.033693, longitude: −84.137314, 50 m a.s.l. |
BCCO 30_2610 SAG 36.98 |
MG652624 | MG652613 | |
| Streptofilum capillatum gen. and sp. nov. | Luk-316a | Czech Republic, near Kamenice nad Lipou, Benešov, sandy soils, arable field, pH 6.4, A. Lukešová, April 2002, altitude: 49.326089, longitude: 15.033993105, 602 m a.s.l. |
BCCO 30_0050 SAG 2559 |
MG652626 | – | MG652615 |
The strains were maintained on solid medium (1.5% agar with 3N BBM and vitamins) (Starr and Zeikus 1993) at 20 °C with 25 μmol photons m–2 s–1 (Osram Lumilux Cool White lamps L36W/840) under a light/dark cycle of 12:12 h L:D. Morphological examinations of unialgal cultures were performed using a Olympus BX51 light microscope with Nomarski DIC optics. Photomicrographs were taken with digital camera Olympus UC30 attached to the microscope and processed by software cell Sens Entry. Zoospore formation was induced by transferring the cultures into liquid BBM without nitrogen – 0N BBM and keeping them under dark conditions for several days. This procedure was done for all investigated strains, but zoospores were obtained in Hormidiella and some Streptosarcina strains (AL-63 and Prim-3-3) only. Mucilage of algal cells was stained with an aqueous solution of methylene blue.
Samples were fixed for transmission electron microscopy (TEM) using a standard chemical fixation protocol (2.5% glutaraldehyde, 1% OsO4 in 10 mM caccodylate buffer, pH = 6.8) according to Holzinger et al. (2009). Samples were dehydrated in increasing ethanol concentrations, transferred to modified Spurrś resin and heat polymerized. For TEM, ultrathin sections were prepared, counterstained with uranyl acetate and Reynold’s lead citrate, and investigated in Zeiss LIBRA 120 transmission electron microscopes at 80 kV. Images were captured with a TRS 2k SSCCD camera and further processed using Adobe Photoshop software (Adobe Systems Inc., San José, California, USA).
DNA isolation, PCR and sequencing
Genomic DNA of all investigated strains was extracted using the DNeasy Plant Mini Kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer’s instructions. Nucleotide sequences of the 18S rRNA gene together with ITS-1-5.8S-ITS-2 region were amplified using a set of Taq PCR Mastermix Kit (Qiagen GmbH) and algal-specific primers G800R, G500F and G730F (T. Pröschold, personal communication) or standard primers EAF3, ITS055R, NS1F and 18LR (Hamby et al. 1988; Marin et al. 1998, 2003). Nucleotide sequences of partial rbcL gene were obtained using specific primers for Klebsormidiophyceae rbcL-KF2, rbcL-KR2 and KF590 (Ryšánek et al. 2015; Škaloud and Rindi 2013). PCR reactions were made in a thermocycler T gradient Thermoblock (Biometra, Germany). The PCR for ribosomal operon comprised 30 cycles: initial denaturation at 96 °C for 3 min, the cyclic repeating denaturation 1 min at 96 °C, annealing 2 min at 55 °C, elongation fragment – 3 min at 68 °C, followed by a final elongation of the fragment – 7 min at 68 °C. The PCR for rbcL gene were made using conditions published in Škaloud and Rindi (2013). PCR products were cleaned using a Qiagen PCR purification kit (Qiagen GmbH) according to the manufacturer’s instructions. Cleaned PCR products were sequenced commercially by Qiagen Company using primers G800R, NS1F, N82F, 536R, 920R, 1400R, 920F, 1400F, GF, ITS2F and ITS05R (Hamby et al. 1988; Marin et al. 1998, 2003; Pröschold et al. 2005) for ribosomal operon and rbcL-KF2, rbcL-KR2 and KF590 for rbcL gene. The resulting sequences were assembled and edited using Geneious software (version 8.1.8; Biomatters). They were deposited at GenBank under the accession numbers MG652611–MG652615 and MG652622–MG652626.
Phylogenetic analyses
Sequences of our isolates were compared to those from reference strains at NCBI (http://www.ncbi.nlm.nih.gov) using BLASTn queries (Altschul et al. 1997) for searching of the closest relatives. For comparison with original strains, we used nucleotide sequences available in GenBank (NCBI) of representatives of the Klebsormidiophyceae, Coleochaetophyceae, Chlorokybophyceae, Mesostigmatophyceae, Zygnematophyceae, Charophyceae, Embryophytes, Chlorophyta and Glaucophyta as out-group. Multiple alignments of the nucleotide sequences of the 18S rRNA and rbcL genes were made using Mafft web server (version 7, Katoh and Toh, 2008) followed by manually editing in the program BioEdit (version 7.2). The evolutionary model that is best suited to the used database was selected on the basis of the lowest AIC value (Akaike 1974) and calculated in MEGA (version 6, Tamura et al. 2013). Phylogenetic trees were constructed in the program MrBayes 3.2.2 (Ronquist and Huelsenbeck 2003), using an evolutionary model GTR + G + I, with 5,000,000 generations. Two of the four runs of Markov chain Monte Carlo were made simultaneously, with the trees, taken every 500 generations. Split frequencies between runs at the end of calculations were below 0.01. The trees selected before the likelihood rate reached saturation were subsequently rejected. The reliability of tree topology verified by the maximum likelihood analysis (ML, GTR + I + G) were made using the program GARLI 2.0 (Zwickl 2006), and bootstrap support was calculated with 1,000 replicates.
Analysis of the ITS-2 secondary structure
The models of the secondary structure of ITS-2 region together with 5.8S–28S rRNA stem were predicted for species of Hormidiella and Streptosarcina. Helices were folded with the online software mfold (Zuker 2003) and visualized in the online tool Pseudoviewer (Byun and Han 2009). Compensatory base changes (CBCs), base-pair indels (mismatches, deletions, single or unpaired bases) and position of conservative regions of ITS-2 (15 base pairs of the 5.8S-18S rRNA stem, the first 5 base pairs of Helix I, the first 11 base pairs of Helix II (including the pyrimidine-pyrimidine mismatch), and all base pairs of Helix III) were estimated using recommendations published in Demchenko et al. (2012).
Acknowledgements
This study was supported by a Georg-Forster research fellowship from the Alexander von Humboldt Foundation and a Grant for Young Scientist Fellowship extended by INTAS (Ref. No. 06-1000014-6216) (T.M.). We thank the Deutsche Forschungsgemeinschaft (DFG) for financial support (KA899/16 (U.K.) and SPP 1991 Taxonomics (KG)). The study was supported by the Austrian Science Fund (FWF) project P 24242-B16 and I 1951-B16 to A.H. Our sincere thanks are extended to Dr. Thomas Pröschold (University of Innsbruck, Austria) for permission of using algal specific primers designed by him and Dr. Martin Albrecht (University of Rostock, Germany) for valuable help in purification of investigated strains. We thank Beatrix Jungwirth, University of Innsbruck, for help in cultivation of the algal strains and Dr. Maike Lorenz, University of Göttingen, for help during strain deposition to SAG.
References
- Akaike H. A new look at the statistical model identification. IEEE Trans Cont. 1974;19:716–723. [Google Scholar]
- Altschul SF, Madden TL, Schäffer AA. Gapped BLAST and PSIBLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker B, Marin B. Streptophyte algae and the origin of embryophytes. Ann Bot. 2009;103:999–1004. doi: 10.1093/aob/mcp044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya D, Medlin L. Algal phylogeny and the origin of land plants. Plant Physiol. 1998;116:9–15. [Google Scholar]
- Büdel B, Dulić T, Darienko T, Rybalka N, Friedl T. Cyanobacteria and algae of biological soil crusts. In: Weber B, Büdel B, Belnap J, editors. Biological Soil Crusts: Structure, Function, and Management. Springer; Berlin Heidelberg: 2016. pp. 55–80. [Google Scholar]
- Byun Y, Han K. PseudoViewer3: generating planar drawings of large-scale RNA structures with pseudoknots. Bioinformatics. 2009;25:1435–1437. doi: 10.1093/bioinformatics/btp252. [DOI] [PubMed] [Google Scholar]
- Cocquyt E, Verbruggen H, Leliaert F, De Clerck O. Evolution and cytological diversification of the green seaweeds (Ulvophyceae) Mol Biol Evol. 2010;27:2052–2061. doi: 10.1093/molbev/msq091. [DOI] [PubMed] [Google Scholar]
- Coleman AW. Is there a molecular key to the level of ‘biological species’ in eukaryotes? A DNA guide. Mol Phylogent Evol. 2009;50:197–203. doi: 10.1016/j.ympev.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Cook ME. Structure and asexual reproduction of the enigmatic charophycean green alga Entransia fimbriata (Klebsormidiales, Charophyceae) J Phycol. 2004;40:424–431. [Google Scholar]
- Darienko T, Pröschold T. Toward a monograph of non-marine Ulvophyceae using an integrative approach (Molecular phylogeny and systematics of terrestrial Ulvophyceae II.) Phytotaxa. 2017;324:1–41. [Google Scholar]
- Darienko T, Gustavs L, Mudimu O, Menendes C, Schumann R, Karsten U, Friedl T, Pröschold T. Chloroidium, a common terrestrial coccoid green alga previously assigned to Chlorella (Trebouxiophyceae, Chlorophyta) Eur J Phycol. 2010;45:79–95. [Google Scholar]
- Delwiche CF, Karol KG, Cimino MT, Sytsma KJ. Phylogeny of the genus Coleochaete (Coleochaetales, Charophyta) and related taxa inferred by analysis of the chloroplast gene rbcL. J Phycol. 2002;38:394–403. [Google Scholar]
- Demchenko E, Mikhailyuk T, Coleman AW, Pröschold T. Generic and species concepts in Microglena (previously the Chlamydomonas monadina group) revised using an integrative approach. Eur J Phycol. 2012;47:264–290. [Google Scholar]
- Domozych DS, Korbusieski TJ. The disruption of dictyosome structure, polarity and secretory activity in the scale-producing green alga, Pyramimonas inconstans. J Exp Bot. 1985;36:1304–1312. [Google Scholar]
- Domozych DS, Wells B, Shaw PJ. The basket scales of the green alga, Mesostigma viride: chemistry and ultrastructure. J Cell Sci. 1991;100:397–407. [Google Scholar]
- Domozych DS, Wells B, Shaw PJ. Scale biogenesis in the green alga Mesostigma viride. Protoplasma. 1992;167:19–32. [Google Scholar]
- Duncan TM, Renzaglia KS, Garbary DJ. Ultrastructure and phylogeny of the spermatozoid of Chara vulgaris (Charophyceae) P1 Syst Evol. 1997;204:125–140. [Google Scholar]
- Ettl H, Gärtner G. Syllabus der Boden-, Luft und Flecht-enalgen. 2nd edn. Spektrum Akademischer Verlag; Munich: 2014. p. 772. [Google Scholar]
- Finet C, Timme RE, Delwiche CF, Marlétaz F. Multigene phylogeny of the green lineage reveals the origin and diversification of land plants. Curr Biol. 2010;20:2217–2222. doi: 10.1016/j.cub.2010.11.035. [DOI] [PubMed] [Google Scholar]
- Freitas LC, Loverde-Oliveira SM. Checklist of green algae (Chlorophyta) for the state of Mato Grosso, Central Brazil. Check List. 2013;9:1471–1483. [Google Scholar]
- Friedl T, Rybalka N. Systematics of the green algae: a brief introduction to the current status. Progr Bot. 2012;73:259–280. [Google Scholar]
- Gontcharov AA, Melkonian M. Unusual position of the genus Spirotaenia (Zygnematophyceae) among streptophytes revealed by SSU rDNA and rbcL sequence comparisons. Phycologia. 2004;43:105–113. [Google Scholar]
- Gontcharov AA, Marin B, Melkonian M. Molecular phylogeny of conjugating green algae (Zygnemophyceae, Streptophyta) inferred from SSU rDNA sequence comparisons. J Mol Evol. 2003;56:89–104. doi: 10.1007/s00239-002-2383-4. [DOI] [PubMed] [Google Scholar]
- Graham LE. Coleochaete and the origin of land plants. Am J Bot. 1984;71:603–608. [Google Scholar]
- Graham LE, Delwiche CF, Mishler BD. Phylogenetic connections between the «green algae» and the «bryophytes». Adv Bryol. 1991;4:213–244. [Google Scholar]
- Hamby RK, Sims LE, Issel LE, Zimmer EA. Direct RNA sequencing: optimization of extraction and sequencing techniques for work with higher plants. Plant Mol Biol Rep. 1988;6:179–197. [Google Scholar]
- Herburger K, Karsten U, Holzinger A. Entransia and Hormidiella, sister lineages to Klebsormidium (Streptophyta) respond differently to light and temperature gradients. Protoplasma. 2016;253:1309–1323. doi: 10.1007/s00709-015-0889-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzinger A, Roleda MY, Lütz C. The vegetative arctic green alga Zygnema is insensitive to experimental UV exposure. Micron. 2009;40:831–838. doi: 10.1016/j.micron.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Iyengar MOP, Kanthamma S. Hormidiella, a new member of the Ulothrichaceae. J Indian Bot Soc. 1940;19:157–166. [Google Scholar]
- Karol KG, McCourt RM, Cimino MT, Delwiche CF. The closest living relatives of land plants. Science. 2001;294:2351–2353. doi: 10.1126/science.1065156. [DOI] [PubMed] [Google Scholar]
- Kaštovský J, Veselá J, Bohunická M, Fučíková K, Štenclová L, Brewer-Carías C. New and unusual species of cyanobacteria, diatoms and green algae, with a description of a new genus Ekerewekia gen. nov. (Chlorophyta) from the table mountain Churš-tepui, Chimantý Massif (Venezuela) Phytotaxa. 2016;247:153–180. [Google Scholar]
- Katoh K, Toh H. Recent developments in the MAFFT multiple sequence alignment program. Brief Bioinform. 2008;9:286–298. doi: 10.1093/bib/bbn013. [DOI] [PubMed] [Google Scholar]
- Kranz HD, Miks D, Siegler ML, Capesius I, Sensen CW, Huss VA. The origin of land plants: phylogenetic relationships among charophytes, bryophytes, and vascular plants inferred from complete small-subunit ribosomal RNA gene sequences. J Mol Evol. 1995;41:74–84. doi: 10.1007/BF00174043. [DOI] [PubMed] [Google Scholar]
- Leliaert F, Smith DR, Moreau H, Herron MM, Verbruggen H, Delwiche CF, De Clerck O. Phylogeny and molecular evolution of the green algae. Crit Rev Plant Sci. 2012;31:1–46. [Google Scholar]
- Lemieux C, Otis C, Turmel M. A clade uniting the green algae Mesostigma viride and Chlorokybus atmophyticus represents the deepest branch of the Streptophyta in chloroplast genome-based phylogenies. BMC Biology. 2007;5 doi: 10.1186/1741-7007-5-2. art no. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux C, Otis C, Turmel M. Comparative chloroplast genome analyses of Streptophyte green algae uncover major structural alterations in the Klebsormidiophyceae, Coleochaetophyceae and Zygnematophyceae. Front Plant Sci. 2016;7 doi: 10.3389/fpls.2016.00697. art no. 697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis LA, McCourt RM. Green algae and the origin of land plants. Am J Bot. 2004;91:1535–1556. doi: 10.3732/ajb.91.10.1535. [DOI] [PubMed] [Google Scholar]
- Lokhorst GM. Comparative taxonomic studies on the genus Klebsormidium (Charophyceae) in Europe. Cryptogamic Studies, 5. Gustav Fischer Stuttgart. 1996:132. [Google Scholar]
- Lokhorst GM, Star W. Ultrastructure of mitosis and cytokinesis in Klebsormidium mucosum nov. comb., formerly Ulothrix verrucosa (Chlorophyta) J Phycol. 1985;21:466–476. [Google Scholar]
- Lokhorst GM, Sluiman HJ, Star W. The ultrastructure of mitosis and cytokinesis in the sarcinoid Chlorokybus atmophyticus (Chlorophyta, Charophyceae) revealed by rapid freeze fixation and freeze substitution. J Phycol. 1988;24:237–248. [Google Scholar]
- Lokhorst GM, Star W, Lukešová A. The new species Hormidiella attenuata (Klebsormidiales), notes on morphology and reproduction. Algol Stud. 2000;100:11–27. [Google Scholar]
- Maden AR, Renzaglia KS, Whittier DP. Ultrastructure of the spermatozoid of Lycopodium obscurum (Lycopodiaceae) Am J Bot. 1996;83:419–429. [Google Scholar]
- Manton I. Observations on scale production in Pyramimonas amylifera Conrad. J Cell Sci. 1966;1:429–438. doi: 10.1242/jcs.1.4.429. [DOI] [PubMed] [Google Scholar]
- Manton I, Ettl H. Observations on the fine structure of Mesostigma viride Lauterborn. J Linn Soc (Bot) 1965;59:175–184. [Google Scholar]
- Marin B, Melkonian M. Mesostigmatophyceae, a new class of streptophyte green algae revealed by SSU rRNA sequence comparisons. Protist. 1999;150:399–417. doi: 10.1016/S1434-4610(99)70041-6. [DOI] [PubMed] [Google Scholar]
- Marin B, Klingberg M, Melkonian M. Phylogenetic relationships among the Cryptophyta: Analyses of nuclear-encoded SSU rRNA sequences support the monophyly of extant plastid-containing lineages. Protist. 1998;149:265–276. doi: 10.1016/S1434-4610(98)70033-1. [DOI] [PubMed] [Google Scholar]
- Marin B, Palm A, Klingberg M, Melkonian M. Phylogeny and taxonomic revision of plastid-containing euglenophytes based on SSU rDNA sequence comparisons and synapomorphic signatures in the SSU rRNA secondary structure. Protist. 2003;154:99–145. doi: 10.1078/143446103764928521. [DOI] [PubMed] [Google Scholar]
- Mattox KR, Stewart KD. Classification of the Green Algae: A Concept Based on Comparative Cytology. In: Irvin DEG, John DM, editors. Systematics of the Green Algae. Acad Press; London: 1984. pp. 29–72. [Google Scholar]
- McCourt RM, Delwiche CF, Karol KG. Charophyte algae and land plant origins. Trends Ecol Evol. 2004;19:661–666. doi: 10.1016/j.tree.2004.09.013. [DOI] [PubMed] [Google Scholar]
- McFadden GI, Preisig HR, Melkonian M. Golgi apparatus activity and membrane flow during scale biogenesis in the green flagellate Scherffelia dubia (Prasinophyceae) II. Cell wall secretion and assembly. Protoplasma. 1986;131:174–184. [Google Scholar]
- Melkonian M. Effect of divalent cations on flagellar scales in the green flagellate Tetraselmis cordiformis. Protoplasma. 1982;111:221–233. [Google Scholar]
- Melkonian M. Flagellar apparatus ultrastructure in Mesostigma viride (Prasinophyceae) Pl Syst Evol. 1989;164:93–122. [Google Scholar]
- Melkonian M, Becker B, Becker D. Scale formation in algae. J Electron Microsc Techn. 1991;17:165–178. doi: 10.1002/jemt.1060170205. [DOI] [PubMed] [Google Scholar]
- Mikhailyuk T, Holzinger A, Massalski A, Karsten U. Morphology and ultrastructure of Interfilum and Klebsormidium (Klebsormidiales, Streptophyta) with special reference to cell division and thallus formation. Eur J Phycol. 2014;49:395–412. doi: 10.1080/09670262.2014.949308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhailyuk T, Glaser K, Holzinger A, Karsten U. Biodiversity of Klebsormidium (Streptophyta) from alpine biological soil crusts (Alps, Tyrol, Austria, and Italy) J Phycol. 2015;51:750–767. doi: 10.1111/jpy.12316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhailyuk TI, Sluiman H, Massalski A, Mudimu O, Demchenko E, Kondratyuk S, Friedl T. New streptophyte green algae from terrestrial habitats and an assessment of the genus Interfilum (Klebsormidiophyceae, Streptophyta) J Phycol. 2008;44:1586–1603. doi: 10.1111/j.1529-8817.2008.00606.x. [DOI] [PubMed] [Google Scholar]
- Moestrup Ø. The fine structure of mature spermatozoids of Chara corallina, with special reference to microtubules and scales. Planta. 1970;93:295–308. doi: 10.1007/BF00384103. [DOI] [PubMed] [Google Scholar]
- Moestrup Ø. Scale structure in Mantoniella squamata, with some comments on the phylogeny of Prasinophyceae. Phycologia. 1990;29:437–442. [Google Scholar]
- Moshkova NO. Vyznacnyk prisnovodnych vodorostej Ukrainskoj RSR. VI. Nauk. Dumka; Kiev: 1979. Ulotrichales, Cladophorales; p. 498. [in Ukrainian] [Google Scholar]
- Paczuska B, Paczuski R. Small water ponds as reservoirs of algae biodiversity. Oceanolog Hydrobiol Stud. 2015;44:480–486. [Google Scholar]
- Patrick R. The Eastern and Southeastern States. III. John Wiley and Sons, Inc; New York: 1996. Rivers of the United States; p. 829. [Google Scholar]
- Pickett-Heaps JD. Cell division in Klebsormidium subtilissimum (formely Ulothrix subtilissima) and its possible phylogenetic significance. Cytobiosis. 1972;6:167–183. [PubMed] [Google Scholar]
- Pröschold T, Harris EH, Coleman AW. Portrait of a species: Chlamydomonas reinhardtii. Genetics. 2005;170:1601–1610. doi: 10.1534/genetics.105.044503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renzaglia KS, Johnson TH, Gates HD, Whittier DP. Architecture of the sperm cell of Psilotum. Am J Bot. 2001;88:1151–1163. [PubMed] [Google Scholar]
- Rindi F, Allali HA, Lam DW, López-Bautista M. An overview of the Biodiversity and Biogeography of Terrestrial Green Algae. In: Rescingo V, et al., editors. Biodiversity Hotspots. Nova Science Publ Inc; 2009. pp. 105–122. [Google Scholar]
- Rindi F, Mikhailyuk TI, Sluiman HJ, Friedl T, López-Bautista JM. Phylogenetic patterns and phylogenetic relationships in Interfilum and Klebsormidium (Klebsormidiophyceae, Streptophyta) Mol Phyl Evol. 2011;58:218–231. doi: 10.1016/j.ympev.2010.11.030. [DOI] [PubMed] [Google Scholar]
- Ronquist F, Huelsenbeck JP. MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Ryšánek D, Hrčková K, Škaloud P. Global ubiquity and local endemism of free-living terrestrial protists: phylogeographic assessment of the streptophyte alga Klebsormidium. Environ Microbiol. 2015;17:689–698. doi: 10.1111/1462-2920.12501. [DOI] [PubMed] [Google Scholar]
- Škaloud P, Rindi F. Ecological differentiation of cryptic species within an asexual protist morphospecies: a case study of filamentous green alga Klebsormidium (Streptophyta) J Eukaryot Microbiol. 2013;60:350–362. doi: 10.1111/jeu.12040. [DOI] [PubMed] [Google Scholar]
- Sluiman HJ. Comparative studies on the ultrastructure, phylogeny and classification of green algae. VU Uitgeverij; Amsterdam: 1985. p. 155. Academisch proefschrift. [Google Scholar]
- Sluiman HJ, Guihal C, Mudimu O. Assessing phylogenetic affinities and species delimitations in Klebsormidiales (Streptophyta): nuclear-encoded rDNA phylogenies and ITS secondary structure models in Klebsormidium, Hormidiella, and Entransia. J Phycol. 2008;44:183–195. doi: 10.1111/j.1529-8817.2007.00442.x. [DOI] [PubMed] [Google Scholar]
- Starmach K. Flora sladkowodna Polski. Vol. 10. Panstwowe Wyadwnictwo Naukowe; Warsaw, Poland: 1972. Chlorophyta III. Zielenice nitkowate: Ulotrichales, Ulvales, Prasiolales, Sphaeropleales, Cladophorales, Chaetophorales, Trentepohliales, Siphonales, Dichotomosiphonales; p. 750. [Google Scholar]
- Starr RC, Zeikus JA. UTEX the culture collection of algae at the University of Texas at Austin. J Phycol. 1993;29(Suppl):1–106. [Google Scholar]
- Stewart KD, Floyd GL, Mattox KR, Davis ME. Cytochemical demonstration of a single peroxisome in a filamentous green algae. J Cell Biol. 1972;54:431–434. doi: 10.1083/jcb.54.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subrahmanyan A. Structure and reproduction of Hormidiella bharatiensis sp. nov. Hydrobiologia. 1976;48:33–36. [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turmel M, Otis C, Lemieux C. The chloroplast genome sequence of Chara vulgaris sheds new light into the closest green algal relatives of land plants. Mol Biol Evol. 2006;23:1324–1338. doi: 10.1093/molbev/msk018. [DOI] [PubMed] [Google Scholar]
- Turmel M, Otis C, Lemieux C. An unexpectedly large and loosely packed mitochondrial genome in the charophycean green alga Chlorokybus atmophyticus. BMC Genomics. 2007;8:137. doi: 10.1186/1471-2164-8-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Hoek C, Mann DG, Jahns HM. Algae: An Introduction to Phycology. Cambr Univ Press; Cambridge: 1995. p. 623. [Google Scholar]
- Wickett NJ, Mirarab S, Nguyen N, Warnow T, Carpenter E, Matasci N, Ayyampalayam S, Barker MS, Burleigh JG, Gitzendanner MA, Ruhfel BR, et al. Phylotranscriptomic analysis of the origin and early diversification of land plants. Proc Natl Acad Sci USA. 2014;111:E4859–E4868. doi: 10.1073/pnas.1323926111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodniok S, Brinkmann H, Glockner G, Heidel A, Philippe H, Melkonian M, Becker B. Origin of land plants: Do conjugating green algae hold the key? BMC Evol Biol. 2011;11:104–114. doi: 10.1186/1471-2148-11-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucl Acid Res. 2003;31:3406–3416. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwickl DJ. Genetic algorithm approaches for the phylogenetic analysis of large biological sequence datasets under the maximum likelihood criterion. Ph.D. dissertation. The University of Texas at Austin; 2006. [Google Scholar]