 The trypanocidal activity of N-hexyl naphthoimidazoles (and other N-alkyl-naphthoimidazoles) is demonstrated for the first time, supporting further studies for rational chemical optimization.
The trypanocidal activity of N-hexyl naphthoimidazoles (and other N-alkyl-naphthoimidazoles) is demonstrated for the first time, supporting further studies for rational chemical optimization.
Abstract
The QSAR study of 34 2-aryl-naphthoimidazoles screened so far revealed that σi is the most important factor for their lytic activity on the bloodstream trypomastigote forms of T. cruzi, the etiologic agent of Chagas disease. Based on this result, 16 new N-alkyl-naphthoimidazoles derived from 6,6-dimethyl-3,4,5,6-tetrahydrobenzo[7,8]chromene[5,6-d]imidazole (the product of the reaction of β-lapachone with paraformaldehyde) by its reaction with halo-alkanes were prepared and evaluated against the parasite and peritoneal macrophages. The N1-n-hexyl and N3-n-hexyl naphthoimidazoles were 2.2 and 3.2 times more active than the standard drug benznidazole with selectivity indices of 2.7 and 13.4, respectively.
1. Introduction
Chagas disease, with an estimated global prevalence of 5–7 million people,1 is caused by Trypanosoma cruzi. It is transmitted mainly to humans by triatomine vectors, blood transfusions, and oral and congenital transmission and, less commonly, by direct transmission from T. cruzi reservoirs, ingestion of uncooked meat from infected animals, organ transplantation, and laboratory accidents.2 This disease, classically associated with poor and rural populations, underwent an urbanization process in the 1970s and 1980s to Latin American cities and later beyond endemic countries, creating new epidemiological, economic, social and political challenges.3 Chagas disease results from the establishment of T. cruzi in host tissues, involving an initial acute phase followed by a chronic phase, classified as indeterminate, cardiac, or digestive.4 The current treatment is restricted to two nitroheterocyclic drugs, benznidazole and nifurtimox, and their efficacy varies according to the phase of the disease, with the effectiveness decreasing with the advancement of the infection.5 The high incidence of collateral effects, especially for adults, has led to the abandonment of treatment in several instances.6 In this context, there is an urgent need for alternative drugs, and our group is investigating the trypanocidal activity of naphthoquinones and their derivatives.7 The molecular structures of naphthoquinones endow them with redox properties, allowing interference in different biological oxidative processes. Previous reports demonstrate the trypanocidal activity of β-lapachone through the generation of reactive oxygen species, leading to lipid peroxidation and inhibition of nucleic acid and protein synthesis.8
For the past 20 years, our group has been working on experimental chemotherapy for Chagas disease and special emphasis was given to naphthoquinonoid prototypes. By the use of three synthetic strategies – C-ring, A-ring and redox centre modifications – different classes of heterocyclic compounds were prepared and screened against the parasite.9 For example, redox centre modifications led to imidazoles, oxazoles, phenazines/quinoxalines, phenoxazines, pyrroles and carbocycles10 and the molecular hybridisation between a naphthoquinoidal moiety and a heterocyclic 1,2,3-triazole led to bioactive quinone-based triazoles.11 Among the derivatives investigated so far, three compounds derived from β-lapachone (1), containing a phenyl group (2), a 3-indolyl (3) group and a para-methyl phenyl (4) group attached to the naphthoimidazolic moiety, were the most active against T. cruzi (Fig. 1).10 However, all compounds including 2–4 showed considerable toxicity against host cells, indicating the need for further research to find substituents which might alleviate the toxicity issue.
Fig. 1. 2-Aryl-naphthoimidazoles obtained from the reaction of β-lapachone (1), which are active against the bloodstream forms of T. cruzi.10.
The imidazole moiety is present in the structures of many bioactive substances of both biological and chemical interest such as the neurotransmitter histamine – which, in turn, is a metabolite of the amino acid histidine – the antifungal azoles, miconazole and ketoconazole, and the bactericidal metronidazole.12 These azoles are heterocycles found in substances with a variety of biological activities,13 and it is important to note that several substances described as trypanocidal agents, such as benznidazole, contain imidazolic moieties.14
In this work, adding to the previous 29 related compounds,10 five new 2-aryl-naphthoimidazoles were obtained from β-lapachone (1), their activity against the infective bloodstream trypomastigote form of T. cruzi was also determined and the structure/activity correlation was studied using all the 34 compounds synthesized so far. This study directed us to the synthesis of new alkyl-naphthoimidazoles, that were also assayed against the parasite, and the most active ones were tested for their toxicity to mammalian host cells.
2. Results and discussion
Chemistry
Lapachol was extracted from the scraps left from commercial use of the heartwood of Tabebuia sp. (Tecoma) and purified by a series of recrystallizations. Through the cyclization of its isoprenyl lateral chain, by the nucleophilic attack of oxygen, induced by sulfuric acid, β-lapachone (1) was obtained.15 From the reaction of 1 with aromatic aldehydes (using ammonium acetate supported on basic alumina and microwave activation, which allows a much shorter reaction time, compared with the traditional method),10 new aryl-naphthoimidazoles (5–9), with arylic substituents at C2 of the imidazole ring, were obtained and their activity against T. cruzi was investigated (Scheme 1).
Scheme 1. Synthesis of the 2-aryl-naphthoimidazoles 5–9 derived from β-lapachone.
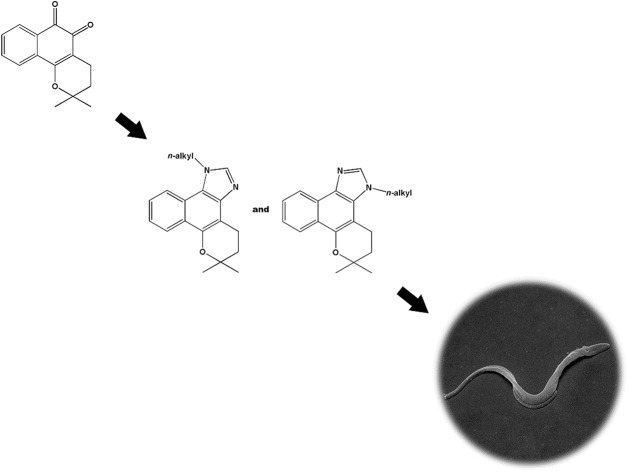
The correlation of the chemical structure and trypanocidal activity of 34 β-lapachone-derived aryl-naphthoimidazoles (Table S1‡) revealed the important role of the inductive factor (σi) in the trypanocidal activity of these naphthoimidazoles, pointing to the potential effect of alkyl substitution on the imidazole ring (see the ESI‡). Efforts were then directed to the synthesis of new analogues – naphthoimidazoles derived from β-lapachone – alkylated at the imidazole ring. Also using microwave activation, 10 (BLI-H), the simplest imidazole derivative of 1, was obtained by the reaction of 1 with paraformaldehyde, using the same procedure mentioned above (ammonium acetate supported on basic alumina and microwave activation).16 In sequence, 10 was treated with a base and 8 linear alkylating agents, leading to two series of derivatives, N1-n-alkyl and N3-n-alkyl (11–26, Scheme 2).
Scheme 2. Synthesis of BLI-H, N1-n-alkyl and N3-n-alkyl naphthoimidazoles 10–26 derived from β-lapachone.
The NMR data and spectra for compounds 11–26, as well as details about the discrimination between the N1- and N3-alkyl regioisomers, are presented in the ESI.‡ Shortly, if the alkyl group is attached to the N1 nitrogen, there will be a NOESY correlation between the first (and maybe the second, third, etc.) methylene group hydrogens of the side chain and the nearest aromatic methyne (3JCH HMBC coupling between this methylene and the aromatic quaternary carbon adjacent to N1 will also be evident). If the alkyl group is attached to the N3 nitrogen, the allylic methylene of the dihydropyran ring will show a NOESY correlation with the hydrogens in the side chain (the nearby quaternary carbon adjacent to N3 will show 3JCH HMBC coupling with the first methylene of the side chain). In the 11–26 series, the methylenes directly attached to nitrogen will correspond to the triplets at 4.3–4.6 ppm in the 1H spectra. In general, given a pair of N1 and N3 regioisomers, the one with the more deshielded triplets will be the N1-alkyl regioisomer.
Biological activity
All the 5 new 2-aryl-naphthoimidazoles (5–9) synthesized were less active than the standard drug benznidazole (Bz) against T. cruzi17 (Table 1). A characteristic of 5–9 is the presence of an aromatic group attached to the imidazole ring at the C2 carbon of the imidazole ring (Scheme 1). Comparison of 6, 7 and 9 with 2, the unsubstituted benzaldehyde derivative,10a showed that substituents on the phenyl group led to a decreased biological response. The QSAR study using these new derivatives together with the 29 aryl-naphthoimidazoles with electron-releasing or electron-withdrawing groups at positions 2, 3 and 4 of the phenyl ring linked to C2 of the imidazole moiety previously reported10 revealed no obvious correlation between the substituent polar electronic effects and the trypanocidal activity. The only trend observed with these 34 aryl-naphthoimidazoles was a correlation with σi (inductive effect substituent constant; see the ESI‡).
Table 1. Activity of the synthesized aryl- and alkyl-naphthoimidazoles against the trypomastigote forms of T. cruzi.

| ||
| Cpd | Description | IC50 a /24 h (μM) |
| 1 | Fig. 1, β-lapachone | 391.5 ± 16.5 b |
| 2 | R1 = R3 = H, R2 = phenyl | 37.0 ± 0.7 b |
| 3 | R1 = R3 = H, R2 = 3-indolyl | 15.5 ± 2.9 c |
| 4 | R1 = R3 = H, R2 = p-methylphenyl | 37.5 ± 12.8 d |
| 5 | R1 = R3 = H, R2 = 2-thienyl | 170.2 ± 20.4 |
| 6 | R1 = R3 = H, R2 = 2,6-dichlorophenyl | 2118.8 ± 546.1 |
| 7 | R1 = R3 = H, R2 = 2,4-dichlorophenyl | >4000 |
| 8 | R1 = R3 = H, R2 = 1-naphthyl | 1680.7 ± 47.5 |
| 9 | R1 = R3 = H, R2 = 2-methoxy-4-hydroxyphenyl | 251.8 ± 28.0 |
| 10 | R1 = R2 = R3 = H | 89.5 ± 5.1 |
| 11 | R1 = n-propyl, R2 = R3 = H | 99.7 ± 4.4 |
| 12 | R1 = R2 = H, R3 = n-propyl | 172.2 ± 12.6 |
| 13 | R1 = n-butyl, R2 = R3 = H | 106.0 ± 8.8 |
| 14 | R1 = R2 = H, R3 = n-butyl | 123.0 ± 2.2 |
| 15 | R1 = n-pentyl, R2 = R3 = H | 92.7 ± 9.4 |
| 16 | R1 = R2 = H, R3 = n-pentyl | 123.7 ± 16.7 |
| 17 | R1 = n-hexyl, R2 = R3 = H | 42.5 ± 7.3 |
| 18 | R1 = R2 = H, R3 = n-hexyl | 31.5 ± 5.3 |
| 19 | R1 = n-heptyl, R2 = R3 = H | 406.6 ± 11.5 |
| 20 | R1 = R2 = R3 = n-heptyl | 99.0 ± 10.3 |
| 21 | R1 = n-octyl, R2 = R3 = H | 376.1 ± 23.4 |
| 22 | R1 = R2 = R3 = n-octyl | 180.1 ± 3.2 |
| 23 | R1 = n-nonyl, R2 = R3 = H | 194.6 ± 20.5 |
| 24 | R1 = R2 = R3 = n-nonyl | 294.8 ± 15.0 |
| 25 | R1 = n-dodecyl, R2 = R3 = H | >500 |
| 26 | R1 = R2 = R3 = n-dodecyl | 191.7 ± 10.4 |
| Bz | Benznidazole | 103.6 ± 0.6 e |
The trypanocidal activity of the 2-aryl-naphthoimidazoles 2–9 and S1–S26 (Table S1, ESI‡) demonstrates no systematic influence of the substituent polar effects: both 2-methoxy and 2-cyano (cpds. S1 and S21) were inactive (IC50 > 6500 μM), 4-methoxy, 4-nitro and 4-trifluoromethyl (S3, S6, S20) displayed IC50 around 250 μM, while 4-methyl (cpd. 4) presented one of the three smallest IC50 values (15.5 μM); 3-trifluoromethyl and 3-bromo (S19, S14) have similar IC50 larger than that of 3-methyl (S17) (37.5 μM) but much smaller than those of 3-fluoro, 3-nitro, 3-chloro and 3-methoxy (S8, S5, S11, S2, in that order). The simple phenyl substituent (cpd. 2) gives one of the best activities, whereas 1-naphthyl (8) is almost fifty times worse. So there was no surprise when QSAR analysis showed the overriding importance of σi, as seen in the correlation equation (eqn (1), experimental section), and that the most active compounds have very small or negative σi values. This, in turn, directed us to the synthesis of 16 N-alkyl-naphthoimidazoles derived from 10 (BLI-H, an imidazole unsubstituted at the nitrogen atom), by treatment with a base followed by an alkyl halide.
The biological activities of simple N-alkyl-imidazoles have been studied for many years and are believed to be directly related to the hydrophobic character of these compounds.18 Modification in the chain of a biologically active substance, which leads to the formation of more lipophilic products, can be an important strategy to obtain more active products. The N-alkyl-imidazoles also showed antibacterial and antifungal potentials, which are also related to the hydrophobic character of these substances; however, it has been observed that the optimal size for the aliphatic chains is nine18c and twelve18d carbon atoms, respectively.
Our results revealed that with the exception of the aryl-naphthoimidazoles 2 and 3, all the most active compounds against T. cruzi have alkyl substituents at N1 and N3 of the imidazole ring, in accordance with the results of the QSAR study. The N-hexyl derivatives (17 and 18) were the most active, followed by 10, 11, 13–16 and 20 with IC50 values in the range of 90 to 120 μM, similar to Bz (IC50 of 103.6 μM) (Table 1). From all the naphthoimidazoles synthesized from 1, the most active ones are the aryl derivatives 2–4 (ref. 10) and alkyl derivatives 17 and 18, which showed similar activities, that were 2.2 and 3.2 times more active than the standard drug, Bz. Comparison between 10 (BLI-H, IC50 of 89 μM) and 2 (IC50 of 37 μM) revealed that the addition of an aromatic group to the imidazole ring increased the trypanocidal activity by about 2.4 times. Cytotoxicity assays using primary cultures of mouse peritoneal macrophages led to LC50 values of 116.6 ± 5.0 and 422.1 ± 17.4 μM for 17 and 18, respectively, leading to selectivity indices of 2.7 and 13.4. The correlations between trypanocidal activity (IC50/24 h) and the number of carbon atoms for N1- and N3-alkyl-naphthoimidazoles are plotted in Fig. 2. The effect of aliphatic chains attached to nitrogens N1 or N3 of the imidazole ring was not very pronounced, when compared to the parent compound, 10. But some trends seem clear: IC50 values increase for long chains (n > 7) and are minimal for n = 6 (n-hexyl attached to either N1 or N3). Since the alkyl substituents, from n-propyl to n-dodecyl, have similar and close to zero (∼0.01–0.03) values of σi, constants related to the substituent size,19 MV (molecular volume) and MR (molecular refractivity) – calculated using the Spartan 4 (Wavefunction, U.S.A.) and ACD/Labs 6.00 (Canada) programs – were considered. The compounds benzene, indole and toluene (present as substituents in cpds. 2, 3 and 4) have MV = 99.5, 132.8 and 117.7 Å3 and MR = 25.73, 37.21 and 30.35 cm3 mol–1 (Table S2‡), respectively. Pentane, present as an n-pentyl substituent in cpds. 15 and 16, has MV = 107.0 Å3 and MR = 25.21 cm3 mol–1, and hexane (n-hexyl in 17 and 18) has MV = 125.4 Å3 and MR = 29.84 cm3 mol–1. These values of MV and MR are in the same range as those for cpds. 2–4 (above), which are very active. But propane (cpds. 11 and 12) and butane (13 and 14) have MV and MR values (70.3 and 88.7 Å3; 15.94 and 20.58 cm3 mol–1, respectively) which are too low, whereas for heptane (19 and 20), octane (21 and 22), nonane (23 and 24) and dodecane (25 and 26), the MV and MR values (143.7, 162.1, 180.4 and 235.4 Å3; 34.47, 39.11, 43.74 and 57.64 cm3 mol–1, respectively) are too high. So, there seems to be a steric motive for the peak in activity for cpds. 15 and 16 (N1- and N3-n-pentyl) and 17 and 18 (N1- and N3-n-hexyl).
Fig. 2. Correlation between trypanocidal activity (IC50/24 h) and the number of carbon atoms for 1-N- and 3-N-alkyl-naphthoimidazoles.
Naphthoimidazoles with a phenylic group attached to the imidazole ring, with smaller lipophilic phenyl substituted groups, could act through intercalation within apolar regions of proteins. On the other hand, for those with a heterocycle group attached to the ring (3, S24 and S25), the planar substituted heterocyclic ring at the 2-position could facilitate a polar–polar interaction of the compounds with macromolecules through the non-bonding electron pair of the nitrogen atom. Previous studies pointed to the T. cruzi mitochondrion as the main target of 2, 3 and 4,20 but phenotypic and molecular assays also supported the involvement of autophagy in their mode of trypanocidal action.20c The proteomic analysis of epimastigote and bloodstream trypomastigote forms confirmed the susceptibility of the T. cruzi mitochondrion to the treatment with these three active naphthoimidazoles, due to the high number of mitochondrial proteins differentially expressed in treated parasites. The proteomic approach also revealed multiple pathways including redox metabolism, energy production, ergosterol biosynthesis, cytoskeleton assembly, protein metabolism and chaperone modulation.21 Despite the structural and chemical similarities between 2, 3 and 4 and the compounds investigated here, a detailed evaluation of their mechanisms of action must be performed in order to better characterize the trypanocidal effect as well as the host toxicity.
The present work on alkyl-naphthoimidazoles points to new prototypes for bioactive β-lapachone derivatives based on the aliphatic substituents at N1 and N3 in the imidazole ring with 5 out of 16 compounds being more active than Bz against T. cruzi. For the aryl-naphthoimidazoles synthesized so far, 4 out of 35 compounds were more active than the standard drug (Tables 1 and S1‡).
3. Conclusions
The redox system of lapachones is involved in a variety of molecular modifications, leading to carbocyclic, heterocyclic and acyclic compounds and facilitating the development of scaffolds with steric and electronic properties that are distinct from those of β-lapachone. By comparing the activities of the original naphthoquinones to those of the synthesized compounds, we concluded that the structural features involved in the increase in lipophilicity, such as a furan moiety or the presence of a methoxyl group or an aliphatic side chain, led to an increase in trypanocidal activity. The aim of the derivatisation is the generation of novel molecules that inhibit cellular organelles/processes, generate reactive oxygen species and increase lipophilicity to enhance their penetration through the plasma membrane. We have previously associated the trypanocidal activity of C-allyl lawsone and juglone derivatives with alterations in the mitochondrial physiology interfering with the oxygen uptake and the membrane potential in this organelle, pointing to modified lapachones as promising prototypes for the development of drugs against neglected diseases.22 The present work reinforces studies in the literature of sterol biosynthesis inhibitors23 and nitroimidazoles (Bz, fexinidazole, megazol, metronidazole and MK-436),24 pointing to the strong correlation of the trypanocidal activity with the imidazolic skeleton. As a hypothesis, it can be stated that this moiety is an architectural appendage group for the delineation of molecules of potential value for the chemotherapy of Chagas disease.
4. Experimental section
Melting points were obtained using a Reichert micro hot stage and are uncorrected. Analytical grade solvents were used. Column chromatography was performed using silica gel (Acros Organics, 0.035–0.070 mm, pore diameter ca. 6 nm). Infrared spectra were recorded on a Perkin-Elmer FT-IR spectrometer. 1H- and 13C-NMR were recorded at room temperature using a Bruker Advance 400 or a Bruker Advance 500 instrument, in the solvents indicated, with TMS as the internal standard. Chemical shifts (δ) are given in ppm and coupling constants (J) in hertz. A Shimadzu, Model OMINI spectrometer was used for ultraviolet-visible spectra.
Electron-impact mass spectra (70 eV) were obtained using a MAT8500 or an Agilent 6890n/5973 instrument and a VG Autospec apparatus (Micromass, Manchester, UK). GC-MS analysis was performed using a Varian Saturn 2000 instrument with an electron-impact (70 eV) detector. High resolution mass spectra were obtained using a Bruker MicroTOF II (IPPN-UFRJ) electrospray ionization instrument. The main fragments were described as a relation between the atomic mass units and the charge (m/z) and the relative abundance in percentage of the base peak intensity.
General procedure for the synthesis of aryl-naphthoimidazoles 5–9 and of 6,6-dimethyl-3,4,5,6-tetrahydrobenzo[7,8]chromene[5,6-d]imidazole (10)
Supported ammonium acetate on basic alumina (SAA-BA) was prepared by grinding together a mixture of 9.3 g of basic alumina (Aluminiumoxid S, Riedel-de Haën) and 4.3 g of ammonium acetate and stored before use in a desiccator. For 5–9, β-lapachone (1) (0.5 mmol) and the desired aldehyde (0.5 mmol) were dissolved in a minimum quantity of dichloromethane (2 mL), then SAA-BA (2.5 g) was added, and the solvent was evaporated at room temperature. For 10, SAA-BA (2.5 g) and paraformaldehyde (3 mmol) were ground together and transferred to a small beaker. β-Lapachone (1, 2 mmol) was dissolved in a minimum quantity of dichloromethane (2 mL) and added to the beaker and the solvent was evaporated at room temperature.16 The reaction mixtures were subjected for 15 min to irradiation in a domestic microwave oven (Panasonic, model NN-S42BH) adjusted to potency 1 corresponding to 84.1 W from calibration runs.25 The crude product was extracted from the support by washing the mixture with ethyl acetate. These extractions were monitored by TLC and the solvent was removed by rotoevaporation. The naphthoimidazoles were separated by column chromatography using silica and a mixture of hexane and ethyl acetate as the eluent with an increasing polarity gradient from 4 : 1 to 100% ethyl acetate.
General procedure for the synthesis of alkyl-naphthoimidazoles 11–26
The alkylating agents used were linear 1-bromo-alkanes (R-Br for propyl, butyl, pentyl, hexyl, heptyl, nonyl and dodecyl) and 1-tosyl-octane. In a 10 mL glass reaction tube, 25.2 mg (0.1 mmol) of 10, 5 mg of sodium hydride, 60% suspension in paraffin (0.125 mmol), and 3 mL of acetonitrile were added. The tube was closed and left under stirring for 3 h at room temperature. The alkylating agent was added (see Table 2 for quantities), the tube was sealed, and the mixture was stirred at 80 °C for 48 h. The reaction mixtures were analysed by TLC (Merck Kieselgel 60 F254 aluminum plates, visualized with UV, at 254 and 366 nm). The products were isolated by preparative TLC (2 mm silica (VETEC) plates), using hexane/ethyl acetate (1 : 3) as the eluent and extracted from the plates with ethyl acetate (see Table 2, for yields).
Table 2. Experimental conditions and results for the synthesis of compounds 11–26.
| Cpd | Alkylating agent (R-X) | Molar ratio 10 : R-X | N1 product (%) | N3 product (%) | Total yield (%) |
| 11, 12 | 1-Bromo-propane | 1 : 1 | 11 (42) | 12 (49) | 91 |
| 13, 14 | 1-Bromo-butane | 1 : 1 | 13 (42) | 14 (48) | 90 |
| 15, 16 | 1-Bromo-pentane | 1 : 1 | 15 (50) | 16 (47) | 97 |
| 17, 18 | 1-Bromo-hexane | 1 : 1 | 17 (46) | 18 (46) | 92 |
| 19, 20 | 1-Bromo-heptane | 1 : 1 | 19 (51) | 20 (46) | 97 |
| 21, 22 | n-Octyl tosylate | 1 : 4 | 21 (44) | 22 (44) | 88 |
| 23, 24 | 1-Bromo-nonane | 1 : 1 | 23 (40) | 24 (49) | 92 |
| 25, 26 | 1-Bromo-dodecane | 1 : 2 | 25 (43) | 26 (46) | 89 |
UV-vis spectroscopy was not very useful for the structure confirmation of these products since all compounds had spectra very similar to those of 10. Infrared spectra were also very similar for these products, except that, in comparison with 10, they lacked the 3409 cm–1 N–H stretching band.
Trypanocidal activity
Stock solutions of the naphthoimidazoles were prepared in dimethylsulfoxide (DMSO), with the final concentration of the solvent never exceeding 0.1%. Experiments showed that in concentrations of up to 0.5%, DMSO had no deleterious effect on the parasites. Bloodstream trypomastigotes (Y strain)26 were resuspended in Dulbecco's modified Eagle's (DME) medium plus 10% blood to a concentration of 5 × 106 cells per ml. This suspension (100 μl) was added to the drugs in a volume ratio of 1 : 1 and also diluted in DME medium at twice the final desired concentration, and the mixture was then incubated at 4 ° C. Some experiments were performed in DMES without the addition of blood. Cell counts were performed after 24 h of incubation and the drug concentration corresponding to 50% parasite lysis was expressed as IC50.27 All experiments with animals were carried out in accordance with the guidelines established by the FIOCRUZ Committee of Ethics for the Use of Animals (License LW 16/13).
Cytotoxicity to mammalian cells
The cytotoxicity assays were performed using primary cultures of peritoneal macrophages obtained from Swiss mice. For the experiments, 2.5 × 104 cells in 200 μL of RPMI-1640 medium (pH 7.2) plus 10% foetal bovine serum and 2 mM glutamine were added to each well of a 96-well microtiter plate and incubated for 24 h at 37 °C. The treatment of the cultures was performed in fresh supplemented medium (200 μL per well) for 24 h at 37 °C. After this period, 110 μL of the medium was discarded and 10 μL of PrestoBlue (Invitrogen) was added to complete the final volume of 100 μL. Thus, the plate was incubated for 2 h and the measurement was performed at 560 and 590 nm, as recommended by the manufacturer. The results were expressed as the difference in the percentage of reduction between treated and untreated cells being the LC50 value corresponding to the concentration that leads to damage of 50% of the mammalian cells.28
Quantitative structure–activity relationship
Physical–chemical and steric descriptors such as log P (lipophilicity), MR (molar refractivity) and η (hardness, calculated from frontier orbital energies: η = (ELUMO – EHOMO)/2) were determined for each compound using the ACDLabs 6.0 software package;29 substituent electronic constants (including σi inductive constants) were collected from the literature.30 These parameters are shown in Table S2.‡ Compounds S1 and S21 (Table S1‡) showed IC50 > 6500 μM and were not considered. The 2-D QSAR models were derived from multiple regression analysis of data in Table S2,‡ using BuildQSAR software31 for the determination of the correlation coefficients of the equations. n is the number of points, r is the correlation factor between the observed and the calculated values obtained from the equation, s is the model standard deviation, q2 is the cross validation, and F is the Fisher's test of statistical significance. Principal component analysis was also performed using BuildQSAR software31 using the descriptors shown in Table S2;‡ the data were pre-processed by autoscaling to unit variance.
Multiple linear regression analysis showed a dependency of the trypanocidal activity on the following substituent parameters: MR and σi. The established QSAR (Hansch model, eqn (1)) presented a modest correlation coefficient (r2 = 0.76) but provided expressive information, revealing that σi is the most important factor for the trypanocidal activity displayed by the naphthoimidazoles.
| log1/IC50 = – 10.271(±2.667)σi – 0.0716(±0.032)MR + 1.399(±1.170) | 1 |
(n = 26; r2 = 0.76; s = 0.335; F = 36.48; q2 = 0.717). Outliers (excluded from the correlation): entries S2, S6, S10, S12, S25 and S26 (Table S2‡).
It can be observed by the Hansch model that electron donating groups, which have small volumes and low polarity, linked to the imidazole ring increase the trypanocidal activity, while MR is an ambiguous parameter, that reflects both volume and polarity according to Verma & Hansch.32 Due to the inexistence of models using the molecular volume, this suggests the preponderance of the polarity factor.
We tried to establish models involving log P for the group linked to the imidazolic ring and log P of the total molecule, but the results obtained were poor with regard to the statistical parameters (r2 < 0.7), which is a peculiar fact, since most of the QSAR models involve lipophilicity.33 The correlation between the calculated values and the observed ones is depicted in Fig. S1.‡
Supplementary Material
Acknowledgments
This research was supported by grants from the Fundação Carlos Chagas Filho de Amparo à Pesquisa do Rio de Janeiro (FAPERJ), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação Oswaldo Cruz (FIOCRUZ) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). Useful discussions with Drs. Eufranio N. da Silva-Junior (UFMG) and Martha Teresa P. Oliveira Castro (UFRRJ) are gratefully acknowledged.
Footnotes
†The authors declare no competing interests.
‡Electronic supplementary information (ESI) available: All the experimental details, spectroscopic data, QSAR tables and figure. See DOI: 10.1039/c7md00069c
References
- WHO, Weekly Epidemiol. Rec, 2015, vol. 90, pp. 33–43. [Google Scholar]
- (a) Altclas J. D., Barcan L., Nagel C., Lattes R., Riarte A. JAMA, J. Am. Med. Assoc. 2008;299:34–35. doi: 10.1001/jama.299.10.1134-a. [DOI] [PubMed] [Google Scholar]; (b) Dias J. C. P., Amato N. V. Rev. Soc. Bras. Med. Trop. 2011;44:68–72. doi: 10.1590/s0037-86822011000800011. [DOI] [PubMed] [Google Scholar]
- (a) Schmunis G. A., Yadon Z. E. Acta Trop. 2010;115:14–21. doi: 10.1016/j.actatropica.2009.11.003. [DOI] [PubMed] [Google Scholar]; (b) Jackson Y., Varcher-Herrera M., Gascon J. Bull. W. H. O. 2014;92:771–772. doi: 10.2471/BLT.13.134072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias J. C. P. Epidemiol. Serv. Saude. 2016;25:7–86. doi: 10.5123/S1679-49742016000500002. [DOI] [PubMed] [Google Scholar]
- (a) Coura J. R., de Castro S. L. Mem. Inst. Oswaldo Cruz. 2002;97:3–24. doi: 10.1590/s0074-02762002000100001. [DOI] [PubMed] [Google Scholar]; (b) Coura J. R., Borges-Pereira J. Mem. Inst. Oswaldo Cruz. 2011;106:641–645. doi: 10.1590/s0074-02762011000600001. [DOI] [PubMed] [Google Scholar]
- (a) Pinazo M. J., Munoz J., Posada E., López-Chejade P., Gállego M., Ayala E., del Cacho E., Soy D., Gascon J. Antimicrob. Agents Chemother. 2010;54:4896–4899. doi: 10.1128/AAC.00537-10. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Jackson Y., Alirol E., Getaz L., Wolff H., Combescure C., Chappuis F. Clin. Infect. Dis. 2010;51:e69–e75. doi: 10.1086/656917. [DOI] [PubMed] [Google Scholar]
- (a) Pinto A. V., Menna-Barreto R. F. S. and De Castro S. L., in: Recent Progress in Medicinal Plants, ed. J. N. Govil, Studium Press, Houston, Texas, 2007, pp. 112–127. [Google Scholar]; (b) Pinto A. V., de Castro S. L. Molecules. 2009;14:4570–4590. doi: 10.3390/molecules14114570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docampo R., De Souza W., Cruz F. S., Roitman I., Cover B., Gutteridge W. E. Z. Parasitenkd. 1978;57:189–198. doi: 10.1007/BF00928032. [DOI] [PubMed] [Google Scholar]
- (a) de Castro S. L., Emery F. S., Silva-Junior E. N. Eur. J. Med. Chem. 2013;69:678–700. doi: 10.1016/j.ejmech.2013.07.057. [DOI] [PubMed] [Google Scholar]; (b) Silva-Junior E. N., Jardim G. A. M., Menna-Barreto R. F. S., de Castro S. L. J. Braz. Chem. Soc. 2014;25:1780–1798. [Google Scholar]
- (a) Pinto A. V., Neves Pinto C., Pinto M. C. F. R., Santa Rita R., Pezzella C. A. C., de Castro S. L. Arzneim. Forsch. 1997;47:74–79. [PubMed] [Google Scholar]; (b) Neves-Pinto C., Dantas A. P., Moura K. C. G., Emery F. S., Polequevitch P. F., Pinto M. C. F. R., de Castro S. L., Pinto A. V. Arzneim. Forsch. 2000;50(II):1120–1128. doi: 10.1055/s-0031-1300337. [DOI] [PubMed] [Google Scholar]; (c) Moura K. C. G., Salomão K., Menna-Barreto R. F. S., Emery F. S., Pinto M. C. F. R., Pinto A. V., de Castro S. L. Eur. J. Med. Chem. 2004;39:639–645. doi: 10.1016/j.ejmech.2004.02.015. [DOI] [PubMed] [Google Scholar]
- (a) da Silva Júnior E. N., Menna-Barreto R. F. S., Pinto M. C. F. R., Silva R. S. F., Teixeira D. V., Souza M. C. B. V., de Simone C. A., de Castro S. L., Ferreira V. F., Pinto A. V. Eur. J. Med. Chem. 2008;43:1774–1780. doi: 10.1016/j.ejmech.2007.10.015. [DOI] [PubMed] [Google Scholar]; (b) da Silva Júnior E. N., Melo I. M. M., Emilay B. T., Diogo E. B. T., Verenice A., Costa V. A., Souza Filho J. D., Valença W. O., Camara C. A., Oliveira R. N., Araujo A. S., Emery F. S., Santos M. R., de Simone C. A., Menna-Barreto R. F. S., de Castro S. L. Eur. J. Med. Chem. 2012;52:304–312. doi: 10.1016/j.ejmech.2012.03.039. [DOI] [PubMed] [Google Scholar]; (c) Diogo E. B. T., Dias G. G., Rodrigues B. L., Guimarães T. T., Valença W. O., Camara C. A., Oliveira R. N., Silva M. G., Ferreira V. F., Paiva Y. G., Goulart M. O. F., Menna-Barreto R. F. S., de Castro S. L., da Silva Júnior E. N. Bioorg. Med. Chem. 2013;21:6337–6348. doi: 10.1016/j.bmc.2013.08.055. [DOI] [PubMed] [Google Scholar]
- Heeres J., Meerpoel L., Lewi P. Molecules. 2010;15:4129–4188. doi: 10.3390/molecules15064129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Moura K. C. G., Carneiro P. F., Pinto M. C. F. R., da Silva J. A., Malta V. R. S., de Simone C. A., Dias G. G., Jardim G. A. M., Cantos J., Coelho T. S., Silva P. E. A., da Silva Jr E. N. Bioorg. Med. Chem. 2012;20:6482–6488. doi: 10.1016/j.bmc.2012.08.041. [DOI] [PubMed] [Google Scholar]; (b) Del Prete S., Vullo D., De Luca V., Carginale V., Ferraroni M., Osman S. M., AlOthman Z., Supuran C. T., Capasso C. Med. Chem. Res. 2015;24:1155–1161. doi: 10.1016/j.bmc.2016.01.037. [DOI] [PubMed] [Google Scholar]; (c) Cantos J., Coelho T. S., Carneiro P. F., Moura K. C. G., Pinto M. C. F. R., da Silva Jr E. N., Silva P. E. A. Lat. Am. J. Pharm. 2012;31:507–513. [Google Scholar]
- (a) McCabe R. E., Araujo F. G., Remington J. S. Am. J. Trop. Med. Hyg. 1983;32:960–962. doi: 10.4269/ajtmh.1983.32.960. [DOI] [PubMed] [Google Scholar]; (b) Nothenberg M. S., Takeda G. K., Najjar R. J. Inorg. Biochem. 1991;42:217–229. doi: 10.1016/0162-0134(91)84008-w. [DOI] [PubMed] [Google Scholar]; (c) Caterina M. C., Perillo I. A., Boiani L., Pezaroglo H., Cerecetto H., González M., Salerno A. Bioorg. Med. Chem. 2008;16:2226–2234. doi: 10.1016/j.bmc.2007.11.077. [DOI] [PubMed] [Google Scholar]; (d) Silva R. B., Loback V. B., Salomão K., de Castro S. L., Wardell J. L., Wardell S. M., Costa T. E., Penido C., Henriques M., Carvalho S. A., da Silva E. F., Fraga C. A. M. Molecules. 2013;18:3445–3457. doi: 10.3390/molecules18033445. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Papadopoulou M. V., Bloomer W. D., Rosenzweig H. S., Wilkinson S. R., Kaiser M. Eur. J. Med. Chem. 2014;87:79–88. doi: 10.1016/j.ejmech.2014.09.045. [DOI] [PubMed] [Google Scholar]; (f) Gopi E., Kumar T., Menna-Barreto R. F. S., Valença W. O., da Silva Júnior E. N., Namboothiri I. N. Org. Biomol. Chem. 2015;13:9862–9871. doi: 10.1039/c5ob01444a. [DOI] [PubMed] [Google Scholar]
- (a) Hooker S. C. J. J. Chem. Soc., Trans. 1892;61:611–650. [Google Scholar]; (b) Pinto A. V., Pinto M. C. F. R., Oliveira C. G. T. An. Acad. Bras. Cienc. 1982;54:107–114. [Google Scholar]
- Silva A. R., Silva A. M., Ferreira A. B. B., Bernardes B. O., Costa R. L. J. Braz. Chem. Soc. 2008;19:1230–1233. [Google Scholar]
- Silva Jr E. M., Menna-Barreto R. F. S., Pinto M. C. F. R., Silva R. S. F., Teixeira D. V., Souza M. C. B. V., De Simone C. A., de Castro S. L., Ferreira V. F., Pinto A. V. Eur. J. Med. Chem. 2008;43:1774–1780. doi: 10.1016/j.ejmech.2007.10.015. [DOI] [PubMed] [Google Scholar]
- (a) Wilkinson C. F., Hetnarski K., Cantwell G. P., Di Carlo F. J. Biochem. Pharmacol. 1974;23:2377–2386. doi: 10.1016/0006-2952(74)90227-5. [DOI] [PubMed] [Google Scholar]; (b) Rogerson T. D., Wilkinson C. F., Hetarski K. Biochem. Pharmacol. 1977;26:1039–1042. doi: 10.1016/0006-2952(77)90241-6. [DOI] [PubMed] [Google Scholar]; (c) Khabnadideh S., Rezaei Z., Khalafi-Nezhad A., Bahrinajafi R., Mohamadi R., Farrokhroz A. A. Bioorg. Med. Chem. Lett. 2003;13:2863–2865. doi: 10.1016/s0960-894x(03)00591-2. [DOI] [PubMed] [Google Scholar]; (d) Khabnadideh S., Shadzi S. H., Alipour E., Mirmohammad-Sadeghi M. Pazhuhesh dar Oloome Pezeshki. 2003;7(supl. 2):126–130. [Google Scholar]
- Martin Y. C., Quantitative Drug Design, CRC Press, USA, 2nd edn, 2010, p. 64. [Google Scholar]
- (a) Menna-Barreto R. F. S., Henriques-Pons A., Pinto A. V., Morgado-Diaz J. A., Soares M. J., de Castro S. L. J. Antimicrob. Chemother. 2005;56:1035–1041. doi: 10.1093/jac/dki403. [DOI] [PubMed] [Google Scholar]; (b) Menna-Barreto R. F. S., Corrêa J. R., Pinto A. V., Soares M. J., de Castro S. L. Parasitol. Res. 2007;101:895–905. doi: 10.1007/s00436-007-0556-1. [DOI] [PubMed] [Google Scholar]; (c) Menna-Barreto R. F. S., Corrêa J. R., Cascabulho C. M., Fernandes M. C., Pinto A. V., Soares M. J., de Castro S. L. Parasitology. 2009;136:499–510. doi: 10.1017/S0031182009005745. [DOI] [PubMed] [Google Scholar]
- (a) Menna-Barreto R. F. S., Beghini D. G., Ferreira A. T. S., Pinto A. V., de Castro S. L., Perales J. J. Proteomics. 2010;73:2306–2315. doi: 10.1016/j.jprot.2010.07.002. [DOI] [PubMed] [Google Scholar]; (b) Brunoro G. V., Faça V. M., Caminha M. A., Ferreira A. T., Trugilho M., Moura K. C., Perales J., Valente R. H., Menna-Barreto R. F. S. PLoS Neglected Trop. Dis. 2016;10:e0004951. doi: 10.1371/journal.pntd.0004951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Fernandes M. C., Silva Jr E. M., Pinto A. V., de Castro S. L., Menna-Barreto R. F. S. Parasitology. 2012;139:26–36. doi: 10.1017/S0031182011001612. [DOI] [PubMed] [Google Scholar]; (b) Salomão K., Santana N. A., Molina M. T., de Castro S. L., Menna-Barreto R. F. S. BMC Microbiol. 2012;13:196. doi: 10.1186/1471-2180-13-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Lepesheva G. I., Hargrove T. Y., Rachakonda G., Wawrzak Z., Pomel S., Cojean S., Nde P. N., Nes W. D., Locuson C. W., Calcutt M. W., Waterman M. R., Daniels J. S., Loiseau P. M., Villalta F. J. Infect. Dis. 2015;212:1439–1448. doi: 10.1093/infdis/jiv228. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Suryadevara P. K., Racherla K. K., Olepu S., Norcross N. R., Tatipaka H. B., Arif J. A., Planer J. D., Lepesheva G. I., Verlinde C. L., Buckner F. S., Gelb M. H. Bioorg. Med. Chem. 2013;23:6492–6499. doi: 10.1016/j.bmcl.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) De Vita D., Moraca F., Zamperini C., Pandolfi F., Di Santo R., Matheeussen A., Maes L., Tortorella S., Scipione L. Eur. J. Med. Chem. 2016;113:28–33. doi: 10.1016/j.ejmech.2016.02.028. [DOI] [PubMed] [Google Scholar]
- Salomão K., Menna-Barreto R. F. S., de Castro S. L. Curr. Top. Med. Chem. 2016;16:2266–2289. doi: 10.2174/1568026616666160413125049. [DOI] [PubMed] [Google Scholar]
- Barboza A. C. R. N., Cruz C. V. M. S., Graziani M. B., Lorenzetti M. C. F., Sabadini E. Quim. Nova. 2001;24:901–904. [Google Scholar]
- Silva L. H. P., Nussenszweig V. Folia Clin. Biol. 1953;20:191–208. [Google Scholar]
- de Castro S. L., Pinto M. C. F. R., Pinto A. V. Microbios. 1994;78:83–90. [PubMed] [Google Scholar]
- Jardim G. A. M., Reis W. J., Ribeiro M. F., Ottoni F. M., Alves R. J., Silva T. L., Goulart M. O. F., Braga A. L., Menna-Barreto R. F. S., Salomão K., de Castro S. L., da Silva Júnior E. N. RSC Adv. 2015;5:78047–78060. [Google Scholar]
- Spessard G. O. J. Chem. Inf. Comput. Sci. 1998;38:1250–1253. [Google Scholar]
- Hansch C., Leo A. and Hoekman D., Exploring QSAR – Hydrophobic, electronic and Steric constants, ACS, Washington, 1995. [Google Scholar]
- Oliveira D. D., Gaudio A. C. Quant. Struct.-Act. Relat. 2000;19:599–601. [Google Scholar]
- Verma R. P., Hansch C. J. Pharm. Sci. 2007;97:88–109. doi: 10.1002/jps.21087. [DOI] [PubMed] [Google Scholar]
- Kubinyi H. Drug Discovery Today. 1997;2:457–467. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






