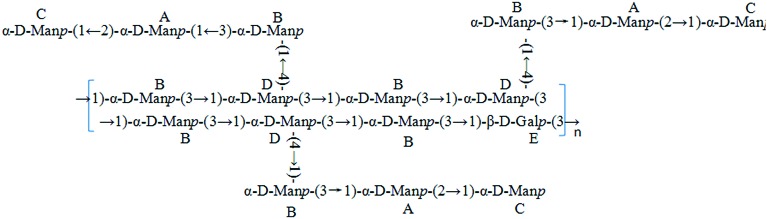 Carboxymethylated and sulfated polysaccharides (CLEP and SLEP) were prepared from an exopolysaccharide previously obtained from Lachnum YM240 (LEP) by chemical modifications.
Carboxymethylated and sulfated polysaccharides (CLEP and SLEP) were prepared from an exopolysaccharide previously obtained from Lachnum YM240 (LEP) by chemical modifications.
Abstract
Carboxymethylated and sulfated polysaccharides (CLEP and SLEP) were prepared from an exopolysaccharide previously obtained from Lachnum YM240 (LEP) by chemical modifications. Two doses (50 mg kg–1 and 200 mg kg–1 b. w.) of LEP, CLEP and SLEP were orally administered to normal mice and type 2 diabetic mice (T2DM) that were induced by streptozotocin (STZ) and a high fat diet, respectively. The hypoglycemic effect was evaluated by testing the oral glucose tolerance, fasting blood glucose (FBG) levels, fasting serum insulin (FINS), glycosylated hemoglobin A1c (HbA1c), and the hypolipidemic effect was evaluated by the body, spleen, pancreas, liver and kidney weights, as well as serum triglycerides (TG), cholesterol (TC) and free fatty acids (FFA). After four weeks of administration, LEP, CLEP and SLEP showed a marked FBG fall rate of 11.2%, 44.0% and 42.5% for the high-dose and 7.43%, 38.5% and 33.1% for the low-dose, respectively, as compared to the DC group. Moreover, compared with DC mice, TC concentrations in the high-dose groups of LEP, CLEP and SLEP were significantly decreased by 29.6%, 38.7% (P < 0.05), 33.0% (P < 0.05), and TG concentrations decreased by 18.9%, 43.9% (P < 0.01), 29.0% (P < 0.05), respectively. In addition, LEP and the derivatives significantly upregulated the expression of glucokinase (GK) and adenosine monophosphate-activated protein kinase (AMPK) in the liver, AMPK and glucose transporter 4 (Glut4) in skeletal muscle and peroxysome proliferator-activated receptor (PPAR-γ) in adipose tissue, whereas downregulated the expression of glucose-6-phosphatase (G6P) in the liver; these were examined using ELISA detection kits. These results for FBG and serum lipids indicate that LEP and its derivatives possess significant hypoglycemic and hypolipidemic effects and carboxymethylation improved the hypoglycemic and hypolipidemic effects more effectively than sulfation. Therefore, the carboxymethylated and sulfated modifications were effective ways to enhance the hypoglycemic and hypolipidemic activities of polysaccharides.
Introduction
Diabetes mellitus (DM), a chronic metabolic disorder characterized by abnormalities in carbohydrate, lipid, and lipoprotein metabolism, has been considered a major health risk in modern society.1,2 The number of diabetic patients is increasing year by year and will be about 300 million by 2025, according to the prediction of the World Health Organization.3 T2DM accounts for more than 90% of the total diabetes patients, which could not only lead to hyperglycemia but also cause many complications, such as hyperlipidemia, hyperinsulinemia, hypertension and atherosclerosis, due to an imbalance between endocrine pancreatic function and peripheral insulin sensitivity in skeletal muscle, the liver and adipose tissue.4,5 Therefore, simultaneous control of hyperglycemia and hyperlipidemia could be greatly meaningful for the treatment of T2DM. Therapeutic options for DM normally involve exercise, diet, oral administration of hypoglycemic drugs, and insulin therapy.6 Chemical medicines including sulfonylureas, biguanide, thiazolidinedione and α-glycosidase inhibitors are widely used for the treatment of DM. However, the chemical medicines are not only associated with drawbacks such as rigid and multiple dosing regimens, high-cost, inaccessibility and untoward effects, but may also pose side effects causing great damage to the health of patients.7 Polysaccharides play a major role in the development of new therapeutic agents for DM because they have fewer toxic side effects than synthetic drugs. Additionally, numerous studies indicate that polysaccharides from plants and fungal sources have strong effects for the treatment of diabetes or hyperlipidemia.8,9
Generally, the chemical modification of polysaccharides provides an opportunity to obtain new pharmacological agents with possible therapeutic properties. Various types of chemical modifications could change polysaccharides in aspects such as molecular structure, molecular weight (Mw), monosaccharide composition, the glycosidic bond of the main chain, the type and degree of substitution, degree of branching and conformation of the main chains and other physicochemical properties such as water solubility, thus allowing a broader range of applications.10 In the last few decades, the biological properties of polysaccharides and their chemical derivatives, especially carboxymethylated and sulfated derivatives, have attracted much attention.11,12 Carboxymethylation has been used to modify the structure of polysaccharides to provide diverse physiological functions. Wang et al. (2015) investigated the antioxidant and moisture-preserving activities of Tremella fuciformis polysaccharides and carboxymethylated derivatives, and they reported that carboxymethylated derivatives exhibited stronger antioxidant and moisture-preserving activities than unmodified polysaccharides, which indicated that chemical modification might have increased those activities.12 It was reported that the Ulva pertusa polysaccharide and its high sulfate content derivative possessed hypolipidemic activity, and the high sulfate content derivative exhibited stronger hypolipidemic activity than Ulva pertusa polysaccharide, which was likely caused by the fact that the sulfate content had a significant effect on the hypolipidemic activity.13 Therefore, carboxymethylated and sulfated modifications may be used as methods to improve the biological activities of some polysaccharides and obtain more effective polysaccharide derivatives.
Lachnum, a saprophytic fungi distributed around the world, belongs to the Hyaloscyphaceae family of the Helotiales order.14 It has been reported that polysaccharides isolated from Lachnum have preventive effects against ethanol-induced gastric ulcer, and anti-hypoxia activity.15,16 Therefore, polysaccharides from Lachnum are considered an important class of bioactive natural products, which have been widely studied for their biochemical and medical properties. In our previous study, LEP was purified from an exopolysaccharide produced by Lachnum YM240. LEP, having the molecular weight of 1.68 × 103 kDa, is composed of mannose and galactose in the approximate molar ratio of 16.3 : 1.0. The structure of the LEP was elucidated by a combination of chemical methods and GC-MS as well as FT-IR and NMR (Fig. 1). The carboxymethylated polysaccharide (CLEP) and sulfated polysaccharide (SLEP) were prepared from LEP by molecular modification, and the results of FT-IR and 13C NMR analysis confirmed the successful modification. Carboxymethyl groups –CH2COOH were substituted at C-3 of → 1)-α-d-Manp-(2 →, C-4 of → 1)-α-d-Manp-(3 → and C-6 of α-d-Manp-(1 →, while sulfate groups –SO3H were mainly at C-4 of → 1)-α-d-Manp-(2 → and C-6 of → 1)-β-d-Galp-(3 →. In this study, the hypoglycemic and hypolipidemic effects of LEP, CLEP and SLEP on T2DM mice induced by high fat diet and low dose STZ were investigated. We also investigated the glucose metabolism in vivo underlying the hypoglycemic effect of LEP and its derivatives, which is expected to allow a better understanding of the functional effects of LEP and its derivatives and should be beneficial in exploring more new bioresources.
Fig. 1. The repeating unit structure of the LEP from Lachnum YM240.
Materials and methods
Chemicals and reagent kits
STZ and 1,1-dimethylbiguanide hydrochloride were purchased from Sigma (St. Louis, MO, USA). Test kits for the following compounds were obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China): liver glycogen, HbA1c, serum TG, TC and FFA. ELISA detection kits for serum FINS, GK, AMPK, Glut4, PPAR-γ and G6P were provided by Shanghai Yansheng Industrial Co. Ltd. (Shanghai, China). Haematoxylin and eosin (H&E) were obtained from Shanghai 101 Lanji Technological Development Co. Ltd. (Shanghai, China). The Blood Glucose Assay kit was obtained from Rongsheng Biotech Co. (Shanghai, China). All other reagents were of analytical grade and were obtained from commercial sources.
The high-fat diet (HFD) composition was as follows: 70.0% standard laboratory diet, 3.00% egg yolk, 10.0% lard, 15.0% sucrose, 1.00% cholesterol and 1.00% sodium cholate.
Preparation of LEP and its derivatives
The fruiting bodies of Lachnum YM240 collected from Yunnan Province (China) were separated and preserved in the Microbial Resource and Application Laboratory of the Hefei University of Technology. Preparation and purification of LEP were carried out according to the previous report of our research team with little modification.16 Carboxymethylated polysaccharide and sulfated polysaccharide were obtained according to the methods of He et al. (2015) and Chen et al. (2014).17,18
Animal model6
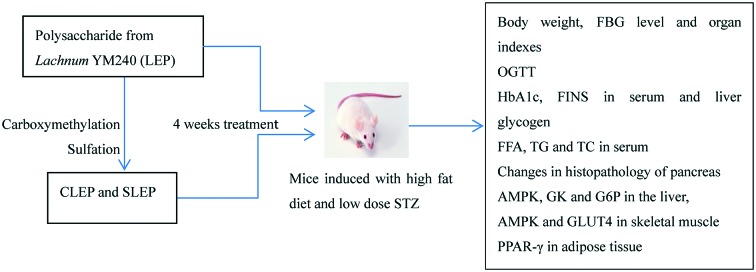
Male mice (18–22 g) were purchased from the Experimental Animal Center of Anhui Medical University (certificate number: no. 1 license of the Medical Laboratory Animal of Anhui). They were acclimated in a controlled environment (temperature of 22 ± 1 °C; humidity of 60 ± 10%; and a 12 h/12 h light/dark cycle) with free access to water and food. The mice were randomly divided into 2 groups. One group (n = 6, normal control) was fed with a standard laboratory diet and another group (n = 60) was fed with the HFD. Apart from the group fed with the standard laboratory diet, another group of mice were fasted for 12 h and were intraperitoneally injected three times (day 20/day 27 and day 34) with 30 mg kg–1 per day of streptozotocin (0.1 M sodium citrate and 0.1 M citric acid, pH 4.2–4.5) after being fed with high-fat food for 19 days (from day 1). HFD-fed mice with FBG levels above 7.8 mM were considered as successfully induced type 2 diabetic mice and were fed with HFD during the experiment period.7 Type 2 diabetic mice were divided into 8 groups. One group was used as a diabetic control and one group was given metformin (100 mg kg–1 b. w.) by oral administration. The other 6 groups were orally gavaged with LEP, CMLEP, SLEP at the doses of 200 and 50 mg kg–1 b. w., respectively. The animals were grouped as follows: group I, normal control mice treated with vehicle alone (NC); group II, diabetic control mice treated with vehicle alone (DC); group III, diabetic mice were given metformin, 100 mg kg–1 b. w. (DM); group IV, diabetic mice were given LEP, 200 mg kg–1 b. w. (HLEP); group V, diabetic mice were given LEP, 50 mg kg–1 b. w. (LLEP); group VI, diabetic mice were given CLEP, 200 mg kg–1 b. w. (HCLEP); group VII, diabetic mice were given CLEP, 50 mg kg–1 b. w. (LCLEP); group VIII: diabetic mice were given SLEP, 200 mg kg–1 b. w. (HSLEP); group IX, diabetic mice were given SLEP, 50 mg kg–1 b. w. (LSLEP).
Respective treatments were intragastrically given to mice daily for 4 consecutive weeks. Accordingly, the mice in the NC and DC groups received equivalent volumes of normal saline. Blood glucose levels (12 hours after fasting) and weight were determined weekly. At the end of the experiment, blood was collected from the orbital sinus and then mice were sacrificed by cervical dislocation, followed by the collection and weighing of the spleen, pancreas, liver, kidney, skeletal muscle and adipose tissue. Whole pancreases were preserved for pathological histology using hematoxylin and eosin (H&E) staining. The rest of the liver and serum were stored at 70 °C until analyses. Organ indexes were calculated as follows: organ index = average weight of organ (g)/body weight (g) × 100%.
The study was approved by the Committee for the Protection of Animal Care at the Hefei University of Technology. All experimental protocols were in accordance with the Guidelines for Experimental Animal Administration published by the State Committee of Science and Technology of People's Republic of China.
Oral glucose tolerance test (OGTT)
After 4 weeks treatment, all mice were fasted for 12 h and then orally administered with glucose (2.0 g kg–1 b. w.). Blood glucose levels were measured at 0, 30, 60, 90, and 120 min, respectively, via blood drops obtained by clipping the tail of the mice after glucose administration.
Blood biochemical measurement
After 4 weeks of treatment, the mice were fasted for 12 h, and blood samples were then collected under anaesthetized conditions and placed into prechilled tubes. The samples were immediately centrifuged at 3000 rpm for 5 min at 4 °C and the serum was separated for further analyses. FINS quantification was assayed using a mouse insulin enzyme-linked immunosorbent assay (ELISA) kit. FFA content and levels of serum, HbA1c and lipids including TC, TG, were measured using commercial kits, according to the guidelines. All analyses were performed in accordance with the manuals provided by the manufacturers.
Preparation and analysis of liver, skeletal muscle and adipose tissue
After the administration, tissue samples from the liver, the thigh skeletal muscles as well as epididymal adipose were excised and weighed after washing with 0.9% saline and homogenized. Homogenized tissue was centrifuged at 3000 rpm for 10 min at 4 °C and then stored at –70 °C until use. The supernatant was collected and analyzed immediately. Liver glycogen contents were measured by test kits and the GK, G6P, AMPK, GLUT4 and PPAR-γ contents were examined by ELISA detection kits.
Statistical analysis
The data are represented as mean ± SD. The single factor analysis of variance was used to analyze the differences between groups with the DPS software. P < 0.05 and P < 0.01 were considered statistically significant.
Results
Effect of LEP and its derivatives on FBG level, body weight and organ indexes
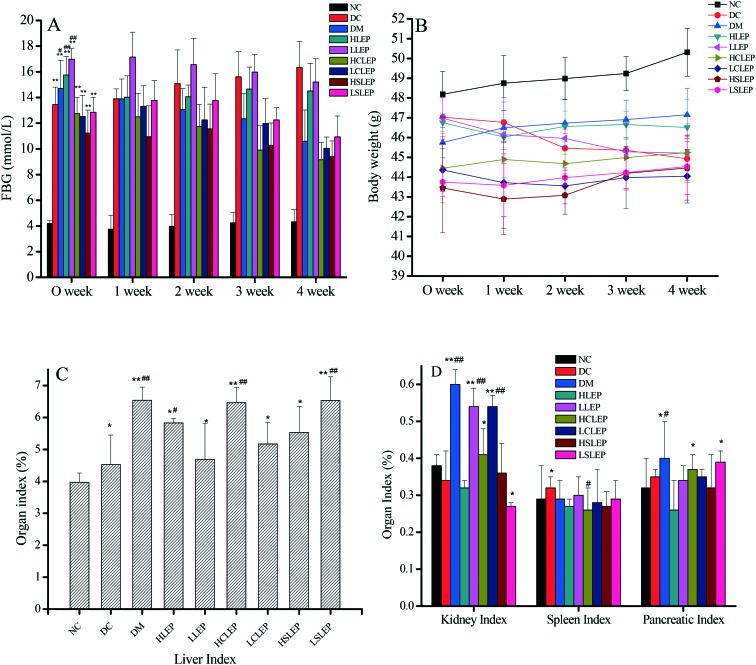
After 4 weeks of treatment, FBG levels in the DC group and treatment group mice were significantly higher than in the NC group mice (Fig. 2A). The diabetic mice administered with CLEP and SLEP for 4 weeks exhibited a significant decrease in FBG levels (P < 0.01 and P < 0.05 respectively) and the level further decreased by 44.0% and 42.5% for the high-dose and 38.5% and 33.1% for the low-dose, respectively, (P < 0.05 or P < 0.01) compared to the DC group (FBG fall rate% = ((DC group – treatment group)/DC group) × 100%). Treatment with LEP showed a lesser hypoglycemic effect and the percentage fall in FBG levels in the high-dose and low-dose groups was 11.2% and 7.43% (P < 0.01), respectively, as compared to the DC group. The hypoglycemic effects on diabetic mice decreased in the order of CLEP > SLEP > LEP at the same dosage. The changes in body weight in the different experimental groups are presented in Fig. 2B. Our study showed that the body weight of diabetic mice was badly restricted after STZ administration and was lower than that of the normal mice. After treatment with metformin, LEP, CLEP and SLEP, the body weight of diabetic mice was reduced (P < 0.05 or P < 0.01), while, the body weight of the DC group had a small weight gain (P < 0.01, Fig. 2B), compared with the initial weight. Therefore LEP, CLEP and SLEP could dose-dependently improve the bodyweight loss, but the effect was inferior to that of metformin (P < 0.05) (Fig. 2B). In addition, liver, spleen, kidney and pancreatic indexes in the DC group and treated group increased significantly, as compared to the NC group (P < 0.05 or P < 0.01, Fig. 2C); the higher organ indexes may be due to the weight loss in these mice.19 The data suggest that CLEP had a more positive effect on FBG levels, body weight and organ indexes than SLEP in diabetic mice, and the high-dose treatment resulted in the greatest hypoglycemic effects.
Fig. 2. FBG level (A), body weight (B), and organ indexes (C and D) of mice in different groups. FBG level and body weight were recorded every week and the organ indexes were recorded at the end of the research. *P < 0.05, **P < 0.01 compared with NC group. #P < 0.05, ##P < 0.01, compared with the DC group.
Effect of LEP and its derivatives on the oral glucose tolerance test (OGTT)
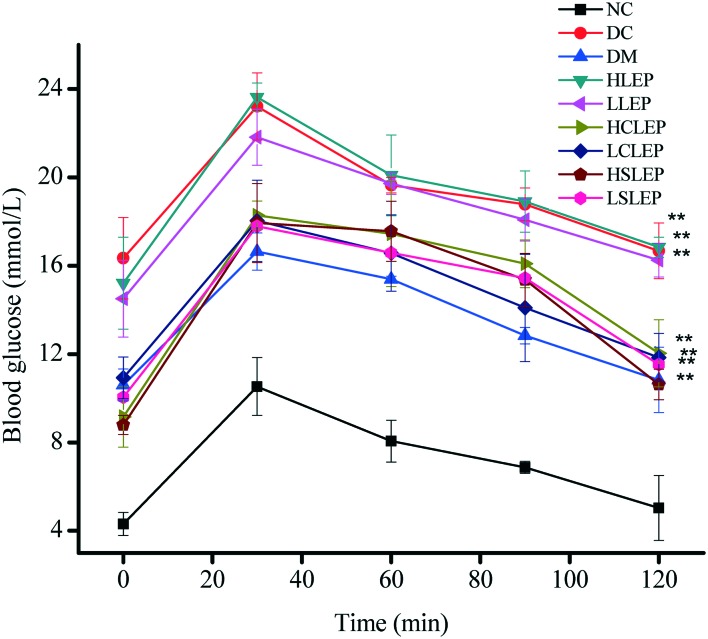
Fig. 3 shows the changes in the levels of blood glucose during OGTT. The blood glucose level in all groups increased to a maximum at 30 min after the oral glucose challenge, and then slowly decreased. The DC group mice showed a remarkably stronger hyperglycemic response to oral glucose administration compared to NC group mice. The blood glucose level of treatment groups was significantly lower (P < 0.05) than that of the DC group at each time point, which indicated that LEP, CLEP and SLEP showed a remarkable improvement in overall glucose response in a dose-dependent manner. Among the treatment groups, the higher dose-treated mice usually had more potent glucose tolerance than the lower dose-treated mice; glucose tolerance was progressively reduced in the following order: HCLEP > HSLEP > HLEP > LCLEP > LSLEP > LLEP (Fig. 3).
Fig. 3. Effects of LEP, CLEP and SLEP on OGTT in mice. All values represent the means ± SD (n = 6). *P < 0.05, **P < 0.01 compared with NC group. #P < 0.05, ##P < 0.01 compared with DC group.
Effect of LEP and its derivatives on HbA1c and FINS in serum and liver glycogen
Table 1 shows the levels of HbA1c and FINS in the serum and glycogen in the liver for each group. The HbA1c level of the DC group was significantly higher than the NC group (P < 0.01). After 4 weeks of administration, LEP, CLEP and SLEP significantly lowered the HbA1c values (P < 0.01). The percentage fall in the high-dose groups of LEP, CLEP and SLEP was 7.35%, 14.4% and 11.6%, respectively (P < 0.01 or P < 0.05), as compared to the DC group. The DC group mice exhibited much higher (P < 0.05) FINS levels than the NC group mice. Administration of CLEP and SLEP significantly decreased (P < 0.01) the FINS levels of the diabetic mice. On the other hand, the DC group mice, after STZ injection, showed a significant decrease in the level of liver glycogen, compared with the NC group (P < 0.05, Table 1). When treated with CLEP, SLEP (low and high dose) and metformin for 4 weeks, diabetic mice obviously recovered the glycogen content. However, LEP had a lesser impact on HbA1c in serum and glycogen in liver.
Table 1. Effect of LEP and its derivatives on HbA1c, FINS, FFA, TG and TC in serum and liver glycogen.
| Groups | FINS (mU L–1) | Liver glycogen (mg g–1) | HbA1c (%) | TG (mmol L–1) | TC (mmol L–1) | FFA (μmol L–1) |
| NC | 8.52 ± 2.13 | 22.75 ± 0.94 | 5.64 ± 1.06 | 1.18 ± 0.13 | 2.82 ± 0.21 | 256.79 ± 78.92 |
| DC | 18.15 ± 1.89**, ## | 6.13 ± 0.24** | 14.55 ± 0.32** | 2.28 ± 0.20** | 5.61 ± 0.16** | 905.92 ± 174.93** |
| DM | 7.85 ± 3.32 | 19.60 ± 0.86## | 11.97 ± 0.88*, ## | 1.67 ± 0.24*, # | 3.05 ± 0.33*, ## | 318.81 ± 149.72 |
| HLEP | 16.95 ± 2.76*, # | 17.70 ± 0.39**, ## | 13.48 ± 0.47** | 1.85 ± 0.11* | 3.95 ± 0.26* | 765.67 ± 144.65**, # |
| LLEP | 11.56 ± 3.52* | 14.29 ± 0.76**, ## | 13.32 ± 1.21** | 2.05 ± 0.03** | 5.24 ± 0.22** | 817.46 ± 92.38** |
| HCLEP | 17.01 ± 2.18**, ## | 19.05 ± 0.58*, ## | 12.46 ± 0.96*, # | 1.28 ± 0.33## | 3.44 ± 0.17*, # | 526.82 ± 166.46*, ## |
| LCLEP | 11.43 ± 2.73* | 12.36 ± 0.43**, # | 14.54 ± 0.75**, # | 1.74 ± 0.15* | 4.88 ± 0.24** | 711.67 ± 108.09** |
| HSLEP | 18.25 ± 4.17**, ## | 16.13 ± 0.57*, ## | 12.87 ± 0.96**, # | 1.62 ± 0.16*, # | 3.76 ± 0.35*, # | 625.43 ± 177.61* |
| LSLEP | 13.79 ± 3.82* | 9.29 ± 1.03** | 14.37 ± 1.12** | 1.98 ± 0.23** | 5.08 ± 0.32** | 787.45 ± 123.57**, # |
Effect of LEP and its derivatives on FFA, TG and TC in serum
The effect of LEP and its derivatives on serum lipid concentration was also studied after oral administration for 4 weeks. As shown in Table 1, there was a significant increase in the levels of serum FFA, TG and TC of the DC group mice in comparison to NC group mice (P < 0.01 or P < 0.05). Administration of LEP, CLEP and SLEP resulted in a significant diminution (P < 0.05) of elevated serum TC and TG levels. Compared to DC mice, TC concentrations in high-dose groups of LEP, CLEP and SLEP were significantly decreased by 29.6%, 38.7% (P < 0.05), 33.0% (P < 0.05), and TG concentrations decreased by 18.9%, 43.9% (P < 0.01), 29.0% (P < 0.05), respectively. Moreover, TC concentrations in the low-dose groups of CLEP and SLEP were significantly decreased by 6.60%, 13.0%, 9.45%, and TG concentrations decreased by 10.1%, 23.7%, 13.2%, respectively, compared to DC mice. It was likely that the LEP, CLEP and SLEP had significant hypolipidemic activity and obvious differences in hypolipidemic activity among LEP, CLEP and SLEP were observed. Moreover, the hypolipidemic activity of CLEP for the high-dose group was stronger than SLEP for the high-dose group. After treatment with LEP, CLEP and SLEP, the serum FFA levels in the fasted mice were reduced (P < 0.05, Table 1) compared to the DC group mice, and presented a dose resistance according to the relationship. Fortunately, we found that LEP, CLEP and SLEP could significantly reduce FFA. Compared with DC mice, the contents of FFA in LEP, CLEP and SLEP groups were reduced by 15.5%, 41.9%, 31.0% (P < 0.01, P < 0.05, P < 0.05) for the high dose and 9.77%, 21.4%, 13.1% (P < 0.01, P < 0.05, P < 0.01) for the low dose, respectively. These results confirmed that LEP could modulate blood lipid disorders and both sulfated and carboxymethylated modifications were effective ways to enhance the hypolipidemic activity of LEP.
Changes in the histopathology of the pancreas
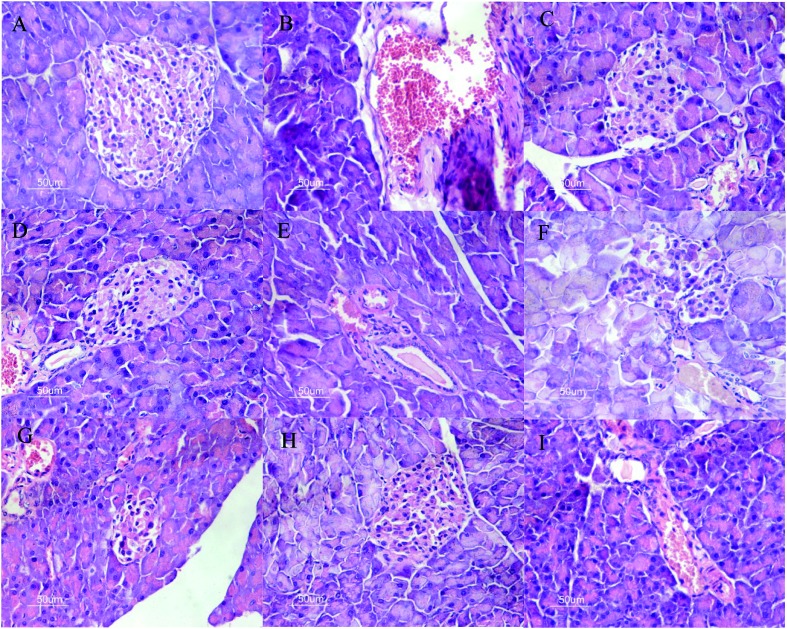
The changes in the pancreas histology of the different groups are presented in Fig. 4. We observed degeneration in the pancreas tissue of STZ-induced diabetic mice, such as hepatic cords, focal necrosis, congestion in the central vein and infiltration of lymphocytes. Das et al. (1996) pointed out that insulin depletion may result in degenerative structural changes in tissue.20 Most of these pathologic morphologies were detected in the pancreas tissues of DC and treated group mice (Fig. 4). According to the microscopic examinations, the severe pancreatic lesions induced by STZ were considerably diminished by the administration of LEP, CLEP and SLEP at low and high doses, but there is no dose-related effect. The results indicated that pancreas tissues of STZ-induced diabetic mice can be protected and repaired by intervention with LEP, CLEP and SLEP. The pancreatic β-cells in CLEP and SLEP of high-dose groups were distributed more uniformly and the amounts were obviously elevated, as well as the islet structure was better than that in the DC group. These results agreed with that reported by Huang et al. (2012), where protection of pancreatic β-cells by polysaccharides may be attributed to anti-diabetic activity.21
Fig. 4. Effects of LEP, CLEP and SLEP on pancreas damage in diabetic mice. A: group I (normal mice); B: group II (untreated diabetic mice); C: group III (administration of metformin in diabetic mice); D: group IV (administration of LEP with high dose in diabetic mice); E: group V (administration of LEP with low dose in diabetic mice); F: group VI (administration of CLEP with high dose in diabetic mice). G: group VI (administration of CLEP with low dose in diabetic mice). H: group VI (administration of SLEP with high dose in diabetic mice). I: group VI (administration of SLEP with low dose in diabetic mice). Hematoxylin/eosin staining; 400× magnification.
Effects of LEP and its derivatives on the expressions of key glucose-metabolic enzymes in liver
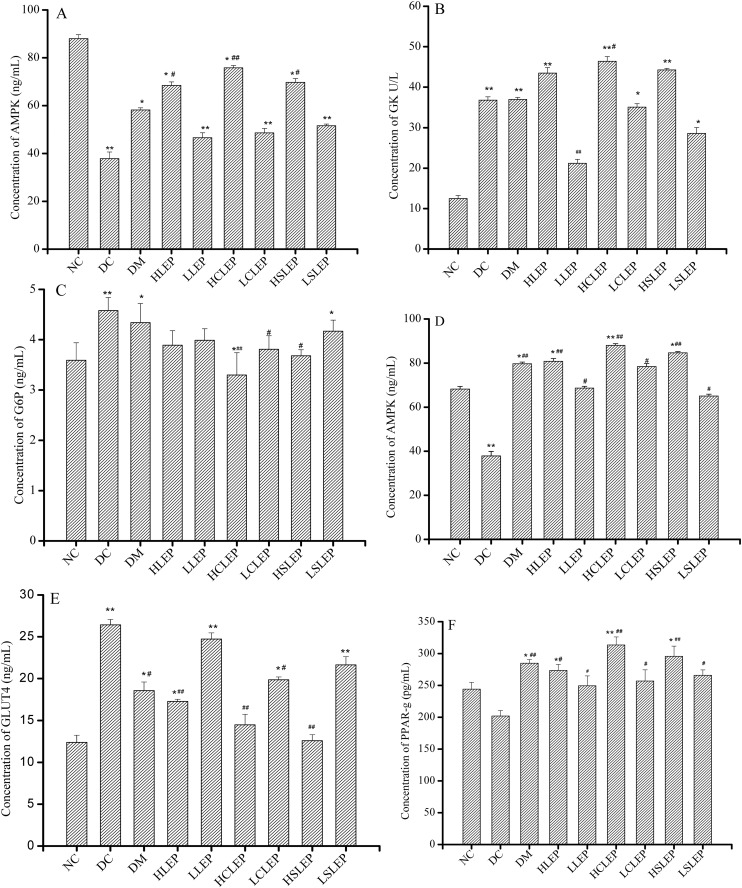
Fig. 5A–C show the concentrations of AMPK, GK and G6P, respectively, in liver tissue in different mice groups. After 4 weeks of treatment, there was a significant increase (P < 0.05 or P < 0.01) in GK and AMPK expression in the liver tissue of diabetic mice supplemented with LEP, CLEP and SLEP, compared with DC group mice, while the expression of G6P was downregulated. However, HCLEP and HSLEP promoted the AMPK expression (P < 0.05) to almost normal levels, which might be an attractive target of CLEP and SLEP. These data suggest that LEP, CLEP and SLEP dose-dependently and significantly promoted glycolysis and glycogenesis in the liver.
Fig. 5. The effects of LEP and its derivatives on AMPK, GK and G6P in the liver, AMPK and GLUT4 in skeletal muscle, and PPAR-γ in adipose tissue. Expressions of AMPK (A), GK (B) and G6P (C) in the liver, AMPK (D) and GLUT4 (E) in skeletal muscle, and PPAR-γ (F) in adipose tissue after treatment with LEP, CLEP and SLEP for 4 weeks. Contents in the supernatant of tissue homogenate were tested using Elisa kits. Each group consisted of 6 mice. *P < 0.05, **P < 0.01 compared with NC group, #P < 0.05, ##P < 0.01 compared with DC group.
Expressions of AMPK, GLUT4 in skeletal muscle and PPAR-γ in adipose tissue
As shown in Fig. 5D, AMPK expression was slightly increased in LEP, CLEP and SLEP groups without any significant difference, compared to the DC group. However, LEP, CLEP and SLEP greatly promoted the GLUT4 expression in skeletal muscle (Fig. 5E, P < 0.05). Administration of 200 mg kg–1 of CLEP (HCLEP group) restored the GLUT4 expression in diabetic mice to the same level as in normal mice. In addition, as shown in Fig. 5F, PPAR-γ protein expression in adipose tissue was slightly lower in the DC group than that in NC group, but no significant difference was observed between them. HLEP significantly increased PPAR-γ protein expression by 35.5%, compared to that of DC group mice (P < 0.01). HCLEP and HSLEP increased PPAR-γ protein expression by 55.3% and 46.5%, compared to that of the DC group mice (P < 0.05). PPAR-γ protein expression increased in adipose tissue in the LEP, CLEP and SLEP groups, compared to the NC group (P < 0.01 or P < 0.05).
Discussion
LEP, CLEP and SLEP promoted the expression of GK and AMPK, and inhibited the expression of G6P; therefore, the balance among glycogenesis, glycogenolysis and glycolysis in the liver was regulated by LEP, CLEP and SLEP. LEP, CLEP and SLEP showed much higher GLUT4 expression so that more glucose could be transported and utilized by the muscle. Mice treated with LEP, CLEP and SLEP could also increase PPAR-γ protein expression in the adipose tissue. A possible schematic diagram of LEP and its derivatives in glycometabolism is shown in Fig. 6.
Fig. 6. Schematic of the putative mechanisms in glycometabolism.
T2DM is one of the most common chronic and progressive diseases, which is defined as hyperglycemia and glucose intolerance caused by insulin resistance or impaired insulin secretion.7 With the increasing incidence rate of T2DM, more in depth study is necessary on the polysaccharides from natural materials for the treatment of diabetes mellitus. Therefore, this study was designed to determine whether LEP and its derivatives had hypoglycemic and hypolipidemic activities in diabetic mice. Herein, the mice model of diabetes was induced by a high-fat diet and low-dose STZ, which simulates the natural pathogenesis of T2DM and is widely used in studies of hyperglycemia and hyperlipidemia drugs.22 The substantial increase in the incidence of T2DM over the past decade is associated with a marked increase in the prevalence of obesity, which contributes greatly to insulin resistance. T2DM develops primarily due to insulin resistance and insulin producing pancreatic β-cell dysfunction, leading to insufficient insulin secretion.23 Insulin resistance, the remarkable feature of T2DM, is defined as the reduced response of cells or tissues to physiological levels of insulin.24 As we all know, diabetic mice are characterized by advanced hyperinsulinemia, so the effect of LEP, CLEP and SLEP administration on FINS was investigated. After 4 weeks of treatment, there was significantly increased FINS and glucose disposal, presumably due to higher insulin sensitivity, compared to those in the DC group. OGTT showed that the ability of LEP, CLEP and SLEP to improve glucose tolerance was also related to an increase in insulin sensitivity.
Additionally, FFA derived from visceral fat lipolysis is released into the portalvein and drained by the liver, which results in hepatic fat accumulation and hepatic insulin resistance.25 The evolution of type 2 diabetes is frequently combined with hyperglycemia, as well as insulin resistance and dyslipidemia, which is often an important determinant of the course and status of the diabetes mellitus.9 It is reported that some polysaccharides from Talinum triangulare,7Enterobacter cloacae Z0206 (ref. 9) and Momordica charantia L.10 have overall positive effects on glucose and lipid metabolism. In this study, there was a marked decline in the serum levels of TG, TC and FFA in treated mice. We similarly found that LEP and its derivatives effectively relieved hyperlipidemia in diabetic mice and presented a dose resistance according to the relationship. Results in the present study are in agreement with our previous studies that polysaccharides from Lachnum calyculiforme and Lachnum YM281 had a significant hypoglycemic effect on diabetic mice and had lipid lowering effects with a dose-effect relationship, respectively.26,27
As previously reported, signaling pathways and AMPK, can lead to the translocation of GLUT4 to the plasma membrane.28 GLUT4, expressed in the skeletal tissues, plays an important role in insulin resistance and glucose uptake as a glucose transporter, and its reduced translocation from the intracellular pool to the plasma membranes is reportedly a possible cause.29 Previous studies have demonstrated that insulin resistance in skeletal muscle is the critical factor leading to reduced whole-body glucose disposal in obesity and T2DM. A central role for GLUT4 in whole-body metabolism is strongly supported by a variety of genetically engineered mouse models, where expression of the transporter is either enhanced or ablated in muscle or adipose tissue or both.30 In the present study, LEP and its derivatives significantly increased GLUT4 protein expression in skeletal muscle.
AMPK is a phylogenetically conserved serine/threonine protein kinase, which not only acts as an integrator of regulatory signals, monitoring the systemic and cellular energy status, but is also investigated as a potential therapeutic target for the treatment of T2DM.31,32 AMPK acts by directly affecting the activity of enzymes involved in carbohydrate, lipid, and protein biosynthesis, and by long-term transcriptional control of key components.33 In glucose and lipid metabolism, the phosphorylation and activation of AMPK leads to GLUT4 translocation and eventually glucose uptake.34 LEP and its derivatives upregulate AMPK protein expressions in liver and skeletal muscle so that more glycogen is decomposed in order to provide energy. After administration, more glucose can be removed from the circulation and transported to skeletal muscle in diabetic mice, which means that LEP and its derivatives accelerate glucose uptake in skeletal muscle and partly contribute to the decrease of fasting blood glucose. Our results indicate that the hypoglycemic and hypolipidemic effects of LEP and its derivatives are related to GLUT4 transposition and AMPK. These results indicate that the hyperglycemia effect of LEP, CLEP and SLEP may be related to GLUT4 transposition, and LEP and its derivatives enhanced GLUT4 translocation by specifically targeting the AMPK pathway. Glucose concentrations are strictly maintained under physiological conditions. Glucose homeostasis is maintained through a balance between glucose uptake by skeletal muscle and adipose tissue, and production by the liver. Hepatic gluconeogenesis is strictly controlled by the activities of rate-limiting enzymes such as G6P,35 which is the regulatory enzyme that catalyzes the final step of glycogen decomposition.36 In contrast, GK is the key enzyme in the phosphorylation of glucose, responsible for glucose storage and conversion to glycogen. Together, they reflect the balance between liver glycogen and glycogen decomposition. LEP and its derivatives downregulate the expression of G6P in the insulin signaling pathway and upregulate GK expression in diabetic mice; overall, these investigational studies indicate the role of LEP and its derivatives in glucose metabolism as increasing glycolysis, while decreasing gluconeogenesis.
It is well known that PPAR-γ plays a crucial role in insulin sensitization. The PPAR-γ receptor is highly expressed in adipose tissues and mainly mediates the transcriptional activation of genes involved in adipogenesis, lipid metabolism, inflammation, and the maintenance of metabolic homeostasis.37 Natural products reported to activate or bind PPAR-γ were therapeutically used to combat hyperglycemia associated with metabolic syndrome and T2DM.38 Alternating the protein content encoded by these genes also confirmed the regulation of PPAR-γ, AMPK, GLUT4, G6P and GK. From these results, we concluded that LEP and its derivatives displayed multi-target regulation effects on glucose metabolism in the liver, muscle and adipose tissue. In diabetes, the administration of LEP and its derivatives promotes the conversion of glucose to glycogen and inhibits glycogen decomposition, which helps in maintaining relatively low blood sugar levels. This research has shown the hypoglycemic activity and insulin-sensitizing effects of polysaccharides from Lachnum YM240 in diabetic mice and suggests that LEP and its derivatives may be used as an adjuvant therapy to control blood glucose and insulin resistance in type 2 diabetic individuals. Further research on LEP and other activities are still under investigation.
There is increasing evidence to support the idea that the antioxidant activity of fungal polysaccharides such as those isolated from Cordyceps militaris and Pleurotus ostreatus play a very important role in the treatment of hyperglycemia and hyperlipidemia.12,39 Studies have also shown that tissue antioxidant status may have been incriminated as a contributory factor of diabetes.32 Moreover, the oxidative damage could accelerate the pathogenic progress of hyperglycemia and hyperlipidemia. Thus, oxidative stress may play an important role in multiple therapeutic interventions and therapeutic synergy. Zhao et al. (2012) suggested that the antioxidant activity of Opuntia dillenii polysaccharides might be relevant to the treatment of diabetes.40 Huang et al. (2012) also indicated that polysaccharides appeared to be more effective at boosting antioxidant status, thereby protecting liver cells against hyperglycemia-induced oxidative damage.41 Xue et al. (2009) also found that sulfated Achyranthes bidentata polysaccharides exerted protective effects in STZ-induced diabetic rats, possibly by reducing oxidative stress and hence protecting the organism from oxidative damage.42 Therefore, the antioxidant activity of polysaccharides plays a very important role in the treatment of hyperglycemia and hyperlipidemia. Our previous study showed that LEP and its derivatives can reduce reactive oxygen free radicals in vitro. We hypothesized that the significant free radical scavenging activity of LEP and its derivatives might be the effective means of treating hyperglycemic and hypolipidemic effects. The results are probably related to the introduction of carboxyl groups and sulfenyl groups, which changed the structure of LEP and decreased the intermolecular/intramolecular hydrogen bonds.43
Conclusion
Polysaccharides from Lachnum YM240 were successfully modified to obtain sulfated and carboxymethylated derivatives in this study. Both carboxymethylated and sulfated modifications are effective ways to enhance hypoglycemic and hypolipidemic activities. Compared with sulfated modification, carboxymethylated modification improved the hypoglycemic and hypolipidemic effects more effectively. LEP, CLEP and SLEP exhibit effective hypoglycemic effects, possibly through promoting glycogenesis and glucose utilization. In order to improve the bioactivities of the derivatives, we can increase DS and change the substitution sites of the polysaccharide in different ways. However, the structure–function relationship of LEP and its derivatives on hyperglycemia and hyperlipidemia needs to be further studied.
Acknowledgments
The authors would like to acknowledge the support provided by the project “National Natural Science Foundation of China (31470146)”.
Footnotes
†The authors declare no competing interests.
References
- Chen C., You L. J., Abbasi A. M., Fu X., Liu R. H., Li C. Food Funct. 2016;71(1):530–539. doi: 10.1039/c5fo01114k. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Fu F., Chen T., Li Z., Shen Q. W. J. Ethnopharmacol. 2016;192:256–263. doi: 10.1016/j.jep.2016.07.002. [DOI] [PubMed] [Google Scholar]
- Jeszka-Skowron M., Flaczyk E., Jeszka J., Krejpcio Z., Król E., Buchowski M. S. J. Funct. Foods. 2014;8(1):9–17. [Google Scholar]
- Liu Z., Li W., Li X., Zhang M., Chen L., Zheng Y. N., Sun G., Ruan C. J. Ethnopharmacol. 2013;145(1):233–240. doi: 10.1016/j.jep.2012.10.058. [DOI] [PubMed] [Google Scholar]
- Jia S., Hu Y., Zhang W., Zhao X., Chen Y., Sun C., Li X., Chen K. Food Funct. 2015;6(3):878–886. doi: 10.1039/c4fo00993b. [DOI] [PubMed] [Google Scholar]
- Kumar R., Arora V., Ram V., Bhandari A., Vyas P. Int. J. Diabetes Mellitus. 2015;3(1):45–50. [Google Scholar]
- Xu W., Zhou Q., Yin J. J., Yao Y., Zhang J. L. Int. J. Biol. Macromol. 2014;72:575–579. doi: 10.1016/j.ijbiomac.2014.09.011. [DOI] [PubMed] [Google Scholar]
- Wang X., Zhang Z., Zhao M. Int. J. Biol. Macromol. 2015;72(2):157–165. doi: 10.1016/j.ijbiomac.2014.08.045. [DOI] [PubMed] [Google Scholar]
- Huang M., Wang F., Zhou X., Yang H., Wang Y. Carbohydr. Polym. 2015;117:91–98. doi: 10.1016/j.carbpol.2014.09.008. [DOI] [PubMed] [Google Scholar]
- Yi C., Hui Z., Wang Y., Nie S., Chang L., Xie M. Food Chem. 2014;186(3):231–238. doi: 10.1016/j.foodchem.2014.10.032. [DOI] [PubMed] [Google Scholar]
- Ktari N., Mnafgui K., Nasri R., Hamden K., Bkhairia I., Ben H. A., Boudaouara T., Elfeki A., Nasri M. Food Funct. 2013;4(11):1691–1699. doi: 10.1039/c3fo60264h. [DOI] [PubMed] [Google Scholar]
- Wang L., Xu N., Zhang J., Zhao H., Lin L., Jia S., Jia L. Carbohydr. Polym. 2015;131:355–362. doi: 10.1016/j.carbpol.2015.06.016. [DOI] [PubMed] [Google Scholar]
- Qi H., Huang L., Liu X., Liu D., Zhang Q., Liu S. Carbohydr. Polym. 2012;87(2):1637–1640. [Google Scholar]
- He Y., Ye M., Du Z., Wang H., Wu Y., Yang L. Carbohydr. Polym. 2014;105(1):169–176. doi: 10.1016/j.carbpol.2014.01.080. [DOI] [PubMed] [Google Scholar]
- Xu P., Yang L., Yuan R. Y., Ye Z. Y., Ye H. R., Ye M. Int. J. Biol. Macromol. 2016;86:10–17. doi: 10.1016/j.ijbiomac.2016.01.036. [DOI] [PubMed] [Google Scholar]
- Du Z., Zong S., Surhio M. M., Xu P., Yang L., Ye M. Process Biochem. 2016;51:1290–1298. [Google Scholar]
- He Y., Ye M., Jing L., Du Z., Surahio M., Xu H. Carbohydr. Polym. 2015;117:788–796. doi: 10.1016/j.carbpol.2014.10.046. [DOI] [PubMed] [Google Scholar]
- Chen Y., Zhang H., Wang Y., Nie S., Li C., Xie M. Food Chem. 2014;156(3):279–288. doi: 10.1016/j.foodchem.2014.01.111. [DOI] [PubMed] [Google Scholar]
- Lu J.J. M.M., Wang Y. F., Yan H. L., Lin P., Gu W., Yu J. J. Ethnopharmacol. 2015;179:291–300. doi: 10.1016/j.jep.2015.12.057. [DOI] [PubMed] [Google Scholar]
- Das A. V., Padayatti P. S., Paulose C. S. Indian J. Exp. Biol. 1996;34:341–345. [PubMed] [Google Scholar]
- Huang M., Zhang S., Zhang M., Ou S., Pan Z. Appl. Microbiol. Biotechnol. 2012;94(3):763–771. doi: 10.1007/s00253-011-3711-7. [DOI] [PubMed] [Google Scholar]
- Liu Y., Dong M., Yang Z., Pan S. Int. J. Biol. Macromol. 2016;89:484–488. doi: 10.1016/j.ijbiomac.2016.05.015. [DOI] [PubMed] [Google Scholar]
- Mahmoud A. M., Ahmed O. M., Ashour M. B., Abdel-Moneim A. Int. J. Diabetes Dev. Countries. 2015;35(3):250–263. [Google Scholar]
- Savage D. B., Petersen K. F., Shulman G. I. Physiol. Rev. 2007;87(2):507–520. doi: 10.1152/physrev.00024.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis G. F., Carpentier A., Adeli K., Giacca A. Endocr. Rev. 2002;23(2):201–229. doi: 10.1210/edrv.23.2.0461. [DOI] [PubMed] [Google Scholar]
- Ye M., Qiu T., Peng W., Chen W. X., Ye Y. W., Lin Y. R. Carbohydr. Polym. 2011;86(86):285–290. [Google Scholar]
- Qiu T., Ma X., Ye M., Yuan R., Wu Y. Carbohydr. Polym. 2013;98(1):922–930. doi: 10.1016/j.carbpol.2013.07.014. [DOI] [PubMed] [Google Scholar]
- Govers R. Diabetes Metab. 2014;40(6):400–410. doi: 10.1016/j.diabet.2014.01.005. [DOI] [PubMed] [Google Scholar]
- Ueda-Wakagi M., Mukai R., Fuse N., Mizushina Y., Ashida H. Int. J. Mol. Sci. 2014;16(7):16288–16299. doi: 10.3390/ijms160716288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Czech M. P. Cell Metab. 2007;5(4):237–352. doi: 10.1016/j.cmet.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Andreelli F., Viollet B., Vaulont S. J. Bone Miner. Res. 2013;28(4):936–947. [Google Scholar]
- Hardie D. G. J. Intern. Med. 2014;276(6):543–559. doi: 10.1111/joim.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana S., Blowers E. C., Natarajan A. J. Med. Chem. 2015;58(1):2–29. doi: 10.1021/jm401994c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. T., Ussher J. R., Mohammad A., Lam A., Lopaschuk G. D. Can. J. Physiol. Pharmacol. 2014;92(4):307–314. doi: 10.1139/cjpp-2013-0107. [DOI] [PubMed] [Google Scholar]
- Kim H. J., Jee H. J., Yun J. Acta Biochim. Biophys. Sin. 2011;43(8):589–594. doi: 10.1093/abbs/gmr053. [DOI] [PubMed] [Google Scholar]
- Haeusler A. R., Donnelly C. J., Periz G., Simko E. A., Shaw P. G., Kim M. S., Maragakis N. J., Troncoso J. C., Pandey A., Sattler R., Rothstein J. D., Wang J. Nature. 2014;507(7491):195–200. doi: 10.1038/nature13124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dussault I., Forman B. M. Prostaglandins Other Lipid Mediators. 2000;62(1):1–13. doi: 10.1016/s0090-6980(00)00071-x. [DOI] [PubMed] [Google Scholar]
- Wang L., Waltenberger B., Pferschy-Wenzig E. M., Blunder M., Liu X., Malainer C., Blazevica T., Schwaigerb S., Rollingerb J. M., Heissa E. H., Schusterd D., Koppa B., Bauerc R., Stuppnerb H., Dirscha V. M., Atanasov A. G. Biochem. Pharmacol. 2014;92(1):73–89. doi: 10.1016/j.bcp.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu K., Nie S., Li C., Lin S., Xing M., Li W., Gong D., Xie M. Int. J. Biol. Macromol. 2013;57(6):142–150. doi: 10.1016/j.ijbiomac.2013.03.009. [DOI] [PubMed] [Google Scholar]
- Zhao L. Y., Huang W., Yuan Q. X., Cheng J., Huang Z. C., Ouyang L. J., Zeng F. Food Chem. 2012;134(2):964–971. doi: 10.1016/j.foodchem.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Huang H. Y., Korivi M., Chaing Y. Y., Chien T. Y., Tsai Y. C. Evid. Based Complement. Alternat. Med. 2012;2012:1–8. doi: 10.1155/2012/856381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue S., Chen X., Lu J., Jin L. Carbohydr. Polym. 2009;75(3):415–419. [Google Scholar]
- Wu Y., Ye M., Du Z., Jing L., Surahio M., Yang L. Carbohydr. Polym. 2014;114:190–195. doi: 10.1016/j.carbpol.2014.07.075. [DOI] [PubMed] [Google Scholar]