 Stereoselective synthesis of (–)-kunstleramide and its amide derivatives has been achieved and they have been evaluated for their in vitro anti-proliferative activities against various cell lines.
Stereoselective synthesis of (–)-kunstleramide and its amide derivatives has been achieved and they have been evaluated for their in vitro anti-proliferative activities against various cell lines.
Abstract
Stereoselective total synthesis of (–)-kunstleramide, a cytotoxic dienamide from the bark of Beilschmiedia kunstleri gamble, has been accomplished by using Keck's asymmetric allylation and Trost isomerization as key reactions. Application of the developed strategy for the synthesis of a series of amide analogues (8–22) was also reported. Furthermore, the synthesized compounds were evaluated for their in vitro anti-proliferative activities against human epithelial lung carcinoma (A549), human epithelial cervical cancer (HeLa), human breast adenocarcinoma (MCF7) and human neuroblastoma (IMR32) cell lines using the SRB assay. All the compounds show moderate anti-proliferative activity against all cell lines. Some of the piperazine derivatives (17–22) strongly inhibit the growth of breast cancer cells with IC50 values of 8–20 μM.
Introduction
The unsaturated amide moiety is a widespread functionality found in numerous naturally occurring plant-based alkaloids. These natural products contain a diene moiety as the main carbon backbone, differing from each other by chain length and/or the number of functional groups.1 During the past decade, several natural products with a diene structural motif such as lobatamides,2 salicylihalamides,3 apicularens,4 saliniketals,5 scyphostatins6 and crocacins7 have been isolated and have been shown to possess significant biological activities.
The genus Piper is a rich source of dienamides and most of these compounds exhibit a wide range of biological activities, such as immunomodulatory,8 anticarcinogenic,9 antiulcer,10 antidepressant,11 antifungal,12 and anticancer activities.13 The biological importance of unsaturated amides and their pharmacological potential have made them extremely attractive targets for both synthetic and biological researchers to study their synthesis and medicinal properties.14 As a part of our ongoing research program aimed at developing stereoselective syntheses of biologically interesting molecules15 originating from natural sources, we developed an enantioselective route to (–)-kunstleramide.
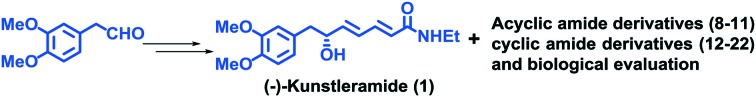
(–)-Kunstleramide, an unsaturated amide, was isolated from the bark of Beilschmiedia kunstleri gamble and displayed moderate cytotoxic activity.16 To the best of our knowledge, only one synthesis has been reported in the literature.17 With the aim of developing a new synthetic route and structural modifications of (–)-kunstleramide, we would like to report herein an efficient access to the construction of new derivatives 8–22 with improved anti-proliferative activity, by employing Keck's allylation and Trost isomerization as key steps and evaluate their antiproliferative activities against human tumor cell lines such as cervical (HeLa), breast (MCF7), lung (A549) and neuroblastoma (IMR32).
Results and discussion
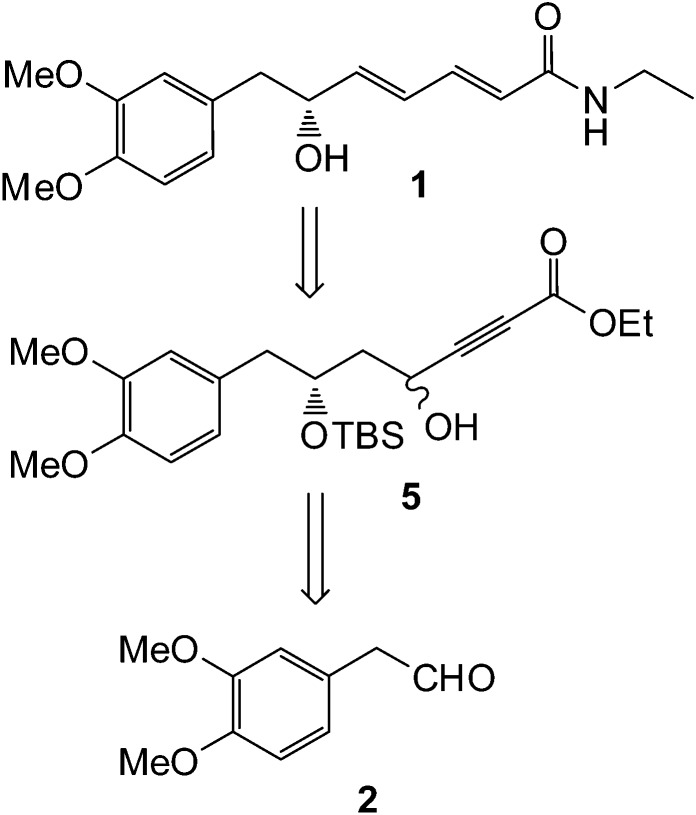
The retrosynthetic analysis of 1 is outlined in Scheme 1. A TPP-mediated allene type rearrangement of 5 and amide coupling would provide the target molecule, whereas, the alkyne precursor 5 could be made from 2 by Keck's allylation, oxidative cleavage of olefin, followed by alkynylation.
Scheme 1. Retrosynthetic analysis of (–)-kunstleramide (1).
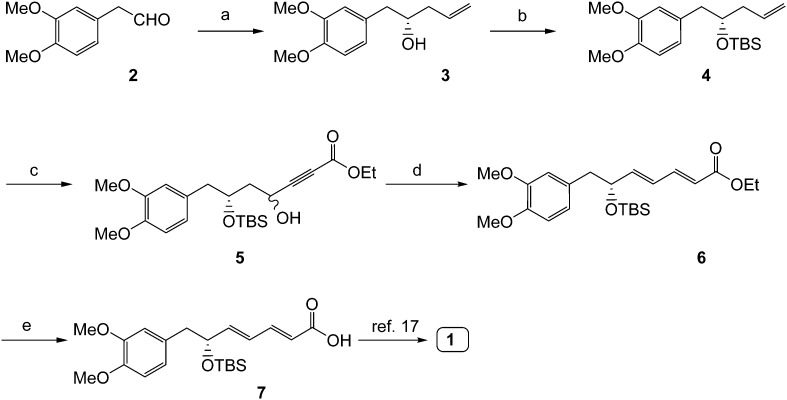
The synthetic route for key intermediate 7 is shown in Scheme 2. An improved synthesis of kunstleramide was accomplished in seven steps from commercially available 3,4-dimethoxy phenyl acetaldehyde. The key diene acid fragment 7 was prepared on a 3.19 g scale in five steps with a 54% overall yield.
Scheme 2. Synthesis of (–)-kunstleramide: reagents and conditions: (a) (S)-BINOL, 4 Å molecular sieves, Ti(OiPr)4, allyl(tributyl)stannane, anhydrous CH2Cl2, –78 °C to –20 °C, 48 h; (b) TBDMS-Cl, imidazole, CH2Cl2, 0 °C to rt, 8 h; (c) i) NMO, OsO4, acetone–water (4 : 1), rt, overnight; ii) NaIO4, THF, rt, 1 h; iii) ethyl propiolate, LiHMDS, THF, –78 °C, 1 h; (d) TPP, benzene, rt, 6 h; (e) LiOH·H2O, THF : MeOH : H2O (4 : 2 : 1), rt, 6 h.
As illustrated in Scheme 2, 3,4-dimethoxy phenyl acetaldehyde (2) was subjected to Keck's asymmetric allylation18 with (S)-Binol, allyl tributyltin and Ti(iOPr)4 to give allylic alcohol 3 (enantiomeric excess 96%, determined by chiral HPLC). Alcohol 3 was converted into TBDMS ether 4 by silylation with TBDMS-Cl.19 Then the silyl ether was dihydroxylated with OsO4, followed by cleavage with NaIO4 to afford the crude aldehyde, which was used for the next step without further purification.20 The aldehyde was subjected to propargylation using ethyl propiolate and LiHMDS to give 5 as a diastereomeric mixture; treatment of 5 with TPP in benzene at room temperature gave the conjugated diene ester 6.21
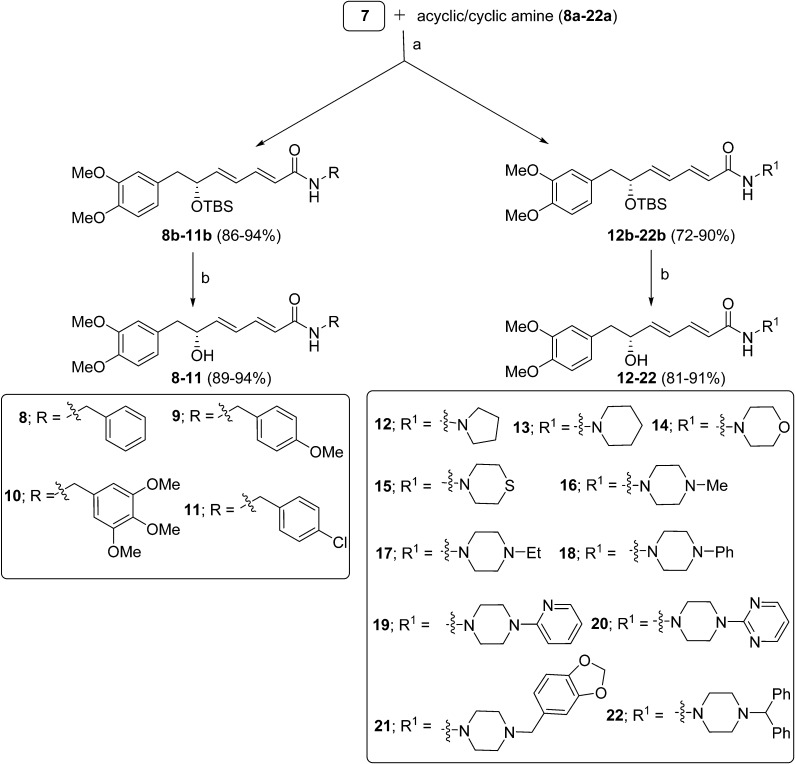
Ester 6 was hydrolyzed with LiOH·H2O to provide the key diene acid fragment 7 in a 3.19 gram scale. Diene acid 7 was converted into amide 1 by using a reported protocol.17 With the intermediate 7 in hand and a diverse class of acyclic and cyclic amines, we have prepared 15 new analogues of (–)-kunstleramide (8–22) with moderate to good yields, as shown in Scheme 3. All the synthesized compounds were characterized by their 1H, 13C NMR, ESI-MS and HRMS spectral data.
Scheme 3. Synthesis of cyclic and acyclic analogues of 1: reagents and conditions: (a) HATU, DIPEA, acyclic/cyclic amine, anhydrous DMF, 0 °C to rt, overnight; (b) PTSA, MeOH, 0 °C, 4 h.
The newly synthesized compounds, (–)-kunstleramide (1) and its derivatives (8–22), were evaluated to determine their antiproliferative activities against a panel of four different human cancer cells from the cervix, breast, lungs and neuroblastoma (HeLa, MCF7, A549, and IMR32, respectively, which were obtained from the American Type Culture Collection) using the SRB assay. The IC50 values (μM) for compounds 1 and 8–22 are summarized in Table 1. Most of the synthesized compounds have shown good antiproliferative activities against these cell lines in a concentration-dependent manner. Compounds derived from substituted piperazines (19–22) exhibited potent activity against the A549 cell line with IC50 values of 8.13, 9.92, 7.58, and 10.6 μM, respectively. Compounds 9, 18 and 21 display significant activity against the human epithelial cervical cancer cell line (HeLa) with IC50 values of 9.45, 9.75 and 8.25 μM, respectively, and the amides prepared from acyclic amines (8 and 10) and cyclic amines (17, 20 and 22) showed moderate activity against the HeLa cell line. Furthermore, the other kunstleramide analogues 17 and 18 exhibited better anti-proliferative activities against the human breast adenocarcinoma (MCF7) cell line with IC50 values 11.51 and 11.97 μM, respectively. Similarly, (–)-kunstleramide 1, 19 and 21 showed potent activity against the human neuroblastoma (IMR32) cell line with IC50 values of 9.43, 9.43 and 8.23 μM, respectively. The IC50 values of all these synthesized compounds were comparatively less than that of the standard drug combretastatin (CA4) and exhibited higher activity than piperine.
Table 1. IC50 values for antiproliferative activities of compounds 1 and 8–22 against cancer cell lines a .
| S. No | IC50 (μM) |
||||
| Compound | A-549 | Hela | MCF-7 | IMR-32 | |
| 1 | 1 | 13.83 ± 0.27 | 14.58 ± 0.13 | 12.87 ± 0.71 | 9.43 ± 0.12 |
| 2 | 8 | 17.12 ± 0.63 | 12.93 ± 0.94 | 33.56 ± 0.1 | 16.59 ± 0.97 |
| 3 | 9 | 16.81 ± 0.74 | 9.45 ± 0.61 | 26.61 ± 0.8.3 | 17.35 ± 0.26 |
| 4 | 10 | 19.19 ± 0.15 | 10.72 ± 0.1 | 15.33 ± 0.91 | 13.41 ± 0.78 |
| 5 | 11 | 16.46 ± 0.2 | 13.36 ± 0.1 | 18.05 ± 0.16 | 15.66 ± 0.93 |
| 6 | 12 | 24.36 ± 0.92 | 14.14 ± 0.94 | 26.23 ± 0.91 | 12.01 ± 0.58 |
| 7 | 13 | 16.37 ± 0.49 | 14.09 ± 0.64 | 39.47 ± 0.83 | 11.02 ± 0.22 |
| 8 | 14 | 17.9 ± 0.65 | 16.41 ± 0.73 | 16.18 ± 0.96 | 20.36 ± 0.74 |
| 9 | 15 | 18.03 ± 0.49 | 20.59 ± 0.93 | 20.15 ± 0.83 | 16.09 ± 0.24 |
| 10 | 16 | 22.17 ± 0.54 | 16.49 ± 0.71 | 17.13 ± 0.96 | 18.87 ± 0.42 |
| 11 | 17 | 15.68 ± 0.33 | 11.68 ± 0.48 | 11.51 ± 0.52 | 10.79 ± 0.4 |
| 12 | 18 | 14.17 ± 0.67 | 9.75 ± 0.17 | 11.97 ± 0.1 | 9.43 ± 0.29 |
| 13 | 19 | 8.13 ± 0.73 | 23.57 ± 0.9 | 23.57 ± 0.9 | 11.06 ± 0.95 |
| 14 | 20 | 9.92 ± 0.98 | 12.61 ± 0.42 | 14.66 ± 0.58 | 10.79 ± 0.45 |
| 15 | 21 | 7.58 ± 0.11 | 8.25 ± 0.54 | 12.3 ± 0.36 | 8.23 ± 0.34 |
| 16 | 22 | 10.6 ± 0.31 | 10.48 ± 0.15 | 12.54 ± 0.23 | 11.86 ± 0.44 |
| 17 | Piperine b | 11.89 ± 0.18 | 19.21 ± 0.73 | 12.96 ± 0.49 | 10.19 ± 0.38 |
| 18 | Combretastatin c | 6.45 ± 0.41 | 5.36 ± 0.12 | 4.63 ± 0.47 | 5.72 ± 0.4 |
aIC50 is defined as the concentration which results in a 50% decrease in cell number compared with that of the control cultures in the absence of an inhibitor and the values were calculated using the respective regression analysis. The values represent the mean ± SE of three individual observations.
bPiperine was employed as a positive control.
cCombretastatin was also employed as a positive control.
Conclusions
In conclusion, we have achieved the total synthesis of (–)-kunstleramide and its analogues from commercially available starting materials using Keck's asymmetric allylation and Trost isomerization chemistry as key maneuvers. The overall yield of the target natural product was 46% in seven steps and the key fragment 7 was synthesized on a 3.19 gram scale. All the compounds were screened for anti-proliferative activity. Most of the synthesized analogues exhibited higher activity than the natural product (–)-kunstleramide. In particular, compounds with piperazine amide moieties (18, 21, and 22) showed superior activity against all cell lines.
Experimental
Chemistry
General experimental: 1H NMR and 13C NMR spectra were recorded in CDCl3 solvent on Bruker 300 MHz (Avance), Varian Unity 500 MHz (Innova) and 600 MHz spectrometers at ambient temperature. Chemical shifts are reported in ppm relative to TMS as the internal standard. FTIR spectra were recorded on a Perkin-Elmer 683 infrared spectrophotometer, as neat or thin films in KBr. Optical rotations were measured on an Anton Paar MLP 200 modular circular digital polarimeter by using a 2 mL cell with a path length of 1 dm. Low-resolution MS were recorded on an Agilent Technologies LC-MSD trap SL spectrometer. All the reagents and solvents were of reagent grade and used without further purification unless otherwise stated. Technical-grade EtOAc, hexanes, CH2Cl2 and EtOAc used for column chromatography were distilled before use. THF, when used as a solvent for the reactions, was freshly distilled from sodium benzophenone ketyl. Column chromatography was carried out on silica gel (60–120 mesh) packed in glass columns. All the reactions were performed under N2 in flame- or oven dried glassware with magnetic stirring.
(S)-1-(3,4-Dimethoxyphenyl)pent-4-en-2-ol (3)
A mixture of (S)-BINOL (3.8 g, 13.32 mmol, 0.4 equiv.), Ti(O-i-Pr)4 (6.66 mL, 6.66 mmol, 1 M in CH2Cl2, 0.2 equiv.), CF3CO2H (333 μL, 0.333 mmol, 0.1 M in CH2Cl2, 0.01 equiv.), and oven-dried 4 Å molecular sieves (10 g) in 150 mL of CH2Cl2 was heated to reflux for 1 h. The resulting red-brown mixture was cooled to rt and 2-(3,4-dimethoxyphenyl)acetaldehyde 2 (6 g, 33.3 mmol, 1 equiv.) was added. This mixture was stirred at rt for 15 min before it was cooled to –78 °C and allyltributylstannane (22.05 g, 20.65 mL, 66.6 mmol, 2 equiv.) was then added. The reaction flask was placed in a –20 °C freezer for 48 h without stirring. The resulting mixture was quenched by the addition of 150 mL of a saturated NaHCO3 solution and stirred for 10 min at rt, then filtered through a plug of Celite. The filtrate was diluted with 150 mL of CH2Cl2 and washed with 100 mL of water. The organic layer was dried over anhydrous Na2SO4 and then concentrated. The residue was purified by flash chromatography on a silica gel column, eluting with EtOAc/hexanes (1 : 10) to give 6.95 g (94%) of the product 3 as a colorless oil. [α]25D: +79.8 (c 0.02, CHCl3); IR (KBr): 3451, 2920, 2851, 1633, 1514, 1461, 1264, 1140, 1024, 770; 1H NMR (300 MHz, CDCl3): δ 6.95–6.85 (m, H), 6.83–6.79 (m, 1H), 6.66–6.58 (m, 1H), 5.85–5.74 (m, 1H), 5.14–4.95 (m, 2H), 3.90 (s, 3H), 3.90–3.85 (m, 1H), 3.87 (s, 3H), 2.81–2.70 (m, 1H), 2.65–2.62 (m, 1H), 1.84–1.79 (m, 2H), 1.58 (s, 1H); 13C NMR (75 MHz, CDCl3): δ 148.3, 147.5, 130.2, 121.5, 115.3, 113.2, 111.0, 71.4, 55.9, 55.8, 43.5, 42.1; ESIMS: 245 [M + Na]+; HRMS (ESI) (m/z): calcd. for C13H18O3Na [M + Na]+ 245.1148; found 245.1152. The enantiomeric purity was determined by chiral HPLC (CHIRALCEL-OJ-H column, 250 × 4.6 mm, 5 mm; mobile phase, 15% IPA in hexane; flow rate, 1 mL min–1; detection, 210 nm; tR 13.196 min); 96% ee.
(S)-tert-Butyl((1-(3,4-dimethoxyphenyl)pent-4-en-2-yl)oxy)dimethylsilane (4)
To a stirred solution of alcohol 3 (5 g, 22.49 mmol, 1 equiv.) in dry DMF (60 mL), imidazole (2.29 g, 33.7 mmol, 1.5 equiv.), DMAP (cat.), followed by tert-butyldimethylsilyl chloride (4.07 g, 27.0 mmol, 1.2 equiv.) were added at 0 °C. The reaction mixture was allowed to warm to room temperature and stirred for 8 h. The reaction mixture was then diluted by the addition of ice cold water (80 mL) and the aqueous phase was extracted with diethyl ether (2 × 70 mL). The combined organic layer was washed with cold water (100 mL) and brine (100 mL), dried over Na2SO4 and the organic solvent was evaporated under reduced pressure. The crude residue was purified by flash column chromatography (silica gel, hexanes : EtOAc = 95 : 05) to give 4 (7.19 g, 95%) as a colorless oil. [α]25D: +3.09 (c 0.13, CHCl3); IR (KBr): 3401, 2925, 2854, 1712, 1513, 1461, 1262, 1139, 1025, 984, 763; 1H NMR (300 MHz, CDCl3): δ 6.74–6.71 (m, 1H), 6.68–6.65 (m, 2H), 5.74–5.72 (m, 1H), 5.10–4.98 (m, 2H), 3.85 (s, 3H), 3.90–3.85 (m, 1H), 3.82 (s, 3H), 2.79–2.76 (m, 1H), 2.59–2.55 (m, 1H), 1.82–1.79 (m, 2H), 0.86 (s, 9H), –0.07 (s, 3H), –0.20 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 149.8, 147.4, 131.2, 121.6, 115.4, 113.5, 111.1, 72.9, 56.1, 56.0, 43.7, 42.2, 25.6, 18.1, –4.8, –5.2; ESIMS: 359 [M + Na]+; HRMS (ESI) (m/z): calcd. for C19H32O3NaSi [M + Na]+ 359.2013; found 359.2017.
(6R)-Ethyl 6-((tert-butyldimethylsilyl)oxy)-7-(3,4-dimethoxyphenyl)-4-hydroxyhept-2-ynoate (5)
To a stirred solution of silyl ether 4 (5 g, 14.86 mmol) and NMO (2.09 g, 17.83 mmol, 1.2 equiv.) in H2O (20 mL) and acetone (100 mL) was slowly added a solution of OsO4 (4% in H2O, 944 μL, 0.149 mmol, 0.01 equiv.), and the mixture was stirred at rt overnight. The reaction was quenched with an aqueous solution of Na2S2O3 (100 mL), and EtOAc (100 mL) was added. The phases were separated, and the aqueous layer was extracted with EtOAc (2 × 80 mL). The organic layers were combined, dried over Na2SO4, and filtered, and the solvents were removed under reduced pressure to give a brown residue (5.3 g), which was used for the next step without further purification.
To a stirred solution of the crude diol compound in THF/H2O (1 : 1, 100 mL) was added NaIO4 (12.71 g, 59.4 mmol, 4 equiv.). The mixture was stirred at rt for 30 min before adding EtOAc (80 mL) and H2O (80 mL). The phases were separated, and the aqueous layer was extracted with EtOAc (2 × 60 mL). The organic layers were combined, dried over Na2SO4, filtered, and the solvents were removed under reduced pressure to give a yellow oil. The crude aldehyde was used for the next step without further purification.
To a solution of ethyl propiolate (1.47 g, 1.51 mL, 14.95 mmol, 1.1 equiv.) in dry THF (80 mL) at –78 °C was added LiHMDS (15.85 mL, 19.02 mmol, 1.2 M solution in THF, 1.4 equiv.), and the mixture was stirred at –78 °C for 1.5 h. The crude aldehyde (4.5 g, 13.59 mmol) in THF (20 mL) was added at –78 °C and stirring was continued for 1 h. The reaction mixture was quenched with a saturated aqueous NH4Cl solution and warmed to room temperature. It was diluted with water and extracted with EtOAc (3 × 60 mL). The combined organic layers were washed with water and brine, dried (Na2SO4) and concentrated. The residue was purified by silica gel column chromatography (hexanes : EtOAc = 80 : 20) to give 5 (4.7 g, 72% overall three steps) as a colorless oil. [α]25D: +13.08 (c 0.01, CHCl3); IR (KBr): 3488, 2931, 2856, 2235, 1713, 1514, 1465, 1256, 1078, 1028, 834, 775; 1H NMR (300 MHz, CDCl3): δ 6.81–6.66 (m, 3H), 4.47–4.62 (m, 1H), 4.28–4.18 (m, 2H), 4.16–4.09 (m, 1H), 3.87 (d, J = 3.0 6H), 2.91–2.82 (m, 1H), 2.77–2.66 (m, 1H), 1.95–1.76 (m, 2H), 1.30 (t, J = 7.1, 3H), 0.90 (d, J = 3.0, 9H), 0.1–0.02 (m, 6H); 13C NMR (75 MHz, CDCl3): δ 153.2, 148.5, 147.4, 130.4, 130.2, 121.5, 121.4, 112.7, 111.2, 111.1, 87.5, 71.4, 70.8, 61.8, 60.3, 60.1, 58.9, 55.6, 43.5, 43.2, 42.7, 41.7, 29.5, 25.6, 17.8, 13.8, –4.6, –4.66, –5.0; ESIMS: 459 [M + Na]+; HRMS (ESI) (m/z): calcd. for C23H36O6NaSi [M + Na]+ 459.2173; found 459.2180.
(R,2E,4E)-Ethyl 6-((tert-butyldimethylsilyl)oxy)-7-(3,4-dimethoxyphenyl)hepta-2,4-dienoate (6)
To a solution of 5 (4.5 g, 10.31 mmol, 1 equiv.) in benzene (80 mL) was added PPh3 (3.24 g, 12.37 mmol, 1.2 equiv.) at room temperature and the resulting mixture was stirred for 6 h. It was then diluted with hexanes/EtOAc (95 : 5, 80 mL) and filtered through a pad of silica gel. The filtrate was concentrated and the residue was purified by silica gel column chromatography (hexanes : EtOAc = 95 : 05) to give 6 (3.86 g, 89%) as a colorless oil. [α]25D: –6.8 (c 0.7, CHCl3); IR (KBr): 2954, 2932, 2856, 1715, 1644, 1515, 1465, 1263, 1237, 1139, 1031, 835, 776; 1H NMR (300 MHz, CDCl3): δ 7.25 (dd, J = 15.3, 10.9 Hz, 1H), 6.81–6.64 (m, 3H), 6.29 (dd, J = 15.3, 10.9 Hz, 1H), 6.11 (dd, J = 15.3, 5.4 Hz, 1H), 5.84 (d, J = 15.3 Hz, 1H), 4.41–4.31 (m, 1H), 4.20 (q, J = 7.1 Hz, 2H), 3.85 (s, 6H), 2.77–2.67 (m, 2H), 1.29 (t, J = 7.1 Hz, 3H), 0.85 (s, 9H), –0.09 (s, 3H), –0.18 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 167.0, 148.4, 147.5, 145.2, 143.9, 130.4, 126.8, 121.7, 121.0, 113.1, 110.9, 73.9, 60.2, 55.8, 55.7, 44.3, 25.7, 18.1, 14.2, –4.9, –5.2; ESIMS: 443 [M + Na]+; HRMS (ESI) (m/z): calcd. for C23H36O5NaSi [M + Na]+ 443.2224; found 443.2228.
(R,2E,4E)-6-((tert-Butyldimethylsilyl)oxy)-7-(3,4-dimethoxyphenyl)hepta-2,4-dienoic acid (7)
To a stirred solution of ester 6 (3.6 g, 8.56 mmol, 1 equiv.) in a 4 : 2 : 1 mixture of THF : MeOH : H2O (35 mL) was added LiOH·H2O (1.08 g, 25.7 mmol, 2 equiv.), and the resulting mixture was stirred for 6 h at room temperature. The reaction mixture was quenched with saturated aqueous KHSO4 (50 mL), and extracted with EtOAc (2 × 80 mL). The combined organic layers were washed with brine (50 mL), dried over Na2SO4, and concentrated and the residue was purified by silica gel column chromatography (silica gel, hexanes : EtOAc = 70 : 30) to give 7 (3.19 g, 95%) as a colorless oil. [α]25D: –50 (c 0.4, CHCl3); IR (KBr): 3447, 2930, 2855, 1685, 1640, 1613, 1512, 1463, 1416, 1257, 1139, 1105, 1001, 831, 771; 1H NMR (300 MHz, CDCl3): δ 7.34 (dd, J = 15.1, 10.5 Hz, 1H), 6.79 (d, J = 8.1 Hz, 1H), 6.73–6.67 (m, 2H), 6.34 (dd, J = 15.1, 10.5 Hz, 1H), 6.17 (dd, J = 15.8, 4.5 Hz, 1H), 5.85 (d, J = 15.1 Hz, 1H), 4.43–4.34 (m, 1H), 3.86 (s, 6H), 2.77–2.70 (m, 2H), 0.86 (s, 9H), –0.08 (s, 3H), –0.17 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 172.1, 148.6, 147.6, 146.5, 146.1, 130.3, 126.6, 121.8, 120.1, 113.3, 111.2, 73.8, 55.8, 55.7, 44.2, 25.7, 18.0, –4.9, –5.1; ESIMS: 415 [M + Na]+; HRMS (ESI) (m/z): calcd. for C21H32O5NaSi [M + Na]+ 415.1911; found 415.1914.
(R,2E,4E)-6-((tert-Butyldimethylsilyl)oxy)-7-(3,4-dimethoxyphenyl)-N-ethylhepta-2,4-dienamide (1a)
To a solution of 7 (200 mg, 0.508 mmol) in dry DMF (20 mL) were added HATU (196 mg, 1.02 mmol) and DIPEA (0.220 mL, 1.27 mmol) at 0 °C, stirred at the same temperature for 5 min. Then the temperature was raised to rt and ethylamine (1 M in THF, 0.104 mL, 1.02 mmol) was added. The mixture was stirred at rt overnight. After completion of the reaction, the diol solution was diluted with EtOAc (20 mL) and washed with water (2 × 30 mL). The organic layer was dried and concentrated and the residue was purified by column chromatography over silica gel using hexanes/EtOAc (80 : 20) to afford 1a (196 mg, 93%) as a light yellow liquid. [α]25D: –18 (c 1, CHCl3); IR (KBr): 3288, 2930, 2856, 1661, 1616, 1515, 1462, 1260, 1150, 834, 773; 1H NMR (300 MHz, CDCl3): δ 7.19 (dd, J = 15.1, 11.3 Hz, 1H), 6.79 (d, J = 8.3 Hz, 1 H), 6.70 (s, 2H), 6.23 (dd, J = 15.1, 11.3 Hz, 1H), 6.06 (dd, J = 15.1, 5.2 Hz, 1H), 5.79 (d, J = 15.8 Hz, 1H), 5.59 (br s, 1H), 4.35 (m, 1H), 3.86 (s, 6H), 3.37 (q, J = 7.5 Hz, 2H), 2.72 (m, 2H), 1.17 (t, J = 7.5 Hz, 3H), 0.85 (s, 9H), –0.09 (s, 3H), –0.16 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 165.9, 148.4, 147.5, 143.7, 140.1, 130.6, 126.9, 123.5, 121.8, 113.2, 111.0, 74.0, 55.8, 55.7, 44.3, 34.4, 25.7, 18.1, 14.8, –4.8, –5.1; ESIMS: 442 [M + Na]+; ESIMS: 415 [M + Na]+; HRMS (ESI) (m/z): calcd. for C23H37NO4NaSi [M + Na]+ 442.2384; found 442.2390.
(R,2E,4E)-7-(3,4-Dimethoxyphenyl)-N-ethyl-6-hydroxyhepta-2,4-dienamide (1)
A solution of 1a (145 mg, 0.253 mmol) in THF (5 mL) was cooled to 0 °C and TBAF (0.38 mL, 0.38 mmol, 1.0 M solution in THF) was added dropwise. The resulting brown solution was stirred at room temperature for 2 h. The reaction was quenched with saturated aqueous NH4Cl (5 mL) and extracted with EtOAc (2 × 10 mL). The combined organic layers were washed with brine (10 mL), dried over Na2SO4 and evaporated to dryness under reduced pressure. The residue was purified by flash column chromatography (silica gel, hexanes : EtOAc = 90 : 10) to give 1 (105 mg, 91%) as a white solid. [α]25D: –54.2 (c 0.042, MeOH); IR (KBr): 3432, 3310, 2922, 1649, 1593, 1536, 1258, 1148, 1027, 991, 805; 1H NMR (300 MHz, CDCl3): δ 7.13 (dd, J = 14.3, 9.0 Hz, 1H), 6.74 (d, J = 8.3 Hz, 1H), 6.67 (d, J = 9.0 Hz, 2H), 6.25 (dd, J = 14.3, 11.3 Hz, 1H), 6.04 (dd, J = 14.3, 5.2 Hz, 1H), 5.75 (d, J = 14.3 Hz, 1H), 5.61 (br s, 1H), 4.36 (m, 1H), 3.79 (s, 6H), 3.30 (q, J = 7.5 Hz, 2H), 2.79 (dd, J = 13.5, 4.5 Hz, 1H), 2.66 (dd, J = 13.5, 4.5 Hz, 1H), 2.03 (br s, 1H), 1.10 (t, J = 7.5 Hz, 3H); 13C NMR (75 MHz, CDCl3): δ 165.9, 148.9, 147.8, 142.3, 139.9, 129.6, 127.6, 124.1, 121.5, 112.7, 111.3, 72.5, 55.8, 43.3, 34.5, 14.8; HRMS (ESI) (m/z): calcd. for C17H23NO4Na [M + Na]+ 328.1519; found 328.1525.
General experimental procedure for amide formation (8b–22b)
To a solution of acid 7 (1 mmol) in dry DMF (5 mL) were added HATU (1 mmol) and DIPEA (1.5 mmol) at 0 °C and the resulting mixture was stirred at the same temperature for 5 min. Then the temperature was raised to rt, and the corresponding amine (8a–22a) (1 mmol) was added. The mixture was stirred at rt overnight. After completion of the reaction, the mixture was diluted with EtOAc and washed with water. The organic layer was dried over anhydrous Na2SO4 and concentrated under reduced pressure to give the crude residue, which was purified by silica gel column chromatography using hexanes/EtOAc as the eluent to give the product (8b–22b).
General experimental procedure for kunstleramide analogues (8–22)
To a cooled (0 °C) solution of TBS ether compound (8b–22b) (1 mmol) in MeOH (5 mL) was added a catalytic amount of PTSA (0.1 mmol) and stirred at the same temperature for 0.5 h. After completion of the reaction, the mixture was quenched with solid sodium bicarbonate and filtered and MeOH was evaporated under reduced pressure to afford a crude product, which was purified by silica gel column chromatography using hexanes/EtOAc as the eluent to give the corresponding amide product (8–22).
The spectral (IR, 1H and 13C NMR and HRMS) data of TBS-protected amide intermediates (8b–22b) and kunstleramide analogues (8–22) are given below.
(R,2E,4E)-N-Benzyl-6-((tert-butyldimethylsilyl)oxy)-7-(3,4-dimethoxyphenyl)hepta-2,4-dienamide (8a)
Pale yellow oil, 94% yield; [α]25D: –12.9 (c 0.2, CHCl3); IR (KBr): 2929, 2855, 1661, 1613, 1515, 1461, 1260, 1150, 1029, 833, 773; 1H NMR (600 MHz, CDCl3): δ 7.36–7.27 (m, 5H), 7.24 (dd, J = 15.0, 11.2 Hz, 1H), 6.78 (d, J = 8.6 Hz, 1H), 6.70 (d, J = 6.4 Hz, 1H), 6.69 (s, 1H), 6.24 (dd, J = 15.0, 11.2 Hz, 1H), 6.08 (dd, J = 15.0, 5.2 Hz, 1H), 5.81 (d, J = 15.0 Hz, 1H), 5.78–5.73 (m, 1H, NH), 4.52 (d, J = 5.6 Hz, 2H), 4.37–4.32 (m, 1H), 3.86 (s, 3H), 3.85 (s, 3H), 2.76–2.67 (m, 2H), 0.84 (s, 9H), –0.10 (s, 3H), –0.18 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 165.9, 148.4, 147.4, 143.9, 140.4, 138.1, 130.5, 128.5, 127.6, 127.2, 126.8, 123.2, 121.7, 113.2, 111.0, 73.9, 55.7, 55.6, 44.3, 43.5, 25.7, 18.0, –4.8, –5.2; ESIMS: 504 [M + Na]+.
(R,2E,4E)-N-Benzyl-7-(3,4-dimethoxyphenyl)-6-hydroxyhepta-2,4-dienamide (8)
Pale yellow solid, 92% yield; [α]25D: –17.3 (c 0.3, CHCl3); IR (KBr): 3426, 3303, 2922, 2853, 1649, 1594, 1540, 1516, 1259, 1235, 1141, 1025, 747, 699; 1H NMR (300 MHz, CDCl3): δ 7.36–7.23 (m, 6H), 6.82 (d, J = 7.9 Hz, 1H), 6.75 (d, J = 8.3 Hz, 1H), 6.73 (s, 1H), 6.34 (dd, J = 15.1, 11.2 Hz, 1H), 6.13 (dd, J = 15.1, 5.1 Hz, 1H), 5.85 (d, J = 15.1 Hz, 1H), 5.83 (br s, 1H), 4.52 (d, J = 4.8 Hz, 2H), 4.47–4.41 (m, 1H), 3.86 (s, 6H), 2.86 (dd, J = 13.5, 5.0 Hz, 1H), 2.74 (dd, J = 13.5, 7.9 Hz, 1H), 1.82 (br s, 1H); 13C NMR (125 MHz, CDCl3): δ 165.9, 148.8, 147.7, 142.7, 140.4, 138.0, 129.6, 128.6, 127.7, 127.5, 127.4, 123.7, 121.6, 112.6, 111.2, 72.4, 55.8, 43.6, 43.2; HRMS (ESI) (m/z): calcd. for C22H26O4N [M + H]+ 368.1856; found 368.1858.
(R,2E,4E)-6-((tert-Butyldimethylsilyl)oxy)-7-(3,4-dimethoxyphenyl)-N-(4-methoxybenzyl)hepta-2,4-dienamide (9a)
Pale yellow oil, 86% yield; [α]25D: –19.3 (c 0.4, CHCl3); IR (KBr): 2930, 2855, 1661, 1612, 1513, 1252, 1031, 833, 775; 1H NMR (300 MHz, CDCl3): δ 7.24 (dd, J = 14.9, 10.7 Hz, 1H), 7.22 (d, J = 8.6 Hz, 2H), 6.86 (d, J = 8.6 Hz, 2H), 6.78 (d, J = 8.6 Hz, 2H), 6.72 (m, 2H), 6.24 (dd, J = 15.2, 11.1 Hz, 1H), 6.07 (dd, J = 15.2, 5.2 Hz, 1H), 5.79 (d, J = 14.9 Hz, 1H), 5.70–5.61 (m, 1H), 4.46 (d, J = 5.6 Hz, 2H), 4.39–4.30 (m, 1H), 3.86 (s, 6H), 3.80 (s, 3H), 2.75–2.70 (m, 2H), 0.84 (s, 9H), –0.09 (s, 3H), –0.17 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 165.8, 158.8, 148.3, 147.3, 143.9, 140.4, 130.5, 130.2, 129.0, 126.8, 123.2, 121.7, 113.8, 113.0, 110.9, 73.9, 55.6, 55.1, 44.2, 43.0, 25.6, 18.0, –4.9, –5.2; ESIMS: 534 [M + Na]+.
(R,2E,4E)-7-(3,4-Dimethoxyphenyl)-6-hydroxy-N-(4-methoxybenzyl)hepta-2,4-dienamide (9)
White solid, 89% yield; [α]25D: –30.0 (c 0.1, CHCl3); IR (KBr): 3311, 2932, 2837, 1650, 1618, 1590, 1516, 1253, 1156, 1026, 991, 805; 1H NMR (300 MHz, CDCl3): δ 7.22 (dd, J = 14.8, 11.1 Hz, 1H), 7.19 (d, J = 8.3 Hz, 2H), 6.83 (d, J = 8.3 Hz, 2H), 6.79 (d, J = 7.9 Hz, 1H), 6.72 (m, 2H), 6.28 (dd, J = 14.9, 11.1 Hz, 1H), 6.09 (dd, J = 14.9, 5.3 Hz, 1H), 6.09–6.05 (m, 1H, NH), 5.82 (d, J = 14.9 Hz, 1H), 4.43–4.37 (m, 1H), 4.40 (d, J = 5.1 Hz, 2H), 3.84 (s, 6H), 3.77 (s, 3H), 2.82 (dd, J = 13.5, 5.0 Hz, 1H), (dd, J = 13.5, 7.7 Hz, 1H), 2.23 (br s, 1H, OH); 13C NMR (75 MHz, CDCl3): δ 165.8, 158.8, 148.6, 147.6, 142.7, 140.2, 130.1, 129.6, 129.1, 127.4, 123.8, 121.4, 113.9, 112.5, 111.1, 72.4, 55.7, 55.1, 43.1, 43.0; HRMS (ESI) (m/z): calcd. for C23H28O5N [M + H]+ 398.1962; found 398.1963.
(R,2E,4E)-6-((tert-Butyldimethylsilyl)oxy)-7-(3,4-dimethoxyphenyl)-N-(3,4,5-trimethoxybenzyl)hepta-2,4-dienamide (10a)
Pale yellow oil, 91% yield; [α]25D: –29.1 (c 0.2, CHCl3); IR (KBr): 2931, 2854, 1661, 1593, 1512, 1462, 1260, 1236, 1128, 1003, 833, 774; 1H NMR (500 MHz, CDCl3): δ 7.25 (dd, J = 14.9, 11.2 Hz, 1H), 6.77 (d, J = 8.5 Hz, 1H), 6.71–6.67 (m, 2H), 6.50 (s, 2H), 6.25 (dd, J = 14.9, 11.2 Hz, 1H), 6.09 (dd, J = 14.9, 5.1 Hz, 1H), 5.83 (d, J = 14.9 Hz, 1H), 5.83–5.79 (m, 1H, NH), 4.44 (d, J = 5.1 Hz, 2H), 4.38–4.33 (m, 1H), 3.85 (s, 6H), 3.84 (s, 6H), 3.82 (s, 3H), 2.77–2.69 (m, 2H), 0.84 (s, 9H), –0.10 (s, 3H), –0.17 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 165.8, 152.9, 148.2, 147.2, 143.9, 140.3, 136.6, 133.9, 130.4, 126.7, 123.2, 121.6, 112.9, 110.8, 104.5, 73.7, 60.5, 55.7, 55.6, 55.5, 44.1, 43.7, 25.5, 17.9, –5.0, –5.3. EIMS: 594 [M + Na]+.
(R,2E,4E)-7-(3,4-Dimethoxyphenyl)-6-hydroxy-N-(3,4,5-trimethoxybenzyl)hepta-2,4-dienamide (10)
White solid, 92% yield; [α]25D: –32.6 (c 0.15, CHCl3); IR (KBr): 3442, 2925, 2853, 1597, 1511, 1454, 1236, 1122, 1023; 1H NMR (300 MHz, CDCl3): δ 7.24 (dd, J = 14.9, 10.9 Hz, 1H), 6.80 (d, J = 8.0 Hz, 1H), 6.74–6.70 (m, 2H), 6.48 (s, 2H), 6.30 (dd, J = 15.1, 11.1 Hz, 1H), 6.16–6.12 (m, 1H, NH), 6.10 (dd, J = 15.1, 5.3 Hz, 1H), 5.85 (d, J = 15.1 Hz, 1H), 4.43–4.38 (m, 1H), 4.41 (d, J = 5.3 Hz, 2H), 3.84 (s, 6H), 3.81 (s, 6H), 3.79 (s, 3H), 2.84 (dd, J = 13.7, 5.0 Hz, 1H), 2.72 (dd, J = 13.7, 7.7 Hz, 1H), 2.10 (br s, 1H, OH); 13C NMR (75 MHz, CDCl3): δ 165.7, 153.3, 148.8, 147.8, 142.7, 140.5, 133.8, 129.5, 127.5, 123.6, 121.4, 112.5, 111.2, 104.8, 72.4, 60.7, 56.0, 55.8, 44.0, 43.2; HRMS (ESI) (m/z): calcd. for C25H32O7N [M + H]+ 458.2173; found 458.2175.
(R,2E,4E)-6-((tert-Butyldimethylsilyl)oxy)-N-(4-chlorobenzyl)-7-(3,4-dimethoxyphenyl)hepta-2,4-dienamide (11a)
Viscous liquid, 93% yield; [α]25D: –13.4 (c 0.2, CHCl3); IR (KBr): 2928, 2855, 1661, 1613, 1514, 1463, 1259, 1149, 1029, 834, 772; 1H NMR (300 MHz, CDCl3): δ 7.38–7.18 (m, 5H), 6.82–6.65 (m, 3H), 6.24 (dd, J = 14.9, 11.3 Hz, 1H), 6.09 (dd, J = 14.9, 4.1 Hz, 1H), 5.82 (d, J = 14.3 Hz, 1H), 5.79 (br s, 1H), 4.49 (s, 2H), 4.40–4.31 (m, 1H), 3.85 (s, 6H), 2.73 (d, J = 4.3 Hz, 2H), 0.85 (s, 9H), –0.09 (s, 3H), –0.16 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 166.0, 148.3, 147.4, 144.2, 140.7, 136.8, 133.0, 130.5, 128.9, 128.5, 126.7, 122.9, 121.7, 113.1, 110.9, 73.8, 55.7, 55.6, 44.2, 42.7, 25.7, 18.0, –4.8, –5.2; EIMS: 538 [M + Na]+.
(R,2E,4E)-N-(4-Chlorobenzyl)-7-(3,4-dimethoxyphenyl)-6-hydroxyhepta-2,4-dienamide (11)
Pale yellow solid, 94% yield; [α]25D: –15.7 (c 0.35, CHCl3); IR (KBr): 3286, 2925, 1659, 1609, 1555, 1513, 1265, 1233, 1138, 1035, 800, 743; 1H NMR (600 MHz, CDCl3): δ 7.29 (d, J = 8.2 Hz, 2H), 7.24 (dd, J = 15.0, 4.1 Hz, 1H), 7.22 (d, J = 8.2 Hz, 2H), 6.81 (d, J = 8.2 Hz, 1H), 6.74 (d, J = 8.2 Hz, 1H), 6.72 (s, 1H), 6.33 (dd, J = 15.0, 11.2 Hz, 1H), 6.13 (dd, J = 15.0, 5.2 Hz, 1H), 5.98–5.94 (m, 1H), 5.84 (d, J = 15.0 Hz, 1H), 4.47 (d, J = 6.0 Hz, 2H), 4.45–4.41 (m, 1H), 3.86 (s, 6H), 2.86 (dd, J = 13.5, 4.8 Hz, 1H), 2.73 (dd, J = 13.5, 7.9 Hz, 1H), 1.79 (br s, 1H); 13C NMR (75 MHz, CDCl3): δ 166.0, 148.7, 147.6, 143.0, 140.6, 136.6, 133.0, 129.5, 129.0, 128.6, 127.3, 123.4, 121.4, 112.5, 111.1, 72.4, 55.7, 43.1, 42.7; HRMS (ESI) (m/z): calcd. for C22H25O4NCl [M + H]+ 402.1466; found 402.1466.
(R,2E,4E)-6-((tert-Butyldimethylsilyl)oxy)-7-(3,4-dimethoxyphenyl)-1-(pyrrolidin-1-yl)hepta-2,4-dien-1-one (12a)
Pale yellow oil, 85% yield; [α]25D: –4.2 (c 0.22, CHCl3); IR (KBr): 2953, 2856, 1625, 1514, 1445, 1261, 1152, 1029, 834, 769; 1H NMR (500 MHz, CDCl3): δ 7.27 (dd, J = 15.2, 11.7 Hz, 1H), 6.77 (d, J = 8.5 Hz, 1H), 6.72–6.67 (m, 2H), 6.26 (dd, J = 15.2, 11.7 Hz, 1H), 6.12 (d, J = 14.8 Hz, 1H), 6.07 (dd, J = 15.2, 5.4 Hz, 1H), 4.38–4.31 (m, 1H), 3.85 (s, 6H), 3.58–3.48 (m, 4H), 2.77–2.67 (m, 2H), 2.00–1.92 (m, 2H), 1.90–1.82 (m, 2H), 0.84 (s, 9H), –0.09 (s, 3H), –0.16 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 164.8, 148.3, 147.3, 143.8, 141.1, 130.5, 127.2, 121.7, 121.4, 113.0, 110.8, 74.0, 55.7, 55.6, 46.4, 45.8, 44.3, 25.9, 25.7, 24.1, 18.0, –4.8, –5.2; EIMS: 468 [M + Na]+.
(R,2E,4E)-7-(3,4-Dimethoxyphenyl)-6-hydroxy-1-(pyrrolidin-1-yl)hepta-2,4-dien-1-one (12)
Pale yellow oil, 88% yield; [α]25D: –12.6 (c 0.3, CHCl3); IR (KBr): 3402, 2924, 1720, 1623, 1591, 1514, 1445, 1623, 1234, 1025, 808, 72; 1H NMR (300 MHz, CDCl3): δ 7.29 (dd, J = 14.9, 11.1 Hz, 1H), 6.81 (d, J = 7.9 Hz, 1H), 6.77–6.69 (m, 2H), 6.37 (dd, J = 15.1, 11.1 Hz, 1H), 6.16 (d, J = 15.1 Hz, 1H), 6.13 (dd, J = 15.1, 5.4 Hz, 1H), 4.48–4.38 (m, 1H), 3.86 (s, 6H), 3.52 (br s, 4H), 2.86 (dd, J = 13.5, 5.2 Hz, 1H), 2.74 (dd, J = 13.5, 7.7 Hz, 1H), 2.04 (br s, 2H), 1.91 (br s, 2H); 13C NMR (75 MHz, CDCl3): δ 164.8, 148.7, 147.6, 142.5, 140.9, 129.7, 127.9, 122.0, 121.4, 112.5, 111.1, 72.5, 55.8, 55.7, 46.4, 45.9, 43.2, 25.9, 24.2; EIMS: 454 [M + Na]+.
(R,2E,4E)-6-((tert-Butyldimethylsilyl)oxy)-7-(3,4-dimethoxyphenyl)-1-(piperidin-1-yl)hepta-2,4-dien-1-one (13a)
Pale yellow oil, 81% yield; [α]25D: –8.7 (c 0.3, CHCl3); IR (KBr): 2932, 2855, 1649, 1623, 1514, 1442, 1259, 1137, 1026, 834, 774; 1H NMR (500 MHz, CDCl3): δ 7.19 (dd, J = 14.8, 11.1 Hz, 1H), 6.76–6.71 (m, 1H), 6.68–6.63 (m, 2H), 6.30–6.18 (m, 2H), 6.01 (dd, J = 15.1, 5.3 Hz, 1H), 4.34–4.27 (m, 1H), 3.81 (s, 6H), 3.57 (br s, 2H), 3.44 (br s, 2H), 2.73–2.63 (m, 2H), 1.64–1.47 (m, 6H), 0.81 (s, 9H), –0.13 (s, 3H), –0.21 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 165.1, 148.2, 147.2, 143.1, 141.6, 130.4, 127.2, 121.6, 120.0, 112.9, 110.7, 73.8, 55.6, 55.5, 46.6, 44.2, 42.9, 26.4, 25.5, 25.3, 24.3, 17.9, –4.9, –5.3; EIMS: 482 [M + Na]+ .
(R,2E,4E)-7-(3,4-Dimethoxyphenyl)-6-hydroxy-1-(piperidin-1-yl)hepta-2,4-dien-1-one (13)
Pale yellow oil, 92% yield; [α]25D: –14.3 (c 0.28, CHCl3); IR (KBr): 3334, 2931, 2849, 1644, 1614, 1568, 1514, 1464, 1258, 1232, 1138, 1002, 803, 763; 1H NMR (300 MHz, CDCl3): δ 7.24 (dd, J = 14.3, 11.3 Hz, 1H), 6.82 (d, J = 8.3 Hz, 1H), 6.75 (d, J = 8.3 Hz, 1H), 6.74 (s, 1H), 6.43–6.30 (m, 2H), 6.10 (dd, J = 15.1, 5.2 Hz, 1H), 4.47–4.38 (m, 1H), 3.87 (s, 6H), 3.54 (br s, 4H), 2.86 (dd, J = 13.5, 5.2 Hz, 1H), 2.75 (dd, J = 13.5, 8.3 Hz, 1H), 1.71–1.51 (m, 6H). 13C NMR (75 MHz, CDCl3): δ 165.2, 148.5, 147.4, 142.1, 141.5, 129.7, 127.9, 121.3, 120.5, 112.4, 111.0, 72.4, 55.7, 55.6, 46.7, 43.2, 43.0, 26.4, 25.4, 24.3; EIMS: 368 [M + NH4]+.
(R,2E,4E)-6-((tert-Butyldimethylsilyl)oxy)-7-(3,4-dimethoxyphenyl)-1-morpholinohepta-2,4-dien-1-one (14a)
Pale yellow oil, 80% yield; [α]25D: –9.3 (c 0.3, CHCl3); IR (KBr): 2928, 2855, 1649, 1621, 1594, 1514, 1460, 1263, 1238, 1114, 1032, 837, 770; 1H NMR (300 MHz, CDCl3): δ 7.28 (dd, J = 15.1, 11.3 Hz, 1H), 6.77 (d, J = 9.0 Hz, 1H), 6.71–6.65 (m, 2H), 6.27 (dd, J = 15.1, 11.3 Hz, 1H), 6.22 (d, J = 15.1 Hz, 1H), 6.09 (dd, J = 15.1, 5.2 Hz, 1H), 4.40–4.31 (m, 1H), 3.85 (s, 6 H), 3.73–3.65 (m, 4H), 3.64–3.55 (br s, 4H), 2.76–2.70 (m, 2H), 0.85 (s, 9H), –0.08 (s, 3H), –0.16 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 165.9, 148.4, 147.4, 143.9, 140.4, 138.1, 130.5, 128.5, 127.6, 127.2, 126.8, 123.2, 121.7, 113.2, 111.0, 73.9, 55.7, 55.6, 44.3, 43.5, 25.7, 18.0, –4.8, –5.2; EIMS: 484 [M + Na]+.
(R,2E,4E)-7-(3,4-Dimethoxyphenyl)-6-hydroxy-1-morpholinohepta-2,4-dien-1-one (14)
Pale yellow oil, 89% yield; [α]25D: –12.9 (c 0.24, CHCl3); IR (KBr): 3410, 2922, 2854, 1649, 1620, 1592, 1514, 1441, 1263, 1238, 1114, 1028, 850, 760; 1H NMR (300 MHz, CDCl3): δ 7.29 (dd, J = 14.7, 11.1 Hz, 1H), 6.81 (d, J = 7.9 Hz, 1H), 6.78–6.70 (m, 2H), 6.38 (dd, J = 15.2, 10.9 Hz, 1H), 6.28 (d, J = 14.7 Hz, 1H), 6.14 (dd, J = 15.2, 5.0 Hz, 1H), 4.49–4.39 (m, 1H), 3.86 (s, 6H), 3.68 (br s, 4H), 3.61 (br s, 4H), 2.87 (dd, J = 13.7, 5.0 Hz, 1H), 2.73 (dd, J = 13.7, 7.9 Hz, 1H); 13C NMR (75 MHz, CDCl3): δ 165.4, 148.7, 147.6, 142.7, 142.4, 129.5, 127.7, 121.4, 119.5, 112.4, 111.1, 72.4, 66.6, 55.7, 45.9, 43.2, 42.2; HRMS (ESI) (m/z): calcd. for C19H26O5N [M + H]+ 348.1805; found 348.1809.
(R,2E,4E)-6-((tert-Butyldimethylsilyl)oxy)-7-(3,4-dimethoxyphenyl)-1-thiomorpholinohepta-2,4-dien-1-one (15a)
Pale yellow oil, 79% yield; [α]25D: –17 (c 0.2, CHCl3); IR (KBr): 2929, 2855, 1649, 1625, 1514, 1461, 1259, 1146, 1028, 834, 774; 1H NMR (500 MHz, CDCl3): δ 7.25 (dd, J = 14.8, 11.1 Hz, 1H), 6.77 (d, J = 8.5 Hz, 1H), 6.70–6.66 (m, 2H), 6.26 (dd, J = 15.1, 10.9 Hz, 1H), 6.23 (d, J = 14.8 Hz, 1H), 6.07 (dd, J = 15.1, 5.3 Hz, 1H), 4.37–4.32 (m, 1H), 3.88 (br s, 2H), 3.84 (s, 6H), 3.80 (br s, 2H), 2.74–2.70 (m, 2H), 2.65–2.59 (m, 4H), 0.84 (s, 9H), –0.09 (s, 3H), –0.17 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 165.4, 148.3, 147.4, 144.0, 142.6, 130.3, 127.0, 121.7, 119.3, 113.0, 110.8, 73.8, 55.7, 55.5, 48.3, 44.6, 44.2, 27.8, 27.1, 25.6, 17.9, –4.9, –5.3; EIMS: 500 [M + Na]+.
(R,2E,4E)-7-(3,4-Dimethoxyphenyl)-6-hydroxy-1-thiomorpholinohepta-2,4-dien-1-one (15)
Pale yellow oil, 86% yield; [α]25D: –21.6 (c 0.24, CHCl3); IR (KBr): 3359, 2925, 2840, 1643, 1612, 1567, 1514, 1461, 1252, 1148, 1028, 807, 765; 1H NMR (500 MHz, CDCl3): δ 7.28 (dd, J = 15.1, 11.1 Hz, 1H), 6.83 (d, J = 8.0 Hz, 1H), 6.77–6.72 (m, 2H), 6.39 (dd, J = 15.1, 11.1 Hz, 1H), 6.29 (d, J = 14.6 Hz, 1H), 6.14 (dd, J = 15.1, 5.3 Hz, 1H), 4.47–4.42 (m, 1H), 3.94 (br s, 2H), 3.87 (s, 6H), 3.83 (br s, 2H), 2.87 (dd, J = 13.7, 5.0 Hz, 1H), 2.74 (dd, J = 13.7, 7.9 Hz, 1H), 2.64 (br s, 4H), 1.74 (br s, 1H, OH); 13C NMR (75 MHz, CDCl3): δ 165.2, 148.5, 147.4, 142.1, 141.5, 129.7, 127.9, 121.3, 120.5, 112.4, 111.0, 72.4, 55.6, 46.7, 43.2, 43.0, 26.4, 25.4; EIMS: 386 [M + Na]+.
(R,2E,4E)-6-((tert-Butyldimethylsilyl)oxy)-7-(3,4-dimethoxyphenyl)-1-(4-methylpiperazin-1-yl)hepta-2,4-dien-1-one (16a)
Thick liquid, 84% yield; [α]25D: –12.3 (c 0.12, CHCl3); IR (KBr): 2928, 2854, 1650, 1624, 1598, 1513, 1457, 1259, 1142, 1032, 833, 773; 1H NMR (300 MHz, CDCl3): δ 7.25 (dd, J = 14.6, 11.2 Hz, 1H), 6.78 (d, J = 8.6 Hz, 1H), 6.70 (s, 2H), 6.30–6.24 (m, 2H), 6.07 (dd, J =15.0, 5.2 Hz, 1H), 4.39–4.33 (m, 1H), 3.85 (s, 6H), 3.72 (br s, 2H), 3.58 (br s, 2H), 2.76–2.70 (m, 2H), 2.43 (br s, 4H), 2.32 (s, 3H), 0.86 (s, 9H), –0.07 (s, 3H), –0.15 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 165.2, 148.2, 147.3, 143.7, 142.2, 130.3, 127.0, 121.6119.3, 113.0, 110.8, 73.8, 55.6, 55.5, 54.8, 54.3, 45.5, 45.1, 44.2, 41.5,25.6, 17.9, –4.9, –5.3; EIMS: 497 [M + Na]+.
(R,2E,4E)-7-(3,4-Dimethoxyphenyl)-6-hydroxy-1-(4-methylpiperazin-1-yl)hepta-2,4-dien-1-one (16)
Pale yellow oil, 88% yield; [α]25D: –18.1 (c 0.22, CHCl3); IR (KBr): 3405, 2933, 2851, 1649, 1619, 1591, 1513, 1446, 1259, 1139, 1000, 771; 1H NMR (500 MHz, CDCl3): δ 7.26 (dd, J = 14.8, 11.1 Hz, 1H), 6.82 (d, J = 7.9 Hz, 1H), 6.75 (d, J = 8.5 Hz, 1H), 6.73 (s, 1H), 6.37 (dd, J = 15.1, 11.2 Hz, 1H), 6.30 (d, J = 14.8 Hz, 1H), 6.12 (dd, J = 15.1, 5.4 Hz, 1H), 4.46–4.39 (m, 1H), 3.86 (s, 6H), 3.71 (br s, 2H), 3.56 (br s, 2H), 2.85 (dd, J = 13.5, 5.1 Hz, 1H), 2.75 (dd, J = 13.5, 7.7 Hz, 1H), 2.42 (br s, 4H), 2.31 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 165.2, 148.6, 147.5, 142.7, 142.1, 129.8, 127.7, 121.3, 119.9, 112.5, 111.1, 72.3, 55.8, 54.8, 54.3, 45.6, 43.2, 41.5; HRMS (ESI) (m/z): calcd. for C20H29O4N2 [M + H]+ 361.2121; found 361.2122.
(R,2E,4E)-6-((tert-Butyldimethylsilyl)oxy)-7-(3,4-dimethoxyphenyl)-1-(4-ethylpiperazin-1-yl)hepta-2,4-dien-1-one (17a)
Pale yellow oil, 81% yield; [α]25D: –16.5 (c 0.28, CHCl3); IR (KBr): 2929, 2855, 1652, 1625, 1514, 1439, 1262, 1155, 1027, 834, 775; 1H NMR (300 MHz, CDCl3): δ 7.26 (dd, J = 14.9, 11.2 Hz, 1H), 6.77 (d, J = 8.5 Hz, 1H), 6.70–6.67 (m, 2H), 6.26 (dd, J = 15.1, 11.1 Hz, 1H), 6.24 (d, J = 14.9 Hz, 1H), 6.08 (dd, J = 15.1, 5.4 Hz, 1H), 4.38–4.32 (m, 1H), 3.85 (s, 3H), 3.84 (s, 3H), 3.80 (br s, 2H), 3.68 (br s, 2H), 2.74–2.70 (m, 2H), 2.63–2.52 (m, 6H), 1.16 (t, J = 7.1 Hz, 3H), 0.85 (s, 9H), –0.09 (s, 3H), –0.16 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 165.4, 148.3, 147.4, 143.9, 142.5, 130.5, 127.2, 121.8, 119.4, 113.1, 110.9, 73.9, 55.8, 55.7, 52.7, 52.0, 45.1, 44.3, 41.5, 29.2, 25.7, 18.1, 11.4, –4.8, –5.1. EIMS: 511 [M + Na]+.
(R,2E,4E)-7-(3,4-Dimethoxyphenyl)-1-(4-ethylpiperazin-1-yl)-6-hydroxyhepta-2,4-dien-1-one (17)
Pale yellow oil, 87% yield; [α]25D: –21.3 (c 0.3, CHCl3); IR (KBr): 3410, 2924, 2850, 1649, 1619, 1592, 1513, 1449, 1261, 1146, 1026, 762; 1H NMR (300 MHz, CDCl3): δ 7.27 (dd, J = 15.1, 10.5 Hz, 1H), 6.82 (d, J = 8.3 Hz, 1H), 6.79–6.71 (m, 2H), 6.38 (dd, J = 15.1, 11.1 Hz, 1H), 6.31 (d, J = 14.3 Hz, 1H), 6.12 (dd, J = 15.1, 5.2 Hz, 1H), 4.48–4.38 (m, 1H), 3.87 (s, 6H), 3.73 (br s, 2H), 3.59 (br s, 2H), 2.86 (dd, J = 13.5, 5.2 Hz, 1H), 2.75 (dd, J = 13.5, 7.5 Hz, 1H), 2.57–2.40 (m, 6H), 1.11 (t, J = 7.5 Hz, 3H); 13C NMR (75 MHz, CDCl3): δ 165.2, 148.6, 147.5, 142.7, 142.1, 129.9, 127.7, 121.3, 119.9, 112.5, 111.0, 72.3, 55.6, 52.7, 51.9, 45.2, 43.2 41.6, 11.5; HRMS (ESI) (m/z): calcd. for C21H31O4N2 [M + H]+ 375.2278; found 375.2283.
(R,2E,4E)-6-((tert-Butyldimethylsilyl)oxy)-7-(3,4-dimethoxyphenyl)-1-(4-phenylpiperazin-1-yl)hepta-2,4-dien-1-one (18a)
Colorless oil, 89% yield; [α]25D: –14.1 (c 0.22, CHCl3); IR (KBr): 2927, 2855, 1599, 1510, 1456, 1229, 1151, 1032, 833, 770; 1H NMR (300 MHz, CDCl3): δ 7.42–7.22 (m 6H), 6.83–6.65 (m, 3H), 6.38–6.23 (m, 2H), 6.10 (dd, J = 15.1, 4.5 Hz, 1H), 4.44 (m, 1H), 3.86 (s, 6H), 3.85 (br s, 2H), 3.24 (br s, 4H), 2.74 (br s, 2H), 2.56–2.44 (m, 1H), 2.34–2.22 (m, 1H), 0.87 (s, 9H), –0.07 (s, 3H), –0.15 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 165.3, 150.7, 148.4, 147.4, 143.9, 142.4, 130.4, 129.0, 127.1, 121.7, 120.3, 119.3, 116.4, 113.1, 111.0, 73.8, 55.7, 55.6, 49.5, 49.3, 45.4, 44.2, 41.7, 25.7, 18.0, –4.8, –5.2. EIMS: 559 [M + Na]+.
(R,2E,4E)-7-(3,4-Dimethoxyphenyl)-6-hydroxy-1-(4-phenylpiperazin-1-yl)hepta-2,4-dien-1-one (18)
Semi solid, 91% yield; [α]25D: –19.0 (c 0.2, CHCl3); IR (KBr): 3370, 2924, 2854, 1642, 1607, 1564, 1463, 1231, 1142, 1027, 762; 1H NMR (300 MHz, CDCl3): δ 7.31 (dd, J = 14.3, 11.4 Hz, 1H), 7.30–7.27 (m, 2H), 7.01–6.89 (m, 3H), 6.82 (d, J = 8.0 Hz, 1H), 6.78–6.72 (m, 2H), 6.41 (dd, J = 15.2, 11.3 Hz, 1H), 6.36 (d, J = 15.2 Hz, 1H), 6.15 (dd, J = 15.2, 5.3 Hz, 1H), 4.48–4.42 (m, 1H), 3.87 (s, 6H), 3.85 (br s, 2H), 3.73 (br s, 2H), 3.20 (br s, 4H), 2.87 (dd, J = 13.7, 5.0 Hz, 1H), 2.75 (dd, J = 13.7, 5.0 Hz, 1H); 13C NMR (75 MHz, CDCl3): δ 165.3, 150.7, 148.8, 147.7, 142.5, 142.3, 129.5, 129.1, 127.9, 121.4, 120.5, 120.0, 116.5, 112.5, 111.1, 72.5, 55.8, 49.8, 49.3, 45.5, 43.3, 41.8. HRMS (ESI) (m/z): calcd. for C25H31O4N2 [M + H]+ 423.2278; found 423.2287.
(R,2E,4E)-6-((tert-Butyldimethylsilyl)oxy)-7-(3,4-dimethoxyphenyl)-1-(4-(pyridin-2-yl)piperazin-1-yl)hepta-2,4-dien-1-one (19a)
Pale yellow oil, 75% yield; [α]25D: –14.2 (c 0.2, CHCl3); IR (KBr): 2927, 2854, 1651, 1624, 1594, 1513, 1433, 1236, 1153, 1031, 833, 773; 1H NMR (300 MHz, CDCl3): δ 8.13 (d, J = 3.3 Hz, 1H), 7.45 (t, J = 6.7 Hz, 1H), 7.23 (dd, J = 14.3, 11.0 Hz, 1H), 6.74–6.54 (m, 5H), 6.25 (d, J = 15.2 Hz, 1H), 6.22 (d, J = 14.3 Hz, 1H), 6.03 (dd, J = 15.2, 5.9 Hz, 1H), 4.33–4.26 (m, 1H), 3.79 (s, 6H), 3.75 (br s, 4H), 3.60 (br s, 2H), 3.47 (br s, 2H), 2.71–2.60 (m, 2H), 0.79 (s, 9H), –0.14 (s, 3H), –0.22 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 165.5, 158.7, 148.3, 147.6, 147.4, 144.0, 142.5, 137.6, 130.4, 127.1, 121.7, 119.3, 113.6, 113.1, 110.9, 107.0, 73.9, 55.7, 55.6, 45.0, 44.3, 41.4, 25.7, 18.0, –4.8, –5.2; EIMS: 560 [M + Na]+.
(R,2E,4E)-7-(3,4-Dimethoxyphenyl)-6-hydroxy-1-(4-(pyridin-2-yl)piperazin-1-yl)hepta-2,4-dien-1-one (19)
Pale yellow oil, 81% yield; [α]25D: –17 (c 0.3, CHCl3); IR (KBr): 3276, 2922, 2844, 1652, 1598, 1518, 1437, 1237, 1157, 1032, 994, 770; 1H NMR (300 MHz, CDCl3): δ 8.20 (d, J = 4.8 Hz, 1H), 7.54–7.49 (m, 1H), 7.32 (dd, J = 14.6, 10.9 Hz, 1H), 6.83 (d, J = 8.0 Hz, 1H), 6.78–6.73 (m, 2H), 6.69–6.64 (m, 2H), 6.42 (dd, J = 15.2, 11.2 Hz, 1H), 6.37 (d, J = 14.8 Hz, 1H), 6.15 (dd, J =15.2, 5.4 Hz, 1H), 4.48–4.43 (m, 1H), 3.87 (s, 6H), 3.83 (br s, 2H), 3.67 (br s, 2H), 3.64 (br s, 2H),3.54 (br s, 2H), 2.88 (dd, J = 13.5, 5.0 Hz, 1H), 2.75 (dd, J = 13.5, 7.9 Hz, 1H); 13C NMR (75 MHz, CDCl3): δ 165.4, 158.7, 148.6, 147.6, 147.5, 142.9, 142.3, 137.6, 129.7, 127.7, 121.3, 119.8, 113.7, 112.5, 111.0, 107.1, 72.4, 55.7, 44.9, 43.2, 41.4; HRMS (ESI) (m/z): calcd. for C24H30O4N3 [M + H]+ 424.2230; found 424.2228.
(R,2E,4E)-6-((tert-Butyldimethylsilyl)oxy)-7-(3,4-dimethoxyphenyl)-1-(4-(pyrimidin-2-yl)piperazin-1-yl)hepta-2,4-dien-1-one (20a)
Pale yellow oil, 72% yield; [α]25D: –6.4 (c 0.26, CHCl3); IR (KBr): 2927, 2856, 1623, 1586, 1506, 1435, 1258, 1148, 1032, 834, 764; 1H NMR (300 MHz, CDCl3): δ 8.36 (d, J = 4.9 Hz, 2H), 7.31 (dd, J = 14.7, 10.9 Hz, 1H), 6.79 (d, J = 8.6 Hz, 1H), 6.74–6.67 (m, 2H), 6.58 (t, J = 4.9 Hz, 1H), 6.37–6.24 (m, 2H), 6.10 (dd, J = 15.2, 5.2 Hz, 1H), 4.42–4.32 (m, 1H), 3.90 (br s, 4H), 3.86 (s, 6H), 3.78 (br s, 2H), 3.67 (br s, 2H), 2.77–2.70 (m, 2H), 0.86 (s, 9H), –0.07 (s, 3H), –0.15 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 165.5, 161.2, 157.5, 148.3, 147.4, 143.9, 142.5, 130.4, 127.1, 121.7, 119.3, 113.1, 110.9, 110.2, 73.8, 55.7, 55.6, 45.2, 44.2, 43.5, 41.6, 25.6, 18.0, –4.9, –5.2; EIMS: 561 [M + Na]+.
(R,2E,4E)-7-(3,4-Dimethoxyphenyl)-6-hydroxy-1-(4-(pyrimidin-2-yl)piperazin-1-yl)hepta-2,4-dien-1-one (20)
Pale yellow oil, 83% yield; [α]25D: –11.8 (c 0.28, CHCl3); IR (KBr): 3418, 2924, 2854, 1651, 1591, 1513, 1443, 1236, 1154, 1030, 797, 768; 1H NMR (600 MHz, CDCl3): δ 8.33 (d, J = 4.5 Hz, 2H), 7.32 (dd, J = 14.6, 11.3 Hz, 1H), 6.82 (d, J = 8.2 Hz, 1H), 6.77–6.72 (m, 2H), 6.55 (t, J = 4.5 Hz, 1H), 6.42 (dd, J = 15.0, 11.3 Hz, 1H), 6.37 (d, J = 15.0 Hz, 1H), 6.15 (dd, J = 15.0, 5.2 Hz, 1H), 4.48–4.43 (m, 1H), 3.87 (s, 6H), 3.86 (br s, 4H), 3.77 (br s, 2H), 3.63 (br s, 2H), 2.87 (dd, J = 13.9, 5.2 Hz, 1H), 2.75 (dd, J = 13.9, 7.9 Hz, 1H); 13C NMR (75 MHz, CDCl3): δ 165.5, 157.7, 148.8, 147.7, 142.6, 142.3, 129.5, 127.9, 121.4, 120.1, 112.4, 111.1, 110.4, 72.5, 55.7, 45.4, 43.6, 43.4, 43.2, 41.7; HRMS (ESI) (m/z): calcd. for C23H29O4N4 [M + H]+ 425.2183; found 425.2183.
(R,2E,4E)-1-(4-(Benzo[d][1,3]dioxol-5-ylmethyl)piperazin-1-yl)-6-((tert-butyldimethylsilyl)oxy)-7-(3,4-dimethoxyphenyl)hepta-2,4-dien-1-one (21a)
Colorless oil, 89% yield; [α]25D: –11.3 (c 0.4, CHCl3); IR (KBr): 2928, 2855, 1652, 1624, 1512, 1441, 1241, 1149, 1036, 1000, 833, 772; 1H NMR (300 MHz, CDCl3): δ 7.25 (dd, J = 14.6, 11.2 Hz, 1H), 6.85 (s, 1H), 6.78 (d, J = 8.2 Hz, 1H), 6.76–6.72 (m, 2H), 6.71–6.68 (m, 2H), 6.29–6.23 (m, 2H), 6.06 (dd, J =15.4, 5.6 Hz, 1H), 5.94 (s, 2H), 4.38–4.33 (m, 1H), 3.85 (s, 6H), 3.69 (br s, 2H), 3.55 (br s, 2H), 3.43 (br s, 2H), 2.78–2.67 (m, 2H), 2.43 (br s, 4H), 0.85 (s, 9H), –0.08 (s, 3H), –0.16 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 165.2, 148.4, 147.5, 147.4, 146.6, 143.6, 142.1, 131.0, 130.4, 127.2, 122.1, 121.7, 119.6, 113.1, 111.0, 109.2, 107.7, 100.7, 73.9, 62.3, 55.7, 55.6, 52.9, 52.4, 45.4, 44.3, 41.8, 25.6, 18.0, –4.8, –5.2; EIMS: 619 [M + Na]+.
(R,2E,4E)-1-(4-(Benzo[d][1,3]dioxol-5-ylmethyl)piperazin-1-yl)-7-(3,4-dimethoxyphenyl)-6-hydroxyhepta-2,4-dien-1-one (21)
Thick liquid, 91% yield; [α]25D: –16.0 (c 0.28, CHCl3); IR (KBr): 3402, 2921, 1645, 1614, 1569, 1514, 1448, 1239, 1144, 1033, 1005, 798; 1H NMR (600 MHz, CDCl3): δ 7.25 (dd, J = 14.3, 11.3 Hz, 1H), 6.86 (s, 1H), 6.81 (d, J= 7.9 Hz, 1H), 6.76–6.71 (m, 4H), 6.37 (dd, J = 15.4, 11.3 Hz, 1H), 6.30 (d, J = 14.3 Hz, 1H), 6.11 (dd, J = 15.4, 5.2 Hz, 1H), 5.94 (s, 2H), 4.45–4.40 (m, 1H), 3.86 (s, 6H), 3.70 (br s, 2H), 3.55 (br s, 2H), 3.45 (s, 2H), 2.85 (dd, J = 13.5, 4.8 Hz, 1H), 2.74 (dd, J = 13.5, 7.9 Hz, 1H), 2.44 (br s, 4H); 13C NMR (75 MHz, CDCl3): δ 165.2, 148.6, 147.6, 147.5, 142.4, 141.9, 131.1, 129.6, 127.9, 122.1 121.4, 120.1, 112.4, 111.0, 109.2, 107.7, 100.8, 72.4, 62.3, 55.7, 52.8, 52.3, 45.5, 43.2, 41.8; EIMS: 481 [M + H]+.
(R,2E,4E)-1-(4-Benzhydrylpiperazin-1-yl)-6-((tert-butyldimethylsilyl)oxy)-7-(3,4-dimethoxyphenyl)hepta-2,4-dien-1-one (22a)
Colorless oil, 90% yield; [α]25D: –4.9 (c 0.2, CHCl3); IR (KBr): 2953, 2929, 2855, 1652, 1624, 1598, 1513, 1446, 1260, 1237, 1148, 999, 834, 754; 1H NMR (500 MHz, CDCl3): δ 7.41 (d, J = 7.4 Hz, 4H), 7.29 (d, J = 7.4 Hz, 4H), 7.24 (dd, J = 15.1, 11.3 Hz, 1H), 7.19 (t, J = 7.4 Hz, 2H), 6.77 (d, J = 8.5 Hz, 1H), 6.70–6.67 (m, 2H), 6.28–6.20 (m, 2H), 6.05 (dd, J = 15.1, 5.4 Hz, 1H), 4.37–4.31 (m, 1H), 4.23 (s, 1H), 3.85 (s, 3H), 3.85 (s, 3H), 3.69 (br s, 2H), 3.54 (br s, 2H), 2.76–2.67 (m, 2H), 2.39 (br s, 4H), 0.84 (s, 9H), –0.10 (s, 3H), –0.17 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 165.9, 148.4, 147.4, 143.6, 142.1, 142.0, 130.5, 128.5, 127.8, 127.3, 127.0, 121.8, 119.7, 113.1, 110.9, 75.8, 74.0, 55.8, 55.7, 52.1, 51.5, 45.8, 44.4, 42.1, 25.7, 18.1, –4.8, –5.1; EIMS: 649 [M + Na]+.
(R,2E,4E)-1-(4-Benzhydrylpiperazin-1-yl)-7-(3,4-dimethoxyphenyl)-6-hydroxyhepta-2,4-dien-1-one (22)
Pale yellow oil, 86% yield; [α]25D: –6.2 (c 0.45, CHCl3); IR (KBr): 3386, 2924, 2853, 1648, 1618, 1591, 1513, 1450, 1236, 1146, 996, 753, 703; 1H NMR (300 MHz, CDCl3): δ 7.41 (d, J = 7.4 Hz, 4H), 7.29 (d, J = 7.4 Hz, 4H), 7.23 (dd, J = 14.8, 11.1 Hz, 1H), 7.19 (t, J = 7.3 Hz, 2H), 6.81 (d, J = 8.0 Hz, 1H), 6.76–6.71 (m, 2H), 6.35 (dd, J = 15.2, 11.1 Hz, 1H), 6.28 (d, J = 14.8 Hz, 1H), 6.10 (dd, J = 15.2, 5.4 Hz, 1H), 4.45–4.39 (m, 1H), 4.24 (s, 1H), 3.86 (s, 6H), 3.69 (br s, 2H), 3.53 (br s, 2H), 2.85 (dd, J = 13.5, 5.0 Hz, 1H), 2.73 (dd, J = 13.5, 7.9 Hz, 1H), 2.39 (br s, 4H), 1.83 (br s, 1H, OH); 13C NMR (75 MHz, CDCl3): δ 165.2, 148.5, 147.5, 142.5, 141.9, 141.8, 129.6, 128.4, 127.8, 127.6, 126.9, 121.3, 119.9, 112.4, 111.0, 75.7, 72.4, 55.6, 51.9, 51.3, 45.6, 43.1, 42.0; HRMS (ESI) (m/z): calcd. for C32H37O4N2 [M + H]+ 513.2747; found 513.2743.
Biology
Cell culture: four different human tumor cell lines from the lungs (A549), cervix (HeLa), breast (MCF7) and neuroblastoma (IMR32) were purchased from the American Type Culture Collection. All the cells were maintained in Dulbecco's modified Eagle's medium (DMEM), supplemented with 10% fetal bovine serum (Gibco, USA) and a 1% penicillin–streptomycin solution (Gibco, USA). The cell lines were maintained at pH 7.2 to 7.5 and 37 °C in a humidified atmosphere containing 5% CO2 in an incubator. Cellular morphology was checked using a Phase Contrast Microscope (Olympus, Japan).
Anti-proliferative activity assay: A549, HeLa, MCF7 and IMR32 cells were grown up to 80% confluence in T75 flasks. The cells were trypsinized and 5 × 104 cells per ml cell suspensions were prepared with a complete medium. 100 μl per well cell suspensions were seeded in 96 well plates and incubated 24 h at 37 °C in 5% CO2. Then the cells were treated with different doses (1, 10, 50, and 100 μM) of kunstleramide derivatives for 48 h at 37 °C. After 48 h, cell monolayers were fixed by the addition of 100 μL of 10% (wt/vol) cold trichloroacetic acid and incubated at 4 °C for 1 h. The supernatant was discarded. The plate was washed four times with tap water and was allowed to air dry. The cells were then stained with 0.057% SRB dissolved in 1% acetic acid for 30 min at room temperature. Unbound SRB was washed away with four washes of 1% acetic acid. The plate was again allowed to air dry and the bound SRB stain, representing surviving cells, was dissolved in 50 μL of Tris base (10 mm). The optical density was determined at 510 nm using a microplate reader (Enspire, Perkin Elmer, USA). And the IC50 values of the compounds were calculated.
Supplementary Material
Acknowledgments
The authors (RV, BC, UR, KP and PS) are thankful to CSIR, New Delhi, India for financial assistance and the Director, CSIR-IICT, Hyderabad for their support in providing NMR equipment.
Footnotes
†The authors declare no competing interests.
‡Electronic supplementary information (ESI) available: 1H and 13C spectra of all new compounds are available. See DOI: 10.1039/c6md00606j
References
- (a) Banerji A., Das C. Phytochemistry. 1989;28:3039–3042. [Google Scholar]; (b) Parmar V. S., Jain S. C., Bisht K. S., Jain R., Taneja P., Jha A., Tyagi O. D., Prasad A. K., Wengel J., Olsen C. E., Boll P. M. Phytochemistry. 1997;46:597–673. [Google Scholar]; (c) Alécio A. C., Bolzani V. d. S., Young M. C. M., Kato M. J., Furlan M. J. Nat. Prod. 1998;61:637–639. doi: 10.1021/np9703656. [DOI] [PubMed] [Google Scholar]
- McKee T. C., Galinis D. L., Pannell L. K., Cardellina J. H., Laakso J., Ireland C. M., Murray L., Capon R. J., Boyd M. R. J. Org. Chem. 1998;63:7805–7810. [Google Scholar]
- Erickson K. L., Beutler J. A., Cardellina J. H., Boyd M. R. J. Org. Chem. 1997;62:8188–8192. doi: 10.1021/jo971556g. [DOI] [PubMed] [Google Scholar]
- Kunze B., Jansen R., Sasse F., Hofle G., Reichenbach H. J. Antibiot. 1998;51:1075–1080. doi: 10.7164/antibiotics.51.1075. [DOI] [PubMed] [Google Scholar]
- Williams P. G., Asolkar R. N., Kondratyuk T., Pezzuto J. M., Jensen P. R., Fenical W. J. Nat. Prod. 2007;70:83–88. doi: 10.1021/np0604580. [DOI] [PubMed] [Google Scholar]
- (a) Pitsinos E. N., Wascholowski V., Karaliota S., Rigou C., Couladouros E. A., Giannis A. ChemBioChem. 2003;4:1223–1225. doi: 10.1002/cbic.200300667. [DOI] [PubMed] [Google Scholar]; (b) Cha J. Y., Burnett G. L., Huang Y., Davidson J. B., Pettus T. R. R. J. Org. Chem. 2011;76:1361–1371. doi: 10.1021/jo102327e. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Reddy M. D., Watkins E. B. J. Org. Chem. 2015;80:11447–11459. doi: 10.1021/acs.joc.5b02138. [DOI] [PubMed] [Google Scholar]
- (a) Kunze B., Jansen R., Hofle G., Reichenbach H. J. Antibiot. 1994;47:881–886. doi: 10.7164/antibiotics.47.881. [DOI] [PubMed] [Google Scholar]; (b) Jansen R., Washausen P., Kunze B., Reichenbach H., Höfle G. Eur. J. Org. Chem. 1999:1085–1089. [Google Scholar]; (c) Reddy M. D., Fronczek F. R., Watkins E. B. Org. Lett. 2016;18:5620–5623. doi: 10.1021/acs.orglett.6b02848. [DOI] [PubMed] [Google Scholar]
- (a) Pradeep C. R., Kuttan G. Clin. Exp. Metastasis. 2002;19:703–708. doi: 10.1023/a:1021398601388. [DOI] [PubMed] [Google Scholar]; (b) Sunila E. S., Kuttan G. J. Ethnopharmacol. 2004;90:339–346. doi: 10.1016/j.jep.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Reen R. K., Wiebel F. J., Singh J. J. Ethnopharmacol. 1997;58:165–173. doi: 10.1016/s0378-8741(97)00104-9. [DOI] [PubMed] [Google Scholar]
- Morikawa T., Matsuda H., Yamaguchi I., Pongpiriyadacha Y., Yoshikawa M. Planta Med. 2004;70:152–159. doi: 10.1055/s-2004-815493. [DOI] [PubMed] [Google Scholar]
- Li S., Wang C., Li W., Koike K., Nikaido T., Wang M. W. J. Asian Nat. Prod. Res. 2007;9:421–430. doi: 10.1080/10286020500384302. [DOI] [PubMed] [Google Scholar]
- Marques J. V., Kitamura R. O. S., Lago J. H. G., Young M. C. M., Guimarães E. F., Kato M. J. J. Nat. Prod. 2007;70:2036–2039. doi: 10.1021/np070347g. [DOI] [PubMed] [Google Scholar]
- (a) Matsuda H., Ninomiya K., Morikawa T., Yasuda D., Yamaguchi I., Yoshikawa M. Bioorg. Med. Chem. 2009;17:7313–7323. doi: 10.1016/j.bmc.2009.08.050. [DOI] [PubMed] [Google Scholar]; (b) Tang G.-H., Chen D.-M., Qiu B.-Y., Sheng L., Wang Y.-H., Hu G.-W., Zhao F.-W., Ma L.-J., Wang H., Huang Q.-Q., Xu J.-J., Long C.-L., Li J. J. Nat. Prod. 2011;74:45–49. doi: 10.1021/np100606u. [DOI] [PubMed] [Google Scholar]; (c) Reddy C. R., Valleti R. R., Reddy M. D. J. Org. Chem. 2013;78:6495–6502. doi: 10.1021/jo400567h. [DOI] [PubMed] [Google Scholar]; (d) Madadi N. R., Ketkar A., Zheng C., Penthala N. R., Janganati V., Bommagani S., Crooks P. A. MedChemComm. 2015;6:788–794. doi: 10.1039/C4MD00478G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Shen R., Porco J. A. Org. Lett. 2000;2:1333–1336. doi: 10.1021/ol005800t. [DOI] [PubMed] [Google Scholar]; (b) Yet L. Chem. Rev. 2003;103:4283–4306. doi: 10.1021/cr030035s. [DOI] [PubMed] [Google Scholar]; (c) Zhang H., Matsuda H., Nakamura S., Yoshikawa M. Bioorg. Med. Chem. Lett. 2008;18:3272–3277. doi: 10.1016/j.bmcl.2008.04.052. [DOI] [PubMed] [Google Scholar]; (d) Andrade R. B. Org. Prep. Proced. Int. 2009;41:359–383. [Google Scholar]; (e) Reddy C. R., Reddy M. D. J. Org. Chem. 2014;79:106–116. [Google Scholar]; (f) Nagesh N., Rajub G., Srinivas R., Ramesh P., Reddy M. D., Reddy C. R. Biochim. Biophys. Acta. 2015;1850:129–140. doi: 10.1016/j.bbagen.2014.10.004. [DOI] [PubMed] [Google Scholar]; (g) Reddy C. R., Krishna G., Reddy M. D. Org. Biomol. Chem. 2014;12:1664–1670. doi: 10.1039/c3ob42396d. [DOI] [PubMed] [Google Scholar]
- (a) Aravind S., Reddy N. Int. Arch. Appl. Sci. Technol. 2011;11:1–5. [Google Scholar]; (b) Yadav J. S., Aravind S., Gundluru M. K., Reddy B. V. S. Synthesis. 2012;44:3077–3084. [Google Scholar]; (c) Yadav J. S., Aravind S., Kumar G. M., Reddy B. V. S. Tetrahedron Lett. 2012;53:6163–6166. [Google Scholar]; (d) Rajaram S., Ramulu U., Aravind S., Babu K. S. Helv. Chim. Acta. 2015;98:650–656. [Google Scholar]
- Mollataghi A., Hadi A. H. A., Cheah S.-C. Molecules. 2012;17:4197–4208. doi: 10.3390/molecules17044197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy S. P., Venkateswarlu Y. Tetrahedron Lett. 2013;54:4617–4619. [Google Scholar]
- (a) Kumar K. S., Reddy C. S. Org. Biomol. Chem. 2012;10:2647–2655. doi: 10.1039/c2ob06940g. [DOI] [PubMed] [Google Scholar]; (b) Mineeva I. V. Russ. J. Org. Chem. 2013;49:1647–1654. [Google Scholar]; (c) Dias L. C., Kuroishi P. K., Polo E. C., de Lucca Jr. E. C. Tetrahedron Lett. 2013;54:980–982. [Google Scholar]; (d) Yadav J. S., Singh V. K., Thirupathaiah B., Reddy A. B. Tetrahedron Lett. 2014;55:4427–4429. [Google Scholar]; (e) Hessler F., Betík R., Kadlčíková A., Belle R., Kotora M. Eur. J. Org. Chem. 2014:7245–7252. [Google Scholar]
- (a) Reddy C. R., Reddy M. D., Dilipkumar U. Eur. J. Org. Chem. 2014:6310–6313. [Google Scholar]; (b) Suman P., Raju B. C. Org. Biomol. Chem. 2014;12:3358–3361. doi: 10.1039/c4ob00323c. [DOI] [PubMed] [Google Scholar]
- Reddy C. R., Dilipkumar U., Reddy M. D., Rao N. N. Org. Biomol. Chem. 2013;11:3355–3364. doi: 10.1039/c3ob27518c. [DOI] [PubMed] [Google Scholar]
- Guo C., Lu X. J. Chem. Soc., Chem. Commun. 1993:394–395. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.