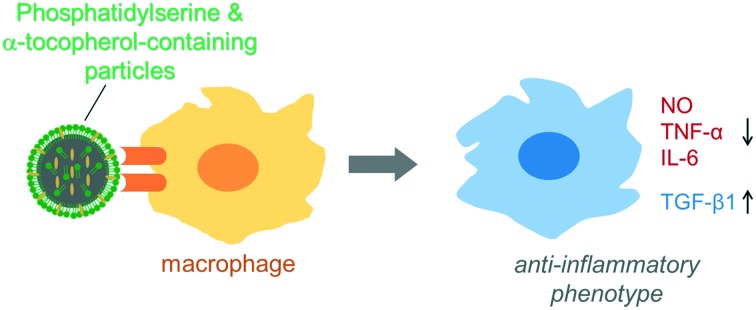
 Bioactive particles directed the macrophage to anti-inflammatory phenotype.
Bioactive particles directed the macrophage to anti-inflammatory phenotype.
Abstract
Inflammatory activation of macrophages is a key factor in chronic inflammatory diseases such as ulcerative colitis. The excessive production of reactive oxygen species (ROS)/reactive nitrogen species (RNS) by macrophages causes oxidative stress during the inflammatory response and exaggerates inflammatory lesions in ulcerative colitis. Inhibition of the inflammatory activation of macrophages is a promising treatment for chronic inflammatory diseases. Here, we prepared self-filling polymer–lipid hybrid nanoparticles (PST-PLNPs) consisting of poly dl-lactic acid as a hydrophobic biodegradable polymer core encapsulating α-tocopherol (T) and phosphatidylserine (PS) both on the surface and interior of the particle. We confirmed the anti-inflammatory response of these hybrid nanoparticles in activated murine macrophages. PS has anti-inflammatory effects on macrophages by modulating the macrophage phenotype, while α-tocopherol is an antioxidant that neutralizes ROS. We found that PS-containing (PS-PLNPs) and PS plus α-tocopherol-containing (PST-PLNPs) polymer–lipid hybrid nanoparticles significantly increased the viability of lipopolysaccharide (LPS)-treated macrophages compared with phosphatidylcholine-containing PLNPs. PST-PLNPs had a better effect than PS-PLNPs, which was attributed to the synergy between PS and α-tocopherol. This synergic action of PST-PLNPs reduced NO and pro-inflammatory cytokine (IL-6) production and increased anti-inflammatory cytokine (TGF-β1) production when incubated with activated macrophages. Thus, these self-filling biodegradable polymer–lipid hybrid nanoparticles (PST-PLNPs) containing anti-oxidant and anti-inflammatory molecules might be potential alternative drug carriers to liposomes and polymeric nanoparticles for the treatment of chronic inflammatory diseases such as ulcerative colitis.
1. Introduction
Ulcerative colitis (UC) is a chronic and non-specific inflammatory disorder of the gastrointestinal tract1 involving the mucosa and sub mucosa of the colon characterized by contiguous inflammation of the colonic lamina propria with subsequent injury and disruption of the mucosal barrier. Multiple factors such as over production of pro-inflammatory mediators including reactive oxygen species (ROS),2,3 nitric oxides, cytokines, and arachidonate metabolites and infiltration of activated macrophages in the lamina propria have been implicated in the pathogenesis of ulcerative colitis.4–6 Infiltrating macrophages secrete many pro-inflammatory cytokines such as IL-1β, IL-6, TNF-α,7 and nitric oxide (NO)6 and generate excess amounts of ROS,8 which overcomes the intestinal defence system, leading to intestinal injury in ulcerative colitis.9 Because the production and release of pro-inflammatory mediators by macrophages is important in the pathophysiology of UC, the inhibition of macrophage activity to reduce inflammatory responses may represent a therapeutic strategy for the treatment of UC.
Drugs primarily used for the treatment of ulcerative colitis include aminosalicylates, steroids and immunosuppressive drugs such as azathioprine, 6-mercaptopurine and monoclonal antibodies,10–13 which aim to decrease inflammation. These drugs exert their anti-inflammatory effects by inhibiting COX activity and NF-kB/MAPK p38 signaling in activated macrophages.14,15 However, these drugs induce side effects such as headache nausea, epigastric pain, diarrhea, moon face, weight gain and dyspepsia.16–19 However, 20–40% of ulcerative colitis patients do not respond to these conventional therapies and may receive secondary drug treatment or colectomy.20 Even after colectomy, patients are predisposed to the risk of complications such as small bowel obstruction, anastomotic stricture, pouchitis and pouch failure.21 Therefore, the search for safer and more effective agents for the management of UC continues.
Recently, antioxidants were shown to exhibit anti-inflammatory activity in an in vitro experimental colitis model. Antioxidants mediate their anti-inflammatory effects by free radical scavenging to neutralize ROS and inhibiting the transcription of NF-kB in activated macrophages.22,23 α-Tocopherol and N-acetylcysteine were reported to attenuate chemically induced colitis24,25 by inhibiting the activation of macrophages.23 Phosphatidylserine (PS) is another promising molecule that has anti-inflammatory effects on activated macrophages.26 PS is a phospholipid found in the inner leaflet of cell membranes, and during apoptosis, it is exposed on the cell surface27 where it binds directly with macrophage receptors or via some bridging molecules (MFG-E8) to enhance the phagocytosis of apoptotic cells.28 After the phagocytic clearance of apoptotic cells, macrophages actively promote anti-inflammatory responses by decreasing the production of pro-inflammatory cytokines.29
The oral delivery of α-tocopherol and PS and their bioavailability in the intestine is a big challenge because of the physiological barriers present in the gastrointestinal tract such as strong acidic conditions and gastric enzymes. The development of composite formulation might help to improve the bioavailability of these molecules. Liposomes and biodegradable nanoparticles comprising polylactic acid30 are predominantly used for oral drug delivery. However, liposomes and general polymer nanoparticles are rapidly degraded because of their weak mechanical stability and the enzymatic effect of the gastrointestinal tract.
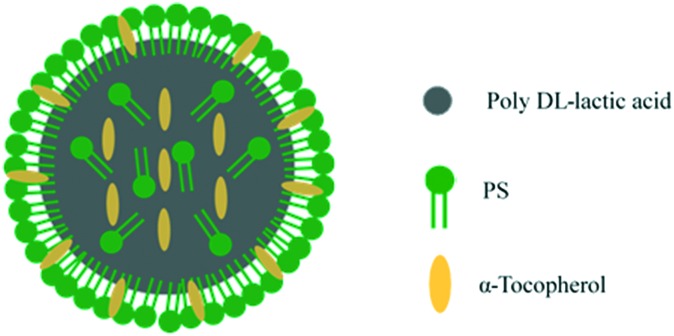
To solve this problem, we propose a novel concept of self-filling biodegradable polymer–lipid hybrid nanoparticles (PLNPs) for the oral delivery of PS and α-tocopherol (Fig. 1). In general, phospholipids such as PS are used as a dispersion in the aqueous phase via O/W emulsion methods for particle preparation because phospholipids act as emulsifiers and linkers for surface modification.31 Here, we dissolved PS and α-tocopherol in the organic phase to distribute them on both the surface and interior of the particles. If surface PS is degraded in the gastrointestinal tract and released, PS incorporated in the particles will enable the self-filling of the surface lipid monolayer to interact with intestinal macrophages. In this study, we prepared a PLA core consisting of self-filling biodegradable PLNPs containing α-tocopherol and PS to decrease oxidative stress and inflammatory responses induced by activated macrophages. These two components demonstrated synergistic anti-inflammatory and anti-oxidative effects on activated RAW264.7 macrophages. These PLNPs might be potential drug carriers and effective tools to target macrophages for the treatment of chronic inflammatory diseases such as ulcerative colitis.
Fig. 1. PS and α-tocopherol-containing polymer–lipid hybrid nanoparticles (PST-PLNPs).
2. Materials and methods
2.1. Materials
3-sn-Phosphatidyl-l-serine sodium from bovine, l-α-phosphatidylcholine from egg yolk and lipopolysaccharide (LPS) from Escherichia coli (serotype 0111; B4) were purchased from Sigma Aldrich (Japan). PLA ester terminated (Mw 18 000–28 000) and polyvinyl alcohol (Mw 89 000–98 000) were also purchased from Sigma Aldrich (Japan). α-Tocopherol was purchased from TCI. ELISA-kits for mouse IL-6 and TGF-β1 were purchased from R&D systems. A nitric oxide assay kit (Griess reagent) was purchased from Dojindo Co. Japan.
2.2. Preparation of PLNPs
Anti-oxidant and anti-inflammatory molecule-containing PLNPs were prepared by an oil-in-water emulsion method. Dichloromethane–toluene mixed (v/v = 1/1; density 1.1 g cm–3) solution (0.53 mL) containing PLA (25 mg mL–1), PS (10 mg mL–1) and α-tocopherol (20 mg mL–1) was prepared. Then, this organic solution was added to an aqueous solution (5.3 mL) containing 2% polyvinyl alcohol (20 mg mL–1). Then the two-phase solution was vigorously mixed by a vortex for 1 min and sonicated for 5 min with a probe sonicator at 40% output (frequency: 20 kHz, output: 200 watts; ultrasonic disrupter UD 201, TOMY, Seiko Co. Ltd, Japan). The organic solvent was evaporated from the solution under reduced pressure using an oil vacuum pump for 2.5 h with shaking. The resulting PLNPs were collected by centrifugation at 10 000 rpm at 4 °C for 10 min. The particles were washed three times with distilled water. PC-, PCT- and PS-PLNPs were prepared similarly. Fluorescence-labelled lipid nanoparticles were prepared by mixing with DiD (6 μg mL–1) in the organic phase.
2.3. Characterization of PLNPs
The size, polydispersity index, and ζ-potential of the PLNPs were measured with a dynamic light-scattering spectrophotometer (Zetasizer Nanoseries, Malvern Instruments, UK) at 25 °C. To measure the size, polydispersity index, and ζ-potential, the samples were suspended in 10 mM HEPES buffer at pH 7.4 by a vortex and the obtained results were reported as the mean of three runs. The morphology of each PLNP was observed by an ultra-high resolution scanning electron microscope SU 8010 (HITACHI, Japan). The encapsulation efficiency of α-tocopherol was determined by absorption at 292 nm after dissolving the PLNPs in ethanol/HEPES buffer (10 : 1).
2.4. Cell culture
RAW264.7 murine macrophages (DS Pharma Biomedical, Japan) were maintained in Dulbecco's Modified Eagle's Medium containing 10% foetal bovine serum (FBS) and 2 mM l-glutamine supplemented with 100 U mL–1 penicillin, 100 μg mL–1 streptomycin, and 0.25 μg mL–1 amphotericin B (all from Gibco Invitrogen Co., NY, USA). The cells were harvested in a humidified atmosphere containing 5% CO2 and 95% air at 37 °C. For the assay of macrophage activation with and without PLNPs, we treated cells with lipopolysaccharide (1 μg mL–1) and interferon-γ (100 U mL–1).32
2.5. Cellular uptake of PLNPs
Internalization of DiD-labelled PLNPs in macrophages was observed by fluorescence microscopy (BZ-8100, Keyence, Japan). Briefly, RAW264.7 macrophages were seeded in a Petri dish with DMEM high glucose containing FBS. After the cells became 70–80% confluent, they were added to a 96-well glass surface plate. Then, 1 × 104 cells were added to 100 μl of DMEM containing FBS per well. After 24 h of incubation, the cells were washed two times with DPBS. The particles and LPS were added to 100 μL of DMEM without FBS. After 3 h of incubation at 37 °C, the cells were observed for the uptake of the particles by fluorescence microscopy. The quantification of the PLNP-positive cells was performed using Tali image-based cytometry.
2.6. Assay for cell survival
Relative cell viability was measured using a cell counting kit-8 (WST-8, Dojindo Laboratories, Inc. Japan). Briefly, 1 × 104 RAW264.7 cells were seeded in 100 μL of DMEM containing FBS in a 96-well plate. After 24 h of incubation, the medium was replaced with FBS-free DMEM high glucose. Then, the particles and LPS were added and incubated for 48 h at 37 °C. Thereafter, 10 μL WST-8 was added to each well after the indicated period and incubated for 3 h at 37 °C. Conversion of WST-8 into formazan by the living cells was measured using a microplate reader (Wallac ARVO.SX 1420 Multilabel Counter) with absorbance at 450 nm. The total number of living cells is expressed as a percentage relative to untreated control samples.
2.7. NO quantification
The accumulation of NO2–, a stable end product extensively used as an indicator of NO production by cultured cells, was assayed using the Griess reaction. Briefly, macrophages (1 × 105 cells per mL) were added to a 24-well plate and kept at 37 °C overnight. Then, the medium was replaced with serum and antibiotic-free fresh medium and PLNPs (125 μg lipid per mL) were added. After 1 h, LPS (1 μg mL–1) and IFN-γ (100 IU mL–1) were added and incubated for 47 h. After a total of 48 h incubation with PLNPs, the cell supernatant was mixed with an equal volume of the Griess reagent and incubated at room temperature for 15 min. The absorbance was measured at 540 nm with a microplate reader (Wallac ARVO.SX 1420 multilabel counter). Nitrite levels were determined using a calibration line prepared from known concentrations of NaNO2 as a standard.
2.8. Cytokine detection by ELISA
The macrophages were seeded in a 24-well plate (1 × 105 cells per mL) and incubated for 24 h at 37 °C. After 24 h, the medium was replaced with serum and antibiotic free-DMEM high glucose and PLNPs (125 μg lipid per mL) were added. After 1 h, LPS (1 μg mL–1) was added and incubated for 47 h. At 48 h after PLNP addition, the cell culture supernatant was collected and cytokines were quantified by ELISA. Briefly, sample supernatants were pipetted onto a monoclonal antibody pre-coated microplate for binding with a specific cytokine. After washing away any unbound substances, an enzyme-linked polyclonal antibody specific for a cytokine was added to the wells to sandwich the cytokine which is immobilized during the first incubation. Following a wash to remove any unbound antibody-enzyme reagent, a substrate solution was added to the wells and colour developed in proportion to the amount of cytokines bound in the initial step. The colour development was stopped by the addition of a stop solution and the intensity of the colour was measured by the microplate reader at 450 nm absorbance.
3. Results and discussion
3.1. Characterization of self-filling PLNPs
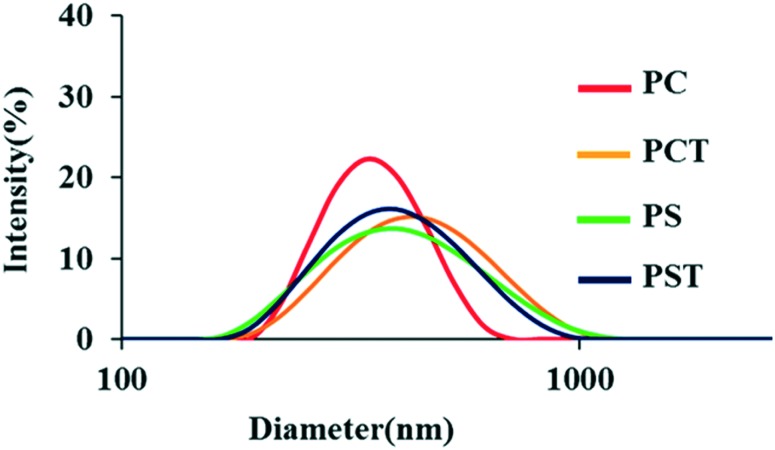
We prepared four types of PLNPs using an emulsification technique. The characteristic values of the PLNPs are summarized in Table 1. The size distributions of the four PLNPs are shown in Fig. 2. The mean diameter of these particles is approximately 400 nm irrespective of the components. As the scanning electron microscopic images are shown in Fig. S1,† the size of each PLNP was almost identical with the result of dynamic light scattering. Their ζ-potentials were almost neutral for PC- and PCT-PLNPs and negative for PS- and PST-PLNPs, indicating the presence of negatively charged PS on the surface of these PLNPs. The encapsulation efficiency of α-tocopherol was not quantitative (Table 1), indicating that degradation of α-tocopherol may take place during the sonication process of the PLNP preparation.
Table 1. Characteristics of PLNPs in 10 mM HEPES buffer solution at pH 7.4.
| Name of PLNP | Composition of organic phase |
Particle size (nm) | ζ-Potential (mV) | Polydispersity index | Encapsulation efficiency of α-tocopherol (%) | |||
| PS | PC | DL-PLA | α-Tocopherol | |||||
| PC | — | 1 mg | 10 mg | — | 411 ± 6.75 | –1.18 ± 0.11 | 0.31 ± 0.03 | — |
| PCT | — | 1 mg | 10 mg | 0.6 mg | 445 ± 9.38 | –6.9 ± 0.23 | 0.21 ± 0.02 | 82 |
| PS | 1 mg | — | 10 mg | — | 410 ± 4.48 | –13.3 ± 1.06 | 0.24 ± 0.01 | — |
| PST | 1 mg | — | 10 mg | 0.6 mg | 390 ± 1.03 | –15.4 ± 1.84 | 0.16 ± 0.01 | 70 |
Fig. 2. Size distributions of the prepared PLNPs.
3.2. Uptake of PLNPs by RAW264.7 macrophages
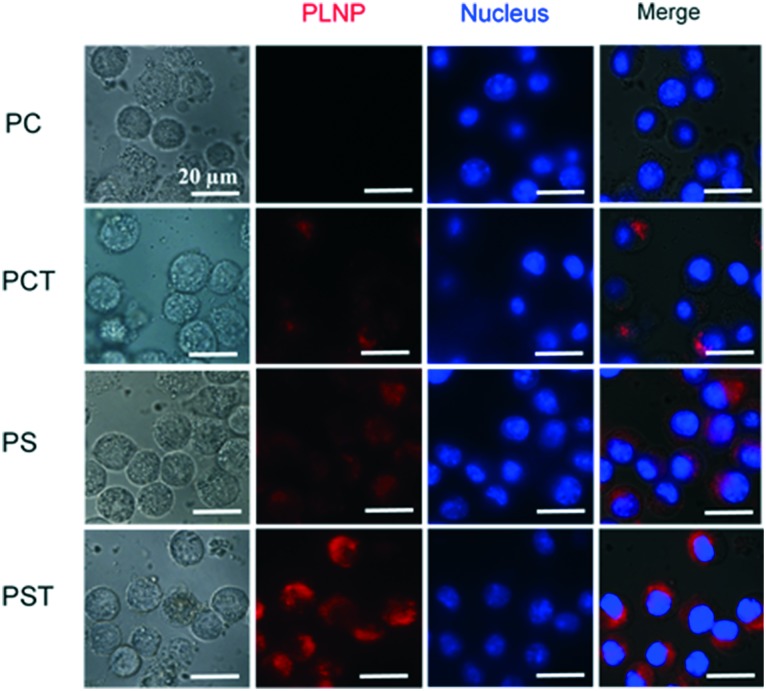
The uptake of PLNPs by the activated RAW264.7 macrophages with LPS stimulation was investigated by fluorescence microscopy. The particles were fluorescently labelled with DiD. As shown in Fig. 3, strong red DiD fluorescence was observed in PS-containing particles (PS- and PST-PLNPs), indicating the engulfment of these PLNPs.
Fig. 3. Uptake of PLNPs by RAW264.7 macrophages. Macrophages were treated with LPS (1 μg ml–1) and PLNPs (125 μg lipid per mL) and incubated for 3 h at 37 °C. The images were taken with a fluorescence microscope (blue and red colors represent nuclei and DiD-labelled particles, respectively).
However, the fluorescence of PC-containing particles (PC- and PCT-PLNPs) in the macrophages was negligible or low. This low rate of engulfment in PCT-PLNPs might be related to the quinone formation of α-tocopherol, which was reported to be taken up by the macrophages.33 The enhanced engulfment of the PS-containing particles was mediated via scavenger receptors and PS-receptors.34–39 The cellular uptake of each PLNP was also quantitated by an image-based cytometer. The percentage of PST-PLNP positive macrophages was much higher than that of PCT-PLNP positive macrophages (Fig. S3†).
3.3. Cell survival assay of macrophages incubated with PLNPs
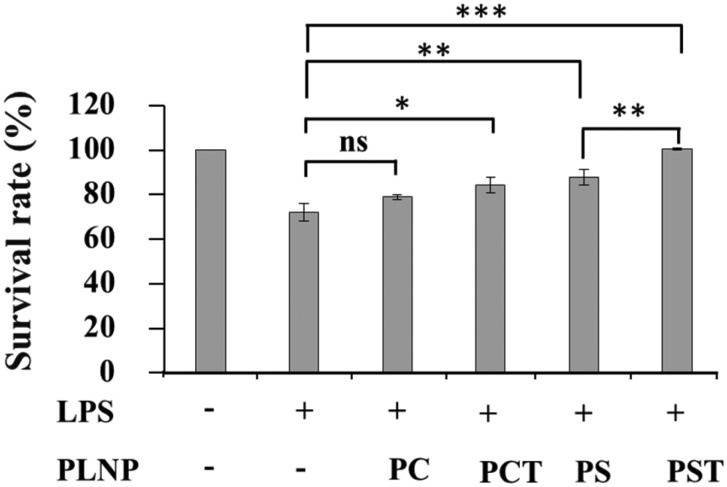
We examined the effect of PLNPs on the ability of the macrophages to resist LPS stimulation. As shown in Fig. 4, the viability of the macrophages improved in the presence of α-tocopherol as well as PS (PCT-, PS- and PST-PLNPs). PST-PLNPs were the most effective at increasing cell viability. The superiority of PST-PLNPs might result from the synergistic effect of α-tocopherol and PS, which both confer resistance against LPS-stimulated cell death.
Fig. 4. RAW264.7 macrophage viability after LPS stimulation. Macrophages (1 × 104 cells per well) were seeded in a 96-well plate, treated with PLNPs (125 μg lipid per ml) and LPS (1 μg ml–1) and incubated for 48 h. Absorbance was measured at 450 nm with a microplate reader at the indicated times. Data are the means ± S.D. ns, not significant; *, p < 0.05; **, p < 0.01; ***, p < 0.001.
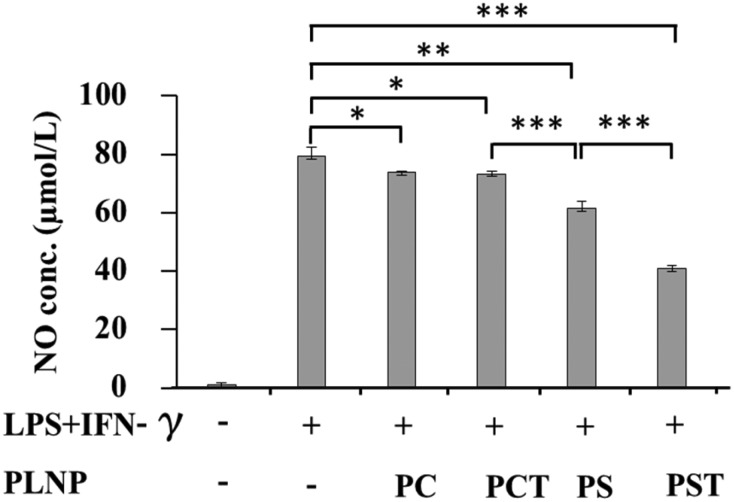
3.4. Suppression of NO production by activated macrophages
Activated macrophages produce NO, which reacts with ROS to propagate injury in colitis tissues.6 Excess production of NO exacerbates the clinicopathological features of UC by direct cytotoxicity and activation of leukocytes.40 Vasodilatation, reduction of smooth muscle tone,41 increased the production of nitrosamines42 and interaction with superoxide to form highly toxic peroxynitrite radicals.43 Thus, the suppression of NO production is important to prevent the oxidative stress and colitis injury in the colon. We examined the inhibitory effects of PLNPs on NO production from activated macrophages. To enhance NO production, the macrophages were stimulated with LPS (1 μg ml–1) and IFN-γ (100 U mL–1).32 As shown in Fig. 5, PC- and PCT-PLNPs had a smaller effect on NO production whereas, in contrast, significant suppression of NO production was observed from PS- and PST-PLNPs treatments. The greatest effect was induced by PST-PLNPs. A previous study reported the NO suppressing effects of PS44 in activated peritoneal macrophages. Our findings confirmed that the combination of PS and α-tocopherol in PST-PLNPs is synergistic for the reduction of NO production from activated macrophages.
Fig. 5. Effects of PLNPs (125 μg lipid per ml) on NO production by RAW264.7 macrophages stimulated with LPS (1 μg ml–1) and IFN-γ (100 U ml–1). Data are the means ± S.D. ns, not significant; *, p < 0.05; **, p < 0.01; ***, p < 0.001.
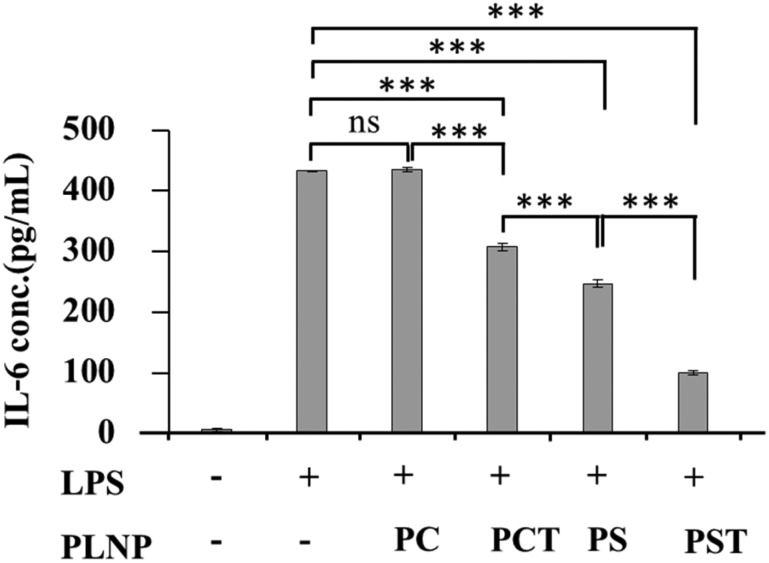
3.5. Reduction of IL-6 production by activated macrophages
To examine the anti-inflammatory activity of each PLNP, we measured the production of IL-6, a typical pro-inflammatory cytokine secreted from macrophages in ulcerative colitis.45 Inflammatory signalling produced by LPS stimulation increased the production of IL-6 from macrophages through trans-signaling by STAT3.46 α-Tocopherol was reported to decrease IL-6 production.47,48
As shown in Fig. 6, PC-PLNPs had no effect on IL-6 production from LPS-activated macrophages. In contrast, significant inhibition of IL-6 production was found in PCT-, PS- and PST-PLNP treatments. The greatest effect on IL-6 production was observed in the PST-PLNP treatment group. Our results indicated a clear synergy between PS and α-tocopherol based on their dominant effect on the reduction of the IL-6 production when compared with PCT- and PS-PLNP treatments.
Fig. 6. Effects of PLNPs (125 μg lipid per ml) on IL-6 production by macrophages stimulated with LPS (1 μg ml–1). Data are means ± SD of three separate experiments. ns, not significant; ***, p < 0.001.
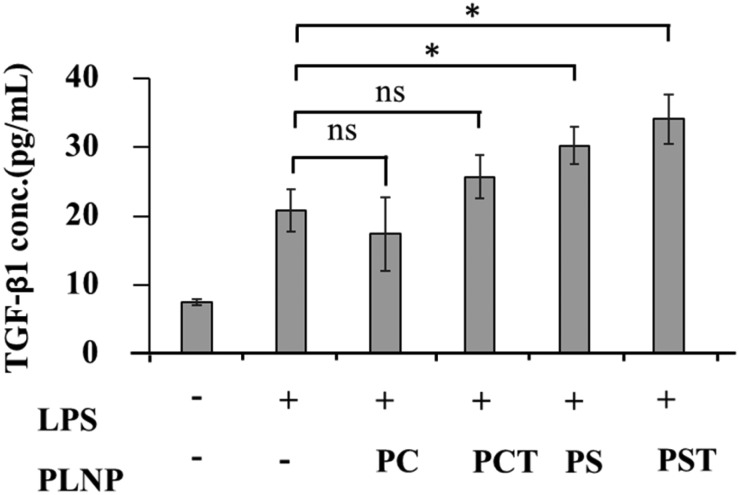
3.6. Enhanced TGF-β1 production by activated macrophages
We next assessed the effects of PST-PLNPs on the production of anti-inflammatory cytokine TGF-β1, a key regulator in the maintenance of immune and inflammatory responses. TGF-β1 together with growth factors protects host tissues from luminal changes and can help mucosal healing in inflammatory bowel diseases.49,50 Inflammation is inhibited by TGF-β1, which acts as a negative regulator of NF-kB activation.51 As shown in Fig. 7, both PS- and PST-PLNPs significantly increased TGF-β1 production from the activated macrophages, whereas PC- and PCT-PLNPs were not effective. Whether α-tocopherol has an additional effect on the production of TGF-β1 in the activated macrophages was not clear in this experiment.
Fig. 7. Effects of PLNPs (125 μg lipid per ml) on TGF-β1 production by macrophages stimulated with LPS (1 μg mL–1). Data are means ± SD of three separate experiments. ns, not significant; *, p < 0.05.
4. Conclusion
Here, we report biodegradable PLNPs containing PS plus α-tocopherol to decrease oxidative stress and inflammatory responses induced by activated macrophages. Because of the incorporation of PS, PLNPs have the potential to refill the cell surface with PS during the degradation process. The two components of PLNPs showed a clear synergy regarding their anti-inflammatory and antioxidative effects on the activated macrophages. PST-PLNPs significantly increased the viability of the activated macrophages. Our data also demonstrated that PST-PLNPs altered the inflammatory properties of the macrophages by reducing the production of NO and inflammatory cytokines when stimulated with LPS. The production of anti-inflammatory cytokines was also enhanced by PST-PLNPs. The potential anti-inflammatory and antioxidative effects of PST-PLNPs were indicated by the inflammatory modulation of the macrophages. However, the self-filling characteristics of the particles might increase the bioavailability of PS and α-tocopherol in the intestinal mucosa. Therefore, α-tocopherol and phosphatidylserine-containing self-filling biodegradable PLNPs might be potential oral drug delivery vehicles and effective tools compared with liposomes and polymeric nanoparticles for the treatment of inflammatory bowel diseases such as ulcerative colitis.
Conflict of interest
The authors declare no competing interest.
Supplementary Material
Acknowledgments
We thank Professor Masahiro Goto for his assistance with the sonicator for PLNP preparation.
Footnotes
†Electronic supplementary information (ESI) available. See DOI: 10.1039/c7md00174f
References
- Geier M. S., Butler R. N., Howarth G. S. Int. J. Food Microbiol. 2007;115:1–11. doi: 10.1016/j.ijfoodmicro.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Araki Y., Andoh A., Fujiyama Y. Int. J. Mol. Med. 2003;12:125–129. [PubMed] [Google Scholar]
- Bitiren M., Karakilcik A. Z., Zerin M., Ozardalı I., Selek S., Nazlıgül Y., Ozgonul A., Musa D., Uzunkoy A. Biol. Trace Elem. Res. 2010;136:87–95. doi: 10.1007/s12011-009-8518-3. [DOI] [PubMed] [Google Scholar]
- Sartor R. B. Gastroenterology. 2004;126:1620–1633. doi: 10.1053/j.gastro.2004.03.024. [DOI] [PubMed] [Google Scholar]
- De Hertogh G., Aerssens J., Geboes K. P., Geboes K. World J. Gastroenterol. 2008;14:845. doi: 10.3748/wjg.14.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelouhab K., Rafa H., Toumi R., Bouaziz S., Medjeber O., Touil-Boukoffa C. Immunopharmacol. Immunotoxicol. 2012;34:590–597. doi: 10.3109/08923973.2011.641971. [DOI] [PubMed] [Google Scholar]
- Mahida Y. R. Inflamm. Bowel Dis. 2000;6:21–33. doi: 10.1097/00054725-200002000-00004. [DOI] [PubMed] [Google Scholar]
- Grisham M. B., Granger D. N. Dig. Dis. Sci. 1988;33:6S–15S. doi: 10.1007/BF01538126. [DOI] [PubMed] [Google Scholar]
- Babbs C. F. Free Radical Biol. Med. 1992;13:169–181. doi: 10.1016/0891-5849(92)90079-v. [DOI] [PubMed] [Google Scholar]
- Stange E. F., Travis S. P. Gut. 2008;57:1029–1031. doi: 10.1136/gut.2007.146761. [DOI] [PubMed] [Google Scholar]
- Choi C. H., Kim Y.-H., Kim Y. S., Ye B. D., Lee K. M., Lee B. I., Jung S., Kim W. H., Lee H., Diseases I. S. G. O. T. K. A. F. T. S. O. I. Intest. Res. 2012;10:1–25. [Google Scholar]
- Kozuch P. L., Hanauer S. B. World J. Gastroenterol. 2008;14:354. doi: 10.3748/wjg.14.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruis W., Schreiber S., Theuer D., Brandes J., Schütz E., Howaldt S., Krakamp B., Hämling J., Mönnikes H., Koop I. Gut. 2001;49:783–789. doi: 10.1136/gut.49.6.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J., Hallett M. Biochem. Pharmacol. 1989;38:149–154. doi: 10.1016/0006-2952(89)90161-5. [DOI] [PubMed] [Google Scholar]
- Goppelt-Struebe M., Wolter D., Resch K. Br. J. Pharmacol. 1989;98:1287. doi: 10.1111/j.1476-5381.1989.tb12676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciriaco M., Ventrice P., Russo G., Scicchitano M., Mazzitello G., Scicchitano F., Russo E. J. Pharmacol. Pharmacother. 2013;4:S94. doi: 10.4103/0976-500X.120975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolk J. N., Boerbooms A. M., de Abreu R. A., de Koning D. G., van Beusekom H. J., Muller W. H., van de Putte L. Arthritis Rheum. 1998;41:1858–1866. doi: 10.1002/1529-0131(199810)41:10<1858::AID-ART19>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Faubion W. A., Loftus E. V., Harmsen W. S., Zinsmeister A. R., Sandborn W. J. Gastroenterology. 2001;121:255–260. doi: 10.1053/gast.2001.26279. [DOI] [PubMed] [Google Scholar]
- Loftus E., Kane S., Bjorkman D. Aliment. Pharmacol. Ther. 2004;19:179–189. doi: 10.1111/j.0269-2813.2004.01827.x. [DOI] [PubMed] [Google Scholar]
- Park S. C., Jeen Y. T. Gut Liver. 2015;9:18. doi: 10.5009/gnl14226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triantafillidis J. K., Merikas E., Georgopoulos F. Drug Des., Dev. Ther. 2011;5:185–210. doi: 10.2147/DDDT.S11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaraj S., Li D., Jialal I. J. Clin. Invest. 1996;98:756. doi: 10.1172/JCI118848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellezzo J. M., Leingang K. A., Bulla G. A., Britton R. S., Bacon B. R., Fox E. S. J. Lab. Clin.J. Lab. Clin. Med.Med. 1998;131:36–44. doi: 10.1016/s0022-2143(98)90075-0. [DOI] [PubMed] [Google Scholar]
- Ardite E., Sans M., Panés J., Romero F. J., Piqué J. M., Fernández-Checa J. C. Lab. Invest. 2000;80:735–744. doi: 10.1038/labinvest.3780077. [DOI] [PubMed] [Google Scholar]
- Yang F., Oz H. S., Barve S., de Villiers W. J., McClain C. J., Varilek G. W. Mol. Pharmacol. 2001;60:528–533. [PubMed] [Google Scholar]
- Vandivier R. W., Fadok V. A., Hoffmann P. R., Bratton D. L., Penvari C., Brown K. K., Brain J. D., Accurso F. J., Henson P. M. J. Clin. Invest. 2002;109:661–670. doi: 10.1172/JCI13572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravichandran K. S. Cell. 2003;113:817–820. doi: 10.1016/s0092-8674(03)00471-9. [DOI] [PubMed] [Google Scholar]
- Hochreiter-Hufford A., Ravichandran K. S. Cold Spring Harbor Perspect. Biol. 2013;5:a008748. doi: 10.1101/cshperspect.a008748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka M., Tsuchiya S., Aramaki Y. Biochem. Biophys. Res. Commun. 2004;324:1400–1405. doi: 10.1016/j.bbrc.2004.09.198. [DOI] [PubMed] [Google Scholar]
- Puri A., Loomis K., Smith B., Lee J.-H., Yavlovich A., Heldman E., Blumenthal R. Crit. Rev. Ther. Drug Carrier Syst. 2009;26:523–580. doi: 10.1615/critrevtherdrugcarriersyst.v26.i6.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker G., Kromp T., Wendel A., Blume A., Zirkel J., Rebmann H., Setzer C., Quinkert R.-O., Martin F., Müller-Goymann C. Pharm. Res. 2010;27:1469–1486. doi: 10.1007/s11095-010-0130-x. [DOI] [PubMed] [Google Scholar]
- Tötemeyer S., Sheppard M., Lloyd A., Roper D., Dowson C., Underhill D., Murray P., Maskell D., Bryant C. J. Immunol. 2006;176:4804–4810. doi: 10.4049/jimmunol.176.8.4804. [DOI] [PubMed] [Google Scholar]
- Rong G., William S., Thomas H., Andreas P., Min Q. Nutr. J. 2002;1:2. [Google Scholar]
- Fukasawa M., Adachi H., Hirota K., Tsujimoto M., Arai H., Inoue K. Exp. Cell Res. 1996;222:246–250. doi: 10.1006/excr.1996.0030. [DOI] [PubMed] [Google Scholar]
- Schroit A. J., Fidler I. J. Cancer Res. 1982;42:161–167. [PubMed] [Google Scholar]
- Allen T., Austin G., Chonn A., Lin L., Lee K. Biochim. Biophys. Acta, Biomembr. 1991;1061:56–64. doi: 10.1016/0005-2736(91)90268-d. [DOI] [PubMed] [Google Scholar]
- Aramaki Y., Akiyama K., Hara T., Tsuchiya S. Eur. J. Pharm. Sci. 1995;3:63–70. [Google Scholar]
- Geelen T., Yeo S. Y., Paulis L., Starmans L., Nicolay K., Strijkers G. J. J. Nanobiotechnol. 2012;10:1. doi: 10.1186/1477-3155-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canton J., Neculai D., Grinstein S. Nat. Rev. Immunol. 2013;13:621–634. doi: 10.1038/nri3515. [DOI] [PubMed] [Google Scholar]
- Ribbons K. A., Zhang X.-J., Thompson J. H., Greenberg S. S., Moore W. M., Kornmeier C. M., Currie M. G., Lerche N., Blanchard J., Clark D. A. Gastroenterology. 1995;108:705–711. doi: 10.1016/0016-5085(95)90442-5. [DOI] [PubMed] [Google Scholar]
- Middleton S., Shorthouse M., Hunter J. Gut. 1993;34:814–817. doi: 10.1136/gut.34.6.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima H., Bartsch H. Mutat. Res., Fundam. Mol. Mech. Mutagen. 1994;305:253–264. doi: 10.1016/0027-5107(94)90245-3. [DOI] [PubMed] [Google Scholar]
- Singer I. I., Kawka D. W., Scott S., Weidner J. R., Mumford R. A., Riehl T. E., Stenson W. F. Gastroenterology. 1996;111:871–885. doi: 10.1016/s0016-5085(96)70055-0. [DOI] [PubMed] [Google Scholar]
- Aramaki Y., Nitta F., Matsuno R., Morimura Y., Tsuchiya S. Biochem. Biophys. Res. Commun. 1996;220:1–6. doi: 10.1006/bbrc.1996.0346. [DOI] [PubMed] [Google Scholar]
- Rogler G., Andus T. World J. Surg. 1998;22:382–389. doi: 10.1007/s002689900401. [DOI] [PubMed] [Google Scholar]
- Carey R., Jurickova I., Ballard E., Bonkowski E., Han X., Xu H., Denson L. A. Inflamm. Bowel Dis. 2008;14:446–457. doi: 10.1002/ibd.20342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca de Oliveira B., Araujo Veloso C., Augusto Nogueira-Machado J., Martins Chaves M. Curr. Aging Sci. 2012;5:148–156. doi: 10.2174/1874609811205020148. [DOI] [PubMed] [Google Scholar]
- Lira F. S., Rosa J. C., Cunha C. A., Ribeiro E. B., do Nascimento C. O., Oyama L. M., Mota J. F. Lipids Health Dis. 2011;10:1186. doi: 10.1186/1476-511X-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa S., Tsunoda T., Onuma E., Majima T., Kagiyama M., Kikuchi K. Am. J. Gastroenterol. 2001;96:822–828. doi: 10.1111/j.1572-0241.2001.03527.x. [DOI] [PubMed] [Google Scholar]
- Lawrance I. C., Maxwell L., Doe W. Inflamm. Bowel Dis. 2001;7:16–26. doi: 10.1097/00054725-200102000-00003. [DOI] [PubMed] [Google Scholar]
- Haller D., Holt L., Kim S. C., Schwabe R. F., Sartor R. B., Jobin C. J. Biolumin. Chemilumin. 2003;278:23851–23860. doi: 10.1074/jbc.M300075200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.