Abstract
Background:
There is growing concern that phthalate exposures may have an impact on child neurodevelopment. Prenatal exposure to phthalates has been linked with externalizing behaviors and executive functioning defects suggestive of an attention-deficit hyperactivity disorder (ADHD) phenotype.
Objectives:
We undertook an investigation into whether prenatal exposure to phthalates was associated with clinically confirmed ADHD in a population-based nested case–control study of the Norwegian Mother and Child Cohort (MoBa) between the years 2003 and 2008.
Methods:
Phthalate metabolites were measured in maternal urine collected at midpregnancy. Cases of ADHD () were obtained through linkage between MoBa and the Norwegian National Patient Registry. A random sample of controls () from the MoBa population was obtained.
Results:
In multivariable adjusted coexposure models, the sum of di-2-ethylhexyl phthalate metabolites () was associated with a monotonically increasing risk of ADHD. Children of mothers in the highest quintile of had almost three times the odds of an ADHD diagnosis as those in the lowest [ (95% CI: 1.47, 5.49)]. When was modeled as a log-linear (natural log) term, for each log-unit increase in exposure, the odds of ADHD increased by 47% [ (95% CI: 1.09, 1.94)]. We detected no significant modification by sex or mediation by prenatal maternal thyroid function or by preterm delivery.
Conclusions:
In this population-based case–control study of clinical ADHD, maternal urinary concentrations of DEHP were monotonically associated with increased risk of ADHD. Additional research is needed to evaluate potential mechanisms linking phthalates to ADHD. https://doi.org/10.1289/EHP2358
Introduction
There is growing concern that phthalate exposures, particularly during the prenatal period, may have an impact on child neurobehavioral development (Bennett et al. 2016). Prenatal exposure to phthalates has been associated with both externalizing (Engel et al. 2010; Kobrosly et al. 2014; Lien et al. 2015) and internalizing (Engel et al. 2010; Whyatt et al. 2012) behaviors using validated behavioral screening instruments, as well as with deficits in executive function as measured by both parental report (Engel et al. 2010) and performance-based assessments (Factor-Litvak et al. 2014), although not all studies have found evidence of associations (Gascon et al. 2015). Among the neurobehavioral domains identified in multiple studies are inattention (Engel et al. 2010; Kobrosly et al. 2014), aggression (Engel et al. 2010; Kobrosly et al. 2014; Lien et al. 2015), conduct problems (Engel et al. 2010; Kobrosly et al. 2014; Lien et al. 2015), and emotional reactivity/regulation (Engel et al. 2010; Whyatt et al. 2012), as well as impairments in working memory (Engel et al. 2010; Factor-Litvak et al. 2014). Sex differences in the associations of phthalates with neurobehavioral end points have often been noted, although some studies have found stronger associations among boys (Engel et al. 2010; Kobrosly et al. 2014), whereas others have found stronger associations among girls (Whyatt et al. 2012). The constellation of phthalate-associated behaviors highlighted across studies has led many researchers to note overlap with symptoms of attention-deficit hyperactivity disorder (ADHD).
Despite the observed overlap in affected neurobehavioral domains, there is less consensus on the specific phthalate responsible for neurodisruptive effects, and no prior study has accounted for the correlation among phthalates by mutual adjustment. Some studies have reported significant associations with dibutyl phthalates (Engel et al. 2010; Kobrosly et al. 2014; Lien et al. 2015; Whyatt et al. 2012) and/or di-2-ethylhexyl phthalate (DEHP) (Kobrosly et al. 2014; Lien et al. 2015); others have highlighted butyl benzyl phthalate (BBzP) (Whyatt et al. 2012). Moreover, as of now there have been no studies with biomarkers of exposure in the prenatal period and access to clinically confirmed neurobehavioral end points, such as ADHD diagnoses from a clinical provider. Rather, the bulk of the literature relies on parent-reported symptoms. Because the ages of the children examined have varied substantially across and within studies, relying solely on parental reports to identify nonnormative behavior may be problematic.
A number of mechanisms have been proposed to explain how phthalates may negatively affect brain development (Miodovnik et al. 2014), although few have been thoroughly examined in humans or in animal models. One prominent concern is phthalate-induced maternal thyroid hormone disruption. Phthalates have been associated with changes in circulating thyroid hormone levels in adults (Huang HB et al. 2017; Meeker et al. 2007; Meeker and Ferguson 2011; Park et al. 2017) and in pregnant women (Huang PC et al. 2007, 2016; Johns et al. 2015, 2016; Kuo et al. 2015; Gao et al. 2017; Yao et al. 2016). The most consistent finding across studies has been an inverse association between metabolites of DEHP and thyroxine and/or free thyroxine (Meeker and Ferguson 2011; Park et al. 2017; Huang PC et al. 2016; Johns et al. 2015; Gao et al. 2017; Yao et al. 2016). Maternal prenatal thyroid hormone is essential for fetal neurodevelopment (Moog et al. 2017; Morreale de Escobar et al. 2004), and clinically diagnosed thyroid hormone disorders (hyperthyroidism and hypothyroidism) in the perinatal period have been linked with ADHD in offspring (Andersen et al. 2014; Instanes et al. 2015). Additionally, both higher and lower levels of thyroid hormone concentrations, even within population reference ranges, have been associated with ADHD-like behaviors (Ghassabian et al. 2011, 2012; Modesto et al. 2015; Päkkilä et al. 2014). Perinatal phthalate exposure has also been associated with preterm delivery (Ferguson et al. 2014a, 2014b), which is itself a risk factor for ADHD (Murray et al. 2016; Sucksdorff et al. 2015).
A true causal association of phthalate exposure with child neurodevelopment would have major public health significance. Phthalates are ubiquitous in consumer products (Schettler 2006), are components of many food processing and packaging materials (Serrano et al. 2014; Sakhi et al. 2014), and can be found in both pharmaceuticals (Kelley et al. 2012; Hernández-Díaz et al. 2013), and personal care products (Calafat et al. 2015; Sakhi et al. 2017). Therefore, to address this critically important public health question, we undertook a prospective, nested case–control study in the Norwegian Mother and Child Study (MoBa) to examine the hypothesis that prenatal biomarkers of phthalate exposure are associated with clinical ADHD in offspring. We further considered whether any associations were mediated by maternal thyroid function or preterm delivery or were modified by child sex.
Methods
Study Population
A total of 112,762 children were born to MoBa enrollees between 1999 and 2008 (Magnus et al. 2006, 2016). Pregnant women were recruited at their first ultrasound appointment, which occurred at approximately 17 wk gestation. Written informed consent was obtained from each MoBa participant upon recruitment. Mothers returned questionnaires three times during pregnancy: a general health and behavior questionnaire at 17 and 30 wk and a food frequency questionnaire at 22 wk gestation. Following the birth of their child, mothers returned questionnaires on child health and development at 6, 18, and 36 months of age. From this originally enrolled population, individuals were eligible for our study if they were pregnant in 2003 or later ( remaining), completed the 36-mo questionnaire ( remaining), did not have Down syndrome or cerebral palsy ( remaining), had maternal urine and blood samples collected ( remaining), had singleton pregnancies ( remaining), and resided in geographic areas eligible for the ADHD substudy (described below ( remaining). The final eligible population was 24,035, from which we randomly sampled our Norwegian Patient Registry (NPR) cases and controls.
Selection of ADHD Cases
Clinically diagnosed cases of ADHD born in 2003 or later were obtained from the NPR. The NPR is a national database containing all persons with diagnoses recorded from 2008 onward, from government-funded facilities, which captures an estimated 90–95% of ADHD diagnoses (Surén et al. 2012). We selected cases if they had at least two registrations of “Hyperkinetic disorder” [The ICD-10 Classification of Mental and Behavioral Disorders: Clinical Descriptions and Diagnostic Guidelines (ICD-10) codes F90, F90.0, F90.1, F90.8 or F90.9; WHO 1993]. We required two registrations in order to exclude erroneous registrations or false diagnoses. Registrations for hyperkinetic disorder before the age of 5 y are exceedingly rare (Surén et al. 2012). The ICD-10 criteria for ADHD are “early onset; a combination of overactive, poorly modulated behavior with marked inattention and lack of persistent task involvement; and pervasiveness over situations and persistence over time of these behavioral characteristics” (WHO 1993). In total, 297 cases were randomly sampled from the eligible registrations.
Selection of Controls
Families with the index child born at one of the larger hospitals in Norway between April 2004 and January 2008 and who completed the 36-mo MoBa questionnaire were eligible to participate in the MoBa Preschool ADHD Substudy (Rohrer-Baumgartner et al. 2014). From this eligible population, we randomly sampled a control population of 553 mother–child pairs. We nested our control group within the population eligible to participate in the Preschool ADHD Substudy so that future studies could utilize the same control population for preschool ADHD cases, which were diagnosed via a systematic neuropsychological evaluation of the child at (Skogan et al. 2014). Apart from eligibility criteria, no other information from the Preschool ADHD Substudy was entered into this analysis.
Phthalate Metabolite Measurements
Maternal urine collected at approximately 17 wk gestation was shipped overnight, unrefrigerated, to the central biorepository in Oslo, Norway for immediate processing. Urine was transported in a commercially available urine transport tube with a preservative to prevent bacterial growth (chlorhexidine plus ethyl paraben and sodium propionate) (UAP Vacutainers; Becton-Dickinson) (Rønningen et al. 2006). In a previous quality control (QC) study in MoBa, no impact was found on the measurement of phthalates from this preservative (Ye et al. 2009). Analysis of urine for phthalate metabolites was conducted at the Norwegian Institute of Public Health. Methods have been previously described (Sabaredzovic et al. 2015). Briefly, on-line column switching liquid chromatography coupled with tandem mass spectrometry was used to measure 12 phthalate metabolites: monoethyl phthalate (MEP), a metabolite of diethyl phthalate; mono-iso-butyl phthalate (MiBP), a metabolite of di-iso-butyl phthalate; mono-n-butyl phthalate (MnBP), a metabolite of di-n-butyl phthalate; monobenzyl phthalate (MBzP), a metabolite of BBzP; mono-2-ethylhexyl phthalate (MEHP), mono-2-ethyl-5-hydroxyhexyl phthalate (MEHHP), mono-2-ethyl-5-oxoyhexyl phthalate (MEOHP), mono-2-ethyl-5-carboxypentyl phthalate (MECPP), and mono-2-methylcarboxyhexyl phthalate (MMCHP), metabolites of DEHP; and mono-4-methyl-7-hydroxyoctyl phthalate (OH-MiNP), mono-4-methyl-7-oxooctyl phthalate (oxo-MiNP), and mono-4-methyl-7-carboxyheptyl phthalate (cx-MiNP), metabolites of di-iso-nonyl phthalate (DiNP). A QC sample of pooled urine was created to assess batch-to-batch variability and assay precision. In each analytic batch, procedural blank samples, two in-house control urine samples and 4–6 QC pooled urine aliquots were included. External reference samples from the National Institute of Standards and Technology [NIST; Standard Reference Material (SRM) 3673] were also analyzed in every fourth analytical batch. Cases and controls were randomly allocated across analytic batches. The analyst was blinded to QC, case, and control samples. To account for urinary dilution, specific gravity was measured using a pocket refractometer (PAL-10S) from Atago. In brief, of the urine sample was placed onto the prism surface, and the specific gravity was measured with the refractometer. The coefficient of variation (CV) was for the in-house control urine samples. In laboratory-blinded QC samples, average batch CVs were .
Prenatal Maternal Thyroid Function
Maternal blood collected in ethylenediamine tetraacetic acid (EDTA) tubes at 17 wk gestation was shipped overnight, unrefrigerated, to a central biospecimen processing lab (Rønningen et al. 2006). Plasma was separated and stored in cryovials at and was shipped frozen on dry ice to ARUP Laboratories (Salt Lake City, Utah) for analysis of thyroid hormone concentrations. Thyroid stimulating hormone (TSH) was measured using a quantitative chemiluminescent immunoassay on a Roche Cobas e602 blood analyzer. Triiodothyronine (T3) and thyroxine (T4) were also measured using quantitative electrochemiluminescent immunoassays on the Roche Cobas e602 analyzer. Intra- and interassay CVs for T3, T4, and TSH were . We previously established the reliability of MoBa maternal plasma for measurement of thyroid hormone concentrations, considering delays in processing and storage and freeze-thaw cycles (Villanger et al. 2017).
Ethics
Data collection for MoBa was approved by the Norwegian Data Inspectorate and the Norwegian Committee for Medical and Health Research Ethics (REC). The present study was approved by the Norwegian REC and the Institutional Review Board at University of North Carolina Chapel Hill.
Statistical Analysis
The QC pool CV for each phthalate metabolite, computed as the ratio of the standard deviation across QC pools to the mean across QC pools, was assessed separately for each batch as well as overall.
Phthalate metabolite concentrations for each participant were standardized to specific gravity using the procedure described by Hauser et al. (2004). Suppose represents the measured value of phthalate metabolite i for participant j. Then, letting represent the corresponding specific gravity adjusted measurement (Equation 1),
| (1) |
where denotes the specific gravity for participant j, and c is a common normalizing constant computed as the geometric mean of specific gravity across all participants minus 1.
To account for potential batch effects, individual measurements were batch-adjusted using a scaled variation of the Ratio-G batch adjustment method (Luo et al. 2010). Consider again the specific gravity–adjusted measurement , and suppose it was processed in batch k. Letting the corresponding specific gravity– and batch-adjusted measurement be denoted (Equation 2),
| (2) |
where represents the geometric mean of phthalate metabolite i across all QC pools, and represents the geometric mean of phthalate metabolite i across the QC pools from batch k. The specific gravity– and batch-adjusted phthalate metabolite measures are used in all tables, figures, and statistical models.
Following specific gravity and batch adjustments of all phthalate metabolites, the molar sums for DEHP and DiNP were computed (hereafter referred to as and , respectively). Each component phthalate metabolite was first converted from to by dividing it by its molecular mass. After conversion, the component phthalates were then summed to produce and measures in .
The present analysis is based on version 9 of the MoBa quality-assured data files. Covariate data were obtained from the 17- and 30-wk prenatal questionnaires. We selected potential confounders a priori by using directed acyclic graphs based on current knowledge of covariates that could influence both phthalate levels and ADHD. In statistical models exploring the relationship between ADHD and maternal urinary phthalate concentrations, adjustments were made for the following covariates: maternal age at delivery, sex of the child, maternal education (obtained from the 17-wk questionnaire), marital status, prenatal maternal smoking in the first or second trimester of pregnancy (self-reported), parity, maternal depression during pregnancy (self-reported on the 30-wk questionnaire), and year of birth.
In order to obtain inference about the relationship between each phthalate metabolite and ADHD after adjustment for all other phthalate levels, we regressed ADHD on (transformations of) all phthalates simultaneously and assumed no interactions among the phthalates. Two participants were excluded from analyses because a single phthalate metabolite was missing because of analytic interference; because our statistical approach involved coadjustment for all phthalate metabolites, they were dropped from the model. One additional participant was excluded because of unusually low values for all phthalate metabolites. The logged phthalate metabolites demonstrated moderate pairwise correlation; therefore, a Bayesian modeling framework, which provides more stable estimates than frequentist models in the presence of correlated exposures (MacLehose et al. 2007), was selected. In all Bayesian models, normal prior distributions with zero mean and 0.5 variance were chosen for each of the regression coefficients. Because pairwise correlations between the logged phthalates were only moderate, mixture prior distributions, which improve estimation with highly correlated predictors, were deemed to be unnecessary (MacLehose et al. 2007).
The associations between the phthalate metabolites and ADHD were first assessed by fitting a Bayesian logistic regression model with binary ADHD status as the outcome and the quintiles of all the phthalates simultaneously as predictors in a complete case analysis framework. As a follow-up, a second Bayesian logistic regression model was fit; this model considered all logged phthalates simultaneously as linear predictors. A model containing the interactions between each of the logged phthalates and child’s sex was fit to investigate effect modification by these factors. We considered effect measure modification by sex to be significant if the 90% credible interval of the phthalate by sex interaction term excluded the null value. Based on the size of our study population, we estimated that we had 90% power to detect an additive interaction term as low as 0.105, corresponding to an interaction term odds ratio (OR) of .
Finally, for phthalate metabolites exhibiting associations with ADHD, mediation analyses were performed for each thyroid function biomarker measure and for preterm delivery (delivery completed weeks of gestation) to explore the possibility that the impact of maternal phthalate exposure on child ADHD is mediated by these factors. Natural direct effect (NDE) and natural indirect effect (NIE) ORs were computed for each phthalate and thyroid hormone combination, and for each phthalate and preterm delivery, using the methods described by Vanderweele and Vansteelandt (Vanderweele and Vansteelandt 2010; Valeri and Vanderweele 2013).
For participant j let represent binary ADHD status, represent continuous log phthalate metabolite measure, represent the mediator under consideration (either a thyroid hormone measure or preterm delivery), represent the set of covariates for the ADHD-phthalate models described above, and represent a relevant set of confounders of the phthalate–mediator relationship. The following two models were fit (Equations 3 and 4):
| (3) |
| (4) |
where the first is called the outcome model, and the second is called the mediator model; g is the appropriate link function, chosen to be E(X) for the continuous thyroid hormone measures and logit(X) for binary preterm delivery. The thyroid hormone measures were square root–transformed to ensure adherence to linear modeling assumptions, and both the log phthalate and the square root thyroid hormone were centered to aid interpretation. For the thyroid hormones, the linear mediator model was fit using inverse probability weights to account for the case–control design of our study.
The NDE and NIE ORs corresponding to a change in log phthalate from its mean to one unit above the mean were calculated by plugging frequentist parameter estimates into exp() and exp() for the thyroid hormones and exp(), respectively, and Equation 5 for preterm delivery:
| (5) |
where represents a chosen set of covariate values. Analogous NDE and NIE ORs were also calculated based on an outcome model containing an interaction between the phthalate and the mediator, as advised by Vanderweele and Vansteelandt (Vanderweele and Vansteelandt 2010). This interaction term increases model flexibility and helps to account for the extent of mediation (Vanderweele 2015). Assumptions of mediation analysis include correct model specification and no unmeasured confounding of the exposure–outcome, mediator–outcome, and exposure–mediator relationships (Vanderweele and Vansteelandt 2010). In preliminary analyses, we found evidence of a U-shaped relationship between T4 and ADHD (data not shown), and the Vanderweele and Vansteelandt method does not easily account for nonlinear mediator forms. Therefore, we conducted a sensitivity analysis wherein we assessed mediation separately among those below and above the median T4 level.
All analyses were performed using R statistical software (R Core Team; Van Buuren and Groothuis-Oudshoorn 2011).
Results
Characteristics of ADHD cases and controls can be found in Table 1. Mothers of cases were slightly younger than mothers of controls, and they were also more likely to report lower educational attainment, less likely to report being married at the time of enrollment, more likely to have reported smoking during their first or second trimester, and more likely to report having experienced depression during pregnancy. Cases were also more likely to be boys.
Table 1.
Characteristics of study population in nested case–control study of attention-deficit hyperactivity disorder in the Norwegian Mother and Child Cohort (MoBa), 2003–2008.
| Characteristic | MoBa Controls or (%) | MoBa NPR ADHD Cases or (%) |
|---|---|---|
| Total N | 553 | 297 |
| Maternal age at delivery (years) | ||
| Missing (n) | 2 | 2 |
| Child Sex | ||
| Boy | 273 (49.6) | 213 (72.2) |
| Girl | 278 (50.4) | 82 (27.8) |
| Missing (n) | 2 | 2 |
| Maternal education | ||
| 123 (22.5) | 160 (59.7) | |
| College | 238 (43.6) | 74 (27.6) |
| 169 (31.0) | 25 (9.3) | |
| Other | 16 (2.9) | 9 (3.4) |
| Missing (n) | 7 | 29 |
| Marital status | ||
| Single/Other | 14 (2.6) | 18 (6.7) |
| Cohabitating | 245 (44.7) | 144 (53.5) |
| Married | 289 (52.7) | 107 (39.8) |
| Missing (n) | 5 | 28 |
| Smoking in 1st or 2nd trimester | ||
| Yes | 78 (14.3) | 94 (34.8) |
| No | 469 (85.7) | 176 (65.2) |
| Missing (n) | 6 | 27 |
| Primiparous | ||
| Yes | 270 (49.0) | 141 (47.8) |
| No | 281 (51.0) | 154 (52.2) |
| Missing (n) | 2 | 2 |
| Reported depression during pregnancy | ||
| Yes | 6 (1.1) | 16 (5.4) |
| No | 547 (98.9) | 281 (94.6) |
| Missing (n) | 0 | 0 |
| Year of Birth | ||
| 2003–2004 | 55 (10.0) | 131 (44.1) |
| 2005 | 130 (23.5) | 87 (29.3) |
| 2006 | 194 (35.1) | 44 (14.8) |
| 2007–2008 | 174 (31.5) | 35 (11.8) |
| Missing (n) | 0 | 0 |
Note: ADHD, attention-deficit hyperactivity disorder; NPR, Norwegian Patient Registry; SD, standard deviation.
The distributions of the maternal urinary phthalate metabolites measured herein are presented in Table 2. The concentrations are adjusted for batch and standardized to the geometric mean of specific gravity. Although all raw measured values were greater than the limit of quantification (LOQ), after adjustment for batch and specific gravity, some of the lower concentrations fell below the analytic LOQ. For all phthalate metabolites except DiNP metabolites, concentrations among controls were lower than among cases, which is explained in part by an imbalance in birth year by case/control status (Table 1). For DiNP metabolites, the reverse pattern was observed. Average exposures were in general highest for MEP and lowest for DiNP metabolites.
Table 2.
Phthalate metabolite distribution in a nested case–control study of attention-deficit hyperactivity disorder in the Norwegian Mother and Child Cohort (MoBa), 2003–2008.
| Exposure and outcome | Geometric mean | Geometric SD | Min | 25% | 50% | 75% | Max | LOQ | % | Average batch-specific CV |
|---|---|---|---|---|---|---|---|---|---|---|
| MEP () | 0.5 | 100.0 | 3.20 | |||||||
| Case | 133 | 4.50 | 5.09 | 40.9 | 132 | 406 | 10,500 | |||
| Control | 98.7 | 4.49 | 0.05 | 32.3 | 98.7 | 297 | 6,760 | |||
| MiBP () | 0.5 | 100.0 | 5.86 | |||||||
| Case | 21.2 | 2.33 | 3.21 | 12.0 | 21.5 | 36.8 | 407 | |||
| Control | 18.0 | 2.58 | 0.02 | 9.61 | 16.5 | 31.4 | 562 | |||
| MnBP () | 0.5 | 100.0 | 4.02 | |||||||
| Case | 25.1 | 2.13 | 4.18 | 14.4 | 25.0 | 43.4 | 214 | |||
| Control | 18.1 | 2.44 | 0.03 | 11.4 | 17.0 | 30.6 | 70,200 | |||
| MBzP () | 0.2 | 100.0 | 4.93 | |||||||
| Case | 7.28 | 2.72 | 0.76 | 3.34 | 6.74 | 14.8 | 151 | |||
| Control | 4.57 | 2.59 | 2.54 | 4.25 | 7.84 | 103 | ||||
| MEHP () | 0.5 | 100.0 | 5.66 | |||||||
| Case | 13.7 | 1.89 | 2.16 | 8.74 | 13.2 | 20.1 | 156 | |||
| Control | 11.2 | 2.21 | 0.02 | 7.11 | 10.3 | 17.2 | 812 | |||
| MEHHP () | 0.4 | 100.0 | 3.74 | |||||||
| Case | 16.9 | 2.25 | 1.71 | 9.52 | 16.6 | 26.5 | 324 | |||
| Control | 13.8 | 2.54 | 0.03 | 8.06 | 12.6 | 20.5 | 1,700 | |||
| MEOHP () | 0.4 | 100.0 | 3.82 | |||||||
| Case | 11.3 | 2.23 | 1.36 | 6.67 | 10.8 | 17.6 | 179 | |||
| Control | 9.29 | 2.51 | 0.02 | 5.51 | 8.41 | 14.0 | 807 | |||
| MECPP () | 2.0 | 100.0 | 1.22 | |||||||
| Case | 23.6 | 1.84 | 7.42 | 15.5 | 21.2 | 32.2 | 327 | |||
| Control | 20.6 | 2.04 | 0.04 | 13.8 | 18.5 | 25.6 | 768 | |||
| MMCHP () | 2.0 | 100.0 | 2.79 | |||||||
| Case | 23.2 | 1.84 | 7.86 | 15.2 | 20.5 | 29.8 | 463 | |||
| Control | 20.6 | 1.95 | 0.03 | 14.0 | 18.1 | 26.1 | 372 | |||
| () | NA | NA | NA | |||||||
| Case | 0.31 | 1.85 | 0.07 | 0.21 | 0.28 | 0.41 | 3.30 | |||
| Control | 0.27 | 2.10 | 0.18 | 0.23 | 0.34 | 14.9 | ||||
| OH-MiNP () | 0.2 | 100.0 | 4.99 | |||||||
| Case | 0.92 | 1.96 | 0.33 | 0.59 | 0.86 | 1.22 | 138 | |||
| Control | 1.06 | 2.10 | 0.69 | 0.95 | 1.42 | 60.7 | ||||
| oxo-MiNP () | 0.2 | 98.5 | 6.58 | |||||||
| Case | 1.09 | 2.18 | 0.33 | 0.62 | 0.95 | 1.58 | 122 | |||
| Control | 1.20 | 2.45 | 0.70 | 1.04 | 1.76 | 201 | ||||
| cx-MiNP () | 1.0 | 100.0 | 2.79 | |||||||
| Case | 3.22 | 1.63 | 1.30 | 2.27 | 2.96 | 4.45 | 36.0 | |||
| Control | 3.61 | 1.80 | 0.01 | 2.50 | 3.49 | 4.73 | 141 | |||
| () | NA | NA | NA | |||||||
| Case | 0.02 | 1.75 | 0.01 | 0.01 | 0.02 | 0.02 | 0.96 | |||
| Control | 0.02 | 1.95 | 0.01 | 0.02 | 0.03 | 1.07 |
Note: Concentrations are expressed to three significant digits. Phthalate metabolites were measured in 297 cases and 552 controls. The LOQ was not available for and because they are molar sums of phthalate metabolites, and therefore were not directly measured. CV, coefficient of variation; cx-MiNP, mono-4-methyl-7-carboxyheptyl phthalate; DEHP, di-2-ethylhexyl phthalate; DiNP, di-iso-nonyl phthalate; LOQ, limit of quantification; MBzP, monobenzyl phthalate; MECPP, mono-2-ethyl-5-carboxypentyl phthalate; MEHHP, mono-2-ethyl-5-hydroxyhexyl phthalate; MEHP, mono-2-ethylhexyl phthalate; MEOHP, mono-2-ethyl-5-oxoyhexyl phthalate; MEP, monoethyl phthalate; MiBP, mono-iso-butyl phthalate; MMCHP, mono-2-methylcarboxyhexyl phthalate; MnBP, mono-n-butyl phthalate; NA, not available; OH-MiNP, mono-4-methyl-7-hydroxyoctyl phthalate; oxo-MiNP, mono-4-methyl-7oxooctyl phthalate. Values were adjusted for batch and standardized to the geometric mean of specific gravity.
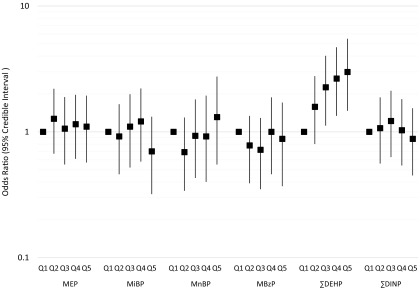
After adjustment for covariates, neither MEP, MiNP, MnBP, MBzP, nor was associated with ADHD in Bayesian multivariable adjusted models, which included adjustment for phthalate coexposures (Table 3, Figure 1; see also Table S1). However, was positively associated with ADHD. Across quintiles of exposure, ORs increased monotonically (Figure 1; see also Table S1). Children of mothers in the highest quintile of had almost three times the odds of an ADHD diagnosis as those in the lowest quintile [ (95% CI: 1.47, 5.49)]. When was modeled as a log-linear term, for each log-unit increase in exposure, the odds of ADHD increased by 47% [ (95% CI: 1.09, 1.94)] (Table 3). Adjustment for year of birth substantially attenuated estimates of association for MEP, MiBP, MnBP, , and ; however, associations for remained statistically significant in year-adjusted models (Table 3; see also Table S1). In sensitivity analyses, we examined associations in single-phthalate models, in models with mutual adjustment only within classes of high- or low-molecular-weight phthalates specifically, and in a model in which specific gravity was instead included as a covariate in the model (see Tables S2 and S3). There was modest confounding by correlated phthalates present in the single-phthalate models, and adjustment for specific gravity as a covariate resulted in a substantial decrease in the precision of estimates. We additionally examined whether adjustment for maternal or paternal income affected our models, and there were no substantial changes. We also constructed alternative models in which we adjusted for month and year of urine collection and found no differences compared with models adjusted for birth year (see Table S4).
Table 3.
Interactions between linear phthalate exposure and child sex in a nested case–control study of attention-deficit hyperactivity disorder in the Norwegian Mother and Child Cohort (MoBa), 2003–2008.
| Phthalate | Combined ()a | Boys ()b | Girls ()b |
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| MEP | 1.02 (0.90, 1.16) | 0.99 (0.85, 1.15) | 1.10 (0.87, 1.36) |
| MiBP | 0.92 (0.70, 1.20) | 0.96 (0.68, 1.29) | 0.87 (0.54,1.29) |
| MnBP | 1.04 (0.77, 1.40) | 0.98 (0.70, 1.34) | 1.33 (0.75, 2.20) |
| MBzP | 1.21 (0.92, 1.55) | 1.21 (0.87, 1.62) | 1.18 (0.75, 1.81) |
| 1.47 (1.09, 1.94) | 1.41 (1.00, 1.95) | 1.62 (0.95, 2.58) | |
| 0.85 (0.61, 1.15) | 0.83 (0.57, 1.18) | 0.85 (0.48, 1.35) |
Note: CI, credible interval; , sum of di-2-ethylhexyl phthalate metabolites; , sum of di-iso-nonyl phthalate metabolites; MBzP, monobenzyl phthalate; MEP, monoethyl phthalate; MiBP, mono-iso-butyl phthalate; MnBP, mono-n-butyl phthalate; OR, odds ratio.
Models adjusted for analytic batch, specific gravity, child sex, mother’s age, mother’s education level, mother’s marital status, mother’s smoking status, parity, maternal depression during pregnancy, and year of birth.
Stratum-specific estimates derived from models that additionally include sex by phthalate interaction terms.
Figure 1.
Odds ratios and 95% credible intervals for quintiles of phthalate metabolite concentrations in a nested case–control study of attention-deficit hyperactivity disorder in the Norwegian Mother and Child Cohort, 2003–2008. This plot displays the results of a multivariable adjusted model, where phthalate metabolite quintiles are mutually adjusted. This model was adjusted for analytic batch, specific gravity, maternal age at delivery, sex of the child, maternal education, marital status, prenatal smoking, parity, maternal depression during pregnancy, and year of birth. , sum of di-2-ethylhexyl phthalate metabolites; , sum of di-iso-nonyl phthalate metabolites; MBzP, monobenzyl phthalate; MEP, monoethyl phthalate; MiBP, mono-iso-butyl phthalate; MnBP, mono-n-butyl phthalate.
We observed no notable effect modification by child sex, with the possible exception of MnBP (Table 3). Among boys, the association between MnBP and ADHD was (95% CI: 0.70, 1.34), whereas among girls, the point estimate was somewhat elevated [ (95% CI: 0.75, 2.20)]; however, neither of the stratum-specific associations excluded the null value, and the 90% credible interval for the phthalate by sex interaction term included the null, suggesting no meaningful interaction. Notably, the association between and ADHD was strong in both boys and girls, as well as overall, although the estimate was slightly stronger, and less precise, among girls (Table 3).
We found no evidence of mediation of the relationship by any of the measured maternal thyroid function biomarkers (Table 4). This finding was true for both mediation models (with and without the exposure–mediator interaction terms). In a sensitivity analysis, we assessed mediation separately among those below and above the median T4 level and again found no mediation (see Table S5). We also investigated mediation of the relationship by preterm delivery and detected no significant mediation in models with and without the thyroid hormone–preterm interaction term (Table 4).
Table 4.
Natural direct effect (NDE) and natural indirect effect (NIE) odds ratios and 95% confidence intervals for mediation of by thyroid hormones and preterm delivery.
| Model parameter | No interaction | Interaction | ||
|---|---|---|---|---|
| NDEa | NIE | NDEa | NIE | |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Thyroid stimulating hormone (TSH)b | 1.45 (1.11, 1.88) | 1.00 (0.99, 1.01) | 1.43 (1.10, 1.86) | 1.00 (0.98, 1.02) |
| Triiodothyronine (T3)b | 1.46 (1.12, 1.90) | 1.00 (0.98, 1.02) | 1.51 (1.14, 2.00) | 1.00 (0.95, 1.05) |
| Thyroxine (T4)b | 1.46 (1.13, 1.90) | 1.00 (0.99, 1.01) | 1.47 (1.13, 1.92) | 0.99 (0.97, 1.02) |
| Preterm deliveryc | 1.45 (1.12, 1.88) | 1.00 (1.00, 1.00) | 1.44 (1.11, 1.87) | 1.00 (0.99, 1.02) |
Note: CI, confidence interval; , sum of di-2-ethylhexyl phthalate metabolites; OR, odds ratio.
The NDE is conditional on values of the mediator model covariates. For the thyroid hormones, the NDEs are computed for the following covariate specifications: iodine deficient (), sample average maternal age, nonsmoking mother, nonprimiparous, and year of birth 2005. For preterm delivery, the NDEs are computed for the following covariate specifications: sample average maternal age, married mother, nonsmoking mother, and year of birth 2005.
Mediation models adjusted for iodine intake (dichotomized at 150), mother’s age, mother’s smoking status, parity, and year of birth and outcome models adjusted for child sex, mother’s age, mother’s education level, mother’s marital status, mother’s smoking status, parity, maternal depression during pregnancy, and year of birth.
Mediation models adjusted for mother’s age, mother’s education level, mother’s smoking status, and year and outcome models adjusted for child sex, mother’s age, mother’s education level, mother’s marital status, mother’s smoking status, parity, maternal depression during pregnancy, and year of birth.
Discussion
In this prospective, nested case–control study, we found evidence that maternal prenatal exposure to was monotonically associated with increased risk of ADHD in offspring. We did not identify evidence of significant heterogeneity in these associations by child sex, nor did we identify evidence that this association was mediated through either maternal thyroid function or preterm delivery.
Among the previous studies that have examined prenatal phthalate exposures in relation to childhood neurobehavioral development (Engel et al. 2010; Kobrosly et al. 2014; Lien et al. 2015; Whyatt et al. 2012; Gascon et al. 2015; Miodovnik et al. 2011; Philippat et al. 2015; Braun et al. 2014), several, but not all, have highlighted potentially troubling associations with DEHP exposures (Kobrosly et al. 2014; Lien et al. 2015; Philippat et al. 2015). Lien et al. (2015) examined measured biomarkers of DEHP exposure during pregnancy in relation to child behavior and found that increased concentration of DEHP metabolites was associated with more externalizing problems. Kobrosly et al. (2014) reported more somatic complaints in children with increased prenatal concentrations of DEHP metabolites. Although individual phthalate metabolite associations were not provided, Engel et al. (2010) reported that increased concentrations of the sum of high-molecular-weight phthalate metabolites (inclusive of DEHP, as well as MBzP and mono-(3-carboxypropyl) phthalate (MCPP), a metabolite of di-n-octyl phthalate) were associated with poorer scores on the adaptability scale of the behavioral assessment scale for children (BASC). Interestingly, a Swedish study of the indoor environment and autism spectrum disorder (ASD) reported that presence of vinyl flooring, a source of DEHP, in the parents’ bedroom during pregnancy significantly increased odds of an ASD diagnosis (Larsson et al. 2009). Furthermore, in a population-based case–control study of ASD and developmental delay, Philippat et al. (2015) found no association between house-dust levels of DEHP and ASD, although they did find that higher DEHP concentrations in house dust were associated with increased odds of developmental delay. However, not all studies have found associations between prenatal DEHP exposure and behavior (Whyatt et al. 2012; Gascon et al. 2015; Miodovnik et al. 2011; Braun et al. 2014). We also note that our study assessed more DEHP metabolites has been than examined in the prior literature. The total DEHP exposure captured by all five metabolites after 24 h and 44 h of an oral dose is 67% and 74%, respectively (Koch et al. 2005). Both MECPP and MMCHP have longer elimination half-lives (MECPP, 12–15 h; MMCHP, 24 h) than MEHHP and MEOHP (10 h for both MEHHP and MEOHP). Owing to their longer elimination half-lives, both MECPP and MMCHP are excellent biomarkers of time-weighted DEHP body burden. In addition, MMCHP is the major metabolite excreted during the second day of exposure to DEHP (Koch et al. 2005). Therefore, including this additional DEHP metabolite may improve the reliability of our estimates of DEHP exposure compared with those in the existing literature, potentially reducing misclassification.
There has been considerable concern about adverse developmental effects of perinatal exposure to DEHP for over a decade (Shelby 2006; Kavlock et al. 2006; CPSC 2014; NRC 2008), particularly in reference to the development of the male reproductive tract (Shelby 2006). Although most of the experimental research on the developmental effects of phthalates has focused on reproductive toxicity, principally relating to antiandrogenic activity, there is a small but growing body of experimental literature exploring the effects of perinatal exposure to DEHP on neurodevelopment. Gestational DEHP exposure in mice has been linked to decreases in neurogenesis and in the proliferation of neural stem cells in the developing neocortex (Komada et al. 2016), although another study found an increase in neurite length but no difference in the number of cortical neurons (Lee et al. 2016). In rats, gestational DEHP exposure was associated with a dose-dependent impairment of learning and spatial memory along with alterations in gene expression in the neonatal rat brain (Lin et al. 2015), particularly highlighting effects on two genes important for neuron proliferation (Lin et al. 2015). Perinatal DEHP exposure in rats has also been associated with an altered brain lipid metabolome (Xu et al. 2007). Moreover, in an in vitro study, MEHP exposure (a metabolite of DEHP) inhibited cell proliferation and differentiation of neuronotypic PC12 cells via cell cycle arrest (Chen et al. 2011). Perinatal DEHP exposure has also been associated with aggravation of anxiety and depression-like behaviors in pubertal and adult mice (Xu et al. 2015; Quinnies et al. 2017). Although little is known about the tissue distribution of DEHP following exposure, phthalates are known to cross the placenta (Jensen et al. 2015; Jensen et al. 2012; Singh et al. 1975). Additionally, although DEHP has low affinity for brain tissue, more radiolabeled DEHP was found in the brain of 3-d old mice than in the brain of older mice, suggesting that the blood–brain barrier may have increased permeability to DEHP in neonates (Miles-Richarson and Bosch 2002). Overall, however, this body of experimental literature is quite small, and experimental designs have differed in the doses administered and in the periods of exposure examined, as well as in the outcomes assessed.
In contrast with prior studies (Engel et al. 2010; Kobrosly et al. 2014; Lien et al. 2015; Whyatt et al. 2012), we found no evidence that prenatal exposure to low-molecular-weight phthalates (e.g., MEP, MiBP, MnBP) was associated with clinically confirmed ADHD and no evidence of interactions by sex for any of our measured phthalates. There are several plausible explanations for these findings. First, it is possible that the differences between our studies are simply attributable to variability around an overall null association between these phthalates and ADHD. Second, it is possible that focusing on clinically diagnosed cases misses subtle behavioral differences that can be more powerfully assessed using instruments that allow for dimensionality in the severity of symptoms. Third, ADHD is a heterogeneous disability (Thapar and Cooper 2016). Established neuropsychological screening approaches assess a range of symptoms and classify individuals into ADHD subtypes on the basis of their presence or absence. We only had access to the binary diagnosis of ADHD; however, it is conceivable that some phthalates are only associated with specific subtypes of ADHD, and this etiological heterogeneity could potentially attenuate associations in our study. Finally, it may also be relevant that the timing of exposure measurement in our study differs from that of most of the previous studies. Our urine sample was taken at 17 wk gestation, whereas most of the prior studies assessed exposure late in the third trimester. If there are sensitive windows of brain development that are critical for the development of ADHD, then timing of exposure measurement may be a key explanatory factor. However, there is at present no literature that specifically examines timing of phthalate exposure in relation to ADHD.
We acknowledge several limitations in our study. First, phthalates are rapidly metabolized and have been shown to exhibit low to moderate reliability across pregnancy in several studies, with the best reliability often found for MEP, MiBP, and MnBP, and the worst reliability often found for DEHP (Adibi et al. 2008; Baird et al. 2010; Braun et al. 2012; Teitelbaum et al. 2008; Townsend et al. 2013). For DEHP specifically, intraclass correlation coefficients for repeated biomarker measurements from urine collected over a period of weeks (Adibi et al. 2008; Baird et al. 2010), months (Braun et al. 2014; Teitelbaum et al. 2008), or years (Townsend et al. 2013) has ranged from a low of 0.08 to a high of 0.38. Our study only had access to one spot urine sample that was collected at approximately 17 wk gestation. Thus, we cannot represent this single measurement as reflective of pregnancy-wide exposures. Second, to some extent, MoBa under-represents young mothers, those living alone, and women who report smoking during pregnancy (Nilsen et al. 2009). However, a prior study found this self-selection to have little impact (Nilsen et al. 2013), and we considered these factors in our models, so we do not expect such under-representation to be a major source of bias in our study. Additionally, although phthalate metabolite and thyroid function biomarker concentrations were measured, covariate data were largely obtained via maternally completed questionnaires, which may have error, particularly for behaviors carrying a social stigma (such as prenatal smoking).
Perhaps not a weakness, but a challenge, is the significant impact of population-level shifts in exposures to phthalates that have occurred in recent years (Koch et al. 2017). DEHP exposures were generally highest in the early years of our study and have dropped over time. Irrespective of exposure, older children have had more opportunity to be identified as having ADHD; this creates the potential for confounding by year (which accounts for both age of the child and exposure period). To address confounding by time, we adjusted for year of birth in our analyses. We also constructed alternative models in which we adjusted for month and year of urine collection and found no differences when comparing them with models adjusted for birth year (see Table S5). However, it is possible that there remains some residual confounding by year that we cannot address within the present design, and accounting for this bias would attenuate our associations. It is also possible that including year in our models results in over-adjustment for population shifts in exposure. Another limitation of this study is that we did not consider the extent to which genetic features may confound or modify these associations or the extent to which other environmental toxicant exposures may confound these estimates. Future studies that examine populations enrolled over a narrower time range, that include multiple urine samples collected during pregnancy to more validly estimate pregnancy-wide phthalate exposures, and that include information on genetic risk factors for ADHD and other toxicant coexposures are required to address these concerns.
Our study also has several strengths. To our knowledge, this is the only study of prenatal exposure to phthalates and risk of ADHD that had a clinically defined end point. Our study was nested in a well-characterized prospective birth cohort with extensive questionnaire data that enabled us to obtain a wide range of relevant covariate information. Linkage with the NPR enabled us to focus on cases that were by definition severe enough to result in clinical identification. Although we believe this is a notable strength of our study, it is also true that we cannot generalize our results to ADHD cases that are not clinically recognized and that clinical recognition itself may suggest that our cases are on the more severe end of the disease spectrum or have more comorbidities that require specialist management. Our study also produced estimates for phthalate associations that were adjusted for the presence of other phthalates in the mixture using a Bayesian approach. Although accounting for the phthalate mixture is a strength, it is possible that alternative statistical methods for analyzing exposure mixtures may produce slightly different estimates. Finally, we examined the potential for mediation of the association between DEHP (the only phthalate with a significant main effect) and ADHD by thyroid hormones and measures of thyroid function, some of which have been associated with ADHD in offspring in previous studies (Ghassabian et al. 2011, 2012; Modesto et al. 2015; Päkkilä et al. 2014). A lack of evidence for mediation of the DEHP association suggests the possibility that this phthalate may be affecting ADHD through another mechanism, although we cannot exclude the possibility that other phthalates may be operating through a thyroid mechanism or that our ability to fully interrogate this pathway may have been negatively influenced by the fact that phthalate metabolites and thyroid function were measured only once during pregnancy. We also considered the potential for mediation by preterm birth, given prior evidence that elevated prenatal DEHP was associated with increased risk of preterm birth (Ferguson et al. 2017), and preterm birth was associated with subsequent risk of ADHD (Murray et al. 2016; Sucksdorff et al. 2015); again, we found no evidence of mediation through this pathway. Although we did evaluate interactions between DEHP and the potential mediators (thyroid function and preterm birth), the validity of our mediation analyses depends on strong assumptions that cannot be verified with certainty, including the absence of uncontrolled confounding of exposure–mediator and mediator–outcome relationships (Valeri and Vanderweele 2013).
Indoor exposure to phthalates is a significant concern, in part because phthalates are not chemically bound and therefore may be released into air or other media over time (Mitro et al. 2016). Some limited regulations pertaining to consumer products in the United States and in the European Union (EU)/European Economic Area (EEA) (which includes Norway) exist, specifically focused on prohibiting DEHP, as well as BBzP, in children’s toys and child care articles at levels , and in the EU/EEA, additionally prohibiting these phthalates and DnBP in both child care articles and cosmetics. The EU/EEA has additional regulations pertaining to the use of DiNP and other phthalates in children’s products, with more regulations covering other phthalates and consumer products set to take effect in the next several years. However, the 2014 Chronic Hazard Advisory Panel on Phthalates and Phthalate Replacements concluded that food, beverages, and drugs, not toys and personal care products, comprised the greatest sources of phthalates to all subpopulations worldwide (CPSC 2014). Although the European Food Safety Authority (EFSA) has set tolerable intakes for DEHP, DnBP, and BBzP (EFSA 2005a, 2005b, 2005c, 2005d, 2005e), dietary exposure to phthalates in the Norwegian adult population is comparable to that in other countries around the world and comes from similar food sources (Sakhi et al. 2014). Therefore, population exposures will continue without more stringent regulations that specifically address food and pharmaceutical sources.
Conclusion
In summary, in this prospective, population-based case–control study, prenatal DEHP exposure was associated with increased risk of ADHD. Population usage of DEHP is on the decline worldwide as a result of health concerns and some regulatory actions; however, it remains a measurable and prevalent exposure in contemporary populations (Koch et al. 2017). Given the accumulating evidence of developmental impacts, both to the male reproductive system and potentially to the developing nervous system, more health-protective regulations may be in order.
Supplemental Material
Acknowledgment
We are grateful to all the participating families in Norway who take part in this ongoing cohort study.
This research was funded in part by National Institutes of Health/National Institute of Environmental Health Sciences (NIH/NIEHS) R01ES021777 and by the Intramural Research Program of the NIH/NIEHS. The Norwegian Mother and Child (MoBa) Cohort Study is supported by the Norwegian Ministry of Health and Care Services and the Ministry of Education and Research, NIH/NIEHS (no. N01-ES-75558), NIH/National Institute of Neurological Disorders and Stroke (NINDS) (no. 1 UO1 NS 047537-01 and no. 2 UO1 NS 047537-06A1). The Preschool ADHD study, a substudy to MoBa, is supported by funds and grants from the Norwegian Ministry of Health, The Norwegian Health Directorate, The South Eastern Health Region, the G&PJ Sorensen Fund for Scientific Research, and from The Norwegian Resource Centre for ADHD, Tourette’s Syndrome and Narcolepsy.
References
- Adibi JJ, Whyatt RM, Williams PL, Calafat AM, Camann D, Herrick R. 2008. Characterization of phthalate exposure among pregnant women assessed by repeat air and urine samples. Environ Health Perspect 116(4):467–473, PMID: 18414628, 10.1289/ehp.10749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, Laurberg P, Wu CS, Olsen J. 2014. Attention deficit hyperactivity disorder and autism spectrum disorder in children born to mothers with thyroid dysfunction: a Danish nationwide cohort study. BJOG 121(11):1365–1374, PMID: 24605987, 10.1111/1471-0528.12681. [DOI] [PubMed] [Google Scholar]
- Baird DD, Saldana TM, Nepomnaschy PA, Hoppin JA, Longnecker MP, Weinberg CR. 2010. Within-person variability in urinary phthalate metabolite concentrations: measurements from specimens after long-term frozen storage. J Expo Sci Environ Epidemiol 20(2):169–175, PMID: 19277068, 10.1038/jes.2009.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett D, Bellinger DC, Birnbaum LS, Bradman A, Chen A, Cory-Slechta DA, et al. 2016. Project TENDR: Targeting Environmental Neuro-Developmental Risks The TENDR Consensus Statement. Environ Health Perspect 124(7):A118–A122, PMID: 27479987, 10.1289/EHP358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kalkbrenner AE, Just AC, Yolton K, Calafat AM, Sjödin A, et al. 2014. Gestational exposure to endocrine-disrupting chemicals and reciprocal social, repetitive, and stereotypic behaviors in 4- and 5-year-old children: the HOME study. Environ Health Perspect 122(5):513–520, PMID: 24622245, 10.1289/ehp.1307261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Smith KW, Williams PL, Calafat AM, Berry K, Ehrlich S, et al. 2012. Variability of urinary phthalate metabolite and bisphenol A concentrations before and during pregnancy. Environ Health Perspect 120(5):739–745, PMID: 22262702, 10.1289/ehp.1104139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Valentin-Blasini L, Ye X. 2015. Trends in exposure to chemicals in personal care and consumer products. Curr Environ Health Rep 2(4):348–355, PMID: 26342608, 10.1007/s40572-015-0065-9. [DOI] [PubMed] [Google Scholar]
- Chen T, Yang W, Li Y, Chen X, Xu S. 2011. Mono-(2-ethylhexyl) phthalate impairs neurodevelopment: inhibition of proliferation and promotion of differentiation in PC12 cells. Toxicol Lett 201(1):34–41, PMID: 21145954, 10.1016/j.toxlet.2010.12.002. [DOI] [PubMed] [Google Scholar]
- CPSC (Consumer Product Safety Commission). 2014. “Chronic Hazard Advisory Panel on Phthalates and Phthalate Alternatives Final Report.” Bethesda MD:US Consumer Product Safety Commission; https://www.cpsc.gov/s3fs-public/CHAP-REPORT-With-Appendices.pdf [accessed 27 Februaury 2018]. [Google Scholar]
- EFSA (European Food Safety Authority). 2005a. Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request from the Commission related to di-butylphthalate (DBP) for use in food contact materials. EFSA J 3(9):242, 10.2903/j.efsa.2005.242. [DOI] [Google Scholar]
- EFSA. 2005b. Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request from the Commission related to bis(2-ethylhexyl)phthalate (DEHP) for use in food contact materials. EFSA J 3(9):243, 10.2903/j.efsa.2005.243. [DOI] [Google Scholar]
- EFSA. 2005c. Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request from the Commission related to butylbenzylphthalate (BBP) for use in food contact materials. EFSA J 3(9):241, 10.2903/j.efsa.2005.241. [DOI] [Google Scholar]
- EFSA. 2005d. Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request from the Commission related to di-isononylphthalate (DINP) for use in food contact materials. EFSA J 3(9):244, 10.2903/j.efsa.2005.244. [DOI] [Google Scholar]
- EFSA. 2005e. Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request from the Commission related to di-isodecylphthalate (DIDP) for use in food contact materials. EFSA J 3(9):245, 10.2903/j.efsa.2005.245. [DOI] [Google Scholar]
- Engel SM, Miodovnik A, Canfield RL, Zhu C, Silva MJ, Calafat AM, et al. 2010. Prenatal phthalate exposure is associated with childhood behavior and executive functioning. Environ Health Perspect 118(4):565–571, PMID: 20106747, 10.1289/ehp.0901470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Factor-Litvak P, Insel B, Calafat AM, Liu X, Perera F, Rauh VA, et al. 2014. Persistent associations between maternal prenatal exposure to phthalates on child IQ at age 7 years. PLoS One 9(12):e114003, PMID: 25493564, 10.1371/journal.pone.0114003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, Chen YH, VanderWeele TJ, McElrath TF, Meeker JD, Mukherjee B. 2017. Mediation of the relationship between maternal phthalate exposure and preterm birth by oxidative stress with repeated measurements across pregnancy. Environ Health Perspect 125(3):488–494, PMID: 27352406, 10.1289/EHP282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, McElrath TF, Ko YA, Mukherjee B, Meeker JD. 2014a. Variability in urinary phthalate metabolite levels across pregnancy and sensitive windows of exposure for the risk of preterm birth. Environ Int 70:118–124, PMID: 24934852, 10.1016/j.envint.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, McElrath TF, Meeker JD. 2014b. Environmental phthalate exposure and preterm birth. JAMA Pediatr 168(1):61–67, PMID: 24247736, 10.1001/jamapediatrics.2013.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Wu W, Xu Y, Jin Z, Bao H, Zhu P, et al. 2017. Effects of prenatal phthalate exposure on thyroid hormone concentrations beginning at the embryonic stage. Sci Rep 7(1):13106, PMID: 29026179, 10.1038/s41598-017-13672-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascon M, Valvi D, Forns J, Casas M, Martínez D, Júlvez J, et al. 2015. Prenatal exposure to phthalates and neuropsychological development during childhood. Int J Hyg Environ Health 218(6):550–558, PMID: 26095249, 10.1016/j.ijheh.2015.05.006. [DOI] [PubMed] [Google Scholar]
- Ghassabian A, Bongers-Schokking JJ, de Rijke YB, van Mil N, Jaddoe VW, de Muinck Keizer-Schrama SM, et al. 2012. Maternal thyroid autoimmunity during pregnancy and the risk of attention deficit/hyperactivity problems in children: the Generation R Study. Thyroid 22(2):178–186, PMID: 22175242, 10.1089/thy.2011.0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghassabian A, Bongers-Schokking JJ, Henrichs J, Jaddoe VW, Visser TJ, Visser W, et al. 2011. Maternal thyroid function during pregnancy and behavioral problems in the offspring: the generation R study. Pediatr Res 69(5 Pt 1):454–459, PMID: 21471776, 10.1203/PDR.0b013e3182125b0c. [DOI] [PubMed] [Google Scholar]
- Hauser R, Meeker JD, Park S, Silva MJ, Calafat AM. 2004. Temporal variability of urinary phthalate metabolite levels in men of reproductive age. Environ Health Perspect 112(17):1734–1740, PMID: 15579421, 10.1289/ehp.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Díaz S, Su YC, Mitchell AA, Kelley KE, Calafat AM, Hauser R. 2013. Medications as a potential source of exposure to phthalates among women of childbearing age. Reprod Toxicol 37:1–5, PMID: 23333816, 10.1016/j.reprotox.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang PC, Kuo PL, Guo YL, Liao PC, Lee CC. 2007. Associations between urinary phthalate monoesters and thyroid hormones in pregnant women. Hum Reprod 22(10):2715–2722, PMID: 17704099, 10.1093/humrep/dem205. [DOI] [PubMed] [Google Scholar]
- Huang HB, Pan WH, Chang JW, Chiang HC, Guo YL, Jaakkola JJ, et al. 2017. Does exposure to phthalates influence thyroid function and growth hormone homeostasis? The Taiwan Environmental Survey for Toxicants (TEST) 2013. Environ Res 153:63–72, PMID: 27907809, 10.1016/j.envres.2016.11.014. [DOI] [PubMed] [Google Scholar]
- Huang PC, Tsai CH, Liang WY, Li SS, Huang HB, Kuo PL. 2016. Early phthalates exposure in pregnant women is associated with alteration of thyroid hormones. PLoS One 11(7):e0159398, PMID: 27455052, 10.1371/journal.pone.0159398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Instanes JT, Halmoy A, Engeland A, Haavik J, Furu K, Klungsoyr K. 2015. Attention-deficit/hyperactivity disorder in offspring of mothers with inflammatory and immune system diseases. Biol Psychiatry 81(5):452–459, PMID: 26809250, 10.1016/j.biopsych.2015.11.024. [DOI] [PubMed] [Google Scholar]
- Jensen MS, Anand-Ivell R, Nørgaard-Pedersen B, Jönsson BA, Bonde JP, Hougaard DM, et al. 2015. Amniotic fluid phthalate levels and male fetal gonad function. Epidemiology 26(1):91–99, PMID: 25384265, 10.1097/EDE.0000000000000198. [DOI] [PubMed] [Google Scholar]
- Jensen MS, Nørgaard-Pedersen B, Toft G, Hougaard DM, Bonde JP, Cohen A, et al. 2012. Phthalates and perfluorooctanesulfonic acid in human amniotic fluid: temporal trends and timing of amniocentesis in pregnancy. Environ Health Perspect 120(6):897–903, PMID: 22398305, 10.1289/ehp.1104522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns LE, Ferguson KK, McElrath TF, Mukherjee B, Meeker JD. 2016. Associations between repeated measures of maternal urinary phthalate metabolites and thyroid hormone parameters during pregnancy. Environ Health Perspect 124(11):1808–1815, PMID: 27152641, 10.1289/EHP170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns LE, Ferguson KK, Soldin OP, Cantonwine DE, Rivera-González LO, Del Toro LV, et al. 2015. Urinary phthalate metabolites in relation to maternal serum thyroid and sex hormone levels during pregnancy: a longitudinal analysis. Reprod Biol Endocrinol 13:4, PMID: 25596636, 10.1186/1477-7827-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavlock R, Barr D, Boekelheide K, Breslin W, Breysse P, Chapin R, et al. 2006. NTP-CERHR expert panel update on the reproductive and developmental toxicity of di(2-ethylhexyl) phthalate. Reprod Toxicol 22(3):291–399, PMID: 17068859, 10.1016/j.reprotox.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Kelley KE, Hernández-Díaz S, Chaplin EL, Hauser R, Mitchell AA. 2012. Identification of phthalates in medications and dietary supplement formulations in the United States and Canada. Environ Health Perspect 120(3):379–384, PMID: 22169271, 10.1289/ehp.1103998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobrosly RW, Evans S, Miodovnik A, Barrett ES, Thurston SW, Calafat AM, et al. 2014. Prenatal phthalate exposures and neurobehavioral development scores in boys and girls at 6-10 years of age. Environ Health Perspect 122(5):521–528, PMID: 24577876, 10.1289/ehp.1307063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch HM, Bolt HM, Preuss R, Angerer J. 2005. New metabolites of di(2-ethylhexyl)phthalate (DEHP) in human urine and serum after single oral doses of deuterium-labelled DEHP. Arch Toxicol 79(7):367–376, PMID: 15700144, 10.1007/s00204-004-0642-4. [DOI] [PubMed] [Google Scholar]
- Koch HM, Rüther M, Schütze A, Conrad A, Pälmke C, Apel P, et al. 2017. Phthalate metabolites in 24-h urine samples of the German Environmental Specimen Bank (ESB) from 1988 to 2015 and a comparison with US NHANES data from 1999 to 2012. Int J Hyg Environ Health 220(2 Pt A):130–141, PMID: 27863804, 10.1016/j.ijheh.2016.11.003. [DOI] [PubMed] [Google Scholar]
- Komada M, Gendai Y, Kagawa N, Nagao T. 2016. Prenatal exposure to di(2-ethylhexyl) phthalate impairs development of the mouse neocortex. Toxicol Lett 259:69–79, PMID: 27472966, 10.1016/j.toxlet.2016.07.019. [DOI] [PubMed] [Google Scholar]
- Kuo FC, Su SW, Wu CF, Huang MC, Shiea J, Chen BH, et al. 2015. Relationship of urinary phthalate metabolites with serum thyroid hormones in pregnant women and their newborns: a prospective birth cohort in Taiwan. PLoS One 10(6):e0123884, PMID: 26042594, 10.1371/journal.pone.0123884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson M, Weiss B, Janson S, Sundell J, Bornehag CG. 2009. Associations between indoor environmental factors and parental-reported autistic spectrum disorders in children 6-8 years of age. Neurotoxicology 30(5):822–831, PMID: 19822263, 10.1016/j.neuro.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KI, Chiang CW, Lin HC, Zhao JF, Li CT, Shyue SK, et al. 2016. Maternal exposure to di-(2-ethylhexyl) phthalate exposure deregulates blood pressure, adiposity, cholesterol metabolism and social interaction in mouse offspring. Arch Toxicol 90(5):1211–1224, PMID: 25995009, 10.1007/s00204-015-1539-0. [DOI] [PubMed] [Google Scholar]
- Lien YJ, Ku HY, Su PH, Chen SJ, Chen HY, Liao PC, et al. 2015. Prenatal exposure to phthalate esters and behavioral syndromes in children at 8 years of age: Taiwan Maternal and Infant Cohort Study. Environ Health Perspect 123(1):95–100, PMID: 25280125, 10.1289/ehp.1307154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Yuan K, Li L, Liu S, Li S, Hu G, et al. 2015. In utero exposure to diethylhexyl phthalate affects rat brain development: a behavioral and genomic approach. Int J Environ Res Public Health 12(11):13696–13710, PMID: 26516888, 10.3390/ijerph121113696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Schumacher M, Scherer A, Sanoudou D, Megherbi D, Davison T, et al. 2010. A comparison of batch effect removal methods for enhancement of prediction performance using MAQC-II microarray gene expression data. Pharmacogenomics J 10(4):278–291, PMID: 20676067, 10.1038/tpj.2010.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLehose RF, Dunson DB, Herring AH, Hoppin JA. 2007. Bayesian methods for highly correlated exposure data. Epidemiology 18(2):199–207, PMID: 17272963, 10.1097/01.ede.0000256320.30737.c0. [DOI] [PubMed] [Google Scholar]
- Magnus P, Birke C, Vejrup K, Haugan A, Alsaker E, Daltveit AK, et al. 2016. Cohort Profile Update: The Norwegian Mother and Child Cohort Study (MoBa). Int J Epidemiol 45(2):382–388, PMID: 27063603, 10.1093/ije/dyw029. [DOI] [PubMed] [Google Scholar]
- Magnus P, Irgens LM, Haug K, Nystad W, Skjaerven R, Stoltenberg C, et al. 2006. Cohort profile: the Norwegian Mother and Child Cohort Study (MoBa). Int J Epidemiol 35(5):1146–1150, PMID: 16926217, 10.1093/ije/dyl170. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Calafat AM, Hauser R. 2007. Di(2-ethylhexyl) phthalate metabolites may alter thyroid hormone levels in men. Environ Health Perspect 115(7):1029–1034, PMID: 17637918, 10.1289/ehp.9852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Ferguson KK. 2011. Relationship between urinary phthalate and bisphenol A concentrations and serum thyroid measures in U.S. adults and adolescents from the National Health and Nutrition Examination Survey (NHANES) 2007-2008. Environ Health Perspect 119(10):1396–1402, PMID: 21749963, 10.1289/ehp.1103582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles-Richarson S, Bosch S. 2002. Toxicological Profile for Di(2-ethylhexyl) phthalate. Atlanta, Georgia:Agency for Toxic Substances and Disease Registry. U.S. Department of Health and Human Services. [Google Scholar]
- Miodovnik A, Edwards A, Bellinger DC, Hauser R. 2014. Developmental neurotoxicity of ortho-phthalate diesters: review of human and experimental evidence. Neurotoxicology 41:112–122, PMID: 24486776, 10.1016/j.neuro.2014.01.007. [DOI] [PubMed] [Google Scholar]
- Miodovnik A, Engel SM, Zhu C, Ye X, Soorya LV, Silva MJ, et al. 2011. Endocrine disruptors and childhood social impairment. Neurotoxicology 32(2):261–267, PMID: 21182865, 10.1016/j.neuro.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitro SD, Dodson RE, Singla V, Adamkiewicz G, Elmi AF, Tilly MK, et al. 2016. Consumer product chemicals in indoor dust: a quantitative meta-analysis of U.S. studies. Environ Sci Technol 50(19):10661–10672, PMID: 27623734, 10.1021/acs.est.6b02023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modesto T, Tiemeier H, Peeters RP, Jaddoe VW, Hofman A, Verhulst FC, et al. 2015. Maternal mild thyroid hormone insufficiency in early pregnancy and attention-deficit/hyperactivity disorder symptoms in children. JAMA Pediatr 169(9):838–845, PMID: 26146876, 10.1001/jamapediatrics.2015.0498. [DOI] [PubMed] [Google Scholar]
- Moog NK, Entringer S, Heim C, Wadhwa PD, Kathmann N, Buss C. 2017. Influence of maternal thyroid hormones during gestation on fetal brain development. Neuroscience 342:68–100, PMID: 26434624, 10.1016/j.neuroscience.2015.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morreale de Escobar G, Obregon MJ, Escobar del Rey F. 2004. Role of thyroid hormone during early brain development. Eur J Endocrinol 151(suppl3):U25–U37, PMID: 15554884, 10.1530/eje.0.151U025. [DOI] [PubMed] [Google Scholar]
- Murray E, Pearson R, Fernandes M, Santos IS, Barros FC, Victora CG, et al. 2016. Are fetal growth impairment and preterm birth causally related to child attention problems and ADHD? Evidence from a comparison between high-income and middle-income cohorts. J Epidemiol Community Health 70(7):704–709, PMID: 26767410, 10.1136/jech-2015-206222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen RM, Surén P, Gunnes N, Alsaker ER, Bresnahan M, Hirtz D, et al. 2013. Analysis of self-selection bias in a population-based cohort study of autism spectrum disorders. Paediatr Perinat Epidemiol 27(6):553–563, PMID: 23919580, 10.1111/ppe.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen RM, Vollset SE, Gjessing HK, Skjaerven R, Melve KK, Schreuder P, et al. 2009. Self-selection and bias in a large prospective pregnancy cohort in Norway. Paediatr Perinat Epidemiol 23(6):597–608, PMID: 19840297, 10.1111/j.1365-3016.2009.01062.x. [DOI] [PubMed] [Google Scholar]
- NRC (National Research Council). 2008. Phthalates and Cumulative Risk Assessment: The Task Ahead., Committee on the Health Risks of Phthalates. Washington, DC:National Academies Press. [PubMed] [Google Scholar]
- Päkkilä F, Männistö T, Pouta A, Hartikainen AL, Ruokonen A, Surcel HM, et al. 2014. The impact of gestational thyroid hormone concentrations on ADHD symptoms of the child. J Clin Endocrinol Metab 99(1):E1–E8, PMID: 24384024, 10.1210/jc.2013-2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C, Choi W, Hwang M, Lee Y, Kim S, Yu S, et al. 2017. Associations between urinary phthalate metabolites and bisphenol A levels, and serum thyroid hormones among the Korean adult population - Korean National Environmental Health Survey (KoNEHS) 2012-2014. Sci Total Environ 584–585:950–957, PMID: 28153396, 10.1016/j.scitotenv.2017.01.144. [DOI] [PubMed] [Google Scholar]
- Philippat C, Bennett DH, Krakowiak P, Rose M, Hwang HM, Hertz-Picciotto I. 2015. Phthalate concentrations in house dust in relation to autism spectrum disorder and developmental delay in the CHildhood Autism Risks from Genetics and the Environment (CHARGE) study. Environ Health 14:56, PMID: 26108271, 10.1186/s12940-015-0024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinnies KM, Harris EP, Snyder RW, Sumner SS, Rissman EF. 2017. Direct and transgenerational effects of low doses of perinatal di-(2-ethylhexyl) phthalate (DEHP) on social behaviors in mice. PLoS One 12(2):e0171977, PMID: 28199414, 10.1371/journal.pone.0171977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer-Baumgartner N, Zeiner P, Egeland J, Gustavson K, Skogan AH, Reichborn-Kjennerud T, et al. 2014. Does IQ influence associations between ADHD symptoms and other cognitive functions in young preschoolers?. Behav Brain Funct 10:16, PMID: 24884579, 10.1186/1744-9081-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rønningen KS, Paltiel L, Meltzer HM, Nordhagen R, Lie KK, Hovengen R, et al. 2006. The biobank of the Norwegian Mother and Child Cohort Study: a resource for the next 100 years. Eur J Epidemiol 21(8):619–625, PMID: 17031521, 10.1007/s10654-006-9041-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabaredzovic A, Sakhi AK, Brantsæter AL, Thomsen C. 2015. Determination of 12 urinary phthalate metabolites in Norwegian pregnant women by core-shell high performance liquid chromatography with on-line solid-phase extraction, column switching and tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 1002:343–352, PMID: 26355271, 10.1016/j.jchromb.2015.08.040. [DOI] [PubMed] [Google Scholar]
- Sakhi AK, Lillegaard IT, Voorspoels S, Carlsen MH, Løken EB, Brantsæter AL, et al. 2014. Concentrations of phthalates and bisphenol A in Norwegian foods and beverages and estimated dietary exposure in adults. Environ Int 73:259–269, PMID: 25173060, 10.1016/j.envint.2014.08.005. [DOI] [PubMed] [Google Scholar]
- Sakhi AK, Sabaredzovic A, Cequier E, Thomsen C. 2017. Phthalate metabolites in Norwegian mothers and children: Levels, diurnal variation and use of personal care products. Sci Total Environ 599-600:1984–1992, PMID: 28558421, 10.1016/j.scitotenv.2017.05.109. [DOI] [PubMed] [Google Scholar]
- Schettler T. 2006. Human exposure to phthalates via consumer products. Int J Androl 29(1):134–139, PMID: 16466533, 10.1111/j.1365-2605.2005.00567.x. [DOI] [PubMed] [Google Scholar]
- Serrano SE, Braun J, Trasande L, Dills R, Sathyanarayana S. 2014. Phthalates and diet: a review of the food monitoring and epidemiology data. Environ Health 13:43, PMID: 24894065, 10.1186/1476-069X-13-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelby MD. 2006. NTP-CERHR monograph on the potential human reproductive and developmental effects of di (2-ethylhexyl) phthalate (DEHP). NTP CERHR MON (18):v, vii-7, II-iii-xiii passim. https://ntp.niehs.nih.gov/ntp/ohat/phthalates/dehp/dehp-monograph.pdf [accessed 27 February 2018], PMID: 19407857. [PubMed] [Google Scholar]
- Singh AR, Lawrence WH, Autian J. 1975. Maternal-fetal transfer of 14C-di-2-ethylhexyl phthalate and 14C-diethyl phthalate in rats. J Pharm Sci 64(8):1347–1350, PMID: 1151708, 10.1002/jps.2600640819. [DOI] [PubMed] [Google Scholar]
- Skogan AH, Zeiner P, Egeland J, Rohrer-Baumgartner N, Urnes AG, Reichborn-Kjennerud T, et al. 2014. Inhibition and working memory in young preschool children with symptoms of ADHD and/or oppositional-defiant disorder. Child Neuropsychol 20(5):607–624, PMID: 24053105, 10.1080/09297049.2013.838213. [DOI] [PubMed] [Google Scholar]
- Sucksdorff M, Lehtonen L, Chudal R, Suominen A, Joelsson P, Gissler M, et al. 2015. Preterm Birth and Poor Fetal Growth as Risk Factors of Attention-Deficit/Hyperactivity Disorder. Pediatrics 136(3):e599–e608, PMID: 26304830, 10.1542/peds.2015-1043. [DOI] [PubMed] [Google Scholar]
- Surén P, Bakken IJ, Aase H, Chin R, Gunnes N, Lie KK, et al. 2012. Autism spectrum disorder, ADHD, epilepsy, and cerebral palsy in Norwegian children. Pediatrics 130(1):e152–e158, PMID: 22711729, 10.1542/peds.2011-3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum SL, Britton JA, Calafat AM, Ye X, Silva MJ, Reidy JA, et al. 2008. Temporal variability in urinary concentrations of phthalate metabolites, phytoestrogens and phenols among minority children in the United States. Environ Res 106(2):257–269, PMID: 17976571, 10.1016/j.envres.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Thapar A, Cooper M. 2016. Attention deficit hyperactivity disorder. Lancet 387(10024):1240–1250, PMID: 26386541, 10.1016/S0140-6736(15)00238-X. [DOI] [PubMed] [Google Scholar]
- Townsend MK, Franke AA, Li X, Hu FB, Eliassen AH. 2013. Within-person reproducibility of urinary bisphenol A and phthalate metabolites over a 1 to 3 year period among women in the Nurses’ Health Studies: a prospective cohort study. Environ Health 12:80, PMID: 24034517, 10.1186/1476-069X-12-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valeri L, Vanderweele TJ. 2013. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods 18(2):137–150, PMID: 23379553, 10.1037/a0031034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Buuren S, Groothuis-Oudshoorn K. 2011. mice: Multivariate-Imputation by Chained Equations in R. J Stat Soft 45(3):1–67, 10.18637/jss.v045.i03. [DOI] [Google Scholar]
- Vanderweele TJ. 2015. Explanation in Causal Inference: Methods for Mediation and Interaction. New York, NY:Oxford University Press. [Google Scholar]
- Vanderweele TJ, Vansteelandt S. 2010. Odds ratios for mediation analysis for a dichotomous outcome. Am J Epidemiol 172(12):1339–1348, PMID: 21036955, 10.1093/aje/kwq332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanger GD, Learner E, Longnecker MP, Ask H, Aase H, Zoeller RT, et al. 2017. Effects of sample handling and analytical procedures on thyroid hormone concentrations in pregnant women’s plasma. Epidemiology 28(3):365–369, PMID: 27984425, 10.1097/EDE.0000000000000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (World Health Organization). 1993. The ICD-10 Classification of Mental and Behavioral Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva, Switzerand: World Health Organization; http://www.who.int/classifications/icd/en/bluebook.pdf [accessed 27 February 2018]. [Google Scholar]
- Whyatt RM, Liu X, Rauh VA, Calafat AM, Just AC, Hoepner L, et al. 2012. Maternal prenatal urinary phthalate metabolite concentrations and child mental, psychomotor, and behavioral development at 3 years of age. Environ Health Perspect 120(2):290–295, PMID: 21893441, 10.1289/ehp.1103705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Agrawal S, Cook TJ, Knipp GT. 2007. Di-(2-ethylhexyl)-phthalate affects lipid profiling in fetal rat brain upon maternal exposure. Arch Toxicol 81(1):57–62, PMID: 16951938, 10.1007/s00204-006-0143-8. [DOI] [PubMed] [Google Scholar]
- Xu X, Yang Y, Wang R, Wang Y, Ruan Q, Lu Y. 2015. Perinatal exposure to di-(2-ethylhexyl) phthalate affects anxiety- and depression-like behaviors in mice. Chemosphere 124:22–31, PMID: 25441928, 10.1016/j.chemosphere.2014.10.056. [DOI] [PubMed] [Google Scholar]
- Yao HY, Han Y, Gao H, Huang K, Ge X, Xu YY, et al. 2016. Maternal phthalate exposure during the first trimester and serum thyroid hormones in pregnant women and their newborns. Chemosphere 157:42–48, PMID: 27208644, 10.1016/j.chemosphere.2016.05.023. [DOI] [PubMed] [Google Scholar]
- Ye X, Pierik FH, Angerer J, Meltzer HM, Jaddoe VW, Tiemeier H, et al. 2009. Levels of metabolites of organophosphate pesticides, phthalates, and bisphenol A in pooled urine specimens from pregnant women participating in the Norwegian Mother and Child Cohort Study (MoBa). Int J Hyg Environ Health 212(5):481–491, PMID: 19394271, 10.1016/j.ijheh.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.