 The C-4 hydroxyl group of sialic acid is not important for its binding with hemagglutinin and could be replaced with hydrophobic moieties.
The C-4 hydroxyl group of sialic acid is not important for its binding with hemagglutinin and could be replaced with hydrophobic moieties.
Abstract
Sialic acid derivatives, analogs, and their conjugates are important pharmacophores. Modification of the C-4 hydroxyl group of sialic acid can lead to derivatives, such as zanamivir, with potent anti-influenza activities. Herein, we described the synthesis of C-4-modified sialic acid derivatives via conjugation with naturally derived pentacyclic triterpenes, which are active ingredients of traditional Chinese medicine, and the evaluation of their in vitro anti-influenza virus activity in MDCK cells. Interestingly, a set of configurational isomers was obtained during the de-O-acetylation reaction of two pentacyclic triterpene–sialic acid conjugates under Zemplén conditions, and a mechanism was proposed. Owing to the attachment of the Neu5Ac2en moiety, all synthesized conjugates displayed lower hydrophobicity than their parent compounds. In comparison with ursane- and lupane-type triterpenes, oleanane-type triterpene-functionalized Neu5Ac2en conjugates were most promising. The insertion of a (1,2,3-triazol-4-yl)-methyl between the amide bond and Neu5Ac2en caused a substantial decrease in activity. Compound 15a exhibited the highest inhibitory activity (IC50 = 8.3 μM) and selectivity index (SI = 22.7). Further studies involving hemagglutination inhibition and neuraminidase inhibition suggested that compound 15a inhibited virus-induced hemagglutination with no effect on the enzymatic activity of neuraminidase, indicating that the antiviral activity appeared to be mediated via interaction with hemagglutinin at the initial stage of viral infection.
1. Introduction
The influenza A virus, which causes a substantial number of deaths during annual epidemics and occasional pandemics, belongs to the family of single-stranded negative-sense RNA viruses or Orthomyxoviridae.1 The most well-known influenza pandemics in the 20th century, namely, Spanish Flu (1918/H1N1), Asian Flu (1957/H2N2) and Hong Kong Flu (1968/H3N2), resulted in the deaths of more than 50 million people globally.2 Currently, influenza viruses still infect 3 to 5 million people worldwide every year.3 Although anti-influenza vaccines are available, their efficacy is limited owing to rapid mutations in the two surface antigens (hemagglutinin (HA) and neuraminidase (NA)), which leave the immune system unable to cope with the essentially new antigens.4 Currently, only two classes of anti-influenza drugs, namely, M2 ion-channel inhibitors (amantadine and rimantadine) and NA inhibitors (oseltamivir, zanamivir and peramivir), have been approved by the FDA for the interruption of specific processes in influenza infections.5 However, frequent clinical applications of the marketed anti-influenza drugs, in combination with the high mutation rate of the RNA genome of the influenza virus, have led to the rapid emergence of drug-resistant strains of the influenza virus.6 Therefore, there is a continuous and urgent need to discover new drugs to overcome resistance and the looming threat of sporadic outbreaks of pandemic influenza A strains.7
The first step in influenza A virus infection involves attachment to cells via the binding of viral HA to cell surface receptors containing sialic acid (also called α-5-N-acetylneuraminic acid, Neu5Ac), which leads to internalization of viral particles into the endosome.8 One approach to combating cellular infection comprises the inhibition of the recognition process by using sialic acid analogues that compete with cell surface sialyl oligosaccharides for viral HA. Unfortunately, sialic acid and its derivatives, such as α-methyl sialoside, bind only weakly to HA with dissociation constants in the millimolar range.9 Since the crystal structure of influenza virus HA complexed with its receptor was first published by Weis et al. in 1988,10 there have been many efforts to search for sialic acid derivatives with high affinity for HA. Knowles et al. reported that the binding affinity of sialic acid can be increased by introducing hydrophobic aglycons at the C-2 position, with dissociation constants in the micromolar range.11 Rudrawar et al. also reported that the introduction of a hydrophobic group at the C-3 position of Neu5Ac could lock open the 150-loop of influenza A virus group 1 sialidase.12 Therefore, four kinds of hydrophobic pentacyclic triterpene, which are widely distributed in the plant kingdom and are generally believed to enhance the immunity of host plants and increase the resistance of plants to pathogens,13 were selected for conjugation with sialic acid via C-2 in our previous study.14 However, those derivatives only exhibited weak anti-influenza entry activity, with IC50 ranging from 41.2 to 95.2 μM, which was possibly because when the sialic acid groups bound to the HA polymer backbone, the linking groups were brought too close to the HA surface.
The structures of influenza HA complexed with several different 2-substituted sialic acid derivatives, such as Neu5Ac2N4 and Neu5Ac2N6, have been determined by X-ray crystallography.15 In all cases the 4-OH of sialic acid points away from the binding site into the solution and does not interact with the HA protein, which indicates that 4-modified sialosides would not interfere with binding to HA. For example, Wu et al.16 reported that a polymeric 4-N-linked sialoside strongly inhibited the activity of influenza virus HA with a Ki of 10–6 M. Moreover, the addition of certain functionalities at the C-4 position of an unsaturated sialic acid analog, such as 4-amino-Neu5Ac2en and 4-guanidino-Neu5Ac2en, should enhance its binding with influenza virus neuraminidase (NA).17 There have been many efforts to develop anti-influenza inhibitors based on Neu5Ac2en.18 However, reports of C-4 triazole-modified analogs of Neu5Ac2en are rare. In 2006, Li et al. reported the synthesis of C-4 triazole analogs of zanamivir and found that an analog derived from 3-hydroxypentyne displayed protective activity of 61% (50 μM) against infection by avian influenza virus.19 One year later, Lu et al. reported the synthesis of C-4 triazole analogs of Neu5Ac2en derivatives as multivalent sialic acid-containing scaffolds.20
Our recent works on the anti-influenza virus activities of pentacyclic triterpenes,14,21 together with published reports,15,17,19 led us to further investigate novel sialic acid derivatives in which pentacyclic triterpenes were attached at the 4-position of sialic acid. To ensure a stable linkage to the 4-hydroxy group under assay conditions, a triazolyl or amide bond was introduced as the linkage between the two moieties. Interestingly, a set of configurational isomers was obtained during the de-O-acetylation reaction of 8a and 8b under Zemplén conditions. In addition, a fivefold increase in anti-influenza activity was found for compound 15, with a better selectivity index (SI = 22.7), when the pentacyclic triterpene was conjugated to sialic acid via the C-4 position. Here, we describe the synthesis and in vitro anti-influenza virus activity of these novel pentacyclic triterpene–Neu5Ac2en conjugates.
2. Results and discussion
2.1. Chemistry
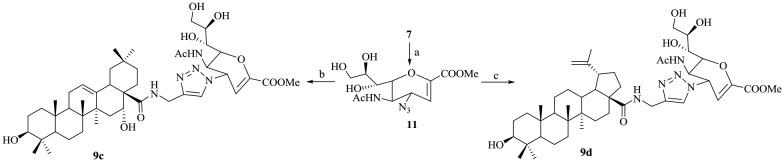
The target pentacyclic triterpene–Neu5Ac2en conjugates were synthesized as described in Schemes 1–5. The pentacyclic triterpenes used for conjugation with Neu5Ac2en included oleanolic acid (OA, 1a), ursolic acid (UA, 1b), echinocystic acid (EA, 1c) and betulinic acid (BA, 1d). Scheme 1 depicts the synthesis of conjugates 8a–d. Firstly, alkynyl-functionalized pentacyclic triterpene derivatives 3a–d were prepared according to published procedures.22 The key intermediate 4-azido-Neu5Ac2en 7 was synthesized from commercially available sialic acid 4 in 48% yield via a three-step process described by Chandler et al.23 The structures of compounds 6 and 7 were confirmed by 1H and 13C NMR (ESI‡). The subsequent conjugation of 3a–c with 7via a copper-catalyzed azide-alkyne 1,3-dipolar cycloaddition reaction (CuAAC) provided the 5,7,8,9-tetra-O-acetyl-protected intermediates 8a–c in good yield. In a similar way, the conjugation of 3d with 7 afforded intermediate 8d in 87% yield.
Scheme 1. Reagents and conditions: (a) TBTU, DIPEA, THF; (b) propargylamine, K2CO3, DMF; (c) H+-exchange resin, RT, MeOH; then Ac2O, pyridine, DMAP; (d) TMSOTf, EtOAc; (e) Me3SiN3, t-BuOH; (f) CuSO4, sodium l-ascorbate, DCM/H2O (1 : 1, v/v).
Scheme 5. Reagents and conditions: (a) PPh3, THF, H2O; (b) EDC, Na2CO3, DMF, 2a, 2b or 2c, 60 °C; (c) EDC, Na2CO3, DMF, 2d, 60 °C; (d) MeONa/MeOH.
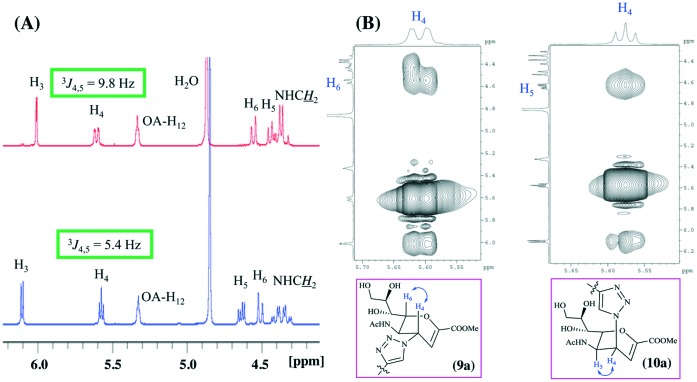
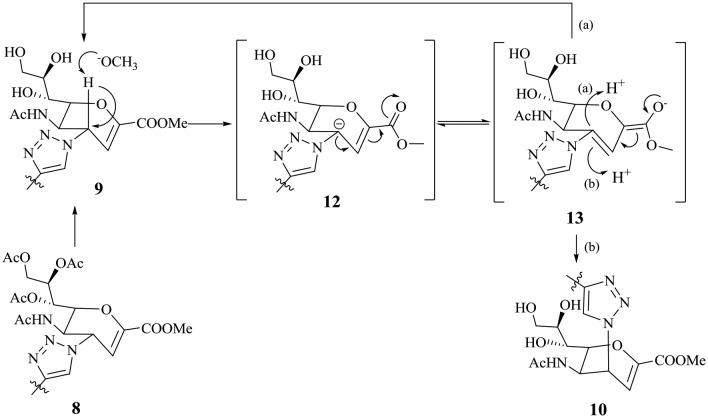
As an initial experiment, de-O-acetylation of 8a was carried out under Zemplén conditions.24 However, two compounds, namely, 9a and its isomer 10a, were separated by standard silica gel chromatography in 52% and 31% yield, respectively (Scheme 2). We observed that 9a was more polar than its isomer 10a (on the basis of its migratory ability on silica gel). The structures of the two isomers were fully ascertained by ESI-HRMS and NMR spectroscopy. Fig. 1(A) presents the characteristic parts (δ = 3.5–6.5 ppm) of the 1H NMR spectra (in CD3OD) of conjugates 9a and 10a. The chemical shifts of the H3 and H5 protons of 10a were significantly shifted downfield in comparison with those of its isomer 9a. In addition, the coupling constant between H4 and H5 of 9a was 9.8 Hz when these two protons were trans, whereas this coupling constant was close to 5.4 Hz when they were cis. To further demonstrate the configurations of the two isomers 9a and 10a, 2D NOE spectra were also acquired (Fig. 1(B)). The NOE experiment on compound 9a showed an NOE effect between the H4 proton and the H6 proton of the carbohydrate ring, whereas this effect was absent in its isomer 10a. In contrast, an obvious NOE effect in 10a between the H4 proton and the H5 proton of the carbohydrate ring was observed, whereas this effect was absent in its isomer 9a. Therefore, we concluded that the H4 protons of 9a and 10a are in the β-orientation and α-orientation, respectively. Similar results were observed for the de-O-acetylation of 8b under Zemplén conditions, and two isomers, namely, 9b and 10b, were also obtained. To the best of our knowledge, there are no other reports about this reaction. We considered whether the triazolyl group facilitated the formation of these isomers. Therefore, de-O-acetylation of 7 was carried out under the same conditions (Scheme 3). We found that the reaction finished quickly, as shown by TLC analysis, and yielded the azido compound 11 exclusively in 88% yield. Subsequent direct conjugation with the alkynyl-functionalized pentacyclic triterpene derivatives 3c and 3d in a similar manner to that described in Scheme 1. The conjugates 9c and 9d afforded in high yields.
Scheme 2. Reagents and conditions: (a) MeONa/MeOH.
Fig. 1. (A) Characteristic portions of the 400 MHz proton spectra of conjugates 9a (red) and 10a (blue). (B) Section of NOESY spectra of compounds 9a and 10a in CD3OD at 25 °C.
Scheme 3. Reagents and conditions: (a) MeONa/MeOH; (b) CuSO4, sodium l-ascorbate, 3c, DCM/H2O (1 : 1, v/v); (c) CuSO4, sodium l-ascorbate, 3d, DCM/H2O (1 : 1, v/v).
On the basis of these results, we propose a plausible mechanism for the formation of the two isomers (Scheme 4). The first step comprises the removal of the acetate protecting groups by sodium methoxide in methanol to afford the triol 9. Removal of the H4 hydrogen by methoxide forms an allylic carbanion, which is further stabilized by resonance with an extended enolate to form the intermediate 13. Racemization occurs when a proton attacks from the upper or lower side of the planar carbanion to generate 9 or 10, respectively.
Scheme 4. Proposed mechanism for the formation of the two isomers during the de-O-acetylation reaction under Zemplén conditions.
Alternatively, reduction of the azide group in 7 was carried out by treatment with Ph3P in THF/H2O (1 : 1) to yield the intermediate 14, which was condensed with intermediates 2a–d in the presence of EDC to yield 15a–d in yields of 62–75%. After de-O-acetylation under Zemplén conditions, 16a–d were afforded quantitatively (Scheme 5).
2.2. Calculated A log P
Lipophilicity governs the interaction of a given molecule with the intestinal membrane. A compound must possess hydrophobic properties for passive transport across intestinal epithelia. It is known that pentacyclic triterpenes are hydrophobic molecules with relatively high lipophilicity. Different substituents such as the sugar moiety at C3–OH and/or C28–COOH contribute to different degrees of lipophilicity. In this study, the A log P values of pentacyclic triterpene–Neu5Ac2en derivatives were calculated using Pipeline Pilot software version 7.5 (Accelrys Corp., San Diego, CA, USA), and the results are presented in Table 1. All the studied conjugates exhibited decreased hydrophobicity in comparison with their parent compounds, with A log P values within the range of 2.52–4.86. Benefiting from the hydrophilic Neu5Ac2en moiety, the A log P value decreased by about 2.8 for each series (for example, OA vs.9a and 16a), which means that their solubilities in water are about 630 times higher than those of their parent compounds.
Table 1. Calculated A log P values of pentacyclic triterpene–Neu5Ac2en conjugates a .
| Compound | A log P | Compound | A log P | Compound | A log P |
| OA | 6.447 | 9a | 3.619 | 15c | 3.673 |
| UA | 6.492 | 9b | 3.664 | 15d | 4.874 |
| EA | 5.345 | 9c | 2.517 | 16a | 3.638 |
| BA | 6.546 | 9d | 3.718 | 16b | 3.683 |
| 8a | 4.756 | 10a | 3.619 | 16c | 2.536 |
| 8b | 4.802 | 10b | 3.664 | 16d | 3.737 |
| 8c | 3.654 | 15a | 4.775 | ||
| 8d | 4.856 | 15b | 4.821 |
a A log P values (Ghose–Crippen octanol–water partition coefficient at 25 °C) were calculated using Pipeline Pilot software version 7.5 (Accelrys Corp., San Diego, CA, USA).
2.3. Cytotoxic activity
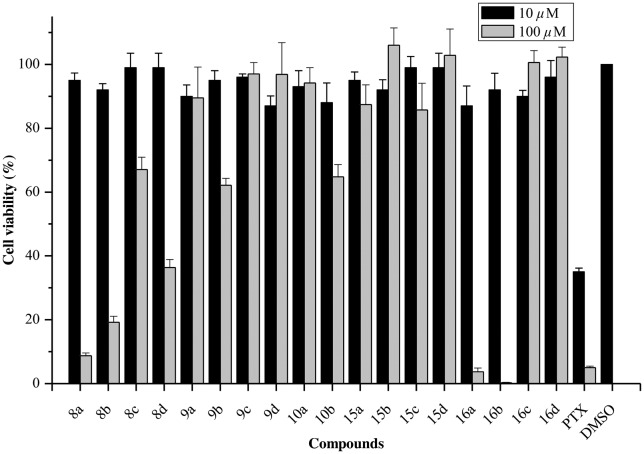
After the completion of synthesis, all synthesized conjugates were tested for their in vitro anti-influenza activity against A/WSN/33 (H1N1) virus in an MDCK cell line. To exclude the possibility that the observed anti-influenza virus activity was due to non-specific cytotoxic activity, preliminary screening of the synthesized conjugates 8a–d, 9a–d, 10a–b, 15a–d and 16a–d was performed to determine their cytotoxic activity in MDCK cells on the basis of a CellTiter-Glo assay.21 The commercial antitumor agent paclitaxel (PTX) was used as a positive control. The results of the cytotoxicity test at two different concentrations (10 μM and 100 μM) are shown in Fig. 2. No significant cytotoxicity was observed at concentrations of less than 10 μM. However, compounds 8a–b, 8d and 16a–b exhibited strong cytotoxicity, whereas compounds 8c, 9b and 10b displayed moderate cytotoxicity, at high concentrations (100 μM). With the exception of compound 15b, most of the UA–Neu5Ac2en conjugates exhibited strong (8b and 16b) to intermediate (9b and 10b) cytotoxicity at the same concentration. Two BA–Neu5Ac2en conjugates (8a and 16a) also displayed strong cytotoxicity in MDCK cells at high concentrations (100 μM).
Fig. 2. Cytotoxicity screening of pentacyclic triterpene–Neu5Ac2en conjugates 8a–d, 9a–d, 10a–b, 15a–d and 16a–d using a CellTiter-Glo® assay. DMSO and paclitaxel were used as negative and positive controls, respectively. The error bars indicate the standard deviations of triplicate experiments.
2.4. In vitro anti-influenza virus activity in MDCK cells
With the exception of compounds 8a–b, 8d and 16a–b, which exhibited strong cytotoxic activity, as described above, the other 13 pentacyclic triterpene–Neu5Ac2en conjugates were tested against the influenza virus strain A/WSN/33 (H1N1), which was propagated in MDCK cells, by a cytopathic effect (CPE) reduction assay.25 Curcumin, which is a small-molecule entry inhibitor that targets the HA1 domain,25,26 was utilized as a positive control. The concentrations required to inhibit viral replication by 50% (IC50) are summarized in Table 2. We also determined the cytotoxic effects by a CellTiter-Glo assay, and the cytotoxicity of each compound was expressed as the concentration required to induce 50% cell death (CC50) of the MDCK cells. As shown in Table 2, we found that: 1) compounds 15a and 15c exhibited appreciable effects against A/WSN/33 influenza virus, with IC50 values of 8.3 and 15.5 μM (SI > 22.7), respectively, which were more potent than or comparable to that of the diarylheptanoid anti-influenza entry inhibitor curcumin. 2) The conjugation of Neu5Ac2en with oleanane-type pentacyclic triterpenes was more effective than with ursane- or lupane-type pentacyclic triterpenes. Most of the conjugates with ursolic acid and betulinic acid scaffolds displayed cytotoxicity but no anti-influenza activity, with the exception of compound 15b, which exhibited potent activity against A/WSN/33 virus, with an IC50 of 19.2 μM. 3) The insertion of a (1,2,3-triazol-4-yl)-methyl linkage between the amide bond and Neu5Ac2en caused a substantial decrease in activity (e.g., 15cvs.8c, 16cvs.9c), which indicated that the linker between the pentacyclic triterpene and Neu5Ac2en played an important role in their antiviral activity. 4) In comparison with our previous study, the anti-influenza activities of conjugates of sialic acid linked to pentacyclic triterpenes via the C-4 position were almost five times greater than those of conjugates linked via the C-2 position (IC50: 8.3 μM vs. 41.2 μM (ref. 14)).
Table 2. In vitro anti-influenza virus activity and cytotoxicity of the active compounds.
| Compd | IC50 a (μM) | CC50 b (μM) | SI c | Compd | IC50 (μM) | CC50 (μM) | SI |
| 8c | >100 | ND | ND | 15a | 8.3 ± 1.3 | 188.1 ± 10.1 | 22.7 |
| 9a | 28.3 ± 2.6 | >200 | >7.1 | 15b | 19.2 ± 3.5 | 135.9 ± 6.3 | 7.1 |
| 9b | >100 | ND | ND | 15c | 15.5 ± 0.5 | >200 | >32.3 |
| 9c | 55.0 ± 4.1 | >200 | >3.6 | 15d | >100 | ND | ND |
| 9d | >100 | ND | ND | 16c | 40.5 ± 3.9 | >200 | >4.9 |
| 10a | >100 | ND | ND | 16d | >100 | ND | ND |
| 10b | >100 | ND | ND | Curcumin | 6.7 ± 1.2 | 48.3 ± 5.7 | 7.2 |
aConcentration inhibiting viral replication by 50%. The values are means of at least three independent determinations; the corresponding standard deviations are noted.
bConcentration causing 50% cytotoxicity.
cSelectivity index, defined as CC50/IC50.
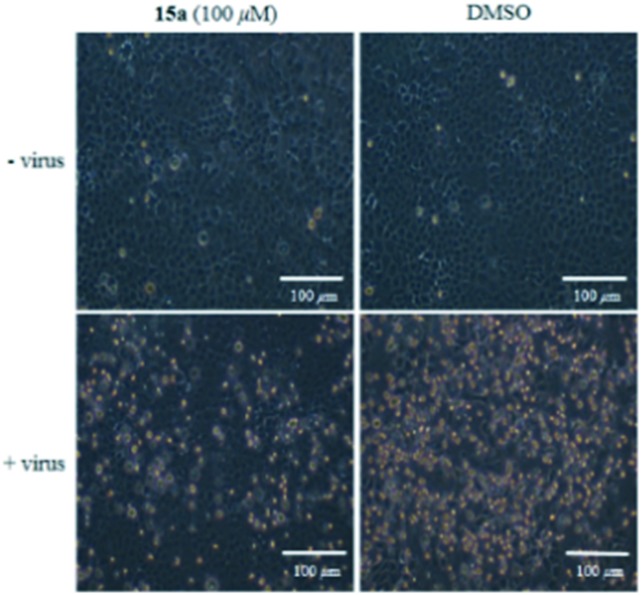
In addition, the CPE in the virus-infected cells was observed microscopically. Morphologically, the influenza virus-infected cells displayed an aggregated appearance and were detached from the dish in the absence of 15a at 40 h post-infection. Compound 15a inhibited the virus-induced CPE significantly, and no cytotoxicity was morphologically observed at a concentration of up to 100 μM (Fig. 3).
Fig. 3. Compound 15a inhibited the virus-induced CPE in MDCK cells. The antiviral efficacy of 15a was observed in terms of cellular morphology at 40 h post-infection.
2.5. Identifying HA as the potential target of compound 15a
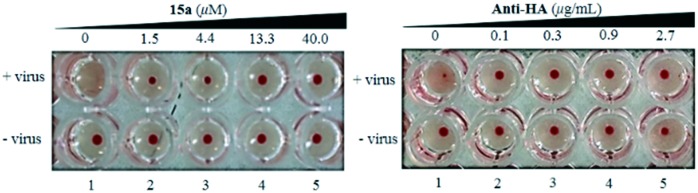
As described in our previous study,14,21 we found that the molecular basis of the anti-influenza activity of pentacyclic triterpenes is likely to be due to their high affinity for HA protein, which is a surface glycoprotein of the influenza virus and is essential for viral attachment to host cells. In this study, a hemagglutination inhibition (HI) assay was performed to determine the potential interaction of the HA envelope protein with 15a. Threefold serial dilutions of compound 15a from 40 to 1.5 μM were added. As was expected, no hemagglutination was elicited by compound 15a alone (lower row, Fig. 4), and the virus alone caused hemagglutination (lane 1, upper row, Fig. 4). However, like anti-HA antibody, compound 15a inhibited the hemagglutination of red blood cells (RBCs) caused by the influenza virus at concentrations of 40 to 1.5 μM (lanes 2–5, upper row, Fig. 4), which implied that 15a and anti-HA antibody had the same target, namely, HA, and the inhibition of viral infection might result from the inhibition of the hemagglutinin–sialic acid receptor interaction.
Fig. 4. Comparison of the behavior of 15avs. anti-HA antibody in the inhibition of the influenza virus-induced aggregation of chicken erythrocytes. 15a exhibited identical activity to that of anti-HA antibody in hemagglutination inhibition in a dose-dependent manner.
On the other hand, Neu5Ac2en was found to be an inhibitor of influenza NA with a Ki of 4 μM in earlier studies.27 To examine whether the designed pentacyclic triterpene–Neu5Ac2en derivatives inhibited the enzymatic activity of NA, A/WSN/33 influenza virus, both untreated and treated with compound 15a, was tested for enzymatic activity with 4-methylumbelliferyl-α-d-N-acetylneuraminic acid sodium salt hydrate solution (MUNANA). Zanamivir, which is a derivative of Neu5Ac2en formed by the introduction of a guanidine group linked to C-4, was used as a positive control. In comparison with zanamivir, compound 15a displayed no inhibition of the enzymatic activity of A/WSN/33 influenza virus (ESI‡ Fig. S1), which implied that NA is not a target of compound 15a in its inhibition of viral infection.
3. Experimental
3.1. Chemistry
All chemicals were used as supplied without further purification. The syntheses of compounds 2a–d, 3a–d, 4–7 and 14 have been reported previously.22b,28 High-resolution mass spectra (HRMS) were obtained with an APEX IV FT-MS (7.0 T) spectrometer (Bruker) in positive ESI mode. NMR spectra were recorded with a Bruker DRX 400 spectrometer at ambient temperature. 1H NMR chemical shifts are referenced to the internal standard TMS (δH = 0.00) or the solvent signal (δH = 3.31 for the central line of CD3OD). 13C NMR chemical shifts are referenced to the solvent signal (δC = 77.00 for the central line of CDCl3, δC = 49.00 for the central line of CD3OD). Reactions were monitored by thin-layer chromatography (TLC) on a precoated 60 F254 silica gel plate (layer thickness 0.2 mm; E. Merck, Darmstadt, Germany) and detected by staining with a yellow solution containing Ce(NH4)2(NO3)6 (0.5 g) and (NH4)6Mo7O24·4H2O (24.0 g) in 6% H2SO4 (500 mL), followed by heating. Flash column chromatography was performed on 60 silica gel (200–300 mesh, Qingdao Haiyang Chemical Co. Ltd.). The calculated values of A log P and water solubility were determined using Pipeline Pilot software version 7.5 (Accelrys Corporation, San Diego, CA, USA).
3.1.1. General procedure a for the CuAAC reaction
To a solution of alkyne (0.45 mmol) and azide (0.30 mmol) in DCM/H2O (1 : 1, v/v, 12 mL) were added CuSO4 (48 mg, 0.30 mmol) and sodium ascorbate (119 mg, 0.60 mmol). The resulting solution was stirred vigorously for 12 hours at room temperature. The reaction mixture was extracted with DCM (3 × 10 mL). The combined organic layer was dried over Na2SO4, filtered and concentrated. The residue was purified by column chromatography over silica gel.
3.1.2. General procedure B for the de-O-acetylation reaction
The per-O-acetylated Neu5Ac2en–pentacyclic triterpene conjugate was dissolved in MeOH (∼5 mL per 100 mg compound), and a solution of MeONa (30% in MeOH, 0.1 equiv. [mol acetate]–1) was added. The solution was stirred at room temperature for 3 hours. After completion (indicated by TLC), the reaction mixture was neutralized with Amberlite IR-120 (H+) ion-exchange resin, filtered and concentrated. The crude product was purified by column chromatography over silica gel.
3.1.3. Synthesis of 5(R)-acetylamino-4(S)-[4-[(3β-hydroxyolean-12-en-28-oyl)amino]methyl-[1,2,3]triazol-1-yl]-6(R)-((1S,2R)-1,2,3-triacetoxypropyl)-5,6-dihydro-4H-pyran-2-carboxylic acid methyl ester (8a)
8a was prepared from 3a and 7 according to general procedure A, and the residue was purified by chromatography (eluent: PE/Act = 3 : 2) over silica gel to afford compound 8a as a white solid in 93% yield. Rf = 0.33 (PE/Act = 3 : 2); 1H NMR (400 MHz, CDCl3): δ 7.79 (s, 1H), 7.31–7.33 (m, 1H, overlap with CDCl3), 6.79 (br s, 1H), 6.03 (s, 1H), 5.69 (d, 1H, J = 9.6 Hz), 5.54 (d, 1H, J = 4.4 Hz), 5.37–5.36 (m, 2H), 4.71 (td, 2H, J = 12.4, 1.9 Hz), 4.46 (dd, 1H, J = 15.1, 5.2 Hz), 4.38–4.31 (m, 2H), 4.21 (dd, 1H, J = 12.4, 7.3 Hz), 3.82 (s, 3H), 3.23–3.18 (m, 1H), 2.56 (d, 1H, J = 10.5 Hz), 2.09 (s, 3H, CH3CO), 2.06 (2 × s, 6H, 2 × CH3CO), 2.02–0.92 (m, other aliphatic ring protons), 1.77 (s, 3H, CH3CO), 1.15, 0.98, 0.90, 0.88, 0.87, 0.78 (s, 3H each, 6 × CH3), 0.72 (d, 1H, J = 11.2 Hz), 0.57 (s, 3H, CH3); 13C NMR (100 MHz, CDCl3): δ 178.61, 170.65, 170.41, 170.31, 170.23, 161.24, 146.11, 145.39, 144.35, 123.15, 122.21, 106.82, 78.91, 77.08, 71.38, 67.85, 62.24, 58.44, 55.12, 52.72, 48.21, 47.53, 46.67, 46.22, 41.95, 41.92, 39.36, 38.79, 38.48, 36.96, 35.13, 34.14, 33.03, 32.64, 32.37, 30.74, 28.16, 27.27, 27.18, 25.85, 23.94, 23.63, 23.48, 22.83, 21.00, 20.83, 20.75, 18.29, 16.68, 15.70, 15.41; ESI-HRMS calcd for C51H75N5NaO12 [M + Na]+: 972.5304, found: 972.5309.
3.1.4. Synthesis of 5(R)-acetylamino-4(S)-[4-[(3β-hydroxyurs-12-en-28-oyl)amino]methyl-[1,2,3]triazol-1-yl]-6(R)-((1S,2R)-1,2,3-triacetoxypropyl)-5,6-dihydro-4H-pyran-2-carboxylic acid methyl ester (8b)
8b was prepared from 3b and 7 according to general procedure A, and the residue was purified by chromatography (eluent: PE/Act = 3 : 2) over silica gel to afford compound 8b as a white solid in 93% yield. Rf = 0.35 (PE/Act = 3 : 2); 1H NMR (400 MHz, CDCl3): δ 7.74 (s, 1H), 7.18 (d, 1H, J = 8.9 Hz), 6.76 (br s, 1H), 6.03 (d, 1H, J = 1.6 Hz), 5.70 (d, 1H, J = 9.5 Hz), 5.53 (dd, 1H, J = 4.7, 1.3 Hz), 5.33–5.38 (m, 2H), 4.70–4.74 (m, 2H), 4.45 (dd, 1H, J = 14.6, 5.2 Hz), 4.27–4.34 (m, 2H), 4.20 (dd, 1H, J = 12.5, 7.2 Hz), 3.82 (s, 3H), 3.20–3.22 (m, 1H), 2.09, 2.07, 2.06 (s, 3H each, 3 × CH3CO), 1.92–0.97 (m, other aliphatic ring protons), 1.78 (s, 3H, CH3CO), 1.09, 0.99, 0.94, 0.89 (s, 3H each, 4 × CH3), 0.86 (d, 3H, CH3, J = 6.3 Hz), 0.78, 0.61 (s, 3H each, 2 × CH3), 0.71 (d, 1H, J = 11.6 Hz); 13C NMR (100 MHz, CDCl3): δ 178.50, 170.70, 170.44, 170.28, 161.28, 146.14, 145.40, 139.27, 126.10, 121.96, 106.85, 78.99, 77.02, 71.38, 67.92, 62.26, 58.35, 55.17, 53.50, 52.77, 48.39, 47.69, 47.56, 42.40, 39.76, 39.58, 39.02, 38.82, 38.66, 37.21, 36.97, 35.12, 32.78, 30.92, 28.22, 27.86, 27.25, 25.02, 23.39, 22.94, 21.28, 21.04, 20.86, 20.79, 18.31, 17.26, 16.75, 15.75, 15.57; ESI-HRMS calcd for C51H76N5O12 [M + H]+: 950.5485, found: 950.5493.
3.1.5. Synthesis of 5(R)-acetylamino-4(S)-[4-[(3β,16α-dihydroxyolean-12-en-28-oyl)amino]methyl-[1,2,3]triazol-1-yl]-6(R)-((1S,2R)-1,2,3-triacetoxypropyl)-5,6-dihydro-4H-pyran-2-carboxylic acid methyl ester (8c)
8c was prepared from 3c and 7 according to general procedure A, and the residue was purified by chromatography (eluent: PE/Act = 3 : 2) over silica gel to afford compound 8c as a white solid in 91% yield. Rf = 0.30 (PE/Act = 3 : 2); 1H NMR (400 MHz, CDCl3): δ 7.74 (s, 1H), 7.49 (d, 1H, J = 8.2 Hz), 7.09 (br s, 1H), 6.04 (s, 1H), 5.68 (br s, 1H), 5.57 (d, 1H, J = 2.3 Hz), 5.54 (br t, 1H), 5.36–5.35 (m, 1H), 4.81 (d, 1H, J = 10.8 Hz), 4.66 (d, 1H, J = 10.5 Hz), 4.57 (dd, 1H, J = 9.5, 4.3 Hz), 4.46–4.39 (m, 1H), 4.29 (d, 1H, J = 12.6 Hz), 4.25–4.20 (m, 2H), 3.82 (s, 3H), 3.24 (br s, 2H), 2.88 (d, 1H, J = 12.4 Hz), 2.14 (t, 1H, J = 13.1 Hz), 2.09 (s, 3H, CH3CO), 2.07 (s, 6H, 2 × CH3CO), 1.91–1.90 (m, 3H), 1.68–0.98 (m, other aliphatic ring protons), 1.72 (s, 3H, CH3CO), 1.33, 0.99, 0.92, 0.90, 0.89, 0.79, 0.70 (s, 3H each, 7 × CH3), 0.74 (d, 1H, J = 11.6 Hz); 13C NMR (100 MHz, CDCl3): δ 178.31, 170.76, 170.64, 170.60, 170.05, 161.06, 146.00, 145.57, 142.99, 123.25, 121.51, 106.75, 78.77, 77.32, 74.34, 71.75, 67.85, 62.09, 58.69, 55.18, 52.66, 49.27, 47.70, 46.77, 46.37, 41.84, 41.34, 39.69, 38.69, 38.50, 36.85, 35.55, 34.98, 34.65, 32.43, 29.97, 27.99, 27.11, 26.90, 25.86, 23.27, 22.60, 20.91, 20.73, 20.68, 18.11, 17.00, 15.61, 15.56; ESI-HRMS calcd for C51H76N5O13 [M + H]+: 966.5434, found: 966.5440.
3.1.6. Synthesis of 5(R)-acetylamino-4(S)-[4-[(3β-hydroxylup-20(29)-en-28-oyl)amino]methyl-[1,2,3]triazol-1-yl]-6(R)-((1S,2R)-1,2,3-triacetoxypropyl)-5,6-dihydro-4H-pyran-2-carboxylic acid methyl ester (8d)
8d was prepared from 3d and 7 according to general procedure A, and the residue was purified by chromatography (eluent: PE/Act = 3 : 2) over silica gel to afford compound 8d as a white solid in 87% yield. Rf = 0.35 (PE/Act = 3 : 2); 1H NMR (400 MHz, CDCl3): δ 7.73 (s, 1H), 6.90 (s, 1H), 6.69 (s, 1H), 6.00 (s, 1H), 5.79 (s, 1H), 5.54 (d, 1H, J = 4.2 Hz), 5.38 (br t, 1H), 4.78 (d, 1H, J = 9.4 Hz), 4.71–4.74 (m, 2H), 4.59 (s, 1H), 4.45 (br s, 2H), 4.24–4.19 (m, 2H), 3.81 (s, 3H), 3.19–3.17 (m, 1H), 3.08 (t, 1H, J = 8.3 Hz), 2.45 (t, 1H, J = 10.5 Hz), 2.09 (s, 3H, CH3CO), 2.06 (s, 6H, 2 × CH3CO), 2.00 (d, 1H, J = 12.8 Hz), 1.80 (s, 3H, CH3CO), 1.79–0.88 (m, other aliphatic ring protons), 1.67, 0.96, 0.95, 0.87, 0.80, 0.76 (s, 3H each, 6 × CH3); 13C NMR (100 MHz, CDCl3): δ 176.89, 170.75, 170.66, 170.50, 170.27, 161.18, 150.78, 146.26, 121.95, 109.60, 106.77, 79.03, 76.84, 71.25, 67.88, 62.15, 58.30, 55.72, 55.44, 52.83, 50.66, 50.11, 48.73, 46.77, 42.52, 40.85, 38.93, 38.80, 38.34, 37.80, 37.25, 34.88, 34.49, 33.49, 30.88, 29.52, 28.10, 27.48, 25.68, 22.99, 21.05 (2C), 20.87, 20.80, 19.49, 18.36, 16.28, 16.22, 15.49, 14.72; ESI-HRMS calcd for C51H75N5NaO12 [M + Na]+: 972.5304, found: 972.5298.
3.1.7. Synthesis of 5(R)-acetylamino-4(S)-[4-[(3β-hydroxyolean-12-en-28-oyl)amino]methyl-[1,2,3]triazol-1-yl]-6(R)-((1R,2R)-1,2,3-trihydroxypropyl)-5,6-dihydro-4H-pyran-2-carboxylic acid methyl ester (9a) and 5-acetylamino-4(R)-[4-[(3β-hydroxyolean-12-en-28-oyl)amino]methyl-[1,2,3]triazol-1-yl]-6(R)-((1R,2R)-1,2,3-trihydroxypropyl)-5,6-dihydro-4H-pyran-2-carboxylic acid methyl ester (10a)
9a and 10a were prepared from 8a according to general procedure B, and the residue was purified by chromatography (eluent: DCM/MeOH = 10 : 1) over silica gel to afford compounds 9a and 10a as white solids in yields of 52% and 31%, respectively. Compound 9a: Rf = 0.18 (DCM/MeOH = 10 : 1); 1H NMR (400 MHz, CD3OD): δ 7.82 (s, 1H), 6.01 (d, 1H, J = 2.4 Hz), 5.61 (dd, 1H, J = 9.8, 2.2 Hz), 5.33 (t, 1H, J = 3.4 Hz), 4.56 (d, 1H, J = 10.9 Hz), 4.46–4.33 (m, 3H), 3.95–3.90 (m, 1H), 3.85–3.81 (m, 4H), 3.68 (dd, 1H, J = 11.5, 6.2 Hz), 3.65 (d, 1H, J = 8.7 Hz), 3.13 (dd, 1H, J = 11.4, 4.5 Hz), 2.80 (dd, 1H, J = 13.0, 3.6 Hz), 2.06 (dt, 1H, J = 13.6, 3.9 Hz), 1.89–1.85 (m, 3H), 1.87 (s, 3H, CH3CO), 1.77 (t, 1H, J = 13.7 Hz), 1.62–0.95 (m, other aliphatic ring protons), 1.15, 0.96, 0.94 (s, 3H each, 3 × CH3), 0.91 (s, 6H, 2 × CH3), 0.77 (s, 3H, CH3), 0.73 (d, 1H, J = 11.1 Hz), 0.53 (s, 3H, CH3); 13C NMR (100 MHz, CD3OD): δ 180.36, 173.76, 163.51, 147.58, 146.68, 145.07, 124.13, 123.29, 107.29, 79.67, 78.44, 71.16, 69.71, 64.78, 59.95, 56.70, 53.07, 50.09, 48.96, 47.63, 47.51, 42.86, 42.52, 40.63, 39.84, 38.10, 35.86, 35.08, 34.21, 33.79, 33.54, 31.61, 28.74, 28.46, 27.85, 26.39, 24.51, 24.03, 22.55, 19.45, 17.75, 16.34, 15.97; ESI-HRMS calcd for C45H70N5O9 [M + H]+: 824.5168, found: 824.5174. Compound 10a: Rf = 0.21 (DCM/MeOH = 10 : 1); 1H NMR (400 MHz, CD3OD): δ 7.80 (t, 1H, J = 5.6 Hz), 7.77 (s, 1H), 6.11 (d, 1H, J = 5.4 Hz), 5.58 (t, 1H, J = 5.4 Hz), 5.33 (br t, 1H), 4.64 (dd, 1H, J = 11.1, 5.4 Hz), 4.51 (dd, 1H, J = 11.1, 1.0 Hz), 4.37 (dq, 2H, J = 15.0, 5.4 Hz), 3.94 (ddd, 1H, J = 8.8, 5.3, 2.8 Hz), 3.85–3.81 (m, 4H), 3.66 (dd, 1H, J = 11.4, 5.4 Hz), 3.61 (dd, 1H, J = 9.4, 0.8 Hz), 3.13 (dd, 1H, J = 11.4, 4.5 Hz), 2.79 (dd, 1H, J = 13.3, 3.4 Hz), 2.07 (dt, 1H, J = 13.6, 3.4 Hz), 1.88 (dd, 1H, J = 8.8, 3.2 Hz), 1.85 (s, 3H, CH3CO), 1.77 (t, 1H, J = 13.6 Hz), 1.64–0.98 (m, other aliphatic ring protons), 1.15, 0.96, 0.93, 0.92, 0.90, 0.78 (s, 3H each, 6 × CH3), 0.73 (d, 1H, J = 11.2 Hz), 0.49 (s, 3H, CH3); 13C NMR (100 MHz, CD3OD): δ 180.46, 173.54, 163.79, 148.09, 145.86, 145.03, 125.51, 124.16, 104.61, 79.66, 73.83, 71.45, 69.77, 64.79, 56.69, 54.48, 53.10, 48.97, 48.36, 47.56, 47.50, 42.85, 42.60, 40.60, 39.83, 38.10, 35.93, 35.05, 34.26, 33.80, 33.53, 31.61, 28.74, 28.45, 27.85, 26.43, 24.51, 24.02, 22.42, 19.45, 17.48, 16.36, 16.00; ESI-HRMS calcd for C45H69N5NaO9 [M + Na]+: 846.4987, found: 846.4993.
3.1.8. Synthesis of 5(R)-acetylamino-4(S)-[4-[(3β-hydroxyurs-12-en-28-oyl)amino]methyl-[1,2,3]triazol-1-yl]-6(R)-((1R,2R)-1,2,3-trihydroxypropyl)-5,6-dihydro-4H-pyran-2-carboxylic acid methyl ester (9b) and 5-acetylamino-4(R)-[4-[(3β-hydroxyurs-12-en-28-oyl)amino]methyl-[1,2,3]triazol-1-yl]-6(R)-((1R,2R)-1,2,3-trihydroxypropyl)-5,6-dihydro-4H-pyran-2-carboxylic acid methyl ester (10b)
9b and 10b were prepared from 8b according to general procedure B, and the residue was purified by chromatography (eluent: DCM/MeOH = 10 : 1) over silica gel to afford compounds 9b and 10b as white solids in yields of 49% and 28%, respectively. Compound 9b: Rf = 0.19 (DCM/MeOH = 10 : 1); 1H NMR (400 MHz, CD3OD): δ 7.83 (s, 1H), 6.02 (d, 1H, J = 2.3 Hz), 5.62 (d, 1H, J = 9.2 Hz), 5.32 (br t, 1H), 4.56 (d, 1H, J = 10.9 Hz), 4.46–4.36 (m, 3H), 3.92 (ddd, 1H, J = 8.6, 5.1, 2.8 Hz), 3.83 (dd, 1H, J = 11.5, 2.8 Hz), 3.81 (s, 3H), 3.64–3.70 (m, 2H), 3.14 (dd, 1H, J = 11.0, 5.0 Hz), 2.14 (d, 1H, J = 5.6 Hz), 2.03–2.10 (m, 1H), 1.87 (s, 3H, CH3CO), 1.68–0.95 (m, other aliphatic ring protons), 1.10 (s, 3H, CH3), 0.96 (s, 6H, 2 × CH3), 0.92 (s, 3H, CH3), 0.89 (d, 3H, J = 6.4 Hz, CH3), 0.77 (s, 3H, CH3), 0.72 (d, 1H, J = 11.0 Hz), 0.56 (s, 3H, CH3); 13C NMR (100 MHz, CD3OD): δ 180.17, 173.72, 163.49, 147.54, 146.63, 139.71, 127.28, 123.19, 107.30, 79.62, 78.40, 71.15, 69.67, 64.76, 59.96, 56.67, 54.11, 53.09, 50.04, 49.85, 48.91, 43.22, 40.82, 40.26, 39.94, 39.82, 38.55, 38.04, 35.80, 34.11, 31.88, 28.92, 28.77, 27.87, 25.25, 24.33, 24.04, 22.58, 21.59, 19.41, 17.74, 17.73, 16.41, 16.13; ESI-HRMS calcd for C45H70N5O9 [M + H]+: 824.5168, found: 824.5163. Compound 10b: Rf = 0.22 (DCM/MeOH = 10 : 1); 1H NMR (400 MHz, CD3OD): δ 7.75 (s, 1H), 6.12 (d, 1H, J = 5.4 Hz), 5.57 (t, 1H, J = 5.4 Hz), 5.31 (br t, 1H), 4.63 (dd, 1H, J = 11.0, 5.4 Hz), 4.51 (d, 1H, J = 11.1 Hz), 4.30–4.39 (m, 2H), 3.95–3.92 (m, 1H), 3.83–3.81 (m, 4H), 3.66 (dd, 1H, J = 11.3, 5.4 Hz), 3.60 (d, 1H, J = 9.3 Hz), 3.14 (dd, 1H, J = 10.8, 4.70 Hz), 2.14–2.04 (m, 2H), 1.85 (s, 3H, CH3CO), 1.72–0.97 (m, other aliphatic ring protons), 1.10 (s, 3H, CH3), 0.96 (s, 6H, 2 × CH3), 0.93 (s, 3H, CH3), 0.89 (d, 3H, J = 6.3 Hz, CH3), 0.78 (s, 3H, CH3), 0.72 (d, 1H, J = 11.1 Hz), 0.51 (s, 3H, CH3); 13C NMR (100 MHz, CD3OD): δ 180.29, 173.56, 163.81, 148.10, 145.77, 139.71, 127.35, 125.51, 104.61, 79.67, 73.85, 71.46, 69.78, 64.80, 56.68, 54.49, 54.22, 53.10, 48.95, 48.88, 48.39, 43.27, 40.83, 40.80, 40.35, 39.98, 39.84, 38.66, 38.06, 35.78, 34.13, 31.87, 28.91, 28.75, 27.89, 25.27, 24.34, 24.00, 22.40, 21.54, 19.42, 17.69, 17.49, 16.42, 16.15; ESI-HRMS calcd for C45H69N5NaO9 [M + Na]+: 846.4987, found: 846.4988.
3.1.9. Synthesis of 5(R)-acetylamino-4(S)-[4-[(3β,16α-dihydroxyolean-12-en-28-oyl)amino]methyl-[1,2,3]triazol-1-yl]-6(R)-((1R,2R)-1,2,3-trihydroxypropyl)-5,6-dihydro-4H-pyran-2-carboxylic acid methyl ester (9c)
To a solution of 3c (229 mg, 0.45 mmol) and 11 (99 mg, 0.30 mmol) in THF/H2O (1 : 1, v/v, 12 mL) were added CuSO4 (48 mg, 0.30 mmol) and sodium ascorbate (119 mg, 0.60 mmol). The resulting solution was stirred vigorously for 12 hours at room temperature. After the removal of THF and water, the residue was purified by chromatography (eluent: DCM/MeOH = 10 : 1) over silica gel to afford compound 9c as a white solid in 85% yield. Rf = 0.15 (DCM/MeOH = 10 : 1); 1H NMR (400 MHz, CD3OD): δ 7.82 (s, 1H), 7.57 (t, 1H, J = 5.2 Hz), 5.99 (d, 1H, J = 2.4 Hz), 5.63 (dd, 1H, J = 9.8, 2.2 Hz), 5.45 (br t, 1H), 4.56 (d, 1H, J = 10.9 Hz), 4.46–4.30 (m, 4H), 3.92 (ddd, 1H, J = 8.4, 5.1, 2.8 Hz), 3.85–3.81 (m, 4H), 3.68 (dd, 1H, J = 11.6, 5.3 Hz), 3.66 (d, 1H, J = 9.4 Hz), 3.14 (dd, 1H, J = 11.0, 4.9 Hz), 2.88 (dd, 1H, J = 13.6, 3.3 Hz), 2.35 (t, 1H, J = 13.2 Hz), 1.98–1.89 (m, 4H), 1.87 (s, 3H, CH3CO), 1.64–1.01 (m, other aliphatic ring protons), 1.36, 0.97, 0.96, 0.92, 0.88, 0.77 (s, 3H each, 6 × CH3), 0.74 (d, 1H, J = 11.2 Hz), 0.56 (s, 3H, CH3); 13C NMR (100 MHz, CD3OD): δ 180.12, 173.73, 163.49, 147.55, 146.56, 144.92, 124.17, 122.96, 107.31, 79.64, 78.45, 75.56, 71.15, 69.69, 64.77, 59.96, 56.79, 53.08, 50.01, 49.96, 48.11, 48.05, 42.77, 42.26, 40.75, 39.92, 39.83, 38.07, 36.38, 36.10, 35.97, 33.93, 33.29, 31.92, 31.23, 28.73, 27.88, 27.29, 25.38, 24.46, 22.57, 19.43, 17.77, 16.35, 16.20; ESI-HRMS calcd for C45H70N5O10 [M + H]+: 840.5117, found: 840.5125.
3.1.10. Synthesis of 5(R)-acetylamino-4(S)-[4-[(3β-hydroxylup-20(29)-en-28-oyl)amino]methyl-[1,2,3]triazol-1-yl]-6(R)-((1R,2R)-1,2,3-trihydroxypropyl)-5,6-dihydro-4H-pyran-2-carboxylic acid methyl ester (9d)
To a solution of 3d (222 mg, 0.45 mmol) and 11 (99 mg, 0.30 mmol) in THF/H2O (1 : 1, v/v, 12 mL) were added CuSO4 (48 mg, 0.30 mmol) and sodium ascorbate (119 mg, 0.60 mmol). The resulting solution was stirred vigorously for 12 hours at room temperature. After the removal of THF and water, the residue was purified by chromatography (eluent: DCM/MeOH = 10 : 1) over silica gel to afford compound 9d as a white solid in 83% yield. Rf = 0.19 (DCM/MeOH = 10 : 1); 1H NMR (400 MHz, CD3OD): δ 7.82 (s, 1H), 6.02 (d, 1H, J = 2.4 Hz), 5.61 (dd, 1H, J = 9.6, 2.0 Hz), 4.70 (s, 1H), 4.58–4.53 (m, 2H), 4.49–4.42 (m, 2H), 4.29 (d, 1H, J = 15.1 Hz), 3.94 (ddd, 1H, J = 8.9, 5.1, 2.9 Hz), 3.78–3.85 (m, 4H), 3.69 (dd, 1H, J = 11.4, 5.2 Hz), 3.66 (d, 1H, J = 9.4 Hz), 3.07–3.13 (m, 2H), 2.51 (dt, 1H, J = 12.7, 3.0 Hz), 2.12 (td, 1H, J = 13.6, 3.0 Hz), 1.88 (s, 3H, CH3CO), 1.86–1.78 (m, 2H), 1.72–0.90 (m, other aliphatic ring protons), 1.68, 0.98, 0.94, 0.87, 0.84, 0.76 (s, 3H each, 6 × CH3), 0.68 (dd, 1H, J = 8.4, 2.7 Hz); 13C NMR (100 MHz, CD3OD): δ 179.27, 173.87, 163.51, 152.28, 147.58, 123.35, 110.02, 107.34, 79.66, 78.52, 71.15, 69.71, 64.78, 59.95, 56.93, 53.10, 52.07, 51.40, 50.10, 48.13, 43.46, 42.08, 40.11, 39.96, 39.20, 39.01, 38.32, 35.65, 35.54, 33.92, 31.95, 30.58, 28.66, 28.05, 27.00, 22.58, 22.16, 19.65, 19.46, 16.99, 16.85, 16.18, 15.12; ESI-HRMS calcd for C45H70N5O9 [M + H]+: 824.5168, found: 824.5173.
3.1.11. Synthesis of 5(R)-acetylamino-4(S)-[(3β-hydroxyolean-12-en-28-oyl)amino]-6(R)-((1S,2R)-1,2,3-triacetoxypropyl)-5,6-dihydro-4H-pyran-2-carboxylic acid methyl ester (15a)
To a solution of 14 (188.5 mg, 0.44 mmol) and 2a (321 mg, 0.56 mmol) in DMF (20 mL), Na2CO3 (201 mg, 1.9 mmol) was added. The resulting mixture was stirred vigorously for 24 hours at 60 °C. After the removal of DMF under vacuum, the residue was purified by chromatography (eluent: PE/Act = 2 : 1) over silica gel to afford compound 15a as a white solid in 66% yield. Rf = 0.33 (PE/Act = 2 : 1); 1H NMR (400 MHz, CDCl3): δ 6.15–6.16 (m, 1H), 6.04 (d, 1H, J = 7.3 Hz), 5.91 (d, 1H, J = 1.9 Hz), 5.49 (d, 1H, J = 4.5 Hz), 5.31–5.30 (m, 2H), 4.70–4.72 (m, 2H), 4.26–4.22 (m, 2H), 4.18 (dd, 1H, J = 12.4, 7.4 Hz), 3.79 (s, 3H), 3.21 (dd, 1H, J = 9.8, 3.3 Hz), 2.86 (dd, 1H, J = 10.4, 3.4 Hz), 2.10, 2.07, 2.06, 1.89 (s, 3H each, 4 × CH3CO), 1.87–0.97 (m, other aliphatic ring protons), 1.13, 0.99, 0.90, 0.89, 0.88, 0.78, 0.76 (s, 3H each, 7 × CH3), 0.73 (d, 1H, J = 11.2 Hz); 13C NMR (100 MHz, CDCl3): δ 178.54, 171.44, 170.55, 170.40, 170.03, 161.82, 144.30, 143.48, 122.62, 110.78, 78.96, 77.32, 71.51, 67.86, 62.21, 55.20, 52.37, 49.19, 47.58, 46.74, 46.14, 45.98, 41.81, 40.97, 39.36, 38.73, 38.40, 37.04, 34.05, 33.37, 32.97, 32.81, 30.61, 28.11, 27.34, 27.15, 25.80, 23.53, 23.41, 23.15, 20.89, 20.75, 20.65, 18.30, 17.23, 15.61, 15.30; ESI-HRMS calcd for C48H72N2NaO12 [M + Na]+: 891.4977, found: 891.4984.
3.1.12. Synthesis of 5(S)-acetylamino-4(S)-[(3β-hydroxyurs-12-en-28-oyl)amino]-6(R)-((1S,2R)-1,2,3-triacetoxypropyl)-5,6-dihydro-4H-pyran-2-carboxylic acid methyl ester (15b)
To a solution of 14 (188.5 mg, 0.44 mmol) and 2b (321 mg, 0.56 mmol) in DMF (20 mL), Na2CO3 (201 mg, 1.9 mmol) was added. The resulting mixture was stirred vigorously for 24 hours at 60 °C. After the removal of DMF under vacuum, the residue was purified by chromatography (eluent: PE/Act = 2 : 1) over silica gel to afford compound 15b as a white solid in 75% yield. Rf = 0.35 (PE/Act = 2 : 1); 1H NMR (400 MHz, CDCl3): δ 6.60 (d, 1H, J = 8.8 Hz), 6.11 (d, 1H, J = 7.2 Hz), 5.88 (s, 1H), 5.50 (d, 1H, J = 4.2 Hz), 5.29 (br t, 1H), 5.25 (s, 1H), 4.72–4.70 (m, 2H), 4.30–4.32 (m, 2H), 4.18 (dd, 1H, J = 12.5, 7.6 Hz), 3.78 (s, 3H), 3.22–3.20 (m, 1H), 2.31 (s, 1H), 2.19–2.11 (m, 2H), 2.10, 2.07, 2.06, 1.90 (s, 3H each, 4 × CH3CO), 1.94–0.91 (m, other aliphatic ring protons), 1.08, 0.99, 0.93, 0.91 (s, 3H each, 4 × CH3), 0.85 (d, 3H, J = 6.1 Hz, CH3), 0.79, 0.78 (s, 3H each, 2 × CH3), 0.72 (d, 1H, J = 11.5 Hz); 13C NMR (100 MHz, CDCl3): δ 178.49, 171.42, 170.53, 170.43, 170.01, 161.78, 144.07, 137.67, 125.77, 110.91, 78.89, 77.25, 71.49, 67.80, 62.22, 55.10, 52.54, 52.31, 49.15, 47.62, 47.42, 46.11, 42.10, 39.55, 39.15, 38.66, 38.51, 37.60, 36.91, 33.09, 30.73, 28.12, 27.72, 27.10, 24.26, 23.36, 23.27, 23.17, 21.10, 20.85, 20.72, 20.64, 18.23, 17.41, 16.98, 15.68, 15.38; ESI-HRMS calcd for C48H73N2O12 [M + H]+: 869.5158, found: 869.5150.
3.1.13. Synthesis of 5(R)-acetylamino-4(S)-[(3β,16α-dihydroxyolean-12-en-28-oyl)amino]-6(R)-((1S,2R)-1,2,3-triacetoxypropyl)-5,6-dihydro-4H-pyran-2-carboxylic acid methyl ester (15c)
To a solution of 14 (188.5 mg, 0.44 mmol) and 2c (330 mg, 0.56 mmol) in DMF (20 mL), Na2CO3 (201 mg, 1.9 mmol) was added. The resulting mixture was stirred vigorously for 24 hours at 60 °C. After the removal of DMF under vacuum, the residue was purified by chromatography (eluent: PE/Act = 2 : 1) over silica gel to afford compound 15c as a white solid in 68% yield. Rf = 0.30 (PE/Act = 2 : 1); 1H NMR (400 MHz, CDCl3): δ 6.80 (d, 1H, J = 6.9 Hz), 6.59 (d, 1H, J = 8.6 Hz), 5.86 (s, 1H), 5.47 (s, 2H), 5.30 (br s, 1H), 4.82–4.75 (m, 2H), 4.29–4.08 (m, 4H), 3.79 (s, 3H), 3.23 (br s, 1H), 3.11 (d, 1H, J = 14.3 Hz), 2.45 (s, 1H), 2.10, 2.06, 2.05, 1.89 (s, 3H each, 4 × CH3CO), 1.95–0.96 (m, other aliphatic ring protons), 1.25, 1.00, 0.95, 0.92, 0.89, 0.84, 0.79 (s, 3H each, 7 × CH3), 0.74 (d, 1H, J = 10.9 Hz); 13C NMR (100 MHz, CDCl3): δ 178.79, 171.19, 170.68, 170.47, 170.05, 161.90, 144.30, 141.77, 123.08, 111.16, 78.86, 77.66, 73.62, 71.77, 68.03, 62.33, 55.32, 52.34, 49.94, 48.79, 47.12, 46.49, 45.67, 42.08, 41.03, 40.00, 38.74, 38.61, 36.94, 36.15, 33.84, 32.65, 32.39, 29.63, 28.04, 27.23, 27.08, 26.61, 23.29, 23.21, 20.84, 20.76, 20.73, 18.21, 17.64, 15.76, 15.66; ESI-HRMS calcd for C48H73N2O13 [M + H]+: 885.5107, found: 885.5108.
3.1.14. Synthesis of 5(R)-acetylamino-4(S)-[(3β-hydroxylup-20(29)-en-28-oyl)amino]-6(R)-((1S,2R)-1,2,3-triacetoxypropyl)-5,6-dihydro-4H-pyran-2-carboxylic acid methyl ester (15d)
To a solution of 14 (188.5 mg, 0.44 mmol) and 2d (321 mg, 0.56 mmol) in DMF (20 mL), Na2CO3 (201 mg, 1.9 mmol) was added. The resulting mixture was stirred vigorously for 24 hours at 60 °C. After the removal of DMF under vacuum, the residue was purified by chromatography (eluent: PE/Act = 2 : 1) over silica gel to afford compound 15d as a white solid in 62% yield. Rf = 0.35 (PE/Act = 2 : 1); 1H NMR (400 MHz, CDCl3): δ 6.35 (d, 1H, J = 7.5 Hz), 6.03 (d, 1H, J = 8.4 Hz), 5.86 (d, 1H, J = 2.0 Hz), 5.50 (d, 1H, J = 3.2 Hz), 5.32–5.28 (m, 1H), 4.83 (t, 1H, J = 8.2 Hz), 4.70–4.74 (m, 2H), 4.58 (s, 1H), 4.30–4.23 (m, 2H), 4.17 (dd, 1H, J = 12.5, 7.5 Hz), 3.80 (s, 3H), 3.22–3.18 (m, 1H), 3.04 (dt, 1H, J = 11.2, 4.1 Hz), 2.40 (dt, 1H, J = 9.5, 3.1 Hz), 2.15 (s, 1H), 2.09, 2.08, 2.06, 1.90 (s, 3H each, 4 × CH3CO), 1.93–0.85 (m, other aliphatic ring protons), 1.66, 0.97, 0.96, 0.93, 0.83, 0.76 (s, 3H each, 6 × CH3), 0.68 (d, 1H, J = 9.0 Hz); 13C NMR (100 MHz, CDCl3): δ 177.06, 171.29, 170.58, 170.43, 170.01, 161.82, 150.58, 144.47, 111.21, 109.46, 78.95, 77.53, 71.54, 67.82, 62.18, 55.73, 55.33, 52.43, 50.57, 50.14, 48.12, 46.74, 46.45, 42.38, 40.71, 38.83, 38.69, 37.89, 37.69, 37.17, 34.31, 33.37, 30.79, 29.37, 27.97, 27.33, 25.52, 23.06, 20.88, 20.75, 20.62, 19.31, 18.25, 16.10, 16.00, 15.43, 14.60; ESI-HRMS calcd for C48H73N2O12 [M + H]+: 869.5158, found: 869.5160.
3.1.15. Synthesis of 5(R)-acetylamino-4(S)-[(3β-hydroxyolean-12-en-28-oyl)amino]-6(R)-((1R,2R)-1,2,3-trihydroxypropyl)-5,6-dihydro-4H-pyran-2-carboxylic acid methyl ester (16a)
16a was prepared from 15a according to general procedure B, and the residue was purified by chromatography (eluent: DCM/MeOH = 10 : 1) over silica gel to afford compound 16a as a white solid in 86% yield. Rf = 0.31 (DCM/MeOH = 10 : 1); 1H NMR (400 MHz, CD3OD): δ 5.83 (d, 1H, J = 2.5 Hz), 5.28 (t, 1H, J = 3.3 Hz), 4.74 (dd, 1H, J = 9.4, 2.5 Hz), 4.55 (s, 1H), 4.31–4.22 (m, 2H), 3.89 (ddd, 1H, J = 8.6, 5.3, 2.9 Hz), 3.82 (dd, 1H, J = 11.4, 2.9 Hz), 3.78 (s, 3H), 3.66 (dd, 1H, J = 11.4, 5.4 Hz), 3.60 (d, 1H, J = 10.0 Hz), 3.15 (dd, 1H, J = 11.1, 4.9 Hz), 2.91 (dd, 1H, J = 9.9, 3.5 Hz), 2.11 (dt, 1H, J = 14.5, 4.0 Hz), 1.96 (s, 3H, CH3CO), 1.93–1.89 (m, 2H), 1.73–0.97 (m, other aliphatic ring protons), 1.17, 0.98, 0.96, 0.93, 0.90, 0.84, 0.78 (s, 3H each, 7 × CH3); 13C NMR (100 MHz, CD3OD): δ 180.69, 174.46, 164.28, 145.66, 145.03, 123.83, 111.77, 79.71, 78.54, 71.17, 70.02, 64.88, 56.77, 52.82, 49.64, 49.00, 48.36, 47.65, 47.33, 42.96, 42.35, 40.71, 39.84, 38.21, 35.11, 34.63, 34.17, 33.54, 31.57, 28.76, 28.60, 27.87, 26.40, 24.56, 23.98, 23.48, 22.82, 19.51, 18.29, 16.33, 15.96; ESI-HRMS calcd for C42H66N2NaO9 [M + Na]+: 765.4661, found: 765.4669.
3.1.16. Synthesis of 5(R)-acetylamino-4(S)-[(3β-hydroxyurs-12-en-28-oyl)amino]-6(R)-((1R,2R)-1,2,3-trihydroxypropyl)-5,6-dihydro-4H-pyran-2-carboxylic acid methyl ester (16b)
16b was prepared from 15b according to general procedure B, and the residue was purified by chromatography (eluent: DCM/MeOH = 10 : 1) over silica gel to afford compound 16b as a white solid in 87% yield. Rf = 0.32 (DCM/MeOH = 10 : 1); 1H NMR (400 MHz, CD3OD): δ 7.49 (d, 1H, J = 7.9 Hz), 5.81 (d, 1H, J = 2.5 Hz), 5.26 (t, 1H, J = 3.2 Hz), 4.73 (dt, 1H, J = 9.8, 2.4 Hz), 4.28 (t, 1H, J = 10.5 Hz), 4.23 (t, 1H, J = 10.7 Hz), 3.89 (ddd, 1H, J = 8.5, 5.2, 2.8 Hz), 3.82 (dd, 1H, J = 11.4, 2.9 Hz), 3.77 (s, 3H), 3.66 (dd, 1H, J = 11.4, 5.4 Hz), 3.59 (d, 1H, J = 10.0 Hz), 3.15 (dd, 1H, J = 11.0, 4.8 Hz), 2.26 (d, 1H, J = 11.0 Hz), 2.12 (dt, 1H, J = 14.0, 3.6 Hz), 1.97 (s, 3H, CH3CO), 1.96–0.98 (m, other aliphatic ring protons), 1.12, 0.98, 0.97, 0.96 (s, 3H each, 4 × CH3), 0.89 (d, 3H, J = 6.4 Hz, CH3), 0.87, 0.78 (s, 3H each, 2 × CH3); 13C NMR (100 MHz, CD3OD): δ 180.64, 174.36, 164.23, 145.58, 139.38, 127.02, 111.83, 79.61, 78.52, 71.09, 69.90, 64.82, 56.70, 53.83, 52.82, 49.05, 48.18, 43.28, 40.92, 40.55, 40.17, 39.98, 39.82, 38.93, 38.11, 34.47, 31.93, 28.97, 28.82, 27.87, 24.65, 24.43, 24.06, 22.95, 21.62, 19.47, 18.47, 17.73, 16.44, 16.15; ESI-HRMS calcd for C42H67N2O9 [M + H]+: 743.4841, found: 743.4846.
3.1.17. Synthesis of 5(R)-acetylamino-4(S)-[(3β,16α-dihydroxyolean-12-en-28-oyl)amino]-6(R)-((1R,2R)-1,2,3-trihydroxypropyl)-5,6-dihydro-4H-pyran-2-carboxylic acid methyl ester (16c)
16c was prepared from 15c according to general procedure B, and the residue was purified by chromatography (eluent: DCM/MeOH = 10 : 1) over silica gel to afford compound 16c as a white solid in 84% yield. Rf = 0.29 (DCM/MeOH = 10 : 1); 1H NMR (400 MHz, CD3OD): δ 7.39 (d, 1H, J = 7.8 Hz), 5.78 (d, 1H, J = 2.4 Hz), 5.40 (br t, 1H), 4.68–4.70 (m, 1H), 4.23–4.24 (m, 3H), 3.89 (ddd, 1H, J = 8.6, 5.2, 2.8 Hz), 3.82 (dd, 1H, J = 11.3, 2.9 Hz), 3.76 (s, 3H), 3.66 (dd, 1H, J = 11.5, 5.4 Hz), 3.59 (d, 1H, J = 9.4 Hz), 3.16 (dd, 1H, J = 11.0, 5.0 Hz), 3.09 (dd, 1H, J = 14.1, 3.4 Hz), 2.20 (t, 1H, J = 13.5 Hz), 1.97 (s, 3H, CH3CO), 1.92 (dd, 1H, J = 5.9, 2.4 Hz), 1.84–1.01 (m, other aliphatic ring protons), 1.34, 0.98, 0.97, 0.95, 0.89, 0.86, 0.78 (s, 3H each, 7 × CH3); 13C NMR (100 MHz, CD3OD): δ 180.43, 174.29, 164.18, 145.59, 144.34, 123.68, 111.61, 79.62, 78.47, 74.78, 71.10, 69.95, 64.83, 56.86, 52.82, 50.48, 49.77, 48.43, 48.33, 47.58, 42.91, 41.89, 40.98, 39.97, 39.83, 38.14, 36.42, 36.03, 34.17, 33.26, 31.00, 30.69, 28.76, 27.89, 27.59, 26.26, 24.49, 22.90, 19.47, 18.24, 16.37, 16.27; ESI-HRMS calcd for C42H67N2O10 [M + H]+: 759.4790, found: 759.4787.
3.1.18. Synthesis of 5(R)-acetylamino-4(S)-[(3β-hydroxylup-20(29)-en-28-oyl)amino]-6(R)-((1R,2R)-1,2,3-trihydroxypropyl)-5,6-dihydro-4H-pyran-2-carboxylic acid methyl ester (16d)
16d was prepared from 15d according to general procedure B, and the residue was purified by chromatography (eluent: DCM/MeOH = 10 : 1) over silica gel to afford compound 16d as a white solid in 81% yield. Rf = 0.32 (DCM/MeOH = 10 : 1); 1H NMR (400 MHz, CD3OD): δ 7.69 (d, 1H, J = 8.5 Hz), 5.76 (d, 1H, J = 2.4 Hz), 4.83–4.86 (m, 1H, overlap with water), 4.69 (br s, 1H), 4.59 (br s, 1H), 4.25 (t, 1H, J = 10.6 Hz), 4.22 (t, 1H, J = 10.6 Hz), 3.90 (ddd, 1H, J = 8.6, 5.2, 2.8 Hz), 3.82 (dd, 1H, J = 11.4, 2.9 Hz), 3.78 (s, 3H), 3.66 (dd, 1H, J = 11.4, 5.4 Hz), 3.60 (d, 1H, J = 9.4 Hz), 3.13 (dd, 1H, J = 10.9, 5.1 Hz), 3.07 (dt, 1H, J = 11.1, 4.3 Hz), 2.59 (dt, 1H, J = 12.8, 3.2 Hz), 2.14 (d, 1H, J = 10.9 Hz), 1.96 (s, 3H, CH3CO), 1.87–0.92 (m, other aliphatic ring protons), 1.68 (s, 3H, CH3), 1.00 (2 × s, 6H, 2 × CH3), 0.96, 0.87, 0.76 (s, 3H each, 3 × CH3), 0.71 (d, 1H, J = 8.7 Hz); 13C NMR (100 MHz, CD3OD): δ 179.32, 174.31, 164.19, 152.18, 145.80, 112.11, 110.05, 79.63, 78.60, 71.10, 69.90, 64.84, 57.10, 56.91, 52.85, 52.11, 51.45, 48.69, 48.66, 48.11, 43.49, 42.02, 40.13, 39.95, 39.16, 38.91, 38.35, 35.64, 33.75, 31.98, 30.56, 28.66, 28.05, 26.98, 22.78, 22.20, 19.68, 19.47, 16.84, 16.79, 16.16, 15.16; ESI-HRMS calcd for C42H66N2NaO9 [M + Na]+: 765.4661, found: 765.4667.
3.2. Bioassays
3.2.1. Cytotoxicity assay
The assay was performed as previously described with some modifications.29 Cells were seeded into 96-well plates in DMEM supplemented with 10% FBS and cultured overnight at 37 °C in 5% CO2. Then, the test compounds were added and the cells were further incubated at 37 °C in 5% CO2 for 40 hours. Cell viability was assessed using the CellTiter-Glo assay kit as recommended by the supplier, and the plates were read using a plate reader (Tecan Infinite M2000 PRO; Tecan Group Ltd., Männedorf, Switzerland). Viability was calculated using the background-corrected absorbance as follows:Viability (%) = (A of experiment well/A of control well) × 100.
3.2.2. CPE reduction assay
MDCK cells (ATCC CCL-34, Manassas, VA, USA) were seeded into 96-well plates in DMEM supplemented with 10% FBS and incubated overnight at 37 °C under 5% CO2. The culture medium was replaced with the test compound and influenza virus (MOI = 0.1)-DMEM supplemented with 1% FBS and 2 μg mL–1 TPCK-treated trypsin. The final concentration of DMSO was 1%. After incubation for 40 hours, CellTiter-Glo reagent (Promega Corp., Madison, WI, USA) was added, and CPE was assessed by a CellTiter-Glo assay as described above.
3.2.3. Hemagglutination inhibition (HI) assay
The HI assay was performed as described previously.30 In brief, a compound from a threefold serial dilution in saline was mixed with an equal volume of influenza virus (2 HA units) in V-bottomed 96-well microplates. Subsequently, 50 μL freshly prepared chicken red blood cells (cRBCs) (1% v/v in saline) (Beijing Yuabio Biotechnology Co., Ltd, China) was added to each well. The mixture was incubated for 30 min at room temperature before the aggregation of cRBCs was observed on the plate.
3.2.4. Neuraminidase (NA) inhibition assay
The NA inhibition assay was performed utilizing NAs from the A/WSN/33 influenza virus. The activity of influenza virus NA was determined by quantifying the fluorescent product resulting from the cleavage of the substrate 4-methylumbelliferyl-α-d-N-acetylneuraminic acid sodium salt hydrate solution (MUNANA; Sigma, Saint Louis, MO, USA) by NA. The reaction mixture consisted of the tested compounds, the virus (as the source of NAs) and 20 μM 4-MUNANA in 32.5 mM MES buffer (containing 4 mM CaCl2, pH = 6.5) in a 96-well plate. After incubation for 30 min at 37 °C, the reaction was terminated with 150 μL of 34 mM NaOH, and the fluorescence of the mixture was recorded for an excitation wavelength of 360 nm and an emission wavelength of 460 nm.
Conclusions
A series of pentacyclic triterpene–Neu5Ac2en derivatives were synthesized and tested for their anti-influenza virus activity by a CPE assay. During the de-O-acetylation reaction of 8a or 8b under Zemplén conditions, a pair of configurational isomers were separated, and their configurations were characterized by detailed NMR and HRMS analysis. Benefiting from the Neu5Ac2en group, all the new conjugates exhibited decreased hydrophobicity with A log P values within the range of 2.52–4.86. The most potent inhibitory activity against the A/WSN/33 (H1N1) influenza virus was displayed by compounds 15a and 15c, with IC50 values of 8.3 and 15.5 μM, respectively, which were comparable to that of the known anti-influenza entry inhibitor curcumin, but these compounds were almost 2.7–5.0 times more potent than their analogs in our previous study.14 Compound 15a inhibited the hemagglutination of red blood cells whereas no inhibition of NA activity was observed, which implied that compound 15a targeted hemagglutinin-related functions such as hemagglutinin–sialic acid interactions during viral entry. These findings indicate that compound 15a could be a potential anti-influenza entry inhibitor, although more studies are necessary to investigate its anti-influenza effects in vivo.
Conflict of interest
The authors declare no competing interests.
Supplementary Material
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grants No. 81373271, 81361168002, 81573269 and 21572015). The authors gratefully acknowledge Dr. Hongwei Jin (State Key Laboratory of Natural and Biomimetic Drugs, Peking University) for carrying out the A log P determinations.
Footnotes
†Dedicated to Professor Lihe Zhang on the Occasion of His 80th Birthday.
‡Electronic supplementary information (ESI) available. See DOI: 10.1039/c7md00245a
References
- Horimoto T., Kawaoka Y., Kilbourne E. D. Nat. Rev. Microbiol. Emerging Infect. Dis. 2005;2006;312:591–600. 9. doi: 10.1038/nrmicro1208. [DOI] [PubMed] [Google Scholar]
- Cox N. J., Subbarao K., Loregian A., Mercorelli B., Nannetti G., Compagnin C., Palu G. Annu. Rev. Med. Cell. Mol. Life Sci. 2000;2014;5171:407–421. 3659. doi: 10.1146/annurev.med.51.1.407. [DOI] [PubMed] [Google Scholar]
- Potter C. W. J. Appl. Microbiol. 2001;91:572. doi: 10.1046/j.1365-2672.2001.01492.x. [DOI] [PubMed] [Google Scholar]
- Bedford T., Riley S., Barr I. G., Broor S., Chadha M., Cox N. J., Daniels R. S., Gunasekaran C. P., Hurt A. C., Kelso A., Klimov A., Lewis N. S., Li X., McCauley J. W., Odagiri T., Potdar V., Rambaut A., Shu Y., Skepner E., Smith D. J., Suchard M. A., Tashiro M., Wang D., Xu X., Lemey P., Russell C. A. Nature. 2015;523:217. doi: 10.1038/nature14460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zu M., Li C., Fang J. S., Lian W. W., Liu A. L., Zheng L. S., Du G. H. Molecules. 2015;20:19735. doi: 10.3390/molecules201119653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista E., Chotpitayasunondh T., Gao Z., Harper S. A., Shaw M., Uyeki T. M., Zaki S. R., Hayden F. G., Hui D. S., Kettner J. D., Kumar A., Lim M., Shindo N., Penn C., Nicholson K. G., N. Engl. J. Med., 2010, 362 , 1708 , Writing Committee of the WHO Consultation on Clinical Aspects of Pandemic (H1N1) 2009 Influenza Moscona A., N. Engl. J. Med., 2009, 360 , 953, Cheng P. K. C., To A. P. C., Leung T. W. C., Leung P. C. K., Lee C. W. C., Lim W. W. L., Emerging Infect. Dis., 2010, 16 , 155, Deyde V. M., Xu X. Y., Bright R. A., Shaw M., Smith C. B., Zhang Y., Shu Y. L., Gubareva L. V., Cox N. J., Klimov A. I., J. Infect. Dis., 2007, 196 , 249 . [DOI] [PubMed] [Google Scholar]
- Das K., Das K., Aramini J. M., Ma L. C., Krug R. M., Arnold E. J. Med. Chem. Nat. Struct. Mol. Biol. 2012;2010;5517:6263–6277. 530. [Google Scholar]
- Skehel J. J., Wiley D. C. Annu. Rev. Biochem. 2000;69:531. doi: 10.1146/annurev.biochem.69.1.531. [DOI] [PubMed] [Google Scholar]
- Sauter N. K., Bednarski M. D., Wurzburg B. A., Hanson J. E., Whitesides G. M., Skehel J. J., Wiley D. C. Biochemistry. 1989;28:8388. doi: 10.1021/bi00447a018. [DOI] [PubMed] [Google Scholar]
- Weis W., Brown J. H., Cusack S., Paulson J. C., Skehel J. J., Wiley D. C. Nature. 1988;333:426. doi: 10.1038/333426a0. [DOI] [PubMed] [Google Scholar]
- Toogood P. L., Galliker P. K., Glick G. D., Knowles J. R., Weinhold E. G., Knowles J. R. J. Med. Chem. J. Am. Chem. Soc. 1991;1992;34114:3138–3140. 9270. doi: 10.1021/jm00114a025. [DOI] [PubMed] [Google Scholar]
- Rudrawar S., Dyason J. C., Rameix-Welti M. A., Rose F. J., Kerry P. S., Russell R. J. M., van der Werf S., Thomson R. J., Naffakh N., von Itzstein M. Nat. Commun. 2010;1:113. doi: 10.1038/ncomms1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liby K. T., Yore M. M., Sporn M. B. Nat. Rev. Cancer. 2007;7:357. doi: 10.1038/nrc2129. [DOI] [PubMed] [Google Scholar]
- Han X., Shi Y., Si L., Fan Z., Wang H., Xu R., Jiao P., Meng K., Tian Z., Zhou X., Jin H., Wu X., Chen H., Zhang Y., Zhang L., Xiao S., Zhou D. MedChemComm. 2016;7:1932. [Google Scholar]
- Watowich S. J., Skehel J. J., Wiley D. C. Structure. 1994;2:719. doi: 10.1016/s0969-2126(00)00073-3. [DOI] [PubMed] [Google Scholar]
- Wu W. Y., Jin B., Krippner G. Y., Watson K. G. Bioorg. Med. Chem. Lett. 2000;10:341. doi: 10.1016/s0960-894x(00)00007-x. [DOI] [PubMed] [Google Scholar]
- Burmeister W. P., Henrissat B., Bosso C., Cusack S., Ruigrok R. W. H. Structure. 1993;1:19. doi: 10.1016/0969-2126(93)90005-2. [DOI] [PubMed] [Google Scholar]
- Smith P. W., Starkey I. D., Howes P. D., Sollis S. L., Keeling S. P., Cherry P. C., von Itzstein M., Wu W. Y., Jin B., Andrews D. M., Cherry P. C., Humber D. C., Jones P. S., Keeling S. P., Martin P. F., Shaw C. D., Swanson S., Rudrawar S., Kerry P. S., Rameix-Welti M. A., Maggioni A., Dyason J. C., Rose F. J., van der Werf S., Thomson R. J., Naffakh N., Russell R. J. M., von Itzstein M., Pascolutti M., Bohm R., Maggioni A., Dyason J. C., Thomson R. J., von Itzstein M. Eur. J. Med. Chem. Eur. J. Med. Chem. Org. Biomol. Chem. Glycobiology. 1996;1999;2012;2014;31341024:143. 563, 8628, 1127. [Google Scholar]
- Li J., Zheng M. Y., Tang W., He P. L., Zhu W. L., Li T. X., Zuo J. P., Liu H., Jiang H. L. Bioorg. Med. Chem. Lett. 2006;16:5009. doi: 10.1016/j.bmcl.2006.07.047. [DOI] [PubMed] [Google Scholar]
- Lu Y., Gervay-Hague J. Carbohydr. Res. 2007;342:1636. doi: 10.1016/j.carres.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W., Zhao J., Yu H., Li X., Sun S., Li Y., Xia Q., Zhang C., He Q., Gao X., Zhang L., Zhou D. PLoS One. 2014;9:e111911. doi: 10.1371/journal.pone.0111911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Yu F., Wang Q., Zhang Z., Peng Y., Qiu Y., Shi Y., Zheng Y., Xiao S., Wang H., Huang X., Zhu L., Chen K., Zhao C., Zhang C., Yu M., Sun D., Zhang L., Zhou D. J. Med. Chem. 2013;56:4300. doi: 10.1021/jm301910a. [DOI] [PubMed] [Google Scholar]; (b) Xiao S., Wang Q., Si L., Shi Y., Wang H., Yu F., Zhang Y., Li Y., Zheng Y., Zhang C., Wang C., Zhang L., Zhou D. ChemMedChem. 2014;9:1060. doi: 10.1002/cmdc.201300545. [DOI] [PubMed] [Google Scholar]
- Chandler M., Bamford M. J., Conroy R., Lamont B., Patel B., Patel V. K., Steeples I. P., Storer R., Weir N. G., Wright M., Williamson C. J. Chem. Soc., Perkin Trans. 1. 1995:1173. [Google Scholar]
- Zemplen G., Pascu E. Ber. Dtsch. Chem. Ges. 1929;62:1613. [Google Scholar]
- Yang J., Li M. M., Shen X. T., Liu S. W. Viruses. 2013;5:352. doi: 10.3390/v5010352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou J. L., Mizushina Y., Wang S. Y., Chuang D. Y., Nadar M., Hsu W. L. FEBS J. 2013;280:5829. doi: 10.1111/febs.12503. [DOI] [PubMed] [Google Scholar]
- Holzer C. T., Vonitzstein M., Jin B., Pegg M. S., Stewart W. P., Wu W. Y. Glycoconjugate J. 1993;10:40. doi: 10.1007/BF00731185. [DOI] [PubMed] [Google Scholar]
- Yu F., Peng Y., Wang Q., Shi Y., Si L., Wang H., Zheng Y., Lee E., Xiao S., Yu M., Li Y., Zhang C., Tang H., Wang C., Zhang L., Zhou D., Kuhn R., Lutz P., Macdonald D. L., Ikeda K., Nagao Y., Achiwa K. Eur. J. Med. Chem. Chem. Ber./Recl. Carbohydr. Res. 2014;1966;1992;7799224:258. 611, 123. doi: 10.1016/j.ejmech.2014.03.017. [DOI] [PubMed] [Google Scholar]
- Hamilton B. S., Whittaker G. R., Daniel S. Viruses. 2012;4:1144. doi: 10.3390/v4071144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M., Si L., Wang Y., Wu Y., Yu F., Jiao P., Shi Y., Wang H., Xiao S., Fu G., Tian K., Wang Y., Guo Z., Ye X., Zhang L., Zhou D. J. Med. Chem. 2014;57:10058. doi: 10.1021/jm5014067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.