 We describe the use of the ortho-nitrophenyl-β-galactoside (ONPG) assay developed by Lehrer et al. to which a new mathematical data treatment was applied.
We describe the use of the ortho-nitrophenyl-β-galactoside (ONPG) assay developed by Lehrer et al. to which a new mathematical data treatment was applied.
Abstract
We describe the use of the ortho-nitrophenyl-β-galactoside (ONPG) assay developed by Lehrer et al. to which a new mathematical data treatment was applied. In this simplified assay, only one enzymatic assay is needed to provide direct evidence of the kinetics of Escherichia coli membrane permeabilization induced by different concentrations of benzalkonium chloride (BAC). Analysis of the data obtained from the revised ONPG assay with our adapted mathematical formula indicates that BAC induces permeabilization of the bacterial outer and inner membranes in a two-step process. The two effective concentration (EC50) values obtained in this study, combined with the results from an outer membrane permeabilization assay, suggest that the two steps observed in the permeabilization process are related to the two different bacterial membranes. We show that membrane permeabilization occurs very fast upon the addition of bacterial cells to the BAC solutions and demonstrate that sub-lethal concentrations of BAC disturb the integrity of the Gram-negative bacterial membranes. Overall, our work broadens our knowledge on the mode of action of BAC on bacterial cells and emphasizes that BAC, and quaternary ammonium compounds in general, should not be used at sub-lethal concentrations in order to lower the risk of bacterial tolerance and resistance to antibiotics.
The emergence of antibiotic resistance is a serious worldwide threat, primarily due to the widespread use of antibiotics.1 Quaternary ammonium compounds (QACs) are topical antibacterial compounds that permeabilize bacterial membranes.2 QACs are active both on Gram-positive and Gram-negative bacterial pathogens.3 While Gram-positive bacterial cells are surrounded by an inner membrane and a thick layer of cell wall, Gram-negative bacterial cells are protected by a more complex and impermeable envelope. This envelope contains an inner membrane made of a phospholipid bilayer with incorporated and associated proteins, a thin layer of cell wall mainly constituted of a network of polysaccharides and peptides called peptidoglycan, and an asymmetric outer membrane containing phospholipids in its inner leaflet, lipopolysaccharides in its outer leaflet and also harboring proteins such as porins.4 This multi-layered structure forms a physical barrier that renders Gram-negative bacterial cells naturally resistant to many antibacterial agents.5,6
QACs have been actively deployed since the 1930s and are now widely used in hospitals, industry and cosmetics.7 They have played a major role in reducing the number of bacterial infections through effective hygiene. However, the widespread and inappropriate use of QACs could compromise their effectiveness.8,9 This is a general concern based on the association between the use of low doses of antimicrobial agents and their potential to select for less sensitive bacteria that can eventually also become resistant to other antibiotics.7,10,11 Most QAC formulations do not require rinsing with water after application. Bacterial cells thus remain exposed to small concentrations of QACs over long periods of time. Long-term exposure to a QAC selects for the survival of bacterial clones that are more resistant and that could only be treated effectively with higher doses of the antimicrobial agent.12 Several studies have shown that exposure to sub-inhibitory concentrations of biocides can change the antimicrobial susceptibility of bacterial cultures.9 Changes in the minimum inhibitory concentrations (MICs) of QACs have often been associated with the acquisition or hyperexpression of plasmid-encoded multi-drug efflux pumps (i.e. QAC genes).13 Such efflux pumps can actively secrete QACs through the bacterial envelope and release them into the extracellular environment, thereby reducing their effectiveness. These multi-drug efflux pumps have also been associated with changes in the MIC of therapeutically important third-party antibiotics that are substrates of the pumps.14 Long-term exposure to sub-MICs of QACs could thus be the preoccupying selective pressure that drives the emergence of multi-resistant bacterial clones.14
The most common QAC is benzalkonium chloride (BAC), a mixture of alkyl-benzyl-dimethyl-ammonium chlorides with various alkyl chains, typically ranging from 8 to 18 carbons (Fig. 1).15 The antibacterial effect of BAC was proposed to be based on the association between the positively charged quaternary nitrogen and the negatively charged polar groups of acidic phospholipids in bacterial membranes.16 This association would drive the integration of the hydrophobic tail of BAC into the bacterial membrane, at the level of the hydrophobic fatty acids of phospholipids. At high concentrations, BAC solubilizes hydrophobic membrane components by forming micellar aggregates.3 The antibacterial activity of BAC may also involve the disruption and denaturation of structural membrane proteins.17
Fig. 1. Chemical structure of benzalkonium chloride (BAC).

The outer membrane of Gram-negative bacterial cells constitutes a semi-permeable barrier that prevents many otherwise effective antibacterial agents from reaching their molecular targets in bacteria.5,6 The action of QACs on bacterial cells alters the permeability of the outer membrane and thus affects their antibiotic susceptibility. Restricted outer membrane permeability works in synergy with co-determinant resistance mechanisms, such as active efflux mechanisms, bringing about antibiotic resistance.18 QACs also permeabilize the bacterial inner membrane, and the overall effect generated by sub-lethal concentrations of QACs on the bacterial envelope contributes to broad-spectrum antibiotic resistance.14 The most influential factors that impact the emergence of resistance to QACs, and especially to BAC, are the kinetics of the treatment, the time of exposure to the drug, the quantity of bacterial cells, and their growth conditions. Although in vitro assays with phospholipids and artificial membranes provide valuable information on the antibacterial activity of QACs,2 studying the effects of QACs on bacterial cells is necessary to get a better understanding of their impact on bacterial membranes and their contribution to the development of antibiotic resistance. To reach this goal, we quantitatively evaluated the effect of BAC on bacterial membranes at sub-lethal concentrations or around its MIC over time.
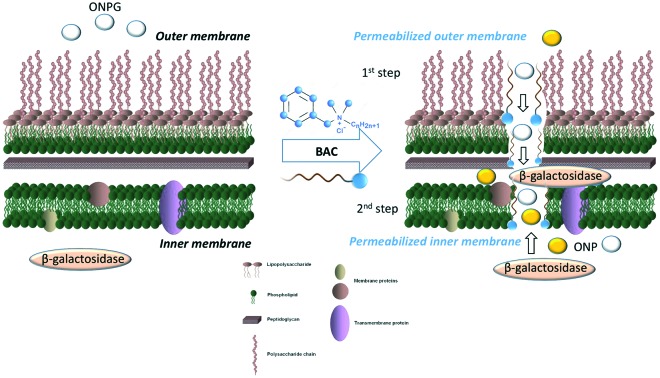
Lehrer et al. developed a dual spectrophotometric assay to measure the permeabilization of bacterial membranes. They studied the permeabilization of the Gram-negative outer membrane according to the activity of a constitutively expressed β-lactamase enzyme present in the periplasmic space, sandwiched between the outer and inner membranes. This assay is based upon a β-lactamase substrate that is impermeable to the other membrane.19 Lehrer et al. simultaneously monitored the permeabilization of the bacterial inner membrane as the chromogenic substrate ortho-nitrophenyl-β-galactoside (ONPG) diffuses through the phospholipid barrier in the absence of the galactoside permease LacY and is hydrolyzed to ortho-nitrophenol (ONP) by the constitutively expressed cytoplasmic β-galactosidase enzyme LacZ.19 In this assay, ONPG has to cross first the outer membrane through non-specific porins before passing through the inner membrane to access cytoplasmic LacZ.
Here, we hypothesized that Lehrer's assay can be adapted for the study of both outer and inner membrane permeabilization kinetics using an appropriate protocol and mathematical data treatment to analyse ONPG hydrolysis data. We developed this optimized assay in a wild-type strain of the bacterium Escherichia coli, a recognized model system to study the Gram-negative bacterial envelope. The wild-type strain E. coli MG1655 contains the native lactose operon, including the gene coding for the inner membrane galactoside permease LacY.
The mathematical treatment we propose here is based on the standardization of each kinetic experiment using a maximal and minimal enzymatic reaction rate of ONPG hydrolysis. Lehrer et al. demonstrated that bacterial cell lysis by sonication is the most efficient way to measure the total activity of the β-galactosidase LacZ.20 We thus used this method to lyse bacterial cells and release the entire pool of cytoplasmic β-galactosidase into the extracellular medium in order to obtain the maximal enzymatic rate of ONPG hydrolysis. Each experiment was standardized using eqn (1). This ensures that native levels of passive diffusion and potential active transport of ONPG through the bacterial membranes are not taken into account. Reaction rates were determined as the slope of the curve for the first 360 s of ONPG hydrolysis. It should be noted that, in our assay, bacterial cells were added to the ONPG-containing buffer immediately prior to data acquisition. Under these conditions, the concentration of ONPG in the periplasmic space can be considered as null and ONPG must pass through both the outer and inner membranes to be in contact with the cytoplasmic β-galactosidase enzyme for hydrolysis to occur. Each curve presented in this article thus represents the kinetics of the enzymatic hydrolysis of ONPG in ONP induced by the presence of a membrane-permeabilizing agent.
 |
1 |
where: RRx is the reaction rate of ONPG hydrolysis at [permeabilizing agent] = x; RR0 is reaction rate of ONPG in the absence of a permeabilizing agent; RRmax: is the reaction rate of ONPG hydrolysis in the cellular lysate and RRr is reaction rate ratio.
Our adapted membrane permeabilization assay was first tested by monitoring the enzymatic hydrolysis of ONPG at different concentrations of polymyxin B (see ESI‡ Fig. S6). Polymyxin B is a cationic nonapeptide known to increase the permeability of the bacterial outer membrane by interacting with the lipopolysaccharide components.21 The MIC value of polymyxin B has been previously reported to be 1 μg mL–1 for the strain E. coli IH3080 (EM40),22 but it was shown that polymyxin B permeabilizes the bacterial outer membrane at concentrations from 0.3 to 1 μg mL–1.21 To measure the MIC of polymyxin B for the wild-type strain E. coli MG1655, bacterial cells were incubated with different concentrations of polymyxin B and the MIC was determined as the lowest concentration that inhibited bacterial growth at 37 °C. The MIC of polymyxin B for E. coli MG1655 was established to be 5 ± 1 μg mL–1.
Lehrer et al. demonstrated that polymyxin B treatment leads to a maximal readout for β-lactamase activity, whereas it did not have a significant effect on β-galactosidase activity in their assay.19 Studying the dose–response relationship of polymyxin B with our adapted assay and mathematical data treatment showed a concentration-dependent rate of ONPG hydrolysis that does not exceed 40%, even at 60-fold its MIC (Fig. 2).
Fig. 2. Dose–response relationship between the concentration of polymyxin B and the ONPG hydrolysis rate for a bacterial population of 7.2 × 107 colony forming units (CFU) mL–1 (OD600nm = 0.27). The inserted box represents a zoom of the sub-MIC concentrations.
As mentioned before, the initial ONPG concentration in the bacterial periplasm should be null in our assay. The monitored reaction observed with polymyxin B is thus probably related to the destabilization of the outer membrane that directly facilitates the influx of ONPG in the periplasm and further indirectly increases ONPG access to the cytoplasm. The reaction rate must be limited by the passage of ONPG through the inner membrane.
The MIC of BAC for the wild-type strain E. coli MG1655 was determined to be 45 ± 5 μg mL–1. Kinetics of ONPG hydrolysis in real time, upon the exposure of E. coli cells to BAC, revealed key information about the permeabilization process. With the mathematical approach that we described above, we were able to determine the minimal BAC concentration that has an immediate effect on the bacterial envelope. This concentration of BAC is below the MIC; it does not inhibit bacterial growth nor kill bacterial cells. However, exposure to this sub-lethal concentration of BAC has a membrane-permeabilizing effect and might thus give rise to resistant bacterial clones.
E. coli membrane permeabilization was observed by monitoring the enzymatic hydrolysis of ONPG at different concentrations of BAC (see ESI‡ Fig. S7–S11, for different bacterial populations). While membrane permeabilization by polymyxin B was observed as a one-step process (Fig. 2), all the curves obtained for BAC show a biphasic dose–response function. This indicates that the permeabilization process induced by BAC is a two-step process. The inflexion points of each phase of the curve in Fig. 3, also called effective concentrations (EC50), were calculated using the biphasic dose–response function analysis implemented in OriginPro 8. The results are reported in Fig. 3 and Table 1.
Fig. 3. Dose–response relationship between the concentration of BAC and the ONPG hydrolysis rate for bacterial populations of 2.5 × 107 CFU mL–1 (OD600nm = 0.11, ■ black squares), 5.4 × 107 CFU mL–1 (OD600nm = 0.21,  red circles), 7.8 × 107 CFU mL–1 (OD600nm = 0.29,
red circles), 7.8 × 107 CFU mL–1 (OD600nm = 0.29,  blue triangles), 1.0 × 108 CFU mL–1 (OD600nm = 0.37,
blue triangles), 1.0 × 108 CFU mL–1 (OD600nm = 0.37,  green triangles) and 1.4 × 108 CFU mL–1 (OD600nm = 0.51,
green triangles) and 1.4 × 108 CFU mL–1 (OD600nm = 0.51,  pink triangles). Each data point is the average of three different experiments. Error bars represent standard deviations. Curves were fitted using the biphasic dose–response function analysis with OriginPro 8.
pink triangles). Each data point is the average of three different experiments. Error bars represent standard deviations. Curves were fitted using the biphasic dose–response function analysis with OriginPro 8.
Table 1. EC50 of BAC determined from the biphasic dose–response analysis.
| Bacterial population (CFU mL–1) | 1st EC50 (μg mL–1) | 2nd EC50 (μg mL–1) |
| 2.5 × 107 | 31 ± 1 | 61 ± 2 |
| 5.4 × 107 | 31.1 ± 0.8 | 72 ± 1 |
| 7.8 × 107 | 30.5 ± 0.8 | 78 ± 1 |
| 1.0 × 108 | 33 ± 1 | 94 ± 2 |
| 1.4 × 108 | 31.3 ± 0.5 | 101 ± 2 |
The 1st EC50 value represents the minimal critical concentration of BAC necessary to induce considerable ONPG hydrolysis. Above this value of about 30 μg mL–1BAC, the reaction rate ratio keeps increasing up to a concentration of about 40 μg mL–1BAC before it reaches a plateau (Fig. 3). The BAC concentration that significantly leads to bacterial membrane permeabilization is thus lower than the determined MIC value of 45 μg mL–1. After the plateau, the reaction rate ratio of ONPG hydrolysis increases up to 100% (Fig. 3). This represents the total activity of the β-galactosidase LacZ in the absence of any membrane barrier. Our data thus suggest that high concentrations of BAC can disrupt both the outer and inner membranes at the levels of which these envelope layers no longer restrict the access of ONPG to cytoplasmic LacZ. The effect of BAC on the bacterial inner membrane might also allow the β-galactosidase LacZ to leak out of the bacterial cytoplasm.
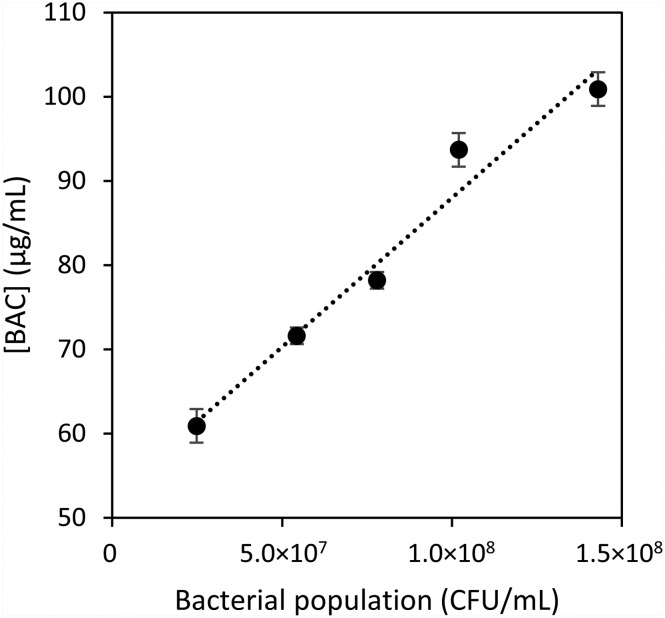
As shown in Table 1 and Fig. 3 and 4, the 1st EC50 value is independent of the bacterial population, whereas the 2nd EC50 shows a linear relationship with the number of bacterial cells used in the experiments. As the isopropyl-β-d-1-thiogalactopyranoside (IPTG) used to induce lacZ expression is a non-metabolizable analogue of lactose, its concentration remains stable in a bacterial population, and the number of β-galactosidase enzymes accumulates as the number of bacterial cells increases. This is supported by the fact that the maximal values of β-galactosidase activity increase in the cell lysate controls as the number of bacterial cells increases (see ESI‡ Fig. S7–S11). Our data thus indicate that the number of β-galactosidase enzymes is not a limiting factor for the 1st EC50 value that is independent of the bacterial population. The 1st EC50 value must be mainly limited by the amount of ONPG that reaches the cytoplasm. However, the 2nd EC50 is dependent on the number of β-galactosidases available in the experiments, and this suggests that ONPG freely passes the bacterial membranes at these concentrations of BAC.
Fig. 4. Linear relationship between the 2nd EC50 and the bacterial population. Error bars represent the calculated error from the curve fitting. y = 3.54 × 107x + 52.6; R2 = 0.9674.
In order to test our model according to which the 1st EC50 value obtained in the ONPG assay is related to the effect of BAC on the outer membrane of E. coli cells, we performed an outer membrane permeabilization assay using the hydrophobic fluorescent probe 1-N-phenylnaphthylamine (NPN) (Fig. 5).20 NPN is largely excluded by intact Gram-negative bacteria. However, enhanced uptake of NPN occurs in cells with a damaged outer membrane. NPN has a low fluorescence quantum yield in aqueous solution, but becomes strongly fluorescent in a hydrophobic environment. Therefore, its interaction with phospholipids in the bacterial envelope increases its fluorescence signal that can be used as an indication of the compromised bacterial outer membrane.23,24 To study the mode of action of BAC, we exposed E. coli cells to different concentrations of BAC in the presence of NPN. At higher concentrations, BAC can form micelles (above 150 μg mL–1),25 and NPN insertion into their hydrophobic core could occur. The dose–response curves were obtained using the maximum of fluorescence at 3000 s, where the equilibrium was reached for all the experiments (see ESI‡ Fig. S17–S21). For the mathematical data treatment, the maximum of fluorescence was arbitrarily considered the one induced by a BAC concentration of 150 μg mL–1 and the minimal response was the one obtained in the absence of BAC in order to take into account all the parameters that can affect NPN fluorescence. As previously observed for polymyxin B, NPN insertion shows a monophasic BAC concentration dependence. As shown in Fig. 5, NPN started to become fluorescent at a BAC concentration of 30 μg mL–1, which corresponds to the 1st EC50 value obtained in the ONPG assay (Fig. 3 and Table 1). At this concentration, the outer membrane is permeable enough to allow ONPG diffusion and enzymatic hydrolysis and NPN insertion into the phospholipid hydrophobic environment. Furthermore, no difference was observed in the relative fluorescence intensity of NPN when different bacterial populations were used. These two observations support the hypothesis that the 1st EC50 value corresponds to the permeabilization of the outer membrane.
Fig. 5. Relative fluorescence of NPN induced by different concentrations of BAC. Data were recorded at 3000 s at different bacterial populations: 2.5 × 107 CFU mL–1 (OD600nm = 0.11,  blue circles); 6.0 × 107 CFU mL–1 (OD600nm = 0.23,
blue circles); 6.0 × 107 CFU mL–1 (OD600nm = 0.23,  red squares); 7.5 × 107 CFU mL–1 (OD600nm = 0.28,
red squares); 7.5 × 107 CFU mL–1 (OD600nm = 0.28,  green triangles); 1.1 × 108 CFU mL–1 (OD600nm = 0.41, ♦ black diamonds). Each data point is the average of three different experiments. Error bars represent standard deviations.
green triangles); 1.1 × 108 CFU mL–1 (OD600nm = 0.41, ♦ black diamonds). Each data point is the average of three different experiments. Error bars represent standard deviations.
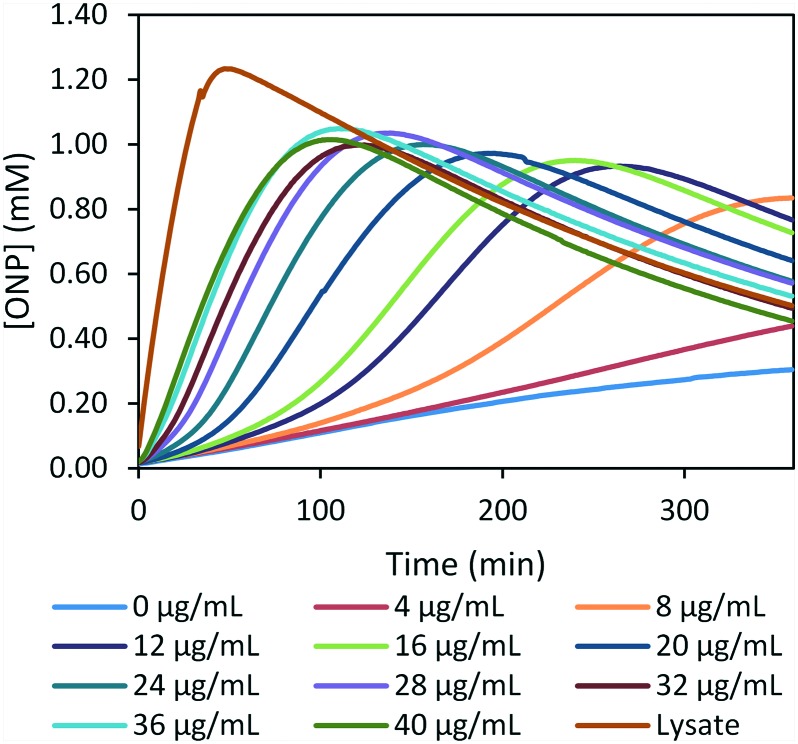
To assess if sub-lethal concentrations of BAC can permeabilize both bacterial membranes, we studied the effect of low sub-MIC concentrations of BAC on bacterial cells over time (Fig. 6). In this case, we monitored ONP production over time. As bacterial cells were kept in PBS buffer, we did not observe any bacterial growth over the period of these experiments. Under these conditions, most sub-lethal concentrations of BAC lead to complete ONPG hydrolysis over time, but the permeabilization process is slower when compared to the lysate control. The time lag needed to reach full ONPG consumption is dependent on the BAC concentration. These results indicate that BAC affects the integrity of both bacterial membranes over time, even at very low concentrations.
Fig. 6. Long-term membrane permeabilization induced by BAC at sub-MIC concentrations at a bacterial population of 2.2 × 107 CFU mL–1 (OD600nm = 0.10). Each curve is the average of three different experiments. The observed decrease in ONP's absorption is attributed to its slow ionization in the buffered solution (see ESI‡ Fig. S22).
Conclusions
Here, we adapted the assay developed by Lehrer et al. for the study of bacterial membrane permeabilization kinetics in a wild-type strain of the Gram-negative bacterial species E. coli, using a single enzymatic assay and developing a novel mathematical analysis to treat data obtained from ONPG hydrolysis by the β-galactosidase LacZ. This assay gives the possibility to extract quantitative values and should allow scientists to compare the efficacy of different permeabilizing agents. It should also allow a deeper understanding of the mode of action of these permeabilizing agents on bacterial membranes. This is necessary to avoid their usage at inappropriate concentrations, as this can compromise their effectiveness and drive the emergence of bacterial resistance.
Using our adapted assay, we studied the effect of different BAC concentrations on the E. coli cell membranes. We show that membrane permeabilization occurs very fast, immediately after the addition of bacteria to the BAC solutions. Our data demonstrate that the most common QAC, BAC, induces the permeabilization of the outer and inner membranes of Gram-negative bacterial cells in a two-step process. Analysis of the two EC50 values of BAC obtained from our adapted ONPG assay, combined with the data of the NPN assay that detects outer membrane permeability defects, enabled us to dissect the steps of the permeabilization process. BAC seems to affect Gram-negative bacterial cells by first disrupting their outer membrane, and then permeabilizing their inner membrane. Further investigation should allow us to verify this hypothesis and to study the membrane permeabilization kinetics of other permeabilizing agents.
Quite importantly, we observed that BAC concentrations lower than the MIC value permeabilize both bacterial membranes almost completely over time. This effect of sub-lethal concentrations of BAC, and of QACs in general, on Gram-negative bacterial membranes must be considered when using these antibacterial agents. The permeabilization of bacterial membranes by low-doses of QACs probably activates envelope stress responses in bacteria and therefore leads to the development of bacterial tolerance and resistance to antibiotics. Exposure of bacteria to sub-lethal concentrations of BAC should thus be avoided, especially in the current context of the emergence of multi-resistant bacterial pathogens.
Supplementary Material
Acknowledgments
We gratefully acknowledge the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Université de Montréal for financial support (JG is a NSERC scholar and ARS is the PI for grant 03866). We thank professors J. N. Pelletier and K. J. Wilkinson for the access to their laboratories and instruments. We also thank our colleagues from the Département de Microbiologie, Infectiologie et Immunologie – Université de Montréal for the use of instruments.
Footnotes
†The authors declare no competing interests.
‡Electronic supplementary information (ESI) available: Detailed experimental procedures and data treatment. See DOI: 10.1039/c7md00113d
References
- World Health Organization, http://www.who.int/mediacentre/factsheets/fs194/en/ (accessed February 17, 2017).
- Buffet-Bataillon S., Tattevin P., Bonnaure-Mallet M., Jolivet-Gougeon A. Int. J. Antimicrob. Agents. 2012;39:381–389. doi: 10.1016/j.ijantimicag.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Gilbert P., Moore L. E. J. Appl. Microbiol. 2005;99:703–715. doi: 10.1111/j.1365-2672.2005.02664.x. [DOI] [PubMed] [Google Scholar]
- Dufresne K., Paradis-Bleau C. Adv. Exp. Med. Biol. 2015;883:41–76. doi: 10.1007/978-3-319-23603-2_3. [DOI] [PubMed] [Google Scholar]
- Delcour A. H. Biochim. Biophys. Acta. 2009;1794:808–816. doi: 10.1016/j.bbapap.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H. Drug Resist. Updates. 1998;1:93–98. doi: 10.1016/s1368-7646(98)80023-x. [DOI] [PubMed] [Google Scholar]
- Gilbert P., McBain A. J. Surg. Infect. 2002;3(Suppl 1):S55–S63. doi: 10.1089/sur.2002.3.s1-55. [DOI] [PubMed] [Google Scholar]
- Bloomfield S. F. J. Appl. Microbiol. 2002;92:144S–157S. [PubMed] [Google Scholar]
- Braoudaki M., Hilton A. C. J. Clin. Microbiol. 2004;42:73–78. doi: 10.1128/JCM.42.1.73-78.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert P., Al-taae A. Lett. Appl. Microbiol. 1985;1:101–104. [Google Scholar]
- Gilbert P., McBain A. J. Clin. Microbiol. Rev. 2003;16:189–208. doi: 10.1128/CMR.16.2.189-208.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBain A. J., Rickard A. H., Gilbert P. J. Ind. Microbiol. Biotechnol. 2002;29:326–330. doi: 10.1038/sj.jim.7000324. [DOI] [PubMed] [Google Scholar]
- Heir E., Sundheim G., Holck A. L. J. Appl. Microbiol. 1999;86:378–388. doi: 10.1046/j.1365-2672.1999.00672.x. [DOI] [PubMed] [Google Scholar]
- Poole K. Clin. Microbiol. Infect. 2004;10:12–26. doi: 10.1111/j.1469-0691.2004.00763.x. [DOI] [PubMed] [Google Scholar]
- Walker E. B., in Handbook of topical antimicrobials: industrial applications in consumer products and pharmaceuticals, ed. D. S. Paulson, NY: Marcel Dekker, New York, 2003, pp. 99–116. [Google Scholar]
- Ioannou C. J., Hanlon G. W., Denyer S. P. Antimicrob. Agents Chemother. 2007;51:296–306. doi: 10.1128/AAC.00375-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredell D. L., in Cationic surfactants, ed. J. Cross and E. J. Singer, NY: Marcel Dekker, New York, 1994, pp. 31–60. [Google Scholar]
- Kumar A., Schweizer H. P. Adv. Drug Delivery Rev. 2005;57:1486–1513. doi: 10.1016/j.addr.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I., Barton A., Ganz T. J. Immunol. Methods. 1988;108:153–158. doi: 10.1016/0022-1759(88)90414-0. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I., Barton A., Daher K. A., Harwig S. S., Ganz T., Selsted M. E. J. Clin. Invest. 1989;84:553–561. doi: 10.1172/JCI114198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martti V. Microbiol. Rev. 1992;53:395–411. [Google Scholar]
- Martti V., Timo V. Antimicrob. Agents Chemother. 1983;24:107–113. doi: 10.1128/aac.24.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh B., Grant C., Hancock R. E. Antimicrob. Agents Chemother. 1984;26:546–551. doi: 10.1128/aac.26.4.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson M., Nielsen P. E., Good L. J. Biol. Chem. 2002;277:7144–7147. doi: 10.1074/jbc.M106624200. [DOI] [PubMed] [Google Scholar]
- Deutschle T., Porkert U., Reiter R., Keck T., Riechelmann H. Toxicol. In Vitro. 2006;20:1472–1477. doi: 10.1016/j.tiv.2006.07.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.