Abstract
Background:
Radon is the second most important cause of lung cancer, ranked by the World Health Organization as the fifth leading cause of mortality in 2010. An updated database of national radon exposures for 66 countries allows the global burden of lung cancer mortality attributable to radon to be estimated.
Objective:
Our goal was to estimate the global population attributable burden of lung cancer mortality in 2012 from residential radon.
Methods:
Estimates of the population attributable risk (PAR) of lung cancer mortality from radon were determined using the attributable fraction approach, using three models for excess relative risk of lung cancer from radon.
Results:
The estimates of the median PAR of lung cancer mortality from residential radon in 2012 for the 66 countries having representative national radon surveys were consistent, as 16.5%, 14.4%, and 13.6% for the exposure–age–concentration (EAC) model (BEIR VI), the Hunter model, and the Kreuzer model, respectively. The mean PAR using the EAC model ranged from 4.2% (95% CI: 0.9, 11.7) for Japan, to 29.3% (95% CI: 22.9, 35.7) for Armenia, with a median for the 66 countries of 16.5%. Radon-attributable lung cancer deaths for all 66 countries totaled 226,057 in 2012 and represent a median of 3.0% of total cancer deaths.
Conclusions:
Consistent findings between the three models used to estimate excess relative risks of lung cancer from radon, and between the attributable fraction methodology and the life table analysis, confirm that residential radon is responsible for a substantial proportion of lung cancer mortality worldwide. https://doi.org/10.1289/EHP2503
Introduction
Radon is the second most important cause of lung cancer, after smoking (WHO 2006). Radon-222 and its decay products were classified as a known cause of human cancer by the International Agency for Research on Cancer (IARC) in 1988 (IARC 2012). Radon-222 is a colorless, odorless, radioactive gas that occurs naturally when the uranium-238 present in rock and soil undergoes radioactive decay. The short-lived radon progeny (or decay products) are also radioactive. The alpha radiation emitted by radon progeny inhaled into the lungs can damage cellular DNA, which can eventually result in clinically evident lung cancer. Radon can accumulate in poorly ventilated buildings, especially in basements, when radon from the ground seeps in through entry points such as cracks in the foundations.
Strong and complementary evidence of the risks of lung cancer from cumulative exposure to radon and its progeny via inhalation have been determined from studies of occupational exposures of uranium miners and residential exposures of the public (Tirmarche et al. 2010). An inverse dose–rate effect was reported in the analysis of pooled data from 11 cohort studies of radon-exposed miners reviewed by Lubin et al. (1995). The risk to miners exposed at lower doses initiated studies of the risk to the general public from residential radon. Evaluation of the lower residential radon exposures of the pooled case–control studies yielded results consistent with those from the miner studies, and showed that the odds ratio of lung cancer generally increased with radon concentration and was consistent with linearity. After adjustments for uncertainties in radon measurements, the excess risk ratio (ERR) was 0.16 [95% confidence interval (CI): 0.05, 0.31] for European pooling (Darby et al. 2006) and the excess odds ratio (EOR) was 0.18 (95% CI: 0.02, 0.43) per for North American pooling (Krewski et al. 2006). Recent estimates of ERR per working level month (ERR/WLM) were derived from studies of miners having low radon exposure rates and low cumulative exposures similar to those from residential radon: (95% CI: 0.009, 0.0035) from three European nested case–control miner studies restricted to cumulative exposures WLM (Hunter et al. 2013); and (95% CI: 0.009, 0.0035) from a German miner cohort with mean cumulative exposure of 17 WLM (range: 0–334 WLM) (Kreuzer et al. 2015). WLM, working level month, is a cumulative exposure defined as breathing a concentration of 1 working level (WL; ) for a working month of 170 h. ( per hour for equilibrium equivalent concentration of radon.
The global burden from lung cancer mortality is significant and steadily increasing. The World Health Organization (WHO) Global Burden of Disease (GBD) reported that trachea, bronchus, and lung cancers represented 19% of all cancer deaths and ranked as the fifth leading cause of death worldwide (Lozano et al. 2012). Although the age-standardized lung cancer mortality rate decreased by 9%, the number of lung cancer deaths increased by 57% between 1990 and 2013, primarily due to the aging of the population in low- and middle-income countries (Naghavi et al. 2015). The goal of this research was to compare estimates of the number, the percentage of total cancer deaths, and the population attributable risk (PAR) for lung cancer mortality in 2012 due to residential radon using an updated global radon database.
Methods
Study Design
The global burden of lung cancer mortality attributed to residential radon was estimated for the 66 countries that have conducted a representative national radon survey. The lung cancer mortality attributable to radon was estimated from the attributable fraction approach, using three different models to calculate the excess rate ratio from radon: the BEIR VI (sixth Committee on Biological Effects of Ionizing Radiation) exposure–age–concentration (EAC) model (NRC 1999), the Hunter et al. (2013) model, and the Kreuzer et al. (2015) model. Estimates of the mean and 95% CIs were determined for the PAR and the number of lung cancer deaths attributable to radon, with the uncertainty assessed using Monte Carlo simulation with 10,000 replications to sample the national distributions for residential radon and adult smoking prevalence for men and women and probabilistic distributions for the excess rate ratio models.
National Assessment of Lung Cancer Mortality Attributed to Residential Radon
National radon exposure.
The International Atomic Energy Agency (IAEA) has summarized the methodology and measurement techniques suitable for indoor radon surveys that are representative of a national or regional population (IAEA 2013). An estimate of either arithmetic or geometric mean (GM) national residential radon exposure was included in the updated database if it resulted from a systematic survey using a reliable radon measurement technique. The Medline database was searched on 20 May 2017, using MeSH terms and keywords “Radon Daughters/or Radon/” and “residential.mp.”, published between 2000 and 2017. Of the 1,599 papers identified, 27 were used to update the United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) 2000 and 2006 databases (original sources referenced when provided). Radon distributions for the 66 countries included in the analysis are shown in Table 1 and the references listed in Table S1. The lognormal distribution [defined by the geometric mean (GM) and geometric standard deviation (GSD)] characterizing the residential radon for each country is sampled in the Monte Carlo simulation to provide a probabilistic estimate of the mean and 95% CIs of the lung cancer mortality attributable to radon. For the 22 countries with incomplete radon data, the geometric standard deviation (GSD) was imputed from the mean based on linear regression of the data from countries reporting both a mean and a GSD.
Table 1.
Radon population attributable risk (PAR) of lung cancer mortality.
| Country | Radona [GM (GSD)] () | PAR (%), BEIR VIb [mean (95% CI)] | PAR (%), Hunterc [mean (95% CI)] | PAR (%), Kreuzerd [mean (95% CI)] | Attributable number lung cancer deaths, BEIR VIb [mean (95% CI)] | Total Cancer Deathse (%) |
|---|---|---|---|---|---|---|
| Median | 38 | 16.5 | 14.4 | 13.6 | 630 | 3.0 |
| Albania | 75 (2.2) | 23.5 (8.1, 36.6) | 24.4 (6.8, 37.5) | 23.2 (12.5, 32.1) | 246 (85, 383) | 5.2 |
| Algeria | 22 (2.2) | 15.8 (3.4, 37.0) | 9.0 (2.2, 15.1) | 8.3 (4.1, 12.3) | 376 (82, 882) | 1.7 |
| Argentina | 27 (2.2) | 13.6 (3.1, 30.7) | 10.8 (2.6, 18.0) | 10.0 (4.9, 14.7) | 1,434 (324, 3,234) | 2.2 |
| Armenia | 101 (1.3) | 29.3 (22.9, 35.7) | 30.1 (9.1, 44.6) | 28.9 (16.2, 39) | 415 (325, 508) | 6.1 |
| Australia | 8.7 (2.1) | 4.7 (0.9, 13.7) | 3.7 (0.8, 6.5) | 3.5 (1.6, 5.2) | 384 (77, 1,128) | 0.9 |
| Austria | 61 (2.7) | 20.9 (1.4, 53.4) | 21.0 (5.6, 32.9) | 19.8 (10.4, 27.8) | 764 (51, 1,954) | 3.7 |
| Belarus | 23 (2.2) | 13.4 (2.9, 32.0) | 9.5 (2.3, 15.9) | 8.8 (4.3, 12.9) | 459 (99, 1,096) | 2.4 |
| Belgium | 38 (2.0) | 15.2 (4.4, 29.1) | 14.3 (3.5, 23.3) | 13.5 (6.8, 19.4) | 1,093 (318, 2,088) | 3.7 |
| Brazil | 30 (2.2) | 15.9 (3.7, 33.7) | 11.8 (2.9, 19.5) | 11 (5.5, 16.1) | 4,503 (1,044, 9,541) | 2.0 |
| Bulgaria | 80 (2.1) | 25.8 (9.4, 39.3) | 25.8 (7.5, 39.3) | 24.4 (13.3, 33.6) | 943 (343, 1,437) | 5.2 |
| Canada | 42 (2.8) | 16.3 (2.6, 32.4) | 15.5 (3.8, 25.1) | 14.6 (7.4, 20.9) | 3,277 (518, 6,521) | 4.4 |
| Chile | 21 (1.8) | 9.4 (3.0, 21.0) | 8.5 (2.0, 14.4) | 7.9 (3.9, 11.7) | 279 (89, 627) | 1.1 |
| China | 34 (2.0) | 15.9 (1.5, 38.1) | 13.1 (3.2, 21.5) | 12.4 (6.2, 18.0) | 94,931 (8,999, 227,236) | 4.3 |
| Croatia | 50 (2.3) | 19.3 (4.7, 33.2) | 18.0 (4.7, 28.7) | 16.9 (8.7, 24.0) | 538 (132, 928) | 4.0 |
| Cuba | 5.2 (3.3) | 4.3 (0.3, 20.1) | 2.3 (0.5, 4.0) | 2.1 (1.0, 3.2) | 248 (15, 1,158) | 1.0 |
| Cyprus | 7.0 (2.6) | 4.9 (0.5, 18.6) | 3.1 (0.7, 5.3) | 2.8 (1.3, 4.2) | 13 (1, 48) | 0.9 |
| Czech Republic | 94 (1.8) | 24.3 (11.6, 32.9) | 28.9 (8.4, 43.3) | 27.5 (15.2, 37.2) | 1,271 (605, 1,720) | 4.7 |
| Denmark | 39 (2.2) | 16.0 (3.8, 30.5) | 14.6 (3.6, 23.8) | 13.7 (6.9, 19.7) | 607 (144, 1,162) | 3.9 |
| Ecuador | 70 (2.2) | 23.3 (7.9, 35.9) | 23.0 (6.2, 35.6) | 22.2 (11.9, 30.8) | 247 (83, 380) | 1.8 |
| Egypt | 6.6 (2.2) | 5.9 (1.1, 18.0) | 2.9 (0.7, 5.0) | 2.6 (1.2, 4.0) | 266 (47, 808) | 0.4 |
| Estonia | 44 (2.2) | 16.3 (4.1, 29.8) | 16.3 (4.1, 26.3) | 15.3 (7.8, 21.9) | 108 (27, 198) | 3.0 |
| Finland | 84 (2.1) | 21.6 (7.8, 34.6) | 26.5 (7.3, 40.3) | 25.3 (13.8, 34.6) | 462 (168, 740) | 4.1 |
| France | 50 (2.0) | 19.4 (6.2, 32.5) | 17.8 (4.6, 28.4) | 16.9 (8.7, 24.0) | 6,084 (1,957, 10,202) | 3.9 |
| Germany | 37 (2.2) | 14.9 (3.6, 29.8) | 14.0 (3.4, 22.9) | 13.1 (6.6, 18.9) | 6,449 (1,548, 12,950) | 3.0 |
| Greece | 44 (2.4) | 15.5 (3.3, 29.2) | 16.1 (4.0, 26.1) | 15.2 (7.8, 21.8) | 996 (213, 1,876) | 3.5 |
| Hungary | 62 (2.1) | 23.3 (7.8, 36.0) | 21.4 (5.8, 33.4) | 20.1 (10.6, 28.2) | 1,880 (633, 2,904) | 6.2 |
| Iceland | 7.3 (2.2) | 4.2 (0.7, 13.1) | 3.2 (0.7, 5.5) | 2.9 (1.4, 4.4) | 6 (1, 19) | 1.1 |
| India | 42 (2.2) | 23.8 (6.6, 41.7) | 15.6 (4.2, 25.1) | 14.7 (7.5, 21.1) | 15,175 (4,211, 26,612) | 2.2 |
| Indonesia | 35 (1.2) | 17.5 (13.1, 22.7) | 13.5 (3.4, 22.0) | 12.5 (6.3, 18.2) | 5,418 (4,047, 7,001) | 2.8 |
| Iran | 61 (2.2) | 24.8 (8.0, 38.0) | 20.8 (5.7, 32.6) | 19.9 (10.5, 28.0) | 1,080 (350, 1,657) | 2.0 |
| Ireland | 51 (2.4) | 17.6 (3.9, 30.9) | 18.1 (4.6, 29.0) | 17.1 (8.9, 24.3) | 313 (70, 550) | 3.7 |
| Israel | 23 (2.2) | 11.1 (2.3, 27.4) | 9.2 (2.1, 15.4) | 8.6 (4.2, 12.7) | 217 (45, 536) | 2.0 |
| Italy | 52 (2.1) | 15.9 (4.5, 27.3) | 18.3 (4.6, 29.3) | 17.5 (9.1, 24.8) | 5,327 (1,495, 9,147) | 3.1 |
| Japan | 10.4 (2.0) | 4.2 (0.9, 11.7) | 4.4 (0.9, 7.6) | 4.1 (2.0, 6.2) | 3,117 (688, 8,784) | 0.8 |
| Kazakstan | 11 (2.2) | 7.9 (1.5, 23.1) | 4.7 (1.1, 8.2) | 4.3 (2.1, 6.5) | 334 (63, 973) | 1.4 |
| Kuwait | 27 (1.9) | 18.1 (0.4, 57.1) | 10.5 (2.6, 17.5) | 9.9 (4.9, 14.5) | 15 (0, 48) | 1.9 |
| Lithuania | 37 (2.5) | 16.9 (1.1, 46.1) | 13.9 (3.4, 22.7) | 12.9 (6.5, 18.7) | 219 (15, 596) | 2.6 |
| Luxembourg | 70 (2.0) | 21.9 (8.2, 32.4) | 23.2 (6.3, 36.0) | 22.1 (11.8, 30.7) | 48 (18, 71) | 4.7 |
| Malaysia | 10 (2.2) | 8.3 (1.6, 23.9) | 4.4 (1.0, 7.6) | 4.1 (1.9, 6.1) | 342 (65, 990) | 1.6 |
| Mexico | 82 (1.3) | 26.7 (20.3, 32.9) | 25.9 (7.3, 39.5) | 24.9 (13.6, 34.2) | 2,030 (1,541, 2,503) | 2.6 |
| Montenegro | 50 (3.2) | 24.4 (3.4, 51.8) | 18.0 (5.0, 28.5) | 16.9 (8.7, 24.0) | 84 (12, 178) | 6.2 |
| Netherlands | 12 (2.0) | 7.3 (0.9, 23.2) | 5.2 (1.2, 8.9) | 4.8 (2.3, 7.2) | 773 (97, 2,466) | 1.8 |
| New Zealand | 20 (1.6) | 10.2 (1.8, 27.3) | 8.0 (1.8, 13.5) | 7.4 (3.6, 11.0) | 170 (30, 452) | 2.0 |
| Norway | 38 (3.2) | 15.4 (1.7, 34.7) | 14.3 (3.5, 23.4) | 13.4 (6.8, 19.3) | 341 (37, 769) | 3.2 |
| Pakistan | 52 (2.2) | 24.0 (7.0, 39.2) | 18.6 (5.1, 29.4) | 17.4 (9.0, 24.6) | 1,443 (420, 2,355) | 1.4 |
| Paraguay | 21 (2.2) | 11.5 (2.4, 29.1) | 8.4 (2.0, 14.1) | 7.8 (3.8, 11.5) | 76 (16, 192) | 1.5 |
| Peru | 24 (2.2) | 13.1 (2.9, 30.5) | 9.4 (2.2, 15.8) | 8.9 (4.3, 13.0) | 328 (72, 765) | 1.3 |
| Philippines | 22 (1.1) | 12.3 (10.0, 15.0) | 9.0 (2.2, 15.0) | 8.3 (4.0, 12.2) | 1,275 (1,033, 1,553) | 2.2 |
| Poland | 133 (1.9) | 28.4 (15.8, 43.5) | 36.1 (11.6, 51.8) | 34.8 (20.3, 45.7) | 6,639 (3,691, 10,174) | 7.0 |
| Portugal | 45 (2.2) | 18.4 (4.8, 32.9) | 16.5 (4.2, 26.5) | 15.5 (8.0, 22.2) | 633 (166, 1,132) | 2.6 |
| Rep. of Korea | 49 (2.0) | 16.8 (5.3, 29.1) | 17.5 (4.4, 28.1) | 16.6 (8.5, 23.6) | 2,993 (939, 5,197) | 3.7 |
| Romania | 84 (2.5) | 26.3 (7.6, 48.4) | 26.7 (7.8, 40.4) | 25.4 (13.9, 34.7) | 2,650 (762, 4,879) | 5.5 |
| Russian Fed. | 35 (2.2) | 16.9 (3.9, 33.8) | 13.6 (3.4, 22.3) | 12.7 (6.4, 18.4) | 8,583 (1,994, 17,191) | 2.9 |
| Saudi Arabia | 16 (2.2) | 12.2 (2.5, 31.7) | 6.7 (1.6, 11.4) | 6.2 (3.0, 9.3) | 90 (18, 233) | 1.0 |
| Slovakia | 48 (3.3) | 19.7 (2.4, 45.8) | 17.5 (4.5, 28.0) | 16.3 (8.4, 23.3) | 391 (47, 907) | 3.3 |
| Slovenia | 60 (2.2) | 21.5 (6.2, 34.3) | 20.7 (5.5, 32.5) | 19.6 (10.3, 27.6) | 243 (70, 388) | 4.1 |
| Spain | 53 (2.5) | 18.5 (4.2, 32.4) | 18.7 (4.8, 29.8) | 17.8 (9.2, 25.1) | 3,914 (883, 6,849) | 3.8 |
| Sweden | 67 (2.2) | 19.2 (6.0, 30.3) | 22.4 (5.9, 34.9) | 21.2 (11.3, 29.6) | 710 (220, 1,119) | 3.2 |
| Switzerland | 51 (2.3) | 19.6 (1.4, 49.5) | 18.2 (4.7, 29.0) | 17.2 (8.9, 24.4) | 627 (46, 1,580) | 3.8 |
| Syria | 33 (2.2) | 16.3 (4.0, 33.8) | 12.6 (3.2, 20.7) | 11.7 (5.9, 17.1) | 298 (74, 617) | 2.1 |
| Thailand | 16 (1.2) | 9.4 (6.7, 12.6) | 6.7 (1.6, 11.3) | 6.2 (3.0, 9.2) | 1,660 (1,191, 2,228) | 2.0 |
| Tunisia | 34 (2.2) | 17.5 (4.2, 34.8) | 13.0 (3.3, 21.2) | 12.1 (6.1, 17.6) | 271 (66, 541) | 3.7 |
| Turkey | 57 (2.3) | 24.7 (6.9, 40.0) | 20.1 (5.6, 31.4) | 18.9 (9.9, 26.6) | 5,422 (1,509, 8,773) | 5.9 |
| United Kingdom | 14 (3.2) | 8.0 (0.6, 26.5) | 5.8 (1.3, 10.1) | 5.4 (2.6, 8.1) | 2,858 (219, 9,419) | 1.8 |
| USA | 25 (3.1) | 12.5 (1.3, 30.6) | 9.9 (2.3, 16.7) | 9.3 (4.6, 13.6) | 20,925 (2,137, 51,296) | 3.4 |
| Venezuela | 39 (2.2) | 19.4 (4.9, 36.1) | 14.5 (3.7, 23.6) | 13.7 (6.9, 19.7) | 746 (188, 1,391) | 3.2 |
Note: BEIR VI, sixth Committee on Biological Effects of Ionizing Radiation exposure–age–concentration model; CI, confidence interval; GM, geometric mean; GSD, geometric standard deviation; PAR, population attributable risk.
Sources of the national radon GMs and GSDs listed in column 2 are described in Table S1.
Estimate of PAR based on the BEIR VI EAC model for excess rate ratio.
Estimate of PAR based on the Hunter et al. (2013) model for excess rate ratio.
Estimate of PAR based on the Kreuzer et al. (2015) model for excess rate ratio.
Percentage of total cancer deaths represented by the mean radon-attributable number of lung cancer deaths estimated using the BEIR VI EAC model for excess rate ratio.
Lung cancer mortality by smoking status.
National lung cancer mortality is reported at the detailed age- and sex-specific country-level in the WHO/IARC GLOBOCAN project for 2012: Estimated Cancer Incidence Mortality and Prevalence Worldwide in 2012 (IARC 2012). The 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10) codes for malignant neoplasm of bronchus and lung are C33–34. National smoking prevalence in 2012 among adult men and women, of age, was obtained from the WHO Global Health Observatory data for prevalence of tobacco smoking (WHO 2012) and is shown in Table S2. The smoking prevalence for the nine countries missing adult data was imputed from similar countries using linear regression, and thus expected to contribute little to overall uncertainty. Lung cancer mortality was adjusted for smoking using the relative risk of lung cancer mortality for smoker versus nonsmokers and the adult smoking prevalence in each country (Tirmarche et al. 2010; Villeneuve and Mao 1994). The relative risk of lung cancer mortality for smokers versus nonsmokers () by sex and age category reported by the American Cancer Society Cancer Prevention Study (CPS)-II (Thun et al. 1997) was used for all countries.
Excess rate ratio.
Three different models are used to calculate the excess rate ratio from radon derived from the miner cohort studies because they are characterized by very good radon exposure assessment and annual measurements over long periods of occupational exposure. The BEIR VI EAC model is based on over one million person-years of observation, and the recent Hunter and Kreuzer models are based on low radon exposure rates and cumulative doses that approximate those resulting from lifetime residential exposures. The models used to calculate the excess rate ratio from radon include: a) the BEIR VI EAC model (NRC 1999) based on 11 miner cohorts, the most complete model that also fits low cumulative exposures; b) the model derived from three European nested case–control miner studies restricted to exposures WLM (Hunter et al. 2013); and c) the model derived from a German miner cohort having low radon exposures rates, characterized by a mean cumulative exposure of 17 WLM, mean individual average radon exposure rate of 0.2 WL, and mean duration of employment of 10 y (Kreuzer et al. 2015).
The ERR/WLM is 0.08 () for the BEIR VI EAC model, modified by factors for smoking status, effective exposure duration, exposure-rate effect, and attained age. Central estimates of the parameter values for the modifying factors were derived from an overall fit of the BEIR VI EAC model to 11 miner cohort studies taken from Krewski et al. (1999), as described in greater detail in Brand et al. (2005). For the Hunter et al. (2013) model restricted to WLM, the ERR/WLM is 0.043 (95% CI: 0.022, 0.08) and 0.007 (95% CI: 0.002, 0.018), for time since exposure of 5–25 y and , respectively, and is also modified by a factor for attained age. The ERR/WLM is 0.013 (95% CI: 0.007, 0.021) for the unrestricted Kreuzer et al. (2015) model, based on cumulative radon exposures ranging from 0 to 334 WLM.
The three excess rate ratio models of lung cancer mortality used in this analysis are applied to lifetime radon exposure and assessed for each age group in the population, assuming constant residential radon exposure and a 5-y latency period. The residential radon exposure [in becquerels per cubic meter ()] is converted into working level months for use in the miner excess rate ratio models, assuming an equilibrium factor of 0.4 and 7,000 h per year indoors at home lead to radon exposure for 1 y equaling 0.0044 WLM at home. The BEIR VI EAC model uses relative weights to progressively diminish the contribution of radon exposure and prior to lung cancer, and the Hunter et al. (2013) model reported a contribution at one-sixth for exposure prior to the outcome ( for exposure prior). Although double the risk for nonsmokers than current smokers was reported in the Hunter model and a higher risk estimate for non/light smokers than for moderate/heavy smokers was reported in the Kreuzer model, neither model includes a factor for smoking status because the differences were not statistically significant.
Estimates of PAR are derived from the pooled residential studies (adjusted for uncertainties in radon measurement) by first converting each reported ERR to an excess rate ratio using cumulative radon exposure, ERR/WLM. The excess rate ratio for the residential studies was assumed to be contributed primarily from the period 5–25 y prior to lung cancer, with the model extended to lifetime exposure using the estimate reported by Hunter et al. (2013) for exposures prior to lung cancer of . The estimate for the period 5–25 y prior to lung cancer from the pooled North American study was , based on of radon from Krewski et al. (2006), and from the pooled European study was , based on radon from Darby et al. (2006).
Attributable number and risk of lung cancer deaths.
The excess rate ratio attributable to radon is calculated for each sex–age–smoking category and the attributable fraction is calculated using Equation 1:
| (1) |
The attributable fraction is multiplied by the number of lung cancer deaths for each age–sex–smoking category to determine the number of radon-attributable lung cancer deaths. For each country, the PAR of lung cancer mortality is given by the ratio of the sum of the attributable lung cancer deaths (over all age–sex–smoking categories) to the total number of lung cancer deaths.
Validation
The validity of the approach was assessed by comparing the results for Canada for radon PAR of lung cancer mortality from the attributable fraction approach to an alternative life-table analysis. The abridged period life-table approach will use 5-y-age intervals, and age- and sex-specific all-cause and lung cancer mortality rates will be calculated from the census and death database. The probabilities of death reported in the death database were first converted into mortality rates, so that an exposed person’s risk can be modeled as the sum of the baseline risk and the risk due to radon exposure, expressed in terms of an intensity ratio. The excess rate ratio used to model the risk due to radon exposure in the life-table analysis is the BEIR VI EAC model described earlier. The probability of surviving to the beginning of each age interval, the probability of dying during the age interval, and the intensity ratio between all-cause and lung cancer mortality are used to determine baseline and exposed life expectancy (LE; ) and lifetime risk (LR; ) of lung cancer. The PAR (Equation 2) is calculated from the population-averaged lifetime risk in the exposed () and the baseline lifetime risk for a theoretical unexposed population (LR) as described in Brand et al. (2005):
| (2) |
Results
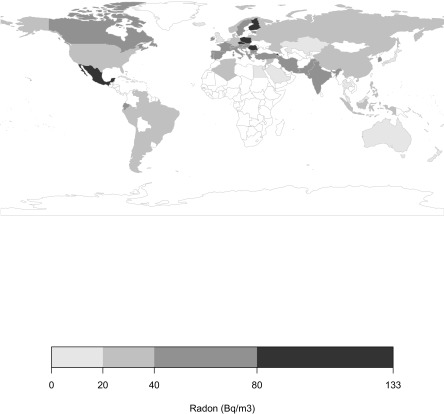
The GMs of the national residential radon distributions around the world are shown in Figure 1. The national residential radon distributions for the 66 countries around the world are listed in Table 1 and the national adult male and female smoking prevalence distributions in Table S2. Geometric mean radon exposures for the 66 countries ranged from for Poland to for Cuba, with a median of . The median adult smoking prevalence was 35% for males and 18% for females.
Figure 1.
World map of geometric means of national residential radon exposures [in becquerels per cubic meter ()]. For more information, see Table S1. (Plotted using R package rworldmap; R Core Team.)
The estimates of the national radon PAR of lung cancer mortality for the 66 countries included in the present analysis are listed in Table 1 for the BEIR VI EAC model, the Hunter et al. (2013) model restricted to WLM, and the Kreuzer et al. (2015) model for excess rate ratio. The mean PAR using the BEIR VI EAC model ranged from 4.2% (95% CI: 0.9, 11.7) for Japan to 29.3% (95% CI: 22.9, 35.7) for Armenia, with a median for the 66 countries of 16.5%. The three estimates are consistent, with the median for the 66 countries of 16.5%, 14.4%, and 13.6% for the BEIR VI EAC model, the Hunter model and the Kreuzer model, respectively. One difference between the three estimates results from the higher excess rate ratio for nonsmokers compared to smokers in the BEIR VI EAC model; a higher mean PAR is estimated for Mexico, at 26.7%, than for Romania, at 26.3%, despite Mexico having a slightly lower GM radon than Romania, at 82 and , respectively, because the smoking prevalence for males and females are lower in Mexico than in Romania. A lower mean PAR is estimated for Mexico than for Romania using the Hunter model, at 25.9% for Mexico and 26.7% for Romania, and using the Kreuzer model, at 24.9% for Mexico and 25.4% for Romania.
Estimates of the number radon-attributable lung cancer deaths using the BEIR VI EAC model for the 66 countries totaled 226,057 in 2012 are also listed in Table 1. The most populous country in the world, China, is the country with the highest estimated number of radon-attributable lung cancer deaths, at 94,931 (95% CI: 8,999, 227,236). The United States is ranked second, with 20,925 (95% CI: 2,137, 51,296) radon-attributed lung cancer deaths in 2012, and India is ranked third, with 15,175 (95% CI: 4,211, 26,612). Radon-attributable lung cancer deaths represented a median of 3.0% of total cancer deaths for the 66 countries, ranging from 7.0% for Poland down to 0.4% for Egypt.
Validation
An alternative life table analysis was conducted for Canada using the 2012 age- and sex-specific all-cause and lung cancer mortality rates, using 10,000 simulations of the BEIR VI EAC model for the excess rate ratio from radon. The mean PAR in Canada for lung cancer mortality from radon was estimated to be 14.7% (95% CI: 2.0, 30.0) from the life table analysis, which was similar to the PAR estimate of 16.3% (95% CI: 2.6, 32.4) from the main analysis for Canada derived from the attributable fraction approach using the BEIR VI EAC model. The life table analysis for Canada estimated PAR for males at 13.2% (95% CI: 1.7, 26.7), for male nonsmokers at 19.8% (95% CI: 2.7, 40.0), and for male smokers at 10.9% (95% CI: 1.4, 22.3) and PAR for females at 16.5% (95% CI: 2.2, 33.3), for female nonsmokers at 21.2% (95% CI: 3.0, 41.9), and for female smokers at 13.4% (95% CI: 1.7, 27.7).
Published estimates of national PAR of lung cancer mortality from residential radon exposure for another eight countries are compared to the estimates from this analysis in Table 2. The methodologies used in these studies included the attributable fraction approach, the period life table analysis, and one study that used the cohort life table analysis; whereas the excess relative risk of lung cancer from radon was modeled using the estimate from pooled case–control residential studies in Europe and North America based on a 30-y exposure period and the BEIR VI EAC model, which is based on lifetime exposure from extrapolation of miner studies.
Table 2.
Comparison of national estimates of PAR of lung cancer mortality from radon.
| Country | PAR estimates (%) [mean (95% CI)]a | Published estimates | ||||
|---|---|---|---|---|---|---|
| BEIR VI EAC | Hunter | Kreuzer | PAR (ERR model)b | Methodology | Reference | |
| France | 19.4 (6.2, 32.5) | 17.8 (4.6, 28.4) | 16.9 (8.7, 24) | 13 (EAC) 5 (Euro. pooled) 2 (N. Am. pooled) 11 (N. Am. pooled cor.) | Attributable fraction, regional analysis | Catelinois et al. 2006 |
| Germany | 14.9 (3.6, 29.8) | 14.0 (3.4, 22.9) | 13.1 (6.6, 18.9) | 12 (EAC) 3.1 (Euro. pooled) 7.3 (Euro. pooled cor.) | Period life table | Menzler et al. 2008 |
| Netherlands | 7.3 (0.9, 23.2) | 5.2 (1.2, 8.9) | 4.8 (2.3, 7.2) | 3 (2-mutation carc.) | Cohort life table | Leenhouts and Brugmans 2001 |
| Sweden | 19.2 (6.0, 30.3) | 22.4 (5.9, 34.9) | 21.2 (11.3, 29.6) | 19 (2-mutation carc.) | Cohort life table | Leenhouts and Brugmans 2001 |
| Switzerland | 19.6 (1.4, 49.5) | 18.2 (4.7, 29.0) | 17.2 (8.9, 24.4) | 8.3 (Euro. pooled) | Period life table | Menzler et al. 2008 |
| South Korea | 16.8 (5.3, 29.1) | 17.5 (4.4, 28.1) | 16.6 (8.5, 23.6) | 22 (EAC) 8.3 (Euro. pooled) | Attributable fraction, regional analysis | Lee et al. 2015 |
| United States | 12.5 (1.3, 30.6) | 9.9 (2.3, 16.7) | 9.3 (4.6, 13.6) | 13.9 (EAC) | Period life table | NRC 1999 |
| United Kingdom | 8.0 (0.6, 26.5) | 5.8 (1.3, 10.1) | 5.4 (2.6, 8.1) | 6.0 (EAC) 3.3 (Euro. pooled cor.) | Period life table | HPA 2009 |
Note: BEIR VI EAC, sixth Committee on Biological Effects of Ionizing Radiation exposure–age–concentration model; CI, confidence interval; ERR, excess risk ratio; Euro. pooled, European pooled study; Euro. pooled cor., European pooled study corrected for random uncertainties in radon measurement; N. Am. pooled, North American pooled study; N. Am. pooled cor., North American pooled study corrected for random uncertainties in radon measurement (restricted to lower residential mobility); PAR, population attributable risk; 2-mutation carc., two-mutation carcinogenesis model (a biologically based carcinogenesis model).
Brief information about the three models for excess rate ratio used in this analysis: BEIR IV EAC, BEIR VI EAC model; Hunter, Hunter et al. (2013) model; Kreuzer, Kreuzer et al. (2015) model.
Information about the models used in published studies: Euro. pooled: radon; N. Am. pooled: radon; N. Am. pooled cor. radon; Euro. pooled cor. radon.
Discussion
The estimates of PAR for lung cancer mortality from residential radon for the 66 countries are very similar for the three excess rate ratio models used in this analysis: the median PAR was 16.5% using the BEIR VI EAC model, 14.4% using the Hunter et al. (2013) restricted model, and 13.6% using the Kreuzer et al. (2015) model. The estimates support the use of models based on miner studies, with the two miner models based on low radon exposure rates and cumulative doses that approximate those resulting from lifetime residential exposures yielding PARs close to those from the BEIR VI EAC model. The BEIR VI EAC model is the most complete excess rate ratio model, including factors for smoking status, time since exposure, radon exposure rate, and attained age. The three excess rate ratio models used in this analysis to estimate PAR were based on lifetime exposure, calculated from lifetime risk as presented in Equation 2. Four excess rate ratio models, including the three in this analysis, were used in Hunter et al. (2015) to estimate risk of exposure-induced death from residential radon exposure between 30 and 75 y of age. The fourth excess rate ratio model included in Hunter et al. (2015) was the model derived from the European pooled residential study; after corrections for uncertainties in radon measurement, the European pooled residential radon study estimate of radon exposure 5–35 y prior to lung cancer (Darby et al. 2006) was found to be equivalent to an , very close to the derived from the Kreuzer et al. (2015) miner model based on low radon exposure rates (Hunter et al. 2015).
The studies of radon-attributed lung cancer mortality in miners were characterized by far superior radon exposure assessment, based on annual measurements of radon for each year a miner was exposed, whereas the residential radon studies relied on only one or two measurements for the entire 30-y period of exposure considered. It is reassuring that estimates of PAR derived from extension of the pooled residential studies, after restriction to the better radon exposure measurement, were comparable though lower (see Table S3) than those derived from the miner cohort studies. Any degree of residential mobility has a severe impact on the precision of residential radon exposure in the pooled case–control studies. The excess relative risk per radon increased from 8% to 16% when the European analysis was adjusted for the random uncertainties from using limited measurements of residential radon (Darby et al. 2006). Similarly, after consideration of spatial mobility of participants, the ERR per radon in the North American pooled residential study increased from 10% to 18% when the analysis was restricted to individuals with measurements for at least 20 of the 25 y of radon exposure (Krewski et al. 2006). As noted by Field and Withers (2012), any remaining nondifferential radon exposure measurement error would tend to bias the observed association toward the null and it is quite possible that more precise radon measurement in residential studies (such as based on annual radon levels) would result in a higher estimate for ERR. The policy implications from basing decisions on epidemiologic studies with significant measurement error in environmental exposure and outcomes have been discussed recently by Edwards and Keil (2017).
The Global Burden of Disease Study 2010 (Lozano et al. 2012) reported that the number of lung cancer deaths globally has increased by 47% between 1990 and 2010, to 1,527.1 (95% CI: 1,126.3, 1,779.4) thousand deaths. Tobacco use remains the most important risk factor for lung cancer, but characterizing the contribution of other modifiable risk factors, such as exposure to radon and to outdoor air particulates, is necessary to reduce the burden of lung cancer mortality. The estimates of the global PAF of lung cancer mortality in 2012 from radon, ranging from 16.5% to 13.6%, were comparable to the 12.8% attributable to outdoor air particulates () (Evans et al. 2013). This analysis demonstrates that residential radon is a significant and modifiable risk factor for lung cancer mortality worldwide.
The demographics of a country affect the national number of lung cancer deaths. Lung cancer mortality increases with age, so countries with higher life expectancies have a greater number of lung cancer deaths. In 2012, life expectancy was 79 y in the United States and 68 y in India (World Bank 2014). Even though the radon exposure is higher and the population is much greater in India than in the United States, there are more lung cancer deaths attributed to radon in the United States (20,925) than in India (15,175).
The validity of estimating the radon ERR of lung cancer mortality from the attributable fraction approach is supported by the results from the attributable fraction approach and the life table analysis for Canada, with PARs of 16.3% (95% CI: 2.6, 32.4) and 14.7% (95% CI: 2.0, 30.0), respectively. Similar results were reported for the Canadian province of Ontario from a life table analysis (Peterson et al. 2013) using the same BEIR VI EAC model for excess rate ratio: an overall PAR of 13.6%, with PARs of 21.9% for never smokers and 12.3% for ever smokers [radon distribution GM (GSD): for Ontario and for Canada]. A life table analysis for Canada also based on a BEIR VI model for excess rate ratio reported a comparable estimate of radon PAR of lung cancer mortality of 16% (Chen et al. 2012).
The estimates determined in this analysis were compared with published national estimates of PARs for eight other countries in Table 2. The estimates in Table 2 determined using the excess rate ratio derived from the pooled residential studies were roughly half those using the BEIR VI models, following the pattern observed for the estimates of PAR determined in this analysis. The PARs determined using the excess rate ratio from pooled residential studies were closer when corrected for uncertainties in the exposure assessment to those using the BEIR VI EAC model for both France (Catelinois et al. 2006), at 11% and 12%, respectively, and for Germany (Menzler et al. 2008), at 7% and 12%, respectively. Use of the ERR estimate from the residential studies assumes that radon exposure prior does not contribute to lung cancer. The BEIR VI EAC model uses relative weights to progressively diminish the contribution of radon exposure 15–24 y prior and prior to the outcome, and the Hunter et al. (2013) model diminishes the contribution to one-sixth for exposure prior to the outcome. However, it is not surprising that PAR estimates of lung cancer mortality from radon based on lifetime exposure are higher than those limited to exposure 30 y prior to lung cancer mortality.
The studies listed in Table 2 also used various measures of radon exposure: the arithmetic mean national exposure for the Netherlands and Sweden (Leenhouts and Brugmans 2001) and the radon distribution for the United Kingdom (HPA 2009) and the United States (NRC 1999), whereas regional radon distributions were used for the estimate for France (Catelinois et al. 2006), Germany and Switzerland (Menzler et al. 2008), and South Korea (Lee et al. 2015). The published regional analysis for France reported a smaller PAR estimate (13% vs. 19.4%), whereas the regional analysis for South Korea reported a larger PAR estimate of 22% versus 16.8% when compared with the results from the national analysis used in this study (using the EAC risk model). The estimates from published studies that also used a national analysis were much closer to the results from this study (for the EAC radon model), the United States (13.9% vs. 12.5%), and the United Kingdom (6% vs. 8%).
The results of this analysis must be interpreted cautiously due to the uncertainty associated with the national radon exposures. Many countries have not conducted a population representative national survey, and very few countries have conducted more than one representative national radon survey. A population representative national radon survey requires long-term radon measurement, 3–12 months in duration, in several hundred to several thousand randomly selected dwellings. Some uncertainty is also associated with the excess rate ratio model for lung cancer mortality from radon, as indicated by the range in PAR values resulting from the different excess rate ratio models used in this analysis. The use of the BEIR VI EAC model resulted in the highest estimate of median PAR (16.5%) and the use of excess rate ratios based on the pooled residential studies resulted in the lowest estimates (10.4% for North American; 8.4% for European), whereas the excess rate ratio models from the recent low-dose miner cohort resulted in intermediate estimates of PAR [14.4% for Hunter et al. (2013); 13.6% for Kreuzer et al. (2015)].
Representative national surveys of residential radon are required in all countries to better characterize the global burden of lung cancer mortality attributed to radon. Reducing the burden of lung cancer mortality and morbidity attributed to radon will depend on future research into the practical implementation and cost effectiveness of interventions to reduce radon exposures and improved education and awareness about radon. Many countries could benefit from a radon reduction intervention such as the installation of a soil gas membrane at the time of construction, as determined for Norway (Stigum et al. 2003), the United Kingdom (Gray et al. 2009), and Ireland (Pollard and Fenton 2014). A highly effective intervention, such as active sub-slab depressurization, might prove cost effective in countries or regions with higher burdens from radon-attributed lung cancer mortality and substantial investment in healthcare.
Conclusions
Representative national surveys of residential radon exposures were available for 66 countries around the world. The estimates of the PAR of lung cancer mortality from residential radon exposure for 2012 were very consistent for the 66 countries, with a median PAR of 16.5%, 14.4%, and 13.6% from the three excess rate ratio models [BEIR VI EAC, Hunter et al. (2013) restricted, and Kreuzer et al. (2015) unrestricted, respectively]. An alternative methodological approach, the period life table analysis, was conducted for Canada, and yielded an estimate of PAR of 14.7% (95% CI: 2.0, 30.0) that was very close to the PAR of 16.3% (95% CI: 2.6, 32.4) determined from the attributable fraction approach. This study provides an estimate of the global burden from lung cancer attributable to radon, pending more complete data on national radon exposures.
Supplemental Material
References
- Brand KP, Zielinski JM, Krewski D. 2005. Residential radon in Canada: an uncertainty analysis of population and individual lung cancer risk. Risk Anal 25(2):253–269, PMID: 15876202, 10.1111/j.1539-6924.2005.00587.x. [DOI] [PubMed] [Google Scholar]
- Catelinois O, Rogel A, Laurier D, Billon S, Hemon D, Verger P, et al. 2006. Lung cancer attributable to indoor radon exposure in France: impact of the risk models and uncertainty analysis. Environ Health Perspect 114(9):1361–1366, PMID: 16966089, 10.1289/ehp.9070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Moir D, Whyte J. 2012. Canadian population risk of radon induced lung cancer: a re-assessment based on the recent cross-Canada radon survey. Radiat Prot Dosimetry 152(1–3):9–13, PMID: 22874897, 10.1093/rpd/ncs147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby S, Hill D, Deo H, Auvinen A, Barros-Dios JM, Baysson H, et al. 2006. Residential radon and lung cancer—detailed results of a collaborative analysis of individual data on 7148 persons with lung cancer and 14,208 persons without lung cancer from 13 epidemiologic studies in Europe. Scand J Work Environ Health 32(suppl 1):1–84. [PubMed] [Google Scholar]
- Edwards JK, Keil AP. 2017. Measurement error and environmental epidemiology: a policy perspective. Curr Environ Health Rep 4(1):79–88, 10.1007/s40572-017-0125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J, van Donkelaar A, Martin RV, Burnett R, Rainham DG, Birkett NJ, et al. 2013. Estimates of global mortality attributable to particulate air pollution using satellite imagery. Environ Res 120:33–42, PMID: 22959329, 10.1016/j.envres.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Field RW, Withers BL. 2012. Occupational and environmental causes of lung cancer. Clin Chest Med 33(4):681–635, 10.1016/j.ccm.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray A, Read S, McGale P, Darby S. 2009. Lung cancer deaths from indoor radon and the cost effectiveness and potential of policies to reduce them. BMJ 338:a3110, PMID: 19129153, 10.1136/bmj.a3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HPA (Health Protection Agency). 2009. RCE-11: Radon and Public Health RCE 11. Report of the Independent Advisory Group on Ionising Radiation. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/335102/RCE-11_for_website.pdf [accessed 7 May 2018].
- Hunter N, Muirhead C, Bochicchio F, Haylock R. 2015. Calculation of lifetime lung cancer risks associated with radon exposure, based on various models and exposure scenarios. J Radiol Prot 35(3):539–546, PMID: 26083042, 10.1088/0952-4746/35/3/539. [DOI] [PubMed] [Google Scholar]
- Hunter N, Muirhead CR, Tomasek L, Kreuzer M, Laurier D, Leuraud K, et al. 2013. Joint analysis of three European nested case-control studies of lung cancer among radon exposed miners: exposure restricted to below 300 WLM. Health Phys 104(3):282–292, PMID: 23361424, 10.1097/HP.0b013e3182765857. [DOI] [PubMed] [Google Scholar]
- IAEA (International Atomic Energy Agency). 2013. National and Regional Surveys of Radon Concentration in Dwellings: Review of Methodology and Measurement Techniques. IAEA Analytical Quality in Nuclear Applications Series No. 33. Vienna, Austria:IAEA. [Google Scholar]
- IARC (International Agency for Research on Cancer). 2012. GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. Version 1-0-2012. http://globocan.iarc.fr/Pages/age-specific_table_sel.aspx [accessed 1 October 2016].
- Kreuzer M, Fenske N, Schnelzer M, Walsh L. 2015. Lung cancer risk at low radon exposure rates in German uranium miners. Br J Cancer 113(9):1367–1369, PMID: 26393888, 10.1038/bjc.2015.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krewski D, Lubin JH, Zielinski JM, Alavanja M, Catalan VS, Field RW, et al. 2006. A combined analysis of North American case-control studies of residential radon and lung cancer. J Toxicol Environ Health A 69(7–8):533–597, 10.1080/15287390500260945. [DOI] [PubMed] [Google Scholar]
- Krewski D, Rai SN, Zielinski JM, Hopke PK. 1999. Characterization of uncertainty and variability in residential radon cancer risks. Ann N Y Acad Sci 895:245–272, PMID: 10676422, 10.1111/j.1749-6632.1999.tb08090.x. [DOI] [PubMed] [Google Scholar]
- Lee HA, Lee WK, Lim D, Park SH, Baik SJ, Kong KA, et al. 2015. Risks of lung cancer due to radon exposure among the regions of Korea. J Korean Med Sci 30(5):542–548, PMID: 25931783, 10.3346/jkms.2015.30.5.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leenhouts HP, Brugmans MJP. 2001. Calculation of the 1995 lung cancer incidence in the Netherlands and Sweden caused by smoking and radon: risk implications for radon. Radiat Environ Biophys 40(1):11–21, PMID: 11357706, 10.1007/s004110000084. [DOI] [PubMed] [Google Scholar]
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. 2012. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380(9859):2095–2128, PMID: 23245604, 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubin JH, Boice JD Jr, Edling C, Hornung RW, Howe GR, Kunz E, et al. 1995. Lung cancer in radon-exposed miners and estimation of risk from indoor exposure. J Natl Cancer Inst 87(11):817–827, PMID: 7791231, 10.1016/0169-5002(95)90522-7. [DOI] [PubMed] [Google Scholar]
- Menzler S, Piller G, Gruson M, Rosario AS, Wichmann H-E, Kreienbrock L. 2008. Population attributable fraction for lung cancer due to residential radon in Switzerland and Germany. Health Phys 95(2):179–189, PMID: 18617799, 10.1097/01.HP.0000309769.55126.03. [DOI] [PubMed] [Google Scholar]
- Naghavi M, Wang H, Lozano R, Davis A, Liang X, Zhou M, et al. 2015. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 385(9963):117–171, PMID: 25530442, 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC (National Research Council). 1999. Health Effects of Exposure to Radon: BEIR VI. Washington, DC:National Academies Press. [PubMed] [Google Scholar]
- Peterson E, Aker A, Kim J, Li Y, Brand K, Copes R. 2013. Lung cancer risk from radon in Ontario, Canada: how many lung cancers can we prevent? Cancer Causes Control 24(11):2013–2020, PMID: 23982909, 10.1007/s10552-013-0278-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard D, Fenton D. 2014. Use of health economics in the development of a national radon control strategy in Ireland. Radiat Prot Dosimetry 160(1–3):30–34, PMID: 24723196, 10.1093/rpd/ncu106. [DOI] [PubMed] [Google Scholar]
- Stigum H, Strand T, Magnus P. 2003. Should radon be reduced in homes? A cost-effect analysis. Health Phys 84(2):227–235, PMID: 12553653, 10.1097/00004032-200302000-00011. [DOI] [PubMed] [Google Scholar]
- Thun MJ, Myers DG, Day-Lally C, Namboodiri MM, Calle EE, Flanders WD, et al. 1997. “Age and the exposure–response relationships between cigarette smoking and premature death in Cancer Prevention Study II.” Smoking and Tobacco Control Monograph No 8. NIH Publication No. 97-4213. Bethesda, MD:National Institutes of Health. [Google Scholar]
- Tirmarche M, Harrison JD, Laurier D, Paquet F, Blanchardon E, Marsh JW. 2010. ICRP Publication 115. Lung cancer risk from radon and progeny and statement on radon. Ann ICRP 40(1):1–64, PMID: 22108246, 10.1016/j.icrp.2011.08.011. [DOI] [PubMed] [Google Scholar]
- Villeneuve P, Mao Y. 1994. Lifetime probability of developing lung cancer, by smoking status, Canada. Can J Public Health 85(6):385–388, PMID: 7895211. [PubMed] [Google Scholar]
- WHO (World Health Organization). 2006. “Report of the 2nd meeting of the WHO International Radon Project.” Zeeb H, ed. Geneva, Switzerland:WHO; http://www.who.int/ionizing_radiation/env/radon/Mar06MeetingReport.pdf [accessed 7 May 2018]. [Google Scholar]
- WHO. 2012. Global Health Observatory (GHO) data. Prevalence of tobacco smoking. http://www.who.int/gho/tobacco/use/en/ [accessed 1 October 2016].
- World Bank. 2014. Life expectancy at birth, total (years). http://data.worldbank.org/indicator/SP.DYN.LE00.IN [accessed 10 October 2016].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.