Abstract
Background:
Exposure to triclosan, an endocrine disrupting chemical, may affect thyroid hormone homeostasis and adversely affect neurodevelopment.
Objective:
Using a longitudinal pregnancy and birth cohort, we investigated associations between triclosan exposures during different time windows, and cognitive test scores at 8 y of age in 198 children from the HOME Study.
Methods:
We quantified triclosan in urine samples from mother–child pairs up to nine times between the second trimester of gestation and 8 y of age. The Wechsler Intelligence Scale for Children-IV [i.e., Full-Scale Intelligence Quotient (IQ)] assessment was administered to HOME Study children at 8 y of age. We estimated covariate-adjusted triclosan–IQ associations at each visit. We also tested whether associations between triclosan concentrations and cognitive test scores varied among exposure at different time periods.
Results:
Full-Scale IQ was not significantly associated with urinary triclosan concentrations during gestation or childhood but was significantly associated with a 10-fold increase in maternal urinary triclosan concentration at delivery [ (95% CI: , )]. Perceptual Reasoning Index (PRI) scores were significantly decreased in association with urinary triclosan concentrations at delivery and at 2 y of age. Associations between repeated triclosan concentrations and cognitive test scores significantly varied among exposure at different time periods for Full-Scale IQ, PRI, Verbal Comprehension Index, and Working Memory (triclosan–visit interaction ).
Conclusion:
Urinary triclosan concentrations at delivery, but not during mid to late pregnancy and childhood, were associated with significantly lower children’s cognitive test scores at 8 y of age in this cohort of U.S. children. https://doi.org/10.1289/EHP2777
Introduction
Triclosan is an antimicrobial compound used in personal care and household products including some deodorants, soaps, toothpastes, mouthwashes, cosmetics, shower gels, cleaning products, and kitchen utensils (Rodricks et al. 2010). Exposure to triclosan occurs primarily through oral intake (e.g., toothpaste and mouthwash) and dermal absorption (e.g., soaps and cosmetics) (Rodricks et al. 2010). Triclosan exposure is ubiquitous among persons in the United States, including pregnant women and children (Calafat et al. 2008; Stacy et al. 2017a; Woodruff et al. 2011). After ingestion or absorption, triclosan is primarily excreted in the urine and has an estimated biological half-life of about 21 h (Sandborgh-Englund et al. 2006). Results from epidemiological and animal studies suggest that triclosan could adversely affect neurodevelopment by disrupting thyroid hormone homeostasis (Braun et al. 2017a; Johnson et al. 2016); however, few studies have examined the association between early-life triclosan exposure and neurodevelopment in either animals or humans.
In rodents, triclosan exposure decreases circulating thyroxine levels in both the pregnant damn and her fetus during gestation (Johnson et al. 2016; Paul et al. 2012) and may interfere with other hormonal pathways (Dann and Hontela 2011). During gestation, thyroid hormones transferred from the mother to the embryo and fetus are critical for proper neurodevelopment (Haddow et al. 1999). Even small deficiencies in thyroid hormone concentrations during pregnancy have been associated with lower IQ scores in children (Haddow et al. 1999; Henrichs et al. 2013; Korevaar et al. 2016). In humans, a previously published study from our group and a prospective birth cohort study in Shanghai, China, both reported that increasing urinary triclosan concentrations during gestation or at delivery were associated with decreased maternal or neonatal thyroid hormone concentrations (Braun et al. 2017b; Wang et al. 2017). In the present study, we hypothesized that early-life triclosan exposure may reduce circulating thyroid hormone concentrations, which may in turn adversely affect fetal, infant, or childhood cognitive outcomes. Although we previously did not find an association between gestational urinary triclosan concentrations and visual-spatial abilities at 8 y of age (Braun et al. 2017b), we did not consider omnibus measures of cognitive abilities or examine childhood as a window of heightened cognitive vulnerability to triclosan. In this study, we investigated the associations between up to nine repeated measures of triclosan exposure time periods from mid-pregnancy through 8 y of age with child cognitive test scores at 8 y of age.
Methods
Study Participants
We used data collected from the Health Outcomes and Measures of the Environment (HOME) study, a prospective pregnancy and birth cohort in Cincinnati, Ohio, designed to examine health effects associated with early-life exposure to environmental chemicals (Braun 2017; Johnson et al. 2016). We recruited women from nine prenatal care clinics in the Cincinnati, Ohio, metropolitan area from March 2003 to January 2006. Eligibility criteria included being age 18 y or older, being 13–19 wk pregnant, living within the study area, having no history of thyroid or mental health disorders, and having continued prenatal care and delivery at participating clinics and/or hospitals. Of 1,263 eligible women, 468 agreed to participate. Of 389 mothers who remained in the study until delivering singleton infants, we excluded children with congenital or genetic anomalies (), those missing all triclosan measurements (), and those without 8-y-of-age cognitive test results (). Our final analysis included 198 mother–child pairs (51% of mothers who delivered singletons) (Braun et al. 2017b; Wang et al. 2017).
The institutional review boards (IRBs) at Cincinnati Children’s Hospital Medical Center (CCHMC) and participating delivery hospitals approved this study (Braun et al. 2017a). The Centers for Disease Control and Prevention (CDC) deferred to CCHMC IRB as the IRB of record. All women provided written informed consent for themselves and their children.
Triclosan Measurements
Women provided up to two urine samples during their prenatal care clinic visits at around 16 and 26 wk of pregnancy and another at the delivery hospital, usually within 48 h after delivery. We denote these exposure periods as “16-wk,” “26-wk,” and “at delivery” hereafter (Braun et al. 2017c). We collected urine samples from children during annual clinic or home visits from 1 to 5 y of age, and again at 8 y of age (see Table S1). Urine samples were collected from 2003 through 2014 and stored at until shipment to the CDC, where they were stored at or below until analysis. The CDC lab has previously published results regarding lab practices to track method performance for the quantification of analytes such as triclosan and has pioneered such practices (Ye et al. 2013).
To reduce the potential for contamination of our urine samples with exogenous sources of triclosan, we followed recommendations for collecting, storing, and processing biospecimens for analysis of environmental chemical biomarkers (Ye et al. 2008, 2013). In addition, the CDC laboratory where our urine samples were analyzed is licensed by the Clinical Laboratory Improvement Act (CLIA) of 1988. Analytical measurements are conducted following strict quality assurance/quality control (QA/QC) guidelines, CLIA guidelines, and frequent proficiency testing. Each analytic batch included reagent blanks and low- and high-concentration QC materials, which are evaluated using standard statistical probability rules. QC procedures are available online and Table S2 (see Table S2) includes the coefficients of variation (CVs) of the QC materials analyzed with National Health and Nutrition Examination Survey (NHANES) samples for five NHANES cycles that encompassed the time periods during which we analyzed most (if not all) HOME Study urine samples (all ). The HOME Study maternal urine samples were analyzed between 2007 and 2009, whereas child samples from 1 to 5 y of age were analyzed during 2010–2012, and child samples collected at 8 y of age were analyzed in 2015. Finally, we analyzed a subset of urine samples with and without enzymatic deconjugation, and the results suggested that triclosan was mostly conjugated, thus further ruling out external contamination.
The concentrations of total (free plus conjugated) triclosan for all maternal urine samples and 1- to 5-y-of-age child urine samples had a limit of detection (LOD) of ; the LOD for child urine samples collected at the 8-y-of-age visit was . Concentrations below the LOD were assigned the value of the LOD divided by the square root of 2 (Hornung and Reed 1990). To account for urine dilution, we measured urinary creatinine concentrations using previously described methods (Larsen 1972).
Cognitive Tests
To evaluate children’s cognitive development, trained research assistants who were blinded to the women and children’s urinary triclosan concentrations administered the Wechsler Intelligence Scale for Children-IV (WISC-IV) to children at 8 y of age (range: 7.5–10 y; see Table S1) (Wechsler 2004). The WISC-IV provides a measure of global cognitive abilities [i.e., Full-Scale Intellectual Quotient (FSIQ)], verbal abilities [i.e., Verbal Comprehension Index (VCI)], perceptual reasoning and organization skills [i.e., Perceptual Reasoning Index (PRI)], speed of mental and motor processing [i.e., Processing Speed Index (PSI)], and working memory [i.e., Working Memory Index (WMI)]. The FSIQ and all index scores are normalized to a mean of 100 and standard deviation of 15. Lower scores on the WISC-IV are indicative of poorer cognitive performance on these tests. We analyzed all scores as continuous variables.
Covariates
In regression models, we adjusted for variables that may be associated with both triclosan exposure and child cognitive abilities based on the results of previous studies and a directed acyclic graph (see Figure S1; Textor et al. 2016) (Bellinger 2004; Stacy et al. 2017a, 2016). We adjusted for the mother’s education (high school or less, some college, or college graduate or more), household income (continuous), marital status (married or unmarried), FSIQ (continuous), and tobacco smoke exposure during pregnancy (continuous serum cotinine concentrations), as well as child sex (male or female) and race/ethnicity (white non-Hispanic, black non-Hispanic, or other), and the caregiving environment at 1 y of age (continuous). Children’s sex was abstracted from hospital medical charts, whereas sociodemographic covariates, including child’s race/ethnicity and mother’s education, household income, and marital status, were obtained from standardized questionnaires. Mother’s FSIQ was assessed using the Wechsler Abbreviated Intelligence Scale (Wechsler 2008). We assessed the mother’s active and secondhand tobacco smoke exposure using mean serum cotinine concentrations quantified in serum samples collected at the 16- and 26-wk gestational visits (Braun et al. 2010, 2011a). We administered the HOME during the 1-y-of-age home visit to assess the quantity and quality of the caregiving environment (Bradley et al. 2003). We included creatinine concentrations as continuous covariates in our models to account for urine dilution.
Statistical Analysis
We explored the distribution of urinary triclosan concentrations across visits by comparing univariate statistics (e.g., medians) and examining bean plots of the distribution of urinary triclosan concentrations by visit. We then calculated geometric mean (GM) gestational (average of 16- and 26-wk of gestation and delivery) and childhood (average of 1 to 8 y of age) urinary triclosan concentrations and mean child FSIQ scores by covariates.
We used a multiple informants model to examine the associations between repeated urinary triclosan concentrations and cognitive test scores. This model allowed us to use the nine repeated urinary triclosan concentrations to jointly estimate the exposure–outcome associations at each exposure period and determine whether triclosan–IQ associations differed across exposure periods. Joint estimation imposes constraints on the regression coefficients across exposure periods and tests whether the exposure coefficients are equal across time periods (Sánchez et al. 2011). This method utilizes generalized estimating equations (GEEs) for parameter estimation that embed separate linear regression models for each time period into a unified set of estimating equations. Some of the assumptions of this model are that a) all participants have the same timing of exposure, b) there are predefined exposure periods, c) there is a homogenous exposure effect within each exposure period, d) there is at least one exposure sample per participant, and e) no substantial collinearity occurs between exposures. This method has been previously described (Sánchez et al. 2011). To determine whether there were unique periods of vulnerability, we examined the interaction between triclosan concentration and exposure period and considered for this coefficient to be evidence that associations significantly differed by exposure period. The null hypothesis for this test is that the associations are the same at all exposure periods. We performed all analyses using R (version 3.2.4; R Development Core Team).
Secondary and Sensitivity Analyses
We previously observed that children’s sex modified the association between neurobehavior and bisphenol A, a phenol and endocrine disruptor, in this cohort (Braun et al. 2009, 2011b; Stacy et al. 2017b). Because triclosan is also a phenol and a suspected endocrine disrupting chemical, we performed exploratory analyses including three-way interactions between urinary triclosan concentrations, children’s sex, and exposure periods to separately estimate the association of repeated triclosan measures with children’s FSIQ by children’s sex and to test whether the pattern of triclosan–FSIQ associations differed by children’s sex in our sensitivity analyses.
Because medical interventions in response to neonatal distress may be associated with both triclosan exposure and neurodevelopment, or the practices of different delivery hospitals could be associated with higher triclosan exposure at delivery, we further adjusted regression models for child admission to the neonatal intensive care unit (NICU) and delivery hospital. Finally, urinary triclosan concentrations were categorized for examining potential dose–response relationships between FSIQ scores and quartiles of urinary triclosan concentration at delivery, controlling for potential covariates. For this analysis, we estimated mean covariate-adjusted FSIQ scores across urinary triclosan concentration quartiles.
Results
Among participants who completed the WISC-IV at 8 y of age and had complete covariate data, 128 (at 4 y of age) to 198 (at the 16th wk of gestation) were included in our final analyses (Figure 1). The distribution of covariates was similar among HOME Study participants included in the final analyses cohort to those in the full cohort (see Table S3).
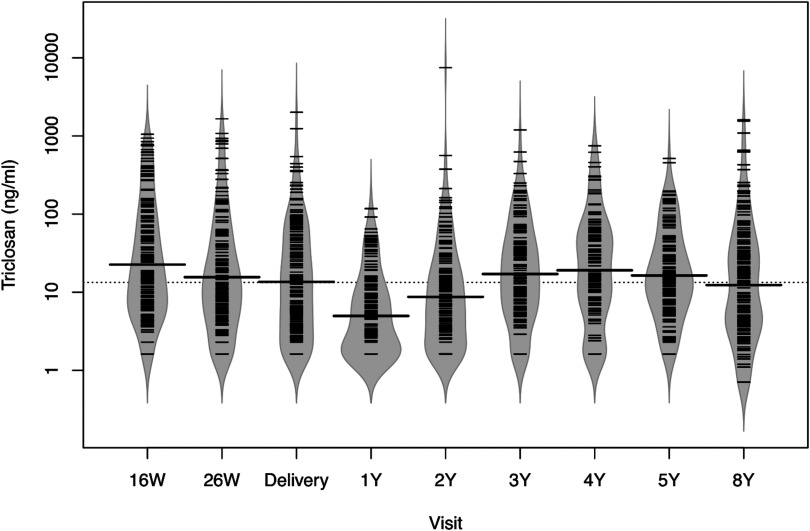
Figure 1.
Bean plots of urinary triclosan concentrations from 16 wk of pregnancy until children completed the 8-y-of-age study visit among mother–child pairs from the HOME Study. HOME, Health Outcomes and Measures of the Environment study; W, weeks of gestation; Y, age in years. Dotted line represents overall median urinary triclosan concentration and solid line in each bean plot represents the median urinary triclosan concentration for that visit. Fringes within each plot represent individual observations, and shaded area the density function of triclosan concentrations; , 192, 181, 174, 155, 159, 128, 156, and 198, respectively.
Predictors and Patterns of Triclosan Concentrations
Median urinary triclosan concentrations between 16-wk and delivery decreased from to (see Table S4). Median child urinary triclosan concentrations increased from 5 to between 1 and 4 y of age and then decreased to at 8 y of age (Figure 1; see also Table S4).
We found differences in maternal and child GM urinary triclosan concentrations according to sociodemographic and maternal factors (Table 1). Specifically, participants in the lowest category of household income and education had the lowest maternal and child GM urinary triclosan concentrations. Higher maternal and childhood GM urinary triclosan concentrations were observed in participants in higher caregiving environment categories. Both maternal and child GM triclosan was lowest in the lowest maternal FSIQ category (). Mothers in higher education, household income, IQ, and caregiving environment categories had children with higher mean FSIQ scores at 8 y of age (Table 1).
Table 1.
Geometric mean maternal and child urinary triclosan concentrations (ng/mL) and mean child FSIQ scores at 8 y of age according to sociodemographic, caregiving, and maternal factors among mother–child pairs in the HOME Study.
| Characteristic | (%) | GM maternal triclosana (GSD) | GM child triclosana (GSD) | Mean child FSIQ (SD) |
|---|---|---|---|---|
| Overall | 198 | 17 (3.6) | 11 (2.7) | 104 (16) |
| Child sex | ||||
| Female | 110 (56) | 15 (3.2) | 12 (2.8) | 102 (17) |
| Male | 88 (44) | 19 (4.0) | 11 (2.6) | 102 (15) |
| Child race/ethnicity | ||||
| White, non-Hispanic | 124 (62) | 17 (3.8) | 12 (2.5) | 108 (13) |
| Black, non-Hispanic | 64 (34) | 17 (3.2) | 10 (3.1) | 91 (16) |
| Other | 10 (4) | 18 (3.9) | 9 (2.6) | 107 (15) |
| Maternal Education | ||||
| College graduate or more | 95 (48) | 17 (4.0) | 13 (2.8) | 109 (13) |
| Some college | 57 (29) | 17 (3.6) | 13 (2.4) | 100 (14) |
| High school or less | 46 (23) | 16 (2.7) | 7 (2.5) | 91 (17) |
| Household income | ||||
| 53 (27) | 19 (3.8) | 15 (2.9) | 111 (12) | |
| 65 (33) | 17 (4.1) | 13 (2.5) | 104 (13) | |
| 31 (15) | 18 (3.5) | 10 (2.7) | 102 (12) | |
| 49 (24) | 15 (2.8) | 8 (2.5) | 90 (16) | |
| Marital status | ||||
| Married | 126 (64) | 18 (4.1) | 13 (2.7) | 108 (13) |
| Unmarried | 72 (36) | 15 (2.7) | 9 (2.7) | 93 (16) |
| Caregiving environmentb | ||||
| 40 (20) | 15 (3.0) | 10 (3.3) | 91 (14) | |
| 35–40 | 53 (27) | 16 (3.4) | 10 (2.5) | 99 (16) |
| 105 (53) | 18 (3.9) | 13 (2.6) | 108 (13) | |
| Maternal FSIQ | ||||
| 58 (29) | 15 (2.8) | 8 (2.5) | 91 (15) | |
| 96–109 | 47 (24) | 17 (3.1) | 12 (3.0) | 102 (14) |
| 42 (21) | 20 (4.8) | 15 (2.4) | 109 (11) | |
| 51 (26) | 17 (4.0) | 11 (2.7) | 110 (14) | |
| Neonatal intensive care unit | ||||
| Yes | 9 (5) | 23 (5.3) | 12 (2.7) | 89 (20) |
| No | 189 (95) | 17 (3.5) | 7 (1.7) | 103 (15) |
| Serum cotinine (ng/mL)c | ||||
| (Unexposed) | 88 (44) | 17 (3.4) | 12 (2.7) | 104 (15) |
| LOD–3.0 (SHS) | 92 (47) | 18 (3.8) | 11 (2.8) | 100 (16) |
| (active) | 18 (9) | 12 (3.0) | 11 (2.7) | 105 (16) |
Note: FSIQ, Full-Scale Intelligence Quotient; GM, geometric mean; GSD, Geometric Standard Deviation; HOME, Health Outcomes and Measures of the Environment study; LOD, limit of detection; SHS, secondhand smoke.
Geometric mean urinary triclosan concentrations were calculated by averaging maternal (16- and 26-wk gestation and delivery) and child (1–8 y of age) urinary triclosan concentrations.
Administered at 1 y of age; higher scores indicate greater quality and quantity of caregiving.
. Threshold of for active smoking was chosen based on results from the 1999–2004 National Health and Nutrition Examination Survey, which compared self-reported smoking status and serum cotinine levels among a representative sample of the U.S. population (Benowitz et al. 2009).
Urinary Triclosan Concentrations and Cognitive Test Scores
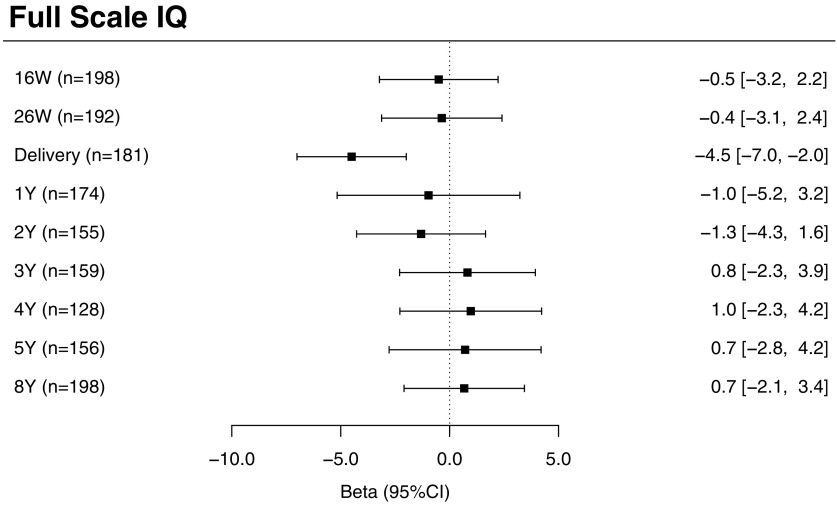
Before adjusting for covariates, there were no significant associations between urinary triclosan concentrations at any measured exposure time period and FSIQ scores (see Table S5). After adjusting for covariates, we observed a significant inverse association between increasing urinary triclosan concentrations at delivery and FSIQ scores, but not at other exposure periods. Specifically, for each 10-fold increase in maternal urinary triclosan concentration at delivery, there was on average a 4.5-point decrease in FSIQ score [95% confidence interval (CI): , ] at 8 y of age (Figure 2). The association between repeated urinary triclosan concentrations and FSIQ significantly differed by the period of exposure (triclosan concentration–exposure period interaction ).
Figure 2.
Adjusted differences in child FSIQ scores at 8 y of age per 10-fold increase in gestational and childhood urinary triclosan concentrations. Betas and 95% confidence intervals derived from a multiple informants model. CI, confidence interval; FSIQ, Full-Scale Intelligence Quotient; W, weeks of gestation; Y, age in years. Multiple informant regression model adjusted for urinary creatinine concentrations and serum cotinine concentrations (continuous), child sex (male vs. female), child race/ethnicity (white-non-Hispanic, black-non-Hispanic, or other), household income (continuous), marital status (married vs. unmarried), maternal education (high school or less, some college, or college graduate or more), caregiving environment scores (continuous), and maternal FSIQ (continuous). Error bars represent 95% confidence intervals. Interaction term for urinary triclosan concentration by exposure period.
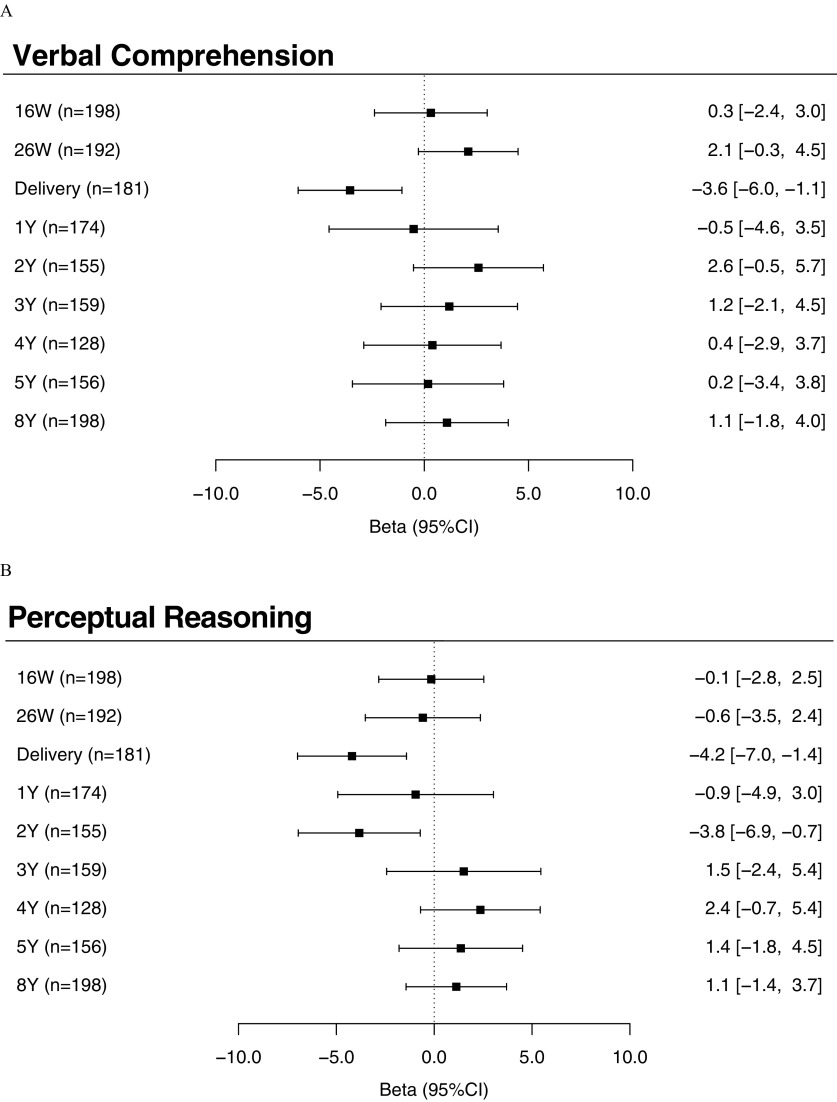
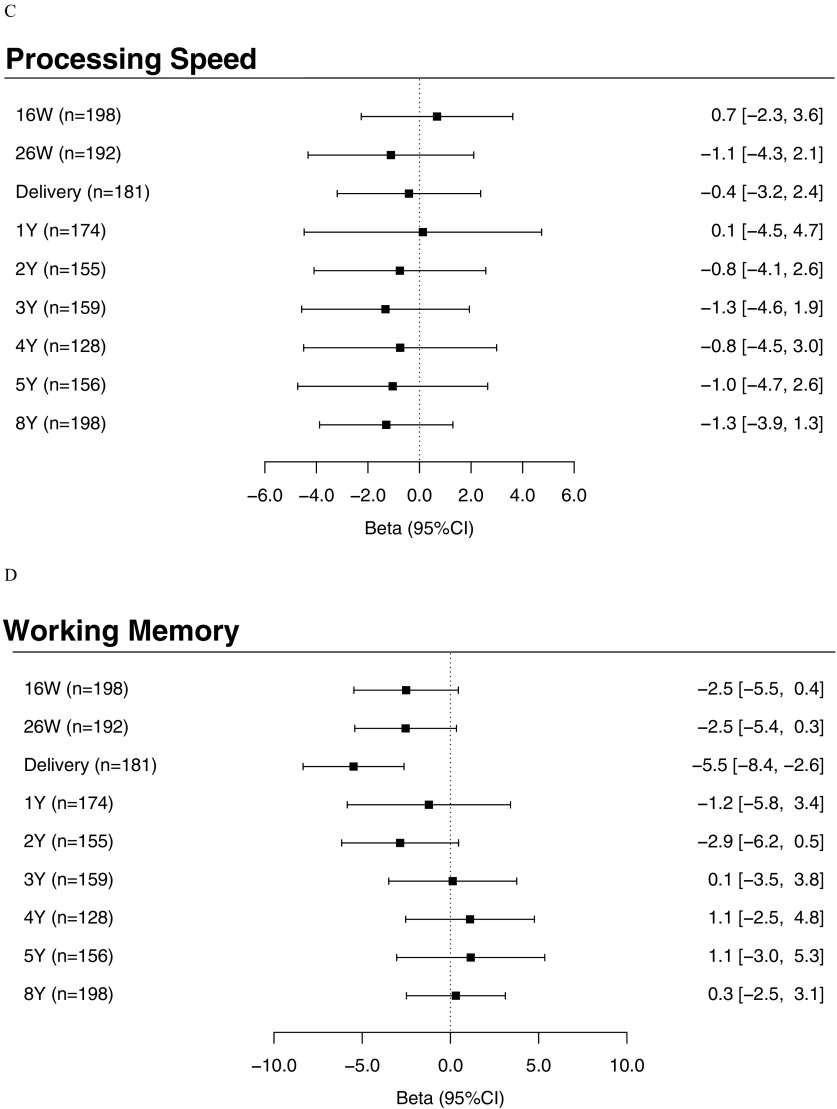
Similar to our findings for FSIQ scores, we also observed significant inverse associations between maternal urinary triclosan at delivery with VCI [ (95% CI: , )], PRI [ (95% CI: , )], and WMI [ (95% CI: , )] scores (Figure 3A,B,D; see also Table S5). The associations between repeated urinary triclosan concentrations and these subscales also significantly differed depending on the time period of exposure [triclosan concentration–exposure period interaction (VCI), 0.03 (PRI), and 0.02 (WMI)]. In addition, we found a significant inverse association between children’s urinary triclosan concentrations at 2 y of age and PRI scores [ (95% CI: , )] (Figure 3B; see also Table S5).
Figure 3.
Adjusted differences in FSIQ subscales: (A) Verbal Comprehension Index; (B) Perceptual Reasoning Index; (C) Processing Speed Index; and (D) Working Memory Index scores at 8 y of age with 10-fold increases in gestational and childhood urinary triclosan concentrations. Betas and 95% confidence intervals derived from a multiple informants model. CI, confidence interval; FSIQ, Full-Scale Intelligence Quotient; W, weeks of gestation; Y, age in years. Adjusted for urinary creatinine concentrations and serum cotinine concentrations (continuous), child sex (male vs. female), child race/ethnicity (white-non-Hispanic, black-non-Hispanic, or other), household income (continuous), marital status (married vs. unmarried), maternal education (high school or less, some college, or college graduate or more), caregiving environment scores (continuous), and maternal FSIQ (continuous). Error bars represent 95% confidence intervals. Urinary triclosan by exposure period interaction term , 0.03, 0.82, and 0.02, for Verbal Comprehension, Perceptual Reasoning, Processing Speed, and Working Memory, respectively.
Secondary and Sensitivity Analyses
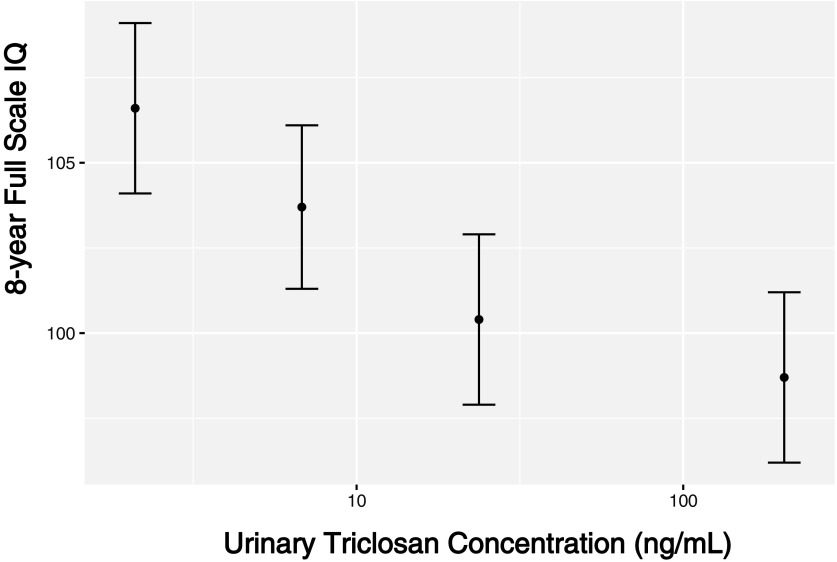
Children’s sex did not significantly modify the association between repeated triclosan measures and 8-y-of-age cognition test scores (FSIQ, VCI, PRI, PSI, and WMI period , 0.2, 0.8, 0.7, and 0.6, respectively; see Table S6). The association between maternal urinary triclosan concentrations at delivery and 8-y-of-age FSIQ did not meaningfully change after adjusting for NICU admission or by delivery hospital (see Figure S2). We observed a monotonic decrease in average FSIQ score across increasing triclosan concentration quartiles at delivery (Figure 4). FSIQ scores were on average 3.0 (95% CI: , 10), 6.3 (95% CI: , 14), and 8.0 (95% CI: 0.6, 15) points lower among children born to mothers with urinary triclosan concentrations in the second, third, and fourth quartiles at delivery, respectively, compared with children born to women with urinary triclosan concentrations at delivery in the first quartile.
Figure 4.
Adjusted least squared mean child FSIQ scores at 8 y of age by quartile of urinary triclosan concentrations at delivery (). Betas and 95% confidence intervals derived from a multiple informants model. FSIQ, Full-Scale Intelligence Quotient; max, maximum; min, minimum. Least mean squared linear regression model adjusted for urinary creatinine concentrations and serum cotinine concentrations (continuous), child sex (male vs. female), child race/ethnicity (white-non-Hispanic, black-non-Hispanic, or other), household income (continuous), marital status (married vs. unmarried), maternal education (high school or less, some college, or college graduate or more), caregiving environment scores (continuous), and maternal FSIQ (continuous). Error bars represent 95% confidence intervals. Median (min–max) urinary triclosan concentrations in 1st (), 2nd (), 3rd (), and 4th () quartiles were (), 6.1 (3.6–13), 22 (13–44), and 90 (47–2013) ng/mL, respectively.
Discussion
We estimated associations between early-life urinary triclosan concentrations and cognitive test scores among 8-y-old children born in Cincinnati, Ohio. We observed that the association of urinary triclosan concentrations with FSIQ and three of the four subscales of the WISC-IV significantly varied by the timing of triclosan exposure assessment. Specifically, triclosan concentrations at delivery were significantly inversely associated with FSIQ and several domains of children’s cognitive test scores at 8 y of age. In addition, urinary triclosan concentrations at 2 y of age were inversely associated with PRI scores at 8 y of age. In contrast, urinary triclosan concentrations during pregnancy and other times during childhood were not significantly associated with children’s cognitive test scores.
The significant association between cognitive test scores and maternal urinary triclosan concentrations at delivery, but not at other exposure periods, is an intriguing finding and should be interpreted cautiously given the lack of prior literature with which to compare and the limitations we describe below. Notably, this association remained after further adjustment for NICU admission and delivery hospital.
We are aware of one epidemiological study examining the neurotoxicity of triclosan in humans that was also based on the HOME Study cohort (Braun et al. 2017a). In this prior study, gestational urinary triclosan concentrations (averaged for the 16- and 26-wk maternal samples) were not associated with visual-spatial abilities at 8 y of age. However, we did not evaluate triclosan exposures at delivery or during childhood in this prior study. The triclosan exposure measurement time period differences between our two studies (gestational only vs. gestational, at delivery, and childhood) may explain why we did not find a significant association between triclosan exposure and visual-spatial scores in our previous study. In addition, it is possible that our current outcome measurement, the WISC-IV, is a more sensitive measure of neurodevelopmental processes that are adversely affected by early-life triclosan exposure compared with our previous outcome, visual-spatial abilities.
There are at least two possible biological mechanisms by which early-life triclosan exposure could adversely affect neurodevelopment. First, in experimental studies, triclosan exposure can reduce circulating levels of thyroxine () in pregnant, infant, and adult mice or rats (Johnson et al. 2016; Louis et al. 2017). Consistent with our hypothesis that triclosan may adversely affect neurodevelopment via disruptions in thyroid hormone homeostasis and these prior rodent studies, a study of 397 Chinese women and their infants found an inverse association of gestational urinary triclosan concentrations with free in maternal serum and free triiodothyronine () in cord serum (Wang et al. 2017). In the HOME Study, we previously observed that urinary triclosan concentrations at delivery were inversely associated with cord serum total (Braun et al. 2017b). Decreases in thyroxine concentrations during pregnancy have consistently been associated with poorer neurodevelopmental outcomes in both human and animal studies (Klein et al. 2001; Lazarus 1999; Murphy et al. 2015). Specific to our findings of a potential period of heightened vulnerability at delivery, previous studies have observed that children with congenital hypothyroidism (a clinical disorder where the fetus and child produces an insufficient amount of thyroxine) have poorer neurobehavioral outcomes (Amino and Ide 2016; Klein et al. 2001; Lazarus 1999). Second, in vitro studies have shown that triclosan exposure can induce neocortical neuron apoptosis by activating and stimulating apoptotic signaling pathways and inducing aryl hydrocarbon receptor (AhR)-dependent apoptosis through impairment of Cyp1a1 signaling and transcriptional activity of AhR (Szychowski et al. 2015, 2016).
Our prospective cohort design enabled us to examine associations between child cognitive test scores and triclosan exposure during nine separate time periods of fetal and child development. We used a multiple informant model to capitalize on these repeated exposure assessments and formally tested differences in associations with concentrations measured at different times during gestation and childhood. This statistical model has been previously used to investigate periods of vulnerability to other environmental exposures, including lead and bisphenol A (Sánchez et al. 2011; Stacy et al. 2017a). The multiple informant model avoids some of the disadvantages of fitting multiple regression models for each exposure period, but it has low power to detect exposure–time period interactions and to identify sex-specific associations. Despite the limited statistical power of this method, we were still able to detect significant modification of associations between urinary triclosan concentrations and child cognitive test scores by exposure period. Another strength of our study was the use of a well-validated measure of cognitive abilities (the WISC-IV) and adjustment for sociodemographic, maternal, and child factors that may confound the association between triclosan exposure and cognitive test scores.
There are several important limitations of this study. First, the within-person variation of urinary triclosan concentrations during gestation and childhood could have introduced exposure misclassification. However, this within-person variation was critical for us to identify potential time periods of heightened vulnerability. Urinary triclosan concentrations were relatively constant and had fair reproducibility [intraclass correlation coefficients ] during gestation and at delivery in this cohort (Stacy et al. 2017a). In contrast, childhood triclosan concentrations increased from 1 to 4 y of age, stabilized from 4 to 8 y of age, and had poor reproducibility (). Thus, there is the potential for triclosan exposure misclassification, particularly for childhood measurements, which if nondifferential could attenuate our estimates towards the null.
Second, it is possible that our observed associations were due to confounding by other chemical exposures or unknown confounders. However, we observed negative confounding of the association between triclosan concentrations at delivery and cognitive test scores where unadjusted estimates were closer to the null than adjusted ones. Thus, any residual confounding would have to be due to factors associated with both higher urinary triclosan concentrations and lower child cognitive test scores. Although we tried to account for factors that may contribute to confounding at delivery by adjusting for NICU admission and delivery hospital, it is possible that there are other unknown and unmeasured factors associated with higher delivery triclosan concentrations and lower child cognitive test scores. Finally, these findings may not be generalizable to other populations; however, median urinary triclosan concentrations among pregnant women () and children () in this cohort were similar to median concentrations observed in the U.S. general population (Calafat et al. 2008) and other previous cohorts of pregnant women and children () (Philippat et al. 2015; Stacy et al. 2017a; Teitelbaum et al. 2008; Wolff et al. 2010).
Conclusions
Maternal urinary triclosan concentrations at delivery, but not at other times, were associated with significant decreases in cognitive test scores among 8-y-old children in the HOME Study cohort. Future studies to replicate these findings would benefit from using serial urine samples with larger and more diverse cohorts to reduce triclosan exposure misclassification and to enhance the generalizability of these findings, respectively. Finally, future investigations should examine potential mechanisms underlying the association between early-life triclosan exposure and child neurodevelopment.
Supplemental Material
Acknowledgments
We acknowledge the technical assistance of X. Ye, Z. Zhou, P. Dwivedi, and J. Tao (Centers for Disease Control and Prevention) in measuring the urinary concentrations of triclosan. This work was supported by the National Institutes of Health/National Institute of Environmental Health Sciences (NIH/NIEHS; grants R01 ES024381, ES020349, P01 ES011261, and R01 ES014575).
References
- Amino N, Ide A. 2016. Maternal thyroid function and child IQ. Lancet Diabetes Endocrinol 4(1):18, PMID: 26724593, 10.1016/S2213-8587(15)00470-2. [DOI] [PubMed] [Google Scholar]
- Bellinger DC. 2004. What is an adverse effect? A possible resolution of clinical and epidemiological perspectives on neurobehavioral toxicity. Environ Res 95(3):394–405, PMID: 15220073, 10.1016/j.envres.2003.07.013. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Bernert JT, Caraballo RS, Holiday DB, Wang J. 2009. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol 169(2):236–248, PMID: 19019851, 10.1093/aje/kwn301. [DOI] [PubMed] [Google Scholar]
- Bradley RH, Caldwell BM, Corwyn RF. 2003. The Child Care HOME Inventories: assessing the quality of family child care homes. Early Child Res Q 18(3):294–309, 10.1016/S0885-2006(03)00041-3. [DOI] [Google Scholar]
- Braun JM. 2017. Early-life exposure to EDCs: role in childhood obesity and neurodevelopment. Nat Rev Endocrinol 13:161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Bellinger DC, Hauser R, Wright RO, Chen A, Calafat AM, et al. 2017a. Prenatal phthalate, triclosan, and bisphenol A exposures and child visual-spatial abilities. Neurotoxicology 58:75–83, PMID: 27888119, 10.1016/j.neuro.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Chen A, Hoofnagle A, Papandonatos GD, Jackson-Browne M, Hauser R, et al. 2017b. Associations of early life urinary triclosan concentrations with maternal, neonatal, and child thyroid hormone levels. Horm Behav S0018-506X(17)30362-8, PMID: 29154791, 10.1016/J.YHBEH.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Daniels JL, Poole C, Olshan AF, Hornung R, Bernert JT, et al. 2010. A prospective cohort study of biomarkers of prenatal tobacco smoke exposure: the correlation between serum and meconium and their association with infant birth weight. Environ Health 9(1):53, PMID: 20799929, 10.1186/1476-069X-9-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kalkbrenner AE, Calafat AM, Bernert JT, Ye X, Silva MJ, et al. 2011a. Variability and predictors of urinary bisphenol a concentrations during pregnancy. Environ. Health Perspect 119:131–137, 10.1289/ehp.1002366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kalkbrenner AE, Calafat AM, Yolton K, Ye X, Dietrich KN, et al. 2011b. Impact of early-life bisphenol A exposure on behavior and executive function in children. Pediatrics 128(5):873–882, PMID: 22025598, 10.1542/peds.2011-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kalloo G, Chen A, Dietrich KN, Liddy-Hicks S, Morgan S, et al. 2017c. Cohort profile: the Health Outcomes and Measures of the Environment (HOME) study. Int J Epidemiol. 46(1):1–10, PMID: 27006352, 10.1093/ije/dyw006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Yolton K, Dietrich KN, Hornung R, Ye X, Calafat AM, et al. 2009. Prenatal bisphenol A exposure and early childhood behavior. Environ Health Perspect 117(12):1945–1952, PMID: 20049216, 10.1289/ehp.0900979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. 2008. Urinary concentrations of triclosan in the U.S. population: 2003–2004. Environ Health Perspect 116(3):303–307, PMID: 18335095, 10.1289/ehp.10768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dann AB, Hontela A. 2011. Triclosan: environmental exposure, toxicity and mechanisms of action. J Appl Toxicol 31(4):285–311, PMID: 21462230, 10.1002/jat.1660. [DOI] [PubMed] [Google Scholar]
- Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, et al. 1999. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med 341(8):549–555, PMID: 10451459, 10.1056/NEJM199908193410801. [DOI] [PubMed] [Google Scholar]
- Henrichs J, Ghassabian A, Peeters RP, Tiemeier H. 2013. Maternal hypothyroxinemia and effects on cognitive functioning in childhood: how and why? Clin Endocrinol (Oxf) 79(2):152–162, PMID: 23600900, 10.1111/cen.12227. [DOI] [PubMed] [Google Scholar]
- Hornung RW, Reed LD. 1990. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg 5(1):46–51, 10.1080/1047322X.1990.10389587. [DOI] [Google Scholar]
- Johnson PI, Koustas E, Vesterinen HM, Sutton P, Atchley DS, Kim AN, et al. 2016. Application of the Navigation Guide systematic review methodology to the evidence for developmental and reproductive toxicity of triclosan. Environ Int 92–93:716–728, PMID: 27156197, 10.1016/j.envint.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RZ, Sargent JD, Larsen PR, Waisbren SE, Haddow JE, Mitchell ML. 2001. Relation of severity of maternal hypothyroidism to cognitive development of offspring. J Med Screen 8(1):18–20, PMID: 11373843, 10.1136/jms.8.1.18. [DOI] [PubMed] [Google Scholar]
- Korevaar TIM, Muetzel R, Medici M, Chaker L, Jaddoe VWV, de Rijke YB, et al. 2016. Association of maternal thyroid function during early pregnancy with offspring IQ and brain morphology in childhood: a population-based prospective cohort study. Lancet Diabetes Endocrinol 4(1):35–43, PMID: 26497402, 10.1016/S2213-8587(15)00327-7. [DOI] [PubMed] [Google Scholar]
- Larsen K. 1972. Creatinine assay by a reaction-kinetic principle. Clin Chim Acta 41:209–217, PMID: 4645233. [DOI] [PubMed] [Google Scholar]
- Lazarus JH. 1999. Thyroid hormone and intellectual development: a clinician’s view. Thyroid 9(7):659–660, PMID: 10447010, 10.1089/thy.1999.9.659. [DOI] [PubMed] [Google Scholar]
- Louis GW, Hallinger DR, Braxton MJ, Kamel A, Stoker TE. 2017. Effects of chronic exposure to triclosan on reproductive and thyroid endpoints in the adult Wistar female rat. J Toxicol Environ Heal. Part A 80:236–249, 10.1080/15287394.2017.1287029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy NC, Diviney MM, Donnelly JC, Cooley SM, Kirkham CH, Foran AM, et al. 2015. The effect of maternal subclinical hypothyroidism on IQ in 7- to 8-year-old children: a case–control review. Aust N Z J Obstet Gynaecol 55(5):459–463, PMID: 26058422, 10.1111/ajo.12338. [DOI] [PubMed] [Google Scholar]
- Paul KB, Hedge JM, Bansal R, Zoeller RT, Peter R, DeVito MJ, et al. 2012. Developmental triclosan exposure decreases maternal, fetal, and early neonatal thyroxine: a dynamic and kinetic evaluation of a putative mode-of-action. Toxicology 300(1–2):31–45, PMID: 22659317, 10.1016/j.tox.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippat C, Bennett D, Calafat AM, Picciotto IH. 2015. Exposure to select phthalates and phenols through use of personal care products among Californian adults and their children. Environ Res 140:369–376, PMID: 25929801, 10.1016/j.envres.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodricks JV, Swenberg JA, Borzelleca JF, Maronpot RR, Shipp AM. 2010. Triclosan: a critical review of the experimental data and development of margins of safety for consumer products. Crit Rev Toxicol 40(5):422–484, PMID: 20377306, 10.3109/10408441003667514. [DOI] [PubMed] [Google Scholar]
- Sánchez BN, Hu H, Litman HJ, Téllez-Rojo MM. 2011. Statistical methods to study timing of vulnerability with sparsely sampled data on environmental toxicants. Environ Health Perspect 119(3):409–415., PMID: 21362588, 10.1289/ehp.1102453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandborgh-Englund G, Adolfsson-Erici M, Odham G, Ekstrand J. 2006. Pharmacokinetics of triclosan following oral ingestion in humans. J Toxicol Environ Health Part A 69(20):1861–1873, PMID: 16952905, 10.1080/15287390600631706. [DOI] [PubMed] [Google Scholar]
- Stacy SL, Eliot M, Calafat AM, Chen A, Lanphear BP, Hauser R, et al. 2016. Patterns, variability, and predictors of urinary bisphenol A concentrations during childhood. Environ Sci Technol 50(11):5981–5990, PMID: 27152530, 10.1021/acs.est.6b00794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacy SL, Eliot M, Etzel T, Papandonatos G, Calafat AM, Chen A, et al. 2017a. Patterns, variability, and predictors of urinary triclosan concentrations during pregnancy and childhood. Environ Sci Technol 51(11):6404–6413, PMID: 28516781, 10.1021/acs.est.7b00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacy SL, Papandonatos GD, Calafat AM, Chen A, Yolton K, Lanphear BP, et al. 2017b. Early life bisphenol A exposure and neurobehavior at 8 years of age: identifying windows of heightened vulnerability. Environ Int 107:258–265, 10.1016/j.envint.2017.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szychowski KA, Sitarz AM, Wojtowicz AK. 2015. Triclosan induces Fas receptor-dependent apoptosis in mouse neocortical neurons in vitro. Neuroscience 284:192–201, PMID: 25313001, 10.1016/j.neuroscience.2014.10.001. [DOI] [PubMed] [Google Scholar]
- Szychowski KA, Wnuk A, Kajta M, Wójtowicz AK. 2016. Triclosan activates aryl hydrocarbon receptor (AhR)-dependent apoptosis and affects Cyp1a1 and Cyp1b1 expression in mouse neocortical neurons. Environ Res 151:106–114, PMID: 27474938, 10.1016/j.envres.2016.07.019. [DOI] [PubMed] [Google Scholar]
- Teitelbaum SL, Britton JA, Calafat AM, Ye X, Silva MJ, Reidy JA, et al. 2008. Temporal variability in urinary concentrations of phthalate metabolites, phytoestrogens and phenols among minority children in the United States. Environ Res 106(2):257–269, PMID: 17976571, 10.1016/j.envres.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Textor J, van der Zander B, Gilthorpe MS, Liśkiewicz M, Ellison GTH. 2016. Robust causal inference using directed acyclic graphs: the R package “dagitty.” Int J Epidemiol 45(6):1887–1894, PMID: 28089956, 10.1093/ije/dyw341. [DOI] [PubMed] [Google Scholar]
- Wang X, Ouyang F, Feng L, Wang X, Liu Z, Zhang J. 2017. Maternal urinary triclosan concentration in relation to maternal and neonatal thyroid hormone levels: a prospective study. Environ Health Perspect 125(6):067017, PMID: 28669941, 10.1289/EHP500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. 2004. WISC-IV: Wechsler Intelligence Scale for Children, Integrated: Technical and Interpretive Manual. San Antonio, TX:Harcourt Brace and Company. [Google Scholar]
- Wechsler D. 2008. Wechsler Adult Intelligence Scale—Fourth Edition (WAIS–IV). San Antonio, TX:NCS Pearson. [Google Scholar]
- Wolff MS, Teitelbaum SL, Pinney SM, Windham G, Liao L, Biro F, et al. 2010. Investigation of relationships between urinary biomarkers of phytoestrogens, phthalates, and phenols and pubertal stages in girls. Environ Health Perspect 118(7):1039–1046, PMID: 20308033, 10.1289/ehp.0901690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff TJ, Zota AR, Schwartz JM. 2011. Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environ Health Perspect 119(6):878–885, PMID: 21233055, 10.1289/ehp.1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Bishop AM, Needham LL, Calafat AM. 2008. Automated on-line column-switching HPLC-MS/MS method with peak focusing for measuring parabens, triclosan, and other environmental phenols in human milk. Anal Chim Acta 622(1–2):150–156, PMID: 18602546, 10.1016/j.aca.2008.05.068. [DOI] [PubMed] [Google Scholar]
- Ye X, Zhou X, Hennings R, Kramer J, Calafat AM. 2013. Potential external contamination with bisphenol A and other ubiquitous organic environmental chemicals during biomonitoring analysis: an elusive laboratory challenge. Environ Health Perspect 121(3):283–286, PMID: 23458838, 10.1289/ehp.1206093. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.