Abstract
Despite the advance of knowledge about the factors and potential mechanisms triggering the ichthyotoxicity in microalgae, these remain unclear or are controversial for several species (e.g. Heterosigma). Neither typical toxicity tests carried out with cell extracts nor direct exposure to harmful species were proved suitable to unravel the mechanism of harm. Ichthyotoxic species show a complex harmful effect on fish, which is mediated through various mechanisms depending on the species. In this work, we present a method to study sub-lethal effects triggered by reactive oxygen species of a population of harmful algae in vivo over a fish cell line. To that end, Transwell co-cultures in which causative and target species are separated by a 0.4 μm pore membrane were carried out. This allowed the evaluation of the effect of the released molecules by cells in a rapid and compact test. In our method, the harmful effect was sensed through the transcriptional activation of sub-lethal marker Hsp70b in the CHSE214 salmon cell line. The method was tested with the raphidophyte Heterosigma akashiwo and Dunaliella tertiolecta (as negative control). It was shown that superoxide intracellular content and its release are not linked in these species. The methodology allowed proving that reactive oxygen species produced by H. akashiwo are able to induce the transcriptional activation of sub-lethal marker Hsp70b. However, neither loss of viability nor apoptosis was observed in CHSE214 salmon cell line except when exposed to direct contact with the raphidophyte cells (or their extract). Consequently, ROS was not concluded to be the main cause of ichthyotoxicity in H. akashiwo.
Introduction
Raphidophyceae include unicellular algae related to considerable mortalities in wild and cultured fish populations, causing substantial losses for the aquaculture industry worldwide [1,2]. Some of the main ichthyotoxic species of this class are Fibrocapsa japonica, Chattonella spp., Pseudochattonella and Heterosigma akashiwo [3]. Particularly, H. akashiwo is one of the harmful algal species that impacts in Japan [4], but it has also been detected in many other countries [5–7].
The toxicological mechanisms responsible for the ichthyotoxic properties of H. akashiwo are currently under debate [8]. For raphidophytes, the following three main mechanisms have been proposed: i) production of neurotoxins (e.g. brevetoxins) [9–11], ii) high free fatty acids content [12], and iii) production of reactive oxygen species (ROS) [13,14]. ROS such as superoxide (•O2-) and hydrogen peroxide (H2O2) are constitutively generated by microalgae [13,15], although important differences between species and growth phases have been shown [16,17]. ROS generated during harmful algal blooms have been linked to gill tissue injuries in fish, including epithelial lifting, cell necrosis, and the alteration of chloride cells [14]. These injuries, in turn, produce massive mucus secretion from the gills and physiological responses such as hypoxia and subsequent asphyxia [14]. However, the current understanding is that the observed effects are attributable to a synergistic relation between ROS, polyunsaturated fatty acids (PUFA) and biotoxins [12], and that the causes of ichthytoxicity are strongly species-dependent [8]. So far, most of the tests carried out were based in direct contact of cells, or the extracts thereof, with cell lines [1,12,18–20] or fish [12,21]. Dorantes-Aranda et al. in a pioneering study used a Transwell plate to expose gill cells to living ichthyotoxic microalgae [22]. In their assay, they intended to mimic a fish gill, so gill cells and microalgae were able to have physical contact. Then, released (allelopathic, ROS, etc) molecules and those constitutive of the cell membrane were evaluated together.
Despite of providing useful information, these tests arise two important concerns: i) direct contact hinders the effect of diffusible released molecules and, ii) in the case of using cell extracts, the effect on the target cell or biomarker cannot be extrapolated to toxicity of living cells in their environment unless massive microalgae lysis occurs. It is our belief that toxicity tests where living causative cells could be assayed separately are able to provide a different perspective of ichthyotoxic mechanisms. Heat-Shock proteins (Hsp) are relevant to understand cellular stress response in fish [23]. In particular, the Hsp70b has not only a role in the response to heat shock, but also to other stressors such as heavy metals [24] or ROS [25].
Consequently, the aim of this work was to propose an in vitro assay to elucidate the toxic mechanisms of living ichthyotoxic microalgae. Transwell plates were used to allow co-culturing microalgae and fish cell without the possibility of physical contact. The short time of exposure in the assays, permits that only the released biomolecules can be sensed by the selected fish cell line. The potential of this method was tested with the ichthyotoxic microalga Heterosigma akashiwo.
Materials and methods
Cell cultures and maintenance
Strains CCMP302 (New Zealand) and CCMP2425 (Spain) of H. akashiwo were used for this study. The ichthyotoxic microalga was obtained from the Provasoli-Guillard National Center for Culture of Marine Phytoplankton, Maine, USA (NCMA). The green microalga Dunaliella tertiolecta (strain UTEX999) was obtained from the Culture Collection of Algae, Austin, Texas, USA (UTEX) and was used as a nontoxic control. These strains were supplied from the Chilean Microalgae Culture Collection COPAS SUR Austral (CCM-UdeC) and were stored and maintained at the center FICOLAB (University of Concepción).
All strains were grown in 100 mL Erlenmeyer flasks containing 50 mL of L1 medium (Guillard and Hargraves, 1993). Culture medium were prepared with natural seawater from Chilean coast (35°S) with a salinity of approximately 33.5 PSU. The cultures were initiated at 1,000 cells mL-1 of exponentially growing cells previously acclimated to experimental conditions for 10 days. Cultures were maintained in a culture chamber at 15 ± 2°C, with 50 μmol·photon ·m-2·s-1 in a 16:8 (Light:Dark) photoperiod, and without aeration. Cultures were shaken manually once a day. Cell density was assessed by cell counting in 1 mL Utermöhl chambers. H. akashiwo extracts were prepared as described in Astuya et al. [11].
CHSE-214 cell line (Chinook salmon embryo) from Oncorhynchus tshawytscha embryo was obtained from the European Collection of Cell Cultures (ECACC). This cell line has been previously used in other ichthyotoxicity studies (e.g. [26]). The cells were maintained in Minimal Essential Medium (MEM; Gibco) supplemented with 10% fetal bovine serum (FBS; Invitrogen), 2 mM glutamine (Invitrogen), 100 U·mL-1 penicillin (Invitrogen), 100 μg·mL-1 streptomycin (Invitrogen), 2.5 μg·mL-1 fungizone (Invitrogen). Cultures were maintained at 20°C without CO2 injection.
ROS determination
Intracellular ROS determination using a fluorescent probe
Relative levels of ROS were measured using 2´,7´dichlorodihydrofluorescein diacetate (H2DCFDA; Invitrogen), which in the presence of ROS is transformed into the highly fluorescent compound 2´,7´dichlorofluorescein (DCF). This probe has been previously used to study causes of ROS in raphidophytes [1] and dinoflagellates [27]. The assay was performed according to a previously described method [28] with the following modifications: microalga cells in the exponential (7 days of culturing) and stationary (14 days of culturing) phases were inoculated in 96-well microplates at different cell densities (1,000–15,000 cells·mL-1) with 10 mM of H2DCFDA in L1 medium. Control wells were inoculated with just microalga cells or the H2DCFDA solution. Emitted fluorescence was measured over a 6 h time-course assay using a FLx800 Multi-Detection Microplate Reader (BioTek Instruments, Inc.) with excitation and emission filters of 485/20 and 528/20 nm, respectively. DCF fluorescence data was expressed as absolute fluorescent units, and background fluorescence of t = 0 was subtracted from each determination. Additionally, cellular fluorescence was photographed with a laser confocal Nikon Eclipse TE2000-U microscope.
Quantitative intracellular superoxide determination
Quantitative determination of intracellular superoxide was performed using the nitroblue tetrazolium (NBT) reduction assay as previously described [29], but with slight modifications. NBT is a cell permeable substrate that is reduced by intracellular superoxide, thus generating an insoluble blue-colored formazan precipitate, which can be dissolved and quantified. Briefly, 5·105 cells were pooled and incubated with 1 mg·mL-1 NBT in L1 medium and incubated at 18°C for 1, 2, and 3 h. Cells were then centrifugated at 1,000 rpm for 1 min, washed with L1 medium, and centrifuged to obtain hard pellet at 5,000 rpm for 1 min. Unreduced NBT was eliminated through dehydration by applying 70% methanol for 15 min followed by air drying. Dry pellets were then treated with KOH (2 M) and dimethyl sulfoxide to dissolve formazan aggregates. Data quantification was obtained by measuring absorbance at 630 nm (FLx800 Microplate Reader, BioTek Instruments, Inc.) and using a calibration curve (1–10 nmol NBT). Controls included cells without NBT treatment and nontoxic microalga.
Quantitative extracellular superoxide determination
Extracellular superoxide was quantified by monitoring the reduction of cytochrome C. Cell suspensions (0.05, 0.1, 0.25, and 0.5·106 cells) in 1 mL of L1 culture medium and containing cytochrome C at a concentration of 0.5 mg·mL-1 were incubated for 6 h at 18°C. Cells were then centrifuged at 1,000 g, and 200 μL of the supernatants were used to measure cytochrome C reduction in a 96-well microplate reader (FLx800, BioTek Instruments, Inc) by determining the change in absorbance at 550 nm. An extinction coefficient of 21,000 M-1·cm-1 was used. The absorbance values from determinations without cells, corresponding to each time point used, were subtracted from the data. Controls included the omission of cytochrome C and the nontoxic microalga mentioned above.
Measurements in CHSE-214 cells with Transwell co-culture
Experimental design
CHSE-214 cells were seeded at a density of 1·106 cells per well in sterile 6-well culture plates 24 h before the assays. The ichthyotoxic microalga (H. akashiwo) was co-cultured with the CHSE-214 cell line by using a Transwell culture system with 0.4 μm pore polyester membrane inserts (Corning HTS Transwell). The inserts were placed on each well, where the microalgae (1·104 cells·mL-1), at either the exponential or stationary phase, were added to a final volume of 3 mL (1.5 mL of MEM and 1.5 mL of L1) and incubated for 6 hours. As a positive control, 0.1 and 1 μM H2O2 was used. Negative controls were the medium MEM: L1 without microalgae. In addition, we also assayed the effect of the cells without Transwell, supernatant and microalgae extract. Cultures of H. akashiwo were harvested through filtration using 0.25 mm GF/F filters (Millipore). Supernatants were immediately put in ice and used within 10 to 15 minutes. Also, the cell line was exposed to the supernatant in the presence antioxidant ascorbic acid (50uM). The filters were sonicated during 10 min at 47 kHz (Branson 1210 Ultrasonic Cleaner, USA) in absolute methanol. The crude microalga methanolic extracts were first evaporated until dryness under N2 flux at 40ºC (TurboVap Concentration System, Caliper, Hopkinton, USA) and then dissolved in MEM:L1. Experiments were performed four times and determinations were performed in triplicate. After the exposure to microalga extracts CHSE-214 cells were subjected to cell health evaluation with MTT (3-(4,5- dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide), percentage of apoptosis with acridine orange staining, also the good condition of the microalgae was observed with microscopy, ensuring morphogenic integrity and swimming movements. Finally, CHSE-214 cells were used to quantitative RT-PCR assays.
Quantitative RT-PCR assays
The mRNA levels of the endogenous genes Hsp70b (sub-lethal marker), Catalase, superoxide dismutase (both oxidation markers) and β-actin (housekeeping gene) in CHSE-214 cells were determined by quantitative real-time PCR (qPCR). Total RNA from CHSE-214 cells was prepared using the TRIzol Reagent (Invitrogen) following the manufacturer's instructions. Then, RNA was treated with DNase I (Thermo Scientific Waltham, MA, USA) and used for the cDNA synthesis reaction with the Revertaid H Minus First Strand cDNA Synthesis Kit. (Thermo Scientific) according to the manufacturer's instructions. The qPCR assays were carried out using the using the Maxima SYBR Green qPCR Master Mix (Thermo Scientific) in an Eco Real-Time PCR System (Illumina San Diego, CA, USA). The primers used in qPCR reactions were Hsp70b (Heat shock protein 70b; NM_001124745) (forward: 5’-AGGCCCAACCATTGAAGAGA-3’; reverse: 5’-GCAATGTCCAGCAATGCAATA-3’); Catalase (XM_024379116) (forward: 5’ -GGTTCAGACCCTACTCAACAA- 3’; reverse: 5’–GGTGGAAGTTAAGGCATCAC- 3’); Superoxide dismutase (XM_024398220.1; Superoxide dismutase [Cu-Zn]-like or Superoxide dismutase I) (forward: 5’–CCGTATTCTTTGAGCAGGAG- 3’; reverse: 5’–AGCCGTTGGTGTTGTCTC- 3’) and β-actin (forward:5’- CTGGACTTTGAGCAGGAGAT -3’; reverse: 5’- GGAGTTGTAGGTGGTCTCG -3’). The relative expressions of the analysed genes were determined with the comparative ΔΔCt method [30] and normalized with β-actin.
Statistical analysis
All experimental data are presented as means ± SD of at least three independent experiments performed with no less than triplicate determinations. When possible, the paired Student's t-test was used to assess significances between experimental data using Prism 4.0 software (GraphPad Inc.). p<0.05 (*) and p<0.01 (**) were considered significant and highly significant, respectively.
Results
Growth curves of Heterosigma were followed to establish the different growth phases. The cells grew exponentially between days 1 and 10 (S1 Fig). Then, the strain showed long stationary phase, and cell density started to decline from day 40. In this study, 7-day and 14-day old cultures were used for exponential and stationary phase cells, respectively.
Evaluation of the toxic potential of H. akashiwo diffusible factors on the salmon cell line CHSE-214 through in vitro Transwell co-incubation assays
CHSE-214 cells exhibited an epithelial cell phenotype with polygonal morphology. Immunocytochemical analysis showed a strong signal for the intermediate filament protein vimentin (S2 Fig), which supported the observation of morphological changes during exposure to the ichthyotoxic microalga. In order to assess if released ROS could elicit detectable cellular damage to fish cells, the effect of exposure to 0.1 μM of H2O2 on the expression of Hsp70b mRNA in CHSE-214 cells was evaluated at different time points. The results from the time-course assays showed increasing levels of Hsp70b mRNA expression after 1 h, and a plateau was reached between 2–6 h after hydrogen peroxide exposure. A seven-fold increase in mRNA levels was finally observed, as assessed by quantification relative to β-actin expression (S3 Fig).
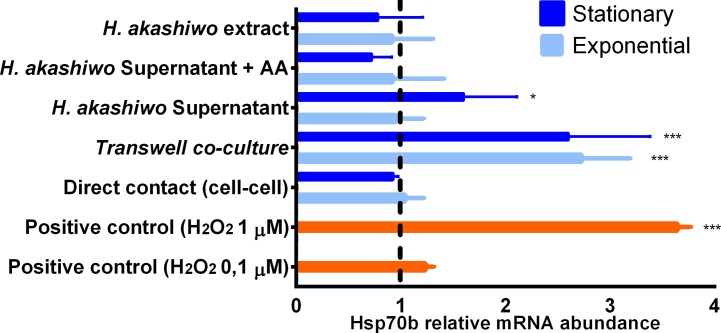
The effect that soluble compounds released by stationary and exponential H. akashiwo had on CHSE-214 cells was analyzed using an in vitro Transwell co-incubation system. qPCR assays showed an increase in the expression of Hsp70b when the fish cells were co-incubated with H. akashiwo using the Transwell. H2O2 treated cells were used as positive control showing concentration dependent response (Fig 1). The ichthyotoxic strain (H. akashiwo CCMP302) produced a significative increase (p<0.01) when data were relativized to negative control (D. tertiolecta). There were not significant differences among growth phases. Additionally, CHSE-214 cells were exposed to microalga without Transwell, supernatants and microalga extract. Significative effect in Hsp70b expression was only measured for exponential supernatants, although relative abundance was lower than for the Transwell assay (Fig 1). In this assay, ROS-scavenger ascorbic acid (AA) was used to prove that the expression of Hsp70b mRNA in CHSE-214 cells induced by H. akashiwo supernatant was due to ROS.
Fig 1.
Relative Hsp70b mRNA expression in CHSE-214 cells exposed to H. akashiwo (CCMP302) (10,000 cell/mL) for 6 h in the exponential (A) and stationary (B) growth phases. Data were relativized to Transwell exposure to D. tertiolecta.
Viability of CHSE-214 cells remained over 90% for more than 6 h for all the treatments (S4 Fig). After 6h, a viability decrease was observed in all samples, including control cultures. However, when we analyzed the acridine orange-stained cells prepared for the apoptosis test, we observed that the cell populations that were directly exposed to the microalga (living cells or total cell extract) showed a considerable increase in their morphological apoptotic-associated characteristics (e.g. condensed chromatin) in comparison to the other assays (Transwell co-culture, supernatant or the H2O2 controls) (S5 Fig). Similar conclusions can be obtained from the Hoechst staining which revealed nuclear fragmentation (S6 Fig).
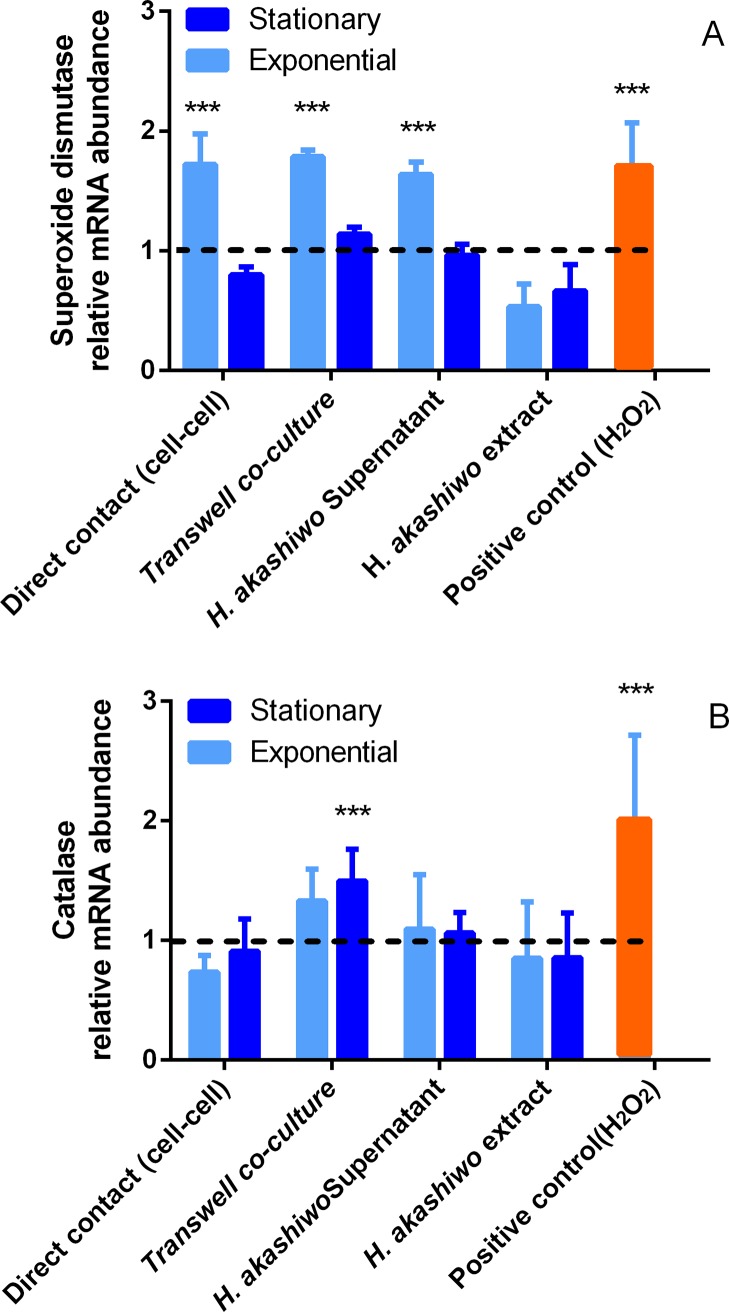
Since transcriptional activation of Hsp70b could be produced by different triggers [25,31], ROS scavengers production (SOD and Catalase) were evaluated. In Fig 2 values for these enzymes are compared with control cultures and co-cultures. In the case of catalase, there was an increment only in Transwell co-culture with H. akashiwo (no differences between growth phases). On the other hand, for SOD, besides Transwell co-culture, direct exposure and supernatant of exponential H. akashiwo had similar effect on the overproduction of this ROS scavenger. Additionally, the total cell extract provoked a significant SOD activity decrease.
Fig 2. Effect in the expression of SOD and Catalase in CHSE-214 cells exposed to H. akashiwo microalga (CCMP302).
Evaluation of different strains of H. akashiwo through in vitro Transwell co-incubation with the salmon cell line CHSE-214
The effect that soluble compounds released by different strains of H. akashiwo had on CHSE-214 cells was analyzed using the in vitro Transwell co-incubation system. qPCR assays showed an increase in the expression of Hsp70b when the fish cells were co-incubated with H. akashiwo using the Transwell. H2O2 treated cells were used as positive control showing concentration dependent response. Short times (30 min) were enough to show an increase in the expression of Hsp70b when the fish cells were co-incubated with H. akashiwo (Fig 3). The ichthyotoxic strains (CCMP302 and CCMP2425) produced a significative increase compared to the negative control (D. tertiolecta). There were differences among growth phases and strains. In both strains, exponential cells had a greater impact on Hsp70 than stationary cells. Strain CCMP2425 showed greater mRNA levels that CCMP302 in both growth phases.
Fig 3. Relative Hsp70b mRNA expression in CHSE-214 cells in Transwell co-culture with to H. akashiwo (CCMP302 and CCMP2425) (10,000 cell/mL) for 30 min in the exponential and stationary growth phases.
Data were relativized to unexposed CHSE-214 cells.
Quantitative analyses of intracellular and extracellular ROS of H. akashiwo strains
A quantitative NBT assay was used for intracellular ROS (superoxide) analysis with H. akashiwo cells. D. tertiolecta was used as a non-ichthyotoxic control. Three-hour time course assays showed higher superoxide levels in the exponential growth phases of H. akashiwo strain compared to stationary cells (Fig 4A). On the contrary, D. tertiolecta showed higher intracellular superoxide during the stationary phase (Fig 4A). Concentrations of intracellular superoxide were similar between D. tertiolecta and H. akashiwo CCMP2425 (although with an opposite pattern between exponential and stationary phases). H. akashiwo CCMP302 showed much less superoxide per cell than CCMP2425. In order to establish a correlation between intracellular superoxide generation and extracellular-released superoxide, extracellular superoxide was analyzed using non-permeable cytochrome C in experiments with increasing quantities of microalgae. In Fig 4B it can be observed that the two Heterosigma strains released extracellular superoxide in higher quantities than D. tertiolecta. Superoxide release between growth phases showed no significant differences.
Fig 4.
Quantitative determinations of intracellular (A) and extracellular (B) superoxide in H. akashiwo (CCMP302 and CCMP2425) and D. tertiolecta as a non-ichthyotoxic control.
Analysis of ROS production with DCF of H. akashiwo strains
Cells of the H. akashiwo strains analyzed with the fluorescent probe DCF emitted fluorescence in both exponential and stationary growth phases, which was checked under a confocal microscope (S7 Fig). In general, fluorescence was distributed around the whole area of H. akashiwo cells and had a normal, healthy ovoid morphology in the exponential and stationary growth phases, showing a fluorescent signal, indicative of intracellular ROS generation. The fluorescence level measured in different growth phases from Heterosigma strain showed significant differences, with lower production during the exponential growth phase only for CCMP302 (Fig 5). The non-ichthyotoxic species, D. tertiolecta, showed much lower fluorescence levels compared to H. akashiwo strain and values were indistinguishable between growth phases (Fig 5).
Fig 5. ROS-associated fluorescence emission of H. akashiwo (CCMP302 and CCMP2425) cells and D. tertiolecta cells in exponential and stationary phases, after incubation with H2DCFDA for different cell concentrations.
Discussion
In recent years, novel and sensitive in vitro approaches have been developed for the study of ichthyotoxicity mechanisms using fish cell lines [8,22,26]. The effect of ROS and other diffusible molecules can be assessed using this approach. In this study we determined the effect of H. akashiwo on Hsp70b transcription in fish CHSE-214 cells. These fish cells were selected due to their high cell adhesion and growth as a monolayer [26,32]. The expression of heat shock proteins (Hsps) is a widely used sub-lethal marker for environmental monitoring [33] that shows transcriptional changes induced by ROS and other stressors [25,31,34]. Our results indicated that the induced expression of Hsp70b by H2O2 was fast and clearly detected after 30 min. Furthermore, co-incubation assays between CHSE-214 cells and microalga were viable, which allowed for qPCR-based comparisons of effects of exposition to different microalga strains.
Thus, the effect of living H. akashiwo cells was detected through the increased expression of Hsp70b mRNA in Transwell co-culture with the fish cell line (Fig 1). Hydrogen peroxide (1μM) showed comparable expression to living H. akashiwo cells. Other assays were carried out to rule out the possibility that released toxins or bioactives could be contributing to the induced expression of Hsp70b. We did not observe any increase expression of Hsp70b mRNA for methanolic extract nor direct contact with living microalga (without Transwell). However, exponential supernatant of H. akashiwo also induced the overexpression of Hsp70b mRNA. In addition, SOD and catalase production (mRNA abundance) in fish cells exposed to H. akashiwo through the Transwell insert demonstrated that the increase expression of Hsp70b mRNA was due to ROS and not to biotoxins released after cell lysis. SOD activity was also higher when exponential cells assays were evaluated.
Fish cell line viability was not affected in our assays. Similarly, a previous study comparing several ichthyotoxic microalgae (including H. akashiwo) only found correlation among superoxide production, SOD activity and loss of viability for C. marina and A. catenella [8]. On the other hand, in our study, apoptotic cells were observed for direct contact and the total cell extract assays. There are previous evidence of apoptosis induction in Sf9 cells after exposure to H. akashiwo supernatants [19]. Although, in this case extracellular organic compounds were concentrated (x50). When direct-contact experiments were carried out, H. akashiwo cells rapidly stop swimming and settled to the bottom. This could be attributed to the change in osmolality that the presence of the Transwell membrane buffered. Then, this could be followed by passive (cell lysis) or active releasing of other molecules (including ROS, toxins, PUFAs). In this regard, Dorantes-Aranda et al. found loss of viability for gills cells exposed to an organic solvent extract of H. akashiwo but not for an aqueous one [8]. Thus, it was suggested that the toxic mechanism was due to hydrophobic compounds.
The proposed Transwell assay with the CHSE cells were used to compare two strains of H. akashiwo (Fig 3). As was shown, H. akashiwo CCMP2425 had a greater impact than the CCMP302 on the Hsp70 mRNA abundance. This could suggest that the former produced more ROS than the latter. Superoxide, hydrogen peroxide and hydroxyl radicals are produced by H. akashiwo and have been pointed out as the cause of its ichthyotoxicity [35]. In other studies, production of ROS is just one of the mechanisms, together with PUFAs and toxins, that has been proposed for explaining the harmful effect of H. akashiwo [35,36]. Heterosigma akashiwo has been found to produce a number of neurotoxins not completely identified [11,19]. ROS are extremely reactive and labile biomolecules whose determination is, therefore, troublesome [14]. Extracellular superoxide concentration has been generally used to assess and compare the ichthyotoxicity of different microalgae [8,37]. H. akashiwo releases lower quantities of superoxide than species such as Chattonella or Karenia but more than conventional microalgae [8]. In our study, extracellular superoxide production was greater for the ichthyotoxic species (both strains) than for the non-toxic microalga without differences among growth phases. To our best knowledge only exponential phase Heterosigma cells had been evaluated for superoxide production. With other raphidophytes there is conflicting evidence about whether ROS generation depends on the growth phase. On the one hand, Oda et al. (Oda et al., 1995) and Kim et al. (Kim, 2004) determined that superoxide production in Chattonella spp. was higher during the exponential growth phase and decreased during the stationary phase. On the other hand, Kim et al. (Kim, 2004) reported that H2O2 production by Chattonella antiqua was similar in both growth phases.
Our results showed a similar (CCMP2425) or lower (CCMP302) intracellular superoxide content for the ichthyotoxic species compared to non-toxic microalga (Fig 4A). Interestingly, superoxide levels through NBT assays were higher in the exponential growth phase for H. akashiwo strains. On the contrary, analysis in the non-toxic microalga D. tertiolecta showed elevated intracellular superoxide levels (higher than in H. akashiwo strain) in the stationary phase. As D. tertiolecta released lower levels of superoxide, this suggests a different role of superoxide among species (Fig 4B). Indeed, Twiner and Trick concluded that H2O2 released by H. akashiwo is not directly linked to photosynthesis [36]. According to our results, it is possible to conclude that there are differences in intracellular superoxide levels between growth phases, but no significant differences in extracellular superoxide release between growth phases. H. akashiwo strains clearly released more superoxide than the non-ichthyotoxic microalga. Consequently, it can be concluded that superoxide intracellular content and its release are not linked.
The fluorescent DCF probe, which accounts for intracellular ROS, offers some key advantages: for example, it can be oxidized by hydrogen peroxide and other ROS, such as peroxyl or hydroxyl radicals [38]. The approach used does not require cell lysis, thus allowing for in vivo detections [1]. In addition, the method only requires small sample volumes and short analysis times [38,39]. The obtained results with H. akashiwo and D. tertiolecta using the DCF probe for detecting intracellular ROS were not comparable to those from the intracellular NBT reduction analysis. The DCF assay showed higher ROS levels in the stationary phase of H. akashiwo (CCMP302) while NBT assay results indicated higher superoxide levels in the exponential phase (Fig 4). Strain CCMP2425 showed no differences between growth phases. Surprisingly, D. tertiolecta showed around 5 and 15 times lower level of ROS when compared to exponential CCMP302 cells or the rest of H. akashiwo cells evaluated, respectively. The DCF assay is generally used for ROS detection, but this probe can also be oxidized by reactive nitrogen species such as peroxynitrite and nitric oxide [38,40]. The observed differences might also be due to a differential penetration of DCF and NBT into subcellular compartments. Further experimentation is required to explain these observations. However, as was discussed for intracellular superoxide (NBT analysis), intracellular ROS is not a good indicative of extracellular ROS.
Conclusions
The use of live microalgae and fish cell lines in Transwell co-cultures separated by a permeable membrane allowed for demonstrating the diffusion of soluble ROS inducing expression of SOD and the sub-lethal marker Hsp70b in a 30-min assay. The methodology avoided microalgae cell lysis and loss of viability in fish cell line allowing for co-culture up tp 6h. Biotoxins (evaluated as methanolic cell extract) did not induce expression of Hsp70b. Consequently, the methodology constitutes a valuable tool for assessing the ROS-mediated ichthyotoxic effect of microalgae. The evaluated H. akashiwo strains produced considerable intracellular ROS compared to non-toxic microalga, although it was not mostly superoxide. Superoxide release in ichthyotoxic strains was higher than in the non-ichthyotoxic microalga. It was demonstrated that living H. akashiwo cells in the Transwell assay caused Hsp70b induction which did not result in an acute loss of viability or apoptosis induction. On the other hand, direct contact of fish cells and H. akashiwo was followed by the appearance of apoptotic cells. Consequently, release of intracellular cell content (other than superoxide) can be pointed out as the main cause of ichthyotoxicity in H. akashiwo.
Supporting information
(TIF)
Immunofluorescence images of CHSE-214 cells, (A) expressing vimentin (red) (B) TOPRO-3 (blue) for nuclear visualization. (C) Merge Vimentin (red) / Topro (blue), (D) Nomarsky (E) Phase contrast and counterstained with vimentin (red) (F) Phase contrast and TOPRO-3 (blue) for nuclear visualization. (F) Phasecontrast, stained with vimentin (red) and counterstained with Topro (blue).
(TIF)
Legend. Results revealed increasing levels of Hsp70b mRNA expression after 1 h, and a plateau was reached between 2–6 h. A seven-fold increase in mRNA levels was finally observed (quantification relative to ß-actin expression).
(TIF)
Microalga and fish cell culture media were used as negative control. H2O2 was used to prove that loss of viability was not due to oxidative stress.
(TIF)
Negative Control (A-B). Direct-contact to stationary (C) and exponential (D) H. akashiwo. Transwell co-culture with stationary (E) and exponential (F) H. akashiwo. Arrows indicate the cell undergoing apoptosis and nuclear fragmentation.
(TIF)
Negative Control (A-B). Direct-contact to stationary (C) and exponential (D) H. akashiwo. Transwell co-culture with stationary (E) and exponential (F) H. akashiwo. Arrows indicate the cell undergoing apoptosis and nuclear fragmentation.
(TIF)
(A) and (B): H. akashiwo in stationary phase; (C) and (D): H. akashiwo in exponential phase. (E) and (F): D. tertiolecta in exponential phase. (A), (C) and (E): images obtained by using contrast phase microscopy. (B), (D) and (F): images obtained with laser confocal microscopy and H2DCFDA stain. (A—D scale bar: 50 μm; E and F scale bar: 100 μm).
(TIF)
Acknowledgments
This research was supported by COPAS Sur Austral Programs (PFB-31), Universidad de Concepción, Chile. We also thank to Chilean National Science and Technology Research Fund (FONDECYT 1170515), the University of Concepción (grant VRID 216.096.067-1.OIN) and Dra. Patricia Gómez and Ms. Ingrid Inostroza (FICOLAB-University of Concepción).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported by the Chilean National Science and Technology Research Fund (FONDECYT 1170515; www.conicyt.cl/fondecyt) and the University of Concepción (grant VRID 216.096.067-1.OIN; www.udec.cl/vrid). We also thank COPAS Sur Austral (http://www.sur-austral.cl), Program PFB-31.
References
- 1.Kim D, Nakashima T, Matsuyama Y, Niwano Y, Yamaguchi K, Oda T. Presence of the distinct systems responsible for superoxide anion and hydrogen peroxide generation in red tide phytoplankton Chattonella marina and Chattonella ovata. J Plankton Res. 2007;29: 241–247. 10.1093/plankt/fbm011 [DOI] [Google Scholar]
- 2.Landsberg JH. The Effects of Harmful Algal Blooms on Aquatic Organisms. Rev Fish Sci. Taylor & Francis; 2002;10: 113–390. 10.1080/20026491051695 [DOI] [Google Scholar]
- 3.Fu M, Koulman A, Van Rijssel M, Lützen A, De Boer MK, Tyl MR, et al. Chemical characterisation of three haemolytic compounds from the microalgal species Fibrocapsa japonica (Raphidophyceae). Toxicon. 2004;43: 355–363. 10.1016/j.toxicon.2003.09.012 [DOI] [PubMed] [Google Scholar]
- 4.Nishibori N, Fujihara S, Nishijima T. Changes in intracellular polyamine concentration during growth of Heterosigma akashiwo (Raphidophyceae). Fish Sci. Blackwell Publishing Asia; 2006;72: 350–355. 10.1111/j.1444-2906.2006.01156.x [DOI] [Google Scholar]
- 5.Ki J-S, Han M-S. Nuclear rDNA and chloroplast rbcL, rbcS and IGS sequence data, and their implications from the Japanese, Korean, and North American harmful algae, Heterosigma akashiwo (Raphidophyceae). Environ Res. 2007;103: 299–304. 10.1016/j.envres.2006.08.014 [DOI] [PubMed] [Google Scholar]
- 6.O’Halloran C, Silver MW, Holman TR, Scholin CA. Heterosigma akashiwo in central California waters. Harmful Algae. 2006;5: 124–132. 10.1016/j.hal.2005.06.009 [DOI] [Google Scholar]
- 7.Clement AL. Phytoplankton monitoring program in the fish farming region of south Chile In: Smayda TJS, editor. Toxic Phytoplankton Blooms in the Sea. Amsterdam: Elsevier Science Publishers B.V.; 1993. pp. 223–258. [Google Scholar]
- 8.Dorantes-Aranda JJ, Seger A, Mardones JI, Nichols PD, Hallegraeff GM. Progress in understanding algal bloom-mediated fish kills: The role of superoxide radicals, phycotoxins and fatty acids. PLoS One. 2015;10 10.1371/journal.pone.0133549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Endo M, Onoue Y, Kuroki A. Neurotoxin-induced cardiac disorder and its role in the death of fish exposed to Chattonella marina. Mar Biol. 1992;112: 371–376. 10.1007/BF00356281 [DOI] [Google Scholar]
- 10.Khan S, Arakawa O, Onoue Y. Neurotoxins in a toxic red tide of Heterosigma akashiwo (Raphidophyceae) in Kagoshima Bay, Japan. Aquac Res. Blackwell Publishing Ltd; 1997;28: 9–14. 10.1046/j.1365-2109.1997.t01-1-00823.x [DOI] [Google Scholar]
- 11.Astuya A, Ramírez AE, Aballay A, Araya J, Silva J, Ulloa V, et al. Neurotoxin-like compounds from the ichthyotoxic red tide alga Heterosigma akashiwo induce a TTX-like synaptic silencing in mammalian neurons. Harmful Algae. 2015;47: 1–8. 10.1016/j.hal.2015.04.006 [DOI] [Google Scholar]
- 12.Marshall JA, Nichols PD, Hamilton B, Lewis RJ, Hallegraeff GM. Ichthyotoxicity of Chattonella marina (Raphidophyceae) to damselfish (Acanthochromis polycanthus): The synergistic role of reactive oxygen species and free fatty acids. Harmful Algae. 2003;2: 273–281. 10.1016/S1568-9883(03)00046-5 [DOI] [Google Scholar]
- 13.Oda T, Nakamura A, Shikayama M, Kawano I, Ishimatsu A, Muramatsu T. Generation of Reactive Oxygen Species by Raphidophycean Phytoplankton. Biosci Biotechnol Biochem. 1997;61: 1658–1662. 10.1271/bbb.61.1658 [DOI] [PubMed] [Google Scholar]
- 14.Marshall JA, Salas M, Oda T, Hallegraeff G. Superoxide production by marine microalgae. II. Towards understanding ecological consequences and possible functions. Mar Biol. 2005;147: 533–540. 10.1007/s00227-005-1596-7 [DOI] [Google Scholar]
- 15.Kim D, Nakamura A, Okamoto T, Komatsu N, Oda T, Iida T, et al. Mechanism of superoxide anion generation in the toxic red tide phytoplankton Chattonella marina: Possible involvement of NAD(P)H oxidase. Biochim Biophys Acta—Gen Subj. 2000;1524: 220–227. 10.1016/S0304-4165(00)00161-6 [DOI] [PubMed] [Google Scholar]
- 16.Kim D. Deleterious effect of Chattonella marina on short-necked clam (Ruditapes philippinarum); possible involvement of reactive oxygen species. J Plankton Res. 2004;26: 967–971. 10.1093/plankt/fbh085 [DOI] [Google Scholar]
- 17.Kwok C-T, van de Merwe JP, Chiu JMY, Wu RSS. Antioxidant responses and lipid peroxidation in gills and hepatopancreas of the mussel Perna viridis upon exposure to the red-tide organism Chattonella marina and hydrogen peroxide. Harmful Algae. 2012;13: 40–46. 10.1016/j.hal.2011.10.001 [DOI] [Google Scholar]
- 18.Kim D, Oda T, Muramatsu T, Kim D, Matsuyama Y, Honjo T. Possible factors responsible for the toxicity of Cochlodinium polykrikoides, a red tide phytoplankton. Comp Biochem Physiol—C Toxicol Pharmacol. 2002;132: 415–423. 10.1016/S1532-0456(02)00093-5 [DOI] [PubMed] [Google Scholar]
- 19.Twiner MJ, Chidiac P, Dixon SJ, Trick CG. Extracellular organic compounds from the ichthyotoxic red tide alga Heterosigma akashiwo elevate cytosolic calcium and induce apoptosis in Sf9 cells. Harmful Algae. 2005;4: 789–800. 10.1016/j.hal.2004.12.006 [DOI] [Google Scholar]
- 20.Kim D, Cha S-H, Sato E, Niwano Y, Kohno M, Jiang Z, et al. Evaluation of the potential biological toxicities of aqueous extracts from red tide phytoplankton cultures in in vitro and in vivo systems. J Toxicol Sci. 2010;35: 591–9. 10.2131/jts.35.591 [DOI] [PubMed] [Google Scholar]
- 21.Tang JYM, Wong CKC, Au DWT. The ichthyotoxic alga Chattonella marina induces Na+, K+-ATPase, and CFTR proteins expression in fish gill chloride cells in vivo. Biochem Biophys Res Commun. 2007;353: 98–103. 10.1016/j.bbrc.2006.11.126 [DOI] [PubMed] [Google Scholar]
- 22.Dorantes-Aranda JJ, Waite TD, Godrant A, Rose AL, Tovar CD, Woods GM, et al. Novel application of a fish gill cell line assay to assess ichthyotoxicity of harmful marine microalgae. Harmful Algae. 2011;10: 366–373. 10.1016/j.hal.2011.01.002 [DOI] [Google Scholar]
- 23.Ojima N, Yamashita M, Watabe S. Quantitative mRNA expression profiling of heat-shock protein families in rainbow trout cells. Biochem Biophys Res Commun. 2005;329: 51–57. 10.1016/j.bbrc.2005.01.097 [DOI] [PubMed] [Google Scholar]
- 24.Aït-Aïssa S, Ausseil O, Palluel O, Vindimian E, Garnier-Laplace J, Porcher JM. Biomarker responses in juvenile rainbow trout (Oncorhynchus mykiss) after single and combined exposure to low doses of cadmium, zinc, PCB77 and 17beta-oestradiol. Biomarkers. 2003;8: 491–508. 10.1080/13547500310001640037 [DOI] [PubMed] [Google Scholar]
- 25.Gupta SC, Siddique HR, Mathur N, Vishwakarma AL, Mishra RK, Saxena DK, et al. Induction of hsp70, alterations in oxidative stress markers and apoptosis against dichlorvos exposure in transgenic Drosophila melanogaster: Modulation by reactive oxygen species. Biochim Biophys Acta—Gen Subj. 2007;1770: 1382–1394. 10.1016/j.bbagen.2007.05.010 [DOI] [PubMed] [Google Scholar]
- 26.Skjelbred B, Horsberg TE, Tollefsen KE, Andersen T, Edvardsen B. Toxicity of the ichthyotoxic marine flagellate Pseudochattonella (Dictyochophyceae, Heterokonta) assessed by six bioassays. Harmful Algae. Elsevier B.V.; 2011;10: 144–154. 10.1016/j.hal.2010.08.007 [DOI] [Google Scholar]
- 27.Gallardo Rodríguez JJ, Sánchez Mirón A, García Camacho F, Cerón García MC, Belarbi EH, Chisti Y, et al. Causes of shear sensitivity of the toxic dinoflagellate Protoceratium reticulatum. Biotechnol Prog. 2009;25: 792–800. 10.1002/btpr.161 [DOI] [PubMed] [Google Scholar]
- 28.Knauert S, Knauer K. The role of reactive oxygen species in copper toxicity to two freshwater green algae. J Phycol. 2008;44: 311–319. 10.1111/j.1529-8817.2008.00471.x [DOI] [PubMed] [Google Scholar]
- 29.Choi HC, Kim J. W, Cha Y-N, Kim C. A quantitative nitroblue tetrazolium assay for determining intracellular superoxide anion production in phagocytic cells. J Immunoass Immunochem. 2006;27: 31–44. 10.1080/15321810500403722 [DOI] [PubMed] [Google Scholar]
- 30.Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods. 2001;25: 402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 31.Aït-Aïssa S, Pandard P, Magaud H, Arrigo AP, Thybaud E, Porcher JM. Evaluation of an in vitro hsp70 induction test for toxicity assessment of complex mixtures: Comparison with chemical analyses and ecotoxicity tests. Ecotoxicol Environ Saf. 2003;54: 92–104. 10.1016/S0147-6513(02)00026-X [DOI] [PubMed] [Google Scholar]
- 32.Mori M, Wakabayashi M. Cytotoxicity evaluation of synthesized chemicals using suspension-cultured fish cells. Fish Sci. 2000;66: 871–875. Available: https://www.scopus.com/inward/record.uri?eid=2-s2.0-0000456457&partnerID=40&md5=825a888deade350d6ee14aa2fdb9aaea [Google Scholar]
- 33.Mukhopadhyay I, Nazir A, Saxena DK, Kar Chowdhuri D. Heat shock response: hsp70 in environmental monitoring. J Biochem Mol Toxicol. 2003;17: 249–254. 10.1002/jbt.10086 [DOI] [PubMed] [Google Scholar]
- 34.Gravel A, Vijayan MM. Non-steroidal anti-inflammatory drugs disrupt the heat shock response in rainbow trout. Aquat Toxicol. 2007;81: 197–206. 10.1016/j.aquatox.2006.12.001 [DOI] [PubMed] [Google Scholar]
- 35.Yang CZ, Albright LJ, Yousif AN. Oxygen-radical-mediated effects of the toxic phytoplankter Heterosigma carterae on juvenile rainbow trout Oncorhynchus mykiss. Dis Aquat Organ. 1995;23: 101–108. 10.3354/dao023101 [DOI] [Google Scholar]
- 36.Twiner MJ, Trick CG. Possible physiological mechanisms for production of hydrogen peroxide by the ichthyotoxic flagellate Heterosigma akashiwo. J Plankton Res. 2000;22: 1961–1975. 10.1093/plankt/22.10.1961 [DOI] [Google Scholar]
- 37.Marshall JA, De Salas M, Oda T, Hallegraeff G. Superoxide production by marine microalgae: I. Survey of 37 species from 6 classes. Mar Biol. 2005;147: 533–540. 10.1007/s00227-005-1596-7 [DOI] [Google Scholar]
- 38.Wang H, Joseph JA. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader1. Free Radic Biol Med. 1999;27: 612–616. 10.1016/S0891-5849(99)00107-0 [DOI] [PubMed] [Google Scholar]
- 39.Godrant A, Rose AL, Sarthou G, David Waite T. New method for the determination of extracellular production of superoxide by marine phytoplankton using the chemiluminescence probes MCLA and red-CLA. Limnol Oceanogr Methods. 2009;7: 682–692. Available: https://www.scopus.com/inward/record.uri?eid=2-s2.0-77950274061&partnerID=40&md5=06e85946e99451b15877b5b09f69fd96 [Google Scholar]
- 40.Crow JP. Dichlorodihydrofluorescein and Dihydrorhodamine 123 Are Sensitive Indicators of Peroxynitritein Vitro:Implications for Intracellular Measurement of Reactive Nitrogen and Oxygen Species. Nitric Oxide. 1997;1: 145–157. 10.1006/niox.1996.0113 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
Immunofluorescence images of CHSE-214 cells, (A) expressing vimentin (red) (B) TOPRO-3 (blue) for nuclear visualization. (C) Merge Vimentin (red) / Topro (blue), (D) Nomarsky (E) Phase contrast and counterstained with vimentin (red) (F) Phase contrast and TOPRO-3 (blue) for nuclear visualization. (F) Phasecontrast, stained with vimentin (red) and counterstained with Topro (blue).
(TIF)
Legend. Results revealed increasing levels of Hsp70b mRNA expression after 1 h, and a plateau was reached between 2–6 h. A seven-fold increase in mRNA levels was finally observed (quantification relative to ß-actin expression).
(TIF)
Microalga and fish cell culture media were used as negative control. H2O2 was used to prove that loss of viability was not due to oxidative stress.
(TIF)
Negative Control (A-B). Direct-contact to stationary (C) and exponential (D) H. akashiwo. Transwell co-culture with stationary (E) and exponential (F) H. akashiwo. Arrows indicate the cell undergoing apoptosis and nuclear fragmentation.
(TIF)
Negative Control (A-B). Direct-contact to stationary (C) and exponential (D) H. akashiwo. Transwell co-culture with stationary (E) and exponential (F) H. akashiwo. Arrows indicate the cell undergoing apoptosis and nuclear fragmentation.
(TIF)
(A) and (B): H. akashiwo in stationary phase; (C) and (D): H. akashiwo in exponential phase. (E) and (F): D. tertiolecta in exponential phase. (A), (C) and (E): images obtained by using contrast phase microscopy. (B), (D) and (F): images obtained with laser confocal microscopy and H2DCFDA stain. (A—D scale bar: 50 μm; E and F scale bar: 100 μm).
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.