Demonstration of the theranostic potential of a porphyrin derivative: as a PET agent and a photosensitizer in PDT.
Demonstration of the theranostic potential of a porphyrin derivative: as a PET agent and a photosensitizer in PDT.
Abstract
Porphyrins, owing to their inherent tendency to accumulate in tumorous lesions, are considered suitable for developing agents for theranostic applications involving tumor diagnosis and targeted tumor therapy. The aim of the present work is to study the potential of a porphyrin derivative namely, 5,10,15,20-tetrakis(p-carboxymethyleneoxyphenyl)porphyrin (SPTA) as a theranostic agent for applications in positron emission tomography (PET) and photodynamic therapy (PDT). SPTA was synthesized in-house following a three-step reaction process and characterized by using spectroscopic techniques, viz. UV-vis, FT-IR, 1H-NMR and 13C-NMR spectroscopy, as well as by mass spectrometry. SPTA was labeled with 68Ga, a generator produced PET radioisotope, and the radiolabeled product was characterized by HPLC. The 68Ga-SPTA complex was prepared with a radiochemical purity of >95% under optimized conditions. The diagnostic potential of 68Ga-SPTA was evaluated by cell uptake studies in two different tumor cell lines (HT1080 and A549) which revealed the affinity of 68Ga-SPTA towards the cancer cells. Biodistribution studies carried out in Swiss mice bearing fibrosarcoma tumors exhibited the accumulation of the radiotracer in the tumor. The therapeutic potential of SPTA was evaluated by determining its photo-cytotoxicity employing the MTT assay in HT1080 and A549 cell lines using three different light doses, which indicated the significant cytotoxicity of SPTA in the presence of light. The present study indicates the possible potential of SPTA in radionuclide imaging as well as in photodynamic therapy (PDT) thus confirming the promising theranostic nature of this porphyrin derivative.
Introduction
Porphyrins offer excellent potential for developing tumor-avid agents as they are biocompatible, their metal complexes are thermodynamically stable and kinetically inert, and they exhibit preferential affinity for tumors, both with and without the presence of a metal ion in its central core.1–3 These properties of porphyrin derivatives have been well-exploited in designing and exploring several tetrapyrrolic macrocyclic derivatives for their possible usage in diagnosis and therapy of tumorous lesions.3–11 However, with the porphyrin moiety being sufficiently lipophilic in nature, porphyrin-based agents usually exhibit high non-specific uptake, which eventually limits the scope of their wide-spread clinical applications. This problem can be circumvented, at least partially, by the introduction of hydrophilic substituents on the peripheral positions of the porphyrin core, as the presence of such substituents not only increases the hydrophilicity of the porphyrin derivatives, which in turn helps in improving their pharmacokinetic behavior; but also makes them water soluble, a preferred prerequisite for clinical applications.9,12–15
The suitability of the porphyrin core to accommodate various metal ions viz. Cu, Ga, Pd, etc. makes it a useful targeting agent for developing radiometallated porphyrin derivatives by incorporating the radioactive counterparts of the metal ions in the porphyrin moiety (64/67Cu, 67/68Ga, 109Pd, etc.).9,11,16–19 Upon radiolabeling these metal ions become an integral part of the porphyrin structure and this rules out the requirement of any structural modification or attachment of a bi-functional chelating agent (BFCA), which may affect the tumor-affinity exhibited by the porphyrin derivative. In addition to their tumor avidity, porphyrin derivatives also act as excellent photosensitizers and this property has long been utilized in the field of photodynamic therapy (PDT).2,14–16,20,21 PDT employs the effects of light-mediated in situ generation of singlet oxygen, through an efficient energy transfer mechanism from a photosensitizer to the triplet oxygen, for exerting the therapeutic action.2,17,21 These dual characteristics of the porphyrin derivatives i.e. core radiometallation and photosensitizing action, combined with their natural tumor targeting ability, make them an excellent choice for developing agents for theranostic applications, wherein in their unlabeled form, they can be used as photosensitizers in PDT for targeted tumor therapy while diagnosis of tumorous lesions can be undertaken by employing a radionuclide imaging technique using the same porphyrin derivative labeled with a suitable diagnostic radionuclide (64Cu, 67Ga, 68Ga).4
In the present study, an attempt was made to synthesize a symmetrically substituted porphyrin derivative, namely 5,10,15,20-tetrakis(p-carboxymethyleneoxyphenyl)porphyrin (SPTA) and to evaluate its potential towards theranostic application. The rationale behind choosing this porphyrin derivative is the presence of four carboxyl substituents in the periphery of the porphyrin ring which is expected to reduce the lipophilicity and thus improve the pharmacokinetic behavior exhibited by the porphyrin. For evaluating the diagnostic potential of the porphyrin derivative, it was radiolabeled with 68Ga. Gallium-68 (T½ = 68 min) is a positron emitting radioisotope and is used for radiolabeling a variety of molecules for the preparation of various PET (positron emission tomography) imaging agents for clinical applications,22–26 such as 68Ga-DOTA-TATE (DOTA: 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid, TATE: Tyr3-octreotate), 68Ga-DOTA-TOC (TOC: d-Phe1-Tyr3-octreotide) and 68Ga-DOTA-NOC (NOC: Nal3-octreotide) for imaging of neuroendocrine tumors; 68Ga-citrate for quantification of pulmonary vascular permeability; 68Ga-BPAMD (4-{[(bis(phosphonomethyl))carbamoyl]methyl}-7,10-bis(carboxymethyl)-1,4,7,10-tetraazacyclododec-1-yl)acetic acid for imaging of bone metastases and 68Ga-PSMA-11 (prostate specific membrane antigen) for imaging of prostate cancer.22–26 In vitro cell uptake studies were carried out in two different cell lines viz. HT1080 (human fibrosarcoma) and A549 (human lung carcinoma) to evaluate the tumor affinity of 68Ga-labeled SPTA. The radiolabeled agent was also administered in fibrosarcoma tumor bearing Swiss mice to investigate its potential as a PET imaging agent. An attempt was also made to determine the photo-cytotoxicity of the porphyrin derivative which was measured in the same tumor cell lines in the absence as well as in the presence of light using different doses to evaluate its suitability for application in PDT.
Experimental
Materials and methods
Pyrrole, 4-hydroxybenzaldehyde and ethylbromoacetate used for the synthesis of the porphyrin derivative were purchased from Aldrich Chemical Company (USA). Propionic acid and nitrobenzene were obtained from S.D. Fine Chemicals (India). The silica gel (60–120 mesh size) used for column chromatography and silica gel plates (silica gel 60 F254) required for analytical thin-layer chromatography (TLC) were procured from Merck (India). All other chemicals used in the present study were purchased from reputable local manufacturers and were of analytical grade.
Ultraviolet-visible (UV-vis) spectra were recorded using a Jasco V-530 UV/vis spectrophotometer (Japan). Photoluminescence measurements were carried out using a Hitachi F-4500 spectrofluorimeter at room temperature. Fourier transform infrared (FT-IR) spectra were recorded on a Jasco FT/IR-420 spectrophotometer (Japan). Proton nuclear magnetic resonance (1H-NMR) spectra were acquired using a 300 MHz Varian VXR 300 s spectrometer (USA). Mass spectra were recorded using a 410 Prostar Binary LC mass spectrometer (Varian, USA) using electron spray ionization (ESI). Gallium-68 was eluted from a 1.11 GBq (30 mCi) 68Ge/68Ga radionuclide generator obtained from Eckert & Ziegler (Germany) and purified, prior to its use, by using a Strata™ X-C cation exchange column, purchased from Phenomenex (India). All radioactive counting, associated with the radiochemical studies, was carried out by using a well-type NaI(Tl) scintillation counter, obtained from Electronic Corporation of India Limited (ECIL, India). The high performance liquid chromatography (HPLC) system (PU 1580) was obtained from Jasco (Japan). Elution profiles were monitored by detecting the radioactivity signal using a well-type NaI(Tl) detector (Jasco, Japan) coupled with the HPLC system. All the solvents used for HPLC studies were degassed and filtered prior to use and were of HPLC grade. Final purification of the 68Ga-labeled porphyrin was carried out using C-18 reversed phase Sep-Pak® cartridges procured from Waters (India).
Chemicals for biological assays were purchased from Sigma Chemical Inc. (USA) unless stated otherwise. HT1080 (human fibrosarcoma) and A549 (human lung carcinoma) cells were obtained from the National Center for Cell Sciences (NCCS, India). These cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% fetal calf serum (Invitrogen Carlsbad, USA) and antibiotic solution (10 mL L–1 of 100× solution) and incubated at 37 °C in a humidified 5% CO2 atmosphere of an incubator. For the measurement of photo-cytotoxicity, cells were exposed to light generated by a setup which included LED bulbs (9 W and 14 W, Philips, India) as the light source. The irradiation was continued for a period of 4 h and the light doses received by the cells were measured using an irradiance meter (PVM210, Taiwan). The fibrosarcoma cell line used for growing the tumors in the animals was also purchased from NCCS (India). Radioactive counting, associated with the animal studies, was carried out using a flat-type NaI(Tl) scintillation counter (ECIL, India). The animal studies reported in the present article were approved by the Institutional Animal Ethics Committee (IAEC) and all the animal experiments were carried out in strict compliance with the institutional (IAEC) guidelines, following the relevant national laws related to the conduct of animal experimentation (Prevention of Cruelty to Animals Act, 1960).
Synthesis of 5,10,15,20-tetrakis(p-carboxymethyleneoxyphenyl)porphyrin (SPTA)
5,10,15,20-Tetrakis(p-carboxymethyleneoxyphenyl)porphyrin (SPTA) was synthesized by following a three-step procedure briefly described below.
Synthesis of 4-carboethoxymethyleneoxybenzaldehyde (i)
4-Carboethoxymethyleneoxybenzaldehyde was synthesized by following the procedure reported in the literature.8 The crude product was purified by silica gel column chromatography using CHCl3 as eluting solvent whereby pure 4-carboethoxymethyleneoxybenzaldehyde (i) was obtained as a colorless viscous liquid (5.7 g, yield 70%): Rf = 0.5 (CHCl3); 1H-NMR (300 MHz, CDCl3): δ = 1.24 (t, 3H, J = 7.8 Hz, –COOCH2CH3), 4.20 (q, 2H, –COOCH2CH3), 4.75 (s, 2H, –OCH2), 6.97 (d, 2H, J = 7.2 Hz, ArH), 7.85 (d, 2H, J = 7.1 Hz, ArH), 9.90 ppm (s, 1H, >CHO); 13C-NMR (75 MHz, [D6]DMSO): δ = 14.02 (CH2–CH3), 60.84 (O–CH2–CH3), 64.75 (O–CH2–C O), 115.06 (m-phenyl), 130.16 (p-phenyl), 131.72 (o-phenyl), 162.48 (p-O-phenyl), 168.21 (O C–O), 191.36 ppm (O CH–); FT-IR (neat, ν cm–1) = 1754; ESI-MS (m/z): calcd for C11H12O4: 208.21, found: [M + 1] 209.15.
Synthesis of 5,10,15,20-tetrakis(p-carboethoxymethyleneoxyphenyl)porphyrin (ii)
5,10,15,20-Tetrakis(p-carboethoxymethyleneoxyphenyl)porphyrin was synthesized by the dropwise addition of pyrrole (1.30 g, 19.2 mmol) to a refluxing mixture of (i) (3 g, 14.4 mmol), propionic acid (25 mL) and nitrobenzene (8 mL).27 The refluxing was continued for an additional hour and the resultant reaction mixture was stored overnight at 4 °C. Subsequently nitrobenzene and propionic acid were removed from the reaction mixture by distillation to obtain the crude product which was purified by silica gel column chromatography using 1% MeOH in CHCl3 as eluting solvent and thus 294 mg of pure (ii) was obtained (yield 2%). Rf = 0.8 (CHCl3/MeOH 9.7 : 0.3); 1H-NMR (300 MHz, CDCl3): δ = 1.41 (t, 12H, J = 7.5 Hz, –COOCH2CH3), 4.42 (q, 8H, J = 4.5 Hz, –COOCH2CH3), 4.91 (s, 8H, –OCH2), 7.50–7.51 (d, 8H, J = 7.5 Hz, m-ArH), 8.12–8.13 (d, 8H, J = 8.0 Hz, o-ArH), 8.84 ppm (s, 8H, pyrrole). 13C-NMR (75 MHz, [D]CHCl3): δ = 9.63 (CH2–CH3), 61.57 (O–CH2–CH3), 65.71 (O–CH2–C O), 112.94 (o-phenyl), 119.8 (β-pyrrole), 124.10 ( C<), 128.98 (p-phenyl), 137.94 (α-pyrrole), 157.70 ( C–O), 172.12 ppm (O C<). FT-IR (neat, ν cm–1): 3291, 3258, 3198, 2976, 1758, 1666. ESI-MS (m/z): calcd for C60H54O12N4: 1022.37, found: [M] 1022.3.
Synthesis of 5,10,15,20-tetrakis(p-carboxymethyleneoxyphenyl)porphyrin (SPTA) (iii)
5,10,15,20-Tetrakis(p-carboxymethyleneoxyphenyl)porphyrin was prepared by room temperature stirring of (ii) (0.1 g, 0.09 mmol) in 10 mL of 2 N NaOH for 2 days. Compound (iii) in its purified form was obtained by removing unreacted (ii) via repetitive extractions with CHCl3 (62 mg, yield 70%). 13C-NMR (75 MHz, D2O): δ = 77.87 (O–CH2–COOH), 114.21 (o-phenyl), 122.28 (β-pyrrole), 125.67 (p-phenyl), 129.28 (α-pyrrole), 181.61 ppm (–COOH); FT-IR (neat, ν cm–1): 3448, 1666, 1599, 1483, 1178, 1064. UV/vis (H2O): (λmax, nm): 416, 516, 553, 591, 649; ESI-MS (m/z): calcd for C52H38N4O12: 909.8, found: [M + Na] 932.6.
Elution and purification of 68Ga
Ga-68, used for the present study, was eluted from a 1.11 GBq (30 mCi) 68Ge/68Ga radionuclide generator. The generator was eluted with 5 mL of 0.1 N HCl. 68GaCl3, thus obtained, was purified by using a Strata™ X-C cation exchange column following the procedure mentioned below. The column was pre-conditioned by passing 0.5 mL CH3OH followed by 0.5 mL of 0.1 N HCl prior to the loading of 68GaCl3 onto the column. The column was washed with 20% 0.1 N HCl in acetone and finally 68Ga was eluted using 0.2% 0.05 N HCl in acetone.
Preparation, purification and characterization of 68Ga-labeled SPTA
Ga-68-labeled SPTA was prepared by incubating SPTA (0.5 mg, 0.5 μmol), dissolved in ammonium acetate buffer (300 μL, pH = 5.0), with 68GaCl3 (200 μL, 0.4 mCi) in a boiling water bath for a period of 45 min. The 68Ga-SPTA complex, thus obtained, was further purified using a Sep-pak® cartridge following the protocol mentioned below. The cartridge was pre-conditioned by passing 4 mL of ethyl alcohol followed by 2 mL of double distilled water before loading the 68Ga–SPTA complex onto the column. Uncomplexed 68Ga, if any, was eluted using 5 mL of double distilled water, subsequent to which the pure 68Ga–SPTA complex was eluted by washing the column with 1 mL of ethyl alcohol. The alcohol was slowly evaporated by gentle warming under an inert gas flow and the purified 68Ga-labeled-SPTA complex was reconstituted in normal saline for the subsequent studies.
The radiolabeled porphyrin complex was characterized by reversed phase HPLC using water (A) and acetonitrile (B), both mixed with 0.1% trifluoroacetic acid, as the mobile phase employing the gradient elution technique (0–4 min 5% B, 4–15 min 5% B to 95% B, 15–25 min 95% B, 25–30 min 95% B to 5% B). About 20 μL of the radiolabeled preparation was injected into the column and the elution was monitored by observing the radioactivity profile using a well-type NaI(Tl) scintillation detector attached to the HPLC system. The flow rate was maintained at 1 mL min–1 during the experiment.
Preparation of natgallium-labeled SPTA (iv)
The inactive gallium complex of the porphyrin derivative (SPTA) was also synthesized in order to have an idea about the position occupied by 68Ga in the 68Ga-SPTA complex. The procedure involved the overnight refluxing of an equimolar amount of SPTA (10 mg, 0.01 mmol) and gallium nitrate (28 mg, 0.1 mmol) in ammonium acetate buffer of pH = 5.0 for a period of 12 h. The product, thus obtained, was further purified by removing unreacted gallium, if any, using a Sep-pak® cartridge following the same protocol used for the purification of the corresponding radiolabeled complex. The formed Ga-SPTA complex was characterized by UV-vis spectroscopy as well as by mass spectrometry.
UV/vis (H2O): (λmax, nm): 421, 559, 603; ESI-MS (m/z): calcd for C52H36GaN4O12: 977.16, found: (M+) 977.3.
Determination of partition coefficient (log Po/w)
The partition coefficient of the 68Ga-SPTA complex was determined in an octanol–water solvent system in order to ascertain the lipophilicity/hydrophilicity of the complex. For this, the radiolabeled preparation (200 μL) was added to a mixture of water (800 μL) and octanol (1 mL) and the resulting solution was vortexed thoroughly. This mixture was subsequently centrifuged at 3000 rpm for 5 min. Aliquots of 100 μL were withdrawn from both water and octanol layers and counted separately using the well-type NaI(Tl) counter.
In vitro stability studies
The in vitro stability of the radiolabeled preparation (68Ga-SPTA complex) was determined by following a generalized procedure. In brief, an aliquot (200 μL) was withdrawn from the radiolabeled complex and incubated with human serum (300 μL) at 37 °C for up to 2 h. Acetonitrile (500 μL) was added to the reaction mixture post-incubation, for precipitating serum proteins. The resultant mixture was centrifuged at 10 000 rpm for 4 min for the separation of the pellet from the supernatant. The supernatant was analyzed by HPLC using the protocol mentioned above.
Determination of photoluminescence quantum (PL) yield of SPTA (iii) and natgallium-labeled SPTA (iv)
The photoluminescence quantum (PL) yield (QY) of the samples was determined based on a comparative method by using eqn (1). An ethanolic solution of coumarin 153 (Φ = 0.544) was used as the standard reference for the present study.
 |
1 |
where, ‘Φ’ is the quantum yield, ‘A’ is the integrated PL intensity, ‘OD’ is the optical density, and ‘n’ is the refractive index. The subscripts ‘S’ and ‘R’ refer to the sample and reference, respectively. The wavelength, where the OD (or absorbance) of both the sample and reference matched with each other, was chosen as the excitation wavelength for performing the study. Excitation was carried out at 418 nm for SPTA whereas 405 nm was selected as the excitation wavelength for natGa-labeled-SPTA. The emission was recorded to be mainly at 680 and 730 nm for the former whereas the latter was found to exhibit emission at 500 and 680 nm.
In vitro cell uptake and viability studies
In vitro cell uptake studies were carried out in HT1080, A549 and PBL (peripheral blood lymphocyte) cells. For determination of cell uptake in cancer cell lines viz. HT1080 and A549, about 1 × 106 cells were plated in 6 well-plates overnight, approximately 10 000 and 20 000 cpm of radioactivity (68Ga-SPTA) were added separately to both the cell types and the cells were incubated for 1 h at 4 °C. On the other hand, for determining the uptake of 68Ga-SPTA in the healthy cell line, PBL cells were isolated from healthy human blood using a solution containing polysucrose and sodium diatrizoate equivalent to Ficoll-Paque™ Plus and Histopaque® 1077. Mononuclear cells (lymphocytes and monocytes) were washed twice with phosphate buffered saline (PBS) and finally re-suspended in RPMI media. Cells were counted and around 1 × 106 cells were added to tubes containing 10 000 and 20 000 cpm of radioactivity. After incubation, cells were washed thrice with ice-cold PBS and the radioactivity associated with the cell pellets was measured using the NaI(Tl) detector. The cell uptake of the radiolabeled porphyrin was calculated as the percentage of total radioactivity associated with the cell pellet. An attempt was also made to determine the viability of healthy PBL cells post-incubation with 68Ga-SPTA. For this study, PBL cells were isolated by following the procedure mentioned above and PHA (phytohemagglutinin) was added to the plated cells. 20 000 cpm of 68Ga-SPTA activity was added to the cells after an incubation period of 24 h and the cell viability was determined using the MTT assay.
Biodistribution studies
The tumor targeting potential as well as biological distribution of the 68Ga-SPTA complex was studied by carrying out biodistribution studies in Swiss mice bearing fibrosarcoma tumors. About 106 HSDM1C1 murine fibrosarcoma cells (100 μL) were injected subcutaneously on the dorsum of each animal. The tumors were allowed to grow until they were approximately 10 mm in diameter subsequent to which the animals were used for the experiment. Each animal, weighing 20–25 g, was intravenously injected with ∼100 μL of the radiolabeled preparation (∼50 μCi, 1.85 MBq) through one of the lateral tail veins. The animals were maintained under a normal laboratory atmosphere and sacrificed through CO2 asphyxia after the designated post-administration time-points, e.g. 30 min, 1 h, and 2 h. All the vital organs/tissues of the animals were excised, washed with normal saline, dried, and weighed in a weighing balance and the radioactivity associated with each organ/tissue was measured using a flat-type NaI(Tl) counter. The activity accumulated in various organs/tissues was calculated from these data and expressed as the percentage injected activity per gram of organ/tissue (% IA per g). For each time point, 3 animals were used. The activity excreted through urine was indirectly calculated by subtracting the activity accounted in all the organs/tissues from the total injected activity. For this purpose, the activity accumulated in the skeleton, blood and muscles was calculated by considering that 10%, 7%, and 40% of the animal body weight are constituted by these organs/tissues, respectively.28,29
Study of cytotoxicity of SPTA by MTT assay in HT1080, A549 and PBL cell lines
Cell toxicity was determined by the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay which was carried out following the protocol mentioned below. Approximately, 2 × 103 cells were plated separately in 96 well plates and incubated at 37 °C in a humidified 5% CO2 atmosphere of an incubator overnight. Different concentrations (2–1000 nM) of SPTA were added to the cells and the cells were incubated for 48 h at 37 °C in a humidified 5% CO2 atmosphere. Subsequently, 10 μL of MTT solution (5 mg mL–1) was added to each well and incubated again for 4 h. The supernatant solution was removed post-incubation and 100 μL of solubilizing buffer (20% SDS in 50% DMF) was added to dissolve the formazan formed after reduction of the MTT dye in living cells. The color was quantified at a 570 nm wavelength with reference to 630 nm using a BioTek universal microplate reader (BioTek USA, Winooski, VT). The percent cell viability was calculated {ratio of OD (optical density) of the treated to that of the control sample, multiplied by 100} to find the magnitude of cell proliferation. The MTT assay was also used to determine the cytotoxicity of SPTA in the healthy cell line (PBL). For this study, PBL cells were isolated from human blood and incubated for 24 h after the addition of PHA. Different concentrations of SPTA ranging from 2 to 1000 nM were added to the cells followed by incubation for 48 h at 37 °C in a humidified 5% CO2 atmosphere. The percent cell viability was determined by the MTT assay following the same protocol described above.
Determination of photo-cytotoxicity of SPTA by MTT assay in HT1080 and A549 cell lines
The photo-cytotoxicity of SPTA was determined in HT1080 and A549 cancer cell lines by following the procedure mentioned below. Approximately, 2 × 105 cells were plated separately in culture dishes and incubated at 37 °C in a humidified 5% CO2 atmosphere in an incubator overnight. Three sets of cells were prepared for the assay. The first set of cells was incubated without porphyrin and without exposure to light and considered as ‘control’, while the second set of cells was incubated in the presence of light but without porphyrin and was considered as ‘vehicle control’. On the other hand, the third set of cells exposed to light in the presence of 1000 nM SPTA was considered as ‘treated’. Cells were exposed to light corresponding to three different light doses viz. 0.02, 0.03 and 0.06 kJ cm–2. Irradiation was continued for 4 h and the light dose received by the cells was calculated by multiplying the light intensity (in W m–2) with the time of exposure. Before exposing to light, the third set of cells was incubated with SPTA for 24 h and subsequently exposed to light for a period of 4 h. The treated cell lines were incubated for another 20 h post-irradiation. Cells were harvested and about 2 × 103 cells were plated separately in 96 well-plates and 10 μL of MTT solution (5 mg mL–1) was added to each well followed by incubation for 4 h. The MTT assay was carried out as described in the previous section.
Results
Synthesis and characterization of SPTA
The symmetrically substituted porphyrin derivative namely, 5,10,15,20-tetrakis(p-carboxymethyleneoxyphenyl)porphyrin (SPTA) was synthesized following a three-step procedure (Fig. 1). All the intermediates [(i) and (ii)] as well as the final product [(iii), SPTA] were characterized employing the usual spectroscopic techniques viz. UV-vis, FT-IR, 1H-NMR and 13C-NMR spectroscopy. The observation of proton and carbon signals in the expected positions in the 1H-NMR and 13C-NMR spectra of all the intermediates as well as the final compound provided the evidence in favour of the formation of desired compounds. This was further confirmed by observing the molecular ion peaks of the compounds at the expected values in their mass spectra.
Fig. 1. Scheme for synthesis of SPTA (iii): (a) K2CO3, dry acetone, reflux, 8 h, (b) propionic acid, nitrobenzene, reflux, (c) 2 N NaOH, stirring, R.T., 48 h.
Preparation and characterization of 68Ga-labeled-SPTA
SPTA was labeled with 68Ga using an ‘integrated approach’ where 68Ga becomes a part of the porphyrin structure without the need for any structural modification which is possible due to the suitability of the ionic radius of Ga(iii) to the porphyrin core.5 The radiolabeled complex, 68Ga-SPTA, was obtained with radiochemical purity ∼80% under the optimized reaction conditions. The radiochemical purity of the 68Ga-SPTA complex was improved further to >95% through purification using a Sep-pak cartridge. The radiolabeled complex was characterized by HPLC where it exhibited a retention time of ∼15 min, while the uncomplexed 68Ga eluted from the column within 4 min under identical conditions. A typical HPLC chromatogram of the 68Ga-SPTA complex is shown in Fig. 2.
Fig. 2. HPLC profile of (a) free 68Ga and (b) 68Ga-SPTA.
Preparation and characterization of natGa-labeled SPTA
Preparation of a cold gallium complex of SPTA was attempted in order to ascertain the position occupied by the gallium metal ion in the porphyrin moiety. The cold Ga-SPTA complex was synthesized following a simple procedure and characterized by UV-vis spectroscopy as well as by mass spectrometry. Fig. 3 shows the overlaid UV-vis spectra of SPTA and the cold Ga-SPTA complex. The presence of only two Q-bands (instead of four bands seen in the case of SPTA) observed in the longer wavelength region of the UV-vis spectrum of the cold Ga-SPTA complex clearly indicates the presence of a Ga metal ion in the core of the porphyrin moiety.30 The structure of the Ga-SPTA complex was further confirmed by obtaining the molecular ion peak of the complex in the desired position of the mass spectrum.
Fig. 3. Overlaid UV-vis profile of SPTA (iii) and natGa-labeled SPTA (iv).
Partition coefficient (log Po/w)
The partition coefficient (log Po/w) for the 68Ga-SPTA complex was determined to be –0.25 which indicates the hydrophilic nature of the complex.
In vitro stability studies
The 68Ga-SPTA complex was found to maintain its radiochemical purity (>90%) till the 2 h post-incubation period, up to which the study was continued.
Determination of photoluminescence quantum (PL) yield of SPTA (iii) and natgallium-labeled SPTA (iv)
Photoluminescence quantum yields were found out to be 0.105 for SPTA (iii) and 0.109 for natGa-labeled SPTA (iv). The similar quantum yields observed for both unmetallated SPTA and Ga-labeled-SPTA indicated the absence of quenching of the PL quantum yield in the presence of metal atoms. The present observation is in a similar line to that documented by Azad et al. where unmetallated and natGa-metallated protoporphyrin IX–peptide conjugates exhibited similar PL quantum yields.31
In vitro cell uptake and viability studies
In vitro cell uptake studies for 68Ga-SPTA were carried out in HT1080, A549 and PBL cells, and the cell bound activity was found to be ∼7% in both cancer cell lines (HT1080, A549) whereas only 0.42% bound activity was observed in PBL cells. The results of cell uptake studies are depicted in Fig. 4(a) and (b). The higher uptake of 68Ga-SPTA in both cancer cell lines relative to that observed in the healthy cell line (PBL) is indicative of the preferential affinity of the radiotracer towards cancer cells. No impact of inhibition of cell uptake was observed in the presence of excess SPTA which indicates the probable non-specific passive diffusion of the radiolabeled porphyrin complex across the cell membranes of all three cell lines.13 Also, cell viability studies carried out in the healthy cell line revealed the absence of any significant difference in the cell viability between the control cells (PBL incubated without 68Ga-SPTA) and the PBL cells incubated with 68Ga-SPTA (p > 0.056).
Fig. 4. Graphical representation of percent cell uptake exhibited by 68Ga-SPTA (a) in two different cancer cell lines viz. HT1080 and A549 corresponding to two different amounts of radioactivity (A) 10 000 and (B) 20 000 cpm, and (b) in peripheral blood lymphocytes (PBL) corresponding to (C) 10 000 and (D) 20 000 cpm of added radioactivity.
Biodistribution studies
The tumor targeting potential as well as the pharmacokinetic behaviour of 68Ga-SPTA was evaluated by carrying out bio-distribution studies in Swiss mice bearing fibrosarcoma tumors. The study revealed accumulation (1.82 ± 0.65% IA per g) of radiolabeled porphyrin in the tumor within 30 min post-injection (p.i.) which increased to 2.49 ± 0.16% IA per g at 1 h p.i. and was retained therein till 2 h p.i. (2.50 ± 0.66% IA per g), up to which the study was continued. The radiolabeled agent also exhibited significant accumulation in various organs/tissues such as blood, lungs, heart, liver and spleen within 30 min p.i. However, the initially accumulated activities in these organs/tissues exhibited gradual clearance with time as the uptake in these organs/tissues was found to decrease at 1 h and 2 h p.i. thus resulting in the gradual improvement in the tumor to non-target uptake ratios (2.71 ± 0.37 at 30 min p.i. to 7.49 ± 1.03 at 2 h p.i. for tumor to muscle and 0.13 ± 0.05 at 30 min p.i. to 0.28 ± 0.12 at 2 h p.i for tumor to blood). The results of the biodistribution studies are tabulated in Table 1. The radiolabeled porphyrin exhibited significant urinary clearance as a % injected activity of 41.53 ± 1.58 was cleared through the renal pathway at 2 h post-administration.
Table 1. Results of biodistribution studies of 68Ga-labeled SPTA in fibrosarcoma bearing Swiss mice (n = 3) at three different time points viz. 30 min, 1 h, and 2 h.
| Organ | Injected activity per gram (% IA per g) of organ/tissue |
||
| 30 min | 1 h | 2 h | |
| Blood | 13.44 ± 0.16 | 11.29 ± 0.67 | 9.19 ± 1.86 |
| Lung | 7.21 ± 0.54 | 5.70 ± 1.27 | 4.64 ± 1.20 |
| Heart | 4.48 ± 0.15 | 3.91 ± 0.52 | 3.62 ± 0.68 |
| Stomach | 1.13 ± 0.21 | 1.46 ± 0.09 | 3.83 ± 0.57 |
| GIT | 1.74 ± 0.20 | 3.15 ± 0.16 | 5.10 ± 2.23 |
| Liver | 18.08 ± 0.26 | 17.28 ± 0.69 | 13.07 ± 3.43 |
| Spleen | 3.27 ± 0.02 | 2.65 ± 0.01 | 2.30 ± 0.04 |
| Kidney | 3.88 ± 0.17 | 3.41 ± 0.08 | 2.38 ± 0.93 |
| Muscle | 0.67 ± 0.04 | 0.61 ± 0.09 | 0.33 ± 0.04 |
| Bone | 3.56 ± 0.20 | 1.07 ± 0.39 | 2.65 ± 0.85 |
| Tumor | 1.82 ± 0.65 | 2.49 ± 0.16 | 2.50 ± 0.66 |
| Tumor/muscle ratio | 2.71 ± 0.37 | 4.67 ± 1.20 | 7.49 ± 1.03 |
| Tumor/blood ratio | 0.13 ± 0.05 | 0.22 ± 0.00 | 0.28 ± 0.12 |
| Excretion | 21.05 ± 1.52 | 34.5 ± 2.54 | 41.53 ± 1.58 |
Study of cytotoxicity of SPTA by MTT assay in HT1080, A549 and PBL cell lines
The MTT assay performed using SPTA showed that the porphyrin derivative is not toxic to the cells at lower concentrations (below 1 μM). However, about 13–24% toxicity was noted when the concentration of SPTA was increased to the 1 μM range in both HT1080 and A549 cell lines. The results of the MTT assay performed with different concentrations of SPTA in HT1080 and A549 cell lines are shown in Fig. 5. The MTT assay performed to determine the toxicity of SPTA towards the healthy cell line revealed that even at 1 μM concentration of SPTA, the decrease in % cell proliferation was only 4–5% (Fig. 6). This observation indicates that the porphyrin derivative under study (SPTA) is highly non-toxic to healthy cells.
Fig. 5. Graphical representation of cell proliferation assay (MTT) for determination of toxicity exhibited by SPTA at different concentrations in two different cancer cell lines viz. HT1080 and A549.
Fig. 6. Graphical representation of cell proliferation assay (MTT) for determination of toxicity exhibited by SPTA at different concentrations in the healthy cell line (PBL).
Determination of photo-cytotoxicity of SPTA by MTT assay in HT1080 and A549 cell lines
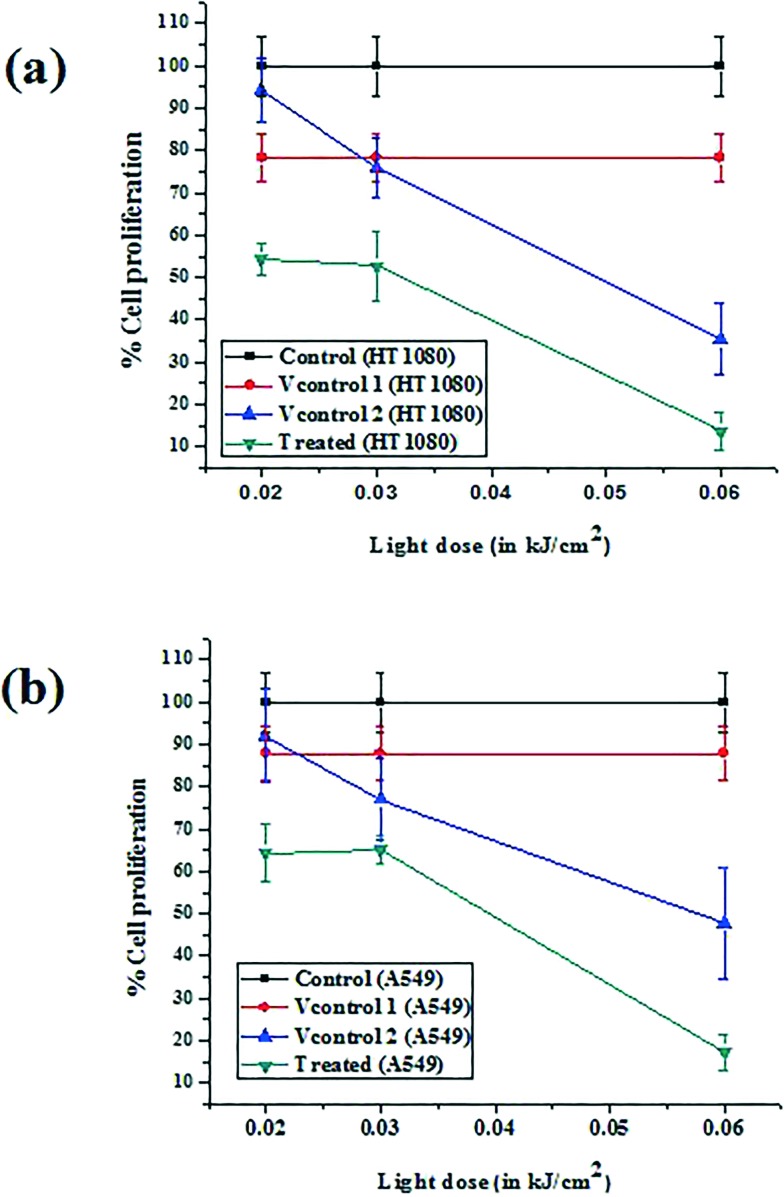
To find out the impact of light induced cytotoxicity of SPTA in HT1080 and A549 cell lines, these cells were treated with 1 μM SPTA and subsequently exposed to light corresponding to different light doses. The light induced cytotoxicity, as determined by the MTT assay, was found out to be increasing with an increase in the light dose received by the cells. Also, the extent of light induced cytotoxicity was found to be slightly higher for the HT1080 cell line in comparison to that observed in the A549 cell line. For the highest light dose received by both cell lines (of the order of 0.06 kJ cm–2), the cell toxicity was observed to be ∼87% in HT1080 and ∼83% in the A549 cell line. The results of photo-induced cell toxicity exhibited by SPTA in comparison with the control (absence of both SPTA and light) as well as the vehicle control [vehicle control1(Vcontrol1) = absence of light and presence of SPTA and vehicle control2(Vcontrol2) = presence of light and absence of SPTA] are shown in Fig. 7. This clearly indicates the potential of SPTA for inducing cytotoxicity when exposed to light. The results obtained in the experiments were found to be statistically significant when analyzed by one way ANOVA (p < 0.05).
Fig. 7. Graphical representation of cell proliferation exhibited by SPTA in two different cancer cell lines viz. (a) HT1080 and (b) A549 with increasing light dose, considering ‘control’ as cells without SPTA and without light, ‘vehicle control/Vcontrol1’ as cells with SPTA and without light, ‘vehicle control/Vcontrol2’ as cells without SPTA and in the presence of light, and ‘treated’ as cells with SPTA in the presence of light.
Discussion
The term ‘theranostics’, coined by John Funkhouser in the year 1998, refers to the application of the same agent for two interdependent applications, namely therapy and diagnosis.32–34 Porphyrins, with their inherent ability to preferentially accumulate in cancerous lesions, can be viewed as crucial substances for developing suitable agents for theranostic applications. Porphyrins and porphyrin based assemblies have been demonstrated as multimodel agents exhibiting theranostic properties.35 However, in radiopharmaceutical applications, there exist two distinct possibilities through which porphyrins can be used for theranostic purposes. One obvious way is the use of a suitable porphyrin derivative, radiolabeled separately with a pair of radionuclides, with one being diagnostic and the other therapeutic in nature. On the other hand, theranosis with porphyrins can also be achieved by using a radiolabeled porphyrin for diagnostic purposes, while affecting the therapy through the comparatively more established route of PDT using the same porphyrin derivative in its unmetallated form. In the present work, we have attempted to explore the second route in order to study the theranostic potential of porphyrins.
The prime reasons for selecting SPTA as the porphyrin derivative of choice for the present work include its hydrophilic nature (due to the presence of four carboxylic acid groups in the meso-positions of the porphyrin moiety), its similarity with hematoporphyrin (a native porphyrin derivative present in the body as part of the heme protein) and literature reports claiming entrapment of acid derivatized porphyrins in solid tumors having an intracellular acidic environment.36 Gallium-68, a generator produced PET radionuclide, was incorporated in SPTA in order to make the porphyrin suitable for radiodiagnostic application. Unlike 18F, the widely used PET radioisotope, which requires an on-site cyclotron facility, 68Ga is readily available from a radionuclide generator (68Ge/68Ga) system which makes it possible to perform PET imaging at centers which are far from a cyclotron facility.37,38 Moreover, the long shelf-life of the 68Ge/68Ga radionuclide generator along with the possibility of multiple elutions of 68Ga in a single day makes it an attractive choice for developing PET agents for radiodiagnostic applications.37–39 The radiolabeled product, 68Ga-SPTA, was characterized by HPLC employing the gradient elution technique and it showed a partition coefficient (log Po/w) value of –0.25 in an octanol–water system indicating the hydrophilic nature of the complex. The structure of the radiolabeled SPTA complex was further confirmed by synthesizing the corresponding inactive gallium complex of SPTA, whose UV-vis spectrum indicated the presence of a gallium metal ion within the porphyrin core. Biodistribution studies carried out with 68Ga-SPTA in Swiss mice bearing fibrosarcoma tumors showed encouraging tumor accumulation and retention therein till 2 h post-administration up to which the studies were continued. The increase in tumor uptake and clearance of activity from the non-target organs with time gradually improved the tumor to background ratio, which is important for a good imaging agent.
The potential of using SPTA for therapeutic application through PDT was also evaluated by determining the photo-cytotoxic effect exerted by the unlabeled derivative on human fibrosarcoma (HT1080) and human lung carcinoma (A549) cell lines. In the present study, the light induced cytotoxicity imparted by SPTA, in both cancer cell lines, was found to be quite significant indicating the potential of the porphyrin derivative for therapeutic applications. Although the present study is preliminary in nature, it demonstrated the possibility of using suitable porphyrin derivatives for theranostic applications and thus realizing the goal of ‘Personalized Medicine’.
Conclusions
The present study documents the preparation of a 68Ga-labeled porphyrin derivative (SPTA) and its evaluation as a theranostic agent. 68Ga-SPTA was found to accumulate in tumorous lesions when administered in tumor bearing Swiss mice. In vitro cell studies to investigate the photo-cytotoxic nature of the unmetallated porphyrin derivative revealed the light dependent cytotoxicity in human fibrosarcoma (HT1080) and human lung carcinoma (A549) cell lines which indicated the potential of SPTA towards its application in PDT. These studies revealed the possible potential of using porphyrin derivatives as theranostic agents. However, the relatively slower in vivo clearance of non-specific uptake exhibited by the porphyrin derivative, investigated in the present study, presses the need to explore other porphyrin derivatives with superior characteristics for their theranostic applications involving radionuclide imaging and photodynamic therapy.
Conflicts of interest
There are no conflicts of interest to declare.
Supplementary Material
Acknowledgments
The authors thankfully acknowledge Dr. Apurav Guleria, Radiation and Photochemistry Division, Bhabha Atomic Research Centre (BARC) for carrying out studies related to the determination of photoluminescence quantum yields. The help rendered by the staff members of the Animal House Facility, Radiation Biology and Health Sciences Division, BARC during animal experimentations is also acknowledged.
Footnotes
†Electronic supplementary information (ESI) available. See DOI: 10.1039/c7md00433h
References
- Policard A. C. R. Seances Soc. Biol. Ses Fil. 1924;91:14–32. [Google Scholar]
- Josefsen L. B., Boyle R. W. Theranostics. 2012;2:916–966. doi: 10.7150/thno.4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waghorn P. A. J. Labelled Compd. Radiopharm. 2014;57:304–309. doi: 10.1002/jlcr.3166. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Liang X., Dai Z. Nanoscale. 2017;8:12394–12405. doi: 10.1039/c5nr07849k. [DOI] [PubMed] [Google Scholar]
- Rai P., Mallidi S., Zheng X., Rahmanzadeh R., Mir Y., Elrington S., Khurshid A., Hasan T. Adv. Drug Delivery Rev. 2010;62:1094–1124. doi: 10.1016/j.addr.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simões A. V. C., Pinto S. M. A., Calvete M. J. F., Gomes C. M. F., Ferreira N. C., Castelo-Branco M., Llop J., Pereira M. M., Abrunhosa A. J. RSC Adv. 2015;5:99540–99546. [Google Scholar]
- Zoller F., Riss P. J., Montforts F. P., Kelleher D. K., Eppard E., Rösch F. Nucl. Med. Biol. 2013;40:280–288. doi: 10.1016/j.nucmedbio.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Bhadwal M., Mittal S., Das T., Sarma H. D., Chakraborty S., Banerjee S., Pillai M. R. A. Q. J. Nucl. Med. Mol. Imaging. 2014;58:224–233. [PubMed] [Google Scholar]
- Bhadwal M., Das T., Sarma H. D., Banerjee S. Mol. Imaging Biol. 2015;17:111–118. doi: 10.1007/s11307-014-0760-1. [DOI] [PubMed] [Google Scholar]
- Mittal S., Bhadwal M., Das T., Sarma H. D., Chakravarty R., Dash A., Banerjee S., Pillai M. R. A. Cancer Biother. Radiopharm. 2013;28:651–656. doi: 10.1089/cbr.2013.1512. [DOI] [PubMed] [Google Scholar]
- Das T., Chakraborty S., Sarma H. D., Banerjee S. Radiochim. Acta. 2012;96:427–433. [Google Scholar]
- Kral V., Králová J., Kaplánek R., Bříza T., Martásek P. Physiol. Res. 2006;55(Suppl. 2):S3–S26. doi: 10.33549/physiolres.930000.55.S2.3. [DOI] [PubMed] [Google Scholar]
- Gianferrara T., Bergamo A., Bratsos I., Milani B., Spagnul C., Sava G., Alessio E. J. Med. Chem. 2010;53:4678–4690. doi: 10.1021/jm1002588. [DOI] [PubMed] [Google Scholar]
- Ormond A. B., Freeman H. S. Materials. 2013;6:817–840. doi: 10.3390/ma6030817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Břiza T., Kralova J., Cigler P., Kejik Z., Poučkov P., Vašek P., Moserová I., Martásek P., Král V. Bioorg. Med. Chem. Lett. 2012;22:82–84. doi: 10.1016/j.bmcl.2011.11.066. [DOI] [PubMed] [Google Scholar]
- Shi J., Liu T. W. B., Chen J., Green D., Jaffray D., Wilson B. C., Wang F., Zheng G. Theranostics. 2011;1:363–370. doi: 10.7150/thno/v01p0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali H., van Lier J. E. Chem. Rev. 1999;99:2379–2450. doi: 10.1021/cr980439y. [DOI] [PubMed] [Google Scholar]
- Das T., Chakraborty S., Sarma H. D., Banerjee S. Curr. Radiopharm. 2012;5:340–347. doi: 10.2174/1874471011205040340. [DOI] [PubMed] [Google Scholar]
- Bhalgat M. K., Roberts J. C., Mercer-Smith J. A., Knotts B. D., Vessella R. L., Lavallee D. K. Nucl. Med. Biol. 1997;24:179–185. doi: 10.1016/s0969-8051(96)00215-6. [DOI] [PubMed] [Google Scholar]
- Sylvian I., Zerrouki R., Granet R., Huang Y. M., Lagorce J. F., Guilloton M., Blais J. C., Krausz P. Bioorg. Med. Chem. 2002;10:57–59. doi: 10.1016/s0968-0896(01)00255-3. [DOI] [PubMed] [Google Scholar]
- Bonnett R. Chem. Soc. Rev. 1995;24:19–33. [Google Scholar]
- Velikyan I. Theranostics. 2014;4:47–80. doi: 10.7150/thno.7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martiniova L., De Palatis L., Etchebehere E., Gregory R. Curr. Radiopharm. 2016;9:187–207. doi: 10.2174/1874471009666161028150654. [DOI] [PubMed] [Google Scholar]
- Jalilian A. R. Iran. J. Nucl. Med. 2016;24:1–10. [Google Scholar]
- Velikyan I. J. Labelled Compd. Radiopharm. 2015;58:99–121. doi: 10.1002/jlcr.3250. [DOI] [PubMed] [Google Scholar]
- Banerjee S. R., Pomper M. G. Appl. Radiat. Isot. 2013;76:2–13. doi: 10.1016/j.apradiso.2013.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler A. D., Longo F. R., Finarelli J. D., Goldmacher J., Assour J., Korsakoff L. J. Org. Chem. 1967;32:476. [Google Scholar]
- Panagi Z., Beletsi A., Evangelatos G., Livaniou E., Ithakissios D. S., Avgoustakis K. Int. J. Pharm. 2001;221:143–152. doi: 10.1016/s0378-5173(01)00676-7. [DOI] [PubMed] [Google Scholar]
- Das T., Chakraborty S., Sarma H. D., Tandon P., Banerjee S., Venkatesh M., Pillai M. R. A. Nucl. Med. Biol. 2009;36:561–568. doi: 10.1016/j.nucmedbio.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Dayer M. R., Moosavi-Movahedi A. A., Dayer M. S. Protein Pept. Lett. 2010;17:473–479. doi: 10.2174/092986610790963645. [DOI] [PubMed] [Google Scholar]
- Azad B. B., Cho C. F., Lewis J. D., Luyt L. G. Appl. Radiat. Isot. 2012;70:505–515. doi: 10.1016/j.apradiso.2011.11.054. [DOI] [PubMed] [Google Scholar]
- Idée J. M., Louguet S., Ballet S., Corot Claire. Quant. Imaging Med. Surg. 2013;3:292–297. doi: 10.3978/j.issn.2223-4292.2013.12.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denardo G. L., Denardo S. J. Semin. Nucl. Med. 2012;42:147–150. doi: 10.1053/j.semnuclmed.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Srivastava S. C. Semin. Nucl. Med. 2012;42:151–163. doi: 10.1053/j.semnuclmed.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Lovell J. F., Jin C. S., Huynh E., Jin H., Kim C., Rubinstein J. L., Chan W. C. W., Cao W., Wang L. V., Zheng G. Nat. Mater. 2011;10:324–332. doi: 10.1038/nmat2986. [DOI] [PubMed] [Google Scholar]
- Shirasu N., Nam S. O., Kuroki M. Anticancer Res. 2013;33:2823–2832. [PubMed] [Google Scholar]
- Shetty D., Lee U., Jeong J. M. Nucl. Med. Mol. Imaging. 2010;44:233–240. doi: 10.1007/s13139-010-0056-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosch F. Appl. Radiat. Isot. 2013;76:24–30. doi: 10.1016/j.apradiso.2012.10.012. [DOI] [PubMed] [Google Scholar]
- Roesch F., Riss P. J. Curr. Top. Med. Chem. 2010;10:1633–1668. doi: 10.2174/156802610793176738. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.