Abstract
In order to well identify the 93 wild Cortex Daphnes samples from different species and habitats in western China and develop a standard operating procedure (SOP) for the authentication and quality of them in the future, a comprehensive and efficient identification system based on DNA barcoding and HPLC fingerprint technologies has been developed. The result showed that only 17 samples (18%) were Daphne giraldii Nitsche (DG), which is recorded in Chinese Pharmacopeia, while the others (82%) might have safety hazards. Additionally, the result of HPLC fingerprint analysis indicated that samples in the same species origins and wild distributions could be clustered together, which was consistent with DNA barcoding analysis. The study can provide a significant system for the authentication and quality of commercial Cortex Daphnes herbs. Undoubtedly, this study undoubtedly confirmed that the chemical compositions of Cortex Daphnes herbs were affected by both species origins and ecological environments, which is required more in-depth research.
Introduction
Cortex Daphnes (Cirald Daphne Bark), a kind of traditional Chinese medicine, is the dried root bark and stem bark of DG recorded in the Chinese Pharmacopeia. However, many Daphne Linn plants, such as Daphne gracilis E. Pritz., Daphne limprichtii H. Winkl. and Edgeworthia Meisn plants, such as Edgeworthia chrysantha Lindl., etc., are also used as Cortex Daphnes in some parts of China [1–4]. Daphne tangutica Maxim (DT) and Daphne retusa Hemsl (DR) are just contained in some provincial medicinal material codes [5–6]. The Daphne Linn comprises approximately 95 species found in the alpine regions of Asia and Europe. About 44 species are found in China, and mainly distributed in southwest and northwest of China. The three Daphne species mostly is located in Provinces of Gansu, Shaanxi, Qinghai and Sichuan [7].
As a traditional Chinese medicine, Cortex Daphnes is pungent, bitter in taste, warm in nature, and of a little toxicity. Acting on the liver channel, the medical efficacy of Cortex Daphnes is dispelling wind and eliminating dampness, promoting blood circulation to remove blood stasis and scattered stasis pain [8]. Phytochemical studies showed that there are mainly four active classes of constituents in the dried velamen and bark of Daphnes Cortex, including coumarins (e.g. daphnetoxin), flavonoids (e.g. luteolin), lignans (e.g. syringaresinol), triterpenes (e.g. oleanolic acid), it also contains syringin, daucosterol, etc. Daphnetin is not the characteristic constituents but also the major bioactive constituents of them. Modern pharmacological studies have shown that Cortex Daphnes extract exhibits various efficacy, such as anti-inflammation, analgesia, anti-malaria, anti-tumor, antithrombus, anti-fertility. Daphnetin effectively inhibit tumor cell growth and kill the malaria parasite, and also its extract has remarkable therapeutic effect on glomerulonephritis [9–12]. In recent pharmaceutical market, the dosage forms of Cortex Daphnes are mainly injection, tablets and patch, which are used as the Extra-Strength Pain Reliever and largely used for the treatment of rheumatic arthralgia, arthralgia, traumatic injury, arthritis, rheumatoid arthritis and promoting blood circulation, and also can cure some blood vessel diseases, such as cardiovascular and cerebrovascular diseases, especially for thrombosis angitis obliterans.
Cortex Daphnes and its relative products is greatly in the world. Up to the year of 2015 in the survey areas, the wild resources storage of Cortex Daphnes was only 583.62t, the storage of DG was 97.64t (16.7%), the storage of DT was 392.66t (67.3%), the storage of DR was 93.32t (16%), according to the resources survey results from our research group (Beijing University of Chinese Medicine, Beijing, China). Many Daphne plants are used as Cortex Daphnes in folk of China and ordinary people is unable to correctly identify them well. Few Daphne plants have been cultivated and almost all wild materials are obtained from the agricultural markets in different areas[13–14].
The medicinal plant trade is the primary source of income for herbalists, and economic constraints may provide incentives for herbalists to substitute cheaper and more readily available species for rare ingredients and sell them under the same name [15]. The constituents contained in medicinal materials of Cortex Daphnes herbs collected from different habitats and different species origins are varied, which may directly influence the quality and efficacy of corresponding Chinese patent medicine.
Morphological classification is an important method for the identification of medicinal plants, mostly depending on their leaves, flowers and fruits. The harvest time of Cortex Daphnes herbs is in March of each year. However, there is no flower or fruit in March when villagers collect Cortex Daphnes herbs, so bark of the three Daphne species as Cortex Daphnes herbs circulated in the medicinal markets is more difficult to be identified because of the high similarity of their external characteristics [3]. Therefore, more scientific and accurate identification methods are required. Currently, DNA barcoding is recognized as a technology that is able to accurately and efficiently identify the species of medicinal plants. Internal transcribed spacer of ribosome gene (ITS) sequence is able to provide sufficient information for species identification [16–21].
Generally, the curative effects of traditional Chinese medicine (TCM) are the results of multiple bioactive components. Chromatographic fingerprinting provide an entire profile of almost global component of herbal medicines and is considered to be an important method for evaluating the quality of herbal medicines. It has been internationally accepted by the World Health Organization (WHO), the Food and Drug Administration of the USA (FDA), the Chinese State Food and Drug Administration (SFDA) and other authorities. HPLC fingerprinting is the most widely used method for qualitative evaluation and species identification of herbal medicines due to its convenience and efficiency [22–25].
In China, 93 wild Cortex Daphnes samples in different species were collected from different provinces covered the main wild distribution areas. Therefore, the aim of this study was to develop a valid and accurate system based on DNA barcoding and HPLC fingerprint methods, to identify and classify the species origins and habitats of the 93 Cortex Daphnes samples and control quality. Standard compound daphnetin was used as reference component for the qualitative of chromatographic peak by an HPLC- UV method. Samples are compared visually and analyzed using neighbor-joining tree (N-J tree) analysis, similarity analysis (SA), hierarchical cluster analysis (HCA) and principal component analysis (PCA). This study will be helpful in development of strategies for conservation, utilization and quality control of Cortex Daphnes and other herbs. The system could be used by the supervisory departments for the market supervision of commercial Cortex Daphnes herbs in the future, and provide a identification model for other commercial Chinese herbal medicines. More importantly, the classification results can preliminarily lay a foundation for the research that how species origins and ecological environments effect on the chemical components of Cortex Daphnes herbs.
Results
Main characteristics on morphology and habitat of the three Daphne species
According to the previous resources survey results, the plant morphology (S1 File) of DG is different from the other two Daphne species (DT and DR). The plant morphology of DT and DR is quite similar to each other. The flowers of DG are yellow and its leaves are pale green and membranous. However, the flowers of DT and DR are all fuchsia and the leaves of them are dark green and leathery. It is easy to separate DG from DT and DR by comparing the characteristics of their flowers and leaves in the flowering and fruiting stages. The leaves of DT are long, narrow and lanceolate. The leaves of DR are long ovate, and the front-end of leaves are concave down, which is able to be selected as a major characteristic to identify DT and DR. It shows that the genetic relationship between DT and DR is most similar to each other, which is consistent with records about DR in flora of China [26]. However, the identification of plants by the external form is restricted by collection time before blooming and growing environment, and the success of differentiation depending on the difference of the subtle external morphological features is restricted by professional experience.
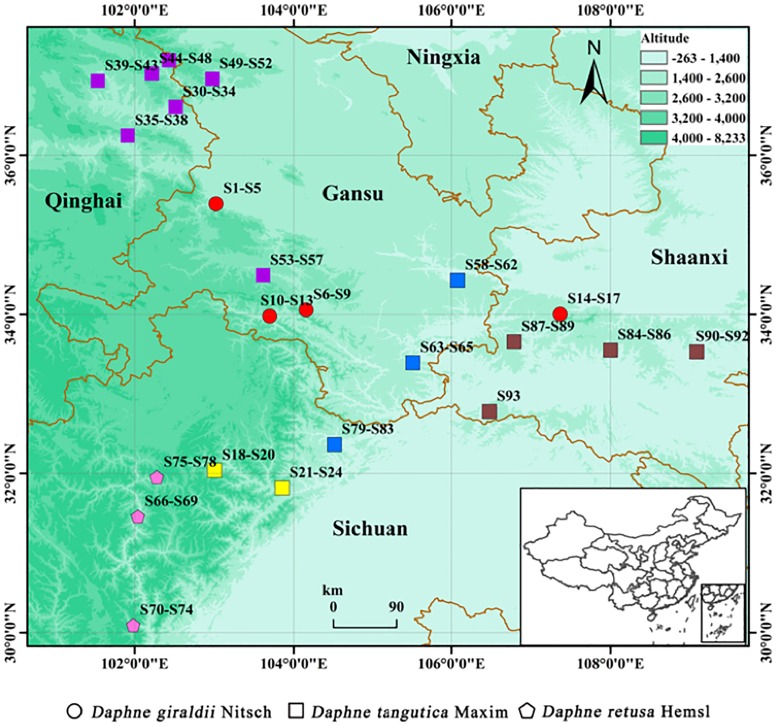
There are obvious differences in habitats of the three Daphne species. DG often grows under the bushes, where the community mostly consists of shrubs and small trees. The soil types are mostly humus with good permeability. The air humidity is relatively modest. Theoretically, the habitat altitude range of DG is 1600∼2600 m, however, we only found them where the altitude range is 2186∼2554 m in the resources survey. DT often grows in the sparse forest, alpine grassland and forest edge, especially on the sunny slope, with the altitude range 1000∼3800 m. The coenotype is relatively diverse. According to Meteorological Data Center of China Meteorological Administration [27], there are large differences in the climate type of each DT habitat, which is able to be divided into four groups. Group 1: the climate types of habitats including Menyuan, Ledu, Hualong, Datong and Zhuoni counties are all plateau continental climate; the climate types of Huzhu and Tianzhu counties are continental cold temperate climate and plateau continental monsoon climate. Characteristics of these climate types in group 1 are more similar. Group 2: the climate types of habitats including Heishui and Mao counties are all plateau monsoon climate. Group 3: the climate types of habitats including Foping, Liuba and Ningqiang counties are all warm temperate humid monsoon climate, the climate type of habitat Zhenan is semi-humid climate. Characteristics of these climate types are relatively mild and more similar. Group 4: the climate types of habitats including Tianshui and Pingwu are temperate monsoon climate and subtropical mountain humid monsoon climate. Kang counties is the excessive area that subtropical transition to warm temperate zone. Characteristics of climate types in group 4 are mild, abundant rainfall and more similar. DR often grows on alpine grass slope, with the altitude range 3000∼3900 m. The climate types of habitats including Jinchuan, Kangding and Maerkang counties are continental plateau climate, alpine plateau climate and low latitude, high altitude special geography and alpine canyon three-dimensional climate. These characteristics of climate types are a bit similar to the plateau monsoon climate. For the same Daphne species, habitat may be the major factor, which result in their chemical composition and gene segment exist differences. The specific habitat distributions of the 93 Cortex Daphnes samples are shown in Fig 1 (generated by the software ESRI ArcGIS Desktop, version: 10.3.0.4322, URL http://www.esri.com/).
Fig 1. Distributions of the 93 collected Cortex Daphnes samples.
This map was generated by the software ESRI ArcGIS Desktop, version: 10.3.0.4322, URL http://www.esri.com/. It was created by author L. G. (The border line is vectorized China map data.).
Genetic taxonomy of Cortex Daphnes samples
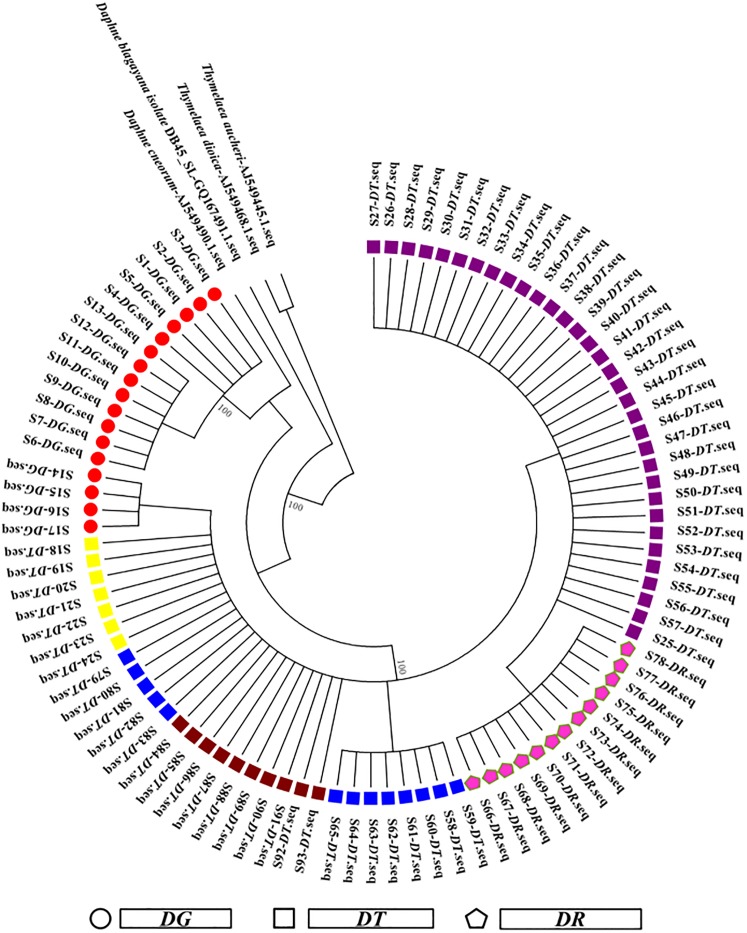
All of the collected samples were identified according to their ITS sequences. To identify the species of the 93 Cortex Daphnes samples more accurately and visually, a phylogenetic tree was constructed based on the ITS sequences (S2 File) obtained from GenBank and the samples. Likewise, the modified ITS sequences were submitted to DNAMAN software to compare similarities of the samples. Among them, 76 Cortex Daphnes samples of DT and DR were identified with the similarities higher than 99%, and the other 17 Cortex Daphnes samples of DG were also identified. The similarity of ITS sequences between DG and DT was 93% and the similarity between DG and DR was 92%. However, the similarity between DT and DR was 99%. The genotype of ITS sequences in DT at 60bp and 156bp were C and G. But, the genotype of ITS sequences in DR at the same locus were all A, based on this, it also able to identify them accurately.
HCA based on ITS sequences was used to analyze the genetic data to characterize the population genetics of the Cortex Daphnes samples and determine their genetic diversity and population differentiation. Thymelaea aucheri (AJ549445.1), Daphne cneorum (AJ549490.1), Thymelaea dioica (AJ549468.1) and Daphne blagayana isolate DB45_SL (GQ167491.1) downloaded from GenBank-NCBI were selected as out-group gene sequences to obtain more accurate branching of the phylogenetic tree. The wild Cortex Daphnes populations distributed in western China were grouped separately through their ITS sequences (as shown in Fig 2).
Fig 2. Results of NJ-tree analysis based on ITS sequences of the 93 Cortex Daphnes samples.
All ITS sequences of Cortex Daphnes samples were merged with Daphne blagayana isolate DB45_SL (GQ167491.1), which indicated that these samples belonged to Daphne Linn. All samples were divided into two main characteristic clusters. The first cluster consisted of DG. The other cluster was made up with DT and DR which was divided into five main branches. The first branch was consisted of DT and contained 33 samples (S25–S48, collected from Qinghai Province; S49–S57 collected from Tianzhu and Zhuoni counties in Gansu Province, the altitude of habitats higher than 2900 m). The second branch including DR contained 13 samples (S66–S78, all gathered from Sichuan Province). The first branch merged with the second branch (DR) to form a larger branch that merged with the other branches. The third branch was consisted of DT and included 8 samples (S58–S65, collected from Tianshui and Kang counties in Gansu Province). The fourth branch was consisted of DT and included 22 samples (S84–S93, all gathered from Shaanxi Province; S79–S83, S18–S24, gathered from Pingwu, Mao and Heishui counties in Sichuan Province). According to plant morphological, the fifth branch contained 4 samples (S14–S17, gathered from Taibai county in Shaanxi Province) was identified to be DG. However, it was merged with DT and DR cluster. Likewise, it was also close to the DG cluster. That may be the species variation of DG caused by specific ecological environment or the coenospecies of DG and DT. The NJ tree obtained from ITS sequences is able to easily distinguish and identify the 93 Daphnes Cortex samples gathered from different species origins and habitats. The results of HCA were in good agreement with the results of the morphological taxonomy. HCA placed all DT samples into one of two main branches. DT samples with the altitude of habitats more than 2900 m were grouped in one branch, and the others with the altitude of habitats below 2900 m merged gradually became one branch. It likely showed that altitude of habitats may have important influence on the ITS sequences of Cortex Daphnes. Accordingly, it revealed that samples from the same species origins, similar geographical environments and nearby regions had similar ITS sequences and were in the same or close clusters. DNA barcoding could successfully identify the Cortex Daphnes samples.
Method validation of HPLC fingerprint
Optimization of extraction and chromatographic conditions
Four different concentrations of methanol and ethanol (60, 70, 80, and 100%) extractions were compared. The best extracting way was ultrasonic and extracted for 45 min in 70% methanol. Under such condition, more chromatographic peaks and more chemical contents were detected. As a result, the best extracting condition was established as follows: the samples were extracted by ultrasonic extraction using 70% methanol as the extracted solvent and the duration was 45min.
To obtain more chromatographic peaks, the optimized gradient elution program was used in this study. To make the chromatograms with better separation and sharper peaks, the mobile phase, column temperature and detection wavelength were all optimized. The elution effect of the mobile phase constitution (methanol / water, methanol / 0.5% FA (formic acid) water, acetonitrile / water, acetonitrile / 0.5% FA water) on the chromatographic separation was compared. It showed that acetonitrile / 0.5% FA water was the best mobile phase composition with higher elution efficiency. In this study, three column temperatures (20, 25, 30°C) were selected to assess their efficiency on gaining higher resolution of the chromatographic peaks. As a result, the best column temperature was 25°C. The wavelength for the detection of compounds was selected by UV detector. Most of the chromatographic peaks could be detected at approximately 327 nm, at which the chromatograms could provide maximum absorption of daphnetin. So 327nm were chosen as detection wavelength for the HPLC fingerprint analysis.
Precision, repeatability and stability tests
The test of precision, repeatability and stability were performed by calculating the relative standard deviations (RSDs) of relative retention times (RRTs) and relative peak areas (RPAs) based on common peaks respectively. The results were showed in Table 1. All RSDs including RRTs and RPAs were < 3%, which indicated that the method was sufficiently accurate, stabilized and sensitive for the fingerprint analysis of the 93 Cortex Daphnes samples.
Table 1. Test results of precision, repeatability and stability for the ten common peaks.
| Peak No. | Precision (n = 6) | Repeatability (n = 6) | Stability (n = 6) | |||
|---|---|---|---|---|---|---|
| RSD of RRT% | RSD of RPA% | RSD of RRT % | RSD of RPA% | RSD of RRT % | RSD of RPA% | |
| 1 | 0.038 | 1.982 | 0.045 | 2.805 | 0.032 | 0.377 |
| 2 | 0.039 | 1.405 | 0.028 | 2.027 | 0.034 | 1.484 |
| 3 | 0.002 | 0.246 | 0.048 | 2.512 | 0.052 | 0.732 |
| 4 | 0.000 | 0.433 | 0.044 | 2.387 | 0.061 | 1.895 |
| 5 | 0.017 | 0.293 | 0.047 | 2.736 | 0.063 | 2.553 |
| 7 | 0.039 | 1.970 | 0.055 | 1.580 | 0.058 | 2.123 |
| 9 | 0.049 | 1.083 | 0.898 | 1.813 | 0.016 | 0.787 |
| 11 | 0.077 | 1.572 | 0.044 | 0.944 | 0.021 | 0.849 |
| 23 | 0.068 | 1.751 | 0.006 | 2.199 | 0.025 | 0.515 |
| 29 | 0.078 | 1.124 | 0.097 | 2.352 | 0.031 | 1.280 |
Establishment of chromatographic fingerprint of Cortex Daphnes samples
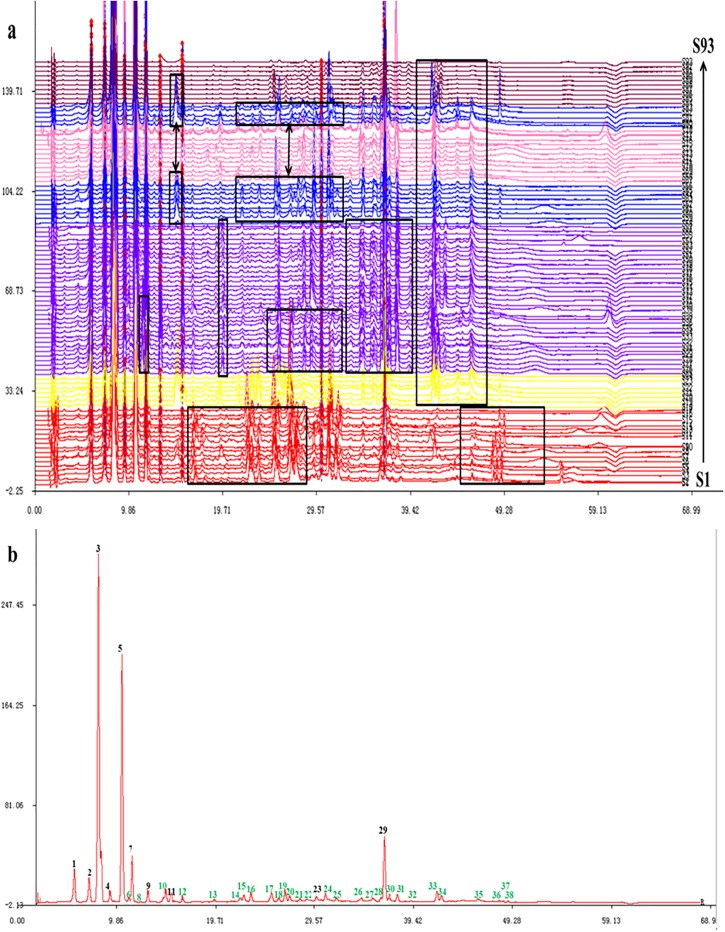
To establish the chromatographic fingerprint, 93 Cortex Daphnes samples from different species and habitats were analyzed under the optimized chromatographic analysis conditions. Sample 1 was the reference sample, and all chromatograms (as shown in Fig 3a) through multipoint correction and free matching were matched and reference chromatogram (as shown in Fig 3b) was generated by the computer-aided Similarity Evaluation System for Chromatographic Fingerprint of TCM (Version 2004A). Peaks in the fingerprint with quite large area and good resolution shared by all the chromatograms of the tested samples were selected as “common characteristic peaks” to represent the characteristics of all the samples. A total of 10 common peaks shared by all samples (black peak No.) which covered more than 90% of the total area and 28 common peaks detected in portion samples (green peak No.) were determined in the reference chromatogram. One component was identified as daphnetin (peak 5) by comparing its retention time and UV spectrum with the standard compound. The other common fingerprint peaks were not identified.
Fig 3. (a) HPLC fingerprints of the 93 Cortex Daphnes samples (S1-S93) and (b) reference chromatogram.
4: Syringoside 5: Daphnetin 9: 7-hydroxycoumarin.
SA of HPLC fingerprint of Cortex Daphnes samples
The similarity values between 93 Cortex Daphnes samples and the reference chromatogram were calculated using the Similarity Evaluation System for Chromatographic Fingerprint of TCM (Version 2004A), and the results were presented in Table 2.
Table 2. Results of similarity evaluation between samples and reference chromatogram.
| Sample | SC | Sample | SC | Sample | SC | Sample | SC | Sample | SC |
|---|---|---|---|---|---|---|---|---|---|
| S1 | 0.975 | S21 | 0.945 | S41 | 0.993 | S61 | 0.947 | S81 | 0.982 |
| S2 | 0.973 | S22 | 0.923 | S42 | 0.991 | S62 | 0.974 | S82 | 0.961 |
| S3 | 0.97 | S23 | 0.946 | S43 | 0.99 | S63 | 0.934 | S83 | 0.955 |
| S4 | 0.975 | S24 | 0.943 | S44 | 0.994 | S64 | 0.98 | S84 | 0.947 |
| S5 | 0.976 | S25 | 0.98 | S45 | 0.994 | S65 | 0.951 | S85 | 0.945 |
| S6 | 0.991 | S26 | 0.987 | S46 | 0.982 | S66 | 0.947 | S86 | 0.925 |
| S7 | 0.979 | S27 | 0.99 | S47 | 0.974 | S67 | 0.978 | S87 | 0.931 |
| S8 | 0.985 | S28 | 0.989 | S48 | 0.992 | S68 | 0.988 | S88 | 0.985 |
| S9 | 0.954 | S29 | 0.981 | S49 | 0.989 | S69 | 0.978 | S89 | 0.989 |
| S10 | 0.983 | S30 | 0.969 | S50 | 0.994 | S70 | 0.991 | S90 | 0.981 |
| S11 | 0.98 | S31 | 0.971 | S51 | 0.99 | S71 | 0.956 | S91 | 0.968 |
| S12 | 0.976 | S32 | 0.922 | S52 | 0.995 | S72 | 0.955 | S92 | 0.974 |
| S13 | 0.983 | S33 | 0.915 | S53 | 0.994 | S73 | 0.922 | S93 | 0.977 |
| S14 | 0.982 | S34 | 0.909 | S54 | 0.958 | S74 | 0.966 | ||
| S15 | 0.963 | S35 | 0.98 | S55 | 0.956 | S75 | 0.95 | ||
| S16 | 0.959 | S36 | 0.961 | S56 | 0.992 | S76 | 0.995 | ||
| S17 | 0.956 | S37 | 0.928 | S57 | 0.986 | S77 | 0.974 | ||
| S18 | 0.98 | S38 | 0.933 | S58 | 0.977 | S78 | 0.941 | ||
| S19 | 0.984 | S39 | 0.954 | S59 | 0.93 | S79 | 0.989 | ||
| S20 | 0.983 | S40 | 0.996 | S60 | 0.978 | S80 | 0.971 |
According to the results, the similarity values were all >0.9. It was indicated that the main chemical compositions among the 93 Cortex Daphnes samples were relatively consistent. However, there were still many differences of chemical compositions among the Cortex Daphnes samples gathered from different habitats and species origins. According to the fingerprint chromatograms, some characteristic peaks especially in the range of 12–55 min only appeared in part of the samples collected from similar ecological environment or the same species origins. These special common characteristic peaks were also marked with green peak No. in the reference chromatogram. Obviously, the types and quantities of chemical components among Cortex Daphnes samples were not completely consistent. The varied chemical profiles of Cortex Daphnes samples may be attributed to the different species origins and habitats, which were the results of plants adapting to the environment.
HCA of HPLC fingerprint of Cortex Daphnes samples
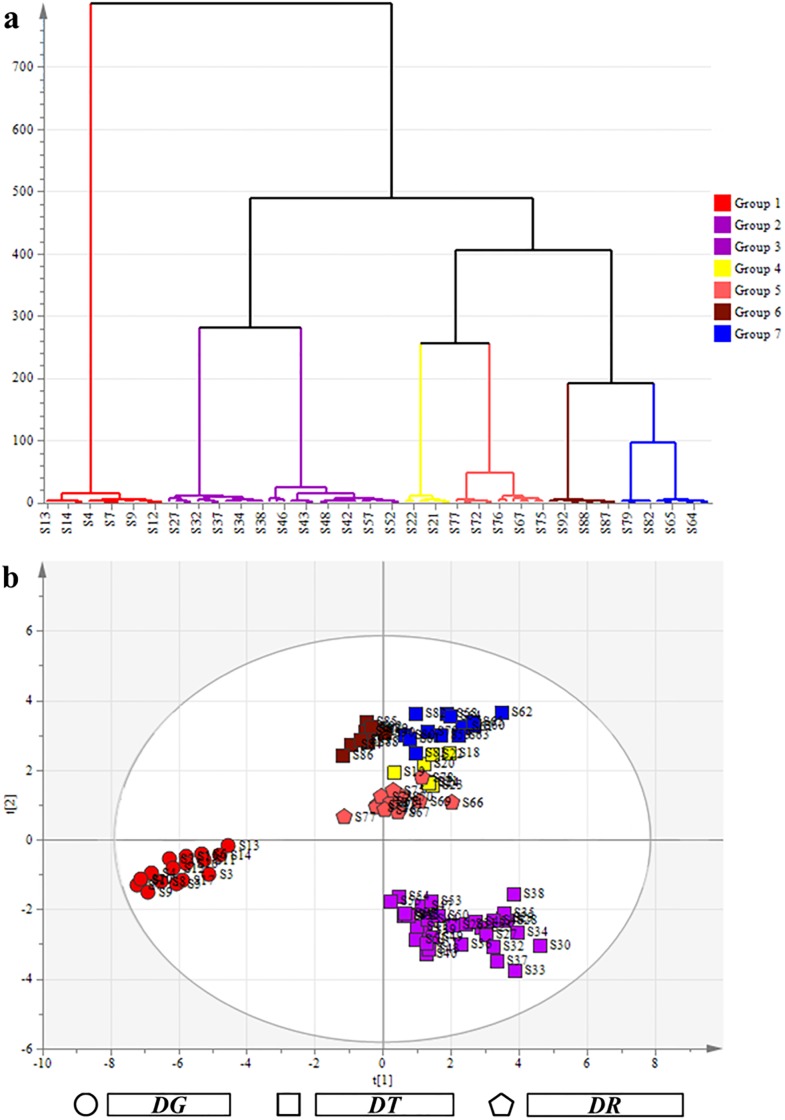
HCA, one of the chemical pattern recognition and classification evaluation methods, is used to set the level of bottom-up decomposition for a given data set until certain conditions are fulfilled. HCA has been commonly applied for fingerprint analysis with standard normal variant transformation of the data, which led to meaningful classification of herbal samples collected from different regions [28–29]. In order to show the degree of similarity and differences among the 93 Cortex Daphnes samples more clearly, the HCA in this study was performed based on the RPAs (S3 File) of all common characteristic peaks (peaks 1–38, as shown in Fig 3b) by the professional analysis software SIMCA 13.0 Demo. The results of HCA were shown in Fig 4a.
Fig 4. (a) Results of HCA based on HPLC fingerprint of the 93 Cortex Daphnes samples and (b) score plot of PCA of the 93 Cortex Daphnes samples.
According to the results, all samples were classified into two main clusters: S1–S17 in cluster 1, S18–93 in cluster 2 (as shown in Fig 4a). Cluster 1 including 17 samples belonged to DG. Additionally, cluster 2 was divided into six groups: S25–S57 in group1and group 2, S18–S24 in group 3, S66–S78 in group 4, S84–S93 in group 5, S58–S65 and S79–S83 in group 6. These five groups including 63 samples belonged to DT. Group 4 including 13 samples belonged to DR (all gathered from Sichuan Province). Group 1 and group 2 including 33 samples were collected from Qinghai and Gansu Province with the altitude of habitats more than 2900 m. Group 3 including 7 samples were gathered from two adjacent counties (Maoxian and Heishui counties in Sichuan Province). Group 5 including 10 samples were all gathered from Shaanxi province. Group 6 including 13 samples were gathered from Sichuan and Gansu Province. The Group 3 (DT) merged with the Group 4 (DR) to form a larger branch1. Maybe it’s because they were gathered from nearby areas. According to the HCA of chemical components and N-J tree of ITS sequences, the DR samples merged with the DT samples consistently which indicated that the chemical compositions and the ITS sequences between the two species were much of a muchness. The results were consistent with the recordation about DR and DT in Flora of China [26]. The Group 5 (DT) merged with the Group 6 (DT) to form a larger branch 2. All samples in the branch 2 were gathered from Qinling and Dabashan mountain areas where the ecological environments were more similar. The branch 1 and branch 2 merged to form a larger branch in which the altitude of the sample collection sites were below 2900 m then merged with group 1and group 2. The classification results of HCA were consistent with the classification of climate types of habitats, and agreed well with the categorized results of the genetic taxonomy and the visual comparisons of their representative chromatograms, which may provide more references for further quality control and evaluation of the commercial Cortex Daphnes herbs.
PCA of HPLC fingerprint of Cortex Daphnes samples
PCA is a multivariate method and widely used in data analysis to summarize variation, which is implemented as a data-reduction technique to generate a visual scatter plot for the qualitative evaluation of resemblances and differences between the studied samples [22]. In order to differentiate all the Cortex Daphnes samples clearly, the PCA was carried out based on the RPAs (S3 File) of all common characteristic peaks (peaks 1–38, as shown in Fig 3b) by the professional analysis software SIMCA 13.0 Demo. The score plot was structured based on the first three principal components which accounted for more than 89.31% of the total variability, and the other principal components which had little effect on the model were discarded.
According to the results of PCA, all samples were divided into seven groups according to their different sources (as shown in Fig 4b). Group 1 contained 17 samples (S1-S17) belonging to DG. Group 2 and group 3 contained 33 samples (S25-S57) belonging to DT. Group 4 contained 13 samples (S66-S78) belonging to DR. Group 5 contained 7 samples (S17-S24) belonging to DT. Group 6 contained 10 samples (S84-S93) belonging to DT. Group 7 contained 13 samples (S58-S65, S79-S83) belonging to DT. Groups excepting group 1, group 2 and group 3 were quite close to each other. The results of PCA were in good agreement with the results of HCA based on the fingerprint. The classification results of the scatter plot adequately showed the noticeable provenance and geographical differences among the samples.
In this study, samples (S14-S17) were divided into DG groups according to the results of phytochemical and morphological taxonomy which was not completely consistent with results of the genetic taxonomic. This conclusion illustrated that the accurate identification of medicinal materials requires comprehensive applications of multifarious identification techniques.
Discussion
In this study, a system based on DNA barcoding and HPLC fingerprint analysis to identify and classify the Cortex Daphnes herbs has been established. Two universal classification techniques include four different analysis methods: SA, HCA and PCA of the chemical components and the NJ-tree analysis of ITS sequences. 93 Cortex Daphnes samples, gathered from different species origins and wild areas in Western China, were identified and classified successfully. The results of genetic taxonomic were greatly consistent with the phytochemical taxonomy results. The morphological taxonomy also played an important role in the identification of Cortex Daphnes samples, such as the distinction of species DR. The conclusions drawn from the classification system were more objective and scientific. The classification system could efficiently identify and control the quality of the three Daphne species. Furthermore, these methods are convenient and suitable for practical use, further pharmacological research and development of the three species. The system is also able to provide a identification model for other commercial Chinese herbal medicines to guarantee their clinical safety.
The results of genetic and phytochemical taxonomic showed that samples belonging to the same species origins and nearby habitats could be clustered together, which demonstrated that the type and composition of the chemical components of the medicinal plants were the combined effect of both genetic materials and habitats. For the same Daphne species, climate types of their habitats may be the decisive factor that make their chemical compositions exist larger differences. The altitude may play an important role, which may be as a classification foundation (as shown in Fig 1). In addition to the altitude, there are many other ecological factors which can influence the type and composition of the chemical components of the medicinal plants, such as temperature, soil, light, moisture and so on. In order to guarantee the quality stability of the Cortex Daphnes herbs, to realize the artificial cultivation and protect wild plants, the effects exerted by the genetic materials and ecological factors to the active ingredients of the three Daphne species is required deep study in future.
Materials and methods
Chemicals and materials
Methanol (analytical grade) and acetonitrile (HPLC grade) were purchased from Fisher Scientific International (Fair Lawn, New Jersey, USA). Ultra-pure water was generated by an Ultrapure Water System (Shanghai Ultrapure Technology, Shanghai, China). Standard daphnetin was purchased from the National Institutes for Food and Drug Control (Beijing, China). The 93 wild Cortex Daphnes samples including 93 stem bark coupled with 93 leaves were collected from four local provinces of China: Gansu, Shaanxi, Qinghai and Sichuan. They were identified as genuine samples of DG, DT and DR by Professor Chunsheng Liu (Beijing University of Chinese Medicine, Beijing, China) through their leaves (as listed in Table 3 [14]). All samples were dried at 22–25°C. The 93 stem bark were comminuted to powder separately, and sieved through a 74μm (or 200 mesh) screen for HPLC analysis and the leaves were used for DNA barcoding analysis.
Table 3. Sources of the 93 Cortex Daphnes samples.
| Sample No. | Location | Latitude | Longitude | Altitude | Origin |
|---|---|---|---|---|---|
| N | E | m | |||
| S1-S5 | Linxia, Gansu | 35.389217 | 103.022983 | 2554 | Daphne giraldii Nitsch |
| S6-S9 | Tanchang, Gansu | 34.056550 | 104.156800 | 2186 | Daphne giraldii Nitsch |
| S10-S13 | Diebu, Gansu | 33.977450 | 103.698217 | 2461 | Daphne giraldii Nitsch |
| S14-S17 | Taibai, Shaanxi | 34.002633 | 107.365500 | 2518 | Daphne giraldii Nitsch |
| S18-S20 | Heishui, Sichuan | 32.039883 | 103.005500 | 2975 | Daphne tangutica Maxim |
| S21-S24 | Maoxian, Sichuan | 31.818083 | 103.855400 | 2000 | Daphne tangutica Maxim |
| S25-S29 | Menyuan, Qinghai | 37.193783 | 102.432317 | 2985 | Daphne tangutica Maxim |
| S30-S34 | Ledu, Qinghai | 36.606717 | 102.515783 | 3032 | Daphne tangutica Maxim |
| S35-S38 | Hualong, Qinghai | 36.247400 | 101.912950 | 3002 | Daphne tangutica Maxim |
| S39-S43 | Datong, Qinghai | 36.934333 | 101.536500 | 3063 | Daphne tangutica Maxim |
| S44-S48 | Huzhu, Qinghai | 37.025333 | 102.215733 | 3293 | Daphne tangutica Maxim |
| S49-S52 | Tianzhu, Gansu | 36.959967 | 102.977033 | 2834 | Daphne tangutica Maxim |
| S53-S57 | Zhuoni, Gansu | 34.491300 | 103.615283 | 3315 | Daphne tangutica Maxim |
| S58-S62 | Tianshui, Gansu | 34.427583 | 106.075817 | 1468 | Daphne tangutica Maxim |
| S63-S65 | Kangxian, Gansu | 33.391550 | 105.514017 | 1524 | Daphne tangutica Maxim |
| S66-S69 | Jinchuan, Sichuan | 31.459533 | 102.040417 | 3108 | Daphne retusa Hemsl |
| S70-S74 | Kangding, Sichuan | 30.093317 | 101.981867 | 3900 | Daphne retusa Hemsl |
| S75-S78 | Maerkang, Sichuan | 31.954117 | 102.277333 | 3076 | Daphne retusa Hemsl |
| S79-S83 | Pingwu, Sichuan | 32.363217 | 104.514000 | 1675 | Daphne tangutica Maxim |
| S84-S86 | Foping, Shaanxi | 33.550000 | 108.000000 | 1584 | Daphne tangutica Maxim |
| S87-S89 | Liuba, Shaanxi | 33.654680 | 106.787400 | 1502 | Daphne tangutica Maxim |
| S90-S92 | Zhenan, Shaanxi | 33.528200 | 109.085100 | 1866 | Daphne tangutica Maxim |
| S93 | Ningqiang, Shaanxi | 32.779200 | 106.477300 | 1617 | Daphne tangutica Maxim |
DNA barcoding analysis
DNA extraction
Genomic DNA from dried leaves of 93 Cortex Daphnes samples which were collected in Beijing University of Chinese Medicine were extracted according to the instructions of the plant DNA extraction kit (Tiangen, Beijing, China). All the DNA were stored at -20°C before analysis [30].
PCR and sequencing
A total of 30μL PCR system contains: template DNA 3μL, Mix-Taq enzyme 15μL, ITS sense primer 1.2μL, ITS antisense primer 1.2μL, ddH2O 9.6μL. PCR amplification was performed: 95°C for 4 min, followed by 35 cycles of 94°C for 30s, 55°C for 1 min, 72°C for 1 min, and final extension 72°C for 10min (Bio-Rad T100™ Thermal Cycler). 5μL of PCR products were examined via electrophoresis in a 1.0% agarose gel, and were sequenced by Shanghai sangong company. To ensure the accuracy, samples were all sequenced in two-way. All the ITS sequences obtained were cut and spliced using DNAMAN and ContigExpress software, then were submitted to GenBank-NCBI for comparison with the deposited sequences of near-source species using the tool BLAST. The modified ITS sequences were aligned and a N-J tree was constructed based on standard parameters with bootstrap testing of 1000 replicates using ClustalX and MEGA version 5.0 software. The N-J tree was used to observe the natural interrelationships and differences for each of the Cortex Daphnes samples by ITS sequences [31].
HPLC analysis
Samples and standard solution preparation
The dried powdered sample (0.500g) was precisely weighed and extracted with 50 mL of 70% v/v methanol by ultrasonic extraction for 45 min. The extraction solution was supplemented to the weight of pre-extracted before analysis when it cooled down to room temperature. The solution was filtered through a 0.45 μm membrane filter and stored at 4°C out of light before HPLC analysis.
The standard daphnetin was prepared for the qualitative of chromatographic peak. The concentration of standard solution was 60μg/mL in methanol and 10μL solution was injected into the HPLC for analysis according to the standard [6]. The standard solution was filtered through 0.45 μm membrane filters and stored at 4°C out of light and brought to room temperature before HPLC analysis.
Instrumentation and analytical conditions
HPLC analyses were performed using a Waters system (Waters e2695/2489/Emp2) with a UV detector. Chromatographic separations were achieved using gradient elution on a C18 reserve-phase column (4.6 mm× 150 mm, 5 μm; Agilent Technologies). The column temperature was maintained at 25°C. The absorption wavelength was 327 nm which was selected by UV detector according to max UV absorption of the reference. The mobile phase consisted of acetonitrile (A) and 0.5% FA water (B) with a linear gradient elution at a flow rate of 1.0 mL/min. The gradient elution program was as follows: 5–27.4% (A) in 0–40 min, 27.4–42%(A) in 40–45 min, 42–53% (A) in 45–58 min, 53–90% (A) in 58–68 min and 90–5% (A) in 68–78 min. The sample injection volume was 10μL.
The daphnetin as the common components of the three species which chromatographic peak was selected to be the referential chromatographic peak, the RRTs and RPAs were analyzed. Method precision was determined by injecting one Cortex Daphnes sample solution (S1) six times continuously. The repeatability was assessed through six independently prepared sample solutions (S1). The stability of the injection solution was determined periodically by injecting samples (S1) stored at 4°C ranging from 0 to 24 h (0, 3, 6, 9, 12, and 24h). The results were all expressed by RSDs of RRTs and RPAs, respectively, of all common peaks (1, 2, 3, 4, 5, 7, 9, 11, 23 and 29) from six chromatographic profiles of Cortex Daphnes sample (S1).
Data analysis
SA analysis
The HPLC analyses of all the samples were carried out under the established experimental condition. The data of all chromatographic profiles was converted into “AIA” form from the software of Empower. SA was performed on the basis of the RRTs and RPAs using the professional software named Similarity Evaluation System for Chromatographic Fingerprint of TCM (2004A), which was recommended by the State Food and Drug Administration of China (SFDA) for calculating the similarity coefficient (SC) of the chromatographic profiles of TCM. The similarities among different chromatograms were quantified by calculating the correlation coefficient or the cosine value of the vectorial angle [32–33].
HCA and PCA analysis
The statistical analysis was performed using the professional analysis software SIMCA 13.0 Demo for HCA and PCA. HCA and PCA were used to show the unsupervised clustering pattern of the Daphne Linn species and discover the differences in samples caused by complex factors. HCA and PCA were used to discover the natural interrelationships among the chemical components for each of the Cortex Daphnes samples [29]. The RPAs of main characteristic peaks were selected as the clustering variable and the critical P value for all analyses in this study was set to 0.05.
All the data were pretreated including background deduction and the chromatograms alignment before SA, HCA, and PCA. The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Supporting information
(PDF)
(PDF)
(PDF)
Acknowledgments
We gratefully thank students Xuezhong Wang, Tianda Zhou, Heng Wang, Wencang Tian, Kunpeng Wang, Sitong Li and Jiwen Li for the collection of wild Cortex Daphnes samples. This study was supported by the Pharmaceutical Co., Ltd., of Qinhuangdao Shanhai Pass (Qinhuangdao 066200, China) which provided great financial support. The funder did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. We would also thank agriculture and forestry sectors in Gansu, Shaanxi, Sichuan and Qinghai provinces for providing great support and help in the research materials.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by the Pharmaceutical Co., Ltd., of Qinhuangdao Shanhai Pass (Qinhuangdao 066200, China) which provided great financial support. The funder did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.State Pharmacopoeia Committee. Pharmacopoeia of the People’s Republic of China Part IV [422] (China Medical Science Press, Beijing, 2015).
- 2.Zhang W, Su J, Hu XJ, Liu RH, Zhang WD. Chemical constituents and pharmacological activities of three origin plants of traditional Chinese medicine zushima. Chinese Journal of Pharmaceuticals. 2007, 38: 233–238. [Google Scholar]
- 3.Wang MW, Li CY, Li B. Progress in research on pharmacognosy of Cortex Daphnes. Journal of TCM University of Hunan. 2007, 27: 81–84. [Google Scholar]
- 4.Li J, Liu JH, Jiang YH. Research overview of species origins and chemical composition of Cortex Daphnes. Guide of China Medicine. 2012, 10: 63–65. [Google Scholar]
- 5.Gansu Provincial Health Bureau Committee. Gansu Province Drug Standard [92] (Gansu People Press, Lanzhou, 1982).
- 6.Hebei Province Food and Drug Administration. Standard Specification for Pieces Processing of Traditional Chinese Medicine in Hebei Province [106–107] (Academy Press, Beijing, 2004).
- 7.Daphne Linn. Flora of China 2017. http://frps.eflora.cn/frps/Daphne (Date of access: 01/12/2017).
- 8.Kang AL, Tang YS, Ma JQ. Optimization of Extraction Technology of Effective Components from DAPHNES CORTEX by Orthogonal Test with Multi-index Comprehensive Evaluation. Medicinal Plant. 2013, 4: 25–27. [Google Scholar]
- 9.Wang P, Liu JP, Zhan N, Li PY, Lu D. Progress in the research on chemical constituents and pharmacological activities of zushima. Special Wild Economic Animal and Plant Research. 2011, 4: 73–76. [Google Scholar]
- 10.Zhou GX, Yang YC, Shi JG. Study of chemical constituents in stem rind of Daphne giraldii. China Journal of Chinese Materia Medica. 2006, 38: 555–557. [PubMed] [Google Scholar]
- 11.Wang YH, Xu HQ, Di LQ, Shan JJ, Gao Q. Analgesic and anti-inflammatory effects of Cortex Daphnes extract. Chinese Traditional and Herbal Drugs. 2007, 38: 1697–1700. [Google Scholar]
- 12.Li SH, Wu LJ, Yin HY. Chemical and pharmacological advances of the study on zushima. China Journal of Chinese Materia Medica. 2002, 27: 401–403. [PubMed] [Google Scholar]
- 13.Kang AL, Li W, Sun CR, Zhang X. Progress in the research on chemical constituents and pharmaceutics of zushima. Northwest Pharmaceutical Journal. 2011, 26: 479–482. [Google Scholar]
- 14.Geng L, Li ST, Yang XH, Chen MY, Ren GX, Li YP, et al. Resource situation investigation and analysis about endangered Chinese herbal medicine Daphnes Cortex. China Journal of Chinese Materia Medica. 2016, 41: 3323–3328. 10.4268/cjcmm20161803 [DOI] [PubMed] [Google Scholar]
- 15.Kool A, Boer HJ, Krüger Å, Rydberg A, Abbad A, Björk L, et al. Molecular identification of commercialized medicinal plants in southern Morocco. PLoS One. 2012, 6, e39459; 10.1371/journal.pone.0039459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen SL, Pang XH, Yao H, Han JP, Luo K. Identification system and perspective for DNA barcoding traditional Chinese Materia Medica. World Science and Technology/Modernization of Traditional Chinese Medicine and Materia Medica. 2011, 13: 747–754. [Google Scholar]
- 17.Techen N, Parveen I, Pan Z, Khan IA. DNA barcoding of medicinal plant material for identification. Current Opinion in Biotechnology. 2014, 25: 103–110. 10.1016/j.copbio.2013.09.010 [DOI] [PubMed] [Google Scholar]
- 18.Li X, Yang Y, Henry RJ, Rossetto M, Wang Y, Chen SL. Plant DNA barcoding: from gene to genome. Biological Reviews. 2015, 90: 157–166. 10.1111/brv.12104 [DOI] [PubMed] [Google Scholar]
- 19.Xin T, Li XJ, Yao H, Lin YL, Ma XC, Cheng RY, et al. Survey of commercial Rhodiola products revealed species diversity and potential safety issues. Scientific Reports. 2015, 5: 8337 10.1038/srep08337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song JY, Shi LC, Li DZ, Sun YZ, Niu YY, Chen ZD, et al. Extensive pyrosequencing reveals frequent intra-genomic variations of internal transcribed spacer regions of nuclear ribosomal DNA. PLoS One. 2012, 7: e43971 10.1371/journal.pone.0043971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xin T, Yao H, Gao HH, Zhou XZ, Ma XC, Xu CQ, et al. Super food Lycium barbarum (Solanaceae) traceability via an internal transcribed spacer 2 barcode. Food Research International. 2013, 54: 1699–1704. [Google Scholar]
- 22.Shu ZH, Li XQ, Rahman K, Qin LP, Zheng CJ. Chemical fingerprint and quantitative analysis for the quality evaluation of Vitex negundo seeds by reversed-phase high-performance liquid chromatography coupled with hierarchical clustering analysis. Journal of separation science. 2016, 39: 279–286. 10.1002/jssc.201500796 [DOI] [PubMed] [Google Scholar]
- 23.Xia PG, Bai ZQ, Liang TY, Yang DF, Liang ZS, Yan XJ, et al. High-performance liquid chromatography based chemical fingerprint analysis and chemometric approaches for the identification and distinction of three endangered Panax plants in Southeast Asia. Journal of separation science. 2016, 39: 3880–3888. 10.1002/jssc.201600460 [DOI] [PubMed] [Google Scholar]
- 24.Liang XR, Ma ML, Su WK. Fingerprint analysis of Hibiscus mutabilis L. leaves based on ultra performance liquid chromatography with photodiode array detector combined with similarity analysis and hierarchical clustering analysis methods. Pharmacognosy Magazine. 2013, 9: 238–243. 10.4103/0973-1296.113277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao YY, Zhang YM, Lin RC, Sun WJ. An expeditious HPLC method to distinguish Aconitum kusnezoffii from related species. Fitoterapia. 2009, 80: 333–338. 10.1016/j.fitote.2009.04.005 [DOI] [PubMed] [Google Scholar]
- 26.Daphne retusa Hemsl. Flora of China 2017. http://frps.eflora.cn/frps/Daphne%20retusa (Date of access: 01/20/2017).
- 27.Meteorological Data Center of China Meteorological Administration 2017. http://data.cma.cn/site/index.html (Date of access: 01/15/2017).
- 28.Gong LL, Xu HY, Wang L, Yin XJ, Yuan HJ, Wang SS, et al. Identification and evaluation of the chemical similarity of Yindan xinnaotong samples by ultra high performance liquid chromatography with quadrupole time-of-flight mass spectrometry fingerprinting. Journal of separation science. 2015, 39: 611–622. [DOI] [PubMed] [Google Scholar]
- 29.Luo HL, Kong WJ, Hu YC, Chen P, Wu XR, Wan L, et al. Quality evaluation of Salvia miltiorrhiza Bge. by ultra high performance liquid chromatography with photodiode array detection and chemical fingerprinting coupled with chemometric analysis. Journal of separation science. 2015, 38: 1544–1551. 10.1002/jssc.201401430 [DOI] [PubMed] [Google Scholar]
- 30.Luo K, Ma P, Yao H, Song JY. Study on DNA extraction method for Chinese herbs. Technology/Modernization of Traditional Chinese Medicine and Materia Medica. 2012, 14: 1433–1439. [Google Scholar]
- 31.Chen SL, Yao H, Han JP, Xin TY, Pang XH, Shi LC, et al. Principles for molecular identification of traditional Chinese materia medica using DNA barcoding. China Journal of Chinese Materia Medica. 2013, 38: 141–148. [PubMed] [Google Scholar]
- 32.Liu ZL, Liu YY, Liu CS, Song ZQ, Li Q, Zha QL, et al. The chemotaxonomic classification of Rhodiola plants and its correlation with morphological characteristics and genetic taxonomy. Chemistry Central Journal. 2013, 7: 118 10.1186/1752-153X-7-118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu YB, Zheng LJ, Yi J, Wu JG, Chen TQ, Wu JZ. Quantitative and Chemical Fingerprint Analysis for the Quality Evaluation of Receptaculum Nelumbinis by RP-HPLC Coupled with Hierarchical Clustering Analysis. International journal of molecular sciences. 2013, 14: 1999–2010. 10.3390/ijms14011999 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.