 The cytotoxic effect of low concentrations of CO against bacteria can be explored using the high-affinity globin, Leg-haemoglobin.
The cytotoxic effect of low concentrations of CO against bacteria can be explored using the high-affinity globin, Leg-haemoglobin.
Abstract
The potential for carbon monoxide-releasing molecules (CO-RMs) as antimicrobials represents an exciting prospective in the fight against antibiotic resistance. Trypto-CORM, a tryptophan-containing manganese(i) carbonyl, is toxic against E. coli following photo-activation. Here, we demonstrate that Trypto-CORM is toxic against Neisseria gonorrhoeae in the absence of photoactivation. Trypto-CORM toxicity was reversed by the high CO affinity globin leg-haemoglobin (Leg-Hb), indicating that the toxicity is due to CO release. Release of CO from Trypto-CORM in the dark was also detected with Leg-Hb (but not myoglobin) in vitro. N. gonorrhoeae is more sensitive to CO-based toxicity than other model bacterial pathogens, and may serve as a viable candidate for antimicrobial therapy using CO-RMs.
Introduction
Carbon monoxide (CO) is well known as a toxic gas, ca. 350–400 ppm, which is responsible for over a third of lethal poisonings in the developed world.1 The toxicity of CO arises as a consequence of binding to haemoglobin, and other metal centres in living systems, particularly cytochrome c oxidase.2 However, CO is a normal part of metabolism during haem turnover,3 and has been shown to display therapeutic potential in a variety of situations, such as cardiovascular disease, inflammation and organ transplantation.4–6
CO is difficult to selectively administer directly as a gas, and thus many chemical CO-releasing molecules (CO-RMs) have been developed, and their potential as therapeutic agents examined.7 A number of recent studies have investigated the validity of using CO-RMs as antimicrobials against a variety of bacteria including Escherichia coli (E. coli), Staphylococcus aureus (S. aureus), Pseudomonas aeruginosa, Helicobacter pylori and Campylobacter jejuni.8–13 The targets of CO-RM action include the respiratory haem-copper oxidases,10 but CO-RM toxicity may also be mediated by reactive oxygen species.14
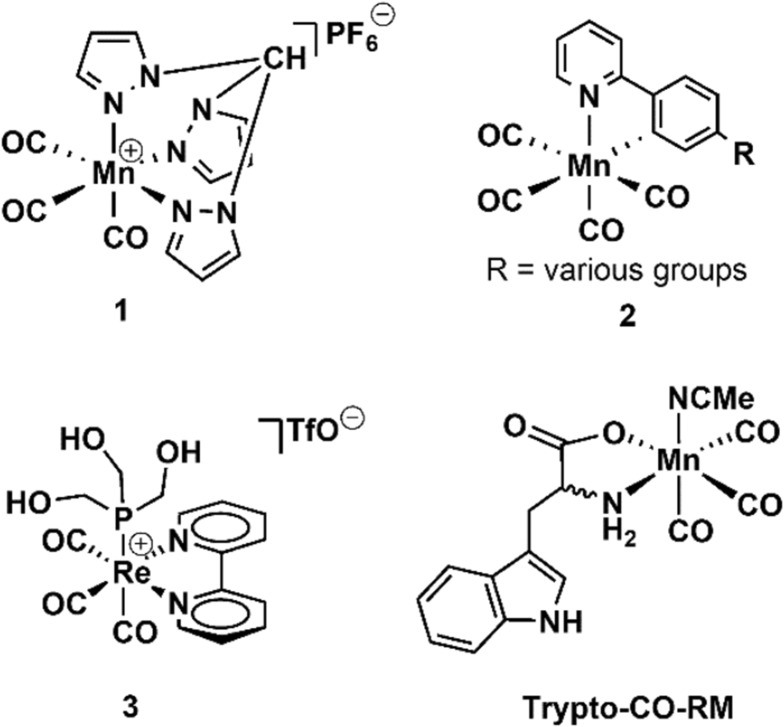
CO-RMs typically consist of a metal centre bearing multiple CO ligands, and an additional multidentate ligand for the tuning of any required physical properties (Fig. 1).7,15 The CO can be released thermally, photolytically or via a hydrolytic (e.g. enzyme-triggered or solvolysis) mechanism.16
Fig. 1. Selected photo-activated CO-RMs.
CO-RMs that release CO in response to photoirradiation are referred to as PhotoCO-RMs – selected examples were reported by Bischof et al.,17 Kretschmer et al.,18 Pfeiffer et al.,19 Rimmer et al.20 and Ward et al.21 These particular PhotoCO-RMs have been shown to inhibit E. coli growth following photoirradiation. This includes irradiation of [Mn(CO)3(tpa-κ3N)]Br at 365 nm, reported by Nagel et al.,22 or following irradiation of Mn(CO)3(CH3CN)(tryptophan) (‘Trypto-CORM’) at 400 nm.23 In the latter case it was demonstrated that the effect of CO-RM was cytotoxic, with >99% of E. coli cells being killed following irradiation of the CO-RM.23
Here we have investigated the toxicity of Trypto-CORM against Neisseria gonorrhoeae which is a globally important bacterial pathogen. N. gonorrhoeae is the causative agent of sexually transmitted infection gonorrhoea, and has recently been identified as a particular danger due to the emergence of hyper-resistant lineages, resistant to the major frontline antibiotics.24–26 New therapies against N. gonorrhoeae are urgently required, and we set out to establish whether CO-RMs might be effective in this respect. As controls, we also investigated the sensitivity of Trypto-CORM against two other model bacterial species, previously used in CO-RM research, E. coli and S. aureus. During this work it has been shown that Trypto-CORM, a thermally stable metal carbonyl compound that only releases CO efficiently following irradiation, caused CO-dependent toxicity in the dark (thermal release of CO) against target pathogenic bacterial species, specifically N. gonorrhoeae.
Results and discussion
Our studies on Trypto-CORM are herein divided into four sections, followed by a discussion of the results and analysis.
Trypto-CORM is toxic to Neisseria gonorrhoeae, in the dark
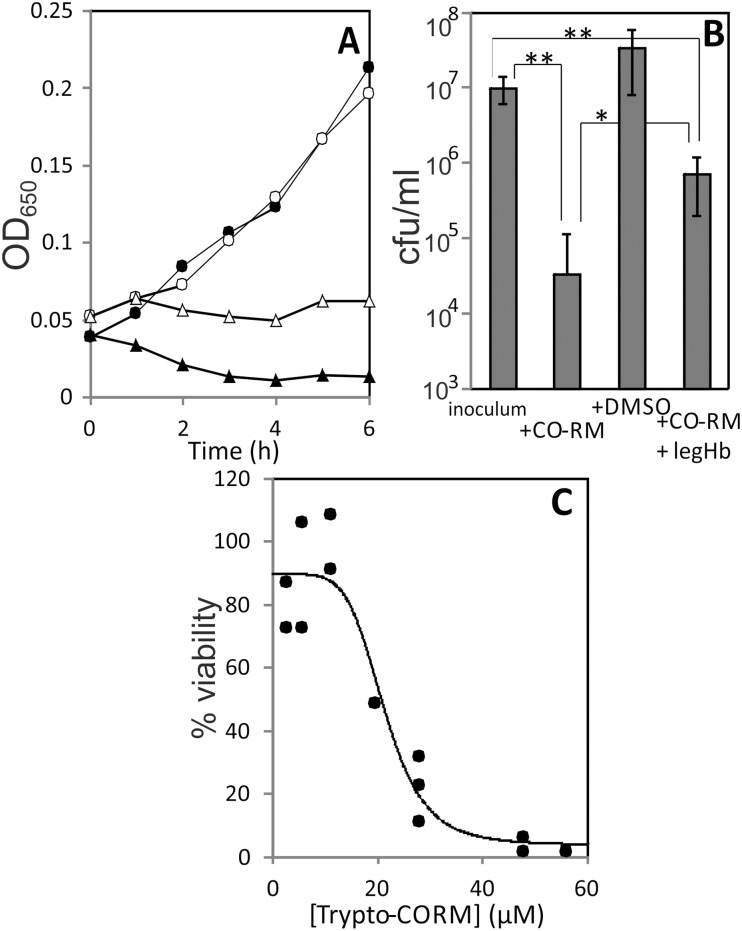
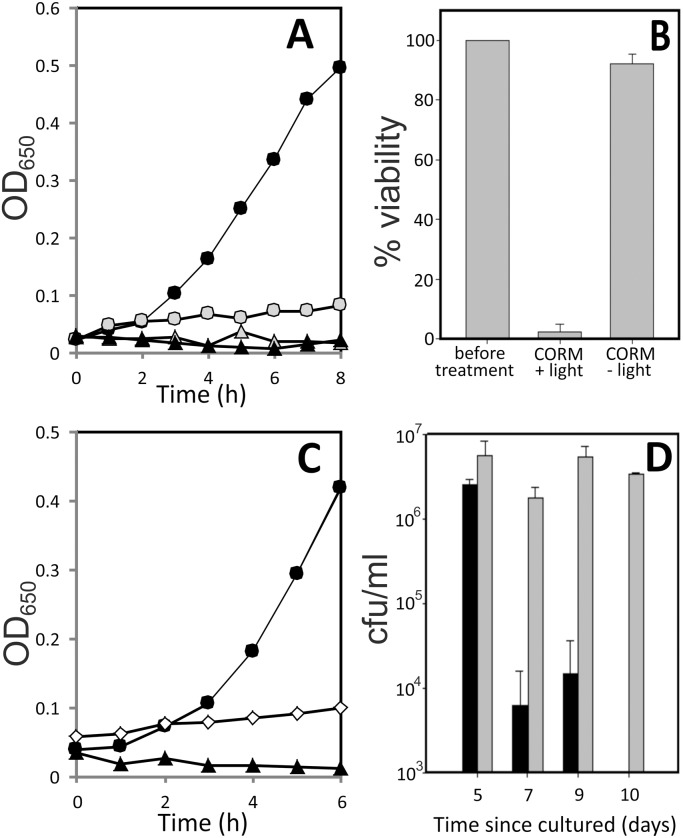
Treatment of N. gonorrhoeae with Trypto-CORM prevented growth (Fig. 2, panel A) and resulted in a loss of >99% cellular viability in the dark (Fig. 2, panel B). These experiments were conducted with fresh (1 to 2 days old) plate cultures. It was not possible to examine the impact of viability of N. gonorrhoeae over longer-term experiments as carried out for E. coli and S. aureus (see below), because N. gonorrhoeae survival on plates is limited to only a few days. The concentration of Trypto-CORM sufficient to reduce viability by 50% (IC50) was 22 μM (Fig. 2, panel C).
Fig. 2. Impact of Trypto-CORM on growth and viability of N. gonorrhoeae. Panel A, growth of N. gonorrhoeae MS11 in RPMI + YAL + 100 μM Trypto-CORM (filled triangles), RPMI + YAL + 0.5% DMSO (filled circles), RPMI + YAL + 100 μM Trypto-CORM + 22 μM Leg-Hb (open triangles) and RPMI + YAL + 0.5% DMSO + 22 μM Leg-Hb (open circles). Panel A data are representative of at least three biological replicates. Panel B, viable numbers (colony forming units per ml (cfu ml–1)) of N. gonorrhoeae at inoculation, after 4 h growth in the presence of 100 μM Trypto-CORM after 4 h growth in the absence of CORM, and after 4 h growth in the presence of 100 μM Trypto-CORM + 22 μM Leg-Hb. Panel B results show the mean and standard deviation from at least four biological replicates. Student's t-tests were performed to assess difference in viability between treatments. ** indicates P < 0.001. * indicates P < 0.05. Panel C, effect of [Trypto-CORM] on N. gonorrhoeae viability. Viability was tested after 4 h with Trypto-CORM at a range of concentrations and the IC50 calculated as 21 ± 2 μM from a logistic fit to the data.
The toxicity of 100 μM Trypto-CORM against N. gonorrhoeae can be partially reversed by treatment with a sub-stoichiometric concentration of Leg-Hb (22 μM). Leg-Hb halts the decline in OD650 over time that is observed in growth curves with Trypto-CORM (Fig. 2, panel A), and also results in a higher recovery of viable numbers of N. gonorrhoeae colony forming units, than following Trypto-CORM treatment in the absence of Leg-Hb (Fig. 2, panel B). Nonetheless, Trypto-CORM continues to exert toxicity against N. gonorrhoeae even in the presence of the high affinity CO scavenger Leg-Hb, indicating that N. gonorrhoeae is exquisitely sensitive to CO-releasing compounds. Deoxy-myoglobin is unable to rescue the toxicity of Trypto-CORM towards N. gonorrhoeae (not shown).
Slow CO release in the dark from Trypto-CORM can be captured using leg-haemoglobin
Trypto-CORM releases CO readily following irradiation, but appears quite stable in the dark, and has thus been termed a photo-activatable CO-RM. The demonstration that Leg-Hb is able to rescue N. gonorrhoeae from Trypto-CORM toxicity in the dark indicates that Trypto-CORM is able to release physiologically significant levels of CO under these conditions. To explore this further we examined the capacity of Leg-Hb to scavenge CO released from Trypto-CORM in vitro.
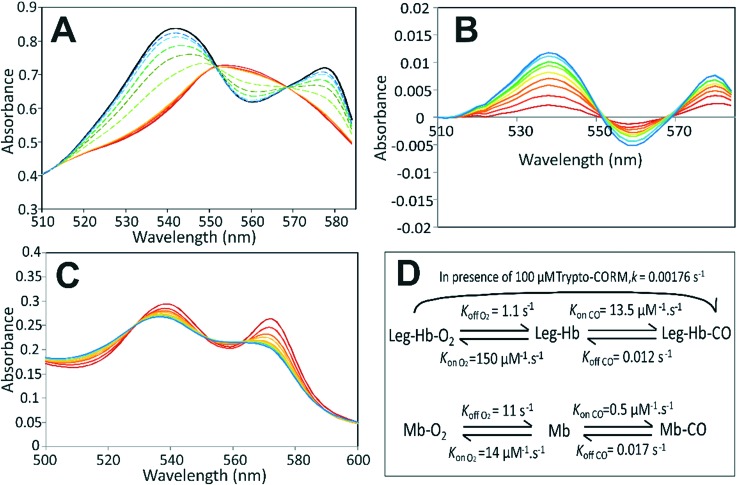
Leg-Hb has a high affinity for O2 (Kd = 6.4 nM) and for CO (Kd = 0.9 nM) compared to other globins, such as horse myoglobin (Mb) (Kd O2 = 780 nM; Kd CO = 34 nM).27,28 In previous work23 it was shown using a myoglobin-based spectroscopic assay29 that Trypto-CORM does not release significant amounts of CO until irradiated. The change in the α/β region for myoglobin in the presence of Trypto-CORM is shown in Fig. 3 (panel A). Very small changes in the visible spectral features are observable prior to irradiation, indicating that some Mb-CO forms in the absence of irradiation. This is seen more clearly in Fig. 3, panel B which shows the difference spectra for time points after adding Trypto-CORM compared to time 0. Over 45 minutes incubation, 5% of ferrous deoxy-Mb is converted to the Mb-CO form. No spectral change occurs to oxygenated myoglobin (Mb-O2) in the presence of Trypto-CORM over a 2 hour period (not shown), indicating that the slow release of CO from Trypto-CORM is unable to displace ligated O2.
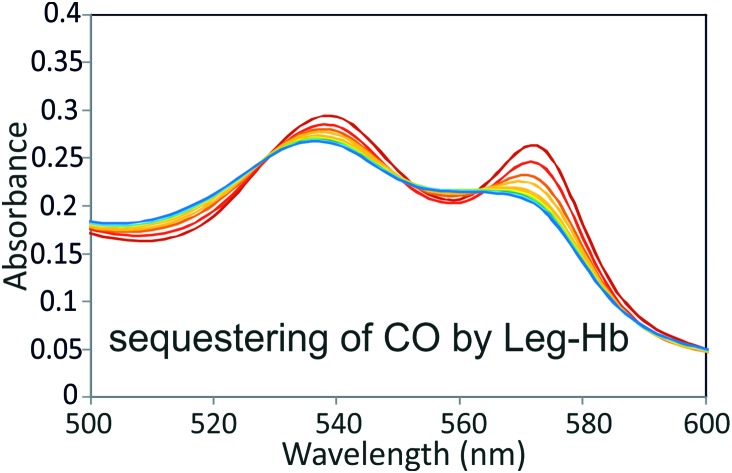
Fig. 3. Spectroscopic analysis of Trypto-CORM reaction with myoglobin and leghaemoglobin. Panel A, spectra of the α/β region of 56 μM horse myoglobin in the presence of 100 μM Trypto-CORM. Deoxy-myoglobin is shown in dark red. Spectra were taken every 10 minutes in the dark (red to orange, solid lines), and then with bursts of 4 min irradiation at 400 nm (2.4 W) (green to blue, dashed lines). Saturated carbonmonoxy form of myoglobin is shown in black. To enable better visualisation of the formation of Mb-CO in the presence of Trypto-CORM in the dark, panel B shows difference spectra of the α/β region of myoglobin at 5 minute intervals following the addition of Trypto-CORM compared to the spectrum at time zero, in the absence of irradiation. Spectra displayed using rainbow colours from red to blue with time. Panel C, spectrum showing the α/β region of leghaemoglobin in the presence of Trypto-CORM, in the absence of irradiation. Spectra were taken at 5 min intervals for 55 min. Spectra displayed using rainbow colours from red to blue with time, as the spectrum shifts from the oxy- towards the carbonmonoxy-form. After 55 min ca. 50% of Leg-HB has assumed the carbonmonoxy form ‘Leg-Hb-CO’ with a pseudo first order exponential rate constant of k = 0.1055 min–1. Panel D, a scheme showing the rates of reaction of myoglobin and leghaemoglobin with CO and O2 (data taken from (Imamura & Riggs, 1972; Wittenberg et al., 1965)).
The reaction of Leg-Hb (as prepared in the oxy form, Leg-Hb-O2) with Trypto-CORM was analysed by measuring the changes in the visible spectrum in the α/β region by UV/vis spectroscopy. Significant conversion of Leg-Hb-O2 to Leg-Hb-CO occurs over a 55 min incubation period (Fig. 3, panel C), such that at this point Leg-Hb is 50% saturated with CO. The rate constant for conversion of Leg-Hb-O2 to Leg-Hb-CO is 0.106 min–1, consistent with an effective steady state [CO] = 0.13 nM in the presence of 100 μM Trypto-CORM (see Fig. 3, panel D). The relatively slow kon CO rate for Mb compared to Leg-Hb (0.5 μM–1 s–1cf. 13.5 μM–1 s–1)28,30 is sufficient to explain the slow rate of conversion of Mb to Mb-CO. koff O2 for Leg-Hb is fast (1 s–1) compared to the observed rate of formation of Leg-Hb-CO from Leg-Hb-O2 in the presence of Trypto-CORM (0.0018 s–1), explaining why Leg-Hb is able to scavenge CO from Trypto-CORM even in the presence of oxygen. This ability of Leg-Hb to scavenge CO from Trypto-CORM, indicates that this CORM does act as a significant source of CO in the dark, but that this can only be detected with a very high affinity CO binding sink. The ability of Leg-Hb to abstract very low concentrations of CO from a system, even when initially present as the oxygen bound form underpins its ability to scavenge CO in the biological systems above, in which oxygen is readily available.
Toxicity of Trypto-CORM against E. coli requires irradiation and depends on culture age
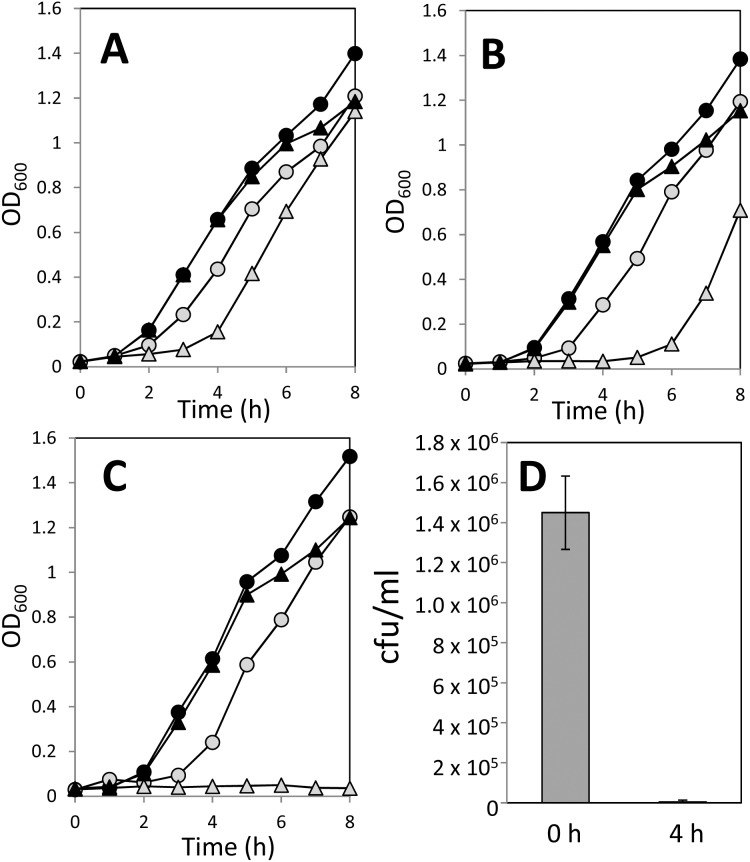
The toxicity of Trypto-CORM against E. coli has been previously reported.23 Here we extend the previous study to confirm that Trypto-CORM toxicity against E. coli requires irradiative CO release, and that the effect depends on E. coli culture age. Trypto-CORM alone had no inhibitory effect on E. coli growth, but the combination of Trypto-CORM and irradiation at 400 nm caused a cessation of growth (Fig. 4, panels A–C). The recovery of growth depends on the age of the plate culture used. Growth resumed following CO-RM irradiation in a 6 day old plate culture (Fig. 4, panel A), but for a 14 day culture, growth was not seen over the 8 hour incubation (Fig. 4, panel C). To test whether the effect of Trypto-CORM is cytostatic or cytotoxic, we determined viable counts before (at t = 0) and after irradiation (at t = 4 h) following liquid culture growth from a 14 day E. coli plate culture (Fig. 4, panel D). Trypto-CORM is cytotoxic, killing greater than 99% of the population following irradiation at 400 nm.
Fig. 4. Impact of Trypto-CORM on growth of E. coli. Growth of E. coli was followed by measuring OD600 over time. Panels A–C show growth curves of E. coli cultured from plates that were 6 days old (panel A), 8 days old (panel B) and 14 days old (panel C). Growth was carried out in LB + 100 μM Trypto-CORM (black triangles) and LB + Trypto-CORM with irradiation (400 nm, 2.4 W, 68 min, started at 1 h after inoculation) (grey triangles), LB + 0.5% DMSO (black circles) and LB + 0.5% DMSO with irradiation (grey circles). Panel D shows the viability of E. coli before (time 0) and after (time = 4 h) treatment with Trypto-CORM and irradiation at 400 nm in the 14 day old E. coli culture used in panel C.
Cytotoxic and cytostatic effect of Trypto-CORM against S. aureus in the dark
The toxicity of Trypto-CORM against Gram positive pathogenic bacterium S. aureus was also tested. Treatment with either CO-RM or irradiation at 400 nm prevents growth of S. aureus (Fig. 5, panel A). Trypto-CORM and 400 nm irradiation in combination kills 95% of bacteria cultured from a 1 day old S. aureus plate culture (Fig. 5, panel B). With Trypto-CORM in the absence of irradiation there is a cytostatic effect with these cultures (Fig. 5, panel B).
Fig. 5. Impact of Trypto-CORM on growth and viability of S. aureus. Growth of S. aureus was followed by measuring OD650 over time. Panel A, the growth of S. aureus 8325-4 in LB + 100 μM Trypto-CORM (black triangles) and LB + Trypto-CORM with irradiation (400 nm, 2.4 W, 68 min, started at 1 h after inoculation) (grey triangles), LB + 0.5% DMSO (black circles) and LB + 0.5% DMSO with irradiation (grey circles). Panel B, the impact of irradiation + CORM on viability of 1–4 day-old cultures of S. aureus in LB + 100 μM Trypto CORM before treatment and after irradiation (CORM + light) (400 nm, 2.4 W, 68 min, started at 1 h after inoculation), and after a prolonged (4–7 h) incubation in the absence of irradiation (CORM – light). 95% of the viability was lost following irradiation, but in the absence of irradiation there is little loss of viability. Panel C, growth of S. aureus in LB + 100 μM Trypto-CORM (black triangles), LB + 0.5% DMSO (black circles) and LB + 100 μM Trypto-CORM + 22 μM Leg-Hb (white diamonds). Panel D, viability of S. aureus following treatment with Trypto-CORM in the dark. X-Axis indicates the number of days since the culture was grown. S. aureus was suspended to an initial OD650 = 0.05 and incubated with 100 μM Trypto-CORM for a prolonged (4–6 h) incubation, at which point suspensions were plated out to determine viable counts (black bars). Trypto-CORM has a >99% cytotoxic effect on cultures over 7 days old. Inclusion of 22 μM Leg-Hb (grey bars) rescues cultures from toxicity of 100 μM Trypto-CORM.
To test whether S. aureus culture age had an impact on the effectiveness of Trypto-CORM, the viability of S. aureus cultures 6 hours after treatment with Trypto-CORM (without irradiation) was determined. The viability of Trypto-CORM treated cultures drops by >99.9% in cultures made from plates that were >5 days old, indicating that Trypto-CORM becomes cytotoxic to S. aureus in an age-dependent way (Fig. 5, panel D). To test whether the toxicity of Trypto-CORM could potentially be reversed by scavenging released CO, cultures were supplemented with 22 μM Leg-Hb. LegHb treated cultures retained viability (Fig. 5, panel D), but did not grow (Fig. 5, panel C). The addition of myoglobin (Mb) had no rescuing effect on the viability of S. aureus in the presence of Trypto-CORM (not shown).
Further discussion
This is the first experimental analysis of the effect of CO-releasing molecules on the important human pathogen Neisseria gonorrhoeae. We find that under the conditions tested, Trypto-CORM is highly toxic to N. gonorrhoeae, even in the absence of irradiative treatment that would cause a more efficient release of CO. N. gonorrhoeae is of particular interest due to recent occurrences of N. gonorrhoeae completely resistant to frontline antibiotics in the clinic.24–26 Previous work has been extended in order to show that the Trypto-CORM is toxic against S. aureus, as well as E. coli. Like with N. gonorrhoeae, Trypto-CORM is able to display toxicity against S. aureus in the absence of irradiation. In both cases, it has been confirmed that even in the absence of irradiation, the toxic effect of Trypto-CORM is at least partially due to CO release itself (as shown by the ability of Leg-Hb to rescue the bacteria from the toxicity of Trypto-CORM). In N. gonorrhoeae experiments, Leg-Hb is not able to completely reverse the toxicity due to Trypto-CORM. Either, N. gonorrhoeae is sensitive to concentrations of CO below the concentration which can be effectively scavenged by Leg-Hb, or, Trypto-CORM is toxic via independent mechanisms in addition to CO release, which are specific to certain bacteria. The Leg-Hb treatment of S. aureus reverses the toxic effect of Trypto-CORM completely, but the cytostatic effect remains.
With both E. coli and S. aureus, a distinctive effect of culture age is observed. Cultures that have been stored for a longer time are more sensitive to Trypto-CORM than “fresh” bacterial cultures. The number of viable cells present in a bacterial inoculum is not sufficient to explain this effect, since when the inoculum from a fresh culture was diluted to give the same viable number as in the inocula from older cultures growth recovered from the freshly cultured bacteria but not the older cultures. The mechanistic basis for this age-dependence of Trypto-CORM toxicity remains to be explored, but might be due to a depletion in the number of active haem-copper oxidase complexes in near-dormant cells. The age-dependent effect is of some potential interest, since persistent bacteria during infection are frequently slow growing or dormant, and it is these bacteria that are often particularly recalcitrant to antibiotic treatment, an example being reported by Rittershaus et al. in 2013.31 A novel antimicrobial that is able to target bacteria in a dormant physiological state could be a useful addition to the antibiotic arsenal.
The finding that Trypto-CORM is able to function in physiologically relevant CO release in the dark is of further interest. This offers a new strategy for targeting the CO released by this molecule, and other molecules like it, to high affinity CO binding sites within particular bacteria. This could provide a selectivity to the infectious agent and added safety to the host (eukaryotic cells in culture have been shown to be insensitive to [Trypto-CORM] of 100 μM in the absence of photoirradiation).23
A novel dimension to our work described here is the development of leghaemoglobin as a useful probe for spectroscopic analysis of CO in biology. Leg-Hb enables us to assess the effect of a lower range of CO concentrations than is possible using other globins, particularly myoglobin which has been used as the routine work-horse for probing CO release from CO-RMs to date.29
The comparative analysis of Trypto-CORM against different bacterial species shows that different bacteria have very different sensitivities to CO release from this molecule, with S. aureus and N. gonorrhoeae considerably more sensitive, since E. coli is completely insensitive to Trypto-CORM except after the rapid release of CO following photoirradiation. It will be of interest to explore the sensitivity of other potential target microorganisms and identify the molecular basis for this differential sensitivity in order to rationally design future CO-based antimicrobials against specific pathogens.
Conclusions
The potential for CO-RMs as antimicrobials is promising for influencing the growth of a range of bacterial species. We conclude that Trypto-CORM is toxic against Neisseria gonorrhoeae in the dark under thermal/cellular control (i.e. absence of photoactivation). The toxicity of Trypto-CORM was reversed by the high CO affinity globin leg-haemoglobin (Leg-Hb), confirming that the toxicity is due to the release of CO. Release of CO from Trypto-CORM in the dark was also detected with Leg-Hb (but not myoglobin) in vitro. It is of further interest to consider that leg-haemoglobin should be used more widely in CORM/CO research, due to its very high CO affinity making it a valuable tool for accessing the physiological effects of CO at low concentrations. In our previous work we have found that Trypto-CORM is toxic to E. coli only following photoactivation, whereas S. aureus was sensitive to CO from Trypto-CORM following irradiative release and, to a certain extent, in the dark. It is evident that N. gonorrhoeae is more sensitive to CO-based toxicity than other model bacterial pathogens, and we suggest that it is an appropriate candidate for antimicrobial therapy using CO-RMs.32,33
Experimental section
Bacterial strains and culture conditions
Specialist bacterial media and components of media were obtained from Oxoid. Other chemicals were obtained from Sigma-Aldrich, except where stated otherwise. Neisseria gonorrhoeae MS11 was stored in 50% glycerol: 50% Mueller Hinton broth at –80 °C. Stocks were streaked onto Columbia agar plus 5% defibrinated horse blood and grown and maintained (for up to three days) at 37 °C in a 95% air: 5% CO2 atmosphere. Liquid cultures were set up from these plates into liquid media RPMI (Gibco) containing Yeast Autolysate Supplement (YAL). The supplement powder was dissolved in 15 ml sterile water and 1.5 ml liquid supplement was added to 50 ml of RPMI medium. Cultures were grown in 3 ml of media in 8 ml Bijou tubes at 37 °C and sampled as described below for E. coli.
Escherichia coli W3110 was cultured in lysogeny broth (LB; 10 g l–1 tryptone, 5 g l–1 yeast extract, 5 g l–1 NaCl) in liquid media, and on plates supplemented with 1.5% bacteriological agar. Streak plates were grown for 16 hours at 37 °C, from glycerol stocks maintained at –80 °C. Plates were subsequently stored at 4 °C for up to one month. Liquid cultures were inoculated to a starting OD of 0.05 (except where stated otherwise) and cultured with shaking at 100 rpm in 3 ml of media in 8 ml Bijou tubes at 37 °C. 300 μl aliquots were withdrawn hourly in order to measure optical density at 600 nm (OD600) using a Jenway 6305 spectrophotometer and/or make serial dilutions in order to determine the number of viable bacteria (colony forming units; cfu) per ml of culture.
Staphylococcus aureus 8325-4 was cultured and maintained as described for E. coli.
Preparation and application of Trypto-CORMs
Trypto-CORM was prepared as described previously.23 The CORM was dissolved in DMSO to generate a 20 mM stock solution. This was diluted 200× into the growth medium to give a working Trypto-CORM concentration of 100 μM. 0.5% DMSO was added to media as a control. The irradiation of Trypto-CORM was carried out using a customised LED arrangement (as described by (Ward et al., 2014)) with each LED has a power of 2.4 W. Irradiation was delivered in “4 minute on, 1 minute off” cycles to prevent the LED from getting hot, beginning 1 h post-inoculation of microbiological cultures. Irradiation in these experiments was typically carried out for a total of 68 minutes (17 full cycles), using a 400 nm LED. Dark controls were maintained in culture vessels wrapped in aluminium foil.
Preparation of leghaemoglobin
Leghaemoglobin from Soybean was purified following its heterologous expression in E. coli, using a previously described expression vector (Jones et al., 199834). E. coli BL21 (λDE3) containing the pET11a derivative containing the Soybean leghaemoglobin gene was grown overnight at 37 °C on an LB agar plate with 100 μg ml–1 ampicillin. A single colony was transferred to 5 ml of LB media with 100 μg ml–1 ampicillin in a Sterilin tube. This was grown at 37 °C on an orbital shaker with 225 rpm rotation for 6 hours. The 5 ml culture was then transferred to 635 ml of auto induction medium in a 2 L baffled conical flask (following the procedure of Bonsor et al.35) and grown at 37 °C overnight with shaking at 120 rpm. The culture was harvested by centrifugation at 5000 rpm for 15 min at 4 °C. The medium was discarded from the red pellet of cells which was subsequently re-suspended in 35 ml 50 mM Tris/HCl (pH 9). The suspension of cells was then sonicated in a 50 ml Falcon tube on ice using a Misonix 3000 sonicator with 10 s on/off cycles for a total sonication time of 3 minutes. Cell debris was then removed by two centrifugation cycles at 11 000 rpm for 15 minutes (4 °C). Supernatant was transferred to a new Falcon tube after the first centrifugation to remove most of the debris.
The protein was purified on a 1.5 cm diameter anionic exchange DEAE sepharose CL6B column, and washed with 50 mM Tris/HCl (pH 9) until the red band indicative of the protein was running very close to the bottom of the column. The protein was then fully eluted using a gradient of 30 mM to 500 mM NaCl in 20 mM K2HPO4 pH 7. 5 ml eluent fractions were retained, and protein purity was confirmed by 15% SDS-PAGE. The protein solution was concentrated 10 fold using a Vivaspin 20 MWCO (PES) 5000 Falcon tube. The resultant protein solution was desalted by diluting 10 fold with 10 mM Tris/HCl pH 7.4 and concentrating the new solution with the Vivaspin tube 10 fold. This dilution with Tris buffer was performed once more to dilute any salt in the solution by a total 100 fold. The protein was divided into aliquots and was stored at –20 °C and was used within 2 months of preparation.
Spectroscopic methods
UV-visible spectroscopy of myoglobin (horse heart Mb, obtained from Sigma) and Leg-Hb was carried out on a JASCO V-560 spectrophotometer. Data was analysed in accordance with the protocol we used previously.29
Acknowledgments
This work was funded by a BBSRC studentship to JSW.
Footnotes
†The authors declare no competing interests.
References
- Omaye S. T. Toxicology. 2002;180:139. doi: 10.1016/s0300-483x(02)00387-6. [DOI] [PubMed] [Google Scholar]
- Prockop L. D., Chichkova R. I. J. Neurol. Sci. 2007;262:122. doi: 10.1016/j.jns.2007.06.037. [DOI] [PubMed] [Google Scholar]
- Sjostrand T. Acta Physiol. Scand. 1952;26:338–344. doi: 10.1111/j.1748-1716.1952.tb00915.x. [DOI] [PubMed] [Google Scholar]
- Abraham N. G., Kappas A. Pharmacol. Rev. 2008;60:79. doi: 10.1124/pr.107.07104. [DOI] [PubMed] [Google Scholar]
- Kohmoto J., Nakao A., Stolz D. B., Kaizu T., Tsung A., Ikeda A., Shimizu H., Takahashi T., Tomiyama K., Sugimoto R., Choi A. M. K., Billiar T. R., Murase N., McCurry K. R. Am. J. Transplant. 2007;7:2279. doi: 10.1111/j.1600-6143.2007.01940.x. [DOI] [PubMed] [Google Scholar]
- Ryter S. W., Alam J., Choi A. M. Physiol. Rev. 2006;86:583. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- Motterlini R., Otterbein L. E. Drug Des. Discovery. 2010;9:728. doi: 10.1038/nrd3228. [DOI] [PubMed] [Google Scholar]
- Davidge K. S., Sanguinetti G., Yee C. H., Cox A. G., McLeod C. W., Monk C. E., Mann B. E., Motterlini R., Poole R. K. J. Biolumin. Chemilumin. 2009;284:4516. doi: 10.1074/jbc.M808210200. [DOI] [PubMed] [Google Scholar]
- Desmard M., Foresti R., Morin D., Dagouassat M., Berdeaux A., Denamur E., Crook S. H., Mann B. E., Scapens D., Montravers P., Boczkowski J., Motterlini R. Antioxid. Redox Signaling. 2012;16:153. doi: 10.1089/ars.2011.3959. [DOI] [PubMed] [Google Scholar]
- Jesse H. E., Nye T. L., McLean S., Green J., Mann B. E., Poole R. K. Biochim. Biophys. Acta. 2013;1834:1693–1703. doi: 10.1016/j.bbapap.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobre L. S., Seixas J. D., Romao C. C., Saraiva L. M. Antimicrob. Agents Chemother. 2007;51:4303. doi: 10.1128/AAC.00802-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H., Mann B. E., Motterlini R., Poole R. K. IUBMB Life. 2011;63:363. doi: 10.1002/iub.476. [DOI] [PubMed] [Google Scholar]
- Tavares A. F., Parente M. R., Justino M. C., Oleastro M., Nobre L. S., Saraiva L. M. PLoS One. 2013;8:e83157. doi: 10.1371/journal.pone.0083157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares A. F., Nobre L. S., Saraiva L. M. FEMS Microbiol. Lett. 2012;336:1. doi: 10.1111/j.1574-6968.2012.02633.x. [DOI] [PubMed] [Google Scholar]
- Johnson T. R., Mann B. E., Clark J. E., Foresti R., Green C. J., Motterlini R. Angew. Chem., Int. Ed. 2003;42:3722. doi: 10.1002/anie.200301634. [DOI] [PubMed] [Google Scholar]
- Romanski S., Kraus B., Schatzschneider U., Neudorfl J. M., Amslinger S., Schmalz H. G. Angew. Chem., Int. Ed. 2011;50:2392. doi: 10.1002/anie.201006598. [DOI] [PubMed] [Google Scholar]
- Bischof C., Joshi T., Dimri A., Spiccia L., Schatzschneider U. Inorg. Chem. 2013;52:9297. doi: 10.1021/ic400746n. [DOI] [PubMed] [Google Scholar]
- Kretschmer R., Gessner G., Gorls H., Heinemann S. H., Westerhausen M. J. Inorg. Biochem. 2011;105:6. doi: 10.1016/j.jinorgbio.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Pfeiffer H., Rojas A., Niesel J., Schatzschneider U. Dalton Trans. 2009:4292. doi: 10.1039/b819091g. [DOI] [PubMed] [Google Scholar]
- Rimmer R. D., Richter H., Ford P. C. Inorg. Chem. 2010;49:1180. doi: 10.1021/ic902147n. [DOI] [PubMed] [Google Scholar]
- Ward J. S., Lynam J. M., Moir J. W., Sanin D. E., Mountford A. P., Fairlamb I. J. S. Dalton Trans. 2012;41:10514. doi: 10.1039/c2dt31588b. [DOI] [PubMed] [Google Scholar]
- Nagel C., McLean S., Poole R. K., Braunschweig H., Kramer T., Schatzschneider U. Dalton Trans. 2014;43:9986. doi: 10.1039/c3dt51848e. [DOI] [PubMed] [Google Scholar]
- Ward J. S., Lynam J. M., Moir J. W., Fairlamb I. J. S. Chem. – Eur. J. 2014;20:15061. doi: 10.1002/chem.201403305. [DOI] [PubMed] [Google Scholar]
- Allen V. G., Mitterni L., Seah C., Rebbapragada A., Martin I. E., Lee C., Siebert H., Towns L., Melano R. G., Low D. E. JAMA, J. Am. Med. Assoc. 2013;309:163. doi: 10.1001/jama.2012.176575. [DOI] [PubMed] [Google Scholar]
- Golparian D., Hellmark B., Fredlund H., Unemo M. Sex. Transm. Infect. 2010;86:454. doi: 10.1136/sti.2010.045377. [DOI] [PubMed] [Google Scholar]
- Shimuta K., Unemob M., Nakayamaa S.-I., Morita-Ishiharaa T., Dorina M., Kawahatac T., Ohnishia M. Antimicrob. Agents Chemother. 2013;57:5225. doi: 10.1128/AAC.01295-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura T., Riggs A. J. Biolumin. Chemilumin. 1972;247:521–526. [Google Scholar]
- Wittenberg B. A., Brunori M., Antonini E., Wittenberg J. B., Wyman J. Arch. Biochem. Biophys. 1965;111:576. doi: 10.1016/0003-9861(65)90237-7. [DOI] [PubMed] [Google Scholar]
- Atkin A. J., Lynam J. M., Moulton B. E., Sawle P., Motterlini R., Boyle N. M., Pryce M. T., Fairlamb I. J. S. Dalton Trans. 2011;40:5755. doi: 10.1039/c0dt01809k. [DOI] [PubMed] [Google Scholar]
- Imamura T., Riggs A. J. Biolumin. Chemilumin. 1972;247:521. [Google Scholar]
- Rittershaus E. S., Baek S. H., Sassetti C. M. Cell Host Microbe. 2013;13:643. doi: 10.1016/j.chom.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobre L. S., Jeremias H., Romão C. C., Saraiva L. M. Dalton Trans. 2016;45:1455. doi: 10.1039/c5dt02238j. [DOI] [PubMed] [Google Scholar]
- García-Gallego S., Bernardes G. J. L. Angew. Chem., Int. Ed. 2014;53:9712. doi: 10.1002/anie.201311225. [DOI] [PubMed] [Google Scholar]
- Jones D. K., Badii R., Rosell F. I., Lloyd E. Biochem. J. 1998;330:983. doi: 10.1042/bj3300983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonsor D., Butz S. F., Solomons J., Grant S., Fairlamb I. J. S., Fogg M. J., Grogan G. Org. Biomol. Chem. 2006;4:1252. doi: 10.1039/b517338h. [DOI] [PubMed] [Google Scholar]