Abstract
Natural products are important leads in drug discovery. The search for effective plant-derived agents or their synthetic analogues has continued to be of interest to biologists and chemists for a long time. Herein, we have synthesized a novel compound, P1C, and P1C-Tit*CAgNPs from chitosan; P1C is a precursor and an anti-inflammatory candidate, which has been validated by molecular docking studies. The synthesized P1C-Tit*CAgNPs showed monodisperse, spherical, and cationic nature and antioxidant properties, protecting destabilization of the erythrocyte membrane by the azo compound 2,2′-azobis(2-amidinopropane)dihydrochloride (AAPH); the involvement of NPs as a protective agent for biomolecules, such as DNA and protein, followed by the treatment of NPs with AAPH was confirmed. The inhibition of cellular damage and leakage of cellular inflammatory agents was confirmed by AFM, SEM, TEM, SDS-PAGE, LDH, and PLA2 enzyme inhibition via in vitro studies. The anti-inflammatory property of P1C was further validated by in silico molecular docking studies and showed that, the P1C best pose aligned to PLA2 compared to standard drug. The significant anticancer property of P1C-Tit*CAgNPs was confirmed against MCF7, U373, and C6 cancer cell lines. Thus, the present study highlights the synthesized P1C in P1C-Tit*CAgNPs as a target PLA2-specific anti-inflammatory candidate, and further tuning of small and development-functionalized nanoparticles has a great future in medicine; hence, their clinical applications are warranted.
Introduction
Nanoparticles (NPs) are promising candidates and have gained significant interest because of their various technological and medical applications including as anticancer, anti-inflammatory, antitumor, antiviral, and anti-angiogenic agents and in wound dressing, drug delivery, combination therapy,1,2 molecular imaging, cosmetics, and food industries; moreover, they play an advanced role in medical devices.3–5 Many noble-metal nanoparticles, such as silver (Ag), gold (Au), copper (Cu), cobalt (Co), and zinc (Zn) nanoparticles, exhibit excellent biological properties; in particular, Ag has attracted the attention of researchers due to its unique properties.6 Due to its wide applications based on its excellent biological activity, significant attention has been paid to its biological chemistry. As reported in literature, AgNPs show biocidal property by slow release of Ag+ ions via many mechanisms such as by interfering with thiol groups in proteins and enzymes, creating oxidative stress or inhibiting DNA replication, making it more complicated for bacteria to become a resistant strain;7 in addition, due to large surface area of AgNPs, the reactivity and adsorption to pathogens are enhanced, and this makes AgNPs more ideal candidates for antibacterial applications.
However, the anti-inflammatory properties of AgNPs have not been investigated extensively; moreover, very recent studies have shown that AgNPs exert cytotoxic, pro-inflammatory, and pro-apoptotic effects via generating reactive oxygen species in both normal and tumor cell lines.8–10 A recent study has explored that the synthesized chitosan-based biodegradable nanoparticles have attracted the attention of a wide range of researchers in the field of medicine due to their nontoxic nature, safe drug delivery, and effective clearance from the body.11
The crustacean exoskeleton is a rich source of chitin, which in turn provides a biodegradable, nontoxic biopolymer called chitosan. Based on its degree of deacetylation, it can have a C2 amino group with a pKa value of 6.5 and can be easily protonated under weakly acidic conditions.12 The polycationic nature of chitosan favors the interaction with cell and cytoplasmic membranes. Owing to its interesting properties, it has been considered as a promising functional biomaterial, which is often incorporated in biopharma to accelerate the transport of polar drugs over epithelial surfaces.13,14
The natural properties, such as nontoxicity and anticancer, hypocholesterolemic, hypotensive, adjuvant, and immunity enhancing properties, of chitosan have attracted the attention of diverse scientific investigators who are now keen to understand the exact relationship of these properties with biomolecules in the system.15 The selection of specific targets with promising candidates will have a great impact on the inflammatory disorder of diseased tissues. To achieve this therapeutic efficacy, inflammatory cells often require high drug doses, which induce unwanted effects on other tissues; in this regard, there is an urgent need to develop nanotechnology-based drug candidates for the treatment of inflammatory and cancer diseases to overcome toxicity and drug-resistant diseases; thus, the current target-based drug design paradigm is specifically searching for new drug candidates that are selective in a molecular sense and cell-type specific to prevent targeted issues effectively at lower doses. Under inflammatory conditions, there are multiple downstream candidates involved in the inflammatory pathway; importantly, phospholipase A2 (PLA2) is the primary and cyclooxygenase 1 (COX1) and cyclooxygenase 2 (COX2) are the secondary candidates involved in this pathway. The inhibition of enzyme PLA2 is very important to revert the inflammatory consequences further. In this regard, a number of studies have been conducted; particularly, piperazine derivatives have been synthesized and studied for the inhibition of enzyme PLA2, but chitosan silver nanoparticle-decorated piperazine-like candidates have not been evaluated to date.
Based on this literature background, herein, we designed a novel and target-specific interesting anti-PLA2 analog, and its efficacy was evaluated by different parameters. Moreover, target specificity was validated by in silico molecular docking studies to predict the nature of the candidate for biomedical applications.
Materials and methods
Synthesis of N-(4-chlorophenyl)-4-(2,3-dihydrobenzo[b][1,4]dioxine-2-carbonyl)piperazine-1-carbothioamide
Typically, 1-(1,4-benzodioxane-2-carbonyl)piperazine (1) (1.0 eq.) was first dissolved in dichloromethane (DCM) and then triethylamine (TEA) (3.0 eq.) followed by stirring and cooling. After 10 min, 4-chlorophenyl isothiocyanate (3) (1.0 eq.) was added, and the mixture was stirred at room temperature for 6–7 h. Then, the solvent was removed, and the residue was extracted with ethyl acetate. The organic layer was dried over anhydrous sodium sulphate and recrystallized from methanol.
FTIR (KBr, cm–1): 3266 (N–H), 1784 (C O), 1530 (C C), 1386 (C–S), 1278 (C–N), 1013 (C–O), 774 (CH2), 598 (C–Cl). 1H NMR (DMSO-d6) δ ppm: 3.32 (piperazine-H, t, 4H), 3.71 (piperazine-H, t, 4H), 4.00 (NH, s, 1H), 4.68 (CH2, d, 2H), 5.14 (CH, t, 1H), 6.40 (aromatic-H, d, 2H), 6.66 (aromatic-H, d, 2H), 6.71 (aromatic-H, t, 2H), 7.02 (aromatic-H, d, 2H). 13C NMR δ ppm: 47.5, 54.2, 66.2, 85.9, 115.0, 121.0, 127.9, 129.2, 130.3, 135.2, 146.7, 168.7, 182.5. MS (ESI) m/z: 418.3578. Anal. calcd for C20H20ClN3O3S (in %): C 57.48, H 4.82, N 10.05; found C 57.60, H 4.71, N 10.18.
Benzodioxane-titivated chitosan silver nanoparticles (P1C-Tit*CAgNPs)
The nanoparticles were synthesized according to the protocol reported by Karthik et al.16 Briefly, a solution of chitosan (0.5 g) in 2% acetic acid and silver nitrate (AgNO3, 0.5 g) in deionized water were mixed. Exactly above 10 mL of chitosan and silver nitrate solutions were mixed in a boiling tube, and then, the mixture was kept in an autoclave at 120 °C for 1 h. Then, the resultant clear orange-yellow solution was mixed with 0.5 g of N-(4-chlorophenyl)-4-(2,3-dihydrobenzo[b][1,4]dioxine-2-carbonyl)piperazine-1-carbothioamide and sonicated for 3 h at 30 °C. The resulting black-colored solution indicates the formation of titivated chitosan-silver nanoparticles (P1C-Tit*CAgNPs).
Characterization of P1C-Tit*CAgNPs
The synthesized novel P1C-Tit*CAgNPs were characterized for their confirmation, size, shape, charge, purity, and crystalline nature by UV-vis spectral analysis, Fourier-transform infrared (FTIR) spectroscopy, dynamic light scattering (DLS), zeta potential, scanning electron microscopy (SEM), transmission electron microscopy (TEM), energy-dispersive X-ray spectroscopic (EDS) analysis, and X-ray powder diffraction (XRD) according to a previous study reported by Karthik et al.16
Quantification of the P1C loading efficiency
The P1C-Tit*CAgNPs were pelleted, and the supernatant was quantified by centrifugation at 12 000 rpm for free P1C according to the protocol reported by Karthik et al.;16 the P1C loading efficiency was calculated as follows:
Antioxidant activity
DPPH radical scavenging assay
The free radical scavenging activity was measured by the 2,2-diphenyl-1-picrylhydrazyl (DPPH) (Sigma Aldrich, Bangalore, India) assay according to a method described earlier.17 Briefly, 4 mg of DPPH was dissolved in 100 mL methanol and stored at 4 °C until use. The working solution was prepared by diluting the DPPH solution with methanol to attain an absorbance of 0.98 at 517 nm using a spectrophotometer. A 2.9 mL aliquot of this solution was mixed with 100 μL of sample at various concentrations (20–100 μg mL–1). The reaction mixture was shaken well and incubated in the dark for 15 min at room temperature. Then, the absorbance was read at 517 nm. The control was prepared as abovementioned without any sample. Ascorbic acid (Vit-C) was used as a positive control, and its scavenging effect was calculated using the following formula: Scavenging effect (%) = [(control absorbance – sample absorbance)/control absorbance] × 100 All the experiments were carried out in triplicates and repeated twice.
Ferrous ion chelating assay
The chelating activity of the samples for ferrous ions (Fe2+) was measured according to the method reported by Mallesha et al.18 Briefly, 0.5 mL of synthesized compounds at different concentrations were added to a solution of 2 mM FeCl2 (0.05 mL). The reaction was initiated by the addition of 5 mM ferrozine (0.2 mL). The mixture was shaken vigorously and left at room temperature for 10 min. Ferrozine reacted with divalent iron to form stable magenta complex species that were soluble in water. The absorbance of the solution was then measured spectrophotometrically at 562 nm using EDTA as a positive control. The percentage of inhibition of ferrozine–Fe2+ complex formation by the compounds was calculated as follows: Percentage of inhibition (%) = [(A0 – A1)/A0] × 100 where A0 is the absorbance of the control and A1 is the absorbance of the test sample.
All the experiments were carried out in triplicates and repeated twice.
Nitric oxide radical scavenging assay
Sodium nitroprusside in an aqueous solution at physiological pH spontaneously generates nitric oxide,18 which in turn reacts with oxygen to produce nitric ions that can be estimated by the Griess reagent. Scavengers of nitric oxide compete with oxygen, leading to reduced production of nitric oxide. Sodium nitroprusside (5 mM) in phosphate-buffered saline was mixed with samples at different concentrations (25, 50, 75, 100, 125, and 150 μg mL–1) and incubated at 25 °C for 150 min. The abovementioned samples were reacted with the Griess reagent (1% sulphanilamide, 2% H3PO4, and 0.1% naphthyl ethylenediamine dihydrochloride). The absorbance of the chromophore, formed during diazotization of nitrite with sulphanilamide and subsequent coupling with naphthyl ethylenediamine, was read at 546 nm using BHT as a standard. The radical scavenging activity was measured using the equation described for the DPPH radical scavenging assay, and the percentage of radical scavenging activity (RSA) was calculated as follows: Percentage of inhibition (%) = [(A0 – A1)/A0] × 100 where A0 is the absorbance of the control and A1 is the absorbance of the test sample.
All the experiments were carried out in triplicates and repeated twice.
Protective effect on the oxidation of biomolecules in vitro
Plasmid DNA strand break assay
The protective efficacy of the synthesized compounds against 2,2′-azobis(2-amidinopropane) dihydrochloride (AAPH) (Sigma Aldrich, Bangalore, India)-induced DNA damage was studied using plasmid pRSET-A plasmid DNA (Invitrogen, Thermo Fisher Scientific, India).19 Briefly, 350 ng of plasmid DNA was dissolved in 10 μL of TE buffer (10 mM Tris–HCl and 1 mM EDTA, pH 8.0), and then, AAPH was added to a final concentration of 10 mM with or without samples followed by incubation for a period of 20 min at room temperature. The plasmid DNA without sample was used as a control, and gallic acid (GA) was used as a standard antioxidant. After treatments, the plasmid DNA was electrophoresed on 1% agarose gel, stained with ethidium bromide, and imaged using a gel documentation system (Bio-Rad Laboratories India Pvt. Ltd).
Protein oxidation
Bovine serum albumin (BSA) (Merck, Bangalore, India) was oxidized by an azo compound, AAPH, which upon decomposition with oxygen generated a peroxyl radical.19 Briefly, BSA (5 μg) was dissolved in phosphate buffer (pH 7.3) and incubated for 2 h with or without AAPH (400 mM final concentration) in the presence or absence of samples. After incubation, the protein samples were subjected to SDS-PAGE electrophoresis (10%); then, gels were stained with 0.15% Coomassie Brilliant Blue R-250, and the amount of protein damage was quantified by measuring the density of each band.
Anti-inflammatory activity
Human erythrocyte suspension
The heparinized human red blood cells (according to the guidance of a medical counsellor from Medical College, Mysore) were obtained in a Vacutainer tube, washed with 0.9% saline, and then centrifuged at 3000 rpm for 10 min to remove plasma. Exactly 40% v/v suspension was made with isotonic phosphate buffer at pH 7.4, and it was used as an RBC stock solution.16
AAPH-induced hemolysis assay
The hemolysis assay was carried out as described by Henkelman et al.20 and Liu and Ng.21 Briefly, erythrocyte hemolysis was induced by an equal volume of 0.2 M AAPH containing test samples at different concentrations (0–100 μg mL–1). Similarly, 0.2% Triton (in PBS) was used as a positive control for 100% hemolysis. The reaction mixture was incubated for 2 h at 37 °C and centrifuged at 2000 rpm for 10 min; then, the supernatant was obtained, and absorbance was read at 540 nm. The percentage of hemolysis was calculated, and the results were expressed in IC50 value in comparison with those of l-ascorbic acid as the standard.
Erythrocyte imaging: atomic force microscopy (AFM) and scanning electron microscopy (SEM) validation
The abovementioned treated blood (0.1 mL) samples were spread onto glass slides and air-fixed. Then, the morphology of erythrocytes was observed using AFM (Park NX 10, Italy),19 and the measurements, such as roughness, thickness, and waviness, of erythrocytes were analyzed using the NOVA 1.1.0.1921 software. Herein, three erythrocyte morphological alterations were observed using a scanning electron microscope (ZEISS).
Lactate dehydrogenase (LDH) assay for erythrocyte damage
The amount of LDH released by damaged erythrocytes was determined according to the method reported by DeBrosse et al.22 with minor modifications. Herein, 500 μL of 10% erythrocytes was incubated for 2 h with an equal volume of 100 mM AAPH solution with samples at different concentrations (20, 40, 60, 80, and 100 μg mL–1) and that without sample served as the blank. After incubation, 200 μL of the whole reaction mixture was diluted in 400 μL of PBS and centrifuged for 5 min at 3000 rpm. Then, 200 μL of the supernatant was used to estimate the amount of LDH released using the LDH estimation kit (Agappe, 11407002, Mysore, India), and the values were expressed as percent LDH released.
Phospholipase A2 (PLA2) inhibition
The concentration of protein in the venom (Sigma Aldrich, India) was calculated using bovine serum albumin fraction (0–75 μg). A semi-quantitative indirect hemolytic assay was employed.23 Briefly, 1 mL egg yolk, packed human erythrocytes, and phosphate-buffered saline were mixed (1 : 1 v/v) and incubated with 60 μg enzyme for 10 min. The amount of haemoglobin released in the supernatant was measured at 540 nm. The assay was carried out at different concentrations (25, 50, 100, and 200 μg mL–1) of samples. Lysis of erythrocytes by the addition of 9 mL of phosphate-buffered saline to a test tube containing the enzyme and the inhibitor without the compound was taken as 100%.
MTT cytotoxicity assay
The effects of samples on cell viability were investigated using the MTT assay,16 which indicated metabolically active cells. A known number of MCF7, U373, and C6 rat glioma cells were procured from the National Center for Cell Sciences, Pune, India, transferred into 96-well plates in a volume of 200 μL of culture medium, and incubated for 48 h before addition of the test compound. Cells were then exposed to known concentrations (200 μM and 400 μM) of the compound to be tested for 24 h at 37 °C. After exposure, the culture medium was removed, and 20 μL of MTT reagent (diluted in a culture medium, 5 mg mL–1) was added. After incubating for 4 h, the MTT/medium was removed, DMSO (100 μL) was added to each well, and the plates were agitated for 1 min. The absorbance of the coloured solution was measured using a 96-well plate reader (Victor3, Perkin Emler) at a wavelength of 570 nm. The results were evaluated by comparing the absorbance of the wells containing the sample in 0.1% DMSO with that of 0.1% DMSO alone as a control. The cell viability was estimated to be 100% in the solvent control, and the assay was performed in triplicates and repeated twice.
In silico molecular docking studies
An entirely in-house-developed drug discovery informatics system, OSIRIS, was used to perform ADMET-based calculations. It is a Java-based library layer that provides reusable chemoinformatics functionality, used to predict the toxicity risks and overall drug score in silico.24 The structure of diclofenac was drawn using the ChemBioDraw tool (ChemBioOffice Ultra 14.0 suite) to assign a proper 2D orientation, and each structure was checked for structural drawing errors. The energy of each molecule was minimized using ChemBio3D (ChemBioOffice Ultra 14.0 suite). The energy-minimized ligand molecules were then used as input for AutoDock Vina to carry out the docking simulation. The protein databank (PDB) coordinate file with the name ; 2B17.pdb was used as a receptor molecule. All the heteromolecules were removed from the receptor, and Swiss PDB Viewer was used automatically to rebuild the missing side chains in the receptor. The Graphical User Interface program MGL Tools was used to set the grid box for docking simulations. The grid was set such that it surrounded the region of interest (active site) in the selected macromolecule.
In the present study, the active site was selected based on the amino acid residues, which were involved in binding with diclofenac in complex with PLA2 obtained from PDB with the accession number 2B17. Therefore, the grid was centered in the region including all the eight amino acid residues Leu2, Ile19, Lys69, Phe5, Cys45, Cys29, Gly30, and His48 that surrounded the active site either with hydrophobic interactions or with hydrogen bonds.
The grid box volume was set to 14, 10, and 12 Å for x, y, and z dimensions, respectively, and the grid center was set to 49.704, 33.31, and 6.528 for x, y, and z centers, respectively, which covered all the 8 amino acid residues in the considered active pocket. AutoGrid 4.0 Program, supplied with AutoDock 4.0, was used to produce grid maps.16 The grid spacing between grid points was 1 Å. The docking algorithm provided with AutoDock Vina was used to search for the best-docked conformation between the ligand and protein. During the docking process, a maximum of 10 conformers were considered for each ligand. All the AutoDock docking runs were performed using the Corei7 Intel processor CPU with 8-GB DDR3l RAM. AutoDock Vina was compiled and the run under Windows 8.0 professional operating system. LigPlot+ was used to deduce the schematic of the interaction between the ligands and the target protein.
Results and discussion
Synthesis of (N-(4-chlorophenyl)-4-(2,3-dihydrobenzo[b][1,4]dioxine-2-carbonyl)piperazine-1-carbothioamide
In this study, (N-(4-chlorophenyl)-4-(2,3-dihydrobenzo[b][1,4]dioxine-2-carbonyl)piperazine-1-carbothioamide compound was synthesized for the first time, and the route of synthesis is depicted in Scheme 1. The predicted structure of the synthesized compound 3 was confirmed by spectral studies, as shown in Scheme 1 (Fig. 1).
Scheme 1.
Fig. 1. Schematic of synthesis of the novel molecule piperazine-1-carbothioamide (P1C).
The designated resonance peak bands in 1H NMR reveal the novel aromatic compound protons appearing in the range of δ 7.84–6.65 ppm as singlets, doublets, triplets, and multiplets in the spectra. The resonance that appeared in the range of δ 3.50–3.46 ppm was attributed to the piperazine protons. The molecular ion peak in the mass spectra confirmed the predicted compound, and the mass spectra showed that the M+ fragmentation peak was in agreement with the molecular formula; the synthesized compound 3 showed a value of 382.41 m/z in the spectrum, which was in accordance with the molecular formula C21H22N2O5. The elemental data matched with the experimental and the theoretically calculated values within ±0.4% (ESI†).
Synthesis of novel P1C-Tit*CAgNPs
The synthesis of novel P1C-Tit*CAgNPs was carried out in a vessel. The water-soluble chitosan reduces AgNO3 to form yellow-coloured chitosan silver nanoparticles (C@AgNPs), and then, P1C is attached to C@AgNPs by ultrasonification of the mixture for 3 h; the transition from colorless to yellow indicates the formation of P1C-Tit*CAgNPs as the predicted novel nanoparticles (Fig. 2A) (ESI†).
Fig. 2. Route of synthesis of P1C-Tit-CAgNPs (A) and UV absorbance (B).
Characterization of P1C-Tit*CAgNPs
UV-visible spectroscopy
The synthesized P1C-Tit*CAgNPs were confirmed using a UV-visible spectrophotometer. The nature of the spectra depends on the morphology, such as size, shape, composition, and stability, of the synthesized nanocomposite. Fig. 2B shows the UV spectra of P1C-Tit*CAgNP, P1C, chitosan, and C@AgNPs. The absorbance at 380 and 310 nm is due to π–π* excitation, indicating the purity of the synthesized piperazine compound. Further, C@AgNP exhibits a peak at 435 nm, indicating the presence of AgNPs. The uniformly dispersed P1C-Tit*CAgNPs reveal a well-defined SPR band at 424 nm, confirming the formation of P1C-Tit*CAgNPs; owing to coherent excitation of the interacting electromagnetic field, a strong absorption band in the visible region is observed, which is attributed to the silver metal NPs, and the abovementioned color transition is absent in the case of the bulk metal.
SEM and TEM
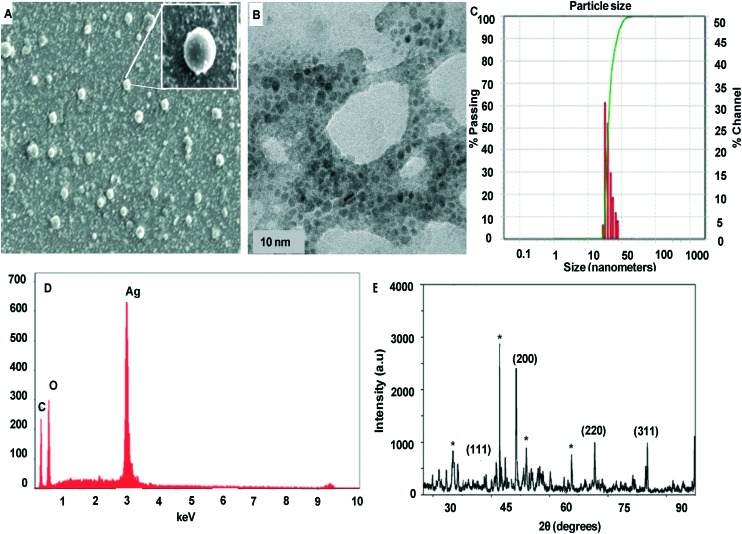
Fig. 3A and B show the surface morphology of P1C*C@AgNPs. It can be seen that the P1C-Tit*CAgNPs are spherical in shape with uniform dispersion. This indicates that the synthesis method employed herein can produce nanoparticles with tunable morphology.
Fig. 3. Characterization of the synthesized P1C-Tit*CAgNPs. The synthesized nanoparticle was characterized: shape by (A) SEM and (B) TEM, size by (C) DLS, (D) purity by EDX, and crystalline nature by (E) XRD.
DLS
The synthesized P1C-Tit*CAgNPs showed a good dispersive nature in the respective solvents. The DLS analysis revealed that the average particle size was 28.56 nm (Fig. 3C).
EDX
The EDX spectrum of P1C-Tit*CAgNPs is depicted in Fig. 3D. A high-intensity peak at 3 keV can be observed, attributed to the presence of AgNPs. Further, the presence of C and O can be attributed to chitosan and the synthesized molecule.
XRD
The crystalline nature of the synthesized P1C-Tit*CAgNPs was analyzed using XRD. Fig. 3E shows the powder XRD pattern for the P1C-Tit*CAgNPs. The spectral peaks at 38.36, 44.54, 64.76, and 77.6 degrees are indexed to be lattice planes with Miller indices (111), (200), (220), and (311), respectively, which are attributed to the presence of AgNPs.
Biology
Antioxidant activity
Herein, the radical scavenging activity (RSA) of the synthesized novel compound and nanoparticles was analyzed to confirm their antioxidant property. The DPPH radical scavenging activity, iron chelating activity, and nitric oxide scavenging capacity were determined for the abovementioned samples. Table 1 represents the neutralizing properties of the samples, indicating their IC50 values as compared to those of the standard. The nanoparticle-linked P1C novel molecule was significantly involved in the RSA as compared to its unbound form (Table 1).
Table 1. Antioxidant activity of the synthesized samples.
| Samples | IC50 value (μg mL–1) |
||
| DPPH assay | Fe2+ ion chelating assay | Nitric oxide assay | |
| P1C | 60.53 ± 0.2 | 19.44 ± 0.1 | 18.90 ± 0.1 |
| CAgNPs | 32.51 ± 0.4 | 40.10 ± 0.3 | 19.32 ± 0.4 |
| P1C-Tit*CAgNPs | 5.20 ± 0.1 | 15.62 ± 0.1 | 10.23 ± 0.1 |
| Standard Vit C | 20.11 ± 0.1 | 26.10 ± 0.4 | 17.60 ± 0.3 |
AAPH-induced hemolysis assay
In the present study, P1C-linked thiourea with chitosan-silver nanoparticles (P1C-Tit*CAgNPs) showed dose-dependent protection as an anti-hemolytic agent against AAPH (100 mM)-induced hemolysis; Table 2 provides information about the samples involved in the protection of RBC membrane destabilization by AAPH. The synthesized P1C-Tit*CAgNPs (55.54 μg mL–1) showed excellent anti-hemolytic property over AAPH as compared to the parental compound P1C in this study. The IC50 value of P1C-Tit*CAgNPs was found to be promising against AAPH as compared to that of the control in the in vitro experiment (Table 2).
Table 2. Anti-hemolytic property of the synthesized samples.
| Samples | IC50 (μg mL–1) |
| P1C | 91.84 ± 0.4 |
| CAgNPs | 73.50 ± 0.1 |
| P1C-Tit*CAgNPs | 55.54 ± 0.3 |
Effect of P1C-Tit*CAgNPs on erythrocyte membranemorphology
Further, the role of P1C-Tit*CAgNPs was studied by investigating the morphological changes in erythrocytes using AFM and SEM analysis; erythrocytes with different treatments, such as untreated RBCs (control), AAPH-treated RBCs, P1C-Tit*CAgNP-treated RBCs, and RBCs treated with AAPH-pretreated P1C-Tit*CAgNPs, were used, and the AFM study revealed that the roughness, thickness, and waviness of erythrocyte surface were controlled by the pretreated P1C-Tit*CAgNPs rather than by AAPH-treated RBCs (Fig. 4). The height, thickness, and average roughness of AAPH-treated erythrocytes (blank) were 35.0 nM, 3.5 nM, and 4.49 nm, respectively (Fig. 4A, a–c), and multiple peaks were found for waviness (Fig. 4Ad). On the other hand, further treatment of erythrocytes with P1C-Tit*CAgNPs and AAPH induction showed that the height was 1.14 μm (Fig. 4B, a); similarly, the average roughness was 0.53 nM (Fig. 4B, b), thickness was 262 nM (Fig. 4B, c), and waviness was found to exhibit a single peak in P1C-Tit*CAgNP-treated samples (Fig. 4B, d). Further, the erythrocytes were observed by SEM, and it revealed a clear difference in their effects on RBC membrane morphology, i.e., erythrocytes after treatment with AAPH at lower and higher doses showed shrinkage, and P1C exhibits membrane protection from the AAPH damage in given time as compared to the control (Fig. 5A–D). The P1C-Tit*CAgNP treatment in the presence of AAPH resulted in a clear unaltered erythrocyte membrane, as observed by SEM analysis (Fig. 5E and F). This shows the anti-hemolytic nature of P1C-Tit*CAgNPs, which is due to the antioxidant and erythrocyte protective properties of P1C on C@AgNPs.
Fig. 4. Morphological observation of erythrocytes by atomic force microscopic (AFM) analysis (park NX 10, Italy). (A) Erythrocytes treated with 100 mM 2,2-azobis(2-amidinopropane) dihydrochloride for 2 h. (B) Erythrocytes treated with P1C-Tit*CAgNPs and 100 mM 2,2-azobis(2-amidinopropane) dihydrochloride induced for 2 h. a–c indicate the height, thickness, and average roughness of the erythrocytes, respectively.
Fig. 5. Morphological observation of erythrocytes by SEM (Ziuss). (A) Indicates control without treatment, (B and C) erythrocytes treated with 50 and 100 mM AAPH for 2 h, (D) erythrocytes treated with P1C with 100 mM AAPH, and (E and F) erythrocytes treated with P1C-Tit*CAgNPs followed by 100 mM AAPH treatment for 2 h.
Lactate dehydrogenase (LDH) assay
Lactate dehydrogenase (LDH) enzyme is found in both animals and humans, which plays an important role in gluconeogenesis; moreover, it is used as a marker in the inflammation process since LDH is released outside the cells upon cell destruction. In this study, AAPH-induced erythrocyte membrane alteration and release of LDH were confirmed, and it was found that the treatment of P1C and P1C-Tit*CAgNPs followed by AAPH treatment controlled the RBC cell membrane stress and damage, which resulted in a decrease in the extracellular LDH level to 52.5 ± 0.6% and 29.8 ± 0.3%, respectively, at a concentration of 100 μg mL–1; the P1C-Tit*CAgNP-treated cells showed a decrease in the level of LDH activity as compared to the control and standard Vitamin C (24.3 ± 0.2%)-treated cells at 100 μg mL–1 (Fig. 4 and Table 3).
Table 3. Effect of nanoparticles on LDH activity.
| Sample | Concentration of compound (μg mL–1) | % inhibition |
| Blank | — | 100.00 ± 0.1 |
| Std (Vit. C) | 100 | 24.30 ± 0.2 |
| P1C | 100 | 52.50 ± 0.6 |
| CAgNPs | 100 | 70.10 ± 0.5 |
| P1C-Tit*CAgNPs | 20 | 94.00 ± 0.6 |
| 40 | 76.60 ± 0.5 | |
| 60 | 57.30 ± 0.4 | |
| 80 | 42.40 ± 0.2 | |
| 100 | 29.80 ± 0.3 |
Phospholipase A2 (PLA2) inhibition: an in vitro study
Phospholipase A2 (PLA2) is an enzyme responsible for hydrolyzing the sn-2 fatty acids of membrane phospholipids, which leads to the production of free fatty acid, in particular arachidonic acid, and a lysophospholipid that acts as an inflammatory mediator like eicosanoids or platelet-activating factor (PAF) to activate the inflammation pathway. This study elucidated the synthesized novel compound as an anti-inflammatory candidate by confirming its PLA2 enzyme inhibition in vitro. The IC50 value of the synthesized novel compound P1Cs was 5.10 ± 0.3 μg mL–1 against the enzyme PLA2 as compared to that of the standard drug diclofenac, having inhibitory action at 70.8 ± 0.3 μg mL–1. Moreover, the synthesized novel P1C-Tit*CAgNPs exhibited significant action against PLA2 activity due to the presence of P1C (Table 4). This indicated that the P1C-Tit*CAgNPs containing P1C had significant action directly on PLA2 and not on the downstream cyclooxygenases.
Table 4. Phospholipase A2 (PLA2) enzyme inhibitory activity.
| Samples | PLA2 IC50 (μg mL–1) |
| P1C | 5.10 ± 0.3 |
| CAgNPs | 27.60 ± 0.6 |
| P1C-Tit*CAgNPs | 18.70 ± 0.4 |
| Diclofenac | 70.80 ± 0.9 |
Anticancer activity
The cytotoxic natures of the samples were analyzed by the MTT assay using MCF7, U373, and C6 rat glioma cells. The survival of the cancer cells was tested against P1C, CAgNPs, P1C-Tit*CAgNPs, and standard doxorubicin after incubation of the cells for 24 h with increasing concentrations of these compounds. All samples showed anticancer property; interestingly, the synthesized P1C-Tit*CAgNPs showed significant action against MCF7, U373, and C6, having IC50 values of 88.6 ± 0.4, 74.6 ± 0.5, and 97.5 ± 0.4 μg mL–1 concentration, which were confirmed as compared to those of the standard drug doxorubicin (IC50 value 12.6 ± 0.2, 22.1 ± 0.3, and 10.4 ± 0.5 μg mL–1, respectively) (Table 5). The potent nature of the synthesized P1C-Tit*CAgNPs is due to the active agent P1C linked to the nanoparticles, as highlighted in this study.
Table 5. Anti-proliferative activity of nanoparticles against cancer cells.
| Compound | IC50 value in μg mL–1 against cancer cell lines |
||
| MCF7 | U373 | C6 | |
| P1C | 105.30 ± 0.8 | 91.20 ± 0.7 | 108.10 ± 0.5 |
| C-AgNPs | 176.21 ± 0.7 | 193.40 ± 0.9 | 159.10 ± 0.8 |
| P1C-Tit*CAgNPs | 88.60 ± 0.4 | 74.60 ± 0.5 | 97.50 ± 0.4 |
| Doxorubicin | 12.60 ± 0.2 | 22.10 ± 0.3 | 10.40 ± 0.5 |
Protective effect on the oxidation of biomolecules: an in vitro study
Plasmid DNA strand break assay
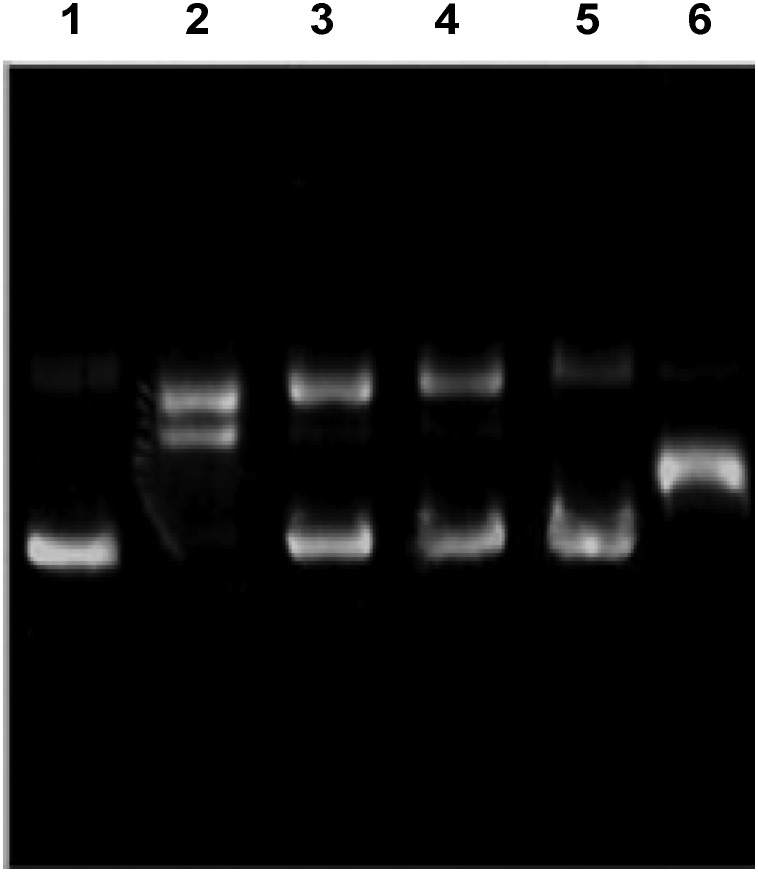
The effects of the synthesized P1C and P1C-Tit*CAgNPs are assessed for their role in AAPH-induced DNA damage on pRSET-A plasmid DNA. Both P1C and P1C-Tit*CAgNPs at different concentrations were treated with DNA followed by AAPH and incubation with the positive control. Agarose gel electrophoresis was carried out, and it showed undamaged native supercoiled circular DNA (ScDNA) moving faster than the faint opened circular DNA (OcDNA). It was confirmed that after treatment of DNA with AAPH, ScDNA was converted into its linear form, indicating a change in the conformation of ScDNA due to hydroxyl radical-mediated damage of DNA, whereas the sample P1C and P1C-Tit*CAgNPs exhibited protection to DNA against the damage induced by AAPH (Fig. 6).
Fig. 6. The DNA protection assay. The ScDNA was treated with P1C and P1C-Tit*CAgNPs followed by AAPH, and then agarose gel electrophoresis confirms the protection nature against AAPH-induced damage, which was plotted. 1. Control (only DNA), 2. DNA treated with AAPH, 3. P1C, 4. CAgNPs, and 5. P1CLt-CAgNPs and 6. DNA treated with gallic acid (GA) followed by AAPH.
Protein oxidation protection assay
The protein protection assay evaluates whether, the biological protein sample is oxidized or not after the suitable treatment was analyzed. In this study, the effects of P1C and P1C-Tit*CAgNPs on protein were determined by oxidation degradation of the BSA protein, which was observed via SDS-PAGE analysis. Fig. 7 indicates the oxidation damage of the protein BSA by AAPH (positive control) by about 80% in lane 2. Lane 1 indicates BSA (negative control), lane 3 indicates P1C, lane 4 indicates CAgNPs, and lane 5 shows the protecting role of P1C-Tit*CAgNPs against the oxidation of protein by AAPH as compared to that of the standard gallic acid (GA) in lane 6 (Fig. 7).
Fig. 7. Protein oxidation protective effect of P1C-Tit*CAgNPs. The azo compound AAPH was given to induce protein oxidation of bovine serum albumin (BSA) and it was further protected by different samples (1 to 6) to understand the role of treated samples on protein oxidation, which was further analyzed by polyacrylamide gel electrophoresis.
Molecular docking studies: in silico validation
Considering the results of the abovementioned in vitro experiments, it was thought worthy to perform molecular docking studies by substantiating the in vivo results by in silico validation. The synthesized novel molecule P1C was subjected to molecular docking studies, and it was demonstrated that the compound P1C showed binding only to the specific receptor PLA2. PLA2 is the target receptor, and comparative and automated docking studies with newly synthesized lead molecules were performed to determine the best in silico conformation. The comparative docking of the receptor PLA2 with diclofenac and the synthesized molecules exhibited well-established bonds with one or more amino acids in the receptor-active pocket. Table 6 shows the binding energies and details of H-bond formation with the target protein molecule. Considering the ligand–protein interactions and binding affinity, molecule 3 (P1C) presented better interactions with PLA2 as compared to the standard diclofenac (Fig. 8 and 9). Fig. 8 represents the extrapolation of 2D interaction of compound 3 (P1C). Comparisons were made on the basis of hydrogen bonding and hydrophobic interactions, as shown in Fig. 8. The common interacting hydrophobic residues are encircled in red colour. The 3D interaction is also represented in Fig. 8 using the educational version of PyMol. The ligands are represented in green colour, H-bonds with their respective distances are represented by black-colored lines, and the interacting residues are represented in the CMYK color model with the ball and stick representation.
Table 6. The molecular docking studies. The synthesized compound P1C showing best pose with target PLA2 interaction and its values are represented.
| Ligand | Affinity (kcal mol–1) | H-bonds | H-bond length (Å) | H-bond between | Hydrophobic residues |
| 7f | –8.1 | 2 | 3.00 | 7f:O2::His48:ND1 | Phe106, Cys45, Leu2, Ile19, Gly6, Tyr22, Gly30, Phe5 |
| 3.21 | 7f:O1::Lys69:NZ |
Fig. 8. The molecular docking best pose. The comparative representation of 2D and 3D interactions of compound 3 P1C (A and B).
Fig. 9. The molecular docking best pose. The comparative representation of 2D and 3D interactions of the compound diclofenac (A and B).
Discussion
Recent investigations have explored a number of natural and synthetic active molecules for the treatment of health complications and diseases such as cancer, inflammatory disorder, diabetes, sepsis, and neurological disorders.25–28 The derivatives of natural compounds are also involved in chemotherapy to cure the major health issues. This study investigated the synthesis of the novel compound P1C and its biological property as an anti-inflammatory candidate. The synthesized P1C-Tit*CAgNPs showed small, cationic, and monodisperse nature, having significant antioxidant properties along with neutralizing properties for free radicals.16 The generation of oxidative stress led to a number of diseases, which in turn damage a number of biomolecules and cause many severe complications. The present study confirmed that AAPH-induced stress, damage to erythrocytes and DNA, and protein oxidation were significantly inhibited by the P1C-Tit*CAgNPs, successfully validated by the LDH assay by studying the LDH enzyme activity. This shows the antioxidant nature of the synthesized nanoparticles P1C-Lt-CAgNPs, correlating with other studies.29–31 AAPH induces RBC membrane lipid peroxidation of polyunsaturated lipids, which in turn leads to leakage of inflammatory agents to induce inflammation. To inactivate the inflammatory agents, many researchers designed a number of synthetic analog targets for PLA2 or cyclooxygenases (COX1 and COX2).32 Herein, we designed and synthesized novel P1C-Tit*CAgNPs, which is significantly inhibited the PLA2 enzyme in vitro. Also, it was validated by molecular docking study for the pure compound P1C, and showed the P1C best pose aligned to PLA2 compared to standard drug. However, according to literature cyclooxygenases COX1 and COX2 involved in the inflammatory pathway but, in this study the synthesized compound did not binding affinity to COX1 and COX2. This confirmed that, the designed compound was specific to the PLA2 which intern suggests the route for synthesis of target drug.33–36 The designed compound 3 (P1C) and P1C-Tit*CAgNPs also showed moderate anticancer properties without affecting the normal physiology, and their inhibitory effects on the inflammatory progression were validated in this study.
Conclusion
Via the present novel approach, we successfully designed a compound, P1C, and successfully synthesized P1C-Tit*CAgNPs with a good compatible nature. The synthesized compound P1C and P1C-Tit*CAgNPs showed significant radical scavenging properties, inhibiting protein oxidation and DNA damage and maintaining the erythrocyte membrane structure in the presence of AAPH stress inducer, which were distinguishable. Particularly, P1C and P1C-Tit*CAgNPs inhibited PLA2 involved in the inflammation progression, and the significant anticancer property was credited to P1C and P1C-Tit*CAgNPs. The synthesized P1C particularly best matching to the PLA2 target has many clinical applications. Thus, the present investigation concludes that the development of nontoxic and target-specific inhibition of enzymes involved in many life-threatening complications can be easily achieved by synthesizing novel molecules, and the use of these molecules in the nano form has been warranted for the future inflammation chemotherapy.
Disclosure statement
The authors declare no competing financial interest. The authors alone are responsible for the content and writing of this article.
Conflicts of interest
The authors declare no competing interests.
Supplementary Material
Acknowledgments
We thank TEQIP-II, SJCE for providing financial assistance, and IOE, University of Mysore, Mysuru for providing instrumentation facility. We are also grateful to the Wuhan applied fundamental research of Wuhan Science and Technology Bureau (Grant NO. 2017060201010216) and Wuhan University of Technology.
Footnotes
†Electronic supplementary information (ESI) available. See DOI: 10.1039/c7md00628d
References
- Friedman A. J., Phan J., Schairer D. O., Champer J., Qin M., Pirouz A., Blecher-Paz K., Oren A., Liu P. T., Modlin R. L., Kim J. J. Invest. Dermatol. 2013;133:1231–1239. doi: 10.1038/jid.2012.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blecher K., Nasir A., Friedman A. Virulence. 2011;2:395–401. doi: 10.4161/viru.2.5.17035. [DOI] [PubMed] [Google Scholar]
- Kohl Y., Kaiser C., Bost W., Stracke F., Fournelle M., Wischke C., Thielecke H., Lendlein A., Kratzand K., Lemor R. Nanomedicine. 2011;7:228–237. doi: 10.1016/j.nano.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Yang F., Jin C., Subedi S., Lee C. L., Wang Q., Jiang Y., Lee J., Di Y., Fu D. Cancer Treat. Rev. 2012;38:566–579. doi: 10.1016/j.ctrv.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Mout R., Moyano D. F., Rana S., Rotello V. M. Chem. Soc. Rev. 2012;41:2539–2544. doi: 10.1039/c2cs15294k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S. J., Yin Y. G., Liu J. F. Environ. Sci.: Processes Impacts. 2013;15:78. doi: 10.1039/c2em30595j. [DOI] [PubMed] [Google Scholar]
- Luoma S. N., Silver Nanotechnologies and the Environment, Woodrow Wilson International Center for Scholars, Washington, DC, USA, 2008, p. 72. [Google Scholar]
- Shamberg M. E., Oldenburg S. J., Monteiro-Riviere N. A. Environ. Health Perspect. 2010;118:407–413. doi: 10.1289/ehp.0901398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E. J., Bae E., Yi J., Kim Y., Choi K., Lee S. H., Yoon J., Lee B. C., Park K. Environ. Toxicol. Pharmacol. 2010;30:162–168. doi: 10.1016/j.etap.2010.05.004. [DOI] [PubMed] [Google Scholar]
- David L., Moldovan B., Vulcu A., Olenic L., Perde-Schrepler M., Fischer-Fodor E., Florea A., Crisan M., Chiorean I., Clichici S., Filip G. A. Colloids Surf., B. 2014;122:767–777. doi: 10.1016/j.colsurfb.2014.08.018. [DOI] [PubMed] [Google Scholar]
- Venkatesan J., Alam M. S., Hong E. J., Kim S. K., Shim M. S. RSC Adv. 2016;6:79307–79316. [Google Scholar]
- Anusha J. R., Fleming A. T. Int. J. Biomater. 2016:1–9. doi: 10.1155/2016/5379424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kean T., Roth S., Thanou M. J. Controlled Release. 2005;103:643–653. doi: 10.1016/j.jconrel.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Friedman A. J., Phan J., Schairer D. O., Champer J., Qin M., Pirouz A., Kim J. J. Invest. Dermatol. 2013;133:1231–1239. doi: 10.1038/jid.2012.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Şenel S., McClure S. J. Adv. Drug Delivery Rev. 2004;56:1467–1480. doi: 10.1016/j.addr.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Karthik C. S., Manukumar H. M., Ananda A. P., Nagashree S., Rakesh K. P., Mallesha L., Qin Hua-Li, Umesha S., Mallu P., Krishnamurthy N. B. Int. J. Biol. Macromol. 2018;108:489–502. doi: 10.1016/j.ijbiomac.2017.12.045. [DOI] [PubMed] [Google Scholar]
- Karthik C. S., Mallesha L., Mallu P. Can. Chem. Trans. 2015;3:199–206. [Google Scholar]
- Mallesha L., Karthik C. S., Kumar C., Mallu P. Chem. Sci. Rev. Lett. 2016;5:183–190. [Google Scholar]
- Kandikattu H. K., Rachitha P., Krupashree K., Jayashree G. V., Abhishek V., Khanum F. Pathophysiology. 2015;22:165–173. doi: 10.1016/j.pathophys.2015.07.001. [DOI] [PubMed] [Google Scholar]
- Henkelman S., Rakhorst G., Blanton J., Oeveren W. Mater. Sci. Eng., C. 2009;29:1650–1654. [Google Scholar]
- Lui F., Ng T. B. Life Sci. 2000;66:725–735. doi: 10.1016/s0024-3205(99)00643-8. [DOI] [PubMed] [Google Scholar]
- DeBrosse C., Nanga R. P. R., Bagga P., Nath K., Haris M., Marincola F., Reddy R. Sci. Rep. 2016;6:19517. doi: 10.1038/srep19517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subhashree G. R., Haribabu J., Saranya S., Yuvaraj P., Anantha Krishnan D., Karvembu R., Gayathri D. J. Mol. Struct. 2017;1145:160–169. [Google Scholar]
- Sander T., Freyss J., Von Korff M., Reich J. R., Rufener C. J. Chem. Inf. Model. 2009;49:232–246. doi: 10.1021/ci800305f. [DOI] [PubMed] [Google Scholar]
- Manukumar H. M., Umesha S., Kumar H. N. Int. J. Biol. Macromol. 2017;102:1257–1265. doi: 10.1016/j.ijbiomac.2017.05.030. [DOI] [PubMed] [Google Scholar]
- Manukumar H. M., Umesha S. Acta Sci. Pol., Technol. Aliment. 2015;14(1):85–90. doi: 10.17306/J.AFS.2015.1.10. [DOI] [PubMed] [Google Scholar]
- Umesha S., Manukumar H. M., Chandrasekhar B. J. Sci. Food Agric. 2017;97(6):1698–1707. doi: 10.1002/jsfa.8144. [DOI] [PubMed] [Google Scholar]
- Manukumar H. M., Thribhuvan K. R. Int. J. Pharma Bio Sci. 2014;5(1):131–141. [Google Scholar]
- Andersen J. K., Oxidative stress in neurodegeneration: cause or consequence?, 2004. [DOI] [PubMed] [Google Scholar]
- Vetrani C., Costabile G., Di Marino L., Rivellese A. A. Int. J. Food Sci. Nutr. 2013;64:312–326. doi: 10.3109/09637486.2012.738651. [DOI] [PubMed] [Google Scholar]
- Kumar V., Sharma M., Lemos M., Shriram V. J. Pharm. Res. 2013;6:620–625. [Google Scholar]
- Simão A. N. C., Suzukawa A. A., Casado M. F., Oliveira R. D., Guarnier F. A., Cecchini R. Life Sci. 2006;78:1202–1210. doi: 10.1016/j.lfs.2005.06.047. [DOI] [PubMed] [Google Scholar]
- Gonçalves I., Edsfeldt A., Ko N. Y., Grufman H., Berg K., Björkbacka H., Adamski J. Arterioscler., Thromb., Vasc. Biol. 2012;32:1505–1512. doi: 10.1161/ATVBAHA.112.249854. [DOI] [PubMed] [Google Scholar]
- Tzeng S. F., Hsiao H. Y., Mak O. T. Curr. Drug Targets: Inflammation Allergy. 2005;4:335–340. doi: 10.2174/1568010054022051. [DOI] [PubMed] [Google Scholar]
- Haefner B. Drug Discovery Today. 2003;8:536–544. doi: 10.1016/s1359-6446(03)02713-2. [DOI] [PubMed] [Google Scholar]
- Manukumar H. M., Chandrasekhar B., Rakesh K. P., Ananda A. P., Nandhini M., Lalitha P., Sumathi S., Qin H. L., Umesha S. MedChemComm. 2017;8(12):2181–2194. doi: 10.1039/c7md00486a. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.