Abstract
Drosophila suzukii (the spotted-wing Drosophila) appears to be unsuitable for the development of most Drosophila larval endoparasitoids, be they sympatric or not. Here, we questioned the physiological bases of this widespread failure by characterizing the interactions between D. suzukii and various parasitoid species (Asobara japonica, Leptopilina boulardi, Leptopilina heterotoma and Leptopilina victoriae) and comparing them with those observed with D. melanogaster, a rather appropriate host. All parasitoids were able to oviposit in L1 and L2 larval stages of both hosts but their propensity to parasitize was higher on D. melanogaster. A. japonica and, to a much lesser extent, L. heterotoma, were the two species able to successfully develop in D. suzukii, the failure of the parasitism resulting either in the parasitoid encapsulation (notably with L. heterotoma) or the host and parasitoid deaths (especially with L. boulardi and L. victoriae). Compared to D. melanogaster, encapsulation in D. suzukii was strongly delayed and led, if successful, to the production of much larger capsules in surviving flies and, in the event of failure, to the death of both partners because of an uncontrolled melanization. The results thus revealed a different timing of the immune response to parasitoids in D. suzukii compared to D. melanogaster with a lose-lose outcome for parasitoids (generally unsuccessful development) and hosts (high mortality and possible reduction of the fitness of survivors). Finally, these results might suggest that some European endoparasitoids of Drosophila interact with this pest in the field in an unmeasurable way, since they kill their host without reproductive success.
Introduction
The spotted-wing Drosophila, Drosophila suzukii Matsumura (Diptera: Drosophilidae), is a Southeast Asian species of the melanogaster subgroup that has expanded rapidly in North-America and Europe since its first record in 2008 [1–3]. Unlike most fruit flies, D. suzukii has a modified ovipositor that allows egg laying through the skin of ripening fruits [4,5]. Damages are mainly due to larval feeding on the fruit pulp and pathogenic infections developing at the prick. D. suzukii is highly polyphagous, larvae developing in a wide range of fruit crops—mainly red fruits (raspberry, strawberry) and stone fruits (cherry, apricot, plum)—as well as wild fruits which act as reservoirs [6–8]. The control of this pest mainly relies on the use of chemical insecticides that increases production costs, results in the presence of residues in harvested fruits, and threaten biodiversity. The development of biological control methods is thus highly desirable in newly invaded countries. However, the use of natural enemies, such as parasitoid wasps, requires a thorough knowledge of their behavioral and physiological interactions with the fly.
Hymenopteran parasitic wasps lay eggs on (ectoparasitoids) or inside (endoparasitoids) arthropod hosts’ larvae or pupae that will be consumed by the developing parasitoid larva. Several endoparasitoid species are well known to use drosophila as hosts, including D. melanogaster, regulating their field populations [9,10]. The main fly immune response to endoparasitoids is encapsulation i.e. the deposit of several layers of specialized immune cells (plasmatocytes and lamellocytes) around the parasitoid egg, together with the activation of the phenoloxidase cascade, leading to the formation of a melanized capsule [11,12]. Most Drosophila parasitoid species nevertheless prevent encapsulation and regulate the host physiology by injecting venom along with the eggs [13–17]. Therefore, the outcome of parasitism is mainly determined by the interaction between the fly immune defense and the wasp venom virulence factors.
D. suzukii interaction with parasitoid wasps has recently received increased interest with a view of developing the biological control for this pest. Identified species reported to be associated with the fly in Asia include Asobara japonica (Braconidae), Ganaspis species (Figitidae), Leptopilina japonica (Figitidae) and pupal parasitoids from the Trichopria genus (Diapriidae) [18–26]. These parasitoids have a wide host range except the Asian Ganaspis species. In Europe and USA, the only species reported to successfully parasitize D. suzukii are pupal parasitoids, Trichopria drosophilae and Pachycrepoideus vindemmiae (Pteromalidae) [27–32]. Larval endoparasitoids of drosophilids, such as Leptopilina boulardi and L. heterotoma, were reported to be unsuccessful to reproduce on D. suzukii in laboratory conditions, despite a high infestation rate [27]. This strong D. suzukii resistance was proposed to result from the high hemocyte load in this species [28,29] since this trait was previously correlated with the percentage of successful encapsulation in species of the melanogaster subgroup [33]. Yet, the time-course and features of encapsulation of the different parasitoid strains (the term “strains” encompassing here and below both inter- and intra-specific variation) by D. suzukii have never been analyzed.
Here, we have investigated in more detail the behavioral and physiological interactions between D. suzukii and several larval parasitoid strains, either allopatric (European origin) or sympatric (Asiatic origin) to the fly. We first evaluated the acceptance and suitability of the fly larval stages for each parasitoid wasp and characterized the outcome of each interaction. Then, we analyzed the time-course of the host immune response to the parasitoid. Results identify precisely when parasitism fails and raise the question of the underlying processes. In addition to the variety of physiological interactions between larval endoparasitoids and D. suzukii, we highlight striking differences between D. suzukii and D. melanogaster in the establishment and time-course of the immune response to parasitoids, with a “delay” in the encapsulation response in the invasive Asian fly.
Materials and methods
Insect strains and rearing
The D. suzukii strain, obtained from a population collected in Sainte-Foy-lès-Lyon (Rhône, France), was kindly provided by Dr. R. Allemand (LBBE, University Lyon 1, France). The D. melanogaster strains Nasrallah (Gif stock no. 1333) and YR (Gif stock no. 1088) are respectively susceptible and resistant to the ISy strain of Leptopilina boulardi [34]. All flies laid eggs on standard Drosophila corn meal-yeast-agar-nipagin medium at 25 °C, and the vials were then transferred to 20 °C (12:12 L: D photoperiod).
The L. boulardi ISy (Lby, Gif stock no. 486) and ISm (Lbm, Gif stock no. 431) isofemale lines were previously described [35]. Briefly, Lby and Lbm were obtained from populations of Brazzaville (Congo) and Nasrallah (Tunisia), respectively. The L. boulardi strain Lb16 was founded in February 2014 from a field population collected in Dordogne (France). The European strain of L. heterotoma (LhGoth, Gif stock no. 548) was obtained from Gotheron (France). The Japanese strains of L. heterotoma (LhJapan) and L. victoriae (Lv) (described in [22]), were provided by Pr. M. T. Kimura (Hokkaido University, Japan). The thelytokous Japanese strain of Asobara japonica (Aj) [20] was kindly provided by the BIPE laboratory (University of Picardie-Jules Verne, France). All strains, except Lb16, had been maintained under laboratory conditions for several years at the time of the experiments. Parasitoids were reared on the susceptible D. melanogaster Nasrallah strain at 25 °C (12:12 L: D), 50–60% humidity. Emerged adult wasps were kept at 20 °C on agar medium with water and honey. Experiments were performed using 3 to 10 days-old females.
Oviposition behavior
D. suzukii females start laying eggs 72–96 hours after emergence and lay less than 10 eggs per day at 20–21 °C. For parasitism experiments, larvae were thus produced by transferring ten to twenty 5 days-old mated females into new vials for 96h (L1) or 120h (L2), respectively. L1 or L2 larvae were then collected manually. D. melanogaster mated adult flies (72-96h old) could lay eggs for 4h, and larvae were collected 48h (L1) and 72h (L2) later, respectively. The duration of the development stages was roughly similar for D. suzukii and D. melanogaster under our rearing conditions, as reported previously [36].
Host parasitism assays were performed in 24 mm (Ø) plastic dishes containing a 3–4 mm layer of standard medium at 25 °C. In each experiment, 20 D. suzukii or D. melanogaster L1 or L2 were exposed to a single naive mated female wasp during 4h. Fly larvae were dissected 48h later to evaluate the wasp propensity to parasitize (PP: proportion of female wasps having oviposited at least one egg) and the infestation rate (IR: percentage of successfully infested hosts whatever their status, dead or alive, or the wasp status, free floating or encapsulated egg, free, partially or completely encapsulated larva). The super-parasitism rate was consistently low (0–6%; mean of 2%) and did not significantly differ between host species. Super-parasitized larvae were thus considered to estimate the infestation rate but not to evaluate the parasitoid encapsulation rate.
Host suitability
Three criteria of host suitability—acceptability for oviposition, development success of the parasite larvae and encapsulation rate—were evaluated as follows. Three pools of 20 to 30 larvae (nl) were independently offered in a vial to batch of 3 female wasps of a given strain during 24 hours at 25 °C. The parasitoid offspring number (np) and the number of emerged flies (na) were counted for each replicate. Emerged flies were then dissected to evaluate the number of hosts containing an encapsulated parasitoid (capsule) (nc). Five control vials were used in parallel to estimate the median survival rate (s) of D. suzukii in the absence of parasitoids.
Two parameters were then calculated for each assay:
- The success of parasitism (SP) defined as the ratio between the number of emerged adult wasps and the estimated number of infested hosts [37]:
- The encapsulation rate (ER) defined as the ratio between the number of hosts containing a capsule and the estimated number of infested hosts:
These two estimates are valid under the assumption that encapsulation is the sole mechanism of host resistance to parasitoids.
Monitoring host-parasitoid immune interaction
A qualitative analysis was first performed to describe the time-course of the immune interaction of all the wasp strains with D. suzukii. To do so, we dissected D. suzukii L1 and L2 larvae 24, 48, 72, 96, 168 and 240 h after parasitism. As a control, we parasitized the D. melanogaster YR strain by the L. boulardi ISy line since the outcome of this interaction is the encapsulation of the parasitoid [38]. Based on this analysis, we identified three main types of interaction outcome: survival of the parasitoid with no encapsulation, death of both partners, and encapsulation. For a more detailed analysis of the interaction outcome, pools of 20 D. suzukii L1 or L2 were exposed to parasitism and dissected 48h (L1 and L2) or 72h later (only L1 since L2 larva were at the pre-pupal stage at that time). The percentages of alive or dead fly larvae containing free floating egg, free parasitoid larva, wasp egg or larva only surrounded by a thin coat of lightly-colored cells or with a few black spots (partial melanization), encapsulated eggs, and completely encapsulated parasitoid larva (fully melanized) were calculated.
Statistical analysis
Data on oviposition behavior and host suitability were analyzed using GLM with a binomial distribution of response variables (proportions). In all cases, a full model was used, including all possible explanatory variables (Parasitoid strain, Female experience, Host species and/or Host stage) and, when possible, their interactions (S1 File). A backward procedure was applied to select the most relevant model according to the Akaike Information Criterion (AIC), the distribution of the residuals being checked visually. An analysis of deviance was then performed to test for the influence of the selected explanatory variables and their interactions. Finally, post-hoc tests were performed in some cases using the Tukey HSD test. All the procedure was performed with the R software (http://www.R-project.org), its graphical interface “R commander” and related packages (in particular “MASS” and “multcomp”).
Results
Host acceptance
Propensity to parasitize (PP)
First, we evaluated the propensity of naive or trained female wasps to oviposit on L1 or L2 larvae of D. suzukii and D. melanogaster (propensity to parasitize, PP; Table 1 and S1 File). Data were analyzed using a full statistical model including all explanatory variables: “Host species”, “Host stage”, “Parasitoid strain”, and “Female training” (i.e. female parasitoids were individually allowed to previously parasitized the same number of hosts) but the “Host stage” variable was not kept in the model. Two variables, “Host species” (χ21df = 6.9; p = 0.008) and “Parasitoid strain” (χ26df = 69.9; p<10−3) had a significant effect. On average, the parasitoids PP was higher on D. melanogaster (87%) than D. suzukii (75%). Asobara japonica was the least “motivated” parasitoid with a lower PP than all other species/strains except Leptopilina victoriae. L. heterotoma Gotheron (LhGoth) and L. boulardi Lbm had a higher PP but it only significantly differed from that of L. victoriae. Finally, the “Female training” variable had no effect (S1 File; χ21df = 2.6; p = 0.108). Surprisingly, trained A. japonica females were reluctant to parasitize (PP = 0) in three out of the four Host species x Host stage combinations (Table 1).
Table 1. Parasitoid propensity to parasitize (PP) and infestation rate (IR).
| D. melanogaster | D. suzukii | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HOST STAGE | L1 | L2 | L1 | L2 | ||||||||||||||||
| n | PP | IR | n | PP | IR | n | PP | IR | n | PP | IR | |||||||||
| PARASITOID | mean | sd | mean | sd | mean | sd | mean | sd | ||||||||||||
| Aj | ||||||||||||||||||||
| naive | 8 | 0.50 | 0.62 | 0.16 | 9 | 0.22 | 0.95 | 0.00 | 11 | 0.36 | 0.40 | 0.20 | 23 | 0.39 | 0.66 | 0.24 | ||||
| trained | 3 | 0.00 | _ | _ | 2 | 1.00 | 0.13 | 0.13 | 3 | 0.00 | _ | _ | 3 | 0.00 | _ | _ | ||||
| Lv | ||||||||||||||||||||
| naive | 4 | 1.00 | 0.80 | 0.29 | 4 | 0.75 | 0.76 | 0.21 | 10 | 0.50 | 0.56 | 0.36 | 8 | 0.25 | 0.80 | 0.05 | ||||
| trained | 2 | 1.00 | 0.96 | 0.01 | 3 | 1.00 | 0.95 | 0.07 | 8 | 0.25 | 0.95 | 0.05 | 3 | 1.00 | 0.94 | 0.09 | ||||
| LhGoth | ||||||||||||||||||||
| naive | 6 | 0.67 | 0.92 | 0.05 | 4 | 1.00 | 0.65 | 0.06 | 10 | 0.90 | 0.81 | 0.07 | 4 | 1.00 | 0.91 | 0.09 | ||||
| trained | 3 | 1.00 | 0.92 | 0.02 | 3 | 1.00 | 0.95 | 0.04 | 3 | 1.00 | 0.95 | 0.00 | 3 | 1.00 | 0.62 | 0.27 | ||||
| LhJapan | ||||||||||||||||||||
| naive | 4 | 1.00 | 0.54 | 0.26 | 4 | 0.75 | 0.48 | 0.09 | 18 | 0.67 | 0.47 | 0.21 | 5 | 0.60 | 0.41 | 0.18 | ||||
| trained | 3 | 1.00 | 0.19 | 0.01 | 2 | 1.00 | 0.71 | 0.13 | 4 | 1.00 | 0.64 | 0.13 | 3 | 1.00 | 0.30 | 0.21 | ||||
| Lbm | ||||||||||||||||||||
| naive | 4 | 1.00 | 0.84 | 0.13 | 4 | 1.00 | 0.76 | 0.22 | 10 | 0.80 | 0.41 | 0.27 | 4 | 1.00 | 0.55 | 0.16 | ||||
| trained | 3 | 1.00 | 0.87 | 0.12 | 3 | 1.00 | 0.98 | 0.02 | 3 | 1.00 | 0.75 | 0.08 | 3 | 0.67 | 0.19 | 0.10 | ||||
| Lby | ||||||||||||||||||||
| naive | 4 | 0.75 | 0.79 | 0.08 | 4 | 0.75 | 0.60 | 0.28 | 10 | 0.60 | 0.51 | 0.33 | 4 | 0.75 | 0.22 | 0.18 | ||||
| trained | 3 | 1.00 | 0.83 | 0.09 | 3 | 1.00 | 0.87 | 0.10 | 4 | 1.00 | 0.71 | 0.07 | 2 | 1.00 | 0.23 | 0.07 | ||||
| Lb16 | ||||||||||||||||||||
| naive | 3 | 1.00 | 0.49 | 0.07 | 2 | 1.00 | 0.85 | 0.05 | 6 | 1.00 | 0.58 | 0.16 | 5 | 0.80 | 0.51 | 0.29 | ||||
| trained | 3 | 1.00 | 0.90 | 0.06 | 3 | 1.00 | 0.98 | 0.03 | 2 | 1.00 | 0.92 | 0.03 | 2 | 1.00 | 0.97 | 0.03 | ||||
Aj, Asobara japonica; Lv, Leptopilina victoriae; LhGoth, L. heterotoma Gotheron; LhJapan, L. heterotoma Japanese strain; Lbm, L. boulardi ISm strain; Lby, L. boulardi ISy strain; L16, L. boulardi strain 16. sd, standard deviation.
Infestation rate (IR)
Parasitoid infestation rates were calculated from all females that initiated oviposition (Table 1 and S1 File). The statistical analysis was performed using naive parasitoid females only, due to the reluctance of trained A. japonica to parasitize. The selection of the model and the analysis of deviance evidenced a complex situation with, notably, a significant triple interaction between the “Parasitoid strain”, the “Host species” and the “Host stage” (χ26df = 48.1; p<10−3). This interaction was also significant when considering only the three parasitoids that were sympatric to D. suzukii in the native area (A. japonica, L. victoriae, L. heterotoma Japan). This suggests that each parasitoid species has its own specificity with regard to the host species and/or stage (χ22df = 9.4; p = 0.009). Interestingly, A. japonica and L. victoriae, the two species with the lowest propensity to parasitize, had fairly high infestation rates on D. suzukii, similar to other parasitoid species.
D. suzukii suitability as a host
Overall parasitism success
A. japonica, and to a lesser extent, L. heterotoma (LhGoth and LhJapan) were the only species that produced offspring from D. suzukii (Table 2; S1 Fig). Accordingly, the “Parasitoid strain” variable was highly significant (χ26df = 40.5; p<10−3) while the “Host stage” (L1 vs L2 larvae) was not kept in the final model (S1 File). We estimated that 41% and 55% of A. japonica eggs successfully developed in mono-parasitized D. suzukii L1 and L2 larvae, respectively, the alternate outcome being the death of both partners.
Table 2. Suitability of D. suzukii L1 and L2 larval stages for the parasitoid species/strains.
| HOST STAGE | L1 | L2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PARASITOID | Ability | Offspring mean (sd) | Success mean (sd) | Sensitivity | Encapsulation mean (sd) |
Ability | Offspring mean (sd) | Success mean (sd) | Sensitivity | Encapsulation mean (sd) | ||
| Aj | 4/4 | 6.8 (3.0) | 0.41 (0.07) | 0/4 | 0.00 (0.00) | 4/4 | 10.3 (2.4) | 0.55 (0.10) | 0/4 | 0.00 (0.00) | ||
| Lv | 0/3 | 0.0 (0.0) | 0.00 (0.00) | 3/3 | 0.29 (0.16) | 0/3 | 0.0 (0.0) | 0.00 (0.00) | 3/3 | 0.16 (0.19) | ||
| LhGoth | 1/3 | 0.3 (0.6) | 0.01 (0.02) | 3/3 | 0.42 (0.25) | 1/3 | 1.0 (1.7) | 0.04 (0.06) | 3/3 | 0.70 (0.15) | ||
| LhJapan | 3/3 | 1.3 (0.6) | 0.07 (0.04) | 3/3 | 0.36 (0.18) | 2/3 | 1.7 (2.9) | 0.06 (0.10 | 3/3 | 0.41 (0.23) | ||
| Lbm | 0/3 | 0.0 (0.0) | 0.00 (0.00) | 3/3 | 0.09 (0.02) | 0/3 | 0.0 (0.0) | 0.00 (0.00) | 3/3 | 0.06 (0.02) | ||
| Lby | 0/4 | 0.0 (0.0) | 0.00 (0.00) | 4/4 | 0.24 (0.20) | 0/3 | 0.0 (0.0) | 0.00 (0.00) | 3/3 | 0.30 (0.20) | ||
| Lb16 | 0/3 | 0.0 (0.0) | 0.00 (0.00) | 3/3 | 0.08 (0.05) | 0/3 | 0.0 (0.0) | 0.00 (0.00) | 3/3 | 0.30 (0.05) | ||
Aj: Asobara japonica; Lv, Leptopilina victoriae; LhGoth, L. heterotoma Gotheron; LhJapan, L. heterotoma Japanese strain; Lbm, L. boulardi ISm strain; Lby, L. boulardi ISy strain; Lb16, L. boulardi strain 16. Ability: ability to develop in D. suzukii (number of successful females/number of tested females); Offspring: mean offspring number per female; Success: mean parasitism success per female; Sensitivity: sensitivity to encapsulation by D. suzukii (number of females with encapsulated offspring/number of tested females); Encapsulation: mean encapsulation rate. sd; standard deviation.
Encapsulation rate
The sensitivity to encapsulation was significantly influenced by the parasitoid strain (χ26df = 44.6; p<10−3; see S1 File), A. japonica being the only one not to be encapsulated at all (Table 2). When this species was discarded, the encapsulation rate (ER) still differed among parasitoid strains (S1 File; χ25df = 114.5, p<10−3). To a lesser extent, ER was also influenced by the host stage (χ21df = 5.7, p = 0.02), this parameter being globally higher for L2 host larva compared to L1 (S1 File; see also S1 Fig). This allowed identifying three different parasitoid groups: (i) L. boulardi strains Lbm and Lb16 with a low encapsulation rate (<30%) and no emergence; (ii) L boulardi Lby strain and L. victoriae with an intermediate encapsulation rate and no emergence (iii) L. heterotoma strains LhJapan and LhGoth with a high encapsulation rate (36–70%) and sporadic emergence of adult offspring (< 10%).
Parasitized D. suzukii experienced mortality in all interactions although with variation between replicates. We observed a high mortality rate when flies were parasitized by L. boulardi and L. victoriae (mean of 80%) compared to those attacked by L. heterotoma or A. japonica (mean of 45%) (S1 Fig).
Time-course of the encapsulation response
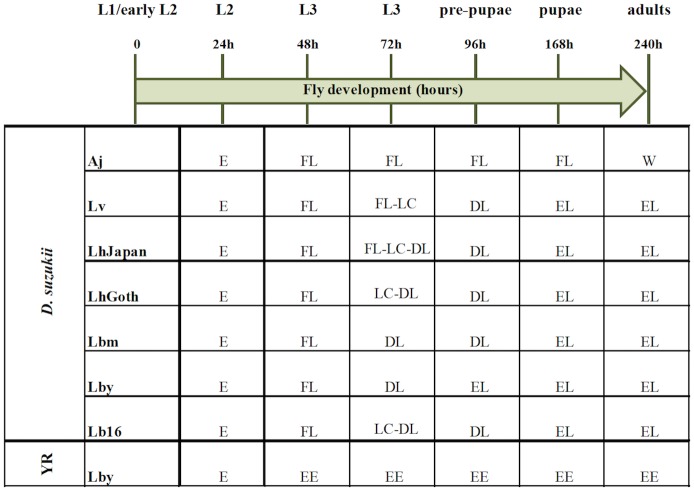
The qualitative time-course of the interaction (parasitism of L1/early L2 D. suzukii hosts) evidenced clear differences between parasitoid strains (Fig 1). The establishment of the immune response also differed from what was observed in the resistant D. melanogaster YR control (L1 or L2 flies parasitized by Lby), i.e. the formation of a complete melanized capsule as earlier as 48h post-parasitism [38] (Fig 2).
Fig 1. Time-course of the wasp-host interaction in parasitized D. suzukii larvae.
Live larvae of D. suzukii parasitized by the different parasitoid strains or D. melanogaster YR (YR) parasitized by L. boulardi ISy were dissected at different time (0h-240h) and the main observed steps of the encapsulation response are reported. E, free parasitoid egg; EE, encapsulated parasitoid egg; FL, free parasitoid larva; LC, free parasitoid larva with a thin coat of light-colored cells; DL, dead parasitoid larva; EL, encapsulated parasitoid larva; W, developing wasp. Aj, Asobara japonica; Lv, Leptopilina victoriae; LhGoth, L. heterotoma Gotheron; LhJapan, L. heterotoma Japanese strain; Lbm, L. boulardi ISm strain; Lby, L. boulardi ISy strain; Lb16, L. boulardi strain 16.
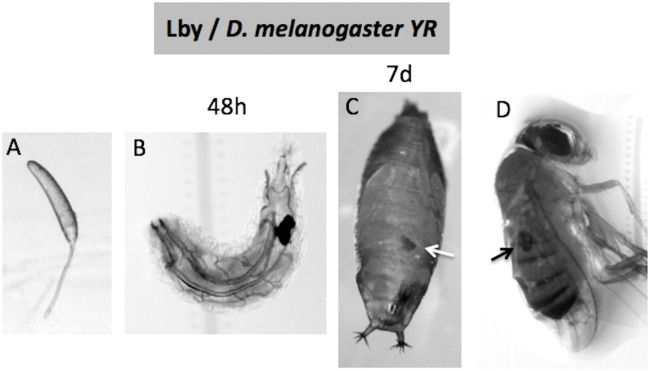
Fig 2. Encapsulation of L. boulardi ISy (Lby) eggs by the D. melanogaster YR strain.
D. melanogaster flies were observed and/or dissected at different times after parasitism: (A) after two hours, only free parasitoid eggs were recovered; (B) after 48h, almost all larvae contained an encapsulated egg. The capsules can be observed in the pupae 7 day (7d) post-parasitism (C) and in newborn flies (D) (arrows).
In D. suzukii, the immune response was only noticeable 72h post-parasitism (Fig 1). Three different outcomes were observed depending on the parasitoid species:
the absence of any visible host reaction leading to either the death of both partners or the successful development of A. japonica;
the hatching of most L. heterotoma eggs and surrounding of larvae by a thin coat of lightly colored cells starting 72h post-oviposition (Fig 3). Most parasitoid larvae were dead 96h post-oviposition and complete melanized capsules were observed at 168h. A small percentage of larvae (<10%) avoided encapsulation and successfully developed;
the hatching of most L. boulardi and L. victoriae eggs followed by the rapid death of the parasitoid larvae and their encapsulation 96h post-parasitism (Lby) or later (Lbm, Lb16) (see example for Lbm in Fig 3), leading to a high mortality rate at pupation.
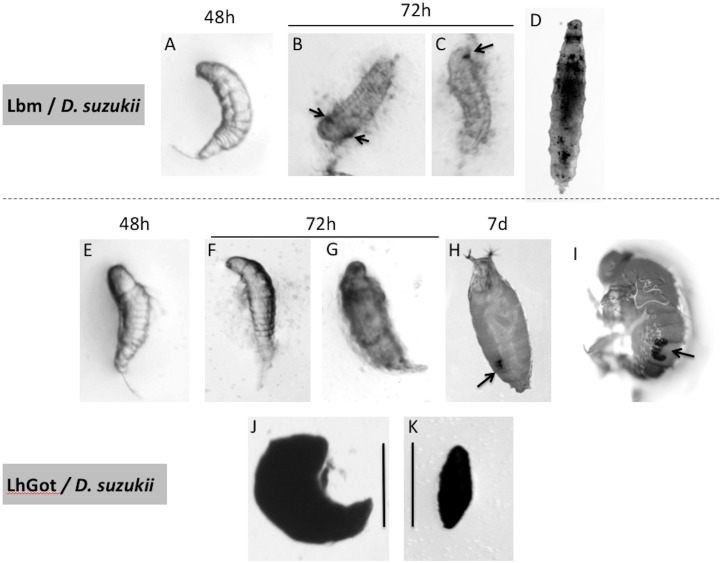
Fig 3. Timing of encapsulation of L. boulardi Ism (Lbm) and L. heterotoma Gotheron eggs in D. suzukii larvae.
Host larvae were observed and/or dissected at different times following parasitism by L. boulardi ISm (Lbm) (A-D) or L. heterotoma Gotheron (E-I). 48h post-parasitism, free-living larvae were mainly observed for the two parasitoids (A and E). At 72h, Lbm larvae were entangled in a thin layer of cells, and a few melanization spots (arrows) were observed (B, C), whereas only a few cells were found on L. heterotoma larvae, without melanization (F, G). Most larvae parasitized by Lbm showed an over-melanization response, and the fly was unable to pupate and died (D). Surviving larvae parasitized by L. heterotoma continued to develop and pupae (H) and emerged adult flies contained a capsule (I). The size of the capsule formed by D. suzukii against L. heterotoma (J) and by D. melanogaster YR against L. boulardi ISy (Lby) (K) are compared. Bar is 0.2 mm.
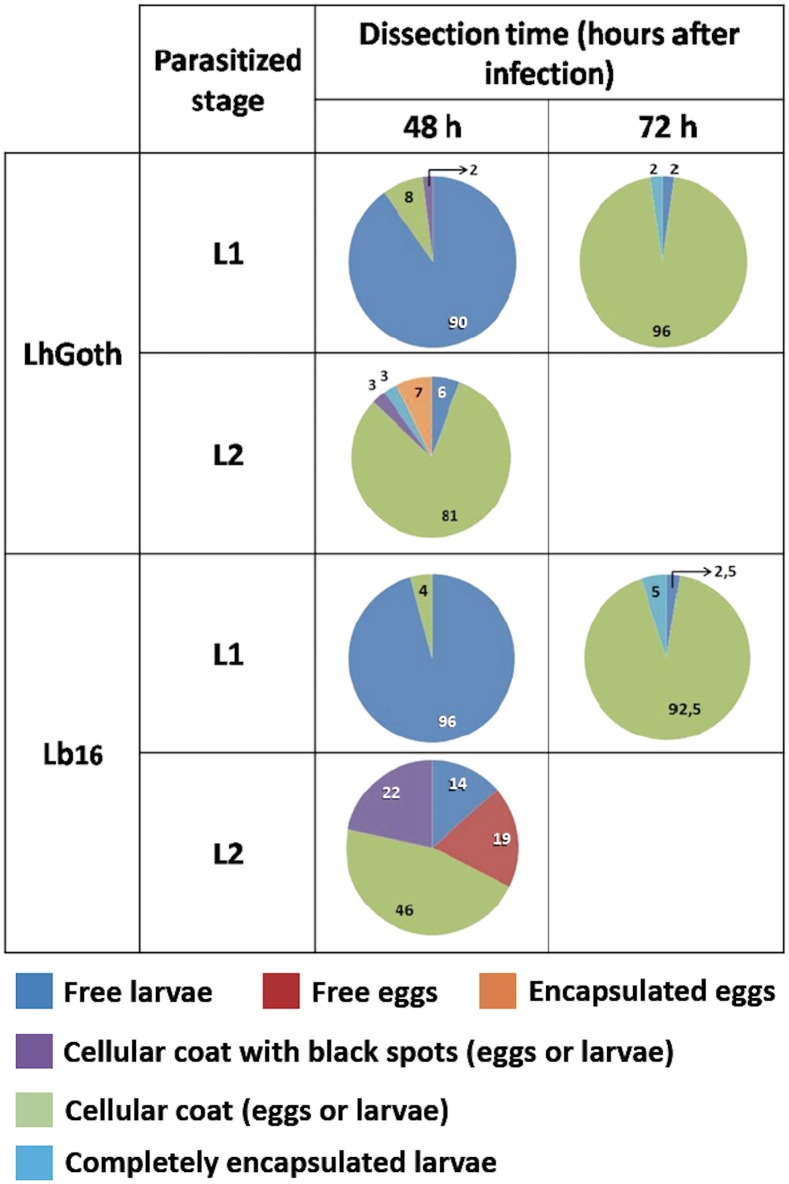
A more detailed and quantitative analysis was performed by dissecting parasitized L1 larvae 48h and 72h post-parasitism, and parasitized L2 larvae 48h post-parasitism. We focus here on the D. suzukii encapsulation response against LhGoth and Lb16 (Fig 4) as examples (see results for the other interactions in S2 Fig). In D. suzukii L1, 90% of the wasp larvae were free 48h post-parasitism, a small percentage being surrounded by a thin coat of lightly colored cells. 72h post-parasitism, a lightly colored cellular coat surrounded almost all larvae, with only 2% of LhGoth and 5% of Lb16 larvae being completely encapsulated. Interestingly, a small percent of LhGoth infested hosts still hosted free wasp larvae, which roughly corresponds to the success rate when parasitizing D. suzukii L1. A more rapid immune response occurred in parasitized L2 larvae as most LhGoth eggs/larvae were surrounded by a cellular coat 48h post-parasitism, but only 7% of larvae contained encapsulated eggs. The percentage of free wasp larvae was also congruent with LhGoth success rate on D. suzukii L2. Results were more complex with Lb16: 48h post-parasitism, about 70% of L2 hosts contained wasp larvae enclosed in a thin coat of lightly colored cells, 22% showing black melanized spots, while other L2 contained almost equally unhatched parasitoid eggs (typically considered as dead) and free larvae that will be encapsulated later.
Fig 4. Analysis of D. suzukii physiological interaction with L. heterotoma and L. boulardi parasitoids.
D. suzukii L1 and L2 larvae parasitized by the French strain of L. heterotoma (LhGoth) or the field strain of L. boulardi (Lb16) were dissected 48h (L1 and L2) or 72h (L1) post-parasitism to evaluate the state of the immune response, as described in Fig 3. Pie charts give the percentage of alive or dead fly larvae containing free parasitoid larvae, free floating eggs, wasp eggs or parasitoid larvae only surrounded by a thin coat of lightly-colored cells, wasp eggs or larvae partially melanized (coat of lightly-colored cells with a few black spots), completely encapsulated parasitoid larvae (fully melanized) and completely encapsulated eggs.
Discussion
Previous investigations on D. suzukii—parasitoids interactions were obtained mainly by quantifying the final outcome (numbers of emerged non-parasitized adult flies, adult flies with capsules or parasitoids [23–25,27–29]), without monitoring in details the physiological interactions. Here, we sought to better understand the success or failure of parasitism of sympatric and allopatric endoparasitoid wasps on the larval stages of D. suzukii and D. melanogaster through qualitative and quantitative analyses of the time course of the parasitoid development and the immune response of the fly larvae. Although the dissection of large numbers of parasitized D. suzukii larvae is time-consuming due to the low fertility of this species under laboratory conditions [39,40], the data obtained have brought new information compared to the emergence counting approach.
As expected, all parasitoid strains tested oviposited in L1 and L2 larvae of both host species and developed successfully on D. melanogaster Nasrallah, without being encapsulated or inducing mortality. A more contrasting situation was observed on D. suzukii. Although the parasitism of A. japonica induced a 35%-50% host mortality, its development was otherwise successful, consistent with previous laboratory data [28, 29]. However, under our conditions, females had a low propensity to parasitize (about 30%) which surprisingly fell to close to 0% for experienced individuals, suggesting a possible inhibition of the oviposition behavior due to wasp handling. Neither the increase in the duration of parasitism exposure (from 4h to 24h, with a day/night period) nor the use of larger boxes (more space) improved the PP, indicating a possible need for other stimuli, possibly associated with the host-plant interaction, for optimal egg-laying in D. suzukii. The parasitism of L. heterotoma (both French and Japanese strains) either induced host mortality or led to the emergence of flies carrying a capsule. However, although infrequent, successful development in D. suzukii was observed more often than reported by Chabert et al. [27] and at a level of 10–20% similar to that recently described for a European L. heterotoma field strain. On the other hand, it has been reported that strains Lh14 and Lhsw (from California and Sweden) have consistently failed on this species [28]. The success of L. heterotoma on D. suzukii thus seems to depend on the parasitoid strain but also possibly on the origin of D. suzukii and the laboratory conditions. Interestingly, the allopatric vs sympatric origin of the parasitoid was not relevant, showing the potential for local L. heterotoma to develop on a host never encountered. We did not observe an emergence of L. victoriae on D. suzukii despite a high infestation rate, especially in the L1 stage, in agreement with the results obtained with two other strains tested [28]. Finally, none of the L. boulardi strains was able to develop in D. suzukii, as previously reported [27,28], but they induced a high host mortality. Altogether, under laboratory conditions, Leptopilina strains parasitized the invasive Asian fly regardless of their origin and probability of success.
The outcome of the interaction of D. suzukii with larval parasitoids depends mainly on the physiological adequacy of the parasitoid and its effectiveness to overcome the immune response. All endoparasitoid species encapsulated by D. suzukii in this study belong to the Leptopilina genus. To be successful in their host, these species rely on active immunosuppressive strategies based on the rapid effect of virulence factors present in the venom they inject at oviposition [11,14,17]. However, despite their close relatedness, they differ widely in their virulence properties, venom components and host physiological targets [17,38,41]. It is therefore unlikely that “late” encapsulation in D. suzukii is mainly related to the effects associated with parasitoid venom. In addition, the medium to high mortality rates of parasitized D. suzukii larvae suggest that none of the parasitoids tested are truly adapted to this fly. A. japonica and L. heterotoma, both considered “generalists” [9,20,42], can produce offspring in D. suzukii but L. heterotoma is mainly unable to overcome the encapsulation response, with little variation between strains. Of the Leptopilina species, L. boulardi only develops in a few species of the D. melanogaster subgroup [43]. Parasitism consistently failed on D. suzukii, and the late L3 and pupal stages of the parasitized host experienced a high mortality rate. The dead host larvae do not contain capsules, but the parasitoid larvae that have died inside showed important signs of melanization, suggesting a strong incompatibility and an uncontrolled systemic immune response after parasitoid death. Interestingly, the Lbm and Lby strains, which differ in their virulence against D. melanogaster and D. yakuba and in their host range [44–45] also behave differently in D. suzukii. Lbm induces higher host mortality (> 90%) than Lby (70%-75% mortality and 25%-30% of emergence of flies with a capsule), in agreement with its narrower host range.
An intriguing question arose from the observation of the time-course of the interaction: why is the encapsulation of parasitoids systematically "delayed" in D. suzukii? Indeed, when parasitoid egg encapsulation occurs in D. melanogaster and in closely-related species, it is completed within 48h of parasitism [11,46–48] (see also Results). The parasitoid egg is killed quickly, usually before the capsule is completely melanized [14]. In contrast, in D. suzukii, hatched wasp eggs and wasp larvae continue to develop for 72 to 96 hours before dying, and encapsulation/melanisation occurs later. The first premise of encapsulation—the presence of immune cells on the parasitoid—is only detected 72h after parasitism.
In D. melanogaster, the recognition of a large foreign body such as the wasp egg is followed by a rapid increase in the number of circulating hemocytes, mainly phagocytic plasmatocytes and lamellocytes. Plasmatocytes first attach to the egg chorion, followed by the deposition of several layers of lamellocytes, the main cells involved in the capsule formation [11,12,49]. Lamellocytes differentiate after parasitism and their presence in the hemolymph has two possible origins: sessile hemocyte islets present under the larval cuticle or the lymph gland which is the larval hematopoietic organ [50–53]. In the hemolymph of D. suzukii, plasmatocyte- and lamellocyte-like cells, as well as larger plasmatocyte type cells called podocytes have been described, and the presence of crystal cells mainly involved in wound healing has been demonstrated [28,29]. The origin of these cells has not yet been studied in D. suzukii. Although variable, the much higher basal number of these hemocytes in this species than in D. melanogaster and other Drosophila species has been proposed as an explanation for the high resistance of this species to larval endoparasitoid wasps [28,29]. According, only lamellocytes appeared to increase in number in response to an immune challenge in D. suzukii [28]. Yet, Poyet et al. [29] reported that parasitism of A. japonica does not cause such an increase in D. suzukii, unlike that of L. heterotoma, which could be related to the success of the first species.
Here, we did not measure the lamellocytes number after parasitization. However, interestingly, we did not observe any important accumulation of hemocytes on the surface of parasitoid eggs during the first 48 hours. This suggests that although many plasmatocytes circulate, they may have a poor ability to recognize the egg and adhere to its surface, or that "activated" plasmatocytes with these properties are not yet present in circulation. It is also possible that the eggs of the different species of parasitoids studied, recognized as non-self in D. melanogaster, are not recognized in D. suzukii contrary to the larva once hatched. The masking of the egg against host immune cells is used by some species of parasitoids to avoid encapsulation [54] but this strategy has not been reported for the species used here. After hatching, the parasitoid larva would move sufficiently to prevent or further slow adhesion of host hemocytes. Accordingly, we observed a slow and late increase in the number of cells attached to the developing parasitoid, and it was mostly dying or dead larvae that were surrounded by cells.
The lag-time is even more noticeable on the melanization process. In D. melanogaster, the main source of the phenoloxidase involved in the melanization of the capsule (PPO3) are the lamellocytes [55,56]. In D. suzukii, the delayed adhesion of plasmatocytes could alter that of the lamellocytes and the subsequent release and activation of the PO. One recognized PO gene (proPO-A; DS10_00003111) and several potential others are present in the D. suzukii genome [57] (http://spottedwingflybase.org). However, there has been no study to assess whether they are equivalent to the three described D. melanogaster POs and what role(s) they may play in the melanization cascade. The late melanization response may also explain the high level of parasitoid-induced mortality we have recorded, particularly with L. boulardi and L. japonica strains, or the occasional parasitic success of L. heterotoma. It has indeed been reported that when the melanic encapsulation response is not fast or strong enough, the developing wasp can escape the capsule or kill the host [58,59]. At last, the presence of large capsules in adult D. suzukii flies due to delayed encapsulation could impact their fitness, as reported for D. melanogaster [60–62], a possible cost that is thus worth to explore.
Our results also question whether local parasitoids could somehow participate in controlling D. suzukii populations in the newly invaded areas. L. heterotoma and L. boulardi, among the most common parasitoid species of Drosophila larvae in Europe [63], show a high acceptance level of D. suzukii. Although L. boulardi never emerges from D. suzukii, it could have a significant impact on its populations since it kills 60 to 90% of the larval hosts. However, this effect will be difficult to assess in the field. L. heterotoma kills about 40% of D. suzukii larvae and is successful at a low rate in laboratory, which probably accounts for the undetected emergence of this parasitoid from field exposed larvae or sampled pupae in fruits [19,25,64,65]. In semi-natural conditions, we observed that L. heterotoma could parasitize D. suzukii within fruits since a low number of flies emerged with a capsule (unpublished observation) and some D. suzukii flies trapped in the field also harbored a capsule. This suggests that L. heterotoma may parasitize this fly in natura at a low level. Altogether, data suggest that local endoparasitoids may be involved in controlling this new pest by inducing non-reproductive host mortality and reducing fitness if they are able to adapt to its specific niche.
Supporting information
Proportions of death of both partners, emerged wasps, emerged flies with a capsule or without capsule (supposedly non-parasitized) following parasitism of L1 or L2 host larvae by Asobara japonica (Aj), Leptopilina heterotoma strains from Japan (LhJapan) and South France (Gotheron, LhGoth), the Leptopilina boulardi strains ISm (Lbm), ISy (Lby), and Lb16 (field strain)), and a Leptopilina victoriae Japanese strain (Lv). CNT: D. suzukii non-parasitized control flies.
(PDF)
Pools of 20 D. suzukii L1 or L2 larvae parasitized either by Asobara japonica (Aj), a Leptopilina heterotoma Japanese strain (LhJapan), the Leptopilina boulardi strains ISm (Lbm) and ISy (Lby), or a Leptopilina victoriae Japanese strain (Lv) were dissected 48h (L1 and L2) or 72h (L1) post-parasitism. Pie charts provide the percentage of alive or dead fly larvae containing free parasitoid larvae, free floating eggs, wasp eggs or larvae only surrounded by a thin coat of lightly-colored cells, wasp eggs or larvae partially melanized (coat of lightly-colored cells with a few black spots), and completely encapsulated parasitoid eggs or larvae (fully melanized).
(PDF)
(PDF)
Acknowledgments
We are highly grateful to Christian Rebuf and Marcel Thaon for help in insects rearing and to Séverine Lemauf for technical help. We also thank Pr. M. T. Kimura (Hokkaido University, Japan), Dr. R. Allemand (Laboratoire de Biométrie et Biologie Évolutive, Université Lyon 1) and the BIPE laboratory (Université Picardie-Jules Verne, Amiens) for providing biological material.
Data Availability
All data necessary can be found in the tables and the supplemental file.
Funding Statement
A. Iacovone was funded by the French Provence Alpes Côte d’Azur region through the Suzukill program and the Department of Plant Health (SPE) (http://www.spe.inra.fr) from the National Institute for Agronomic Research (INRA). This work was supported by the European Union’s Seventh Framework Program for research, technological development and demonstration, under grant agreement N◦ 613678 (DROPSA)(http://dropsaproject.eu) and performed in the context of the “Investments for the Future” LABEX SIGNALIFE: program reference ANR-11-LABX-0028 (http://signalife.unice.fr). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hauser M. A historic account of the invasion of Drosophila suzukii (Matsumura) (Diptera: Drosophilidae) in the continental United States, with remarks on their identification. Pest Manag Sci. 2011;67: 1352–1357. 10.1002/ps.2265 [DOI] [PubMed] [Google Scholar]
- 2.Calabria G, Máca J, Bächli G, Serra L, Pascual M. First records of the potential pest species Drosophila suzukii (Diptera: Drosophilidae) in Europe. J Appl Entomol. 2012;136: 139–147. [Google Scholar]
- 3.Asplen MK, Anfora G, Biondi A, Choi D-S, Chu D, Daane KM, et al. Invasion biology of spotted wing Drosophila (Drosophila suzukii): a global perspective and future priorities. J Pest Sci. 2015;27: 1–26. [Google Scholar]
- 4.Mitsui H, Takahashi KH, Kimura MT. Spatial distributions and clutch sizes of Drosophila species ovipositing on cherry fruits of different stages. Popul Ecol. 2006;48: 233–237. [Google Scholar]
- 5.Atallah J, Teixeira L, Salazar R, Zaragoza G, Kopp A. The making of a pest: the evolution of a fruit-penetrating ovipositor in Drosophila suzukii and related species. Proc Biol Sci. 2014;281(1781): 20132840 10.1098/rspb.2013.2840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walsh DB, Bolda MP, Goodhue RE, Dreves AJ, Lee J, Bruck DJ. Drosophila suzukii (Diptera: Drosophilidae): invasive pest of ripening soft fruit expanding its geographic range and damage potential. J Integr Pest Manag. 2011;2(1): 1–7. [Google Scholar]
- 7.Poyet M, Le Roux V, Gibert P, Meirland A, Prevost G, Eslin P, et al. The wide potential trophic niche of the Asiatic fruit fly Drosophila suzukii: The key of its invasion success in yemperate Europe? PLoS One. 2015;10(11): e0142785 10.1371/journal.pone.0142785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kenis M, Tonina L, Eschen R, van der Sluis B, Sancassani M, Mori N, et al. Non-crop plants used as hosts by Drosophila suzukii in Europe. J Pest Sci. 2016;89: 735–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carton Y, Boulétreau M, van Alphen JJM, van Lenteren JC. The Drosophila parasitic wasps In: Ashburner M, Carson L, Thompson JN, editors. The Genetics and Biology of Drosophila. London: Academic Press; 1986. pp. 347–394. [Google Scholar]
- 10.Godfray HCJ. Parasitoids: Behavioral and evolutionary ecology. Princeton: Princeton University Press; 1994. [Google Scholar]
- 11.Carton Y, Poirié M, Nappi AJ. Insect immune resistance to parasitoids. Insect Sci. 2008;15: 67–87. [Google Scholar]
- 12.Fauvarque M-O, Williams MJ. Drosophila cellular immunity: a story of migration and adhesion. J Cell Sci. 2011;124: 1373–1382. 10.1242/jcs.064592 [DOI] [PubMed] [Google Scholar]
- 13.Rivers DB, Ruggiero L, Hayes M. The ectoparasitic wasp Nasonia vitripennis (Walker) (Hymenoptera: Pteromalidae) differentially affects cells mediating the immune response of its flesh fly host, Sarcophaga bullata Parker (Diptera: Sarcophagidae). J Insect Physiol 2002;48:1053–64. [DOI] [PubMed] [Google Scholar]
- 14.Poirié M, Carton Y, Dubuffet A. Virulence strategies in parasitoid Hymenoptera as an example of adaptive diversity. C R Biol. 2009;332: 311–320. 10.1016/j.crvi.2008.09.004 [DOI] [PubMed] [Google Scholar]
- 15.Goecks J, Mortimer NT, Mobley JA, Bowersock GJ, Taylor J, Schlenke TA. Integrative Approach Reveals Composition of Endoparasitoid Wasp Venoms. PLoS One. 2013;8: e64125 10.1371/journal.pone.0064125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keebaugh ES, Schlenke TA. Insights from natural host-parasite interactions: The Drosophila model. Dev Comp Immunol. 2013;42: 111–123. 10.1016/j.dci.2013.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poirié M, Colinet D, Gatti J-L. Insights into function and evolution of parasitoid wasp venoms. Curr Opin Insect Sci. 2014;6: 52–60. [DOI] [PubMed] [Google Scholar]
- 18.Kanzawa T. Studies on Drosophila suzukii Mats. Yamanashi Agricultural Experiment Station, Kofu, Japan; 1939. [English abstract in: Rev Appl Entomol. 29: 622]. [Google Scholar]
- 19.Mitsui H, Van Achterberg K, Nordlander G, Kimura MT. Geographical distributions and host associations of larval parasitoids of frugivorous Drosophilidae in Japan. J Nat Hist. 2007;41: 1731–1738. [Google Scholar]
- 20.Ideo S, Watada M, Mitsui H, Kimura MT. Host range of Asobara japonica (Hymenoptera: Braconidae), a larval parasitoid of Drosophilid flies. Entomol Sci. 2008;11: 1–6. [Google Scholar]
- 21.Mitsui H, Kimura MT. Distribution, abundance and host association of two parasitoid species attacking frugivorous drosophilid larvae in central Japan. Eur J Entomol. 2010;107: 535–540. [Google Scholar]
- 22.Novković B, Mitsui H, Suwito A, Kimura MT. Taxonomy and phylogeny of Leptopilina species (Hymenoptera: Cynipoidea: Figitidae) attacking frugivorous drosophilid flies in Japan, with description of three new species. Entomol Sci. 2011;14: 333–346. [Google Scholar]
- 23.Kasuya N, Mitsui H, Ideo S, Watada M, Kimura MT. Ecological, morphological and molecular studies on Ganaspis individuals (Hymenoptera: Figitidae) attacking Drosophila suzukii (Diptera: Drosophilidae). Appl Entomol Zool. 2013;48: 87–92. [Google Scholar]
- 24.Nomano FY, Kasuya N, Matsuura A, Suwito A, Mitsui H, Buffington ML, et al. Genetic differentiation of Ganaspis brasiliensis (Hymenoptera: Figitidae) from East and Southeast Asia. Appl Entomol Zool. 2017;63: 742–749. [Google Scholar]
- 25.Girod P, Rossignaud L, Haye T, Turlings TCJ, Kenis M. Development of Asian parasitoids in larvae of Drosophila suzukii feeding on blueberry and artificial diet. J Appl Entomol. 2018;88: 1–12. [Google Scholar]
- 26.Wang X-G, Nance AH, Jones JML, Hoelmer KA, Daane KM. Aspects of the biology and reproductive strategy of two Asian larval parasitoids evaluated for classical biological control of Drosophila suzukii. Biol Control 2018;121: 58–65. [Google Scholar]
- 27.Chabert S, Allemand R, Poyet M, Eslin P, Gibert P. Ability of European parasitoids (Hymenoptera) to control a new invasive Asiatic pest, Drosophila suzukii. Biol Control. 2012;63: 40–47. [Google Scholar]
- 28.Kacsoh BZ, Schlenke TA. High hemocyte load is associated with increased resistance against parasitoids in Drosophila suzukii, a relative of D. melanogaster. PLoS One. 2012;7(4): e34721 10.1371/journal.pone.0034721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poyet M, Havard S, Prevost G, Chabrerie O, Doury G, Gibert P, et al. Resistance of Drosophila suzukii to the larval parasitoids Leptopilina heterotoma and Asobara japonica is related to haemocyte load. Physiol Entomol. 2013;38: 45–53. [Google Scholar]
- 30.Rossi-Stacconi MV, Grassi A, Dalton DT, Miller B, Ouantar M, Loni A, et al. First field records of Pachycrepoideus vindemiae as a parasitoid of Drosophila suzukii in European and Oregon small fruit production areas. Entomologia. 2013;1: 11–16. [Google Scholar]
- 31.Rossi-Stacconi MV, Amiresmaeili N, Biondi A, Carli C, Caruso S, Dindo ML, et al. Host location and dispersal ability of the cosmopolitan parasitoid Trichopria drosophilae released to control the invasive spotted wing Drosophila. Biol Control 2018;117: 188–196. [Google Scholar]
- 32.Wang X-G, Kaçar G, Biondi A, Daane KM. Foraging efficiency and outcomes of interactions of two pupal parasitoids attacking the invasive spotted wing drosophila. Biol Control. 2016;96: 64–71. [Google Scholar]
- 33.Eslin P, Prevost G. Hemocyte load and immune resistance to Asobara tabida are correlated in species of the Drosophila melanogaster subgroup. J Insect Physiol. 1998;44: 807–816. [DOI] [PubMed] [Google Scholar]
- 34.Carton Y, Frey F, Nappi AJ. Genetic determinism of the cellular immune reaction in Drosophila melanogaster. Heredity. 1992;69: 393–399. [DOI] [PubMed] [Google Scholar]
- 35.Dupas S, Brehélin M, Frey F, Carton Y. Immune suppressive virus-like particles in a Drosophila parasitoid: significance of their intraspecific morphological variations. Parasitology. 1996;113(13): 207–212. [DOI] [PubMed] [Google Scholar]
- 36.Lin QC, Zhai Y-F, Zhang AF, Men X-Y, Zhang X-Y, Zalom FG, et al. Comparative Developmental Times and Laboratory Life Tables for Drosophila suzukii and Drosophila melanogaster (Diptera: Drosophilidae). Fla Entomol 2014;97(4): 1434–1442. [Google Scholar]
- 37.Ris N, Allemand R, Fouillet P., Fleury F. The joint effect of temperature and host species induce complex genotype-by-environment interactions in the larval parasitoid of Drosophila, Leptopilina heterotoma (Hymenoptera: Figitidae). Oikos. 2004;106: 451–456. [Google Scholar]
- 38.Dubuffet A, Colinet D, Anselme C, Dupas S, Carton Y, Poirié M. Variation of Leptopilina boulardi success in Drosophila hosts: what is inside the black box? Adv Parasitol. 2009;70: 147–188. 10.1016/S0065-308X(09)70006-5 [DOI] [PubMed] [Google Scholar]
- 39.Emiljanowicz LM, Ryan GD, Langille A, Newman J. Development, Reproductive Output and Population Growth of the Fruit Fly Pest Drosophila suzukii (Diptera: Drosophilidae) on Artificial Diet. J Econ Entomol. 2014; 107(4): 1392–1398. [DOI] [PubMed] [Google Scholar]
- 40.Iacovone A, Girod P, Ris N, Weydert C, Gibert P, Poirié M, et al. Worldwide invasion by Drosophila suzukii: Does being the “cousin” of a model organism really help setting up biological control? Hopes, disenchantments and new perspectives. Rev Ecol Terre Vie 2015;70: 207–214. [Google Scholar]
- 41.Moreau S, Asgari S. Venom Proteins from Parasitoid Wasps and Their Biological Functions. Toxins (Basel) 2015;7: 2385–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jenni W. Beitrag zur morphologie und biologie der cynipide Pseudeucoila bochei weld, eines larvenparasiten von Drosophila melanogaster meig. Acta zool. 1951;32(3): 177–254. [Google Scholar]
- 43.Carton Y, Kitano H. Evolutionary relationships to parasitism by seven species of the Drosophila melanogaster subgroup. Biol J Linn Soc Lond. 1981;16: 227–241. [Google Scholar]
- 44.Dupas S, Carton Y, Poirié M. Genetic dimension of the coevolution of virulence-resistance in Drosophila—parasitoid wasp relationships. Heredity 2003;90: 84–89. 10.1038/sj.hdy.6800182 [DOI] [PubMed] [Google Scholar]
- 45.Dupas S, Poirié M, Frey F, Carton Y. Is parasitoid virulence against multiple hosts adaptive or constrained by phylogeny? A study of Leptopilina spp. (Hymenoptera: Figitidae)/Drosophila (Diptera: Drosophilidae) interactions. Ann Soc Entomol Fr. 2013;49: 222–231. [Google Scholar]
- 46.Nappi AJ, Streams FA. Abortive development of the cynipid parasite Pseudeucoila bochei (Hymenoptera) in species of the Drosophila melanica group. Ann Entomol Soc Am. 1970;63(1): 321–327. [Google Scholar]
- 47.Carton Y, Nappi AJ. Drosophila cellular immunity against parasitoids. Parasitol Today. 1997;13: 218–227. [DOI] [PubMed] [Google Scholar]
- 48.Dubuffet A, Doury G, Labrousse C, Drezen J-M, Carton Y, Poirié M. Variation of success of Leptopilina boulardi in Drosophila yakuba: the mechanisms explored. Dev Comp Immunol. 2008;32(6): 597–602. 10.1016/j.dci.2007.10.009 [DOI] [PubMed] [Google Scholar]
- 49.Rizki TM, Rizki RM. Lamellocyte differentiation in Drosophila larvae parasitized by Leptopilina. Dev Comp Immunol. 1992;16: 103–110. [DOI] [PubMed] [Google Scholar]
- 50.Stofanko M, Kwon SY, Badenhorst P. Lineage Tracing of Lamellocytes Demonstrates Drosophila Macrophage Plasticity. PLoS One. 2010;5: e14051 10.1371/journal.pone.0014051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krzemien J, Oyallon J, Crozatier M, Vincent A. Hematopoietic progenitors and hemocyte lineages in the Drosophila lymph gland. Dev Biol. 2010;346(2): 310–319. 10.1016/j.ydbio.2010.08.003 [DOI] [PubMed] [Google Scholar]
- 52.Honti V, Csordás G, Kurucz E, Márkus R, Andó I. The cell-mediated immunity of Drosophila melanogaster: hemocyte lineages, immune compartments, microanatomy and regulation. Dev Comp Immunol. 2014;42: 47–56. 10.1016/j.dci.2013.06.005 [DOI] [PubMed] [Google Scholar]
- 53.Anderl I, Vesala L, Ihalainen TO, Vanha-aho L-M, Andó I, Rämet M, et al. Transdifferentiation and Proliferation in Two Distinct Hemocyte Lineages in Drosophila melanogaster Larvae after Wasp Infection. PLoS Pathog. 2016;12: e1005746–34. 10.1371/journal.ppat.1005746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eslin P, Prevost G. Racing against host’s immunity defenses: a likely strategy for passive evasion of encapsulation in Asobara tabida parasitoids. J Insect Physiol. 2000;46: 1161–1167. [DOI] [PubMed] [Google Scholar]
- 55.Binggeli O, Neyen C, Poidevin M, Lemaitre B. Prophenoloxidase Activation Is Required for Survival to Microbial Infections in Drosophila. PLoS Pathog. 2014;10: e1004067 10.1371/journal.ppat.1004067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dudzic JP, Kondo S, Ueda R, Bergman CM, Lemaitre B. Drosophila innate immunity: regional and functional specialization of prophenoloxidases. BMC Biol. 2015;13: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chiu JC, Jiang X, Zhao L, Hamm CA, Cridland JM, Saelao P, et al. Genome of Drosophila suzukii, the Spotted Wing Drosophila. G3 (Bethesda). 2013;3(12): 2257–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Strand MR, Pech LL. Immunological basis for compatibility in parasitoid-host relationships. Annu Rev Entomol. 1995;40: 31–56. 10.1146/annurev.en.40.010195.000335 [DOI] [PubMed] [Google Scholar]
- 59.Salazar-Jaramillo L, Paspati A, De Zande Van L, Vermeulen CJ, Schwander T, Wertheim B. Evolution of a Cellular Immune Response in Drosophila: A Phenotypic and Genomic Comparative Analysis. Genome Biol Evol. 2014;6: 273–289. 10.1093/gbe/evu012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carton Y, David JR. Reduction of fitness in Drosophila adults surviving parasitism by a cynipid wasp. Experientia. 1983;39: 231–233. [Google Scholar]
- 61.Fellowes MDE, Kraaijeveld AR, Godfray HCJ. The relative fitness of Drosophila melanogaster (Diptera, Drosophilidae) that have successfully defended themselves against the parasitoid Asobara tabida (Hymenoptera, Braconidae). J Evol Biol. 1999;12: 123–128. [Google Scholar]
- 62.Kraaijeveld AR, Ferrari J, Godfray HCJ. Costs of resistance in insect-parasite and insect-parasitoid interactions. Parasitology. 2002;125: S71–S82. [DOI] [PubMed] [Google Scholar]
- 63.Allemand R, Fleury F, Lemaitre C, Boulétreau M. Population dynamics and competitive interactions in two species of Leptopilina (Hymenoptera: Figitidae) which parasitize Drosophila in the Rhône valley (S-E France). Ann Soc Entomol Fr. 1999;35: 97–103. [Google Scholar]
- 64.Cini A, Anfora G, Escudero-Colomar LA, Grassi A. Tracking the invasion of the alien fruit pest Drosophila suzukii in Europe. J Pest Sci. 2014;87(4): 559–566. [Google Scholar]
- 65.Kremmer L, Thaon M, Borowiec N, David J, Poirié M, Gatti J-L, et al. Field Monitoring of Drosophila suzukii and Associated Communities in South Eastern France as a Pre-Requisite for Classical Biological Control. Insects 2017;8(4): e124 10.3390/insects8040124 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Proportions of death of both partners, emerged wasps, emerged flies with a capsule or without capsule (supposedly non-parasitized) following parasitism of L1 or L2 host larvae by Asobara japonica (Aj), Leptopilina heterotoma strains from Japan (LhJapan) and South France (Gotheron, LhGoth), the Leptopilina boulardi strains ISm (Lbm), ISy (Lby), and Lb16 (field strain)), and a Leptopilina victoriae Japanese strain (Lv). CNT: D. suzukii non-parasitized control flies.
(PDF)
Pools of 20 D. suzukii L1 or L2 larvae parasitized either by Asobara japonica (Aj), a Leptopilina heterotoma Japanese strain (LhJapan), the Leptopilina boulardi strains ISm (Lbm) and ISy (Lby), or a Leptopilina victoriae Japanese strain (Lv) were dissected 48h (L1 and L2) or 72h (L1) post-parasitism. Pie charts provide the percentage of alive or dead fly larvae containing free parasitoid larvae, free floating eggs, wasp eggs or larvae only surrounded by a thin coat of lightly-colored cells, wasp eggs or larvae partially melanized (coat of lightly-colored cells with a few black spots), and completely encapsulated parasitoid eggs or larvae (fully melanized).
(PDF)
(PDF)
Data Availability Statement
All data necessary can be found in the tables and the supplemental file.