Alkylated guanidinium iminosugar derivatives bearing a pH sensitive orthoester moiety are potent and selective β-glucosidase inhibitors.
Alkylated guanidinium iminosugar derivatives bearing a pH sensitive orthoester moiety are potent and selective β-glucosidase inhibitors.
Abstract
Alkylated guanidino derivatives of 1,5-dideoxy-1,5-imino-d-xylitol bearing an orthoester moiety were prepared using a concise synthetic protocol. Inhibition assays with a panel of glycosidases revealed that one of the compounds prepared displays potent inhibition against human β-glucocerebrosidase (GBA) at pH 7.0 with IC50 values in the low nanomolar range. Notably, a significant drop in inhibitory activity is observed when the same compound is tested at pH 5.2. This pH sensitive activity is due to degradation of the orthoester functionality at lower pH accompanied by loss of the alkyl group. This approach provides a degree of control in tuning enzyme inhibition based on the local pH. Compounds like those here described may serve as tools for studying various lysosomal storage disorders such as Gaucher disease. In this regard, the most active compound was also evaluated as a potential pharmacological chaperone by assessing its effect on GBA activity in an assay employing fibroblasts from Gaucher patients.
In recent years, the iminosugars have emerged as a class of promising compounds in medicinal chemistry due to their therapeutic potential in the treatment of a variety of carbohydrate-mediated diseases.1–5 Certain iminosugars are highly potent and selective inhibitors of glycosidases and reversibly bind to their target enzyme through interactions involving the catalytic site or allosteric regions of the enzyme.6,7 Of particular interest are glycomimetics that comprise an endocyclic nitrogen, such as the naturally occurring 1-deoxynojirimycin (DNJ, 1, Fig. 1)8 as well as isofagomine (IFG, 3), 1,5-dideoxy-1,5-imino-d-xylitol (DIX, 4)9 and substituted variants thereof which often possess improved specificities and potent inhibition towards glycosidases.10–21
Fig. 1. A) Chemical structures of selected iminosugar-based glycosidase inhibitors. B) Structures of derivatives previously prepared in our group. C) General structures of orthoester thiourea DNJ compounds previously published (9) and orthoester rich guanidine DIX compounds (10, 11) presented in this work.
Previous investigations in our group have evaluated iminosugar analogues with a partially sp2 hybridized endocyclic nitrogen centre (compounds 6–8, Fig. 1).22,23 This structural feature affects both the conformation and charge delocalization of the endocyclic nitrogen and was found to result in changes in the glycosidase inhibition profile relative to the parent iminosugars. We recently reported the attempted synthesis of a series of alkylated DNJ guanidine compounds 6 and found that they were prone to spontaneous cyclization to generate the corresponding bicyclic isoureas 7.24 Gratifyingly, these compounds proved to be very potent and specific inhibitors of β-glucocerebrosidase (GBA, GCase, β-glucosidase, EC 3.2.1.45)25 an enzyme responsible for the onset of Gaucher disease.26–28 Gaucher disease is the most prevalent lysosomal storage disease (LSD) with 10 000 individuals affected worldwide and can result in the progressive accumulation of the undegraded glucosylceramide substrate leading to a variety of clinical symptoms.29–31
As a therapeutic strategy, iminosugars that bind a misfolded enzyme at neutral pH (pH 7.0) can provide an improvement in its folding and promote proper trafficking from the ER to the lysosome.32 Such inhibitors can be used as pharmacological chaperones, which serve as one of the main options in treating lysosomal storage disorders.33,34 Ideally, an iminosugar-based pharmacological chaperone should have a lower binding affinity for the target glycosidase in the acidic environment of the lysosome (pH 5.2) allowing it to dissociate from the complex after which the enzyme can go on to degrade its substrate. Furthermore, the high initial substrate concentration in the lysosome of a patient with an LSD can further promote dissociation of the pharmacological chaperone–enzyme complex. In this regard, pharmacological chaperone approaches offers promising opportunities in the treatment of a broad range of inherited LSDs.35 To this end, the development of potent, pH-dependent, glycosidase inhibitors presents an attractive target for the development of new therapeutics.36
Recently we described the synthesis and evaluation of a new class of stable, guanidine-modified, DIX analogues 8, both as GBA inhibitors and potential pharmacological chaperones.37 We were able to show that incorporating an N-alkylated guanidino moiety onto the DIX scaffold drastically improved inhibitor potency compared to N-alkylated DIX analogue 5 which is only a moderate glycosidase inhibitor.38 However, while DIX derived compounds such as 8 are potent, selective, and stable inhibitors of GBA at pH 7.0, they also maintain their inhibitory activity at pH 5.2. A similar lack of pH selective inhibition has also been implicated in the disappointing clinical trial failures of many other GBA inhibitors explored as pharmacological chaperones.39 In building upon the GBA inhibitors developed in our group we therefore set out to develop analogues that maintain potent activity at neutral pH but show a significant decrease in activity at acidic pH. In this regard we were drawn to the recent report of Ortiz Mellet and co-workers who described the use of an acid sensitive orthoester functionality, which allowed for pH control in the design of other glycosidase inhibitors.40 Specifically, they prepared the alkylated DNJ thiourea species 9 in which the alkyl was connected to the thiourea unit via the acid labile orthoester. This strategy led to GBA inhibitors with potent activity at pH 7.0 that was virtually abolished at pH 5.2 due to orthoester hydrolysis.40
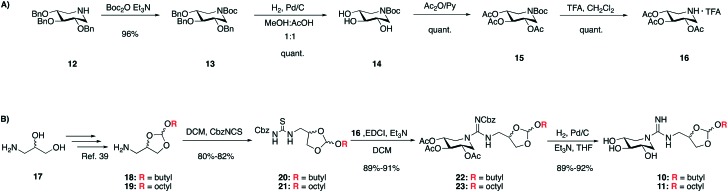
We here apply a similar strategy in modifying the novel class of N-alkylated guanidino DIX analogues recently developed by our group. In doing so we introduced an orthoester moiety between the exocyclic guanidine group and the alkyl to arrive at compounds such as a 10 and 11. The synthetic approach used in preparing analogues 10 and 11 is outlined in Scheme 1. The O-perbenzylated 1,5-dideoxy-1,5-imino-d-xylitol 12 was prepared according to a literature protocol41 and transformed in high yield to the corresponding per-acetylated species 16. Conversion of 12 to Boc-protected 13 was followed by removal of the benzyl groups via hydrogenation under acidic conditions to yield triol 14. Acetylation of 14 with acetic anhydride in pyridine gave 15 followed by treatment with trifluoroacetic acid to yield 16, which served as a common precursor in the preparation of acid sensitive GBA inhibitors 10 and 11. Two orthoester amines 18 and 19, bearing simple linear alkyl chains containing four and eight carbon atoms respectively, were synthesized according to a literature procedure.40,42 Treatment of 18 and 19 with CbzNCS43,44 gave thioureas 20 and 21, which provided a convenient means for incorporation of the DIX moiety. Activation of Cbz-protected thioureas 20 and 21 with EDCI followed by addition of 16 led to clean formation of protected guanidines 22 and 23. Interestingly, in the final deprotection step we observed simultaneous removal of both the Cbz and acetyl groups when performing the hydrogenation under basic conditions. In doing so fully deprotected orthoester-armed guanidine products 10 and 11 were obtained in high yields.45
Scheme 1. A) Synthetic route towards acetylated 1,5-dideoxy-1,5-imino-d-xylitol (16). B) Synthetic route for orthoester enriched guanidine compounds 10 and 11.
The inhibitory potencies of DIX derived guanidine–orthoesters 10 and 11 were determined against a panel of readily available glycosidase enzymes as well as the human recombinant enzymes, β-glucocerebrosidase (GBA) and β-galactocerebrosidase (GALC). Against the plant β-glucosidases screened, IC50 values in the low micromolar range were observed for both compounds 10 and 11. Conversely, no inhibition was seen with the other plant enzymes tested (Table 1). However, when evaluated against human recombinant glycosidase enzymes, both 10 and 11 showed inhibition of GBA and with no inhibition against GALC, indicating a high degree of selectivity among the human enzymes. Strikingly, very potent inhibition was observed against the human recombinant GBA for octyl analogue 11 with an inhibition constant that measured in the low nanomolar range (IC50(pH 7.0): 25 nM). In stark contrast, butyl analogue 10 resulted in a 100-fold weaker inhibitor of GBA (IC50(pH 7.0): 2561 nM) showing that the length of the alkyl appended to the orthoester moiety has a large effect on GBA inhibition as previously found for this enzyme46 and for the DIX series of compounds.37 Compounds 10 and 11 were both found to be very stable in neutral aqueous solution at room temperature with less than 3% degradation after 6 days. Next, we tested the compounds at an acidic pH so as to mimic the environment of the lysosome. Compounds 10 and 11 were preincubated at pH 5.2 for 24 hours and tested under general assay conditions. Gratifyingly, after treatment at acidic pH, both 10 and 11 readily underwent hydrolysis leading to complete loss of inhibitory activity (for detailed time-dependent inhibition studies and structures of the hydrolysis products see supplemental information). As a reference we also assessed the effect of pH on the activity of the commonly used GBA inhibitor NN-DNJ (2). This revealed that at pH 7.0, NN-DNJ has an IC50 against GBA of 532 nM, while at pH 5.2 the IC50 value increases approximately 10-fold. Not only is compound 11 a more potent inhibitor of GBA, it also displays a much more significant pH dependence, a key consideration in the development of pharmacological chaperones.
Table 1. Glycosidase inhibition values obtained for orthoester armed guanidines 10 and 11 a .
| Enzyme | Compound 10 | Compound 11 | NN-DNJ |
| α-glu b | >30 | >30 | >30 |
| α-gal c | >30 | >30 | >30 |
| β-glu d | 19.150 ± 0.536 | 14.570 ± 0.573 | >30 |
| β-gal e | >30 | >30 | >30 |
| Nar f | >30 | >30 | 0.085 ± 0.004 |
| GBA g (pH 7.0) | 2.561 ± 0.233 | 0.025 ± 0.003 | 0.532 ± 0.059 |
| GBA g (pH 5.2) | >30 | >30 | 5.584 ± 0.731 |
| GALC h | >30 | >30 | >30 |
aIC50 values are reported in μM and are averages obtained from triple independent duplicate analysis of each compound. For ease of comparison, the IC50 values obtained for all compounds shown in Table 1 are compared to a reference compound NNDNJ.
bα-Glucosidase (from baker's yeast, Sigma G5003): 0.05 U mL–1, the activity was determined with p-nitrophenyl-α-d-glucopyranoside (0.7 mM final conc. in well) in sodium phosphate buffer (100 mM, pH 7.2).
cα-Galactosidase (from green coffee beans, Sigma G8507): 0.05 U mL–1; α-galactosidase activity was determined with p-nitrophenyl-α-d-galactopyranoside (0.7 mM final conc. in well) in sodium phosphate buffer (100 mM, pH 6.8).
dβ-Glucosidase (from almond, Sigma G4511): 0.05 U mL–1; the activity was determined with p-nitrophenyl-β-d-glucopyranoside (0.7 mM final conc. in well) in sodium acetate buffer (100 mM, pH 5.0).
eβ-Galactosidase (from bovine liver, Sigma G1875): 0.05 U mL–1; activity was determined with p-nitrophenyl-β-d-galactopyranoside (0.7 mM final conc. in well) in sodium phosphate buffer (100 mM, pH 7.2).
fNaringinase (from penicillium decumbens, Sigma N1385): 0.06 U mL–1. The activity was determined with p-nitrophenyl-β-d-glucopyranoside (0.7 mM final conc. in well) in sodium acetate buffer (100 mM, pH 5.0).
gβ-Glucocerebrosidase (GBA) activities were determined using 4-methylumbelliferyl-β-d-glucopyranoside respectively using assay conditions based on those previously reported.53 Samples were preincubated in corresponding buffer for 24 hours before assay was performed.
hβ-Galactocerebrosidase (GALC) activities were determined using 4-methylumbelliferyl-β-d-galactopyranoside respectively using assay conditions based on those previously reported.53
The ability of compounds 10 and 11 to act as a pharmacological chaperone was next investigated. Since permeability is an important property of a successful chaperone, intact cells were used in the assay without lysing the cells. Previous findings suggest that minor increases in mutant GBA activity, caused by chemical chaperoning, may be clinically useful.47,48 Recent findings indicate that even doubling N370S activity may be sufficient to raise activity levels above the critical threshold for the development of disease.49 Assays using Gaucher patient-derived fibroblasts (homozygous for the most prevalent N370S mutation) indicate that 11 possesses a chaperone activity at least on par with that of NN-DNJ (Fig. 2).50 Furthermore, we observed a superior enhancement effect for 11 compared to that exhibited by the known pharmacological chaperone isofagomine (IFG, 3), although the low activity of IFG could be attributable to its poorer permeability.51,52 We also note that the more potent GBA binder 11 is indeed the better chaperone in comparison to 10. This chaperone effect of 11 is in contrast to our previous DIX derivatives, that were good GBA binders, but lacked the cleavable orthoester.37 When assays were performed with patient-derived fibroblasts homozygous for the L444P mutation, a mutation that is harder to rescue, compound 11 showed no significantly enhanced enzyme activity (see ESI,† Fig. S9).50 Taken together, our preliminary findings with Gaucher patient fibroblast are especially encouraging given that the two GBA mutations studied here have generally proven to be among the least responsive to chaperoning approaches.40
Fig. 2. The effect of compounds 10, 11, NNDNJ and IFG on GBA activity in N370S fibroblasts (GM00372) from Gaucher patients. Cells were cultured for 4 days in the absence or presence of increasing concentrations (nM) of the compounds before GBA activity was measured. Experiments were performed in two independent triplicate experiments, and each bar represents the mean ± SD. Enzyme activity is normalized to untreated cells, assigned a relative activity of 1.
In summary, we here report new iminosugar based glycosidase inhibitors bearing an exocyclic guanidinium moiety that is alkylated via an acid labile orthoester. Our investigations revealed that DIX-derived analogue 11 is a particularly potent and selective inhibitor of the human β-glycosidase GBA. Our findings indicate that the addition of the guanidinium moiety leads to more potent GBA inhibition compared to NN-DNJ or the orthoester-linked alkylated DNJ thioureas reported by the group of Ortiz Mellet.40 The inhibitory potency of the DIX analogues explored in our study are very dependent on the length of the alkyl substituent connected to the orthoester with compound 11 among the most potent GBA inhibitors reported to date. Importantly, while 11 was very active at neutral pH (IC50 25.2 ± 2.6 nM), complete inactivation was observed at pH 5.2. These findings suggest that such compounds may have potential for application as pharmacological chaperones in LSDs such as Gaucher disease. More comprehensive studies examining the pharmacological chaperone activities of compound 11 and other guanidino iminosugars bearing an orthoester-linked alkyl will be reported in due course.
Conflicts of interest
The authors declare no competing interests.
Supplementary Material
Acknowledgments
We acknowledge dr. Johan Kemmink for assistance with NMR characterization studies. No competing financial interests are declared. Financial support provided by the Slovenian Human Resources Development and Scholarship for Scientific Research (PhD grant to A. S.). The Utrecht Institute for Pharmaceutical Sciences (UIPS) and Utrecht University are also gratefully acknowledged for their support.
Footnotes
†Electronic supplementary information (ESI) available: Synthetic procedures and analytical data for all new compounds, supporting figures for enzymatic and biological assays. See DOI: 10.1039/c7md00480j
References
- Platt F. M. Nature. 2014;510:68–75. doi: 10.1038/nature13476. [DOI] [PubMed] [Google Scholar]
- Convertino M., Das J., Dokholyan N. V. ACS Chem. Biol. 2016;11:1471–1489. doi: 10.1021/acschembio.6b00195. [DOI] [PubMed] [Google Scholar]
- Parenti G., Andria G., Valenzano K. J. Mol. Ther. 2015;23:1138–1148. doi: 10.1038/mt.2015.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain S., Miller J. L., Harvey D. J., Gu Y., Rosenthal P. B., Zitzmann N., Mccauley J. W. J. Antimicrob. Chemother. 2015;70:136–152. doi: 10.1093/jac/dku349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennekes T., van den Berg R. J. B. H. N., Boot R. G., van der Marel G. A., Overkleeft H. S., Aerts J. M. F. G. Angew. Chem., Int. Ed. 2009;48:8848–8869. doi: 10.1002/anie.200902620. [DOI] [PubMed] [Google Scholar]
- Compain P. and Martin O. R., Iminosugars: From synthesis to therapeutic applications, Wiley, UK, 2007. [Google Scholar]
- Parenti G., Moracci M., Fecarotta S., Andria G. Future Med. Chem. 2014;6:1031–1045. doi: 10.4155/fmc.14.40. [DOI] [PubMed] [Google Scholar]
- Gao K., Zheng C., Wang T., Zhao H., Wang J., Wang Z., Zhai X., Jia Z., Chen J., Zhou Y., Wang W. Molecules. 2016;21:1600–1614. doi: 10.3390/molecules21111600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekioka T., Shibano M., Kusano G. Nat. Med. 1995;49:332–335. [Google Scholar]
- Parmeggiani C., Catarzi S., Matassini C., D'Adamio G., Morrone A., Goti A., Paoli P., Cardona F. ChemBioChem. 2015;16:2054–2064. doi: 10.1002/cbic.201500292. [DOI] [PubMed] [Google Scholar]
- Sanchez-Fernandez E. M., Garcia Fernandez J. M., Mellet C. O. Chem. Commun. 2016;52:5497–5515. doi: 10.1039/c6cc01564f. [DOI] [PubMed] [Google Scholar]
- Ghisaidoobe A. T., Van Den Berg R. J. B. H. N., Butt S. S., Strijland A., Donker-Koopman W. E., Scheij S., Van Den Nieuwendijk A. M. C. H., Koomen G., Van Loevezijn A., Leemhuis M., Wennekes T., Van Der Stelt M., Van Der Marel G. A., Van Boeckel C. A. A., Aerts J. M. F. G., Overkleeft H. S. J. Med. Chem. 2014;57:9096–9104. doi: 10.1021/jm501181z. [DOI] [PubMed] [Google Scholar]
- Hottin A., Wright D. W., Davies G. J., Behr J. B. ChemBioChem. 2015;16:277–283. doi: 10.1002/cbic.201402509. [DOI] [PubMed] [Google Scholar]
- Godin G., Compain P., Martin O. R., Ikeda K., Yu L., Asano N. Bioorg. Med. Chem. Lett. 2004;14:5991–5995. doi: 10.1016/j.bmcl.2004.09.086. [DOI] [PubMed] [Google Scholar]
- Patil N. T., John S., Sabharwal S. G., Dhavale D. D. Bioorg. Med. Chem. 2002;10:2155–2160. doi: 10.1016/s0968-0896(02)00073-1. [DOI] [PubMed] [Google Scholar]
- Pandey G., Kapur M., Khan M. I., Gaikwad S. M. Org. Biomol. Chem. 2003;1:3321–3326. doi: 10.1039/b307455b. [DOI] [PubMed] [Google Scholar]
- Zhu X., Sheth K. A., Li S., Chang H.-H., Fan J.-Q. Angew. Chem., Int. Ed. 2005;44:7450–7453. doi: 10.1002/anie.200502662. [DOI] [PubMed] [Google Scholar]
- Compain P., Martin O. R., Boucheron C., Godin G., Yu L., Ikeda K., Asano N. ChemBioChem. 2006;7:1356–1359. doi: 10.1002/cbic.200600217. [DOI] [PubMed] [Google Scholar]
- Brumshtein B., Aguilar-Moncayo M., García-Moreno M. I., Mellet O., García Fernández J. M., Silman I., Shaaltiel Y., Aviezer D., Sussman J. L. ChemBioChem. 2009;10:1480–1485. doi: 10.1002/cbic.200900142. [DOI] [PubMed] [Google Scholar]
- García-Moreno M. I., Díaz-Pérez P., Mellet O., García Fernández J. M. Chem. Commun. 2002:848–849. doi: 10.1039/b200162d. [DOI] [PubMed] [Google Scholar]
- Compain P. Synlett. 2014;25:1215–1240. [Google Scholar]
- Martin N. I., Woodward J. J., Marletta M. A. Org. Lett. 2006;8:4035–4038. doi: 10.1021/ol061454p. [DOI] [PubMed] [Google Scholar]
- Kooij R., Branderhorst H. M., Bonte S., Wieclawska S., Martin N. I., Pieters R. J. MedChemComm. 2013;4:387–393. [Google Scholar]
- Sevšek A., Čelan M., Erjavec B., Quarles van Ufford L., Sastre Toraño J., Moret E. E., Pieters R. J., Martin N. I. Org. Biomol. Chem. 2016;14:8670–8673. doi: 10.1039/c6ob01735e. [DOI] [PubMed] [Google Scholar]
- Fabrega S., Durand P., Codogno P., Bauvy C., Delomenie C., Henrissat B., Martin B. M., McKinney C., Ginns E. I., Mornon J. P., Lehn P. Glycobiology. 2000;10:1217–1224. doi: 10.1093/glycob/10.11.1217. [DOI] [PubMed] [Google Scholar]
- Zeller J. L. JAMA, J. Am. Med. Assoc. 2007;298:1358. doi: 10.1001/jama.298.11.1358. [DOI] [PubMed] [Google Scholar]
- Jmoudiak M., Futerman A. H. Br. J. Haematol. 2005;129:178–188. doi: 10.1111/j.1365-2141.2004.05351.x. [DOI] [PubMed] [Google Scholar]
- Brady R. O. Baillieres Clin. Haematol. 1997;10:621–634. doi: 10.1016/s0950-3536(97)80031-5. [DOI] [PubMed] [Google Scholar]
- Charrow J., Andersson H. C., Kaplan P., Kolodny E. H., Mistry P., Pastores G., Rosenbloom B. E., Scott C. R., Wappner R. S., Weinreb N. J., Zimran A. Arch. Intern. Med. 2000;160:2835–2843. doi: 10.1001/archinte.160.18.2835. [DOI] [PubMed] [Google Scholar]
- Pastores G. M., Patel M. J., Firooznia H. Curr. Rheumatol. Rep. 2000;2:175–180. doi: 10.1007/s11926-000-0059-x. [DOI] [PubMed] [Google Scholar]
- Beutler E. Acta Paediatr. 2006;95:103–109. doi: 10.1111/j.1651-2227.2006.tb02398.x. [DOI] [PubMed] [Google Scholar]
- Nash R. J., Kato A., Yu C.-Y., Fleet G. W. Future Med. Chem. 2011;3:1513–1521. doi: 10.4155/fmc.11.117. [DOI] [PubMed] [Google Scholar]
- Trapero A., Llebaria A. Future Med. Chem. 2013;5:573–590. doi: 10.4155/fmc.13.14. [DOI] [PubMed] [Google Scholar]
- Trapero A., Llebaria A. Future Med. Chem. 2014;6:975–978. doi: 10.4155/fmc.14.41. [DOI] [PubMed] [Google Scholar]
- Gavrin L. K., Denny R. A., Saiah E. J. Med. Chem. 2012;55:10823–10843. doi: 10.1021/jm301182j. [DOI] [PubMed] [Google Scholar]
- Fan J. Q. Biol. Chem. 2008;389:1–11. doi: 10.1515/BC.2008.009. [DOI] [PubMed] [Google Scholar]
- Sevšek A., Šrot L., Rihter J., Čelan M., van Ufford L. Q., Moret E. E., Martin N. I., Pieters R. J. ChemMedChem. 2017;12:483–486. doi: 10.1002/cmdc.201700050. [DOI] [PubMed] [Google Scholar]
- Wang G.-N., Reinkensmeier G., Zhang S.-W., Zhou J., Zhang L.-R., Zhang L.-H., Butters T. D., Ye X.-S. J. Med. Chem. 2009;52:3146–3149. doi: 10.1021/jm801506m. [DOI] [PubMed] [Google Scholar]
- Shayman J. A., Larsen S. D. J. Lipid Res. 2014;55:1215–1225. doi: 10.1194/jlr.R047167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mena-Barragán T., Narita A., Matias D., Tiscornia G., Nanba E., Ohno Kousaku, Suzuki Y., Higaki K., García Fernández J. M., Ortiz Mellet C. Angew. Chem., Int. Ed. 2015;54:11696–11700. doi: 10.1002/anie.201505147. [DOI] [PubMed] [Google Scholar]
- (a) Boot R. G., Verhoek M., Donker-Koopman W., Strijland A., Van Marle J., Overkleeft H. S., Wennekes T., Aerts J. M. F. G. J. Biol. Chem. 2007;282:1305–1312. doi: 10.1074/jbc.M610544200. [DOI] [PubMed] [Google Scholar]; (b) Wennekes T., Doctoral Thesis, Leiden University, 2008. [Google Scholar]
- Bruyère H., Westwell A. D., Jones A. T. Bioorg. Med. Chem. Lett. 2010;20:2200–2203. doi: 10.1016/j.bmcl.2010.02.035. [DOI] [PubMed] [Google Scholar]
- Martin N. I., Beeson W. T., Woodward J. J., Marletta M. A. J. Med. Chem. 2008;51:924–931. doi: 10.1021/jm701119v. [DOI] [PubMed] [Google Scholar]
- Martin N. I., Liskamp R. M. J. J. Org. Chem. 2008;73:7849–7851. doi: 10.1021/jo801517f. [DOI] [PubMed] [Google Scholar]
- Meier L., Monteiro G. C., Baldissera R. A. M., Sá M. M. J. Braz. Chem. Soc. 2010;21:859–866. [Google Scholar]
- Brumshtein B., Greenblatt H. M., Butters T. D., Shaaltiel Y., Aviezer D., Silman I., Futerman A. H., Sussman J. L. J. Biol. Chem. 2007;282:29052–29058. doi: 10.1074/jbc.M705005200. [DOI] [PubMed] [Google Scholar]
- Beutler E., Kuhl W., Vaughan L. M. Mol. Med. 1995;1:320–324. [PMC free article] [PubMed] [Google Scholar]
- Schueler U. H., Olter T. K., Kaneski C. R., Zirzow G. C. J. Inherited Metab. Dis. 2004;27:649–658. doi: 10.1023/b:boli.0000042959.44318.7c. [DOI] [PubMed] [Google Scholar]
- Sawkar A. R., Adamski-Werner S. L., Cheng W.-C., Wong C.-H., Beutler E., Zimmer K.-P., Kelly J. W. Chem. Biol. 2005;12:1235–1244. doi: 10.1016/j.chembiol.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Sawkar A. R., Cheng W.-C., Beutler E., Wong C.-H., Balch W. E., Kelly J. W. Proc. Natl. Acad. Sci. U. S. A. 2002;99:15428–15433. doi: 10.1073/pnas.192582899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steet R. A., Chung S., Wustman B., Powe A., Do H., Kornfeld S. A. Proc. Natl. Acad. Sci. U. S. A. 2006;103:13813–13818. doi: 10.1073/pnas.0605928103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Liou B., Xu Y. H., Quinn B., Zhang W., Hamler R., Setchell K. D. R., Grabowski G. A. J. Biol. Chem. 2012;287:4275–4287. doi: 10.1074/jbc.M111.280016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapero A., González-Bulnes P., Butters T. D., Llebaria A. J. Med. Chem. 2012;55:4479–4488. doi: 10.1021/jm300342q. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.