Abstract
Ultraviolet B (UVB) radiation induces the production of reactive oxygen species (ROS), resulting in the aging of dermal fibroblasts. Crocin, a bioactive constituent of Crocus sativus, possesses anti-oxidation effects. The purpose of the present study was to evaluate the protective effect of crocin on UVB-induced dermal fibroblast photoaging. Human dermal fibroblasts were isolated and cultured with different concentrations of crocin prior to and following exposure to UVB irradiation. The senescent phenotypes of cells were evaluated, including cell proliferation, cell cycle, senescence-associated β-galactosidase (SA-β-gal) expression, intracellular ROS, expression of antioxidant protein glutathione peroxidase 1 (GPX-1) and extracellular matrix protein collagen type 1 (Col-1). Crocin rescued the cell proliferation inhibited by UVB irradiation, prevented cell cycle arrest and markedly decreased the number of SA-β-gal-positive cells. In addition, crocin reduced UVB-induced ROS by increasing GPX-1 expression and other direct neutralization effects. Furthermore, crocin promoted the expression of the extracellular matrix protein Col-1. Crocin could effectively prevent UVB-induced cell damage via the reduction of intracellular ROS; thus, it could potentially be used in the prevention of skin photoaging.
Keywords: crocin, photoaging, reactive oxygen species, antioxidant, glutathione peroxidase 1
Introduction
Skin photoaging is a cosmetic concern worldwide, and is characterized by atrophy of the skin, coarse wrinkles and leathery skin (1). Sun exposure is the main factor leading to photoaging, primarily due to ultraviolet (UV) radiation. There are three categories of ultraviolet light according to its wavelength, including UVA (320–400 nm), UVB (290–320 nm) and UVC (200–290 nm) (2). UVB is considered to be the most important factor associated with skin photoaging (3). UVB radiation exposure can induce the production of reactive oxygen species (ROS) in dermal fibroblasts, including the hydroxyl free radical, superoxide anion, singlet oxygen and hydrogen peroxide (4). Increased ROS levels can damage dermal fibroblasts, the most important cell type in the dermis that produces extracellular matrix (ECM). Subsequently, the synthesis of ECM could be inhibited and degradation may be accelerated, resulting in skin aging (2). Antioxidants, including vitamins C and E, and coenzyme Q10, could inhibit and neutralize ROS; thus, they have been suggested for the treatment and prevention of skin photoaging (5).
Saffron has been used in traditional Chinese medicine for a number of years. It has been traditionally used for the treatment of many types of disease, including neurodegenerative disorders, coronary artery diseases, respiratory diseases and gastrointestinal diseases (6). The therapeutic effects of saffron are associated with some of its components. Crocin is one of the main and active constituents isolated from saffron. It has been shown previously that crocin exhibits multiple activities, including anti-cancer, anti-inflammatory and anti-oxidation effects in various cell types (7). Lv et al (8) reported that crocin exerts an anti-oxidative effect against H2O2-induced apoptosis in retinal ganglion cells.
Based on the anti-oxidant capacity of crocin, it was speculated that it may prevent UVB-induced skin aging. In the present study, the protective effects of crocin against UVB-induced damage were investigated in cultured human dermal fibroblasts.
Materials and methods
Preparation of crocin
Crocin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was dissolved in sterile PBS solution at a concentration of 10 mM, stored at −20°C and then freshly thawed at room temperature prior to each use.
Antioxidant effects of crocin
To test the antioxidant effects of crocin, 1,1-diphenyl-2-picrylhydrazyl (DPPH; Sigma-Aldrich; Merck KGaA) radical scavenging activity was measured as previously described (9). Reaction mixtures (200 µl) containing DPPH and a serial dilution of crocin (100–800 µM) were placed in a 96-well plate at room temperature in the dark for 30 min, then the absorbance was measured at 515 nm using a Varioskan Flash Spectral Scanning Multimode Reader (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The inhibition rate was determined using the following equation: Inhibition rate (%)=[1-(Asample/Acontrol)] ×100. Where A refers to the absorbance measured at 515 nm.
Cell isolation and culture
Dermal fibroblasts were isolated from human foreskin specimens. Samples were obtained from five donors (age, 6–12 years) who underwent a routine circumcision procedure at Shanghai 9th People's Hospital between January 2016 and January 2017. Written informed consent was obtained. The present study was approved by The Ethics Committee of Shanghai 9th People's Hospital, Shanghai Jiao Tong University School of Medicine (Shanghai, China). A single-cell suspension was obtained as previously described (10). Cells were then suspended in Dulbecco's modified Eagle's medium (Invitrogen; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum (HyClone; GE Healthcare Life Sciences, Logan, UT, USA), 300 µg/ml L-glutamine, 100 U/ml penicillin and 100 µg/ml streptomycin (all Sigma-Aldrich; Merck KGaA). Cells were maintained at 37°C in a humidified atmosphere containing 5% CO2 and passaged by trypsinization with 0.25% trypsin-EDTA (Gibco; Thermo Fisher Scientific, Inc.) every 3–5 days. Cells at passage 3–5 were used in the following experiments.
Crocin toxicity analysis
Fibroblasts were seeded in a 96-well plate at a density of 2,000 cells/well and treated with different concentrations of crocin (0, 12.5, 50 and 100 µM) and subsequently maintained at 37°C in a humidified atmosphere containing 5% CO2 for 72 h. Cell proliferation was then measured using a Cell Counting Kit-8 (CCK-8; Beyotime Institute of Biotechnology, Haimen, China), according to the manufacturer's instructions. Untreated cells served as the control.
UVB irradiation
Fibroblasts were cultured in 96-well (2,000 cells/well) or 6-well plates (1×105 cells/well) in the aforementioned supplemented DMEM mixture, and maintained at 37°C for 24 h. Following a further 24 h of incubation at 37°C with or without crocin (0, 12.5, 50 and 100 µM), culture medium was replaced with PBS. Cells were then exposed to UVB light (Philips 311 nm, TL 20W/01; Philips Lighting Holding B.V., Eindhoven, The Netherlands) at a total dose of 100 mJ/cm2. Following irradiation, the medium was replaced with culture medium with or without crocin, and cells were maintained at 37°C in a humidified atmosphere containing 5% CO2 for 24 or 72 h for further analyses. Cells treated with UVB irradiation and 150 µM vitamin C (Vit C; Sigma-Aldrich; Merck KGaA) served as a positive control. The following experiments were then performed.
Determination of cell proliferation
Cell proliferation was measured at 72 h post-irradiation using a CCK-8 kit (Beyotime Institute of Biotechnology), according to the manufacturer's instructions. Results are expressed as the relative cell proliferation (%) with respect to the control cells (cells without UVB irradiation or crocin treatment).
Cell cycle analysis
At 24 h post-UVB radiation, 5×105 cells were harvested by 0.25% Trypsin-EDTA and then fixed in 70% ethanol overnight at 4°C. Fixed cells were washed twice with PBS and then incubated with 1.5 mg/l RNase A (Sigma-Aldrich; Merck KGaA) for 1 h at 37°C, followed by staining with 5 µl of propidium iodide (Sigma-Aldrich; Merck KGaA) for 20 min on ice. DNA content was assessed using an Epics Altra Flow Cytometer (Beckman Coulter, Inc., Brea, CA, USA), and analyzed with Modi Fit LT v2.0 software (Verity Software House, Inc., Topsham, ME, USA).
β-galactosidase (SA-β-gal) staining
To measure the cell-aging rate, SA-β-gal staining was performed at 72 h post-irradiation using a Senescence β-Galactosidase staining kit purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA; cat. no. 9860). Cells were washed in PBS, fixed for 10 min at room temperature in 4% paraformaldehyde and stained according to manufacturer's instructions. Five random fields from each sample (n=3 samples/group) were selected to observe under a light microscope (Nikon Eclipse 90i; Nikon Corporation, Tokyo, Japan), and the number of SA-β-gal-positive cells was counted using Image-Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA). The aging rate was determined as the percentage of positive cells out of the total number of cells.
Measurement of intracellular ROS
Intracellular ROS levels were determined by 2′,7′-dichlorodihydrofluoresce in diacetate (DCFH2-DA; Sigma-Aldrich; Merck KGaA) staining. Briefly, immediately following UVB irradiation, cells were incubated with DCFH2-DA (10 mM) at 37°C for 20 min. Following this, half of the cells were observed under a fluorescent microscope (Olympus IX70-S1F2; Olympus Corporation, Tokyo, Japan), and the other half of the cells were collected and analyzed with an Epics Altra Flow Cytometer, as described previously (11).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis
Cells (2×106) were collected at 72 h post-irradiation. Total RNA was extracted using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.), and cDNA was synthesized from 2 µg total RNA using 200 U of reverse transcriptase (MMLV-RT) and 20 pM oligo dT (Promega Corporation, Madison, WI, USA) at 42°C for 1 h. The expression of pro-collagen I was determined by qPCR using SYBR Green PCR Master mix (Applied Biosystems, Thermo Fisher Scientific, Inc.). The thermocycling conditions were as follows: Initial denaturation at 95°C for 10 min, followed by 40 cycles at 95°C for 30 sec, 60°C for 30 sec and 72°C for 45 sec, using the Strata Gene Mx3000p (Agilent Technologies, Inc., Santa Clara, CA, USA). Expression was quantified using the 2−∆∆Cq method (12). The primers employed were as follows: Pro-collagen I, forward: 5′-CTCGAGGTGGACACCACCCT-3′ and reverse: 5′-CAGCTGGATGGCCACATCGG-3′. All amplifications were run in triplicate and the results were normalized to the housekeeping gene GAPDH, the primers of which were as follows: Forward: 5′-CAAAAGGGTCATCATCTCTG-3′ and reverse: 5′-CCTGCTTCACCACCTTCTTG-3′. Three independent experiments were performed.
Western blot analysis
Cells (2×106) were collected at 72 h post-irradiation. Total protein was extracted for western blot analysis and the expression of collagen type 1 (Col-1) and glutathione peroxidase 1 (GPX-1) was measured. Proteins were harvested and collected with radioimmunoprecipitation assay lysis buffer (Beyotime Institute of Biotechnology). Protein concentrations were determined with a bicinchoninic acid protein assay. Subsequently, proteins (20 µg/lane) were separated by 12% SDS-PAGE. Following electrophoresis at 100 V for 2 h, proteins were transferred to polyvinylidene difluoride membranes at 350 mA for 90 min. The membranes were blocked with 5% non-fat milk in Tris-buffered saline and Tween 20 (TBST) at room temperature for 2 h, followed by incubation with the following primary antibodies overnight at 4°C: Anti-Col-1 (cat. no. ab6308; 1:1,000; Abcam, Cambridge, UK), anti-GPX-1 (cat. no. ab108427; 1:2,000; Abcam) and β-actin (cat. no. 4970S; 1:2,000; Cell Signaling Technology, Inc.). The membranes were washed three times with TBST and subsequently incubated with horseradish peroxidase-conjugated goat anti-mouse secondary antibody (cat. no. 115-035-062; 1:2,000; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) at room temperature for 2 h. Protein expression levels were analyzed by visualizing the bands with enhanced chemiluminescence (Pierce, Rockford, USA). ImageJ 1.50i software (National Institutes of Health USA) was used for densitometry.
Statistical analysis
Data are expressed as the mean ± standard deviation. Unpaired t-tests were used for direct comparisons between the UVB and the UVB + crocin groups. For multiple comparisons, one-way analysis of variance followed by Tukey's post hoc test was performed. All analyses were performed using SPSS v.13.0 software (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Cytotoxicity of crocin
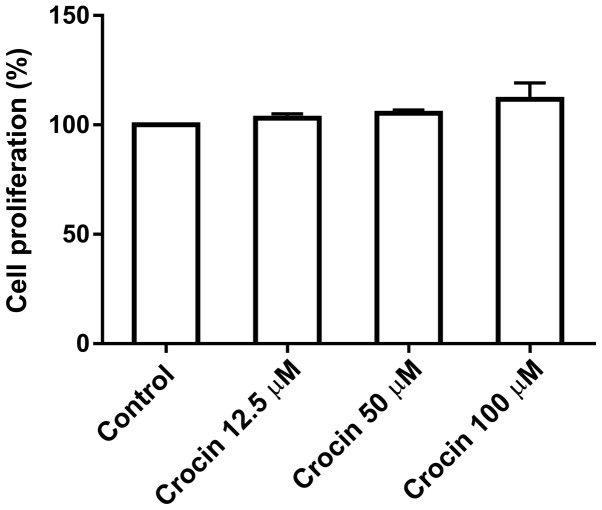
To test the potential cytotoxicity of crocin on dermal fibroblasts, cells were treated with various concentrations (0, 12.5, 50 and 100 µM) of crocin for 72 h prior to measuring the levels of proliferation using a CCK-8 assay. As shown in Fig. 1, crocin did not inhibit the proliferation of cells at the concentrations tested, up to 100 µM, indicating that crocin at this dose range may not be toxic to fibroblasts.
Figure 1.
Effects of crocin (0, 12.5, 50 and 100 µm) on the proliferation of dermal fibroblasts.
Crocin rescues the cell proliferation inhibited by UVB irradiation
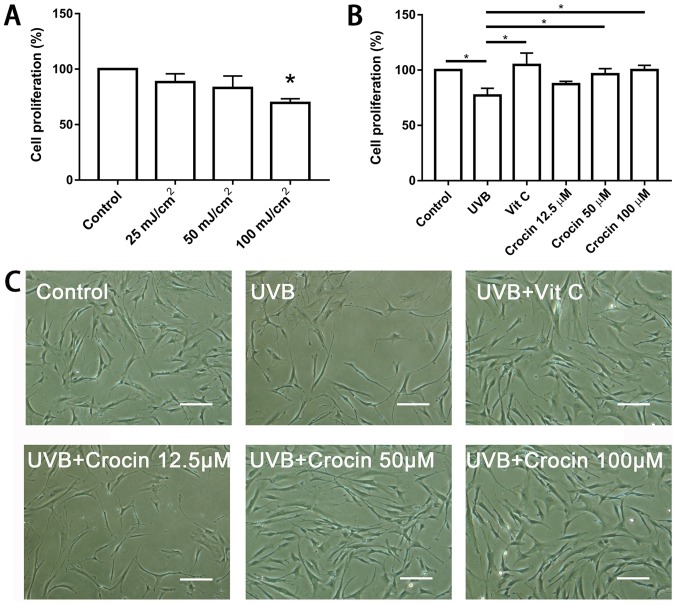
To determine the optimal dose of UVB irradiation, fibroblasts were exposed to a range of UVB doses (0, 25, 50 and 100 mJ/cm2) and a CCK-8 kit was used to measure cell proliferation following 72 h. UVB irradiation significantly inhibited cell proliferation at the dose of 100 mJ/cm2 (Fig. 2A). Therefore, 100 mJ/cm2 was selected for the subsequent experiments. Following pre-incubation with crocin (12.5, 50 and 100 µM) for 24 h, cells were irradiated by UVB and cell proliferation was measured following 72 h. High doses (50 and 100 µM) of crocin significantly rescued the cell proliferation inhibited by UVB irradiation (P<0.05), and the rate was similar to that following Vit C treatment (Fig. 2B and C).
Figure 2.
Crocin rescues the cell proliferation inhibited by UVB irradiation. (A) UVB irradiation inhibited fibroblast proliferation in a dose-dependent manner. *P<0.05 vs. control. (B) Crocin rescued the cell proliferation inhibited by UVB irradiation. *P<0.05, as indicated. (C) Morphology of fibroblasts at 72 h post-UVB irradiation. Scale bars, 150 µm. UVB, ultraviolet B; Vit C, vitamin C.
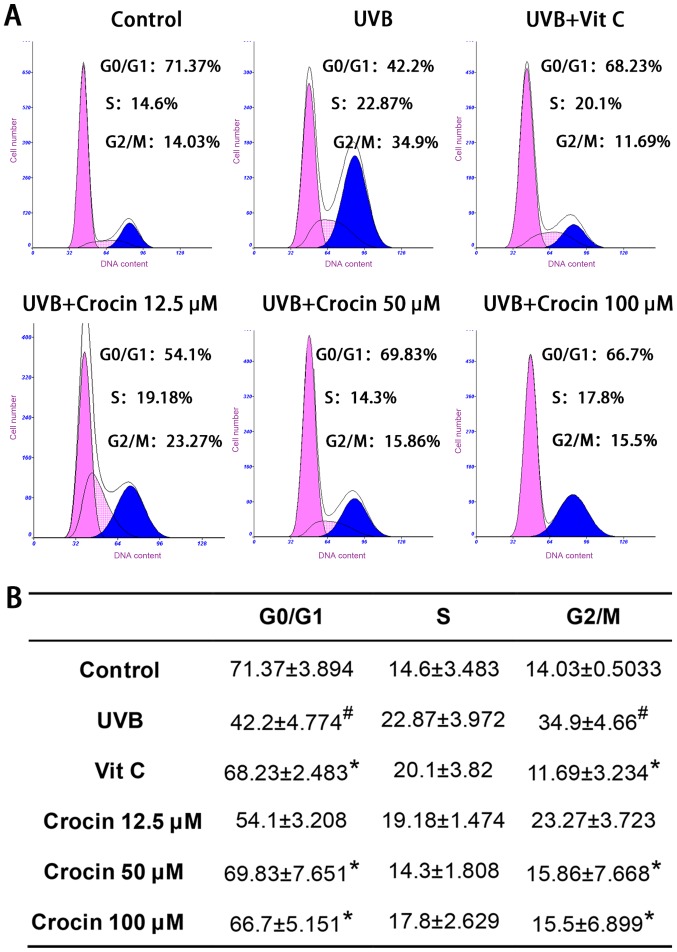
Crocin rescues UVB-induced cell cycle arrest
Flow cytometry analyses of the cell cycle are shown in Fig. 3. Cell populations in the G2/M phases were significantly increased following UVB irradiation, indicating that cells were arrested at the G2/M phase. As a positive control, Vit C treatment rescued cell cycle arrest as expected. High doses (50 and 100 µM) of crocin restored cell cycle arrest to levels similar to those of the control group (without UVB irradiation), indicating that crocin could rescue UVB-induced cell cycle arrest.
Figure 3.
Crocin rescues UVB-induced cell cycle arrest. (A) Representative histograms of the cell cycle analysis, as determined by flow cytometry. (B) Statistical analysis of cell cycle distribution from three independent experiments. #P<0.05 vs. control; *P<0.05 vs. UVB. UVB, ultraviolet B; Vit C, vitamin C.
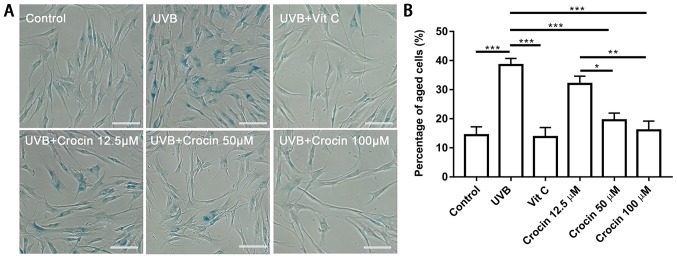
Crocin prevents UVB-induced cell aging
To determine whether crocin could prevent cells from UVB-induced photoaging, SA-β-gal staining was performed following UVB irradiation (Fig. 4A). A significant increase in SA-β-gal-positive cells was observed following UVB irradiation (Fig. 4). A decrease in SA-β-gal-positive cells was observed in the Vit C-treated group as well as in the high dose (50 and 100 µM) crocin-treated groups (Fig. 4). Statistical analysis confirmed that the percentage of SA-β-gal-positive cells was increased in the UVB group, and decreased following Vit C or crocin treatment (Fig. 4B).
Figure 4.
Crocin prevents UVB-induced cell aging. (A) Positive SA-β-gal staining indicated aging cells. Scale bars, 100 µm. (B) Percentage of SA-β-gal-positive cells was determined by counting 200 cells per dish. *P<0.05, **P<0.01 and ***P<0.001, as indicated. UVB, ultraviolet B; Vit C, vitamin C; SA-β-gal, β-galactosidase.
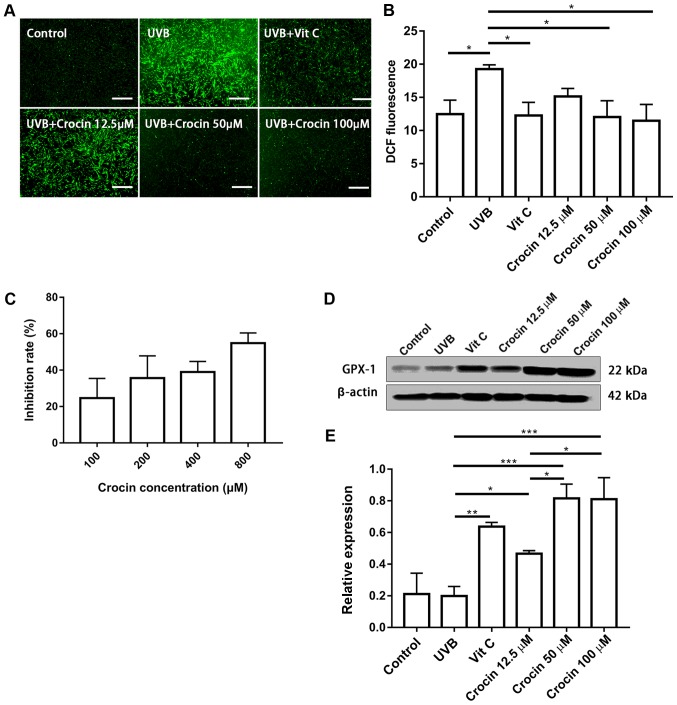
Crocin reduces UVB-induced intracellular ROS
An increase in ROS is one of the most important mechanisms in photoaging (4). In the present study, UVB irradiation significantly increased intracellular ROS when compared with the control group, while the ROS levels were reduced by Vit C and crocin treatment (Fig. 5A). These results were confirmed by flow cytometric analysis of the fluorescent intensity following DCFH2-DA staining (Fig. 5B). To verify the possible underlying mechanisms, free radical scavenging activity of crocin was measured. Crocin inhibited DPPH radial activity in a dose-dependent manner (Fig. 5C), indicating that it could partially neutralize ROS. In addition, the expression of the antioxidant protein GPX-1 was measured by western blot analysis. An increase in GPX-1 expression was observed in the Vit C and crocin-treated groups (Fig. 5D and E), indicating that crocin may reduce UVB-induced ROS, partially via the enhanced expression of antioxidant protein GPX-1.
Figure 5.
Crocin reduces UVB-induced intracellular ROS. (A) Intracellular ROS measured by DCFH2-DA staining at 20 min post-UVB irradiation. Scale bars, 500 µm. (B) Intracellular ROS measured by DCFH2-DA staining and flow cytometry. (C) Antioxidant effect of crocin measured by 1,1-diphenyl-2-picrylhydrazyl radical-scavenging activity. (D) Western blot analysis of (E) GPX-1 expression at 72 h post-UVB irradiation. *P<0.05, **P<0.01 and ***P<0.001, as indicated. UVB, ultraviolet B; Vit C, vitamin C; ROS, reactive oxygen species; GPX-1, glutathione peroxidase 1; DCFH2-DA, 2′,7′-dichlorodihydrofluoresce in diacetate.
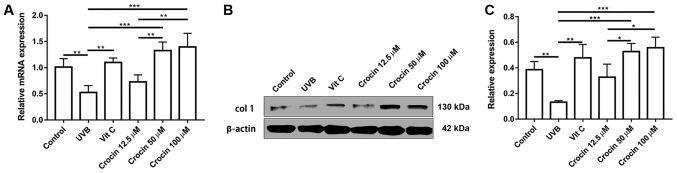
Crocin promotes the expression of ECM protein Col-1
Secretion of ECM is one of the major functions of dermal fibroblasts. A decrease in collagen secretion is commonly observed during skin aging (13). As shown in Fig. 6, a significant decrease in Col-1 expression was observed at the mRNA and protein level following UVB irradiation. In addition, Col-1 expression was upregulated by Vit C and crocin treatment at the mRNA and protein levels, suggesting that cell functions were also restored following Vit C and crocin treatment.
Figure 6.
Crocin promotes the expression of collagen type 1. (A) Reverse transcription-quantitative polymerase chain reaction analysis of pro-collagen I at 72 h post-UVB irradiation. (B) Western blotting analysis of (C) collagen type 1 at 72 h post-UVB irradiation. *P<0.05, **P<0.01 and ***P<0.001, as indicated. UVB, ultraviolet B; Vit C, vitamin C; col 1, collagen type 1.
Discussion
Extrinsic skin aging is caused by environmental factors including smoking, consuming alcohol, UV irradiation and common pollutants, which can lead to dermal fibroblast damage and aging (14,15). UVB is considered to be one of the most important factors that contributes towards aging (16). In the present study, the results confirmed that UVB irradiation induced the production of intracellular ROS, arrested the cell cycle, inhibited cell proliferation and downregulated ECM production. Antioxidants, such as Vit C, which scavenge ROS, are able to protect cells from UVB-induced damage (17).
Crocin, one of the main and active constituents of Crocus sativus, has been reported to have various bioactivities, including immunomodulatory, antitumor, anti-inflammation and antioxidant properties (18–22). Thus, it is not surprising that crocin could protect UVB-induced cell damage in cultured dermal fibroblasts, which was demonstrated by reduced intracellular ROS, rescued cell cycle and proliferation, and upregulated ECM production in the present study. One possible mechanism by which crocin may reduce intracellular ROS is by neutralizing ROS directly, as crocin could inhibit DPPH radical activity in a dose-dependent manner. Another potential mechanism could be that crocin upregulates antioxidant gene expression, which is supported by the upregulated expression of antioxidant protein GPX-1 following crocin treatment observed in the present study. However, an increased protective effect with 100 µM crocin treatment was not observed when compared with 50 µM crocin treatment. Intracellular ROS measurement revealed that ROS levels returned to basal (control) levels following 50 and 100 µM crocin treatment, indicating that 50 µM crocin may be sufficient to neutralize the UVB-induced ROS accumulation. Although GPX-1 expression was upregulated following crocin treatment, the regulatory pathway is still not clear. Therefore, it is worthy of further investigation in the future.
As a natural product, crocin has been widely used as a spice and a food colorant. Previous studies have demonstrated that crocin does not cause damage to any major organ in experimental animals (23,24). Mohamadpour et al (25) evaluated the safety of crocin in healthy volunteers and confirmed that it is relatively safe. In the present study, the proliferation of dermal fibroblasts was not affected by crocin treatment at the tested concentrations; in fact, slight stimulation of cell growth was observed following crocin treatment. These results strongly suggest that crocin could be used in the clinic without significant toxicity at pharmacological doses.
In the present study, the dose of UVB irradiation was optimized. Cells did not undergo apoptosis or necrosis at this exposure dose (data not shown), and the cell cycle was arrested at the G2/M phases. Cell senescence was observed by SA-β-gal staining, which produced similar results to those observed in previous studies (26–28). One limitation of the present study was that the protective effects of crocin against a higher dose of UVB-irradiation were not investigated. The anti-apoptotic potential of crocin has been demonstrated in previous studies (29,30). Jia et al (11) reported that crocin protected retinal pigment epithelial cells from H2O2-induced damage through the upregulation of the anti-apoptotic genes B-cell lymphoma 2 (Bcl-2) and Bcl-2-associated X protein. Thus, it is possible that crocin may exhibit protective effects at higher doses of irradiation via its anti-apoptotic activity. Another limitation of the present study was that cells were pre-treated with crocin 24 h prior to UVB irradiation. It remains to be investigated whether the application of crocin following irradiation would still protect the cells. Since crocin reduces intracellular ROS partially via the upregulation of antioxidant gene expression, it was speculated that the protective effects may be weaker than with pre-incubation due to the delayed expression of antioxidant genes. In future research, we aim to determine the anti-photoaging capacity of crocin in nude mice.
The protective effects of plant extracts on UVB-induced skin damage have been reported previously, including extracts from berries and herbs (9,31). In general, the plant extracts contain a mixture of active components. In the present study, it was demonstrated that a single compound with a definite chemical structure possesses anti-photoaging activity. Furthermore, it is superior to the plant extracts that can be industrially synthesized with better quality control. The protective effects of crocin on photoaging fibroblasts suggest that it has potential applications in the protection against UVB-induced skin photoaging.
Acknowledgements
The authors deeply appreciated the contribution of members of the Shanghai Key Laboratory of Tissue Engineering, National Tissue Engineering Center of China (Shanghai, China) for providing valuable suggestions and discussions.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study is included in this published article.
Authors' contributions
GZ, WL, YC and WZ conceived and designed the experiments. MD, DL and YZ performed the experiments. DL analyzed the data. MD, WZ and YC wrote and revised the manuscript.
Ethics approval and consent to participate
The present study was approved by The Ethics Committee of Shanghai 9th People's Hospital, Shanghai Jiao Tong University School of Medicine (Shanghai, China). Written informed consent was obtained from the donors.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Gilchrest BA. Photoaging. J Invest Dermatol. 2013;133:E2–E6. doi: 10.1038/skinbio.2013.176. [DOI] [PubMed] [Google Scholar]
- 2.Kammeyer A, Luiten RM. Oxidation events and skin aging. Ageing Res Rev. 2015;21:16–29. doi: 10.1016/j.arr.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Lee YR, Noh EM, Jeong EY, Yun SK, Jeong YJ, Kim JH, Kwon KB, Kim BS, Lee SH, Park CS, Kim JS. Cordycepin inhibits UVB-induced matrix metalloproteinase expression by suppressing the NF-kappaB pathway in human dermal fibroblasts. Exp Mol Med. 2009;41:548–554. doi: 10.3858/emm.2009.41.8.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poon F, Kang S, Chien AL. Mechanisms and treatments of photoaging. Photodermatol Photoimmunol Photomed. 2015;31:65–74. doi: 10.1111/phpp.12145. [DOI] [PubMed] [Google Scholar]
- 5.Stern RS. Clinical practice. Treatment of photoaging. N Engl J Med. 2004;350:1526–1534. doi: 10.1056/NEJMcp023168. [DOI] [PubMed] [Google Scholar]
- 6.Boskabady MH, Farkhondeh T. Antiinflammatory, antioxidant, and immunomodulatory effects of Crocus sativus L. and its main constituents. Phytother Res. 2016;30:1072–1094. doi: 10.1002/ptr.5622. [DOI] [PubMed] [Google Scholar]
- 7.Samarghandian S, Azimi-Nezhad M, Borji A, Farkhondeh T. Effect of crocin on aged rat kidney through inhibition of oxidative stress and proinflammatory state. Phytother Res. 2016;30:1345–1353. doi: 10.1002/ptr.5638. [DOI] [PubMed] [Google Scholar]
- 8.Lv B, Chen T, Xu Z, Huo F, Wei Y, Yang X. Crocin protects retinal ganglion cells against H2O2-induced damage through the mitochondrial pathway and activation of NF-κB. Int J Mol Med. 2016;37:225–232. doi: 10.3892/ijmm.2015.2418. [DOI] [PubMed] [Google Scholar]
- 9.Chiang HM, Lin TJ, Chiu CY, Chang CW, Hsu KC, Fan PC, Wen KC. Coffea arabica extract and its constituents prevent photoaging by suppressing MMPs expression and MAP kinase pathway. Food Chem Toxicol. 2011;49:309–318. doi: 10.1016/j.fct.2010.10.034. [DOI] [PubMed] [Google Scholar]
- 10.Chen FG, Zhang WJ, Bi D, Liu W, Wei X, Chen FF, Zhu L, Cui L, Cao Y. Clonal analysis of nestin(−) vimentin(+) multipotent fibroblasts isolated from human dermis. J Cell Sci. 2007;120:2875–2883. doi: 10.1242/jcs.03478. [DOI] [PubMed] [Google Scholar]
- 11.Jia C, Lu Y, Bi B, Chen L, Yang Q, Yang P, Guo Y, Zhu J, Zhu N, Liu T. Platelet-rich plasma ameliorates senescence-like phenotypes in a cellular photoaging model. Rsc Adv. 2017;7:3152–3160. doi: 10.1039/C6RA26725D. [DOI] [Google Scholar]
- 12.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 13.Brenneisen P, Sies H, Scharffetter-Kochanek K. Ultraviolet-B irradiation and matrix metalloproteinases: From induction via signaling to initial events. Ann N Y Acad Sci. 2002;973:31–43. doi: 10.1111/j.1749-6632.2002.tb04602.x. [DOI] [PubMed] [Google Scholar]
- 14.Bernhard D, Moser C, Backovic A, Wick G. Cigarette smoke-an aging accelerator? Exp Gerontol. 2007;42:160–165. doi: 10.1016/j.exger.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 15.Wlaschek M, Tantcheva-Poor I, Naderi L, Ma W, Schneider LA, Razi-Wolf Z, Schüller J, Scharffetter-Kochanek K. Solar UV irradiation and dermal photoaging. J Photochem Photobiol B. 2001;63:41–51. doi: 10.1016/S1011-1344(01)00201-9. [DOI] [PubMed] [Google Scholar]
- 16.Adil MD, Kaiser P, Satti NK, Zargar AM, Vishwakarma RA, Tasduq SA. Effect of Emblica officinalis (fruit) against UVB-induced photo-aging in human skin fibroblasts. J Ethnopharmacol. 2010;132:109–114. doi: 10.1016/j.jep.2010.07.047. [DOI] [PubMed] [Google Scholar]
- 17.Farris PK. Topical vitamin C: A useful agent for treating photoaging and other dermatologic conditions. Dermatol Surg. 2005;31:814–818. doi: 10.1111/j.1524-4725.2005.31725. [DOI] [PubMed] [Google Scholar]
- 18.Deslauriers AM, Afkhami-Goli A, Paul AM, Bhat RK, Acharjee S, Ellestad KK, Noorbakhsh F, Michalak M, Power C. Neuroinflammation and endoplasmic reticulum stress are coregulated by crocin to prevent demyelination and neurodegeneration. J Immunol. 2011;187:4788–4799. doi: 10.4049/jimmunol.1004111. [DOI] [PubMed] [Google Scholar]
- 19.Prieto MA, Vázquez JA, Murado MA. Crocin bleaching antioxidant assay revisited: Application to microplate to analyse antioxidant and pro-oxidant activities. Food Chem. 2015;167:299–310. doi: 10.1016/j.foodchem.2014.06.114. [DOI] [PubMed] [Google Scholar]
- 20.Du J, Chi Y, Song Z, Di Q, Mai Z, Shi J, Li M. Crocin reduces aspergillus fumigatus-induced airway inflammation and NF-κB signal activation. J Cell Biochem. 2018;119:1746–1754. doi: 10.1002/jcb.26335. [DOI] [PubMed] [Google Scholar]
- 21.Bolhassani A, Khavari A, Bathaie SZ. Saffron and natural carotenoids: Biochemical activities and anti-tumor effects. Biochim Biophys Acta. 2014;1845:20–30. doi: 10.1016/j.bbcan.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Xu GL, Li G, Ma HP, Zhong H, Liu F, Ao GZ. Preventive effect of crocin in inflamed animals and in LPS-challenged RAW 264.7 cells. J Agric Food Chem. 2009;57:8325–8330. doi: 10.1021/jf901752f. [DOI] [PubMed] [Google Scholar]
- 23.Hosseinzadeh H, Jahanian Z. Effect of Crocus sativus L. (saffron) stigma and its constituents, crocin and safranal, on morphine withdrawal syndrome in mice. Phytother Res. 2010;24:726–730. doi: 10.1002/ptr.3011. [DOI] [PubMed] [Google Scholar]
- 24.Hosseinzadeh H, Karimi G, Niapoor M. Antidepressant effects of Crocus sativus L. stigma extracts and its constituents, crocin and safranal, in mice. Acta Horticulturae. 2004;650:435–445. doi: 10.17660/ActaHortic.2004.650.54. [DOI] [Google Scholar]
- 25.Mohamadpour AH, Ayati Z, Parizadeh MR, Rajbai O, Hosseinzadeh H. Safety evaluation of crocin (a constituent of saffron) tablets in healthy volunteers. Iran J Basic Med Sci. 2013;16:39–46. [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang JA, Yin Z, Ma LW, Yin ZQ, Hu YY, Xu Y, Wu D, Permatasari F, Luo D, Zhou BR. The protective effect of baicalin against UVB irradiation induced photoaging: An in vitro and in vivo study. PLoS One. 2014;9:e99703. doi: 10.1371/journal.pone.0099703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park J, Seok JK, Suh HJ, Boo YC. Gardenia jasminoides extract attenuates the UVB-induced expressions of cytokines in keratinocytes and indirectly inhibits matrix metalloproteinase-1 expression in human dermal fibroblasts. Evid Based Complement Alternat Med. 2014;2014:429246. doi: 10.1155/2014/429246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeng Q, Zhou F, Lei L, Chen J, Lu J, Zhou J, Cao K, Gao L, Xia F, Ding S, et al. Ganoderma lucidum polysaccharides protect fibroblasts against UVB-induced photoaging. Mol Med Rep. 2017;15:111–116. doi: 10.3892/mmr.2016.6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.El-Maraghy SA, Rizk SM, Shahin NN. Gastroprotective effect of crocin in ethanol-induced gastric injury in rats. Chem Biol Interact. 2015;229:26–35. doi: 10.1016/j.cbi.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 30.Thushara RM, Hemshekhar M, Santhosh MS, Jnaneshwari S, Nayaka SC, Naveen S, Kemparaju K, Girish KS. Crocin, a dietary additive protects platelets from oxidative stress-induced apoptosis and inhibits platelet aggregation. Mol Cell Biochem. 2013;373:73–83. doi: 10.1007/s11010-012-1476-7. [DOI] [PubMed] [Google Scholar]
- 31.Bravo K, Duque L, Ferreres F, Moreno DA, Osorio E. Passiflora tarminiana fruits reduce UVB-induced photoaging in human skin fibroblasts. J Photochem Photobiol B. 2017;168:78–88. doi: 10.1016/j.jphotobiol.2017.01.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study is included in this published article.